Abstract
Background
This large‐scale observational study on negative events in a real‐world setting investigated Japanese patients with atrial fibrillation who were not on anticoagulants. This study aims to evaluate the incidence of ischemic stroke and bleeding events (intracranial hemorrhage, gastrointestinal bleeding, others) based on CHA2DS2‐VASc scores in Japanese patients with atrial fibrillation who were not anticoagulated.
Methods and Results
We used health checkups and insurance claim data from a Japanese insurance organization. Altogether, 9733 atrial fibrillation patients were not prescribed anticoagulation during their follow‐up periods. Patients’ risk levels were defined by their CHA2DS2‐VASc scores (range 0–≥3): Men with scores of 0, 1, or ≥2 and women with scores of 1, 2, or ≥3 were considered at low, intermediate, or high risk, respectively. Cox proportional hazards model was used to assess the association between the CHA2DS2‐VASc‐determined risk and the incidence of ischemic stroke and intracranial, gastrointestinal, and other bleeding. The mean 2.5‐year follow‐up revealed 143 ischemic strokes and 332 bleeding events. Annual event rates were 0.58% for ischemic stroke and 1.17% for total bleeding events. Annual incidence of ischemic stroke increased with elevated predicted risks based on CHA2DS2‐VASc scores: 0.18% for low‐risk, 0.44% intermediate‐risk, and 1.29% high‐risk groups (P<0.001 for trend). Annual incidences of total bleeding also increased with elevated predicted risks: 0.51% for low‐risk, 1.28% intermediate‐risk, and 2.02% high‐risk groups (P<0.001 for trend).
Conclusions
Risks of ischemic stroke and bleeding events were high, particularly among those with high CHA2DS2‐VASc scores.
Keywords: atrial fibrillation, bleeding, CHA2DS2‐VASc score, ischemic stroke
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Intracranial Hemorrhage, Risk Factors
Clinical Perspective
What Is New?
This large‐scale observational study on negative events in a real‐world setting investigated Japanese patients with atrial fibrillation who were not on anticoagulants.
The study aimed to evaluate the incidence of ischemic stroke and bleeding events (intracranial hemorrhage, gastrointestinal bleeding, others) based on CHA2DS2‐VASc scores in Japanese patients with atrial fibrillation who were not anticoagulated.
What Are the Clinical Implications?
This study showed a relatively low incidence of ischemic stroke and a relatively high incidence of intracranial bleeding in Japanese patients with atrial fibrillation who were not on oral anticoagulants.
Although present guidelines for managing atrial fibrillation in Japan recommend anticoagulant therapy for patients with a CHADS2 score of ≥2, physicians should be aware that bleeding risk also increases with the elevation of ischemic risk.
Introduction
Atrial fibrillation is one of the most prevalent arrhythmias, with its prevalence increasing with the aging of the population.1, 2, 3 It has been estimated that the number of people with atrial fibrillation would be enormous by 2050 in Japan4 and the United States.3 Patients with atrial fibrillation have been shown to be at high risk of death and various diseases, including ischemic stroke.5, 6 Although the CHA2DS2‐VASc score has been widely used to evaluate the risk of ischemic stroke in patients with atrial fibrillation, there has been limited real‐world evidence of the burden of atrial fibrillation according to CHA2DS2‐Vasc scores in Japan.
The aim of the present large‐scale observational study was to use real‐world data to evaluate not only the incidence of ischemic stroke but also that of total bleeding events (intracranial hemorrhage, gastrointestinal bleeding, others) according to CHA2DS2‐VASc scores in Japanese patients with atrial fibrillation who were not on anticoagulation.
Methods
Study Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. This observational study used health check‐ups and insurance claims data obtained from JMDC Inc, which handles claims data of 90 of the 1400 Japanese health insurance society members (mainly for company employees and their family members aged <75 years). Elderly people aged ≥75 years were not included in this study because at that age they move to the public late‐elderly health insurance system in Japan. Also, our claims data do not include those from small‐ and medium‐scaled enterprises. As for the data validation system in Japan, the Health Insurance Claims Review & Reimbursement services validate the coding. As the coding in Japan is directly linked to payments, the audit is supposed to be rigorous. Informed consent was waived following national guidelines in Japan.
The total number of people contained in the data numbered 4 203 282 from fiscal years 2005 to 2017. Altogether, 22 940 individuals who belonged to the organization during the study period and who had definitive diagnoses of atrial fibrillation or atrial flatter (International Classification of Diseases, Tenth Revision [ICD‐10]: I48) were extracted. After excluding individuals with a mechanical heart valve (n=28) or hematologic disorder (n=1124), those aged ≤20 years (n=207), and patients who had had anticoagulant therapy any time during the study period (n=11 848), we enrolled 9733 participants with atrial fibrillation who had never been prescribed anticoagulation during their follow‐up in our analysis (Figure 1). The study was approved by the Fukuoka University Clinical Research and Ethics Center (2017M008).
Figure 1.
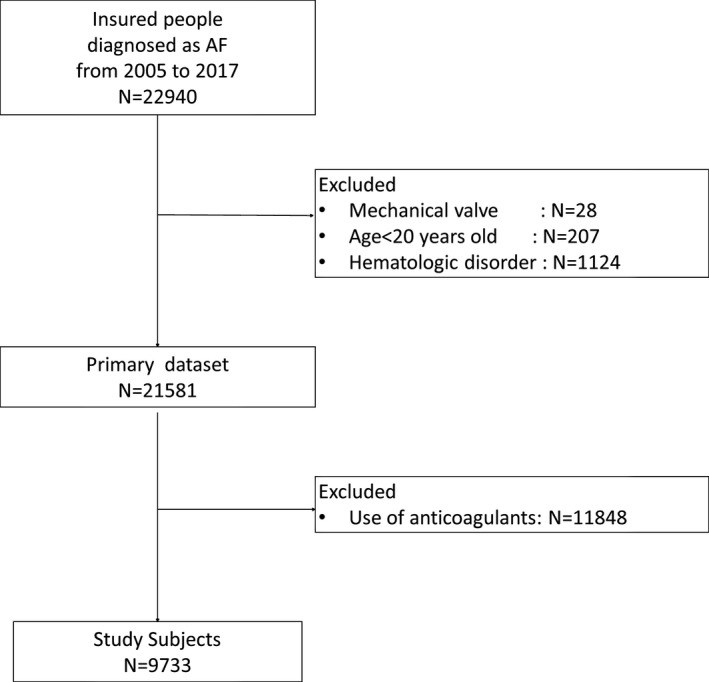
Flow diagram shows the process for selecting our study participants. AF indicates atrial fibrillation.
Follow‐Up and Outcomes
Participants were followed from the first diagnosis of atrial fibrillation to the last visit during the study period (fiscal years 2005–2017). Individuals who died or moved out of the health insurance society because of retirement, job change, or age ≥75 years during the follow‐up were censored. We defined outcomes as admission to a hospital because of ischemic stroke, intracranial hemorrhage, gastrointestinal bleeding, or other non‐traumatic bleeding. Total bleeding was defined as any bleeding (intracranial hemorrhage, gastrointestinal bleeding, and/or other non‐traumatic bleeding). Ischemic stroke was defined as ICD‐10 code I63. Intracranial hemorrhage was defined as ICD‐10 codes I60 (subarachnoid hemorrhage), I61 (intracerebral hemorrhage), and I62 (other and unspecified non‐traumatic intracranial hemorrhage). Gastrointestinal and other non‐traumatic bleeding was identified using claims database disease codes as listed in Tables S1 and S2.
Definition of Explanatory Variables
The CHA2DS2‐VASc score included the patient's age and sex; presence of congestive heart failure, hypertension, diabetes mellitus; history of ischemic stroke/transient ischemic attack, and/or vascular disease. Participants were divided into 2 groups by age (<65 or 65–74 years). Elderly people aged ≥75 years were not included in this study because they move to the public late‐elderly health insurance system in Japan. Congestive heart failure was defined according to the Charlson comorbidity index based on a prior diagnosis using the ICD‐10 code in the claims data.7 Hypertension was defined by ICD‐10 codes I10 to 115 based on the claims data, blood pressure levels of ≥140/90 mm Hg, or use of blood pressure‐lowering medication at the most recent health checkup before the diagnosis of atrial fibrillation. Diabetes mellitus was defined based on the Charlson comorbidity index using the ICD‐10 code in the claims data, a fasting serum glucose level of ≥6.99 mmol/L, a casual serum glucose level of ≥11.1 mmol/L, Hemoglobin A1c ≥6.5%, or use of glucose‐lowering medication at the health checkup. Previous ischemic stroke/transient ischemic attack was defined by ICD‐10 code G45 or I63 based on the claims data, or a disease history according to questionnaires administered at the health checkup. Vascular disease was defined based on the Charlson comorbidity index or questionnaires administered at the health checkup. We did not include suspicious diagnoses on the claims data. Finally, we calculated the CHA2DS2‐VASc score.8 A CHA2DS2‐VASc score of 0 in men or 1 in women classified them as at low risk; 1 in men or 2 in women as at intermediate risk; and ≥2 in men or ≥3 in women as at high risk.5, 9 Using the claims data, we identified the use of antiplatelet medication, which included cyclooxygenase inhibitors, adenosine diphosphate receptor antagonists, or other antiplatelet medications according to the Japanese pharmaceutical classification. We also identified peptic ulcer disease medications, which included proton‐pump inhibitors, histamine H2 receptor antagonists, and others. Antiarrhythmic agents were identified and classified as Ia, Ib, Ic, II, III, or IV, according to the Vaughan Williams classification, and digitalis.
Statistical Analysis
We used the 1‐way ANOVA for continuous variables and the Chi‐squared test for categorical variables. A person‐year approach was used to calculate incidence. We used the Cox proportional hazards model to adjust for competing risks of death from other causes using the Fine and Gray method10 to investigate the association between CHA2DS2‐VASc risk classification and the incidence of each outcome. The multivariable models included the use of antiplatelet agents, antiarrhythmic agents, and anti‐ulcer agents (for the outcomes of gastrointestinal bleeding and total bleeding only). Stratified analysis was also conducted for men and women. STATA release 14 (STATA Corp, College Station, TX) was used for statistical analyses. All reported P values are 2‐tailed, and the level of significance was set at P<0.05.
Results
Descriptive Analysis
In total, 7079 of the 9733 (72.7%) participants were men. Table 1 shows the distribution of the CHA2DS2‐VASc scores for men and women. Most men had CHA2DS2‐VASc scores of ≤3, and most women had scores of <4. In all, 3467 (35.6%) participants were classified as being at low risk (CHA2DS2‐VASc score of 0 in men or 1 in women), 2686 (27.6%) at intermediate risk (scores of 1 in men or 2 in women), and 3580 (36.8%) at high risk (scores of ≥2 in men or ≥3 in women). The results of descriptive analysis according to CHA2DS2‐VASc risk groups are shown in Table 2. The mean (SD) ages of CHA2DS2‐VASc low‐, intermediate‐, and high‐risk groups were 48.2 (11.0), 54.4 (10.6), and 58.2 (10.5), respectively. There was a trend toward older age in accordance with higher estimated CHA2DS2‐VASc risk. Participants aged ≥65 years were most frequently (31.7%) found in the high‐risk group. Participants with prevalent comorbidities were also most frequently observed in the high‐risk group: hypertension 86.5%, congestive heart failure 60.6%, and diabetes mellitus 33.4%. Cyclooxygenase inhibitors, class II antiarrhythmic agents, and proton‐pump inhibitors were the most frequently used antiplatelet, antiarrhythmic, and antiulcer agents, respectively. The frequencies of participants who were on antiplatelet and antiulcer agents increased with heightened CHA2DS2‐VASc risk. HAS‐BLED scores also significantly increased in accordance with increased CHA2DS2‐VASc‐defined risk.
Table 1.
Distribution of CHA2DS2‐VASc Scores
| CHA2DS2‐VASc Score | Total (N=9733) | Men (n=7079) | Women (n=2654) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 0 | 2507 (25.8) | 2507 (35.4) | 0 (0.0) |
| 1 | 2972 (30.5) | 2012 (28.4) | 960 (36.2) |
| 2 | 2138 (22.0) | 1464 (20.7) | 674 (25.4) |
| 3 | 1279 (13.1) | 741 (10.5) | 538 (20.3) |
| 4 | 556 (5.7) | 239 (3.4) | 317 (11.9) |
| 5 | 204 (2.1) | 99 (1.4) | 105 (4.0) |
| 6 | 51 (0.5) | 13 (0.2) | 38 (1.4) |
| 7 | 24 (0.2) | 4 (0.1) | 20 (0.8) |
| 8 | 2 (0.0) | 0 (0.0) | 2 (0.1) |
Table 2.
Analysis According to CHA2DS2‐VASc‐Determined Risk Groups
| CHA2DS2‐VASc* | |||||||
|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | P Value | ||||
| (n=3467) | (n=2686) | (n=3580) | |||||
| Sex | |||||||
| Men, % | 2507 | 72.3 | 2012 | 74.9 | 2560 | 71.5 | 0.009 |
| Age (y) mean, SD | 48.2 | 11.0 | 54.4 | 10.6 | 58.2 | 10.5 | 0.010 |
| ≥65 y, % | 0 | 0.0 | 394 | 14.7 | 1134 | 31.7 | |
| Comorbidities | |||||||
| Congestive heart failure, % | 0 | 0.0 | 610 | 22.7 | 2171 | 60.6 | <0.001 |
| Hypertension, % | 0 | 0.0 | 1330 | 49.5 | 3095 | 86.5 | <0.001 |
| Diabetes mellitus, % | 0 | 0.0 | 240 | 8.9 | 1195 | 33.4 | <0.001 |
| Past history of ischemic stroke /TIA, % | 0 | 0.0 | 0 | 0.0 | 604 | 16.9 | <0.001 |
| Vascular disease, % | 0 | 0.0 | 112 | 4.2 | 676 | 18.9 | <0.001 |
| Medication | |||||||
| Antiplatelet drug, % | |||||||
| Cyclooxygenase inhibitor, % | 271 | 7.8 | 319 | 11.9 | 777 | 21.7 | <0.001 |
| ADP receptor antagonist, % | 27 | 0.8 | 71 | 2.6 | 270 | 7.5 | <0.001 |
| Other antiplatelet, % | 0 | 0.0 | 1 | 0.0 | 9 | 0.3 | 0.002 |
| Antiulcer drug, % | |||||||
| Proton pomp inhibitor, % | 189 | 5.5 | 294 | 10.9 | 741 | 20.7 | <0.001 |
| H2 blocker, % | 172 | 5.0 | 212 | 7.9 | 371 | 10.4 | <0.001 |
| Other antiulcer drug, % | 727 | 21.0 | 600 | 22.3 | 834 | 23.3 | 0.062 |
| Antiarrhythmic drug | |||||||
| Ia, % | 307 | 8.9 | 184 | 6.9 | 223 | 6.2 | <0.001 |
| Ib, % | 19 | 0.5 | 22 | 0.8 | 43 | 1.2 | 0.012 |
| Ic, % | 535 | 15.4 | 421 | 15.7 | 410 | 11.5 | <0.001 |
| II, % | 387 | 11.2 | 308 | 11.5 | 601 | 16.8 | <0.001 |
| III, % | 5 | 0.1 | 10 | 0.4 | 111 | 3.1 | <0.001 |
| IV, % | 41 | 1.2 | 33 | 1.2 | 36 | 1.0 | 0.665 |
| Digitalis, % | 134 | 3.9 | 139 | 5.2 | 218 | 6.1 | <0.001 |
| HAS‐BLED mean, SD | 0.12 | 0.33 | 0.39 | 0.58 | 0.80 | 0.82 | <0.001 |
CHA2DS2‐VASc scores are interpreted as follows: low risk was 0 in men and 1 in women; intermediate risk was 1 in men and 2 in women; high risk was ≥2 in men and ≥3 in women.
Incidence and Crude and Hazard Ratios for Ischemic Stroke and Major Bleeding
During the mean 2.5‐year follow‐up, 143 ischemic strokes and 332 total bleeding events (59 intracerebral hemorrhage, 125 gastrointestinal bleeding, 148 others) were observed. Annual event rates were 0.58% (95% CI 0.49%–0.68%) for ischemic stroke and 1.17% (95% CI 1.03%–1.31%) for total bleeding events; 0.24% (95% CI 0.18%–0.31%) for intracerebral hemorrhage, 0.50% (95% CI 0.42%–0.60%) for gastrointestinal bleeding, and 0.60% (95% CI 0.50%–0.70%) for others.
Table 3 shows incidences and hazard ratios for ischemic stroke, total bleeding events, intracranial hemorrhage, gastrointestinal bleeding, and other hemorrhage according to the CHA2DS2‐VASc risk groups. Annual incidences of ischemic stroke increased with an elevation of predicted risks based on CHA2DS2‐VASc scores: 0.18% (95% CI 0.11%–0.27%) for low‐risk, 0.44% (95% CI 0.39%–0.77%) for intermediate‐risk, and 1.29% (95% CI 1.05%–1.58%) for high‐risk groups (P<0.001 for trend). Annual incidences for total bleeding also increased with elevation of predicted risks: 0.51% (95% CI 0.39%–0.67%) for low‐risk, 1.28% (95% CI 1.02%–1.58%) for intermediate‐risk, and 2.02% (95% CI 1.71%–2.37%]) for high‐risk groups (P<0.001 for trend). Similar findings were observed for each type of bleeding event (intracerebral hemorrhage, gastrointestinal bleeding, others; all P<0.001 for trend). Linear association of the CHA2DS2‐VASc risk group with ischemic stroke and bleeding events (intracranial hemorrhage, gastrointestinal bleeding, others) remained significant even after adjusting for medications, such as antiplatelet and antiarrhythmic agents (all P<0.001 for trend) (Table 3, Figure 2). When participants were stratified by sex, linear associations were still observed for each outcome (all P≤0.001 for trend) except for intracerebral hemorrhage in women (P=0.060 for trend) (Table 4, Figure 3).
Table 3.
Incidences and Crude and Adjusted Hazard Ratios for Ischemic Stroke and All Types of Bleeding/Hemorrhage
| Case/PY | % IR (95% CI) | P for Trend | Crude HR (95% CI) | P Value | P for Trend | Adjusted HR (95% CI) | P Value | P for Trend | |
|---|---|---|---|---|---|---|---|---|---|
| Brain infarction | |||||||||
| Low | 19/10 799 | 0.18 (0.11–0.27) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 29/6636 | 0.44 (0.39–0.77) | 2.25 (1.26–4.01) | 0.006 | 2.14 (1.20–3.84) | 0.010 | |||
| High | 95/7359 | 1.29 (1.05–1.58) | 6.15 (3.75–10.09) | <0.001 | 4.88 (2.90–8.23) | <0.001 | |||
| Any bleeding | |||||||||
| Low | 55/10 723 | 0.51 (0.39–0.67) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 84/6554 | 1.28 (1.02–1.58) | 2.30 (1.64–3.22) | <0.001 | 2.04 (1.45–2.86) | <0.001 | |||
| High | 147/7272 | 2.02 (1.71–2.37) | 3.43 (2.53–4.65) | <0.001 | 2.56 (1.87–3.51) | <0.001 | |||
| Intracranial hemorrhage | |||||||||
| Low | 9/10 818 | 0.08 (0.04–0.16) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 20/6654 | 0.30 (0.18–0.46) | 3.38 (1.56–7.29) | 0.002 | 3.34 (1.53–7.29) | 0.002 | |||
| High | 30/7471 | 0.40 (0.27–0.57) | 4.28 (2.07–8.85) | <0.001 | 4.23 (2.00–8.93) | <0.001 | |||
| Gastrointestinal bleeding | |||||||||
| Low | 21/10 791 | 0.19 (0.12–0.30) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 37/6636 | 0.56 (0.39–0.77) | 2.64 (1.55–4.48) | <0.001 | 2.36 (1.38–4.04) | 0.002 | |||
| High | 67/7399 | 0.91 (0.70–1.15) | 4.06 (2.51–6.57) | <0.001 | 3.18 (1.95–5.19) | <0.001 | |||
| Other bleeding | |||||||||
| Low | 32/10 765 | 0.30 (0.20–0.42) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 39/6644 | 0.59 (0.42–0.80) | 1.85 (1.15–2.95) | 0.010 | 1.81 (1.13–2.90) | 0.013 | |||
| High | 77/7383 | 1.04 (0.82–1.30) | 3.13 (2.08–4.72) | <0.001 | 2.76 (1.83–4.17) | <0.001 | |||
CHA2DS2‐VASc scores are interpreted as follows: low risk was 0 in men and 1 in women; intermediate risk was 1 in men and 2 in women; high risk was ≥2 in men and ≥3 in women. Total bleeding includes those with any following types of bleeding: intracranial, gastrointestinal, and other. Variables used for multivariate analyses were the use of antiplatelet agents and antiarrhythmic agents for all outcomes and use of anti‐ulcer and antiplatelet agents for total bleeding and gastrointestinal bleeding. HR indicates hazard ratio; IR, incident rate; PY, person‐year.
Figure 2.
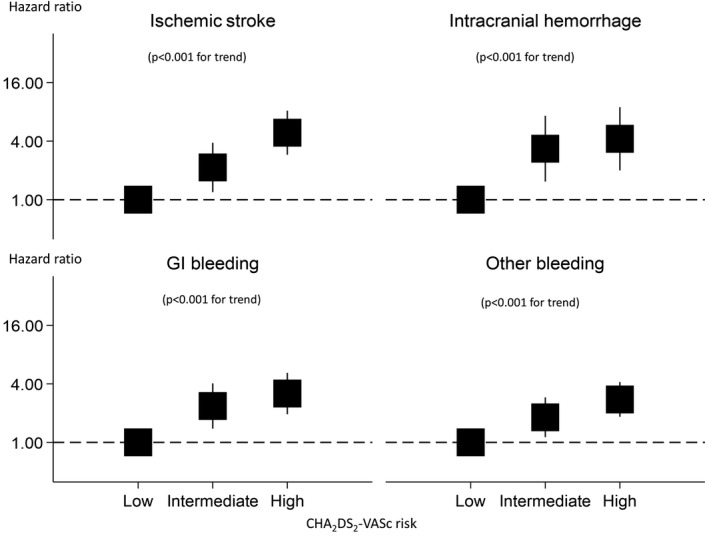
Hazard ratios for the CHA2DS2‐VASc risk score for ischemic stroke and various types of bleeding/hemorrhage. CHA2DS2‐VASc scores are interpreted as follows: low risk was 0 in men and 1 in women; intermediate risk was 1 in men and 2 in women; high risk was ≥2 in men and ≥3 in women. GI indicates gastrointestinal.
Table 4.
Incidences and Crude and Adjusted Hazard Ratios for Ischemic Stroke and All Types of Bleeding/Hemorrhage, by Sex
| Case/PY | % IR (95% CI) | P for Trend | Crude HR (95% CI) | P Value | P for Trend | Adjusted HR (95% CI) | P Value | P for Trend | |
|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||
| Ischemic stroke | |||||||||
| Low | 15/8187 | 0.18 (0.10–0.30 | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 18/5143 | 0.35 (0.21–0.55) | 1.74 (0.88–3.46) | 0.114 | 1.64 (0.82–3.28) | 0.160 | |||
| High | 66/5315 | 1.24 (0.96–1.58) | 5.68 (3.24–9.96) | <0.001 | 4.33 (2.37–7.90) | <0.001 | |||
| Major bleeding | |||||||||
| Low | 43/8134 | 0.53 (0.38–0.71) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 57/5092 | 1.12 (0.85–1.45) | 1.93 (1.30–2.86) | 0.001 | 1.73 (1.16–2.57) | 0.007 | |||
| High | 102/5233 | 1.95 (1.59–2.36) | 3.14 (2.21–4.45) | <0.001 | 2.44 (1.70–3.50) | <0.001 | |||
| Intracranial hemorrhage | |||||||||
| Low | 7/8205 | 0.09 (0.03–0.18) | 0.001 | 1.00 (Reference) | 0.001 | 1.00 (Reference) | 0.001 | ||
| Intermediate | 9/5168 | 0.17 (0.08–0.33) | 1.95 (0.74–5.12) | 0.175 | 2.01 (0.76–5.28) | 0.158 | |||
| High | 21/5384 | 0.39 (0.24–0.60) | 4.13 (1.81–9.40) | 0.001 | 4.22 (1.81–9.82) | 0.001 | |||
| Gastrointestinal bleeding | |||||||||
| Low | 17/8177 | 0.21 (0.12–0.33) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 25/5143 | 0.49 (0.31–0.72) | 2.15 (1.16–3.96) | 0.014 | 1.92 (1.04–3.56) | 0.038 | |||
| High | 49/5324 | 0.92 (0.68–1.21) | 3.81 (2.23–6.53) | <0.001 | 2.95 (1.70–5.14) | <0.001 | |||
| Other bleeding | |||||||||
| Low | 25/8166 | 0.31 (0.20–0.45) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 30/5143 | 0.58 (0.39–0.83) | 1.76 (1.03–3.00) | 0.039 | 1.71 (0.997–2.92) | 0.051 | |||
| High | 49/5321 | 0.92 (0.68–1.22) | 2.61 (1.62–4.23) | <0.001 | 2.33 (1.44–3.77) | 0.001 | |||
| Women | |||||||||
| Ischemic stroke | |||||||||
| Low | 4/2612 | 0.15 (0.04–0.39) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | ||
| Intermediate | 11/1492 | 0.74 (0.37–1.32) | 4.25 (1.35–13.38) | 0.013 | 4.07 (1.28–12.88) | 0.017 | |||
| High | 29/2044 | 1.42 (0.95–2.03) | 7.87 (2.74–22.56) | <0.001 | 6.78 (2.32–19.78) | <0.001 | |||
| Any bleeding | |||||||||
| Low | 12/2591 | 0.46 (0.24–0.81) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | 0.002 | ||
| Intermediate | 27/1462 | 1.85 (1.22–2.68) | 3.72 (1.90–7.29) | <0.001 | 3.16 (1.62–6.18) | 0.001 | |||
| High | 45/2039 | 2.21 (1.61–2.94) | 4.41 (2.35–8.27) | <0.001 | 2.89 (1.50–5.58) | 0.002 | |||
| Intracranial hemorrhage | |||||||||
| Low | 2/2613 | 0.08 (0.01–0.28) | 0.029 | 1.00 (Reference) | 0.041 | 1.00 (Reference) | 0.060 | ||
| Intermediate | 11/1485 | 0.74 (0.37–1.32) | 8.64 (1.93–38.81) | 0.005 | 8.13 (1.78–37.17) | 0.007 | |||
| High | 9/2086 | 0.43 (0.20–0.82) | 4.97 (1.07–23.06) | 0.041 | 4.61 (0.94–22.67) | 0.060 | |||
| Gastrointestinal bleeding | |||||||||
| Low | 4/2614 | 0.15 (0.04–0.39) | 0.001 | 1.00 (Reference) | 0.003 | 1.00 (Reference) | 0.013 | ||
| Intermediate | 12/1492 | 0.80 (0.42–1.40) | 4.85 (1.57–15.00) | 0.006 | 4.20 (1.34–13.13) | 0.014 | |||
| High | 18/2075 | 0.87 (0.51–1.37) | 5.16 (1.75–15.21) | 0.003 | 4.04 (1.35–12.13) | 0.013 | |||
| Other bleeding | |||||||||
| Low | 7/2599 | 0.27 (0.11–0.55) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | 0.001 | ||
| Intermediate | 9/1500 | 0.60 (0.27–1.14) | 2.13 (0.80–5.68) | 0.129 | 2.14 (0.81–5.64) | 0.126 | |||
| High | 28/2062 | 1.36 (0.90–1.96) | 4.81 (2.13–10.86) | <0.001 | 4.04 (1.76–9.27) | 0.001 | |||
CHA2DS2‐VASc scores are interpreted as follows: low risk was 0 in men and 1 in women; intermediate risk was 1 in men and 2 in women; high risk was ≥2 in men and ≥3 in women. Total bleeding includes those with any following types of bleeding: intracranial, gastrointestinal, and other. Variables used for multivariate analyses were the use of antiplatelet agents and antiarrhythmic agents for all outcomes and use of anti‐ulcer and antiplatelet agents for total bleeding and gastrointestinal bleeding. HR indicates hazard ratio; IR, incident rate; PY, person‐years.
Figure 3.
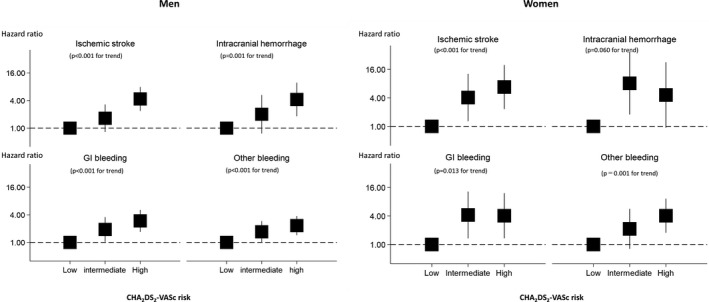
Hazard ratios for the CHA2DS2‐VASc risk score for ischemic stroke and various types of bleeding/hemorrhage, by sex. CHA2DS2‐VASc scores are interpreted as follows: low risk was 0 in men and 1 in women; intermediate risk was 1 in men and 2 in women; high risk was ≥2 in men and ≥3 in women. GI indicates gastrointestinal.
Discussion
This large‐scale observational study in the current real‐world setting in Japan comprehensively evaluated the natural history of patients with atrial fibrillation who were not on anticoagulants. Among them, the annual incidence of ischemic stroke was as high as 0.58%. Patients with atrial fibrillation were also at high risk of bleeding events (annual event rate 1.17%) even though not on oral anticoagulants. The risks of ischemic stroke and bleeding events increased with elevation of the CHA2DS2‐VASc score. The highest risks were observed for the high‐risk group with CHA2DS2‐VASc scores of ≥2 (for men) or ≥3 (for women) (annual incidence of ischemic stroke was 1.29% and that of total bleeding events was 2.02%). Similar findings were observed for different types of bleeding event (intracranial hemorrhage, gastrointestinal bleeding, others) or in stratified analysis according to sex.
The incidence of ischemic stroke among atrial fibrillation patients without anticoagulation for the entire cohort and by the CHA2DS2‐VASc score have been reported at 0.44% to 4.0% for overall,11, 12, 13, 14 0% to 0.95% for low‐risk patients,5, 9, 13, 14, 15, 16, 17 0.10% to 6.6% for intermediate‐risk patients,5, 9, 12, 13, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 and 2.4% to 6.2% for high‐risk patients.14, 16, 17, 26, 27, 28 Evidence from prior studies, however, was mainly derived from Western populations, with only limited evidence from a Japanese population. One Japanese study, which pooled 3 large‐scale atrial fibrillation registries in Japan,12 showed a somewhat lower incidence of ischemic stroke for the entire cohort (0.95 in the Shinken database, 1.39 in the J‐RHYTHM registry, 1.64 in the Fushimi atrial fibrillation registry) than those reported from other countries. Our study confirmed the findings of prior Japanese studies and revealed lower annual incidences of ischemic stroke (0.58% for the entire cohort; 0.18% for low‐, 0.44% for intermediate‐, and 1.29% for high‐risk groups) in Japanese cohorts than in Western populations.
Many prior studies also reported the incidence of total bleeding events in patients with atrial fibrillation. Guo et al23 reported that the annual rate of major bleeding among atrial fibrillation patients without anticoagulation was 2.0%. Olesen et al18 reported annual incidences of total bleeding events at 3.34% overall, with 1.15% for CHA2DS2‐VASc scores indicating low risk, 1.95% for intermediate risk, and 4.14% for high risk in atrial fibrillation patients without anticoagulation. Friberg et al15 reported that annual rates of total major bleeding and intracranial hemorrhage were 2.3% and 0.6%, respectively, although the study included patients on anticoagulation. Banerjee et al19 reported that annual rates of intracranial hemorrhage in patients without anticoagulation were 0.30% overall, 0.05% for low‐risk, 0.10% for intermediate‐risk, and 0.30% for high‐risk scores. Our results showed that the annual incidences of total bleeding and intracranial hemorrhage, respectively, were 1.17% and 0.24% overall, 0.51% and 0.08% for low‐risk, 1.28% and 0.30% for intermediate‐risk, and 2.02% and 0.40% for high‐risk scores. Although the overall risk of bleeding was comparable with those from prior studies, the risk of intracranial bleeding was equivalent or higher than those of prior studies, which were mainly from Western populations. The risks of intracranial hemorrhage among Japanese patients with atrial fibrillation who are not on anticoagulants appears to be higher than that among Western patients, which seems compatible with prior studies that showed higher rates of intracerebral hemorrhage in Japan and East Asian countries than in Western countries.30
Oral anticoagulation is an established strategy to prevent ischemic stroke and other thromboembolic events among patients with atrial fibrillation, although they also increase the risk of bleeding.5, 31 The risks and benefits of oral anticoagulants depend on an absolute reduction in the risk of ischemic stroke and an absolute increase in bleeding events associated with the treatment. This study showed a relatively low incidence of ischemic stroke and a relatively high incidence of intracranial bleeding in Japanese patients with atrial fibrillation who were not on oral anticoagulants. The present guidelines for managing atrial fibrillation in Japan recommend anticoagulant therapy for patients with a CHADS2 score of ≥2. However, physicians should be aware that bleeding risk also increases with the elevation of ischemic risk. Further investigation based on the effects of oral anticoagulants and the absolute risks of both ischemic and bleeding events are needed in Japan to establish the effective antithrombotic therapy for patients with atrial fibrillation.
Limitations
The strengths of this study include the real‐world setting, large sample size, and comprehensive assessment of outcomes, including gastrointestinal bleeding and other bleeding events as well as ischemic stroke and intracranial hemorrhage. There are some weaknesses as well. One weakness was selection bias because of exclusion of old people aged ≥75 years who move to the public insurance system. Another limitation is that outcome events and other comorbidities were identified using disease codes of the claims data. Although some studies32, 33 reported the validity of Charlson comorbidity index or comorbidities based on claims data, there may be some uncertainty about their accuracy.
Conclusions
We evaluated the natural history of Japanese patients with atrial fibrillation in a large‐scale observational study using real‐world data. The risks of ischemic stroke and each type of bleeding event were high among Japanese patients with atrial fibrillation who were not on oral anticoagulants, particularly among those with high CHA2DS2‐VASc scores. Optimal management strategies, blood pressure‐lowering treatment, and glucose‐lowering therapy are required for these high‐risk patients with atrial fibrillation. Further studies are needed to establish the effective management considering racial differences.
Sources of Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 18K17404 and 19H03879.
Disclosures
None.
Supporting information
Table S1. Definition of Gastrointestinal Bleeding
Table S2. Definition of Other Bleeding
Acknowledgments
We thank JMDC Inc. for providing data. We thank Nancy Schatken, BS, MT(ASCP), from Edanz Group (https://jp-author-services.edanzgroup.com/) for editing a draft of this manuscript.
(J Am Heart Assoc. 2020;9:e014574 DOI: 10.1161/JAHA.119.014574.)
References
- 1. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 2. Majeed A. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2002;86:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA. 2003;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 4. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K. Prevalence of atrial fibrillation in the general population of Japan : an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–107. [DOI] [PubMed] [Google Scholar]
- 5. Lip GYH, Skjøth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular af with 0 or 1 stroke risk factor based on the CHA2DS2‐VASc score. J Am Coll Cardiol. 2015;65:1385–1394. [DOI] [PubMed] [Google Scholar]
- 6. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham study. Stroke. 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 7. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 9. Allan V, Banerjee A, Shah AD, Patel R, Denaxas S, Casas JP, Hemingway H. Net clinical benefit of warfarin in individuals with atrial fibrillation across stroke risk and across primary and secondary care. Heart. 2017;103:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 11. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes results from the national registry of atrial fibrillation. JAMA;2001:285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki S, Yamashita T, Okumura K, Atarashi H, Akao M, Ogawa H, Inoue H. Incidence of Ischemic Stroke in Japanese Patients With Atrial Fibrillation Not Receiving Anticoagulation Therapy. Circ J. 2015;79:432–438. [DOI] [PubMed] [Google Scholar]
- 13. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 14. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med.1994; 154:1449–1457. [PubMed] [Google Scholar]
- 15. Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 16. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lip GYH, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation. Stroke. 2010;41:2731–2738. [DOI] [PubMed] [Google Scholar]
- 18. Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, Raunsø J, Tolstrup JS, Hansen PR, Gislason GH, Torp‐Pedersen C. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739–749. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee A, Lane DA, Torp‐Pedersen C, Lip GYH. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107:584–589. [DOI] [PubMed] [Google Scholar]
- 20. Komatsu T, Tachibana H, Satoh Y, Ozawa M, Kunugita F, Ueda H, Nakamura M. Relationship between CHA2DS2‐VASc scores and ischemic stroke/cardiovascular events in Japanese patients with paroxysmal atrial fibrillation without receiving anticoagulant therapy. J Cardiol. 2012;59:321–328. [DOI] [PubMed] [Google Scholar]
- 21. Larsen TB, Lip GY, Skjøth F, Due KM, Overvad K, Hvilsted Rasmussen L. Added predictive ability of the CHA 2 DS 2 VASc risk score for stroke and death in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:335–342. [DOI] [PubMed] [Google Scholar]
- 22. Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GY, Dorian P, Shestakovska O, Connolly SJ. The CHA2DS2‐VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–176. [DOI] [PubMed] [Google Scholar]
- 23. Guo Y, Apostolakis S, Blann AD, Wang H, Zhao X, Zhang Y, Zhang D, Ma J, Wang Y, Lip GY. Validation of contemporary stroke and bleeding risk stratification scores in non‐anticoagulated Chinese patients with atrial fibrillation. Int J Cardiol. 2013;168:904–909. [DOI] [PubMed] [Google Scholar]
- 24. Huang D, Anguo L, Yue WS, Yin L, Tse HF, Siu CW. Refinement of ischemic stroke risk in patients with atrial fibrillation and CHA2DS2‐VASc score of 1. Pacing Clin Electrophysiol. 2014;37:1442–1447. [DOI] [PubMed] [Google Scholar]
- 25. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chen SA. Using the CHA2DS2‐VASc score for refining stroke risk stratification in ‘low‐risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. [DOI] [PubMed] [Google Scholar]
- 26. Forslund T, Wettermark B, Wändell P, von Euler M, Hasselström J, Hjemdahl P. Risks for stroke and bleeding with warfarin or aspirin treatment in patients with atrial fibrillation at different CHA2DS2VASc scores: experience from the Stockholm region. Eur J Clin Pharmacol. 2014;70:1477–1485. [DOI] [PubMed] [Google Scholar]
- 27. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2‐VASc score of 1. J Am Coll Cardiol. 2015;65:225–232. [DOI] [PubMed] [Google Scholar]
- 28. van den Ham HA, Klungel OH, Singer DE, Leufkens HGM, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2‐VASc risk scores predicting stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2015;66:1851–1859. [DOI] [PubMed] [Google Scholar]
- 29. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chen SA. Should atrial fibrillation patients with 1 additional risk factor of the CHA 2 DS 2 ‐VASc Score (Beyond Sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–642. [DOI] [PubMed] [Google Scholar]
- 30. The Japan stroke Society . Japanese Guidelines for the Management of Stroke 2015. Kyōwakikaku, 2017.
- 31. Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non‐valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;20:CD001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimura T, Sugitani T, Nishimura T, Ito M. Validation and recalibration of Charlson and Elixhauser comorbidity indices based on data from a Japanese insurance claims database. Japanese J Pharmacoepidemiol. 2019;24:53–64. [Google Scholar]
- 33. Hara K, Tomio J, Svensson T, Ohkuma R, Svensson AK, Yamazaki T. Association measures of claims‐based algorithms for common chronic conditions were assessed using regularly collected data in Japan. J Clin Epidemiol. 2018;99:84–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of Gastrointestinal Bleeding
Table S2. Definition of Other Bleeding


