The fruitful collaboration between Amsterdam and Aarhus published in this issue of the Journal of the American Heart Association (JAHA)1 provides an important opportunity to address the pragmatic question posed in the title of this editorial. Namely, why can the fractional flow reserve (FFR) value decrease after transcatheter aortic valve implantation (TAVI)?
Caveat Emptor
Before taking up the answer, we must point out 2 caveats. First, current guidelines actively discourage invasive physiologic assessment of coronary lesions being considered for revascularization in patients with severe aortic stenosis (AS). The latest European statement believes that “evidence is insufficient to support the use of invasive functional assessment of coronary lesions in patients with AS … myocardial revascularization based on angiographic assessment of [coronary artery disease] should be maintained.”2 Similarly, a recent American consensus stated that invasive pressure physiology had “No proven value/should be discouraged … in the presence of … valvular heart disease.”3
Second, this skepticism reflects a general lack of high‐quality data regarding the benefits of percutaneous coronary intervention (PCI) for patients with severe AS being considered for TAVI. Very few patients have such severe coronary disease as to become hemodynamically unstable during or after rapid pacing that facilitates TAVI deployment, and teams have become facile at managing patients with a high risk of coronary occlusion identified during preprocedural imaging. A total of 11 cohort studies including 5580 subjects found no statistically significant benefit from TAVI plus PCI compared with TAVI alone on 30‐day mortality, 1‐year mortality, or myocardial infarction—indeed, each end point was numerically lower for isolated TAVI.4 However, because cohort studies remain susceptible to bias, we eagerly await a number of ongoing randomized trials comparing angiographic selection versus medical treatment (ISRCTN registry ISRCTN75836930), FFR versus medical therapy (clinicaltrials.gov NCT03058627), and FFR versus angiographic selection (clinicaltrials.gov NCT03360591). Without a signal of benefit from these studies, it will be difficult to justify PCI before TAVI outside of a limited subgroup with critical aorto‐ostial or proximal disease, since PCI after TAVI almost always remains a viable option.
Twenty Years of Physiologic Observations
Having made clear that guidelines and randomized trials do not currently support physiologic assessment, what can we say about its application to patients with severe AS? To the best of our knowledge, Table summarizes the literature regarding paired physiologic assessment before and after treating severe AS, either surgically or by TAVI. The current article by Vendrik and colleagues1 provides the 12th publication in the past 2 decades,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 now bringing the total number of lesions to about 350 when accounting for overlap, including ≈200 with some assessment of flow.
Table 1.
Literature Review of Coronary Physiology Before vs After Treatment of Severe Aortic Stenosis
| Author | N | Baseline | Immediate | P Value | Long‐Term | P Value | Time | Treatment | Method |
|---|---|---|---|---|---|---|---|---|---|
| Resting perfusion (mL/min per g) or Doppler velocity (cm/s) | |||||||||
| Nemes5 | 21 | 62.2 | 40.1 | <0.01† | 15 mo | SAVR | Echo Doppler (diastolic) | ||
| Hildick‐Smith6 | 27 | 43 | 41 | NS | 6 mo | SAVR | Echo Doppler (diastolic) | ||
| Carpeggiani7 | 8 | 1.01 | 0.92 | >0.05 | 12 mo | SAVR | PET | ||
| Rajappan8 | 22 | 1.08 | 1.01 | 0.27 | 12 mo | SAVR | PET | ||
| Camugila9 | 8 | 22 | 20 | NS | 18 | NS | 12 mo | TAVI | Wire Doppler |
| Vendrik1 | 13 | 19.98 | 19.7 | NS | 21.44 | 0.397 | 6 mo | TAVI | Wire Doppler |
| Wiegerinck10 | 27 | 24.4 | 25.5 | 0.401 | TAVI | Wire Doppler | |||
| Ahmad11 | 30 | 22.13 | 24.84 | 0.1 | TAVI | Wire Doppler | |||
| Instantaneous wave‐free ratio (iFR) | |||||||||
| Vendrik1 | 13 | 0.82 | 0.83 | NS | 0.83 | 0.735 | 6 mo | TAVI | |
| Ahmad11 | 30 | 0.88 | 0.88 | 0.94 | TAVI | ||||
| Scarsini12 | 145 | 0.89 | 0.89 | 0.66 | TAVI | ||||
| Hyperemic perfusion (mL/min per g) or Doppler velocity (cm/s) or mean transit time (s) | |||||||||
| Nemes5 | 21 | 117 | 91.5 | <0.05† | 15 mo | SAVR | Echo Doppler (diastolic) | ||
| Hildick‐Smith6 | 27 | 71 | 108 | <0.01* | 6 mo | SAVR | Echo Doppler (diastolic) | ||
| Carpeggiani7 | 8 | 1.68 | 1.46 | NS | 12 mo | SAVR | PET | ||
| Rajappan8 | 22 | 2.17 | 2.27 | 0.61 | 12 mo | SAVR | PET | ||
| Camugila9 | 8 | 34 | 29 | NS | 39 | NS | 12 mo | TAVI | Wire Doppler |
| Vendrik1 | 13 | 26.36 | 30.78 | <0.001* | 40.2 | <0.001* | 6 mo | TAVI | Wire Doppler |
| Wiegerinck10 | 27 | 44.5 | 51.1 | 0.027* | TAVI | Wire Doppler | |||
| Ahmad11 | 30 | 33.44 | 40.33 | 0.004* | TAVI | Wire Doppler | |||
| Stoller13 | 40 | 0.44 | 0.48 | 0.53 | TAVI | Wire thermo | |||
| CFR | |||||||||
| Nemes5 | 21 | 1.96 | 2.37 | <0.05* | 15 mo | SAVR | Echo Doppler (diastolic) | ||
| Hildick‐Smith6 | 27 | 1.76 | 2.61 | <0.01* | 6 mo | SAVR | Echo Doppler (diastolic) | ||
| Carpeggiani7 | 8 | 1.68 | 1.58 | NS | 12 mo | SAVR | PET | ||
| Rajappan8 | 22 | 2.02 | 2.28 | 0.17 | 12 mo | SAVR | PET | ||
| Camugila9 | 8 | 1.53 | 1.58 | 0.41 | 2.18 | <0.01* | 12 mo | TAVI | Wire Doppler |
| Vendrik1 | 13 | 1.28 | 1.65 | <0.001* | 1.94 | <0.001* | 6 mo | TAVI | Wire Doppler |
| Wiegerinck10 | 27 | 1.9 | 2.1 | 0.113 | TAVI | Wire Doppler | |||
| Stoller13 | 40 | 1.9 | 2 | 0.72 | TAVI | Wire thermo | |||
| FFR | |||||||||
| Stundl14 | 13 | 0.77 | 0.76 | 0.11 | 2 mo | TAVI | |||
| Vendrik1 | 13 | 0.85 | 0.79 | <0.001* | 0.71 | <0.001* | 6 mo | TAVI | |
| Ahmad11 | 30 | 0.87 | 0.85 | 0.0008* | TAVI | ||||
| Stoller13 | 40 | 0.9 | 0.93 | 0.0021† | TAVI | ||||
| Pesarini15 | 133 | 0.89 | 0.89 | 0.73 | TAVI | ||||
*P values statistically significant and supportive of the hypothesis that treating aortic stenosis does not change resting flow but increases hyperemic flow and associated metrics.
†Statistically significant but against this hypothesis.
CFR indicates coronary flow reserve; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; NS, not significant (actual P value not reported); PET, positron emission tomography; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; thermo, bolus thermodilution.
We would like to emphasize 3 observations about this body of literature. First, apart from the large pressure‐wire cohort from Verona with almost 150 lesions, each series alone is of modest size, typically 10 to 40 subjects or lesions, highlighting the difficulty in doing these types of physiology studies. Second, a variety of techniques has been used to study coronary physiology, from noninvasive imaging tools such as echocardiography and positron emission tomography, to invasive wires equipped with Doppler probes or temperature sensors for thermodilution. Their resulting heterogeneity could be viewed as a weakness (harder to combine modestly sized studies) or a strength (true physiologic changes should stand out regardless of measurement technique). Third, any global conclusion should be tempered by noting that neutral or even conflicting results (marked for visual ease) can be seen for almost every section of the table.
Nevertheless, we believe that the literature broadly supports 2 conclusions. First, removing severe AS does not change resting flow or nonhyperemic pressure ratios, either immediately or in the long term. Second, TAVI produces an immediate improvement in hyperemic flow, and can therefore lower FFR and raise coronary flow reserve (CFR) values. Relief from severe AS at least maintains these higher hyperemic flows in the long term or even improves them because of ongoing remodeling of the myocardium.
A Unifying Hypothesis
Assuming these 2 physiologic responses to TAVI (unchanged resting flow, improved hyperemic flow), what implications exist for invasive assessment? Figure provides a visual hypothesis that we now explain in more detail. Let us assume for the moment that the coronary lesion itself remains unchanged—reasonable immediately before versus after TAVI, although less valid over longer time periods because of disease progression and concomitant treatment. A fixed coronary stenosis implies a set relationship between pressure loss and flow with specific viscous friction and expansion/separation coefficients that depend largely on stenosis and vessel geometry. This stenosis curve interacts with the myocardial bed as outlined by Drs. Kirkeeide and Gould in 1986,16 but here expanded to account for acute and chronic remodeling because of TAVI.
Figure 1.
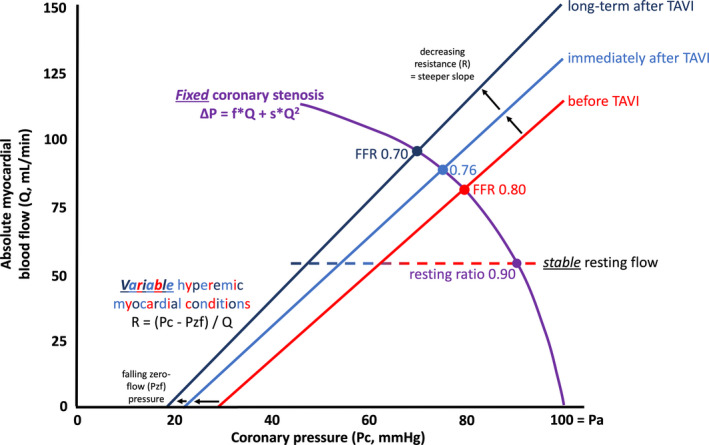
Proposed physiologic framework for concomitant aortic and coronary stenosis. See text for a complete description. FFR indicates fractional flow reserve; TAVI, transcatheter aortic valve implantation.
Under resting conditions, autoregulation maintains a largely constant blood flow and therefore a constant pressure loss, corresponding to a stable nonhyperemic pressure ratio. Both of these aspects agree with the experimental observations in the Table. However, it is worth mentioning that other investigators have reasonably expected that resting myocardial blood flow would be lower after relief of AS because of a reduction in metabolic demand to match a lower left ventricular workload.17 Lack of empiric support in the Table suggests that increased left ventricular pressures contribute only a small amount to total metabolic requirements and/or that existing studies were underpowered (numerically 5 of the 8 had a lower point value for resting flow, although only 1 reached statistical significance).
In response to vasodilation, the flat, horizontal line of autoregulation becomes sloped. Its pressure intercept when flow ceases has been termed the “zero‐flow pressure” (closely related to the coronary wedge pressure measured during proximal balloon occlusion) and its slope relates inversely to the myocardial resistance. The coronary stenosis curve meets the vasodilated myocardial “load line” (a hydraulic or electrical circuit term) at the point where the bed cannot increase flow further, namely, maximum hyperemic flow when the coronary pressure drops to FFR. While this myocardial condition has been naively quantified as a “resistance,” neglecting the pressure offset can cause artifactual changes in “resistance” when in fact the slope remains constant.18 Several techniques exist to measure both components (slope, offset) of this load line invasively, including continuous thermodilution.19 As a result of this widely neglected yet vital aspect of assessing resistance, we have purposely not included “resistance” in the Table although often reported in the literature, including in the article by Vendrik and colleagues.1
TAVI or surgical treatment of AS likely alters both aspects of the vasodilated myocardium. In other words, the “load line” changes in 2 potential ways, likely simultaneously. First, it shifts to the left because of an acute decrease in left ventricular filling pressures after TAVI, perhaps with a further decrease over the long term. Second, it rotates counterclockwise because of a drop in intrinsic myocardial resistance that most likely continues for month(s) during the remodeling phase after TAVI. Together, the net effect produces a changing point of intersection for the same stenosis curve—namely, an increasing hyperemic flow (and CFR) and an associated decreasing FFR. Thus, our model agrees with the experimental observations in the Table. However, the existence and extent of both of these transformations require dedicated mechanistic studies. For example, it would be logical to expect that the myocardial improvement relates to the reduction in left ventricular filling pressures plus the change in the stress aortic valve index that quantifies the improvement in transvalvular flow from TAVI.20
Of course, actual patients will be more complex for the following reasons. First, enormous heterogeneity exists among coronary lesions regarding their relative viscous friction and expansion/separation contributions. Thus, “steep” curves (dominant expansion/separation component) will show larger changes in FFR (and hyperemic flow and CFR), and these lesions in general have lower FFR values even before TAVI. Second, the coronary curve over time might become worse because of disease progression or better because of plaque regression from aggressive treatment and/or positive vessel remodeling. Third, some empiric data suggest a longer‐term return to lower CFR values (mean 2.01) after 3 years despite an improvement at 15 months (CFR 2.57 versus 1.96 at baseline) and stable prothesis function,21 although we are only aware of this single study with such long‐term follow‐up. But the general concept of progressive myocardial disease in elderly patients after TAVI does not require too much imagination.
And What to Do Now?
While waiting for emerging randomized trial evidence regarding PCI in patients being considered for TAVI, we must still treat the patients in our clinics today. Therefore, we would like to conclude by placing the above data and framework into a pragmatic answer to the question posed in our title.
First, the combination of severe AS plus coronary disease behaves like “serial stenoses”22 whereby the presence of an upstream lesion (in this case the severely stenotic aortic valve) reduces flow across the downstream lesion (in this case the coronary stenosis) and makes it appear less severe than when measured in isolation. Just as with serial coronary lesions, it is important to remeasure the coronary stenosis after TAVI, or to anticipate that its FFR value might be lower than before TAVI, depending on the factors in our mechanistic figure. While sophisticated techniques could probably predict the amount of change (measure the current myocardial load line and quantify typical changes in its slope and intercept, assess the pressure loss versus flow curve for the stenosis, and model how their intersection adapts), a more practical solution is to create an aortic stenosis “gray zone” for FFR values between 0.80 and 0.85 before TAVI that might drop to <0.75 after TAVI. Indeed, an ongoing trial notes that “lesions showing ‘borderline’ FFR measurements before TAVI (FFR 0.80–0.83), should be measured again … after TAVI, and the decision of treating or deferring treatment in a given lesion will be based on the FFR value obtained after TAVI” (clinicaltrials.gov NCT03360591).
Second, while awaiting randomized studies, we do have observational data to support FFR‐guided treatment instead of angiography‐based PCI in patients undergoing TAVI.23 In a cohort of 216 patients, treated ≈60% by angiography and 40% by FFR, a lower composite end point was observed over an average 2 years of follow‐up when using physiology. Interestingly, the event curves diverged early because of more periprocedural myocardial infarctions and cardiac death in the angiography‐guided cohort, indicating that the key benefit of FFR was to avoid PCI complications in hemodynamically insignificant lesions.
Third, some investigators including Vendrik and colleagues1 have advocated for resting physiology metrics because of the empiric observation that they remain unaffected by TAVI. In our opinion this insensitivity of resting coronary pressure gradients provides yet another argument against their routine adoption. The importance of any coronary lesion remains intimately intertwined with the status of its downstream myocardium, as obvious when considering extreme cases such as a high‐grade stenosis supplying a transmural scar versus viable tissue. Severe AS and TAVI produce massive changes within the heart. A variable that cannot tell the difference between untreated and treated AS does not merit serious consideration.
In conclusion, FFR values can decrease after TAVI for physiologic reasons hypothesized in the Figure, consistent with the current literature summarized in the Table, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 including the newest study from Vendrik and colleagues.1 Some FFR values 0.80 to 0.85 will drop to <0.75 because of myocardial improvements after relieving AS. Lesions with a low FFR and focal gradient that supply large amounts of myocardium can be considered for PCI depending on clinical circumstances, although the lack of randomized evidence appropriately reduces the certainty of this strategy.
Disclosures
Tonino and Johnson have a patent pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology. Johnson received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; has a patent pending on correcting pressure signals from fluid‐filled catheters; has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm; and has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE‐FLOW, NCT02328820), studies using intracoronary pressure and flow sensors. Zelis has no disclosures to report.
J Am Heart Assoc. 2020;9:e015806 DOI: 10.1161/JAHA.120.015806.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Vendrik J, Ahmad Y, Eftekhari A, Howard JP, Wijntjens GWM, Stegehuis VE, Cook C, Terkelsen CJ, Christiansen EH, Koch KT, Piek JJ, Sen S, Baan J. Long‐term effects of transcatheter aortic valve implantation on coronary haemodynamics in patients with concomitant coronary artery disease and severe aortic stenosis. J Am Heart Assoc. 2020;9:e015133 DOI: 10.1161/JAHA.119.015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 3. Lotfi A, Davies JE, Fearon WF, Grines CL, Kern MJ, Klein LW. Focused update of expert consensus statement: use of invasive assessments of coronary physiology and structure: a position statement of the Society of Cardiac Angiography and Interventions. Catheter Cardiovasc Interv. 2018;92:336–347. [DOI] [PubMed] [Google Scholar]
- 4. Lateef N, Khan MS, Deo SV, Yamani N, Riaz H, Virk HUH, Khan SU, Hedrick DP, Kanaan A, Reed GW, Krishnaswamy A, Puri R, Kapadia SR, Kalra A. Meta‐analysis comparing outcomes in patients undergoing transcatheter aortic valve implantation with versus without percutaneous coronary intervention. Am J Cardiol. 2019;124:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemes A, Forster T, Kovács Z, Thury A, Ungi I, Csanády M. The effect of aortic valve replacement on coronary flow reserve in patients with a normal coronary angiogram. Herz. 2002;27:780–784. [DOI] [PubMed] [Google Scholar]
- 6. Hildick‐Smith DJ, Shapiro LM. Coronary flow reserve improves after aortic valve replacement for aortic stenosis: an adenosine transthoracic echocardiography study. J Am Coll Cardiol. 2000;36:1889–1896. [DOI] [PubMed] [Google Scholar]
- 7. Carpeggiani C, Neglia D, Paradossi U, Pratali L, Glauber M, L'Abbate A. Coronary flow reserve in severe aortic valve stenosis: a positron emission tomography study. J Cardiovasc Med (Hagerstown). 2008;9:893–898. [DOI] [PubMed] [Google Scholar]
- 8. Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. [DOI] [PubMed] [Google Scholar]
- 9. Camuglia AC, Syed J, Garg P, Kiaii B, Chu MW, Jones PM, Bainbridge D, Teefy PJ. Invasively assessed coronary flow dynamics improve following relief of aortic stenosis with transcatheter aortic valve implantation. J Am Coll Cardiol. 2014;63:1808–1809. [DOI] [PubMed] [Google Scholar]
- 10. Wiegerinck EM, van de Hoef TP, Rolandi MC, Yong Z, van Kesteren F, Koch KT, Vis MM, de Mol BA, Piek JJ, Baan J Jr. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e002443. [DOI] [PubMed] [Google Scholar]
- 11. Ahmad Y, Götberg M, Cook C, Howard JP, Malik I, Mikhail G, Frame A, Petraco R, Rajkumar C, Demir O, Iglesias JF, Bhindi R, Koul S, Hadjiloizou N, Gerber R, Ramrakha P, Ruparelia N, Sutaria N, Kanaganayagam G, Ariff B, Fertleman M, Anderson J, Chukwuemeka A, Francis D, Mayet J, Serruys P, Davies J, Sen S. Coronary hemodynamics in patients with severe aortic stenosis and coronary artery disease undergoing transcatheter aortic valve replacement: implications for clinical indices of coronary stenosis severity. JACC Cardiovasc Interv. 2018;11:2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Gottin L, Zanetti C, Faggian G, Ribichini F. Physiologic evaluation of coronary lesions using instantaneous wave‐free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2018;13:1512–1519. [DOI] [PubMed] [Google Scholar]
- 13. Stoller M, Gloekler S, Zbinden R, Tueller D, Eberli F, Windecker S, Wenaweser P, Seiler C. Left ventricular afterload reduction by transcatheter aortic valve implantation in severe aortic stenosis and its prompt effects on comprehensive coronary haemodynamics. EuroIntervention. 2018;14:166–173. [DOI] [PubMed] [Google Scholar]
- 14. Stundl A, Shamekhi J, Bernhardt S, Starke M, Al‐Kassou B, Weber M, Sedaghat A, Treede H, Grube E, Nickenig G, Werner N, Sinning JM. Fractional flow reserve in patients with coronary artery disease undergoing TAVI: a prospective analysis. Clin Res Cardiol. 2019. DOI: 10.1007/s00392-019-01563-2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Pesarini G, Scarsini R, Zivelonghi C, Piccoli A, Gambaro A, Gottin L, Rossi A, Ferrero V, Vassanelli C, Ribichini F. Functional assessment of coronary artery disease in patients undergoing transcatheter aortic valve implantation: influence of pressure overload on the evaluation of lesions severity. Circ Cardiovasc Interv. 2016;9:e004088. [DOI] [PubMed] [Google Scholar]
- 16. Kirkeeide RL, Gould KL, Parsel L. Assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VII. Validation of coronary flow reserve as a single integrated functional measure of stenosis severity reflecting all its geometric dimensions. J Am Coll Cardiol. 1986;7:103–113. [DOI] [PubMed] [Google Scholar]
- 17. Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985). 2009;106:113–121. [DOI] [PubMed] [Google Scholar]
- 18. Aarnoudse W, Fearon WF, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls NH. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110:2137–2142. [DOI] [PubMed] [Google Scholar]
- 19. Fournier S, Colaiori I, Di Gioia G, Mizukami T, De Bruyne B. Hyperemic pressure‐flow relationship in a human. J Am Coll Cardiol. 2019;73:1229–1230. [DOI] [PubMed] [Google Scholar]
- 20. Johnson NP, Zelis JM, Tonino PAL, Houthuizen P, Bouwman RA, Brueren GRG, Johnson DT, Koolen JJ, Korsten HHM, Wijnbergen IF, Zimmermann FM, Kirkeeide RL, Pijls NHJ, Gould KL. Pressure gradient vs. flow relationships to characterize the physiology of a severely stenotic aortic valve before and after transcatheter valve implantation. Eur Heart J. 2018;39:2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nemes A, Forster T, Kovács Z, Csanády M. Is the coronary flow velocity reserve improvement after aortic valve replacement for aortic stenosis transient? Results of a 3‐year follow‐up. Heart Vessels. 2006;21:157–161. [DOI] [PubMed] [Google Scholar]
- 22. De Bruyne B, Pijls NH, Heyndrickx GR, Hodeige D, Kirkeeide R, Gould KL. Pressure‐derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation. 2000;101:1840–1847. [DOI] [PubMed] [Google Scholar]
- 23. Lunardi M, Scarsini R, Venturi G, Pesarini G, Pighi M, Gratta A, Gottin L, Barbierato M, Caprioglio F, Piccoli A, Ferrero V, Ribichini F. Physiological versus angiographic guidance for myocardial revascularization in patients undergoing transcatheter aortic valve implantation. J Am Heart Assoc. 2019;8:e012618 DOI: 10.1161/JAHA.119.012618. [DOI] [PMC free article] [PubMed] [Google Scholar]


