Abstract
Background
Intravascular ultrasound (IVUS) guidance during percutaneous coronary intervention (PCI) offers tomographic images of the coronary vessels, allowing optimization of stent implantation at the time of PCI. However, the long‐term beneficial effect of IVUS over PCI guided by coronary angiography (CA) alone remains under question. We sought to investigate the outcomes of IVUS‐guided compared with CA‐guided PCI.
Methods and Results
We performed a comprehensive search of PubMed, Medline, and Cochrane Central Register, looking for randomized controlled trials and observational studies that compared PCI outcomes of IVUS with CA. Data were aggregated for the primary outcome measure using the random‐effects model as pooled risk ratio (RR). The primary outcomes were the rate of cardiovascular death, need for target lesion revascularization, occurrence of myocardial infarction, and rate of stent thrombosis. A total of 19 studies met the inclusion criteria, comprising 27 610 patients divided into IVUS (n=11 513) and CA (n=16 097). Compared with standard CA‐guided PCI, we found that the risks of cardiovascular death (RR, 0.63; 95% CI, 0.54–0.73), myocardial infarction (RR, 0.71; 95% CI, 0.58–0.86), target lesion revascularization (RR, 0.81; 95% CI, 0.70–0.94), and stent thrombosis (RR, 0.57; 95% CI, 0.41–0.79) were all significantly lower using IVUS guidance.
Conclusions
Compared with standard CA‐guided PCI, the use of IVUS imaging guidance to optimize stent implantation is associated with a reduced risk of cardiovascular death and major adverse events, such as myocardial infarction, target lesion revascularization, and stent thrombosis.
Keywords: coronary imaging, coronary intervention, intravascular ultrasound, optical coherence tomography
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Stent, Meta Analysis, Angiography, Ultrasound
Clinical Perspective
What Is New?
This study demonstrated that use of intravascular ultrasound imaging–guided percutaneous coronary intervention was associated with lower cardiovascular death, myocardial infarction, target lesion revascularization, and stent thrombosis, compared with coronary angiography alone.
Our study encourages the routine use of intracoronary imaging to optimize coronary stent implantation.
What Are the Clinical Implications?
Intracoronary imaging is an innovative technology presented to overcome the limitations of standard routine angiography.
This technique offers more details about the coronary atherosclerotic plaque, vessel wall, and facilitated stent delivery, which subsequently improve the outcomes of percutaneous coronary interventions.
The routine use of intracoronary imaging to optimize coronary stent implantation is encouraged.
Introduction
Percutaneous coronary intervention (PCI) is a mainstay for the treatment of coronary artery disease, a major cause of morbidity and mortality worldwide.1 Although coronary angiography (CA) is the standard imaging modality used for coronary stent implantation, it is limited to 2‐dimensional projections of coronary anatomical characteristics. This limitation can be overcome using high‐resolution intracoronary imaging modalities, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), that offer detailed 3‐dimensional tomographic views of coronary plaque, blood vessel, and stent morphological characteristics, thus enabling greater information to guide optimal stent implantation.2, 3, 4, 5
Several randomized controlled trials (RCTs) and observational studies examining intracoronary imaging during and after stent implantation have demonstrated that IVUS‐guided stent implantation was associated with the reduction of major adverse cardiac events and target vessel revascularization in patients with complex coronary lesions, including long lesions, severe calcification, bifurcations, chronic total occlusions, and unprotected left main disease.3, 4, 5, 6, 7 However, despite accumulating data supporting the use of IVUS to optimize PCI, the adoption of intracoronary imaging to guide stent implantation in real‐world interventional clinical practice remains low, in part because of a perceived lack of supporting clinical evidence.8 Therefore, we sought to synthesize all available data by conducting a comprehensive meta‐analysis exploring the clinical outcomes of PCI guided by angiography with adjunctive IVUS imaging compared with angiography alone.
Methods
Inclusion Criteria
We searched Medline, EMBASE, and Cochrane library for RCTs or observational studies that compared IVUS with CA outcomes as invasive imaging modalities for guiding PCI with stent implantation. Studies using both bare metal stents and drug‐eluting stents (DESs) were eligible. In an attempt to decrease the risk of bias inherent with including observational studies, only nonrandomized studies that used matching algorithms were included. We also excluded all the studies that used intravascular imaging for stent implantation in the presence of flow‐limiting dissections or residual stenosis after plain balloon angioplasty. The analysis was restricted only to studies in which at least 100 patients were enrolled in each treatment arm. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The combinations of medical terms “percutaneous coronary intervention,”” intravascular ultrasound,” “intravascular imaging,” “IVUS,” and/or “coronary angiography” were used to conduct a comprehensive search in the above‐mentioned databases. All searches were restricted to studies conducted in human subjects published from the date of the databases’ inception through April 2019. There was no language restriction or use of additional filters. A cross‐reference check of previously published reviews and/or meta‐analyses on this topic was performed. The literature searches and all analyses were conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement for network meta‐analyses9 (Figure 1).
Figure 1.
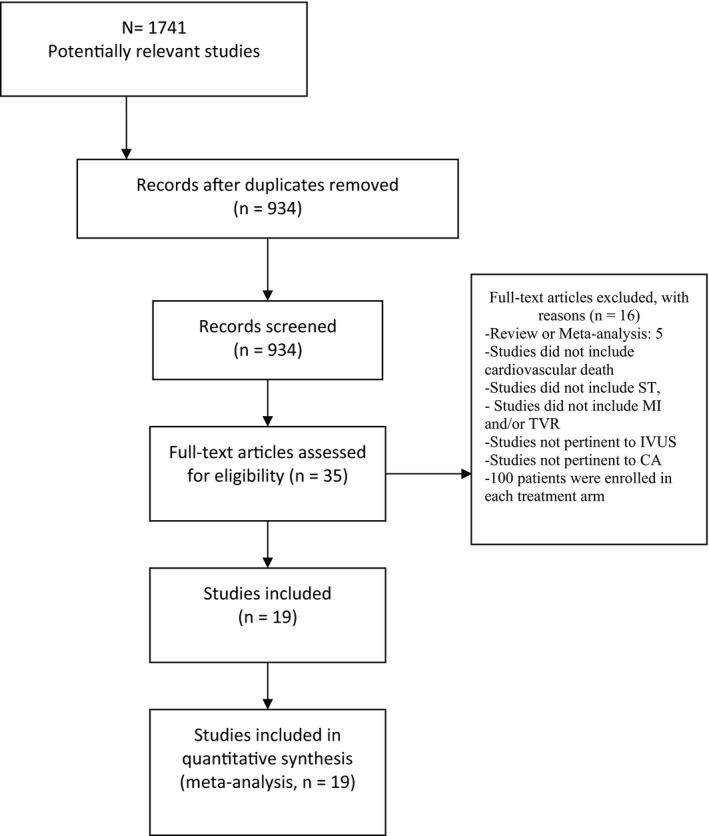
Flow diagram of the literature search. CA indicates coronary angiography; IVUS, intravascular ultrasound; MI, myocardial infarction; ST, stent thrombosis; TVR, target vessel revascularization.
End Point Outcomes
The primary outcome of this meta‐analysis was cardiovascular mortality. Secondary end points were myocardial infarction (MI), target lesion revascularization (TLR), and stent thrombosis (ST).
Data Extraction and Statistical Analysis
After careful title checking and reviewing full texts of all the studies, 2 investigators (E.O. and M.A.) independently verified the inclusion criteria and abstracted the data from all the articles that met the inclusion criteria. Disagreements were resolved by consensus. All extracted data from the included studies were collected into a spreadsheet and verified by a third author (Y.A.). Summarized and weighted means and rates from each individual trial or observation study for baseline characteristics were reported. Data were pooled for the primary and secondary outcomes using summary risk ratios (RRs) and 95% CIs using random effects models, while taking into account the within and between study variance. Two‐sided P values were calculated, with P<0.05 considered significant for all tests. Heterogeneity was assessed by means of the I2 statistic (with an I2 value >50% being considered the result of severe heterogeneity).
Sensitivity analysis was performed for different study design and baseline characteristics to evaluate for the consistency of the main results across all studies that were included in the analysis. Sensitivity analysis and meta‐regression were used to explore the treatment effect and elucidate the relationship between confounding factors and IVUS guidance. We used a linear regression (Littenberg and Moses linear model) approach.10 Random‐effects model was selected because of the difference in the designs of the studies included in this meta‐analysis (observation versus RCTs). A 2‐tailed P<0.05 was considered statistically significant. All data and supporting materials have been provided with the published article, and all supporting data are available within the article.
The methodological quality of observational studies was assessed by the Newcastle‐Ottawa scale, which consists of 3 factors: patient selection, comparability of the study groups, and assessment of outcomes. A score of 0 was considered as an exclusion criterion from a statistical standpoint, and we selected a score cutoff of 0.7 and 1 for observational studies and RCTs, respectively, to ensure the consistency of our meta‐analysis results.11, 12
Results
Nineteen eligible studies, including a total of 27 637 patients, were identified in the current meta‐analysis, with 11 540 patients in the IVUS guidance group and 16 097 patients in the CA guidance group. The detailed study design included in this meta‐analysis is summarized in Table 1.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Patient characteristics are summarized in Table 2.29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Briefly, 16 of 19 studies (84.2%) used DESs as the primary stent type (1 study used a combination of both bare metal stents and DESs). A total of 10 studies were adjusted observational studies with propensity score matching, and 9 studies were RCTs. The follow‐up period ranged from 6 months to a maximum of 64 months. Left main lesions constituted 26.7% of the treated vessels in the CA group and 15.4% of the treated vessels in the IVUS group. A total of 71.6% of the CA‐guided PCI group had a diagnosis of diabetes mellitus, compared with 70.9% in the IVUS‐guided group. Finally, 43.3% and 34.4% of the IVUS and CA groups, respectively, underwent PCI for acute coronary syndromes.
Table 1.
Study Design of the Included Studies
| Study | Year | No. of Patients (CA/IVUS) | Design | Stent Type | Follow‐Up, mo |
|---|---|---|---|---|---|
| AIR‐CTO13 | 2015 | 115/115 | RCT | DES | 12 |
| AVID14 | 2009 | 406/394 | RCT | BMS | 12 |
| Chen et al15 | 2012 | 123/123 | Observational, PSM | DES | 12 |
| Choi et al16 | 2019 | 4331/1674 | Observational | DES | 64 |
| CTO‐IVUS17 | 2015 | 201/201 | RCT | DES | 12 |
| de la Torre Hernandez18 | 2014 | 505/505 | Observational, PSM | DES | 36 |
| DIPOL19 | 2007 | 80/83 | RCT | BMS | 6 |
| EXELLENT20 | 2013 | 463/463 | Observational, PSM | DES | 12 |
| Gao et al21 | 2014 | 291/291 | Observational, PSM | DES | 12 |
| HOME DES IVUS22 | 2010 | 105/105 | RCT | DES | 18 |
| Hong et al23 | 2014 | 201/201 | Observational, PSM | DES | 24 |
| IVUS‐XPL24 | 2015 | 700/700 | RCT | DES | 12 |
| Kim et al (RESET)25 | 2013 | 274/269 | RCT | DES | 12 |
| MATRIX26 | 2011 | 548/548 | Observational, PSM | DES | 24 |
| OPTICUS27 | 2001 | 275/273 | RCT | BMS | 12 |
| Roy et al28 | 2008 | 884/884 | Observational, PSM | DES | 12 |
| ULTIMATE29 | 2018 | 724/724 | RCT | DES | 12 |
| Wakabayashi et al30 | 2012 | 637/637 | Observational, PSM | BMS/DES | 12 |
| Witzenbichler et al31 | 2014 | 5234/3349 | Observational | DES | 12 |
AIR‐CTO indicates Angiographic and Clinical Comparisons of Intravascular Ultrasound‐Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; BMS, bare metal stent; CA, coronary angiography; CTO‐IVUS, Intravascular Ultrasound‐Guided Chronic Total Occlusion Intervention; DES, drug‐eluting stent; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS, intravascular ultrasound; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; REST, Real Safety and Efficacy of a 3‐Month Dual Antiplatelet Therapy Following Zotarolimus‐Eluting Stents Implantation; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; PSM, propensity score matching; RCT, randomized controlled trial; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial.
Table 2.
Baseline Clinical Characteristics of the Included Studies
| Study | Age, y | DM, % | ACS, % | Hypertension, % | Men, % | LVEF, % | LM, % | LAD, % | LCx, % | RCA, % |
|---|---|---|---|---|---|---|---|---|---|---|
| AIR‐CTO13 | 66/67 | 27/30 | 24/29 | 70/75 | 80/89 | 56/55 | 3/0 | 36/44 | 15/21 | 46/35 |
| AVID14 | 63/62 | 17/15 | NA | 45/46 | 68/73 | 55/53 | 1/1 | 37/40 | 18/15 | 32/35 |
| Chen et al15 | 64/63 | 18/19 | 11/15 | 61/67 | 74/81 | 60/61 | 27/42 | 61/40 | 9/14 | 3/4 |
| Choi et al16 | 62/59 | 25/22 | 8/9 | 47/43 | 68/72 | 57/58 | 2/8 | 50/59 | 21/12 | 34/25 |
| CTO‐IVUS17 | 61/61 | 34/35 | 37/28 | 64/63 | 81/81 | 57/57 | 0/0 | 47/42 | 16/14 | 37/44 |
| de la Torre Hernandez18 | 67/66 | 35/36 | 61/59 | 64/68 | 79/80 | 55/55 | 100/100 | NA | NA | NA |
| DIPOL19 | 54/56 | 10/13 | 39/38 | 58/61 | 73/71 | 48/52 | 0/0 | 46/41 | 24/26 | 30/32 |
| EXELLENT20 | 63/63 | 38/37 | 52/51 | 74/73 | 63/66 | NA | 0/0 | 23/54 | 23/20 | 27/27 |
| Gao et al21 | 67/66 | 34/32 | 10/9 | 72/72 | 77/81 | 57/58 | 100/100 | 13/10 | 8/6 | 10/7 |
| HOME DES IVUS22 | 60/59 | 45/42 | 60/72 | 71/67 | 71/73 | NA | 4/3 | 54/56 | 15/11 | 24/29 |
| Hong et al23 | 62/62 | 31/30 | 42/39 | 60/58 | 77/77 | NA | 1/1 | 34/44 | 25/16 | NA |
| IVUS‐XPL24 | 64/64 | 37/36 | 49/49 | 63/65 | 69/69 | 62/63 | 0/0 | 60/65 | 15/14 | 15/14 |
| Kim et al (RESET)25 | 64/62 | 31/30 | 47/49 | 66/61 | 55/66 | 54/55 | 54/55 | 57/50 | 18/21 | 24/29 |
| MATRIX26 | 64/64 | 31/32 | 36/33 | 81/81 | 74/74 | NA | 3/3 | 51/51 | 38/38 | 28/28 |
| OPTICUS27 | 62/60 | 17/17 | 32/36 | 52/48 | 78/77 | 58/56 | 0/0 | 50/51 | 14/18 | 35/30 |
| Roy et al28 | 66/66 | 34/36 | 61/62 | 82/82 | 70/69 | 48/47 | 2.3/2 | 33/3 | 23/25 | 34/34 |
| ULTIMATE29 | 65/66 | 30/31 | 67/64 | 71/72 | 74/73 | 61/60 | 10/9 | 47/47 | 17/17 | 25/28 |
| Wakabayashi et al30 | 67/67 | 40/42 | 56/58 | 90/91 | 69/68 | NA | 4/4 | 25/26 | 23/23 | 32/32 |
| Witzenbichler et al31 | 63/64 | 31/33 | 43/43 | 78/81 | 73/75 | 60/53 | 3/4 | NA | NA | NA |
Data are presented as intravascular ultrasound guidance/coronary angiography guidance. ACS indicates acute coronary syndrome; AIR‐CTO, Angiographic and Clinical Comparisons of Intravascular Ultrasound‐Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound‐Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; DM, diabetes mellitus; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; LAD, left anterior descending artery; LCx, left circumflex artery; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; RCA, right coronary artery; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial.
From the 11 540 patients included in the IVUS group and 16 097 patients included in the CA group, IVUS‐guided PCI reduced the risk of cardiovascular death compared with CA alone (216 [1.9%] versus 627 [3.9%]; RR, 0.63; 95% CI, 0.54–0.73; heterogeneity χ2=15.65; I2=0%; P<0.001). IVUS‐guided PCI also reduced postprocedural MI (314 [2.7%] versus 645 [4.0%]; RR, 0.71; 95% CI, 0.58–0.86), TLR (514 [4.5%] versus 841 [5.2%]; RR, 0.81; 95% CI, 0.70–0.94), and ST (160 [1.4%] versus 360 [2.2%]; RR, 0.57; 95% CI, 0.41–0.79). Statistical heterogeneity for MI was I2=32% (P=0.09); and for TLR, I2=36% (P=0.06). Higher heterogeneity was observed with ST (I2=40%; P=0.04). The primary and secondary outcomes are presented in Figures 2, 3, 4 through 5. Furthermore, meta‐regression analysis revealed that the beneficial effect of IVUS when compared with angiography‐guided PCI remained significant regardless of diabetes mellitus, hypertension, sex, acute coronary syndrome, or left main lesion (Figures S1 through S7).
Figure 2.
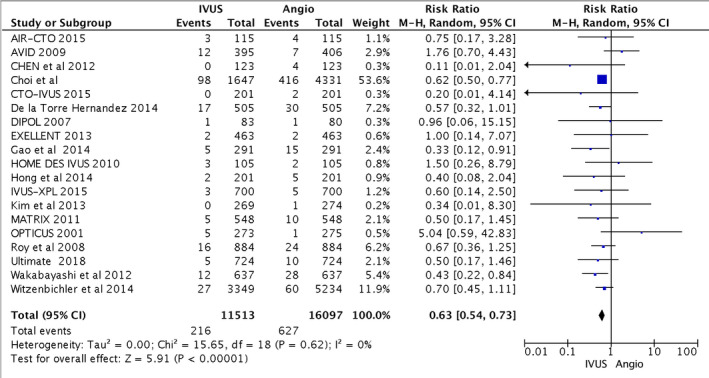
Forest plots for major adverse cardiovascular events. Risk ratio of cardiovascular death associated with intravascular ultrasound (IVUS)–guided compared with angiography (Angio)–guided percutaneous coronary intervention. AIR‐CTO indicates Angiographic and Clinical Comparisons of Intravascular Ultrasound‐Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial; M‐H: Mantel–Haenszel.
Figure 3.
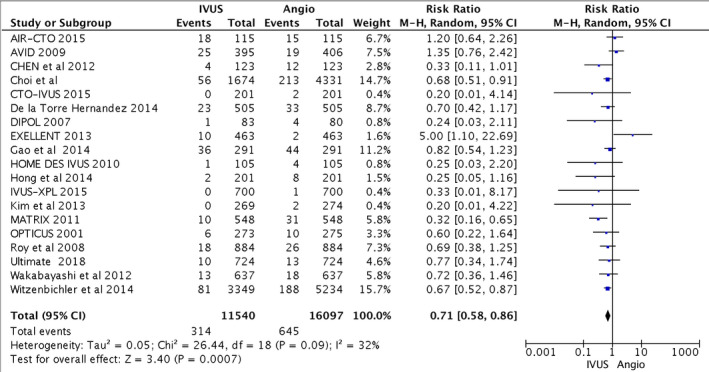
Forest plots for major adverse cardiovascular events. Risk ratio of myocardial infarction associated with intravascular ultrasound (IVUS)–guided compared with angiography (Angio)–guided percutaneous coronary intervention. AIR‐CTO indicates Angiographic and Clinical Comparisons of Intravascular Ultrasound‐Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial; AIR‐CTO indicates, Angiographic and Clinical Comparisons of Intravascular Ultrasound‐ Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial; M‐H, Mantel–Haenszel.
Figure 4.
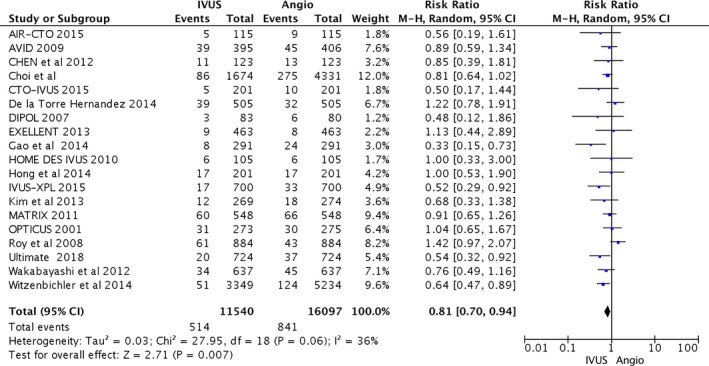
Forest plots for major adverse cardiovascular events. Risk ratio of target lesion revascularization associated with intravascular ultrasound (IVUS)–guided compared with angiography (Angio)–guided percutaneous coronary intervention. AIR‐CTO indicates Angiographic and Clinical Comparisons of Intravascular Ultrasound‐Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial; M‐H, Mantel–Haenszel.
Figure 5.
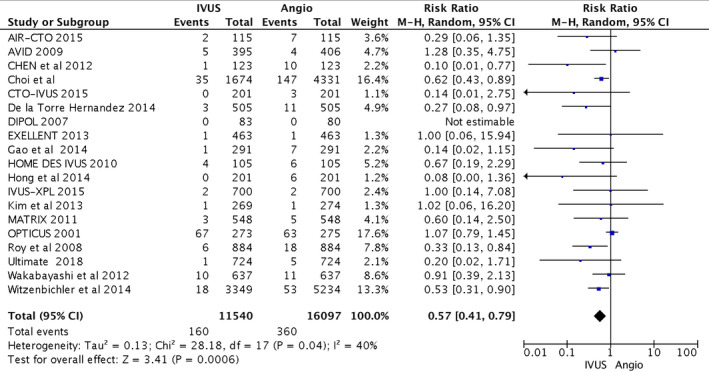
Forest plots for major adverse cardiovascular events. Risk ratio of stent thrombosis with intravascular ultrasound (IVUS)–guided compared with angiography (Angio)–guided percutaneous coronary intervention. AIR‐CTO indicates Angiographic and Clinical Comparisons of Intravascular Ultrasound‐ Versus Angiography‐Guided Drug‐Eluting Stent Implantation for Patients With Chronic Total Occlusion Lesions; AVID, Angiography Versus Intravascular Ultrasound‐Directed Stent Placement; CTO‐IVUS, Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention; DIPOL, Direct Stenting vs Optimal Angioplasty Trial; EXCELLENT, Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting; HOME DES IVUS, Long‐Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance; IVUS‐XPL, Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MATRIX, Comprehensive Assessment of Sirolimus‐Eluting Stents in Complex Lesions; OPTICUS, Optimization With ICUS to Reduce Stent Restenosis; ULTIMATE, Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions trial; M‐H, Mantel–Haenszel.
Discussion
The principal findings from this study are as follows: meta‐analysis of 19 studies showed that, compared with CA alone, IVUS‐guided PCI (1) decreased the risk of cardiovascular death, with a relative risk reduction of 33%; (2) lowered MI risk, with a number needed to treat of 91 to prevent 1 MI; (3) decreased the need for TLR; and (4) was associated with less ST.
Since the inception of PCI decades ago, x‐ray CA has been the standard imaging platform used to guide coronary intervention procedures. However, a major drawback of CA is that it relies on 2‐dimensional projections to define the structure of complex 3‐dimensional coronary artery lumens. In the modern era, through the use of IVUS and OCT, intracoronary imaging can overcome the inherent limitations of CA by providing high‐resolution axial cross‐sectional images with detailed tomographic structural information on lesion and vessel characteristics. Thus, intracoronary imaging promotes an enhanced understanding of coronary anatomical characteristics at the time of PCI, facilitating protocols to optimize coronary stent sizing, avoid stent malapposition and underexpansion, and identify unrecognized complications, such as edge dissection. Overall, by precisely guiding stent implantation at the index procedure, intracoronary imaging aims to improve short‐ and long‐term cardiovascular PCI outcomes.
In the bare metal stent era, a meta‐analysis of 7 randomized trials showed a neutral effect on mortality and MI over a follow‐up period of 6 months to 2.5 years; however, IVUS use was associated with a reduction in both angiographic restenosis at 6 months and the rate of subsequent revascularization.32 These early encouraging results coupled with the introduction of DESs paved the way for IVUS‐guided DESs as an attractive strategy to further improve PCI outcomes. Indeed, the evidence base supporting the theoretical benefits of an IVUS‐guided DES implantation approach has been increasing over the past several years, with several meta‐analyses and randomized studies showing a decrease in major adverse cardiac events,33, 34, 35, 36, 37, 38, 39, 40 particularly in complex lesions and high‐risk patients.
The recently completed all‐comers ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “AllComers” Coronary Lesions) trial, enrolling 1148 patients in the largest randomized IVUS‐guided PCI trial to date, demonstrated that the routine use of IVUS during DES implantation reduced cardiovascular death and ST when compared with CA alone. These findings are reinforced by the current study, the largest IVUS‐guided PCI meta‐analysis performed pooling 19 observational studies and RCTs with a total of 29 637 patients with complex and noncomplex coronary lesions and long‐term follow‐up between 6 and 64 months. Although the variation in the included studies created low to moderate outcome heterogeneity, the overall I2 remained <50%. To investigate the sources of moderated heterogeneity in our study, additional meta‐regression analyses were performed to evaluate the relationship between study characteristics and IVUS use, revealing that IVUS guidance continued to demonstrate benefit over CA‐guided PCI, irrespective of the presence of diabetes mellitus, acute coronary syndrome, hypertension, sex, or left main lesion location.
Overall, accumulating clinical studies support the use of intracoronary imaging to improve outcomes after stent implantation. However, despite increasingly compelling data, the use of intracoronary imaging guidance during PCI procedures continues to be significantly underused in the United States. A recent report showed that intracoronary imaging (IVUS and/or OCT) in the United States increased from 2.1% in 2004 to 6.6% in 2014, heavily weighted toward IVUS (94.3% IVUS versus 6.6% OCT).41 The infrequent use of intracoronary imaging by many operators may be explained by perceived time or cost constraints or a belief that visual assessment of coronary anatomical characteristics with x‐ray angiography is sufficient.42, 43 However, multiple studies have demonstrated that angiographic lesion assessment alone is severely limited, especially in complex lesions,44 and that intracoronary imaging is cost‐effective by preventing the need for repeated procedures.45 Although the data supporting the benefit of intracoronary imaging‐guided PCI on cardiovascular outcomes are limited to IVUS to date, a recent trial comparing IVUS with a protocolized OCT stent implantation algorithm demonstrated similar short‐term procedural results,46 implying that OCT may offer similar long‐term benefits to IVUS. The ongoing ILUMIEN IV (Optical Coherence Tomography [OCT] — Guided Coronary Stent Implantation Compared to Angiography) trial, which will enroll up to 3650 patients, focusing on high‐risk, complex disease at 125 international centers, comparing outcomes after coronary stent implantation using OCT with routine CA will provide further important insight into the generalizability of an intracoronary imaging–guided PCI approach.47
This study has several limitations. First, as specific IVUS criteria for optimal stent implantation were not precisely described or consistent among studies, preintervention imaging assessment and stent postdilation were often at the discretion of the operator, likely leading to variability in the final stent result. Second, studies included in the current analysis used both first‐ and second‐generation DESs as well as bare metal stents, which could affect the outcome. Third, our analysis included a mixture of lesion locations and multivessel disease interventions. Fourth, the definition of cardiovascular death and ST (probable versus definite) varied substantially among included studies and played a role in creating heterogeneity. To mitigate this influence, we avoided unadjusted cohorts and down weighted/excluded observational studies in multiple sensitivity analyses. Finally, we did not have access to individual patient data, and therefore, our findings should be interpreted cautiously in view of the inability to perform specific types of analysis with study‐level data.
Conclusions
IVUS imaging–guided PCI was associated with lower cardiovascular death, MI, TLR, and ST, compared with CA alone. These results encourage the routine use of intracoronary imaging to optimize coronary stent implantation.
Disclosures
None.
Supporting information
Figure S1. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by MI in study population.
Figure S2. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by target lesion revascularization in study population.
Figure S3. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by stent thrombosis in study population.
Figure S4. Fixed effect, risk ratio of cardiovascular death associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S5. Fixed effect, risk ratio of myocardial infarction associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S6. Fixed effect, risk ratio of target lesion revascularization associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S7. Fixed effect, risk ratio of stent thrombosis associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
(J Am Heart Assoc. 2020;9:e013678 DOI: 10.1161/JAHA.119.013678.)
References
- 1. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 2. Ali ZA, Galougahi KK, Maehara A, Shlofmitz RA, Ben‐Yehuda O, Mintz GS, Stone GW. Intracoronary optical coherence tomography 2018: current status and future directions. JACC Cardiovasc Interv. 2017;10:2473–2487. [DOI] [PubMed] [Google Scholar]
- 3. Schiele F, Meneveau N, Vuillemenot A, Gupta S, Mercier M, Danchin N, Bertrand B, Bassand J‐P. Impact of intravascular ultrasound guidance in stent deployment on 6‐month restenosis rate: a multicenter, randomized study comparing two strategies—with and without intravascular ultrasound guidance. J Am Coll Cardiol. 1998;32:320–328. [DOI] [PubMed] [Google Scholar]
- 4. Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the can routine ultrasound influence stent expansion (CRUISE) study. Circulation. 2000;102(5):523–530. [DOI] [PubMed] [Google Scholar]
- 5. Gaster A, Skjoldborg US, Larsen J, Korsholm L, Von Birgelen C, Jensen S, Thayssen P, Pedersen KE, Haghfelt T. Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: insights from a prospective, randomised study. Heart. 2003;89:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oemrawsingh PV, Mintz GS, Schalij MJ, Zwinderman AH, Jukema JW, Wall EE. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation. 2003;107:62–67. [DOI] [PubMed] [Google Scholar]
- 7. Park S‐J, Kim Y‐H, Park D‐W, Lee S‐W, Kim W‐J, Suh J, Yun S‐C, Lee CW, Hong M‐K, Lee J‐H. Impact of intravascular ultrasound guidance on long‐term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–177. [DOI] [PubMed] [Google Scholar]
- 8. Koskinas KC, Nakamura M, Räber L, Colleran R, Kadota K, Capodanno D, Wijns W, Akasaka T, Valgimigli M, Guagliumi G. Current use of intracoronary imaging in interventional practice—results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. Circ J. 2018;82:1360–1368. [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data‐analytic approaches and some additional considerations. Stat Med. 1993;12:1293–1316. [DOI] [PubMed] [Google Scholar]
- 11. Mueller M, D'Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, Scott P. Methods to systematically review and meta‐analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. 2018;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta‐analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian N‐L, Gami S‐K, Ye F, Zhang J‐J, Liu Z‐Z, Lin S, Ge Z, Shan S‐J, You W, Chen L. Angiographic and clinical comparisons of intravascular ultrasound‐versus angiography‐guided drug‐eluting stent implantation for patients with chronic total occlusion lesions: two‐year results from a randomised AIR‐CTO study. EuroIntervention. 2015;10:1409–1417. [DOI] [PubMed] [Google Scholar]
- 14. Russo RJ, Silva PD, Teirstein PS, Attubato MJ, Davidson CJ, DeFranco AC, Fitzgerald PJ, Goldberg SL, Hermiller JB, Leon MB. A randomized controlled trial of angiography versus intravascular ultrasound‐directed bare‐metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv. 2009;2:113–123. [DOI] [PubMed] [Google Scholar]
- 15. Chen SL, Ye F, Zhang JJ, Tian NL, Liu ZZ, Santoso T, Zhou YJ, Jiang TM, Wen SY, Kwan TW. Intravascular ultrasound‐guided systematic two‐stent techniques for coronary bifurcation lesions and reduced late stent thrombosis. Catheter Cardiovasc Interv. 2013;81:456–463. [DOI] [PubMed] [Google Scholar]
- 16. Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, Choi J‐H, Choi S‐H, Gwon H‐C, Hahn J‐Y. Impact of intravascular ultrasound‐guided percutaneous coronary intervention on long‐term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12:607–620. [DOI] [PubMed] [Google Scholar]
- 17. Kim B‐K, Shin D‐H, Hong M‐K, Park HS, Rha S‐W, Mintz GS, Kim J‐S, Kim JS, Lee S‐J, Kim H‐Y. Clinical impact of intravascular ultrasound‐guided chronic total occlusion intervention with zotarolimus‐eluting versus biolimus‐eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8:e002592. [DOI] [PubMed] [Google Scholar]
- 18. de la Torre Hernandez JM, Baz Alonso JA, Gomez Hospital JA, Alfonso Manterola F, Garcia Camarero T, Gimeno de Carlos F, Roura Ferrer G, Recalde AS, Martinez‐Luengas IL, Gomez Lara J, Hernandez Hernandez F, Perez‐Vizcayno MJ, Cequier Fillat A, Perez de Prado A, Gonzalez‐Trevilla AA, Jimenez Navarro MF, Mauri Ferre J, Fernandez Diaz JA, Pinar Bermudez E, Zueco Gil J; IVUS‐TRONCO‐ICP Spanish Study . Clinical impact of intravascular ultrasound guidance in drug‐eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient‐level of 4 registries. JACC Cardiovasc Interv. 2014;7:244–254. [DOI] [PubMed] [Google Scholar]
- 19. Gil RJ, Pawłowski T, Dudek D, Horszczaruk G, Żmudka K, Lesiak M, Witkowski A, Ochała A, Kubica J; Investigators of Direct Stenting vs Optimal Angioplasty Trial (DIPOL) . Comparison of angiographically guided direct stenting technique with direct stenting and optimal balloon angioplasty guided with intravascular ultrasound: the multicenter, randomized trial results. Am Heart J. 2007;154:669–675. [DOI] [PubMed] [Google Scholar]
- 20. Park KW, Kang S‐H, Yang H‐M, Lee H‐Y, Kang H‐J, Cho Y‐S, Youn T‐J, Koo B‐K, Chae I‐H, Kim H‐S. Impact of intravascular ultrasound guidance in routine percutaneous coronary intervention for conventional lesions: data from the EXCELLENT trial. Int J Cardiol. 2013;167:721–726. [DOI] [PubMed] [Google Scholar]
- 21. Gao X‐F, Kan J, Zhang Y‐J, Zhang J‐J, Tian N‐L, Ye F, Ge Z, Xiao P‐X, Chen F, Mintz G. Comparison of one‐year clinical outcomes between intravascular ultrasound‐guided versus angiography‐guided implantation of drug‐eluting stents for left main lesions: a single‐center analysis of a 1,016‐patient cohort. Patient Prefer Adherence. 2014;8:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakabčin J, Špaček R, Bystroň M, Kvašňák M, Jager J, Veselka J, Kala P, Červinka P. Long‐term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance: randomized control trial: HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75:578–583. [DOI] [PubMed] [Google Scholar]
- 23. Hong S‐J, Kim B‐K, Shin D‐H, Kim J‐S, Hong M‐K, Gwon H‐C, Kim H‐S, Yu CW, Park HS, Chae I‐H. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second‐generation drug‐eluting stents for chronic total occlusions (from the Multicenter Korean‐Chronic Total Occlusion Registry). Am J Cardiol. 2014;114:534–540. [DOI] [PubMed] [Google Scholar]
- 24. Hong S‐J, Kim B‐K, Shin D‐H, Nam C‐M, Kim J‐S, Ko Y‐G, Choi D, Kang T‐S, Kang W‐C, Her A‐Y. Effect of intravascular ultrasound–guided vs angiography‐guided everolimus‐eluting stent implantation: the IVUS‐XPL randomized clinical trial. JAMA. 2015;314:2155–2163. [DOI] [PubMed] [Google Scholar]
- 25. Kim J‐S, Kang T‐S, Mintz GS, Park B‐E, Shin D‐H, Kim B‐K, Ko Y‐G, Choi D, Jang Y, Hong M‐K. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography‐guided drug‐eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–376. [DOI] [PubMed] [Google Scholar]
- 26. Claessen BE, Mehran R, Mintz GS, Weisz G, Leon MB, Dogan O, de Ribamar Costa J, Stone GW, Apostolidou I, Morales A. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug‐eluting stents. JACC Cardiovasc Interv. 2011;4:974–981. [DOI] [PubMed] [Google Scholar]
- 27. Mudra H, di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, Wennerblom B, Rutsch W, Voudris V, Regar E. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation. 2001;104:1343–1349. [DOI] [PubMed] [Google Scholar]
- 28. Roy P, Steinberg DH, Sushinsky SJ, Okabe T, Pinto Slottow TL, Kaneshige K, Xue Z, Satler LF, Kent KM, Suddath WO. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug‐eluting stents. Eur Heart J. 2008;29:1851–1857. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen S‐L. Intravascular ultrasound versus angiography‐guided drug‐eluting stent implantation: the ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126–3137. [DOI] [PubMed] [Google Scholar]
- 30. Wakabayashi K, Lindsay J, Laynez‐Carnicero A, Ben‐Dor I, Sardi G, Torguson R, Xue Z, Satler LF, Pichard AD, Waksman R. Utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention for type C lesions. J Interv Cardiol. 2012;25:452–459. [DOI] [PubMed] [Google Scholar]
- 31. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug‐eluting stents: the assessment of dual antiplatelet therapy with drug‐eluting stents (ADAPT‐DES) study. Circulation. 2014;129:463–470. [DOI] [PubMed] [Google Scholar]
- 32. Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta‐analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre–drug‐eluting stent era. Am J Cardiol. 2011;107:374–382. [DOI] [PubMed] [Google Scholar]
- 33. Ahn J‐M, Kang S‐J, Yoon S‐H, Park HW, Kang SM, Lee J‐Y, Lee S‐W, Kim Y‐H, Lee CW, Park S‐W. Meta‐analysis of outcomes after intravascular ultrasound–guided versus angiography‐guided drug‐eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–1347. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Farooq V, Garcia‐Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen S‐L. Comparison of intravascular ultrasound versus angiography‐guided drug‐eluting stent implantation: a meta‐analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855–865. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y‐J, Pang S, Chen X‐Y, Bourantas CV, Pan D‐R, Dong S‐J, Wu W, Ren X‐M, Zhu H, Shi S‐Y. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: a systematic review and meta‐analysis. BMC Cardiovasc Disord. 2015;15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klersy C, Ferlini M, Raisaro A, Scotti V, Balduini A, Curti M, Bramucci E, De Silvestri A. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta‐analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol. 2013;170:54–63. [DOI] [PubMed] [Google Scholar]
- 37. Steinvil A, Zhang Y‐J, Lee SY, Pang S, Waksman R, Chen S‐L, Garcia‐Garcia HM. Intravascular ultrasound‐guided drug‐eluting stent implantation: an updated meta‐analysis of randomized control trials and observational studies. Int J Cardiol. 2016;216:133–139. [DOI] [PubMed] [Google Scholar]
- 38. Jang J‐S, Song Y‐J, Kang W, Jin H‐Y, Seo J‐S, Yang T‐H, Kim D‐K, Cho K‐I, Kim B‐H, Park YH. Intravascular ultrasound‐guided implantation of drug‐eluting stents to improve outcome: a meta‐analysis. JACC Cardiovasc Interv. 2014;7:233–243. [DOI] [PubMed] [Google Scholar]
- 39. Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes with intravascular ultrasound‐guided stent implantation: a meta‐analysis of randomized trials in the era of drug‐eluting stents. Circ Cardiovasc Interv. 2016;9:e003700. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Mintz GS, Gu Z, Qi Y, Liu M, Wu X. Meta‐analysis and systematic review of intravascular ultrasound versus angiography‐guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc Disord. 2018;18:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smilowitz NR, Mohananey D, Razzouk L, Weisz G, Slater JN. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc Interv. 2018;92:E410–E415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nallamothu BK, Spertus JA, Lansky AJ, Cohen DJ, Jones PG, Kureshi F, Dehmer GJ, Drozda JP Jr, Walsh MN, Brush JE Jr. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: the Assessing Angiography (A2) project. Circulation. 2013;127:1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elgendy IY, Conti CR, Bavry AA. Fractional flow reserve: an updated review. Clin Cardiol. 2014;37:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 45. Alberti A, Giudice P, Gelera A, Stefanini L, Priest V, Simmonds M, Lee C, Wasserman M. Understanding the economic impact of intravascular ultrasound (IVUS). Eur J Health Econ. 2016;17:185–193. [DOI] [PubMed] [Google Scholar]
- 46. Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. [DOI] [PubMed] [Google Scholar]
- 47. ClincalTrial.gov, National Library of Medicine , OPtical Coherence Tomography (OCT) Guided Coronary Stent IMplantation Compared to Angiography: a Multicenter Randomized TriaL in PCI, 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT03507777.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by MI in study population.
Figure S2. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by target lesion revascularization in study population.
Figure S3. Meta‐regression of risk for diabetes, hypertension, acute coronary syndrome, sex and left main lesion by stent thrombosis in study population.
Figure S4. Fixed effect, risk ratio of cardiovascular death associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S5. Fixed effect, risk ratio of myocardial infarction associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S6. Fixed effect, risk ratio of target lesion revascularization associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.
Figure S7. Fixed effect, risk ratio of stent thrombosis associated with intravascular ultrasound (IVUS)‐guided compared with angiography‐guided PCI.


