Abstract
Background
As younger patients are being considered for transcatheter aortic valve implantation (TAVI), the assessment and treatment of concomitant coronary artery disease is taking on increased importance.
Methods and Results
Thirteen contemporary lower‐risk patients with TAVI with severe aortic stenosis (AS) and moderate‐severe coronary lesions were included. Patients underwent assessment of coronary hemodynamics in the presence of severe AS (pre‐TAVI), in the absence of severe AS (immediately post‐TAVI), and at longer‐term follow‐up (6 months post‐TAVI). Fractional flow reserve decreased from 0.85 (0.76–0.88) pre‐TAVI to 0.79 (0.74–0.83) post‐TAVI, and then to 0.71 (0.65–0.77) at 6‐month follow‐up (P<0.001 for all comparisons). Conversely, instantaneous wave‐free ratio was not significantly different: 0.82 (0.80–0.90) pre‐TAVI, 0.83 (0.77–0.88) post‐TAVI, and 0.83 (0.73–0.89) at 6 months (P=0.735). These changes are explained by the underlying coronary flow. Hyperemic whole‐cycle coronary flow (fractional flow reserve flow) increased from 26.36 cm/s (23.82–31.82 cm/s) pre‐TAVI to 30.78 cm/s (29.70–34.68 cm/s) post‐TAVI (P=0.012), to 40.20 cm/s (32.14–50.00 cm/s) at 6‐month follow‐up (P<0.001 for both comparisons). Resting flow during the wave‐free period of diastole was not significantly different: 25.48 cm/s (21.12–33.65 cm/s) pre‐TAVI, 24.54 cm/s (20.74–27.88 cm/s) post‐TAVI, and 25.89 cm/s (22.57–28.96 cm/s) at 6 months (P=0.500).
Conclusions
TAVI acutely improves whole‐cycle hyperemic coronary flow, with ongoing sustained improvements at longer‐term follow‐up. This enhanced response to hyperemic stimuli appears to make fractional flow reserve assessment less suitable for patients with severe AS. Conversely, resting diastolic flow is not significantly influenced by the presence of severe AS. Resting indices of coronary stenosis severity, therefore, appear to be more appropriate for this patient population, although large‐scale prospective randomized trials will be required to determine the role of coronary physiology in patients with severe AS.
Keywords: aortic valve stenosis, coronary artery disease, coronary flow, coronary hemodynamic, TAVR/TAVI, ventricular remodeling
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Coronary Circulation, Physiology
Clinical Perspective
What Is New?
Transcatheter aortic valve implantation acutely improves whole‐cycle hyperemic coronary flow, with ongoing sustained improvements at longer‐term follow‐up.
What Are the Clinical Implications?
This enhanced response to hyperemic stimuli appears to make fractional flow reserve assessment less suitable for patients with severe aortic stenosis; therefore, resting indices of coronary stenosis severity appear to be more appropriate for this patient population, although large‐scale prospective randomized trials will be required to determine the role of coronary physiology in patients with severe aortic stenosis.
Transcatheter aortic valve implantation (TAVI) has been demonstrated to provide outcomes at least equivalent to surgical aortic valve replacement in high‐,1 intermediate‐,2, 3 and (more recently) low‐risk4, 5 populations. As younger patients are being considered for TAVI, the assessment and treatment of concomitant coronary artery disease (CAD) is taking on increased importance. Symptomatic assessment is challenging as both severe aortic stenosis (AS) and CAD can commonly cause exertional chest pain and shortness of breath. Noninvasive tests of ischemia have been shown to perform relatively poorly in patients with severe AS.6 Several studies have examined the 2 most commonly used invasive, pressure‐derived indices of coronary perfusion, fractional flow reserve (FFR) and the instantaneous wave‐free ratio (iFR) in patients with severe AS and concomitant CAD.7, 8 However, the complete role of invasive coronary physiology, including coronary flow, has yet to be fully elucidated in patients with severe AS undergoing TAVI.9
The acute effect of TAVI on coronary blood flow has previously been studied,10 demonstrating significant reductions in hyperemic coronary flow and systolic coronary flow in patients with severe AS. TAVI acutely increased hyperemic and systolic flow, which subsequently led to an acute reduction in FFR immediately after TAVI.11The longer‐term effects of TAVI on invasively measured coronary flow in patients with severe AS and concomitant CAD has yet to be studied. It has been hypothesized that as TAVI leads to longer‐term regression of left ventricular (LV) mass and remodeling of the ventricle, there will be further longer‐term changes in coronary blood flow.
In this study, we aim to determine how TAVI affects coronary blood flow and other coronary physiological parameters of coronary stenosis severity in patients with severe AS and concomitant CAD. We assessed the coronary circulation in the presence of severe AS (immediately pre‐TAVI), in the absence of severe AS (immediately post‐TAVI), and after longer‐term follow‐up (6 months post‐TAVI). This allows us to determine whether the acute changes in coronary flow seen immediately after TAVI are sustained or whether they change at longer‐term follow‐up.
Methods
Patient Population
The data, analytic methods, and study materials that support the findings of this study are available from the corresponding author upon reasonable request. Patients with severe, symptomatic AS undergoing TAVI with moderate to severe coronary lesions (≥50% diameter stenosis) were recruited from 2 European centers (Amsterdam Medical Centre, Amsterdam, The Netherlands; and Aarhus University Hospital, Aarhus, Denmark). All patients were scheduled for TAVI on clinical grounds after a decision at a Heart Team meeting. The study protocols were approved by the local institutional review board and patients gave written informed consent (DIVA [Diagnostic and Prognostic Value of Intracoronary Physiologic Indices and Need for Revascularisation in Severe Aortic Valve Disease] study, trialregister.nl identifier: NL6328 [NTR6520] and the FACE (Evaluation of fractional flow reserve of epicarcardial coronary artery disease and aortic stenosis before and after transcatheter aortic valve implantation) study, Central Region Denmark identifier M‐2016‐306‐16). Exclusion criteria were known nonviable myocardium in the area of the corresponding coronary artery being studied, history of coronary artery bypass grafting, severe renal dysfunction (<30 mL/min per 1.73 m2), contraindication to the administration of adenosine, inability to consent, or weight over 200 kg. All patients had prospectively collected combined coronary pressure and flow measurements, with paired measurements immediately pre‐ and post‐TAVI, as well as after 6 months of follow‐up. None of the patients were included in a previously published study.11
TAVI Procedures
All patients were treated using local anesthetic only, via transfemoral access. The used valve types were either Edwards SAPIEN 3 valves (Edwards Lifesciences Corporation) or Medtronic Evolut R valves (Medtronic). Valve choice was at the Heart Team's and operator's discretion, and was decided before study inclusion.
Physiological Assessment Protocol
An intracoronary bolus of nitroglycerin was administered in all patients before intracoronary measurements. A dual pressure and Doppler sensor–equipped 0.014″ guidewire was used for all physiological assessments (ComboWire, Volcano Corporation). The pressure signals were normalized in the aorta before advancing the wire a minimum of 3‐vessel diameters distal to the coronary stenosis. Doppler signals were optimized and stabilized to ensure good tracking profiles. At this stage, resting pressure and flow measurements were recorded. Hyperemia was then induced using an intracoronary bolus of adenosine (respectively 100 μg for right and 200 μg for left coronary system). Physiological measurements under hyperemic conditions were then recorded. At the end of each recording, the pressure sensor was returned to the catheter tip to ensure that there was no pressure drift. When drift was identified (≥0.02), all measurements were repeated. All patients then underwent the TAVI procedure according to standard clinical protocols. Subsequent to the successful TAVI, the entire protocol was repeated with the wire sited in the same location as the preintervention measurements. Patients returned for a follow‐up assessment 6 months following TAVI. The entire physiological protocol was repeated in an identical manner to those conducted during the index assessments during the TAVI procedure.
Analysis of Hemodynamic Data
ECG, pressure, and coronary flow velocity signals were extracted with the dedicated device console (ComboMap, Volcano Corporation). Analog output feeds were taken from the pressure‐velocity console and ECG, fed into a National Instruments DAQ card AI‐16E‐4, and acquired at 1 kHz with LabVIEW. Data were analyzed offline with a custom software package designed with MATLAB (The MathWorks, Inc).
Coronary pressure, flow velocity, and resistance were assessed over the whole cardiac cycle and during the wave‐free period during the diastolic phase of the cardiac cycle. All measurements were performed during resting conditions and during hyperemia and were analyzed accordingly. The wave‐free period was identified using wave‐intensity analysis12 and used to perform phasic analysis.
Definitions of hemodynamic variables were as follows:
where Pa indicates mean aortic pressure; Pd, mean intracoronary pressure distal to a stenosis; PdPa, distal pressure divided by aortic pressure; wfp, the wave‐free period of diastole; vh, mean flow velocity distal to a stenosis during hyperemia; and vb, mean flow velocity distal to a stenosis at baseline.
Statistical Analysis
Continuous variables are presented as median and interquartile range unless otherwise stated. Comparisons for pre‐TAVI, post‐TAVI, and longer‐term follow‐up were performed using Friedman test. In the first instance, we looked for evidence of a significant difference between pre‐, post‐, and follow‐up measurements. In the event that a significant difference was found across all groups, we then compared each individual category in a stepwise fashion, deriving a P value for each comparison (pre‐TAVI versus post‐TAVI, pre‐TAVI versus follow‐up, and post‐TAVI versus follow‐up). We used the Benjamini‐Hochberg procedure to control the false discovery rate.13 The threshold for statistical significance was set at 0.05. All analyses were performed using R version 3.2.1 (R Foundation).
Results
Patient Population
Thirteen patients were recruited for follow‐up measurements after successful TAVI procedures and completion of the baseline physiological protocol. The median age was 77.3 years (75.4–80.8 years) and a predicted surgical risk (Society of Thoracic Surgeons Predicted Risk of Mortality [STS‐PROM]) of 2.11 (1.97–2.60), depicting a more contemporary lower‐risk TAVI population. Baseline clinical characteristics are shown in Table 1. The baseline echocardiographic characteristics are summarized in Table 2. Twelve patients were treated with the SAPIEN 3 prosthesis, and 1 patient was treated with an Evolut R prosthesis. Quantitative coronary angiographic data are summarized in Table 3.
Table 1.
Baseline Clinical Characteristics
| Age, y | 77.3 (75.4–80.8) |
| Men | 6 (46.2) |
| Body mass index, kg/m2 | 27.6 (24.2–31.6) |
| Diabetes mellitus | 1 (7.7) |
| Hypertension | 7 (53.8) |
| Hyperlipidemia | 3 (23.1) |
| Former smoker | 8 (61.5) |
| Previous myocardial infarction | 2 (15.4) |
| Previous percutaneous coronary intervention | 2 (15.4) |
| History of atrial fibrillation | 3 (23.1) |
| STS‐PROM, % | 2.11 (1.97–2.60) |
| EuroSCORE II, % | 1.73 (1.55–2.55) |
| Follow‐up duration | 166 (122–238) |
Data are expressed as median (±interquartile range) or number (percentage). EuroSCORE indicates European System for Cardiac Operative Risk Evaluation; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality score.
Table 2.
Echocardiographic Characteristics
| Pre‐TAVI | Post‐TAVI | Follow‐Up | P Value | |
|---|---|---|---|---|
| Peak gradient, mm Hg | 75 (59–92) | 14 (7–20) | 22 (17–29) | <0.001a/<0.001a/0.06 |
| Aortic valve area, cm2 | 0.83 (0.70–0.95) | 1.53 (1.46–1.70) | 1.57 (1.40–1.68) | <0.001a/<0.001a/0.76 |
| LV systolic function | ||||
| Normal | 10 (76.9) | 11 (84.6) | 11 (84.6) | NS |
| Mildly impaired | 2 (15.4) | 2 (15.4) | 2 (15.4) | |
| Moderately impaired | 1 (7.7) | 0 | 0 | |
| Severely impaired | 0 | 0 | 0 | |
| Paravalvular leak | ||||
| None | … | 10 (76.9) | 12 (92) | NS |
| Mild | … | 2 (15.4) | 1 (7.7) | |
| Moderate | … | 1 (7.7) | 0 | |
| Severe | … | 0 | 0 | |
Data are expressed as median (interquartile range) or number (percentage) analyzed with chi‐square test. LV indicates left ventricular; TAVI, transcatheter aortic valve implantation.
P<0.05.
Table 3.
Quantitative Coronary Angiographic Data
| Target vessel | |
| LAD | 6 (46.2) |
| RCx | 3 (23.1) |
| RCA | 4 (30.8) |
| Stenosis location | |
| Proximal | 6 (46.2) |
| Mid | 3 (23.1) |
| Distal | 4 (30.8) |
| Diameter stenosis by QCA, % | 53.3 (49.04–63.60) |
| Area stenosis by QCA, % | 78.2 (74.02–86.75) |
| Stenosis length, mm | 9.97 (8.07–13.34) |
| Minimum luminal diameter, mm | 1.27 (1.15–1.63) |
| Minimum luminal area, mm2 | 1.27 (1.03–2.58) |
Data are expressed as median (interquartile range) or number (percentage). LAD indicates left anterior descending; QCA, quantitative coronary analysis; RCA, right coronary artery; RCx, ramus circumflexus.
Coronary Hemodynamic Data
A summary of all coronary hemodynamic data is shown in Table 4 and the Figure (Panel A through F) and Figures S1 through S3.
Table 4.
Coronary Hemodynamic Data
| Pre‐TAVI | Post‐TAVI | Follow‐Up | P Valuea | |
|---|---|---|---|---|
| FFR | 0.85 (0.76–0.88) | 0.79 (0.74–0.83) | 0.71 (0.65–0.77) | <0.001/<0.001/<0.001b |
| CFR | 1.28 (1.10–1.51) | 1.65 (1.47–1.85) | 1.94 (1.69–2.25) | <0.001/<0.001/<0.001b |
| iFR | 0.82 (0.80–0.90) | 0.83 (0.77–0.88) | 0.83 (0.73–0.90) | 0.735 |
| PdPa | 0.87 (0.84–0.93) | 0.89 (0.84–0.94) | 0.91 (0.84–0.94) | 0.663 |
| FFR flow, cm/s | 26.36 (23.82–31.82) | 30.78 (29.70–34.68) | 40.20 (32.14–50.00) | 0.012/<0.001/<0.001b |
| iFR flow, cm/s | 25.48 (21.12–33.65) | 24.54 (20.74–27.88) | 25.89 (22.57–28.96) | 0.500 |
| PdPa flow, cm/s | 19.98 (17.51–21.57) | 19.70 (17.49–22.93) | 21.44 (19.80–26.74) | 0.397 |
| BMR, mm Hg/cm per s | 3.55 (3.38–4.99) | 4.26 (3.24–5.03) | 4.05 (3.73–5.38) | 0.397 |
| HMR, mm Hg/cm per s | 2.54 (2.28–2.90) | 2.18 (1.59–2.41) | 1.95 (1.59–2.34) | <0.001/<0.001/<0.001b |
| BSRI, mm Hg/cm per s | 0.36 (0.31–0.44) | 0.37 (0.30–0.44) | 0.32 (0.15–0.52) | 0.397 |
| HSRI, mm Hg/cm per s | 0.50 (0.39–0.87) | 0.51 (0.46–0.63) | 0.46 (0.30–0.69) | 0.397 |
Data are expressed as median (interquartile range). BMR indicates basal microvascular resistance; BSRI, basal stenosis resistance index; CFR, coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; HSRI, hyperemic stenosis resistance index; iFR, instantaneous wave‐free ratio; PdPa, distal pressure divided by aortic pressure.
P value from the Friedman test, the first P value is for a significant difference between all 3 groups. When a significant difference was found across all groups, the 3 stated P values depict the stepwise comparison between all individual groups (pre–transcatheter aortic valve implantation (TAVI) vs post‐TAVI, pre‐TAVI vs follow‐up, and post‐TAVI vs follow‐up). If no significant difference was found using the Friedman test, only this P value is stated.
P<0.05.
Figure 1.
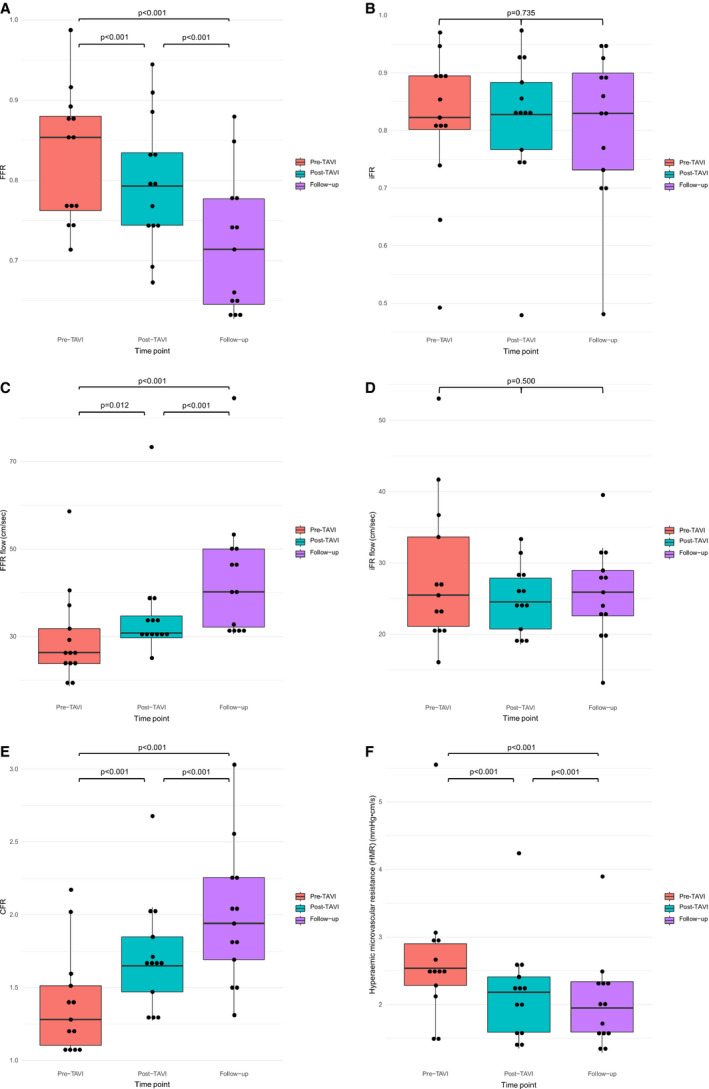
Boxplot of the (A) fractional flow reserve (FFR), (B) instantaneous wave‐free ratio (iFR), (C) FFR flow, (D) iFR flow, (E) coronary flow reserve (CFR), and (F) hyperemic microvascular resistance (HMR) values, for the different time points. Individual values are depicted as the dots. TAVI indicates transcatheter aortic valve implantation.
Indices of Coronary Stenosis Severity
FFR decreased from 0.85 (0.76–0.88) pre‐TAVI to 0.79 (0.74–0.83) post‐TAVI, and then to 0.71 (0.65–0.78) at long‐term follow‐up, with evidence of a significant difference between the groups (P<0.0001). Additional testing showed a significant interaction for each pairwise comparison between the different time points (P<0.0001 for each). Conversely, iFR was unchanged pre‐TAVI (0.82 [0.80–0.89]), post‐TAVI (0.83 [0.77–0.89]), and then at longer‐term follow‐up (0.83 (0.73–0.93), with no evidence of a significant difference between the groups (P=0.735). Figures depicting the FFR and iFR measurements are shown in the Figure (Panel A and B), and the PdPa measurements are disclosed in Figure S1.
Coronary Flow
Hyperemic whole‐cycle coronary flow (FFR flow) increased post‐TAVI (26.36 cm/s pre‐TAVI versus 30.78 cm/s post‐TAVI, with a further increase at 6‐month follow‐up to 40.20 cm/s. There was evidence of a significant difference between the groups (P=0.012), with additional testing showing a significant interaction for each pairwise comparison (P=0.012 for pre‐TAVI versus post‐TAVI; P<0.0001 for pre‐TAVI versus follow‐up and post‐TAVI versus follow‐up).
Resting flow during the wave‐free period of diastole (iFR flow) was unchanged from pre‐TAVI (25.48 cm/s [21.12–33.65]) to post‐TAVI (24.54 cm/s [20.74–27.88]), and then at longer‐term follow‐up (25.89 cm/s [22.58–28.96]), with no evidence of a significant difference between the groups (P=0.500). FFR flow and iFR flow measurements are shown in the Figure (Panel C and D). PdPa flow is shown in Figure S2.
Coronary flow reserve increased from 1.28 (1.10–1.85) pre‐TAVI to 1.65 (1.47–1.85) post‐TAVI, and then to 1.94 (1.69–2.25) at longer‐term follow‐up, with evidence of a significant difference between the groups (P<0.0001). Additional testing showed a significant interaction for each pairwise comparison (P<0.0001 for each).
Microvascular Resistance
Hyperemic microvascular resistance decreased from 2.54 mm Hg/cm per second (2.28–2.90 mm Hg/cm per second) pre‐TAVI to 2.18 mm Hg/cm per second (1.59–2.41 mm Hg/cm per second) post‐TAVI (P<0.01), and then to 1.95 mm Hg/cm per second (1.59–2.34 mm Hg/cm per second) at longer‐term follow‐up, with evidence of a significant difference between the groups (P<0.0001). Additional testing showed a significant interaction for each pairwise comparison (P<0.0001 for each).
Discussion
In this study, we have shown that: (1) hyperemic coronary flow velocity increases acutely post‐TAVI, and continues to rise up to 6‐month follow‐up; (2) this rise in flow causes both acute and long‐term declines in FFR values, leading FFR to underestimate coronary stenosis severity in the presence of severe AS; and (3) resting diastolic flow, and consequently iFR, is not affected by severe AS and remains unchanged pre‐TAVI, post‐TAVI, and at 6‐month follow‐up.
Long‐Term Effects of TAVI on Coronary Flow
It has previously been shown that TAVI causes acute increases in hyperemic flow and systolic flow, leading to an acute reduction in FFR. Scarsini et al14 correlated iFR to FFR values without measuring coronary flow, and showed iFR to be stable before and after the procedure, although depending on the extent of the transaortic gradient drop after TAVI. There were concerns raised regarding iFR values crossing the treatment threshold of 0.89. However, this is dependent on the distributions of values within the study sample, and on interpreting continuous values using a dichotomous cut point.9 Furthermore, it is not yet known whether this 0.89 cut point is applicable and valid for patients with severe AS.
The long‐term effects of TAVI on coronary flow and physiologic parameters, however, have remained unknown and have not previously been studied. In this study, we have demonstrated that there is an ongoing increase in hyperemic coronary flow out to 6 months, and that this leads to a consequent significant drop in the FFR value. Severe AS leads to pathophysiological changes in the LV myocardium, with subsequent hypertrophy and fibrosis.15 These changes cause a fixed compression to the coronary microcirculation and impede its ability to vasodilate in response to hyperemic agents such as adenosine.16, 17 This results in blunted hyperemic flow, as described in this and previous studies. Taking into consideration that Poiseuille and Bernoulli Law (ΔP=fQ+sQ2) states that the pressure gradient over a stenosis is partly determined by the flow over that stenosis, and that severe AS causes a reduction in hyperemic coronary flow velocity, FFR values are likely to be false‐negative in the presence of severe AS.
Following successful treatment of severe AS with TAVI, acute changes in the myocardium lead to increases in coronary flow and reductions in FFR directly post‐TAVI, as shown by the present and previous studies. However, progressive regression of LV mass and other favorable remodeling of the left ventricle may occur far beyond the early phase post‐TAVI. This results in further increases in the ability of the microcirculation to respond to hyperemic stimuli and, thus, in hyperemic coronary flow velocity.18 This is reflected by our data showing both an increase in coronary flow velocity and a reduction in FFR up to 6 months of follow‐up.
In contrast, resting diastolic flow appears to be unaffected by the presence of severe AS. We previously demonstrated that the aortic valve has minimal impact on coronary flow during diastole. In this study, we demonstrated that there are also no longer‐term changes in resting diastolic flow out to 6 months, and therefore no significant changes in the iFR values. This suggests that LV hypertrophy and elevated LV pressures do not have an important impact on iFR. Such resting indices of coronary stenosis severity may, therefore, be used preferentially in patients with severe AS.
Clinical Implications
For patients undergoing TAVI, the optimal way to assess and treat this concomitant coronary disease has not yet been established. There is currently no clear evidence that percutaneous coronary intervention before TAVI improves clinical outcomes,19 and several clinical trials concerning percutaneous coronary intervention in patients with TAVI are still ongoing (ie, the NOTION‐3 [Nordic Aortic Valve Intervention‐3; NCT03058627] and REVIVAL [Revascularization After Transcatheter Aortic Valve Implantation; NCT03283501] trials). Large randomized clinical trials evaluating the use of coronary physiology in these patients will ultimately help to define the optimal treatment strategy. The importance of accurately assessing the significance of coronary disease, and offering percutaneous coronary intervention if appropriate, is increasing as TAVI moves into the lower‐risk realm and is being performed in younger patients.
We have shown that hyperemic indices of coronary stenosis severity, such as FFR, are less able to accurately isolate the functional significance of a coronary lesion in the presence of severe AS. This appears to lead to a systematic underestimation of coronary lesion severity, and therefore will potentially miss flow‐limiting coronary lesions that would benefit from revascularization. For a patient older than 80 years with severe AS, this may be of limited significance, and in such patients a strategy of treating the valve with TAVI and managing the coronary disease medically may well be appropriate. However, for a patient aged 60 years, the situation is different. Last, there may be challenges in accessing the coronary ostia post‐TAVI (especially when higher‐profile self‐expanding valves such as the CoreValve [Medtronic] or Evolut R are used).
Our findings are also potentially of importance for patients undergoing surgical aortic valve replacement. Preoperative coronary angiography and FFR measurement, in the presence of severe AS, is likely to lead to falsely elevated FFR values and therefore the potential to defer concomitant bypass grafting for a patient who might otherwise benefit from surgical coronary revascularization.
Limitations
This is a small prospective 2‐center study. The small sample size deprived us of performing specific subanalyses, such as a correlation of the physiologic indices and angiographic lesion severity or compare results stratified by sex.20 However, this is the only study to demonstrate longer‐term invasive coronary hemodynamic data post‐TAVI (with previous paired measurements immediately before and after TAVI) and is comparable in size to previous physiological studies in the field.21 This was a physiological study examining hemodynamic data, and was not intended to look at clinical outcomes, nor was it powered for this. Ultimately, large prospective randomized trials powered for clinical end points will be required to fully elucidate the role of coronary physiology in guiding the treatment of severe AS.
In this study, adenosine was administered as an intracoronary bolus and not via intravenous infusion. We cannot therefore exclude the possibility that the latter would yield different results. Intravenous adenosine infusion could lead to reductions in aortic pressure destabilizing patients with severe AS, although it has also been shown that intravenous administration is relatively safe.8, 22, 23, 24 Intracoronary adenosine administration is recognized as a valid approach for inducing hyperemia when performing intracoronary measurements and is used in most large trials regarding clinical end points.9 Last, our study only included patients with symptomatic severe AS referred for TAVI. We do not know how milder forms of AS may affect hyperemic coronary flow and the commonly used indices of coronary stenosis severity. This should be the subject of future research.
Conclusions
TAVI acutely improves whole‐cycle hyperemic coronary flow, with ongoing sustained improvements at longer‐term follow‐up. This enhanced response to hyperemic stimuli appears to make FFR assessment less suitable for patients with severe AS. Conversely, resting diastolic flow is not significantly influenced by the presence of severe AS. Resting indices of coronary stenosis severity therefore appear to be more appropriate for this patient population, although large‐scale prospective randomized trials will be required to determine the role of coronary physiology for patients with severe AS.
Disclosures
J.J.P. served as a speaker at educational events organized by Philips‐Volcano, St. Jude Medical, and/or Boston Scientific, manufacturers of sensor‐equipped guidewires. S.S. has attended and conducted teaching sessions supported by Volcano Corporation, St. Jude Medical, Medtronic, Pfizer, and AstraZeneca; has received research grant support from Philips, AstraZeneca, Medtronic, and Pfizer; and has received speaking honoraria from Pfizer and Volcano‐Philips. J.B. receives an unrestricted research grant from Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Boxplot of the distal pressure divided by aortic pressure (PdPa) values, for all of the different time points. Individual values are depicted as the dots. Pa indicates aortic pressure; Pd, distal pressure.
Figure S2. Boxplot of the distal pressure divided by aortic pressure (PdPa) flow values, for all of the different time points. Individual values are depicted as the dots. Pa indicates aortic pressure; Pd, distal pressure.
Figure S3. Boxplot of the HSRi values, for all of the different time points.
(J Am Heart Assoc. 2020;9:e015133 DOI: 10.1161/JAHA.119.015133.)
References
- 1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 3. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators . Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 4. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL III, Forrest JK, Tchetche D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators . Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 5. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators . Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 6. Alli O, Rihal CS, Suri RM, Greason KL, Waksman R, Minha S, Torguson R, Pichard AD, Mack M, Svensson LG, Rajeswaran J, Lowry AM, Ehrlinger J, Tuzcu EM, Thourani VH, Makkar R, Blackstone EH, Leon MB, Holmes D. Learning curves for transfemoral transcatheter aortic valve replacement in the partner‐i trial: technical performance. Catheter Cardiovasc Interv. 2016;87:154–162. [DOI] [PubMed] [Google Scholar]
- 7. Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Barbierato M, Caprioglio F, Vassanelli C, Ribichini F. Coronary physiology in patients with severe aortic stenosis: comparison between fractional flow reserve and instantaneous wave‐free ratio. Int J Cardiol. 2017;243:40–46. [DOI] [PubMed] [Google Scholar]
- 8. Yamanaka F, Shishido K, Ochiai T, Moriyama N, Yamazaki K, Sugitani A, Tani T, Tobita K, Mizuno S, Tanaka Y, Murakami M, Takahashi S, Yamazaki S, Saito S. Instantaneous wave‐free ratio for the assessment of intermediate coronary artery stenosis in patients with severe aortic valve stenosis: comparison with myocardial perfusion scintigraphy. JACC Cardiovasc Interv. 2018;11:2032–2040. [DOI] [PubMed] [Google Scholar]
- 9. Sen S, Ahmad Y, Davies J. Assessing coronary disease in patients with severe aortic stenosis: the need for a ‘valid’ gold standard for validation studies? EuroIntervention. 2018;13:1499–1502. [DOI] [PubMed] [Google Scholar]
- 10. Wiegerinck EM, van de Hoef TP, Rolandi MC, Yong Z, van Kesteren F, Koch KT, Vis MM, de Mol BA, Piek JJ, Baan J Jr. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e002443. [DOI] [PubMed] [Google Scholar]
- 11. Ahmad Y, Gotberg M, Cook C, Howard JP, Malik I, Mikhail G, Frame A, Petraco R, Rajkumar C, Demir O, Iglesias JF, Bhindi R, Koul S, Hadjiloizou N, Gerber R, Ramrakha P, Ruparelia N, Sutaria N, Kanaganayagam G, Ariff B, Fertleman M, Anderson J, Chukwuemeka A, Francis D, Mayet J, Serruys P, Davies J, Sen S. Coronary hemodynamics in patients with severe aortic stenosis and coronary artery disease undergoing transcatheter aortic valve replacement: implications for clinical indices of coronary stenosis severity. JACC Cardiovasc Interv. 2018;11:2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies JE, Whinnett ZI, Francis DP, Manisty CH, Aguado‐Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Evidence of a dominant backward‐propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113:1768–1778. [DOI] [PubMed] [Google Scholar]
- 13. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:298–300. [Google Scholar]
- 14. Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Gottin L, Zanetti C, Faggian G, Ribichini F. Physiologic evaluation of coronary lesions using instantaneous wave‐free ratio (IFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2018;13:1512–1519. [DOI] [PubMed] [Google Scholar]
- 15. Dixon JA, Spinale FG. Myocardial remodeling: cellular and extracellular events and targets. Annu Rev Physiol. 2011;73:47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broyd CJ, Sen S, Mikhail GW, Francis DP, Mayet J, Davies JE. Myocardial ischemia in aortic stenosis: insights from arterial pulse‐wave dynamics after percutaneous aortic valve replacement. Trends Cardiovasc Med. 2013;23:185–191. [DOI] [PubMed] [Google Scholar]
- 17. Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. [DOI] [PubMed] [Google Scholar]
- 18. La Manna A, Sanfilippo A, Capodanno D, Salemi A, Cadoni A, Cascone I, Polizzi G, Figuera M, Pittala R, Privitera C, Tamburino C. Left ventricular reverse remodeling after transcatheter aortic valve implantation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2013;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotronias RA, Kwok CS, George S, Capodanno D, Ludman PF, Townend JN, Doshi SN, Khogali SS, Genereux P, Herrmann HC, Mamas MA, Bagur R. Transcatheter aortic valve implantation with or without percutaneous coronary artery revascularization strategy: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e005960 DOI: 10.1161/JAHA.117.005960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pesarini G, Scarsini R, Zivelonghi C, Piccoli A, Gambaro A, Gottin L, Rossi A, Ferrero V, Vassanelli C, Ribichini F. Functional assessment of coronary artery disease in patients undergoing transcatheter aortic valve implantation: influence of pressure overload on the evaluation of lesions severity. Circ Cardiovasc Interv. 2016;9:e004088. [DOI] [PubMed] [Google Scholar]
- 21. Davies JE, Sen S, Broyd C, Hadjiloizou N, Baksi J, Francis DP, Foale RA, Parker KH, Hughes AD, Chukwuemeka A, Casula R, Malik IS, Mikhail GW, Mayet J. Arterial pulse wave dynamics after percutaneous aortic valve replacement: fall in coronary diastolic suction with increasing heart rate as a basis for angina symptoms in aortic stenosis. Circulation. 2011;124:1565–1572. [DOI] [PubMed] [Google Scholar]
- 22. Tarkin JM, Nijjer S, Sen S, Petraco R, Echavarria‐Pinto M, Asress KN, Lockie T, Khawaja MZ, Mayet J, Hughes AD, Malik IS, Mikhail GW, Baker CS, Foale RA, Redwood S, Francis DP, Escaned J, Davies JE. Hemodynamic response to intravenous adenosine and its effect on fractional flow reserve assessment: results of the adenosine for the functional evaluation of coronary stenosis severity (affects) study. Circ Cardiovasc Interv. 2013;6:654–661. [DOI] [PubMed] [Google Scholar]
- 23. De Bruyne B, Gould KL. Standardized hyperemic stress for fractional flow reserve. Circ Cardiovasc Interv. 2013;6:602–603. [DOI] [PubMed] [Google Scholar]
- 24. Stanojevic D, Gunasekaran P, Tadros P, Wiley M, Earnest M, Mehta A, Lippmann M, Levine M, Dawn B, Gupta K. Intravenous adenosine infusion is safe and well tolerated during coronary fractional flow reserve assessment in elderly patients with severe aortic stenosis. J Invasive Cardiol. 2016;28:357–361. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Boxplot of the distal pressure divided by aortic pressure (PdPa) values, for all of the different time points. Individual values are depicted as the dots. Pa indicates aortic pressure; Pd, distal pressure.
Figure S2. Boxplot of the distal pressure divided by aortic pressure (PdPa) flow values, for all of the different time points. Individual values are depicted as the dots. Pa indicates aortic pressure; Pd, distal pressure.
Figure S3. Boxplot of the HSRi values, for all of the different time points.


