Abstract
Background
ST‐segment–elevation myocardial infarction is associated with an intense acute inflammatory response and risk of heart failure. We tested whether interleukin‐1 blockade with anakinra significantly reduced the area under the curve for hsCRP (high sensitivity C‐reactive protein) levels during the first 14 days in patients with ST‐segment–elevation myocardial infarction (VCUART3 [Virginia Commonwealth University Anakinra Remodeling Trial 3]).
Methods and Results
We conducted a randomized, placebo‐controlled, double‐blind, clinical trial in 99 patients with ST‐segment–elevation myocardial infarction in which patients were assigned to 2 weeks treatment with anakinra once daily (N=33), anakinra twice daily (N=31), or placebo (N=35). hsCRP area under the curve was significantly lower in patients receiving anakinra versus placebo (median, 67 [interquartile range, 39–120] versus 214 [interquartile range, 131–394] mg·day/L; P<0.001), without significant differences between the anakinra arms. No significant differences were found between anakinra and placebo groups in the interval changes in left ventricular end‐systolic volume (median, 1.4 [interquartile range, −9.8 to 9.8] versus −3.9 [interquartile range, −15.4 to 1.4] mL; P=0.21) or left ventricular ejection fraction (median, 3.9% [interquartile range, −1.6% to 10.2%] versus 2.7% [interquartile range, −1.8% to 9.3%]; P=0.61) at 12 months. The incidence of death or new‐onset heart failure or of death and hospitalization for heart failure was significantly lower with anakinra versus placebo (9.4% versus 25.7% [P=0.046] and 0% versus 11.4% [P=0.011], respectively), without difference between the anakinra arms. The incidence of serious infection was not different between anakinra and placebo groups (14% versus 14%; P=0.98). Injection site reactions occurred more frequently in patients receiving anakinra (22%) versus placebo (3%; P=0.016).
Conclusions
In patients presenting with ST‐segment–elevation myocardial infarction, interleukin‐1 blockade with anakinra significantly reduces the systemic inflammatory response compared with placebo.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01950299.
Keywords: acute myocardial infarction, interleukin‐1, heart failure, ST‐segment–elevation myocardial infarction
Subject Categories: Inflammation, Ischemia, Myocardial Infarction, Heart Failure
Clinical Perspective
What Is New?
Interleukin‐1 blockade significantly reduced the acute inflammatory response in patients with ST‐segment–elevation myocardial infarction.
This reduction in inflammatory signaling was accompanied by significant reductions in new‐onset heart failure and hospitalization for heart failure.
What Are the Clinical Implications?
Targeted anti‐inflammatory treatment with interleukin‐1 blockers may be a novel approach to reduce the risk of heart failure after ST‐segment–elevation myocardial infarction.
ST‐segment–elevation myocardial infarction (STEMI) remains a major cause of morbidity and mortality worldwide. Despite considerable advances in STEMI treatment, up to 30% of survivors develop heart failure (HF) and 10% are hospitalized for HF within 1 year.1, 2, 3, 4 This observation suggests that the current treatment paradigm misses ≥1 key pathophysiologic mechanisms. A close interplay exists between inflammation, healing after STEMI, and progression to HF. Acute myocardial ischemia and infarction initiate an intense inflammatory response.5 The degree of the inflammatory response in STEMI is a strong predictor of adverse cardiac remodeling independent of infarct size.5, 6 Likewise, in patients with STEMI, the intensity of the inflammatory response, reflected in levels of circulating biomarkers, predicts adverse cardiac remodeling, HF, and death. 7 Modulation of the inflammatory response, therefore, represents a potential target for intervention. Although many previous attempts to target inflammation have failed,5 interleukin‐1 blockade is a novel and substantially different approach to modulating the inflammatory response.8 Preclinical studies have shown that anakinra, a recombinant interleukin‐1 receptor antagonist, improves myocardial healing, preventing additional cardiomyocyte loss, adverse remodeling, and HF in experimental myocardial infarction.9 Two pilot clinical trials showed preliminary safety and feasibility of treatment with recombinant interleukin‐1 receptor antagonist, anakinra, in STEMI (VCUART [Virginia Commonwealth University Anakinra Remodeling Trial]10 and VCUART211, 12). Moreover, the CANTOS (Canakinumab Atherothrombosis Outcome Trial) showed prevention of recurrent ischemic events and of hospitalizations for HF in patients with prior myocardial infarction treated with canakinumab, an interleukin‐1β blocking antibody.13, 14
The objective of this study was to determine whether anakinra, 100 mg once daily (standard dose) or twice daily (high dose), significantly reduced systemic inflammation (versus placebo) in patients with STEMI (primary end point) and whether this treatment resulted in better preservation of left ventricular (LV) systolic function and/or reduced incidence of HF events (secondary end points).
Methods
The study design and results of the study are posted online on https://clinicaltrials.gov/ct2/show/NCT01950299.
Trial Design
The VCUART3 was a phase 2, multicenter, double‐blinded, randomized, placebo‐controlled clinical trial comparing anakinra, 100 mg once daily (standard dose), alternated with placebo, once daily, every 12 hours, 100 mg twice daily (high dose), or placebo twice daily (in 1:1:1 ratio). The study design has been previously published.15 The trial protocol (available online) and all subsequent amendments received approval by the local Institutional Review Boards.
Screening and Enrollment
We screened consecutive patients, aged ≥21 years, who presented to the hospital with acute STEMI and underwent urgent coronary angiography within 12 hours of symptom onset at the Virginia Commonwealth University (VCU) Health (Richmond, VA), Virginia Cardiovascular Specialists (Richmond, VA), or Medstar Washington Hospital Center (Washington, DC). All subjects provided written informed consent in accordance with the local Institutional Review Board. Patients were excluded from the study if they had contraindications to treatment with anakinra, chronic inflammatory or infectious disease, or preexisting structural or functional severe cardiac abnormalities (Table S1 includes a complete list of inclusion and exclusion criteria).
Randomization and Masking
Anakinra and identical matching placebo syringes were handled by the investigational pharmacy at the coordinating center (VCU). A randomization log was prepared and maintained at the investigational pharmacy at VCU. The syringes were sequentially numbered from 1 to 28 to maintain allocation concealment, and patients were instructed to inject the syringes sequentially so to alternate odd‐numbered syringes in the morning and even‐numbered syringes in the evening. The investigational drug was prepared at VCU and shipped to the other centers in blocks of 5.
Investigational Treatment
Patients were randomly assigned to receive anakinra, 100 mg once daily (standard dose), alternating with placebo, once daily every 12 hours for 14 days; anakinra, 100 mg twice daily, every 12 hours (high dose) for 14 days; or placebo twice daily every 12 hours for 14 days, with the first dose administered within 12 hours of coronary angiography. Patients in the study received guideline‐based medical treatments, as indicated. Compliance was defined as >80% of prescribed doses of investigational drug.
Laboratory Analysis and Biomarkers
Blood samples were used for a complete blood cell count with differential, comprehensive metabolic profile, and plasma levels of hsCRP (high sensitivity C‐reactive protein) (True Health Diagnostics, Richmond, VA). All study personnel were kept blinded to hsCRP levels.
Creatine kinase myocardial band (CK‐MB) levels were determined by direct chemiluminescent technology every 6 hours starting immediately after percutaneous coronary intervention until peak, according to the local clinical standard. We estimated infarct size on the basis of the time‐concentration area under the curve (AUC) of CK‐MB.16, 17 CK‐MB peak value was defined as the maximal CK‐MB level measured during hospitalization. For patients with <6 recorded CK‐MB measurements, we estimated AUC for CK‐MB by a validated log‐normal model incorporating the admission CK‐MB level, the CK‐MB peak level, and the time to CK‐MB peak to reconstruct the typical time course of CK‐MB curve.16, 17 The time to CK‐MB peak, as a measure of duration of myocardial injury, was considered as the time between the initial onset of symptoms (chest pain per patient report) and the CK‐MB peak level.
Doppler Echocardiography
Subjects underwent a transthoracic Doppler echocardiogram within 24 hours of enrollment and at 1‐year follow‐up, as part of their clinical care and as previously described.10, 11, 15 Paired measurements of LV end‐diastolic and end‐systolic volumes and calculation of LV ejection fraction occurred off‐line at the end of the study by a core laboratory with 2 separate operators blinded to group allocations. The average of the 2 measurements was used for measures differing ≤10%, whereas those with >10% difference were rereviewed by a third operator and discussed to achieve consensus.
Primary and Secondary End Points
The primary end point was the AUC for hsCRP, measured at baseline, 72 hours, and day 14. hsCRP is an established prognostic marker in STEMI that reflects systemic interleukin‐1 activity.18 Measuring AUC for hsCRP allowed for integration of measurements across multiple time points to more accurately quantify the acute inflammatory response. The primary end point analysis compared both anakinra groups with placebo (P for significance set at <0.05), followed by a comparison of the anakinra, 100 mg twice daily, group with the placebo group and the anakinra, 100 mg daily, group with the placebo group, separately (P for significance set at <0.025).
Secondary end points included interval changes in LV end‐systolic volume and ejection fraction between baseline and 12 months and the incidence of HF. An independent committee composed of physicians specializing in internal medicine, cardiology, or emergency medicine, who were blinded to treatment allocation and to hsCRP data and not involved in the conduct of the study procedures, adjudicated all events (Protocol). Prespecified HF end points included the combined incidence of death and new onset of HF (defined as hospitalization for HF or need for a new initiation of a loop diuretic in the appropriate clinical setting; D+HF) and the composite of all‐cause death and hospitalization for HF (D+HHF), as specified in a consensus document on the definition of HF end points in STEMI trials.19 Additional adjudicated events included recurrent myocardial infarction, urgent revascularization, and severe infections.
Safety Assessment
All patients underwent a complete physical examination and clinical evaluation at each visit. A Data and Safety Monitoring Board, composed of cardiologists, a general internal medicine specialist, and an infectious disease specialist, reviewed study progress and safety data every 6 months (Protocol).
Sample Size and Statistical Analysis
The sample size for this study was calculated on the basis of the primary outcome of comparing hsCRP‐AUC with anakinra (both groups) versus placebo, to be followed (if significant) by an analysis of each anakinra group (standard and high dose) versus placebo. Given an expected hsCRP‐AUC of 350±250 mg·day/L for placebo‐treated patients with STEMI and 175±150 mg·day/L for anakinra (standard dose), 33 patients per group would provide >99% power (α=0.05) to detect a difference versus placebo and 85% power (2‐tailed α 0.025 considering multiple testing) to detect a further 50% reduction in the anakinra (high dose) (estimated AUC for hsCRP of 88±75 mg·day/L) versus anakinra (standard dose). A 20% loss to follow‐up or withdrawal was expected to retain >80% power for all analyses. No missing data imputation was used.
For statistical analysis, all values are reported as the median and interquartile range for potential deviation from gaussian distribution. All analyses were performed on an intention‐to‐treat basis without imputation for missing data. The differences between treatment groups were computed using the Wilcoxon or Kruskal‐Wallis rank‐sum test for 2 groups (anakinra [both groups] versus placebo) or 3 groups, respectively, for continuous variables. The differences in interval changes between the treatments were compared using random‐effect ANOVA for repeated measures to analyze the effects of time and group allocation. Unadjusted P values are reported throughout, with statistical significance set at the 2‐tailed P of 0.05 for the comparison of both anakinra groups versus placebo and of 0.025 for analysis of each dose group versus placebo. Kaplan‐Meier curves for survival free of HF events between anakinra (combined) and placebo were constructed and compared using the log‐rank (Mantel‐Cox) test. The χ2 or Fisher exact tests were used for discrete variables. All analyses were completed using the Statistical Package for Social Sciences, version 24.0 (SPSS, Chicago, IL). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Screening and Randomization
Between July 2014 and December 2017, 311 patients were screened and 99 were randomized to anakinra once daily (standard dose; N=33 [33%]), anakinra twice daily (high dose; N=31 [31%]), or placebo (N=35 [35%]). The main reasons for exclusion were patient preference (N=53), inability to provide consent (N=39), active or chronic infection (N=27), or active or recent cancer (N=23; Figure 1). All 99 patients (100%) received at least 1 dose of investigational drug, and 77 (78%) had complete biomarker data available for baseline, in‐hospital, and 2‐week follow‐up, allowing for the assessment of the primary end point of hsCRP‐AUC (Figure 1). Clinical follow‐up was available for all 99 subjects (100%), ranging from 3 to 365 days, with a median follow‐up duration of 365 (interquartile range, 287–365) days, with 71 (72%) with 365 days of follow‐up and 87 (88%) with >180 days of follow‐up.
Figure 1.
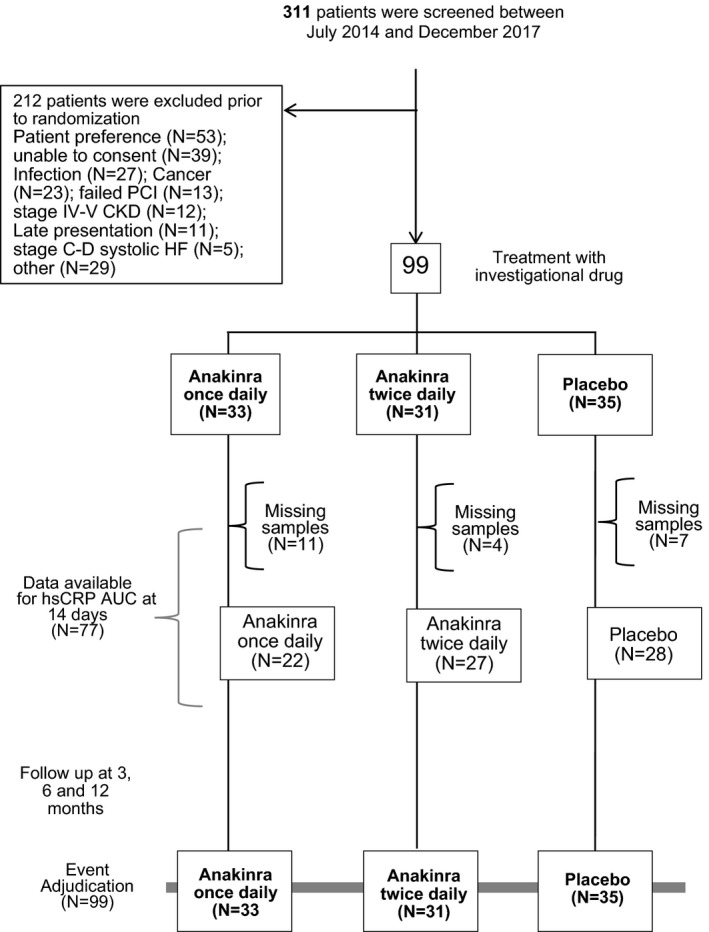
Enrollment, randomization, and follow‐up. AUC indicates area under the curve; CKD, chronic kidney disease; hsCRP, high‐sensitivity C‐reactive protein; HF, heart failure; PCI, percutaneous coronary intervention.
Characteristics of the Patients
Table 1 summarizes the clinical and demographic characteristics of the patients. Guideline‐directed therapies at time of enrollment and at the end of the study are shown in Table S2. There were no clinically meaningful differences between the groups. The estimated infarct size using the AUC for CK‐MB was not statistically different comparing the different groups (Table 1). The number of patients who completed all 14 days of treatment was not different between the groups (24 [73%], 27 [87%], and 24 [69%] for anakinra once daily, anakinra twice daily, and placebo, respectively; P=0.19).
Table 1.
Characteristics of the Patients at Baseline
| Characteristics | Anakinra, Once Daily (N=33) | Anakinra, Twice Daily (N=31) | Placebo (N=35) |
|---|---|---|---|
| Age, y | 53 (49–62) | 55 (45–61) | 56 (51–65) |
| Female sex | 9 (27) | 5 (16) | 5 (14) |
| White/black/Hispanic/other | 19:12:2:3 | 17:9:2:3 | 21:6:3:5 |
| Symptom onset to PCI time, min | 210 (128–280) | 145 (88–435) | 180 (130–347) |
| Symptom onset to investigational drug administration, min | 509 (405–644) | 450 (300–745) | 529 (403–716) |
| Fibrinolytic use before PCI | 1 (3) | 4 (13) | 3 (9) |
| Culprit vessel | |||
| LAD occlusion | 13 (39) | 13 (42) | 11 (30) |
| RCA occlusion | 12 (36) | 13 (42) | 18 (50) |
| LCX occlusion | 7 (21) | 5 (16) | 6 (17) |
| SVG occlusion | 1 (3) | 0 | 1 (3) |
| TIMI flow grade 0/1 pre‐PCI | 30 (91) | 27 (87) | 25 (71) |
| TIMI flow grade 3 post‐PCI | 33 (100) | 31 (100) | 35 (100) |
| PCI type | |||
| Primary PCI | 32 (97) | 27 (87) | 32 (91) |
| PCI after fibrinolysis | 1 (3) | 4 (13) | 3 (9) |
| Coronary stent implantation | 28 (85) | 30 (97) | 35 (100) |
| Use of drug‐eluting stent | 19 (57) | 25 (81) | 30 (86) |
| Use of thrombectomy | 6 (18) | 4 (13) | 6 (17) |
| Use of a P2Y12 inhibitor | 33 (100) | 31 (100) | 35 (100) |
| Clopidogrel | 5 (15) | 4 (13) | 7 (20) |
| Prasugrel | 10 (30) | 12 (39) | 12 (34) |
| Ticagrelor | 18 (55) | 15 (48) | 16 (46) |
| Initial CK‐MB level, ng/mL | 5 (2–21) | 11 (3–58) | 7 (2–28) |
| Peak CK‐MB level, ng/mL | 100 (30–205) | 134 (80–282) | 93 (36–252) |
| CK‐MB AUC at 14 d, ng/mL*d | 2054 (957–3370) | 3051 (1493–4694) | 2351 (765–4668) |
| Time to peak, min | 493 (321–740) | 450 (343–603) | 446 (281–623) |
| Previous diagnosis of CAD | 6 (18) | 8 (26) | 7 (20) |
| Diabetes mellitus | 6 (18) | 9 (29) | 15 (43) |
| Systemic arterial hypertension | 13 (39) | 20 (64) | 23 (66) |
| LVEF (initial assessment), % | 51 (43–58) | 48 (43–58) | 53 (42–57) |
Data are presented as number (percentage) or median (interquartile range) for categorical or continuous variables, respectively. AUC indicates area under the curve; CAD, coronary artery disease; CK‐MB, creatine kinase myocardial band; LAD, left anterior descending artery; LCX, left circumflex artery; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SVG, saphenous venous graft; TIMI, Thrombolysis in Myocardial Infarction.
AUC of hsCRP During the First 14 Days After STEMI
The hsCRP‐AUC values during the first 14 days were available in 22 (67%), 27 (87%), and 28 (80%) of the anakinra once daily, anakinra twice daily, and placebo groups, respectively, without significant difference in the incidence of missing data between groups (P=0.15 comparing the 3 groups, and P=0.80 comparing pooled anakinra group versus placebo). When compared with placebo, the hsCRP‐AUC value for the first 14 days was significantly lower in the pooled anakinra group (both doses combined) versus the placebo group (median, 67 [interquartile range, 39–120] versus 214 [interquartile range, 131–394] mg·day/L; P<0.001; Figure 2, Figure S1). Each anakinra arm analyzed individually versus placebo significantly reduced hsCRP‐AUC (P<0.001) without significant differences between the 2 anakinra arms (median, 60 [interquartile range, 24–139] versus 86 [interquartile range, 43–123] mg·day/L, for once and twice daily anakinra, respectively; P=0.41; Figure 2, Figure S1). The most common reason for missing data was related to lack of blood sample at 72 hours or discharge.
Figure 2.
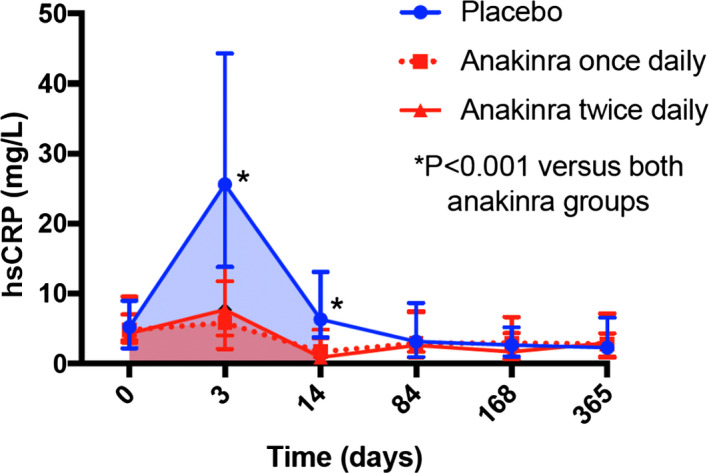
Effects of anakinra on hsCRP (high‐sensitivity C‐reactive protein). Anakinra, once daily or twice daily, significantly reduced the area under the curve for hsCRP at 14 days (shaded areas) (P<0.001 for each anakinra group vs placebo, and P<0.001 for anakinra groups combined vs placebo). We found no significant difference between the once‐daily and twice‐daily anakinra regimens (P=0.41). Data are presented as median and interquartile range.
Effects on LV Dimensions and Systolic Function
Paired echocardiography studies were available in 63 of 99 patients (64%) at a median of 362 days (interquartile range, 336–375 days) after the baseline study: 24 of 35 (69%) placebo patients and 39 of 64 (60%) anakinra patients (both doses combined). Table S3 shows the characteristics of the subjects with paired echocardiographic data. Baseline LV end‐diastolic volume, LV end‐systolic volume, stroke volume, and LV ejection fraction were not significantly different comparing placebo and anakinra (all P>0.05). No significant differences were found between anakinra and placebo groups in the interval changes in LV end‐systolic volume (median, 1.4 [interquartile range, −9.8 to 9.8] versus −3.9 [interquartile range, −15.4 to 1.4] mL; P=0.21) or LV ejection fraction (median, 3.9% [interquartile range, −1.6% to 10.2%] versus 2.7% [interquartile range, −1.8% to 9.3%]; P=0.61).
Effects on Clinical Events
There was 1 death (3%) in the placebo group and none in the anakinra group (Table 2). Compared with placebo, treatment with anakinra was associated with a significant reduction versus placebo in the incidence of the composite end point of all‐cause death and new‐onset or worsening HF (6/64 [9.4%] versus 9/35 [25.7%]; log‐rank χ2=3.995; P=0.046), as well as a reduction in the composite end point of all‐cause death and hospitalization for HF (0/64 [0%] versus 4/35 [11.4%]; log‐rank χ2=6.516; P=0.014), without any significant difference between the 2 anakinra arms (Table 2, Figure 3, Figure S2).
Table 2.
Safety and Efficacy Outcomes
| Outcome | Anakinra, Once Daily (N=33) | Anakinra, Twice Daily (N=31) | Placebo (N=35) | P Value Between Groups | Anakinra Combined (N=64) | P Value Versus Placebo |
|---|---|---|---|---|---|---|
| Death | 0 | 0 | 1 (3) | 0.40 | 0 | 0.17 |
| Death or hospitalization for heart failure | 0 | 0 | 4 (11) | 0.022a | 0 | 0.014a |
| Death or new‐onset or worsening heart failure (including outpatient and hospitalization) | 3 (9) | 3 (10) | 9 (26) | 0.09 | 6 (9) | 0.041a |
| Death, recurrent acute myocardial infarction, or urgent revascularization | 5 (15) | 1 (3) | 5 (14) | 0.24 | 6 (9) | 0.51 |
| Death or stroke | 1 (3) | 1 (3) | 1 (3) | 0.99 | 2 (3) | 0.95 |
| Sepsis or serious infection | 3 (9) | 6 (19) | 5 (14) | 0.24 | 9 (14) | 0.98 |
| Death or serious infections | 3 (9) | 6 (19) | 5 (14) | 0.24 | 9 (14) | 0.98 |
| Injection site reactions | 6 (18) | 8 (26) | 1 (3) | 0.029a | 14 (22) | 0.016a |
| Injection site reaction leading to discontinuation | 3 (9) | 2 (6) | 1 (3) | 0.56 | 6 (9) | 0.42 |
Data are presented as number (percentage). P values reported for χ2 or Fisher exact test.
P<0.05.
Figure 3.
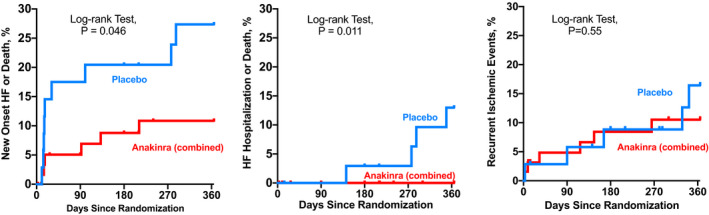
Effects of anakinra on heart failure clinical events. Anakinra‐treated patients had a significantly lower in incidence of heart failure related clinical events than placebo (Log‐rank Mantel‐Cox test). Left, a composite end point of new onset heart failure or death. Middle, a composite end point of hospitalization for heart failure or death. Right, a composite of ischemic events (death, recurrent AMI, or urgent revascularization).
There was no difference in the composite end point of death, recurrent myocardial infarction, or urgent revascularization: 6 in the anakinra group (9%) and 5 (14%) in placebo group (P>0.05; Table 2, Figure 3).
Treatment with anakinra was well tolerated, with no serious unanticipated adverse events. Nine patients in the anakinra group (14%) and 5 in the placebo group (14%; P=0.98) experienced a serious infection requiring prescription of an antimicrobial drug, all considered to be unrelated to the investigational treatment (Table 2). Injection site reactions were significantly more common in the anakinra group than in placebo (16/64 [22%] versus 1/35 [3%]; P=0.016), leading to early interruption of treatment in 6 anakinra‐treated patients (9%) and 1 placebo‐treated patient (3%; P=0.419). There was no significant difference in any of the other end points comparing anakinra, once daily, with anakinra, twice daily (Table 2).
Sensitivity Per‐Protocol Analysis
We performed a per‐protocol analysis in which subjects were analyzed on the basis of treatment received rather than treatment assigned when we found, discovered during the study closure procedures for one of secondary sites, that 3 patients received a treatment different than originally assigned. When we analyzed per protocol comparing anakinra combined (N=65) versus placebo (N=34), the significance in hsCRP‐AUC and HF events (D+HF and D+HHF) was confirmed (P<0.001, P=0.032, and P=0.007, respectively).
Additional Exploratory Analysis on LV Performance
During the recording of the Doppler echocardiogram, using noninvasive 2‐dimensional–derived LV volumes, measures of arterial blood pressure, and Doppler‐derived estimates of LV pressure, we calculated also LV stroke volume and stroke work (stroke work=stroke volume*[mean arterial pressure – left ventricular end diastolic pressure]),20 surrogates for LV performance21 Compared with placebo, anakinra led to a significant improvement in stroke volume (P=0.001 versus baseline and P=0.027 for time×group interaction versus placebo) and stroke work (P=0.001 versus baseline and P=0.004 for time×group interaction versus placebo) (Figure S3).
Discussion
The results of this phase 2 clinical trial support the safety of standard and high‐dose anakinra in patients with STEMI and demonstrate that either dose of anakinra blunts the acute inflammatory response, as measured by the hsCRP‐AUC at 14 days. The degree of hsCRP plasma elevation predicts in‐hospital mortality and long‐term incidence of HF in patients with STEMI.3, 5, 6, 7, 17, 18 The strong reduction in hsCRP levels with anakinra, at both standard and high dose, confirms prior findings of enhanced interleukin‐1 activity in patients with STEMI.8, 9, 10, 11, 12 The lack of differences between standard and high‐dose anakinra suggests that the standard daily dose is sufficient to block the interleukin‐1 receptor and modulate the inflammatory response in STEMI.
Consistent with what was seen in preclinical experimental models,8, 9 the benefits of anakinra appear to be independent of difference of infarct size, supporting an effect of interleukin‐1 blockade on the healing of the myocardium after acute myocardial infarction. We explored the effects of anakinra on interval changes in LV dimensions and function at 12 months. Adverse remodeling is considered to be a primary substrate for HF complicating STEMI3, 4; however, data from this study show minimal changes in LV volumes and systolic function among placebo‐ and anakinra‐treated patients. These data suggest that current strategies of prompt reperfusion and early neurohormonal blockade are sufficient to suppress adverse cardiac remodeling after STEMI. Despite these advances in treatment and the reduction in adverse remodeling, the incidence of subsequent new or worsening HF after STEMI remains unacceptably high, affecting up to 30% of patients at 1 year,1, 2 which may imply an additional pathway for progression to HF independent of traditional measures of cardiac remodeling. In an exploratory analysis, we show that patients treated with anakinra tend to improve LV performance (stroke volume and stroke work21) beyond what is seen in placebo. The role of interleukin‐1 in inhibiting cardiac contractility is well known from experimental studies in cells and animals.8 Previous studies have shown improvement in systolic function with interleukin‐1 blockade in patients with systolic HF and associated improvement in cardiorespiratory fitness.20, 22, 23, 24, 25
Although not powered to detect differences in clinical outcomes, the prespecified analysis of HF outcomes showed a significantly lower incidence of HF hospitalizations and of new or worsening HF with anakinra versus placebo. The results of the VCUART3 are consistent with the findings of the prior pilot feasibility VCUART and VCUART210, 11 that enrolled a total 40 patients with STEMI and demonstrated a nominally lower incidence of HF at 3 months (5% versus 30%; P=0.035) that persisted at long‐term follow‐up.10, 11, 12 The results of the VCUART3 are also in agreement with the analysis of 10 061 patients in the CANTOS clinical trial of canakinumab, an interleukin‐1β blocking antibody, in stable patients with prior myocardial infarction, in whom canakinumab provided a significant reduction in hsCRP and a dose‐dependent reduction in hospitalizations for HF.13, 14
Anakinra was also studied in the MRC‐ILA Heart clinical trial of 182 patients with non–ST‐segment–elevation acute coronary syndrome showing a similar reduction in the hsCRP values with 14 days of once‐daily anakinra treatment,26 but no data on the incidence of HF were provided. In contrast with the MRC‐ILA Heart trial,26 however, treatment with anakinra in this study appeared to have no effect on recurrent ischemic events, whereas an unexpected, and unexplained, excess of late recurrent ischemic events were seen in the MRC‐ILA Heart trial, raising the concern that 2‐week treatment with anakinra was associated with an unexpected untoward late deleterious effect, possibly because of a rebound inflammatory mechanism. Reassuringly, no rebound, or excess, adverse events were seen in the current study, nor in other studies of anakinra used to treat patients with HF.10, 11, 12, 25 Moreover, the primary analysis of CANTOS13 showed a significant 15% reduction in the composite outcome of myocardial infarction, stroke, or cardiovascular death in patients treated with canakinumab that was driven by a 20% reduction in recurrent myocardial infarction. A significant reduction in atherothrombotic complications was also seen in the COLCOT (Cardiovascular Outcome Trial)27 in patients with recent myocardial infarction treated with colchicine, a small molecule able to interfere with the processing and release of interleukin‐1β, further supporting the beneficial role of interleukin‐1 blockade in acute coronary syndromes.
Study Limitations
The major limitations of this phase 2 study are the small sample size and the missing data. Despite these limitations, the statistical significance in the reduction in hsCRP levels is strong, and it paired with a favorable safety profile of the drug. The preliminary signal for reduced incidence of new or worsening HF, on the other hand, requires confirmation and opens the way to a new therapeutic strategy to be further tested in phase 3 clinical trials. Interleukin‐1 blockade may represent the first targeted anti‐inflammatory strategy treatment to prevent and treat HF in patients with STEMI. Confirmatory phase 2B/3 clinical trials are needed. A slight, not statistically significant, imbalance in the number of patients with diabetes mellitus is noted between groups; nevertheless, the effects of anakinra on AUC for hsCRP appeared to be independent of diabetes mellitus status (data not shown).
Conclusions
Among patients with STEMI, interleukin‐1 blockade with anakinra significantly reduces the systemic inflammatory response compared with placebo, without any significant difference between standard or high‐dose regimens. Prespecified analyses on clinical end points observed a reduced incidence of HF and reduced HF hospitalizations, supporting the potential clinical benefit of interleukin‐1 blockade in patients with acute myocardial infarction.
Sources of Funding
The study is supported by a grant from the National Institutes of Health (1R34HL121402‐01) to Dr Abbate and Dr Van Tassell.
Disclosures
Dr Abbate and Dr Van Tassell have served as consultants to Swedish Orphan Biovitrum LLC. The remaining authors have no disclosures to report.
Supporting information
Table S1. Inclusion and Exclusion Criteria
Table S2. Guideline‐Directed Therapy at the Beginning and at the End of the Follow Up
Table S3. Clinical Characteristics of Subjects With Paired Echocardiogram
Figure S1. Individual values of C‐reactive protein area‐under‐the‐curve values. Anakinra once daily or twice daily significantly reduced the C‐reactive protein area‐under‐the‐curve (CRP AUC) at 14 days (P<0.001 for all comparisons), without any significant differences between the once daily and twice daily regimens.
Figure S2. Effects of anakinra once daily and anakinra twice daily on heart failure events. Kaplan–Meier survival curves for anakinra once daily, anakinra twice daily, and placebo are shown for the composite endpoint of death or heart failure (P=0.14 between groups) and for death and hospitalization for heart failure (P=0.038 between groups) (Log‐rank test). No significant differences were found comparing anakinra once daily with anakinra twice daily.
Figure S3. Effects of anakinra on stroke volume and stroke work. Anakinra led to a significant improvement in stroke volume (P=0.001 versus baseline and P=0.027 for time_x_group interaction versus placebo [central panel]) and stroke work (P=0.001 versus baseline and P=0.004 for time_x_group interaction versus placebo [right panel]).
Acknowledgments
Swedish Orphan Biovitrum LLC (Stockholm, Sweden) has provided study medication (anakinra) and matching placebo free of cost, but had no role in the study design, conduct, analysis, or reporting.
(J Am Heart Assoc. 2020;9:e014941 DOI: 10.1161/JAHA.119.014941.)
References
- 1. Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Long‐term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalisation after acute myocardial infarction for Medicare beneficiaries: 1998‐2010. Circulation. 2013;128:2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cung TT, Morel O, Cayla G, Rioufol G, Garcia‐Dorado D, Angoulvant D, Bonnefoy‐Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois‐Randé JL, Unterseeh T, Le Breton H, Béard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 5. Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti‐inflammatory strategies for ventricular remodeling following ST‐segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. [DOI] [PubMed] [Google Scholar]
- 6. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 7. Seropian IM, Sonnino C, Van Tassell BW, Biasucci LM, Abbate A. Inflammatory markers in ST‐elevation acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2016;5:382–395. [DOI] [PubMed] [Google Scholar]
- 8. Buckley LF, Abbate A. Interleukin‐1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. [DOI] [PubMed] [Google Scholar]
- 9. Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi‐Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin‐1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. [DOI] [PubMed] [Google Scholar]
- 10. Abbate A, Kontos MC, Grizzard JD, Biondi‐Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW; VCU‐ART Investigators . Interleukin‐1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU‐ART] Pilot Study). Am J Cardiol. 2010;105:1371–1377. [DOI] [PubMed] [Google Scholar]
- 11. Abbate A, Van Tassell BW, Biondi‐Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin‐1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction. Am J Cardiol. 2013;111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, Oddi C, Carbone S, Trankle CR, Roberts CS, Mueller GH, Gambill ML, Christopher S, Markley R, Vetrovec GW, Dinarello CA, Biondi‐Zoccai G. Comparative safety of interleukin‐1 blockade with anakinra in patients with ST‐segment elevation acute myocardial infarction (from the VCU‐ART and VCU‐ART2 pilot studies). Am J Cardiol. 2015;115:288–292. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 14. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti‐inflammatory therapy with canakinumab for the prevention of hospitalisation for heart failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 15. Van Tassell BW, Lipinski MJ, Appleton D, Roberts CS, Kontos MC, Abouzaki N, Melchior R, Mueller G, Garnett J, Canada J, Carbone S, Buckley LF, Wohlford G, Kadariya D, Trankle CR, Oddi Erdle C, Sculthorpe R, Puckett L, DeWilde C, Shah K, Angiolillo DJ, Vetrovec G, Biondi‐Zoccai G, Arena R, Abbate A. Rationale and design of the Virginia Commonwealth University‐Anakinra Remodeling Trial‐3 (VCU‐ART3): a randomized, placebo‐controlled, double‐blinded, multicenter study. Clin Cardiol. 2018;41:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopes RD, Lokhnygina Y, Hasselblad V, Newby KL, Yow E, Granger CB, Armstrong PW, Hochman JS, Mills JS, Ruzyllo W, Mahaffey KW. Methods of creatine kinase‐MB analysis to predict mortality in patients with myocardial infarction treated with reperfusion therapy. Trials. 2013;14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abouzaki NA, Christopher S, Trankle C, Van Tassell BW, Carbone S, Mauro AG, Buckley L, Toldo S, Abbate A. Inhibiting the inflammatory injury after myocardial ischemia reperfusion with plasma‐derived alpha‐1 antitrypsin: a post hoc analysis of the VCU‐α1RT Study. J Cardiovasc Pharmacol. 2018;71:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ørn S, Manhenke C, Ueland T, Damås JK, Mollnes TE, Edvardsen T, Aukrust P, Dickstein K. C‐reactive protein, infarct size, microvascular obstruction, and left‐ventricular remodelling following acute myocardial infarction. Eur Heart J. 2009;30:1180–1186. [DOI] [PubMed] [Google Scholar]
- 19. Eapen ZJ, Tang WH, Felker GM, Hernandez AF, Mahaffey KW, Lincoff AM, Roe MT. Defining heart failure end points in ST‐segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ Cardiovasc Qual Outcomes. 2012;5:594–600. [DOI] [PubMed] [Google Scholar]
- 20. Buckley LF, Carbone S, Trankle CR, Canada JM, Erdle CO, Regan JA, Viscusi MM, Kadariya D, Billingsley H, Arena R, Abbate A, Van Tassell BW. Effect of interleukin‐1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol. 2018;72:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersen MJ, Borlaug BA. Invasive hemodynamic characterization of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:435–444. [DOI] [PubMed] [Google Scholar]
- 22. Van Tassell BW, Abouzaki NA, Oddi Erdle C, Carbone S, Trankle CR, Melchior RD, Turlington JS, Thurber CJ, Christopher S, Dixon DL, Fronk DT, Thomas CS, Rose SW, Buckley LF, Dinarello CA, Biondi‐Zoccai G, Abbate A. Interleukin‐1 blockade in acute decompensated heart failure: a randomized, double‐blinded, placebo‐controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, Shah K, Canada J, Voelkel NF, Dinarello CA, Abbate A. Enhanced interleukin‐1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7:e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi‐Erdle C, Kadariya D, Christopher S, Viscusi M, Del Buono M, Kontos MC, Arena R, Van Tassell B, Abbate A. Usefulness of canakinumab to improve exercise capacity in patients with long‐term systolic heart failure and elevated c‐reactive protein. Am J Cardiol. 2018;122:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi‐Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin‐1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017;10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin‐1 receptor antagonist therapy on markers of inflammation in non‐ST elevation acute coronary syndromes: the MRC‐ILA Heart Study. Eur Heart J. 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López‐Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and safety and low dose colchicine after myocardial infarction. N Engl J Med. 2019. DOI: 10.1056/NEJMoa1912388. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and Exclusion Criteria
Table S2. Guideline‐Directed Therapy at the Beginning and at the End of the Follow Up
Table S3. Clinical Characteristics of Subjects With Paired Echocardiogram
Figure S1. Individual values of C‐reactive protein area‐under‐the‐curve values. Anakinra once daily or twice daily significantly reduced the C‐reactive protein area‐under‐the‐curve (CRP AUC) at 14 days (P<0.001 for all comparisons), without any significant differences between the once daily and twice daily regimens.
Figure S2. Effects of anakinra once daily and anakinra twice daily on heart failure events. Kaplan–Meier survival curves for anakinra once daily, anakinra twice daily, and placebo are shown for the composite endpoint of death or heart failure (P=0.14 between groups) and for death and hospitalization for heart failure (P=0.038 between groups) (Log‐rank test). No significant differences were found comparing anakinra once daily with anakinra twice daily.
Figure S3. Effects of anakinra on stroke volume and stroke work. Anakinra led to a significant improvement in stroke volume (P=0.001 versus baseline and P=0.027 for time_x_group interaction versus placebo [central panel]) and stroke work (P=0.001 versus baseline and P=0.004 for time_x_group interaction versus placebo [right panel]).


