Abstract
Background
Sex differences have been found in stroke risk factors, incidence, treatment, and outcomes. There are conflicting data on whether diagnostic evaluation for stroke may differ between men and women.
Methods and Results
We performed a retrospective cohort study using inpatient and outpatient claims between 2008 and 2016 from a nationally representative 5% sample of Medicare beneficiaries. We included patients ≥65 years old and hospitalized with ischemic stroke, defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) and ICD‐10‐CM diagnosis codes. Logistic regression was used to determine the association between female sex and the odds of diagnostic testing and specialist evaluation, adjusted for age, race, and number of Charlson comorbidities. Among 78 822 patients with acute ischemic stroke, 58.3% (95% CI, 57.9–58.6%) were women. Female sex was associated with decreased odds of intracranial vessel imaging (odds ratio [OR]: 0.94; 95% CI, 0.91–0.97), extracranial vessel imaging (OR: 0.89; 95% CI, 0.86–0.92), heart‐rhythm monitoring (OR: 0.92; 95% CI, 0.87–0.98), echocardiography (OR: 0.92; 95% CI, 0.89–0.95), evaluation by a neurologist (OR: 0.94; 95% CI, 0.91–0.97), and evaluation by a vascular neurologist (OR: 0.94; 95% CI, 0.90–0.97), after adjustment for age, race, and comorbidities. These findings were unchanged in separate sensitivity analyses excluding patients who died during the index hospitalization or were discharged to hospice and excluding patients with atrial fibrillation diagnosed before their index stroke.
Conclusions
In a nationally representative cohort of Medicare beneficiaries, we found that women with acute ischemic stroke were less likely to be evaluated by stroke specialists and less likely to undergo standard diagnostic testing compared with men.
Keywords: diagnostic evaluation, disparities, ischemic stroke, women
Subject Categories: Cerebrovascular Disease/Stroke, Women, Diagnostic Testing
Clinical Perspective
What Is New?
In a nationally representative cohort of Medicare beneficiaries, women with acute ischemic stroke were less likely than men to undergo standard diagnostic testing and to be evaluated by stroke specialists.
What Are the Clinical Implications?
These results suggest that disparities exist between men and women in diagnostic evaluation after acute stroke and highlight the importance of recognizing potential hidden biases when formulating diagnostic plans.
Introduction
Stroke is a major cause of death and disability in both women and men. Despite a lower incidence rate, women have a higher lifetime prevalence of stroke than men and develop an estimated 55 000 more strokes than men in the United States each year.1 After stroke, women experience higher rates of disability2, 3, 4, 5, 6 and are less likely to be discharged home after hospitalization.2, 5, 7, 8, 9
Recognition has grown about differences between men and women in stroke risk factors, presentation, pathophysiology, and treatment.6, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 There has been conflicting evidence about whether similar disparities exist in diagnostic evaluation after stroke. Although some studies have found no unexplained disparities,12, 15, 20, 21, 22 others have found lower rates of diagnostic testing among women, including brain imaging,2, 3 vessel imaging,23, 24 and echocardiography.3, 23 Existing studies from the United States have been geographically circumscribed and limited by relatively small sample sizes21, 23; few nationally representative data exist on the topic. We used a nationally representative sample of Medicare claims to investigate whether there were differences between men and women in diagnostic evaluation after acute ischemic stroke.
Methods
Design
We performed a retrospective cohort study using inpatient and outpatient claims between 2008 and 2016 from a nationally representative 5% sample of Medicare beneficiaries. The US federal government's Centers for Medicare and Medicaid Services (CMS) provide health insurance to most US residents once they reach 65 years of age. CMS makes available to researchers data on claims submitted by providers and hospitals in the course of Medicare beneficiaries’ clinical care.25 Claims data from hospitals include dates of hospitalization and International Classification of Diseases (ICD) diagnosis and procedure codes. Physician claims include Current Procedural Terminology (CPT) codes, dates of service, and physician specialty. Multiple claims for a given patient can be linked by a unique beneficiary identifier code, allowing for a comprehensive and longitudinal analysis of each beneficiary's care over time. The Weill Cornell Medical College institutional review board approved this study and waived the requirement for informed consent. The data used for this analysis cannot be directly shared by the authors under the terms of their data use agreement, but the data can be obtained by application to CMS.
Patient Population
We included patients aged ≥65 years with continuous coverage in traditional fee‐for‐service Medicare (both Parts A and B) for at least 1 year (or until death, if applicable) between 2008 and 2016. From this sample, we selected patients hospitalized between 2009 and 2016 for acute ischemic stroke. Between January 1, 2009, and September 30, 2015, this condition was defined by an ICD‐9 diagnosis code algorithm previously validated to have sensitivity of 86%, specificity of 95%, and a positive predictive value of 90% compared with medical record review26; after September 30, 2015, we used ICD‐10 code I.63, which has also been validated as highly reliable.27 The start date of January 1, 2009, was chosen because 2008 was used as a run‐in period to ascertain comorbidities and to exclude patients without at least 1 year of Medicare coverage before their first stroke. Although patients may have had multiple ischemic stroke hospitalizations during the study period, we started follow‐up time on the admission date of the first documented stroke hospitalization (index hospitalization) and assessed outcomes through 90 days after discharge from the index hospitalization.
Measurements
We determined whether patients were evaluated by a neurologist during the index stroke hospitalization or within 90 days of discharge. Neurologist evaluation was defined as a physician claim with an evaluation‐and‐management CPT code and a provider specialty code representing neurology. These provider specialty codes have been validated previously to correspond closely to physicians’ specialties, as determined by National Provider Identifier (NPI) files and online physician profiles.28
We also determined whether patients were evaluated by a board‐certified vascular neurologist during the index stroke hospitalization or within 90 days of discharge. Using a Python software script and Medicare providers’ NPI numbers, we searched a publicly available list of US physicians certified in vascular neurology by the American Board of Psychiatry and Neurology29 and determined which of the physicians who had submitted claims for the care of patients in our sample were board‐certified vascular neurologists. We used the same approach to identify which patients were evaluated by board‐certified neurointensivists, who also receive training in stroke management.30
We used CPT codes to establish whether heart‐rhythm monitoring, echocardiography, extracranial vessel imaging, and intracranial vessel imaging were performed during the index stroke hospitalization or within 90 days of discharge. Heart‐rhythm monitoring included the use of Holter monitors, external loop recorders, or implantable loop recorders. Echocardiography included both transthoracic and transesophageal studies. Vessel imaging, both intracranial and extracranial, included ultrasound, computed tomographic angiography, and magnetic resonance angiography.
We used previously validated ICD‐9 and ICD‐10 codes to ascertain Charlson comorbidities31 and the following vascular risk factors and comorbidities that appear in Table 1: atrial fibrillation, hypertension, diabetes mellitus, coronary heart disease, heart failure, peripheral vascular disease, chronic kidney disease, valvular heart disease, chronic obstructive pulmonary disease, tobacco use, and alcohol abuse. These covariates were cumulatively carried forward from the start of Medicare coverage through the discharge date of the index stroke hospitalization.
Table 1.
Characteristics of a National 5% Sample of Medicare Beneficiaries With Acute Ischemic Stroke, Stratified by Sex
| Characteristic | Female (n=45 942) | Male (n=32 880) |
|---|---|---|
| Age, y, mean±SD | 81.0±8.1 | 78.1±7.6 |
| Race | ||
| White | 38 546 (83.9) | 27 809 (84.6) |
| Black | 5221 (11.4) | 3317 (10.1) |
| Other | 2175 (4.7) | 1754 (5.3) |
| Atrial fibrillation | 15 430 (33.6) | 10 504 (31.9) |
| Coronary artery disease | 20 180 (43.9) | 17 557 (53.4) |
| Hypertension | 41 137 (89.5) | 28 034 (85.3) |
| Diabetes mellitus | 20 787 (45.2) | 16 009 (48.7) |
| Heart failure | 14 981 (32.6) | 9765 (29.7) |
| Peripheral vascular disease | 12 920 (28.1) | 10 427 (31.7) |
| Chronic kidney disease | 11 210 (24.4) | 8963 (27.3) |
| Valvular heart disease | 13 371 (29.1) | 8247 (25.1) |
| Tobacco use | 4695 (10.2) | 5286 (16.1) |
Data are presented as number (%) unless otherwise specified.
Statistical Analysis
Descriptive statistics are reported as mean±SD and percentages with 95% CIs, if appropriate. Multiple logistic regression models were used to determine associations of female sex with intracranial vessel imaging, extracranial vessel imaging, echocardiography, heart rhythm monitoring, neurologist evaluation, or vascular neurologist evaluation, after adjusting for age, race, and number of Charlson comorbidities. All models included both a linear term for age and a quadratic term, age2, to account for potentially nonlinear associations between age and the odds of diagnostic testing and specialist evaluation. A sensitivity analysis was performed excluding patients who died during the index hospitalization or were discharged to hospice. We also performed a sensitivity analysis excluding patients with atrial fibrillation diagnosed before their index stroke. Statistical analyses were performed using Stata v14 (StataCorp) and R Environment for Statistical Computing v3.2 (R Core Team). Figures 1 and 2 were created using the R package ggplot2.32
Figure 1.
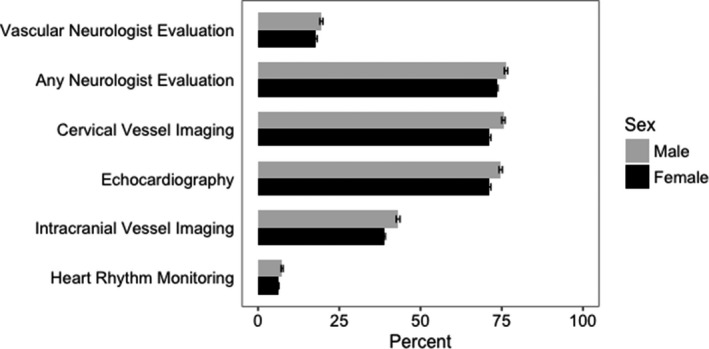
Percentages and 95% CIs of diagnostic testing and specialist evaluation by sex.
Figure 2.
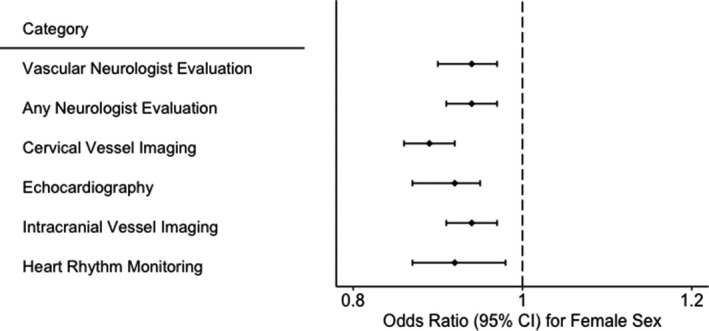
Odds ratio associated with female sex in relation to categories of diagnostic evaluation, adjusted for age, race, and number of Charlson comorbidities.
Results
During the study period, 78 822 patients hospitalized with acute ischemic stroke were identified. The 45 942 women in our sample were slightly older (81.0±8.1 years) than the men (78.1±7.6 years) and had a lower baseline prevalence of coronary heart disease and active smoking but were otherwise broadly similar in terms of baseline characteristics (Table 1).
Compared with men, women less often underwent intracranial vessel imaging (38.7% versus 43.1%), extracranial vessel imaging (71.2% versus 75.4%), echocardiography (71.2% versus 74.7%), and heart rhythm monitoring (6.2% versus 7.4%). Women were also less likely than men to be evaluated by a neurologist (73.4% versus 76.3%) or a vascular neurologist (17.8% versus 19.5%; Figure 1). There was no interaction between female sex and calendar year in relation to any of these diagnostic evaluations.
After adjustment for age, race, and comorbidities, female sex was associated with lower odds of intracranial vessel imaging (odds ratio [OR]: 0.94; 95% CI, 0.91–0.97), extracranial vessel imaging (OR: 0.89; 95% CI, 0.86–0.92), echocardiography (OR: 0.92; 95% CI, 0.89–0.95), heart‐rhythm monitoring (OR: 0.92; 95% CI, 0.87–0.98), evaluation by a neurologist (OR: 0.94; 95% CI, 0.91–0.97), and evaluation by a vascular neurologist (OR: 0.94; 95% CI, 0.90–0.97; Figure 2). These findings were unchanged in a sensitivity analysis excluding patients who died during the index hospitalization or were discharged to hospice (Table 2). Our findings were also similar in a sensitivity analysis excluding patients with atrial fibrillation diagnosed before their index stroke.
Table 2.
Odds Ratiosa and 95% CIs Associated With Female Sex in Relation to Categories of Diagnostic Evaluation in the Full Study Sample and Excluding Patients Who Died in Hospital or Were Discharged to Hospice
| Diagnostic Evaluation | All Patients (N=78 822) | Excluding Death in Hospital and Discharge to Hospice (n=67 893) |
|---|---|---|
| Intracranial vessel imaging | 0.94 (0.91–0.97) | 0.94 (0.92–0.97) |
| Extracranial vessel imaging | 0.89 (0.86–0.92) | 0.90 (0.86–0.93) |
| Echocardiography | 0.92 (0.89–0.95) | 0.92 (0.90–0.97) |
| Heart rhythm monitoring | 0.92 (0.87–0.98) | 0.93 (0.88–0.99) |
| Evaluation by any neurologist | 0.94 (0.91–0.97) | 0.94 (0.91–0.97) |
| Evaluation by vascular neurologist | 0.94 (0.90–0.97) | 0.94 (0.91–0.97) |
Adjusted for age, race, and number of Charlson comorbidities.
Discussion
In this nationally representative sample of Medicare beneficiaries, women were less likely than men to undergo standard diagnostic evaluations for acute ischemic stroke. These differences persisted after adjustment for demographics and comorbidities and were unchanged after excluding patients who died or went to hospice.
Previous studies have reported conflicting findings about comparative rates of diagnostic testing in men versus women with stroke.2, 3, 20, 21, 22, 23, 24 It has recently been suggested that observed differences in diagnostic testing between women and men can be attributed to the older average age of women at time of stroke onset,11 as controlling for age attenuated the results of several studies.11, 20, 24 Our findings suggest that differences in diagnostic evaluations between men and women do not simply reflect residual confounding by age; we adjusted for both linear and nonlinear age effects and found no attenuation in results. In addition, our study provides the additional novel finding that women are less likely to have specialist evaluation after ischemic stroke.
Our findings have important implications because they may suggest hidden bias among practitioners in diagnostic planning after acute stroke. It may be helpful for providers and patients to be aware of at least the possibility of such bias because greater awareness may lead to more appropriate care; however, a number of caveats should temper this interpretation. First, our results do not necessarily provide evidence of differences in quality of care. The evaluations we examined are not recommended by consensus guidelines in all cases of acute stroke, but in practice, they are widely used as part of a standard diagnostic approach, and there is no clear clinical reason for this diagnostic approach to be followed less frequently in women than in men. Second, there may be instances in which certain evaluations may not be appropriate, and thus the discrepancy between men and women may represent overinvestigation in men. Third, because our study was limited to Medicare beneficiaries, our findings may reflect features of the US healthcare system. Although previous large‐scale studies from Europe, Canada, and Australia have found differences between men and women in rates of various diagnostic tests,2, 3, 23, 24 ours adds novel findings on such differences across several categories of diagnostic testing and specialist evaluation. Fourth, because our sample size was large, we had power to detect even small differences between men and women. Indeed, the effect sizes we observed were modest but consistent across all categories of evaluation that we examined.
Our results should be interpreted in the context of several limitations. First, diagnostic and procedure codes were used to define our variables. Although we used previously validated codes if possible, there was still potential for misclassification; however, it is not apparent that such misclassification would occur differentially in men versus women. Second, it is possible that some patients died before they could receive full diagnostic evaluation or that increased testing and specialist assessment may not have been consistent with their goals of care. Our sensitivity analysis, which excluded patients who died in hospital or were discharged to hospice, attempted to account for this possibility and showed no change in results from the main analysis; however, in‐hospital death and discharge to hospice may not perfectly capture the aforementioned phenomena. Third, our study was not equipped to fully adjust for stroke severity and premorbid status, and such variables have previously been associated with sex‐based disparities in diagnostic testing.2, 3, 20 Fourth, we were not able to assess whether patients were treated in stroke units, which may potentially affect diagnostic testing and specialist evaluation. Finally, our data were limited to patients aged ≥65 years. Although the majority of strokes occur in this demographic group,1, 33 our results may not be generalizable to younger age groups.
The limitations of our study were mitigated by strengths such as the large sample size and broadly representative population. The differences we found between men and women may have implications for outcomes. The lower probability of appropriate specialist evaluation, for example, may affect mortality risk for women, as both neurologist evaluation34, 35, 36 and the presence of in‐hospital vascular neurologist consultation37 have been associated previously with a lower risk of short‐term mortality after stroke.
Conclusions
We found that women with ischemic stroke were less likely than men to undergo standard diagnostic testing and to receive specialist evaluation. These results, with modest but consistent effect sizes across modes of diagnostic evaluation, build on previous studies showing sex‐based differences in diagnostic testing. Large, population‐based studies may be warranted to investigate disparities in stroke evaluation between men and women while thoroughly adjusting for potential confounding factors, especially stroke severity and premorbid status. In the meantime, it may be helpful for clinicians to keep our results in mind when considering diagnostic plans for men and women with recent stroke.
Sources of Funding
Kamel is supported by the National Institutes of Health (NIH) and the National Institute of Neurological Disorders and Stroke (NINDS; grant R01NS097443) and the Michael Goldberg Research Fund. Navi is supported by NIH/NINDS (grant K23NS091395) and the Florence Gould Endowment for Discovery in Stroke. Merkler is supported by the American Heart Association (grant 18CDA34110419).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e015625 DOI: 10.1161/JAHA.119.015625.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Roquer J, Campello AR, Gomis M. Sex differences in first‐ever acute stroke. Stroke. 2003;34:1581–1585. [DOI] [PubMed] [Google Scholar]
- 3. Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D; European BSoSCG . Sex differences in the clinical presentation, resource use, and 3‐month outcome of acute stroke in Europe: data from a multicenter multinational hospital‐based registry. Stroke. 2003;34:1114–1119. [DOI] [PubMed] [Google Scholar]
- 4. Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, Heeley E, Otahal P, Vemmos K, Anderson C, Parmar P, Krishnamurthi R, Barker‐Collo S, Feigin V, Bejot Y, Cabral NL, Carolei A, Sacco S, Chausson N, Olindo S, Rothwell P, Silva C, Correia M, Magalhaes R, Appelros P, Korv J, Vibo R, Minelli C, Gall SL. Factors contributing to sex differences in functional outcomes and participation after stroke. Neurology. 2018;90:e1945–e1953. [DOI] [PubMed] [Google Scholar]
- 5. Petrea RE, Beiser AS, Seshadri S, Kelly‐Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke. 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carcel C, Wang X, Sandset EC, Delcourt C, Arima H, Lindley R, Hackett ML, Lavados P, Robinson TG, Munoz Venturelli P, Olavarria VV, Brunser A, Berge E, Chalmers J, Woodward M, Anderson CS. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology. 2019;93:e2170–e2180. [DOI] [PubMed] [Google Scholar]
- 7. Eriksson M, Glader EL, Norrving B, Terent A, Stegmayr B. Sex differences in stroke care and outcome in the Swedish national quality register for stroke care. Stroke. 2009;40:909–914. [DOI] [PubMed] [Google Scholar]
- 8. Glader EL, Stegmayr B, Norrving B, Terent A, Hulter‐Asberg K, Wester PO, Asplund K; Riks‐Stroke C . Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–1975. [DOI] [PubMed] [Google Scholar]
- 9. Holroyd‐Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–1837. [DOI] [PubMed] [Google Scholar]
- 10. Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ, Rundek T. Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab. 2018;38:2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, Chaturvedi S, Madsen TE, Demel SL, Lee SJ, Reeves M. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17:641–650. [DOI] [PubMed] [Google Scholar]
- 12. Arauz A, Serrano F, Pearce LA, Kasner SE, Ameriso SF, Toni D, Bereczki D, Siegler J, Ruiz‐Franco A, Cantu‐Brito C, Czlonkowska A, Lang W, Berkowitz SD, Mundl H, Hart RG. Regional, sex, and age differences in diagnostic testing among participants in the navigate‐esus trial. Int J Stroke. 2019; 1747493019884523. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4:e001967 DOI: 10.1161/JAHA.115.001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gall S, Phan H, Madsen TE, Reeves M, Rist P, Jimenez M, Lichtman J, Dong L, Lisabeth LD. Focused update of sex differences in patient reported outcome measures after stroke. Stroke. 2018;49:531–535. [DOI] [PubMed] [Google Scholar]
- 15. Hung KH, Lai JC, Hsu KN, Hu C, Chang HC, Chen CN, Ku HS, Yang MS, Chen PH. Gender gap and risk factors for poor stroke outcomes: a single hospital‐based prospective cohort study. J Stroke Cerebrovasc Dis. 2018;27:2250–2258. [DOI] [PubMed] [Google Scholar]
- 16. Renoux C, Coulombe J, Li L, Ganesh A, Silver L, Rothwell PM; Oxford Vascular S . Confounding by pre‐morbid functional status in studies of apparent sex differences in severity and outcome of stroke. Stroke. 2017;48:2731–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, Jones EM, Tanner R, Gonzales NR, Beasley TM, Grotta JC, Savitz SI, Martin‐Schild S. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e255–e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asdaghi N, Romano JG, Wang K, Ciliberti‐Vargas MA, Koch S, Gardener H, Dong C, Rose DZ, Waddy SP, Robichaux M, Garcia EJ, Gonzalez‐Sanchez JA, Burgin WS, Sacco RL, Rundek T. Sex disparities in ischemic stroke care: FL‐Pr CReSD study (Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities). Stroke. 2016;47:2618–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu AYX, Penn AM, Lesperance ML, Croteau NS, Balshaw RF, Votova K, Bibok MB, Penn M, Saly V, Hegedus J, Zerna C, Klourfeld E, Bilston L, Hong ZM, Coutts SB; Spec TRASG . Sex differences in presentation and outcome after an acute transient or minor neurologic event. JAMA Neurol. 2019. Available at: https://jamanetwork.com/journals/jamaneurology/article-abstract/2734651. Accessed February 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, Srikanth V, Thrift AG. Sex differences in presentation, severity, and management of stroke in a population‐based study. Neurology. 2010;74:975–981. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe E, Allen NB, Dostal J, Sama D, Claus EB, Goldstein LB, Lichtman JH. Diagnostic evaluation for patients with ischemic stroke: are there sex differences? Cerebrovasc Dis. 2009;27:450–455. [DOI] [PubMed] [Google Scholar]
- 22. Kapral MK, Ben‐Yakov M, Fang J, Gladstone DJ, Saposnik G, Robertson A, Silver FL. Gender differences in carotid imaging and revascularization following stroke. Neurology. 2009;73:1969–1974. [DOI] [PubMed] [Google Scholar]
- 23. Smith MA, Lisabeth LD, Brown DL, Morgenstern LB. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology. 2005;65:855–858. [DOI] [PubMed] [Google Scholar]
- 24. Gattringer T, Ferrari J, Knoflach M, Seyfang L, Horner S, Niederkorn K, Culea V, Beitzke M, Lang W, Enzinger C, Fazekas F. Sex‐related differences of acute stroke unit care: results from the Austrian stroke unit registry. Stroke. 2014;45:1632–1638. [DOI] [PubMed] [Google Scholar]
- 25. Center for medicare and medicaid services . Medicare Limited Dataset Files. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets. Accessed July 5, 2019.
- 26. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 27. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 28. Kamel H, Chung CD, Kone GJ, Gupta A, Morris NA, Fink ME, Navi BB. Medical specialties of clinicians providing mechanical thrombectomy to patients with acute ischemic stroke in the United States. JAMA Neurol. 2018;75:515–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, Chan L. Treatment of acute myocardial infarction and 30‐day mortality among women and men. N Engl J Med. 2000;343:8–15. [DOI] [PubMed] [Google Scholar]
- 30. Gargano JW, Wehner S, Reeves MJ. Do presenting symptoms explain sex differences in emergency department delays among patients with acute stroke? Stroke. 2009;40:1114–1120. [DOI] [PubMed] [Google Scholar]
- 31. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA; Investigators I . Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wickham H. Ggplot2: Elegant graphics for data analysis. New York: Springer‐Verlag; 2016. [Google Scholar]
- 33. Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 34. Gillum LA, Johnston SC. Influence of physician specialty on outcomes after acute ischemic stroke. J Hosp Med. 2008;3:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith MA, Liou JI, Frytak JR, Finch MD. 30‐day survival and rehospitalization for stroke patients according to physician specialty. Cerebrovasc Dis. 2006;22:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldstein LB, Matchar DB, Hoff‐Lindquist J, Samsa GP, Horner RD. VA Stroke Study: neurologist care is associated with increased testing but improved outcomes. Neurology. 2003;61:792–796. [DOI] [PubMed] [Google Scholar]
- 37. Gillum LA, Johnston SC. Characteristics of academic medical centers and ischemic stroke outcomes. Stroke. 2001;32:2137–2142. [DOI] [PubMed] [Google Scholar]


