Abstract
Background
Recommendations have not yet been established for statin therapy in patients on maintenance dialysis. In this study, we aimed to evaluate the effects of statin therapy on all‐cause mortality in patients undergoing maintenance hemodialysis.
Methods and Results
This retrospective cohort study analyzed data from adults, aged ≥30 years, who were on maintenance hemodialysis for end‐stage renal disease. Data on statin use, along with other clinical information between 2007 and 2017, were extracted from the Health Insurance Review and Assessment Service database in Korea. In total, 65 404 patients were included, and 41 549 (73.2%) patients had received statin therapy for a mean duration of 3.6±2.6 years. Compared with statin nonusers before and after the initiation of hemodialysis (entry), patients who initiated statin therapy after entry and patients who continued statins from the pre–end‐stage renal disease to post–end‐stage renal disease period had a lower risk of all‐cause mortality; the adjusted hazard ratios (95% CIs) were 0.48 (0.47–0.50; P<0.001) for post–end‐stage renal disease only statin users and 0.59 (0.57–0.60; P<0.001) for continuous statin users. However, those discontinuing statins before or at entry showed a higher risk of all‐cause mortality. Statin‐ezetimibe combinations were associated with better survival benefits than fixed patterns of statin therapy. These results were consistent across various subgroups, including elderly patients aged >75 years, and were maintained even after propensity score matching.
Conclusions
Our results showed that in adult patients undergoing maintenance hemodialysis, statin therapy, preferably combined with ezetimibe, was associated with a lower risk of all‐cause mortality.
Keywords: all‐cause mortality, end‐stage renal diseases, hemodialysis, statin
Subject Categories: Lipids and Cholesterol, Mortality/Survival, Nephrology and Kidney
Clinical Perspective
What Is New?
Recommendations have not yet been established for statin therapy in patients on maintenance dialysis.
We evaluated the effects of statin therapy on all‐cause mortality in patients undergoing maintenance hemodialysis using a nationwide cohort.
In adult patients on maintenance hemodialysis, statin therapy with appropriate dose adjustment, and preferably in combination with ezetimibe, was associated with decreased all‐cause mortality.
What Are the Clinical Implications?
Our results provide possible answers to multiple critical questions about the effects of statin use in patients on maintenance hemodialysis.
Introduction
Statin therapy in adults with or at risk of atherosclerotic cardiovascular diseases produces relatively consistent proportional reductions in major cardiovascular outcomes and all‐cause mortality.1 Therefore, guidelines issued by major societies recommend moderate‐ to high‐intensity statin therapy for individuals who are at high risk of atherosclerotic cardiovascular diseases, including patients with chronic kidney disease (CKD).2, 3, 4 Because there is insufficient evidence to support recommendations for or against statin treatment in patients on maintenance hemodialysis, recent guidelines have not made any recommendations for the initiation of statins in these patients.2, 4, 5
Indeed, the findings of studies examining the effects of statin use in patients on hemodialysis are inconsistent. For instance, the SHARP (Study of Heart and Renal Protection) showed that moderate‐intensity statin therapy (simvastatin, 20 mg/d) in combination with ezetimibe reduced the risk of major atherosclerotic events in patients with a wide spectrum of CKDs, including those on dialysis.6 In contrast, 2 randomized clinical trials (RCTs) on statin use in patients undergoing maintenance hemodialysis (namely, the AURORA [A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events] and 4D Study [German Diabetes and Dialysis Study]) have failed to show significant benefits of statin therapy in their primary outcomes.7, 8
In addition to established conventional risk factors for atherosclerotic cardiovascular diseases, other factors, including arterial stiffness and arterial calcification, play important roles in the pathophysiological characteristics of cardiovascular diseases in patients with end‐stage renal disease (ESRD).9 Statin therapy has been shown to reduce the pulse wave velocity, a measure of arterial stiffness, independent of changes in the lipid profile and blood pressure.10 The volume of calcified plaques in the coronary arteries may also be reduced by treatment with statins, both dependent and independent of the reduction in low‐density lipoprotein cholesterol levels.11 However, many studies have shown that the use of statins is associated with the progression of coronary artery calcification in ESRD.12, 13 However, the impact of increased coronary calcification on atheroma stabilization remains unclear. If the pathophysiologic factors related to more advanced CKD can be ameliorated by statin therapy, this should translate to improved major clinical outcomes, which, notably, is yet to be proved. Whether inadequate statistical power was responsible for the lack of benefit found in the previous studies is unclear. Furthermore, when evaluating the causes of mortality in patients undergoing dialysis, the causes of death in databases are often uncertain or missing, and are of noncardiovascular origin in a significant proportion of patients.14, 15 Therefore, all‐cause mortality may be a better primary end point in the evaluation of patients with ESRD.
Although RCTs with larger numbers of patients with ESRD than have been used previously would likely be required to prove the above hypothesis, real‐world clinical data may be a useful alternative. The National Health Insurance program in Korea delivers a government‐controlled single‐payer obligatory insurance plan that covers almost the entire Korean population, which was made up of ≈50 million residents in 2014.16, 17, 18 Encrypted customized data tailored for a study protocol may be extracted from the National Health Insurance raw data. These are authorized by 1 of the 2 government organizations in Korea (ie, the Health Insurance and Review Assessment [HIRA] and the Health Insurance Service). As described previously and summarized in Table S1, the HIRA research data consist of 6 major files (namely, the general information, healthcare services [including inpatient prescriptions], diagnoses, outpatient prescriptions, drug master, and provider information).16
In the present study, we aimed to evaluate the effects of statin therapy on all‐cause mortality in patients with ESRD undergoing maintenance hemodialysis by analyzing patient data from the HIRA database.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. The study was reviewed by the Institutional Review Board of the Gachon University Gil Medical Center, which decided the study protocol was qualified for a waiver of ethics approval in compliance with governmental laws and regulations (protocol GCIRB2018‐380); and the requirement for informed consent was also waived as we only accessed deidentified, previously collected data.
Design, Setting, and Participants
This population‐based retrospective cohort study aimed to evaluate the effects of statin therapy on all‐cause mortality in patients with ESRD on maintenance hemodialysis. Customized data of patients who received maintenance hemodialysis from January 1, 2007, to December 31, 2015, were extracted from the HIRA database, and patients were followed up until December 31, 2017. The diagnoses have been coded following the Korean Standard Classification of Diseases Version 6, which is based on the International Classification of Diseases, Tenth Revision (ICD‐10).16
Patients with ESRD undergoing hemodialysis were defined as those who met all 3 of the following criteria: (1) had ICD‐10 codes corresponding to CKD/ESRD or related conditions as a diagnosis (N18, N181–N184, N189, N19, Z490, Z491, Z90, or Z905), (2) had procedure codes related to hemodialysis (O7011–O7018 or O7020), and (3) were supported by a special Korean National Health Insurance program under the copayment assistance policy that covers rare, incurable, malignant, or severe and burdensome diseases (eg, specific code for patients on dialysis: V001 or V003). Although ESRD is one of these diseases, only patients undergoing maintenance hemodialysis, patients undergoing peritoneal dialysis (PD), or recipients of kidney transplantations are supported by copayment in Korea.19 Among the patients who received dialysis therapy, we excluded adult patients with ESRD who were aged <30 years, as younger patients are less likely to have ESRD causally related to cardiometabolic diseases.20 In addition, we excluded those who received renal transplantation or PD and those who received combined hemodialysis and PD during the study period. To ensure a washout period, we also excluded patients who received hemodialysis during 2007.
Measurements
Data on the prescription of statins and other drugs were extracted using drug codes based on the Anatomical Therapeutic Chemical Classification in the claim data of the study period, and were analyzed in conjunction with other information, including demographic parameters, comorbidities, and procedures, on the basis of the patients’ medical records. The lists of statin prescriptions and statin‐ezetimibe combinations captured in the present study are summarized in Table S2. Comorbidities, including diabetes mellitus, hypertension, and coronary heart disease, were defined during the period from 12 months before entry (initiation of hemodialysis) until the end of follow‐up; and the Charlson Comorbidity Index (CCI) was calculated to assess the general health status of the study subjects21 (Tables S3 and S4). Statin use was defined as at least one prescription of statins being recorded in the claim data. The statin intensity was classified according to existing guidelines.5 Some patients who received less than the defined “low‐intensity therapy” were pooled together with the low‐intensity group as the “low‐ or less‐intensity group.” Collectively, statin use was categorized as follows: (1) no statin use before or after entry (nonusers) and statin ever‐use before or after entry (ever‐users), (2) nonuse, continuous (continuous statin use before and after entry), pre‐ESRD only (stopped using statins before or at entry), and post‐ESRD only use (started statin therapy after entry), (3) low‐ or less‐, moderate‐, high‐, and variable‐intensity statin therapies, and (4) proportion of days covered (PDC) per total days of follow‐up after the initiation of hemodialysis to measure statin adherence (PDC categories: <25%, ≥25%–<50%, ≥50%–<75%, and ≥75%). We calculated the PDC by dividing the number of statin prescription days after entry by the total follow‐up period from the initiation of hemodialysis to the end of data collection or mortality event.
The primary outcome of the present study was the all‐cause mortality during the follow‐up period. Mortality was confirmed by using the certificate database (recorded data of reasons for changes in eligibility for National Health Insurance or medical aid, death, or emigration) and the HIRA database. Thus, a death event was defined by any one of the following: (1) the HIRA claim contained a record of death or (2) no medical records of renal replacement for >3 months without kidney transplantation. Patients in each cohort were followed up from the initiation of hemodialysis (entry date) until the occurrence of study outcome or until December 31, 2017, whichever occurred first. This definition is intended to prevent a record of death from being missed during treatment, such as by changing medical service providers.
Statistical Analysis
Baseline characteristics were compared according to the statin use status. Categorical variables are expressed as numbers and percentages, and continuous variables are expressed as the mean±SD. The demographic characteristics of the patients were measured by frequencies and percentages. The overall survival rates were evaluated using a Kaplan‐Meier curve, and the log‐rank test was used to compare the groups. To evaluate the effects of the prognostic variables, the Cox proportional hazards model was used for multivariate analyses; and adjustments were made for age, sex, and CCI. As multiple testing was performed between subgroups, we set a robust cutoff of P<0.01 for statistical significance.
There may be selection bias owing to the difference in the proportion of basic characteristics between the statin users and the nonusers, an issue for which propensity score matching (PSM) provides a reasonable method for addressing. The propensity score was defined as the probability of individuals using statins and was calculated by multiple logistic regression using variables that included age, sex, factors associated with individual patterns of receiving healthcare service, and socioeconomic status (whether participants receive medical aids, whether those were consistently prescribed from the same healthcare facility, and the level of healthcare facility attended), CCI, and specific medical care, such as emergency department visits and hospitalization within 1 year before entry. Statin nonusers and users were matched in a 1:≈2 proportion. We used a greedy nearest neighbor matching on the logit of the propensity score.
After PSM, Cox proportional hazards models were repeated to determine the association between statin use and all‐cause mortality and were reported in terms of hazard ratios and 95% CIs. In the Cox models, age, sex, and CCI were used as covariates, which showed significant differences even after PSM.
SAS, version 9.4 (SAS Institute Inc, Cary, NC), and R, version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria), software programs were used for the analyses. J.H.J. and D.H.L. had full access to all study data and were responsible for data integrity and data analysis accuracy.
Results
Characteristics of the Study Population
In total, there were 115 559 patients with ESRD who were undergoing dialysis between January 1, 2007, and December 31, 2015. As presented in the flowchart in Figure 1, we excluded 50 155 patients on the basis of the above‐mentioned exclusion criteria, including those on hemodialysis during the predetermined washout period in 2007 (n=39 102), patients who received PD, PD combined with hemodialysis, or renal transplantation (n=10 269), and those aged <30 years (n=784). Therefore, 65 404 patients were finally included in the present study. The mean follow‐up duration after entry was 3.4±2.7 years. The baseline characteristics of the study subjects at entry are summarized in Table 1.
Figure 1.
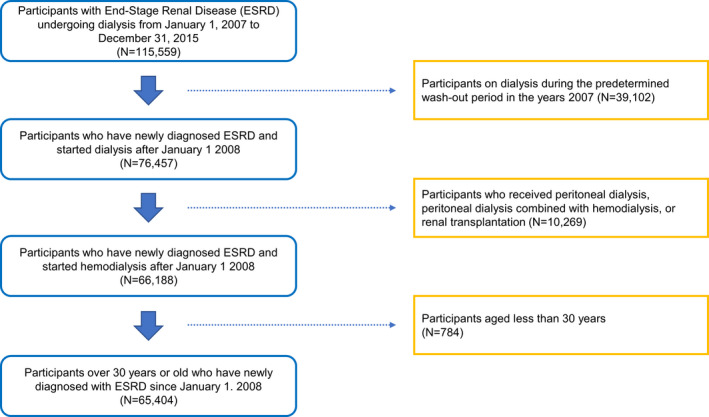
Flow diagram of participant inclusion in the study. ESRD indicates end‐stage renal disease.
Table 1.
Baseline Characteristics of the Study Subjects at Entry (n=65 404)
| Parameters | Groups/Subgroups | Values |
|---|---|---|
| Age at entry, mean (SD), y | Total | 64.9 (12.9) |
| Subgroups by age, N (%) | 30–39 y | 2426 (3.7) |
| 40–49 y | 6496 (9.9) | |
| 50–59 y | 12 529 (19.2) | |
| 60–69 y | 16 522 (25.3) | |
| ≥70 y | 27 431 (41.9) | |
| Sex, N (%) | Men | 38 446 (58.8) |
| Women | 26 958 (41.2) | |
| Statin ever‐use, N (%) | Ever‐users | 47 902 (73.2) |
| Nonusers | 17 502 (26.8) | |
| Statin use over 3 mo, N (%) | Ever‐users | 43 479 (71.3) |
| Nonusers | 17 502 (28.7) | |
| Statin use status during the study period, N (%) | Continuous users | 29 127 (44.5) |
| Nonusers | 17 502 (26.8) | |
| Post‐ESRD only users | 7282 (11.1) | |
| Pre‐ESRD only users | 11 493 (17.6) | |
| Statin use period after entry: PDC, N (%) | PDC <25% | 12 447 (19.0) |
| 25% ≤ PDC < 50% | 5152 (7.9) | |
| 50% ≤ PDC <75% | 4465 (6.8) | |
| PDC ≥75% | 14 345 (21.9) | |
| Nonusers | 17 502 (26.8) | |
| Pre‐ESRD only users | 11 493 (17.6) | |
| Intensity of statin therapy after entry, N (%) | Variable intensity | 13 592 (20.8) |
| High intensity | 405 (0.6) | |
| Moderate intensity | 21 928 (33.5) | |
| Low or less intensity | 484 (0.7) | |
| Nonusers | 17 502 (26.8) | |
| Pre‐ESRD only users | 11 493 (17.6) | |
| Hypertension, N (%) | Yes | 62 630 (95.8) |
| No | 2774 (4.2) | |
| Diabetes mellitus, N (%) | Yes | 51 028 (78.0) |
| No | 14 376 (22.0) | |
| CHD, N (%) | Yes | 31 272 (47.8) |
| No | 34 132 (52.2) | |
| CCI, mean (SD) | Total | 3.31 (1.92) |
| CCI category, N (%) | CCI <3 | 24 839 (38.0) |
| CCI ≥3 | 40 565 (62.0) |
CCI indicates Charlson Comorbidity Index; CHD, coronary heart disease; ESRD, end‐stage renal disease; PDC, proportion of days covered.
The mean age of the study subjects was 64.9±12.9 years at entry, with a mean CCI of 3.3±1.9. In total, 36 409 (55.7%) patients received statin prescriptions after entry, with 29 127 patients being continuous statin users before and after entry and 7282 patients belonging to the post‐ESRD only statin group (Table 1). The mean duration of exposure to statins after entry in the statin user groups was 3.6±2.6 years. Prevalent hypertension, diabetes mellitus, and coronary heart disease were present in 95.8%, 78.0%, and 47.8% of patients, respectively. Interestingly, statin ever‐users showed a higher prevalence of these diseases than nonusers (Tables S5 and S6).
Statin Therapy in Patients on Maintenance Hemodialysis Was Associated With a Lower Risk of All‐Cause Mortality
First, we analyzed all included patients, and statin ever‐users (before or after entry) were then compared with statin nonusers (before and after entry). The survival plots of all‐cause mortality showed a significant benefit of statin therapy in the statin ever‐users compared with the nonusers (Figure S1A). In addition, the statin ever‐users showed a lower risk of all‐cause mortality, according to both the age‐ and sex‐adjusted and age‐, sex‐, and CCI‐adjusted hazard ratios (Table 2 and Figure 2A).
Table 2.
Association Between Statin Use and All‐Cause Mortality in Patients Undergoing Maintenance Hemodialysis
| Parameters Related With Statin Use | All‐Cause Mortality | All Patients | Person‐Years | Adjusted Model 1a | Adjusted Model 2b | Adjusted Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRs | 95% CIs | P Values | HRs | 95% CIs | P Values | HRs | 95% CIs | P Values | ||||
| Nonusers | 11 407 | 17 502 | 50 269.7 | Reference | ··· | ··· | Reference | ··· | ··· | Reference | ··· | ··· |
| Statin ever‐use | ||||||||||||
| Ever‐users | 24 851 | 47 902 | 171 959.9 | 0.68 | 0.66–0.70 | <0.001 | 0.65 | 0.64–0.67 | <0.001 | 0.67 | 0.66–0.69 | <0.001 |
| Over 3 mo | 21 822 | 43 479 | 158 702.1 | 0.65 | 0.64–0.66 | <0.001 | 0.62 | 0.60–0.63 | <0.001 | 0.64 | 0.62–0.65 | <0.001 |
| Statin use status during the study period, N (%) | ||||||||||||
| Pre‐ESRD only users | 7647 | 11 493 | 26 925.0 | 1.17 | 1.14–1.21 | <0.001 | 1.12 | 1.09–1.16 | <0.001 | 1.14 | 1.11–1.18 | <0.001 |
| Continuous users | 13 809 | 29 127 | 109 628.6 | 0.60 | 0.58–0.61 | <0.001 | 0.56 | 0.55–0.68 | <0.001 | 0.59 | 0.57–0.60 | <0.001 |
| Post‐ESRD only users | 3395 | 7282 | 35 406.3 | 0.48 | 0.46–0.50 | <0.001 | 0.48 | 0.46–0.50 | <0.001 | 0.48 | 0.47–0.50 | <0.001 |
| Statin use period after entry: PDC | ||||||||||||
| PDC <25% | 6264 | 12 447 | 53 359.8 | 0.59 | 0.57–0.61 | <0.001 | 0.56 | 0.55–0.58 | <0.001 | 0.57 | 0.56–0.59 | <0.001 |
| 25% ≤ PDC < 50% | 2641 | 5152 | 21 071.3 | 0.61 | 0.59–0.64 | <0.001 | 0.58 | 0.56–0.61 | <0.001 | 0.60 | 0.58–0.63 | <0.001 |
| 50% ≤ PDC < 75% | 2251 | 4465 | 17 904.0 | 0.61 | 0.58–0.64 | <0.001 | 0.58 | 0.56–0.61 | <0.001 | 0.60 | 0.57–0.63 | <0.001 |
| PDC ≥75% | 6048 | 14 345 | 52 699.8 | 0.52 | 0.51–0.54 | <0.001 | 0.50 | 0.48–0.51 | <0.001 | 0.52 | 0.50–0.53 | <0.001 |
| Intensity of statin therapy after entry | ||||||||||||
| Variable intensity | 6309 | 13 592 | 54 490.4 | 0.56 | 0.54–0.58 | <0.001 | 0.53 | 0.51–0.55 | <0.001 | 0.56 | 0.54–0.58 | <0.001 |
| High intensity | 247 | 405 | 1480.3 | 0.76 | 0.67–0.86 | <0.001 | 0.76 | 0.67–0.86 | <0.001 | 0.76 | 0.67–0.86 | <0.001 |
| Moderate intensity | 10 393 | 21 928 | 87 248.8 | 0.57 | 0.55–0.58 | <0.001 | 0.55 | 0.53–0.56 | <0.001 | 0.55 | 0.54–0.57 | <0.001 |
| Low or less intensity | 255 | 484 | 1815.4 | 0.67 | 0.59–0.76 | <0.001 | 0.65 | 0.58–0.74 | <0.001 | 0.70 | 0.62–0.80 | <0.001 |
ESRD indicates end‐stage renal disease; HR, hazard ratio; PDC, proportion of days covered.
Adjusted for age and sex.
Adjusted for age, sex, and Charlson Comorbidity Index.
Adjusted for age, sex, Charlson Comorbidity Index, and ezetimibe.
Figure 2.
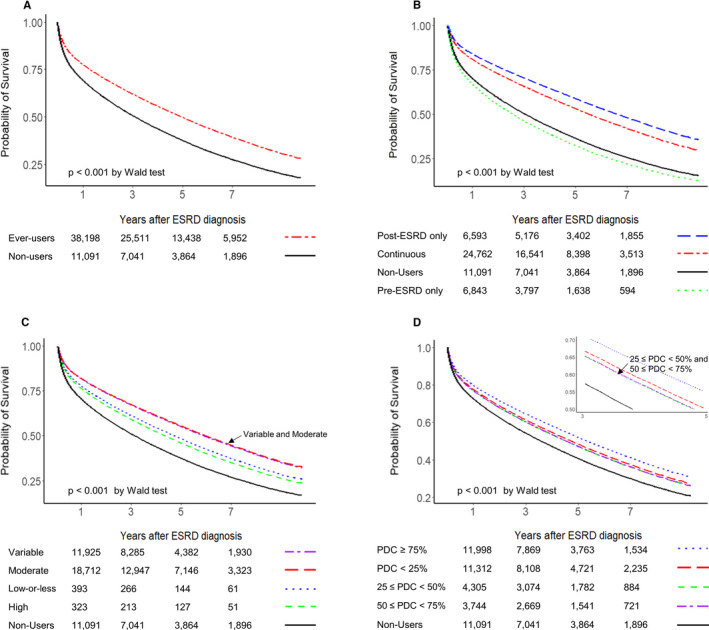
Multiple (age, sex, and Charlson Comorbidity Index) adjusted survival plots for all‐cause mortality related to statin therapy, according to various conditions in adult patients undergoing maintenance hemodialysis. A, Statin ever‐users vs nonusers. B, Statin use before and after entry (the initiation of hemodialysis). C, The intensity of statin therapy. Note that the plot lines for variable and moderate intensities of statin therapies overlap completely. D, Prescription maintenance of statin therapy, as determined by the proportion of days covered (PDC). Note that the plot lines for the 2 PDC groups (25%–<50% and 50%–<75%) overlap completely. ESRD indicates end‐stage renal disease.
Second, we classified the study population into 4 groups (ie, the nonuser, continuous user, pre‐ESRD only user, and post‐ESRD only user groups) (Table S6). Compared with the nonuser group, the pre‐ESRD only user group showed an increased risk of all‐cause mortality, whereas the continuous and post‐ESRD only user groups showed a decreased risk of all‐cause mortality in both the unadjusted and adjusted analyses (Table 2, Figure S1B, and Figure 2B).
When we analyzed the data according to the intensity of statin therapy after entry (Table S7), compared with those in the nonuser group, patients receiving less than high‐intensity statin therapies demonstrated a decrease in both the unadjusted and adjusted hazard ratios for all‐cause mortality. However, high‐intensity statin therapy showed survival benefits after multiple adjustments (Table 2, Figure S1C, and Figure 2C).
We also categorized patients into 4 groups according to their PDC values after entry (<25%, ≥25%–<50%, ≥50%–<75%, and ≥75%) (Table S8). The results showed that patients with a PDC ≥75% had the highest survival benefits among the different PDC groups (Table 2, Figure S1D, and Figure 2D).
Beneficial Effects of Statin Therapy on All‐Cause Mortality Were Consistent Across Various Patient Groups
We also performed sensitivity analyses to determine whether changes in a clinically important variable would influence the effects of statin therapy on all‐cause mortality in patients receiving maintenance hemodialysis. The beneficial effects of statin therapy were maintained across most subgroups (Table S9). Notably, as with nonusers, both older (aged >75 years) and younger (aged <40 years) patients also showed survival benefits with statin therapy. Of the 36 409 statin users undergoing maintenance therapy, 6169 (16.9%) received statin‐ezetimibe combination therapy. The use of statin‐ezetimibe combination therapy after entry was associated with a lower adjusted hazard ratio for all‐cause mortality than statin therapy without ezetimibe (Figure 3A). Further adjustment for ezetimibe combination therapy did not change the results described above and in Figure 2 and Table 2.
Figure 3.
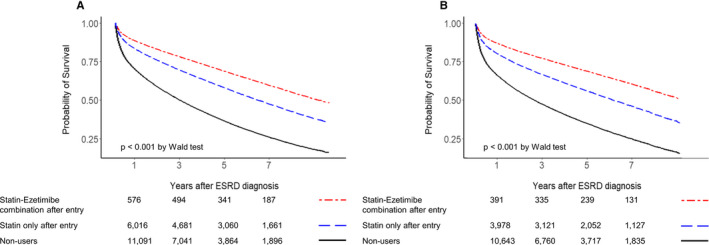
Multiple (age, sex, Charlson Comorbidity Index, and ezetimibe use) adjusted survival plots for all‐cause mortality related to statin therapy, according to ezetimibe combination in adult patients undergoing maintenance hemodialysis. A, Nonmatched survival plot. B, Survival plot after propensity score matching (1:≈2) for statin nonusers and statin users. ESRD indicates end‐stage renal disease.
Beneficial Effects of Statin Therapy on All‐Cause Mortality Were Consistently Observed Even After PSM
After a 1:2 PSM between statin nonuser and user groups, 44 180 patients (16 417 statin nonusers and 27 763 statin users) were included in the analysis. Patients were relatively well matched for factors associated with individual patterns of receiving healthcare service, socioeconomic status, and specific medical care within 1 year before entry. However, further adjustments were needed for age, sex, and CCI for subsequent analyses (Table S10).
The results of the Cox models remained consistent when statin nonusers and users after entry were PSM matched (Tables S11 and S12 and Figure 3B). In patients on maintenance hemodialysis, users of statins, and preferably statin‐ezetimibe combinations, continued to have a reduced risk of all‐cause mortality compared with statin nonusers.
Discussion
In the present study, we found that statin therapy consistently provided beneficial effects in adult patients with ESRD who were on maintenance hemodialysis. Both continuous users and patients who started statin therapy after entry showed lower all‐cause mortality than statin nonusers. In addition, patients who stopped using statins before or at the initiation of hemodialysis showed an increased risk of all‐cause mortality.
These beneficial effects were more evident in patients who continuously received statin prescriptions during the study period (ie, PDC ≥75%), those who received statin‐ezetimibe combination therapy, and patients who received variable‐ or moderate‐intensity statin therapy rather than fixed high‐ or lower‐intensity therapy. The benefits of statin therapy remained significant even after adjusting for age, sex, and CCI. Sensitivity analysis also showed consistent benefits of statin therapy across various subgroups, including older (aged >75 years) and younger (aged <40 years) patients. Furthermore, the main findings in this cohort remained consistently significant even after PSM.
CKD is an important risk‐enhancing factor for atherosclerotic cardiovascular diseases and is a reasonable indication for statin therapy.2, 3 However, an important concern is that the beneficial effects of statin therapy may not be maintained in patients with advanced CKD.6, 7, 8 To the best of our knowledge, for the effects of statin use, the present study is the first to answer multiple key questions in patients on maintenance hemodialysis, including statin ever‐use or nonuse before and after the initiation of hemodialysis, continuation of statins at the initiation of hemodialysis, optimal intensity of statin therapy, benefits of statin therapy in patients aged >75 years, combination of statins with ezetimibe, and the impact of adherence to statins on mortality.
Similar to our results, previous observational studies have shown that statin therapy is associated with reduced mortality in patients receiving hemodialysis.22, 23 The DOPPS (Dialysis Outcomes and Practice Patterns Study), which included 7635 patients receiving hemodialysis, showed that patients on hemodialysis who were prescribed statins had a 31% lower adjusted risk of all‐cause mortality and a 23% lower risk of cardiac mortality than statin nonusers.22 Moreover, Wave 2 (US Renal Data System Dialysis Morbidity and Mortality Study), which included 3700 incident dialysis patients, showed a 32% lower all‐cause mortality and 36% lower cardiovascular mortality with statin therapy.23
In contrast, 2 landmark RCTs in patients undergoing hemodialysis (namely, the AURORA and 4D Study) showed that statin therapy had no significant effects on major clinical outcomes.7, 8 In addition, a recent systematic analysis of 25 studies (8289 participants) showed that statin therapy in adults on dialysis showed little or no beneficial effects on mortality or cardiovascular events.24
The reasons for the discrepancies between the RCTs and the observational study findings and ours are unclear. Some studies have suggested that probably unlike the case of hydrophilic statins, lipophilic statin‐induced enhancement of vascular calcification in patients with ESRD may offset small beneficial effects of statins in these patients with many nontraditional risk factors.12, 13, 25 Although we could not directly evaluate arterial calcification in the present study, we observed that compared with hydrophilic statins, their lipophilic counterparts were associated with a lower risk of all‐cause mortality (Table S13). Further studies are required to address the association between statin and vascular calcification in patients with ESRD. The sample sizes of the previous RCTs may have been too underpowered to prove any significant benefit or harm of statin therapy in patients on dialysis.6, 7, 8 Indeed, the 4D Study showed the beneficial effects of statin therapy on the combined risk of all cardiovascular events.8 Furthermore, a significant proportion of patients in the placebo groups had taken nonstudy statins, and this may have affected the outcomes.6, 8 In the present study, the beneficial effects of statins may be explained by nonrandom effects. Finally, differences in the research designs and study subjects may also explain the discrepant results.26
Also, in patients with ESRD, administering statins with or without ezetimibe may be more beneficial in terms of lowering blood low‐density lipoprotein cholesterol levels with lower‐intensity statins and decreasing the incidence of adverse effects.27 The statin‐ezetimibe combination was found to be beneficial in SHARP, in which 33% of the study population was on maintenance dialysis.6 The results revealed that after weighing for low‐density lipoprotein cholesterol reductions, the proportional effects of the statin‐ezetimibe combination on primary outcomes were similar across patients who were and were not on dialysis.6 Our findings support the results of SHARP, and provide an additional therapeutic implication, in that the statin‐ezetimibe combination was a better option than statin without ezetimibe for decreasing all‐cause mortality in patients on hemodialysis.
The present study has several limitations. First, the customized HIRA data do not provide information pertaining to laboratory data, lifestyle, or family history that may be related to cardiovascular risks.16 Furthermore, the severity or stage of comorbidities was not adequately captured, although we did use the CCI for adjustments. Second, we assessed all‐cause mortality as the only end point, and no information was obtained on the cause of death in the present study for several reasons, which included limitations of the HIRA data. Although cardiovascular mortality is a major cause of death in patients on hemodialysis,7 recent data from the United Kingdom show that the causes of death in patients with ESRD are more diverse.14 The complex nature of ESRD may complicate the assessment of the exact cause of death.13 In addition, several reports have addressed poor concordance (31%–38%) in the causes of death between ESRD registry data and national statistics data based on death certificates.15, 28 Third, adverse effects of statins were not systematically evaluated in the present study. Notably, this issue needs to be further evaluated in the near future with respect to distinct types of statin therapy, including statin‐ezetimibe combinations and high‐intensity therapy in patients receiving dialysis. Finally, our results were based on a single ethnic population within one country.
The strengths of the study include a large number of adults with dialysis‐dependent CKD and the use of records from the HIRA database that covered all patients with ESRD in Korea during the study period.
In conclusion, our observational study using a nationwide cohort showed that in adult patients on maintenance hemodialysis, statin therapy, with appropriate dose adjustment and more favorably in combination with ezetimibe, was associated with decreased all‐cause mortality. Further prospective studies on more diverse cohorts are needed to validate our findings.
Sources of Funding
This work was supported by grants from the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Korea (grant numbers HI14C1135, HI16C1997, and HI18C0331).
Disclosures
None.
Supporting information
Table S1. Variables in the Korean National Health Insurance Claim Data
Table S2. Classification of Statin Therapy Intensity and Prescriptions Captured in the Present Study
Table S3. International Classification of Diseases 10th Revision (ICD‐10) Codes for Major Comorbid Diseases
Table S4. International Classification of Diseases 10th Revision (ICD‐10) Mapping for Charlson Comorbidity Index2
Table S5. Characteristics of Study Subjects Based on Statin Ever‐Use and Non‐Use
Table S6. Baseline Characteristics at Entry Based on Statin Use
Table S7. Characteristics of Study Subjects According to the Intensity of Statin Therapy After Entry
Table S8. Characteristics of Patients Based on the Statin Use Period After Entry: Proportion of Days Covered (PDC) After Entry*
Table S9. All‐Cause Mortality in Relation to Statin Use or Non‐Use After Entry, Based on Various Parameters in Patients Undergoing Maintenance Hemodialysis
Table S10. Characteristics of Study Patients Before and After Propensity Score Matching
Table S11. Association Between Statin Use and All‐Cause Mortality in Patients Undergoing Maintenance Hemodialysis After Propensity Score Matching
Table S12. All‐Cause Mortality According to Various Parameters in Patients Undergoing Maintenance Hemodialysis in Relation to Statin Use or Non‐Use After Entry After Propensity Score Matching
Table S13. All‐Cause Mortality With Lipophilic Statins Versus Hydrophilic Statins, Based on Various Parameters in Patients Undergoing Maintenance Hemodialysis
Figure S1. Kaplan–Meier curves for crude all‐cause mortality related to statin therapy use according to various conditions in adult patients undergoing maintenance hemodialysis
(J Am Heart Assoc. 2020;9:e014840 DOI: 10.1161/JAHA.119.014840.)
References
- 1. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008–2024. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–1309. [DOI] [PubMed] [Google Scholar]
- 4. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–118. [DOI] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 6. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt‐Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen‐Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen‐Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 8. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 9. Guerin AP, Pannier B, Marchais SJ, London GM. Cardiovascular disease in the dialysis population: prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15:105–110. [DOI] [PubMed] [Google Scholar]
- 10. D'Elia L, La Fata E, Iannuzzi A, Rubba PO. Effect of statin therapy on pulse wave velocity: a meta‐analysis of randomized controlled trials. Clin Exp Hypertens. 2018;40:601–608. [DOI] [PubMed] [Google Scholar]
- 11. Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG‐CoA reductase inhibitors on coronary artery disease as assessed by electron‐beam computed tomography. N Engl J Med. 1998;339:1972–1978. [DOI] [PubMed] [Google Scholar]
- 12. Chen Z, Qureshi AR, Parini P, Hurt‐Camejo E, Ripsweden J, Brismar TB, Barany P, Jaminon AM, Schurgers LJ, Heimburger O, Lindholm B, Stenvinkel P. Does statins promote vascular calcification in chronic kidney disease? Eur J Clin Invest. 2017;47:137–148. [DOI] [PubMed] [Google Scholar]
- 13. De Vriese AS. Should statins be banned from dialysis? J Am Soc Nephrol. 2017;28:1675–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Methven S, Steenkamp R, Fraser S. UK renal registry 19th annual report: chapter 5 survival and causes of death in UK adult patients on renal replacement therapy in 2015: national and centre‐specific analyses. Nephron. 2017;137(suppl 1):117–150. [DOI] [PubMed] [Google Scholar]
- 15. Perneger TV, Klag MJ, Whelton PK. Cause of death in patients with end‐stage renal disease: death certificates vs registry reports. Am J Public Health. 1993;83:1735–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, Lee EJ, Ae Shin S. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175:1527–1529. [DOI] [PubMed] [Google Scholar]
- 19. Lim SS, Lee W, Kim YK, Kim J, Park JH, Park BR, Yoon JH. The cumulative incidence and trends of rare diseases in South Korea: a nationwide study of the administrative data from the National Health Insurance Service database from 2011‐2015. Orphanet J Rare Dis. 2019;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foster BJ, Mitsnefes MM, Dahhou M, Zhang X, Laskin BL. Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin J Am Soc Nephrol. 2018;13:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 22. Mason NA, Bailie GR, Satayathum S, Bragg‐Gresham JL, Akiba T, Akizawa T, Combe C, Rayner HC, Saito A, Gillespie BW, Young EW. HMG‐coenzyme a reductase inhibitor use is associated with mortality reduction in hemodialysis patients. Am J Kidney Dis. 2005;45:119–126. [DOI] [PubMed] [Google Scholar]
- 23. Seliger SL, Weiss NS, Gillen DL, Kestenbaum B, Ball A, Sherrard DJ, Stehman‐Breen CO. HMG‐CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61:297–304. [DOI] [PubMed] [Google Scholar]
- 24. Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, Hegbrant J, Strippoli GF. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;9:Cd004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iijima K, Ito Y, Son BK, Akishita M, Ouchi Y. Pravastatin and olmesartan synergistically ameliorate renal failure‐induced vascular calcification. J Atheroscler Thromb. 2014;21:917–929. [DOI] [PubMed] [Google Scholar]
- 26. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 27. Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double‐blind trial. Circulation. 2003;107:2409–2415. [DOI] [PubMed] [Google Scholar]
- 28. Li SQ, Cass A, Cunningham J. Cause of death in patients with end‐stage renal disease: assessing concordance of death certificates with registry reports. Aust N Z J Public Health. 2003;27:419–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variables in the Korean National Health Insurance Claim Data
Table S2. Classification of Statin Therapy Intensity and Prescriptions Captured in the Present Study
Table S3. International Classification of Diseases 10th Revision (ICD‐10) Codes for Major Comorbid Diseases
Table S4. International Classification of Diseases 10th Revision (ICD‐10) Mapping for Charlson Comorbidity Index2
Table S5. Characteristics of Study Subjects Based on Statin Ever‐Use and Non‐Use
Table S6. Baseline Characteristics at Entry Based on Statin Use
Table S7. Characteristics of Study Subjects According to the Intensity of Statin Therapy After Entry
Table S8. Characteristics of Patients Based on the Statin Use Period After Entry: Proportion of Days Covered (PDC) After Entry*
Table S9. All‐Cause Mortality in Relation to Statin Use or Non‐Use After Entry, Based on Various Parameters in Patients Undergoing Maintenance Hemodialysis
Table S10. Characteristics of Study Patients Before and After Propensity Score Matching
Table S11. Association Between Statin Use and All‐Cause Mortality in Patients Undergoing Maintenance Hemodialysis After Propensity Score Matching
Table S12. All‐Cause Mortality According to Various Parameters in Patients Undergoing Maintenance Hemodialysis in Relation to Statin Use or Non‐Use After Entry After Propensity Score Matching
Table S13. All‐Cause Mortality With Lipophilic Statins Versus Hydrophilic Statins, Based on Various Parameters in Patients Undergoing Maintenance Hemodialysis
Figure S1. Kaplan–Meier curves for crude all‐cause mortality related to statin therapy use according to various conditions in adult patients undergoing maintenance hemodialysis


