Abstract
Background
The impact of hyperoxia, that is, supraphysiological arterial partial pressure of O2, on myocardial oxygen balance and function in stable multivessel coronary artery disease (CAD) is poorly understood. In this observational study, we assessed myocardial effects of inhalational hyperoxia in patients with CAD using a comprehensive cardiovascular magnetic resonance exam.
Methods and Results
Twenty‐five patients with stable CAD underwent a contrast‐free cardiovascular magnetic resonance exam in the interval between their index coronary angiography and subsequent revascularization. The cardiovascular magnetic resonance exam involved T1 and T2 mapping for tissue characterization (fibrosis, edema) as well as function imaging, from which strain analysis was derived, and oxygenation‐sensitive cardiovascular magnetic resonance imaging. The latter modalities were both acquired at room air and after breathing pure O2 by face mask at 10 L/min for 5 minutes. In 14 of the 25 CAD patients (56%), hyperoxia induced poststenotic myocardial deoxygenation with a subsequent oxygenation discordance across the myocardium. Extent of deoxygenation was correlated to degree of stenosis (r=−0.434, P=0.033). Hyperoxia‐associated poststenotic deoxygenation was accompanied by ipsiregional reduction of diastolic strain rate (1.39±0.57 versus 1.18±0.65; P=0.045) and systolic radial velocity (37.40±17.22 versus 32.88±13.58; P=0.038). Increased T2, as well as lower cardiac index, and defined abnormal strain parameters on room air were predictive for hyperoxia‐induced abnormalities (P<0.05). Furthermore, in patients with prolonged native T1 (>1220 ms), hyperoxia reduced ejection fraction and peak strain.
Conclusions
Patients with CAD and pre‐existent myocardial injury who respond to hyperoxic challenge with strain abnormalities appear susceptible for hyperoxia‐induced regional deoxygenation and deterioration of myocardial function.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02233634.
Keywords: blood‐oxygen level dependence, coronary artery disease, hyperoxia, oxygen, oxygenation‐sensitive cardiovascular magnetic resonance
Subject Categories: Coronary Circulation, Ischemia, Coronary Artery Disease, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
Hyperoxia induced an oxygenation discordance across the myocardium in 56% of patients with coronary artery disease as detected by cardiovascular magnetic resonance, and regional deoxygenation in poststenotic myocardium was correlated to degree of stenosis.
Only patients with a myocardial oxygenation discordance during hyperoxia showed a decrease in left ventricular ejection fraction and cardiac index.
At room air, several imaging parameters, such as baseline myocardial deformation and edema, were identified as markers that could potentially predict deteriorating myocardial oxygenation and function attributed to hyperoxia.
What Are the Clinical Implications?
Utility of supplemental oxygen in patients with stable coronary artery disease is controversial, with studies reporting both positive and negative results.
Cardiovascular magnetic resonance has the potential to identify predictors of worsening myocardial oxygenation and function during hyperoxia in patients with coronary artery disease.
These results lay the framework for larger studies to assess the prognostic role of these responses and investigate the future role of imaging parameters for tailoring oxygen therapy to the individual patient.
Introduction
Until recently, a supranormal inspired oxygen fraction was perceived to improve safety margins in acute medical care, and its unrestricted use was ubiquitous. In 2010, Moradkhan et al stated that hyperoxia was not perceived by medical staff to be detrimental, because of conflicting evidence and lack of prospective, randomized controlled studies.1 However, recent trials and meta‐analyses suggest not only potential advantages,2 but also negative effects of supraphysiological oxygen concentrations,3 particularly in patients with acute myocardial ischemia.4 Therefore, the European Society of Cardiology has revised previous guidelines for oxygen therapy in patients with acute coronary syndrome and recommends supplemental oxygen in non–ST‐segment–elevation myocardial infarction5 and ST‐segment–elevation myocardial infarction6 only for patients with a peripheral oxygen saturation (SpO2) of <90% or in respiratory distress. Adverse sequelae of hyperoxia may be caused by increased activity of reactive oxygen species, which directly impair tissue function and trigger subsequent cell death.7 Other problems may arise owing to direct vasoconstrictive effects of hyperoxia.8 Although hyperoxia may increase oxygen supply to tissues by the small increment of O2 dissolved in plasma, it has not been conclusively clarified whether, or when, this outweighs the decrement of O2 delivery caused by hyperoxic coronary vasoconstriction. Previous studies have consistently demonstrated a hyperoxia‐induced reduction in cardiac output.9 In an animal study with an acutely induced significant coronary artery stenosis and exposure to normoxemia followed by hyperoxia, functional and oxygenation‐sensitive (OS) cardiac magnetic resonance (CMR) imaging has elucidated some of these mechanisms.10 Hyperoxia decreased coronary flow in this model and failed to compensate for coronary vasoconstriction. Hyperoxia impaired both oxygenation and regional wall motion in the myocardial distal to the stenosis. In addition, hyperoxia impaired left ventricular ejection fraction (LVEF) in the animal group with coronary stenosis to a greater degree than in control animals without coronary stenosis.10 So far, data assessing such effects in patients with chronic nonacute coronary artery disease (CAD) have been lacking. Beyond acute cardiac care, there are various clinical scenarios, particularly in anesthesia and intensive care, where CAD patients may be exposed to supraphysiological oxygen tensions, suggesting the importance of further investigations.
This observational clinical study aims to describe the effects of hyperoxia on global and regional myocardial oxygenation and ventricular function in patients with stable multivessel CAD.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
This was a single‐center project performed at Bern University Hospital, Inselspital, Switzerland (CADOS; Clinical Trials Identifier: NCT02233634). The study was approved by the local ethics board, the Cantonal Ethics Committee of Bern (Swissethics‐ID: PB_2016‐02124), and complies with the Declaration of Helsinki. All subjects had given their written informed consent before enrolment into this study.
The project was designed to investigate the effects of blood gas changes on myocardial tissue oxygenation in patients with stable CAD. Part of this work has already been published in a publication describing the potential diagnostic utility of a patient‐controlled CO2 challenge to detect CAD.11
Patients were recruited to participate if there was at least 1 vascular territory subtended by an untreated stenosis of >50% diameter (percent diameter stenosis [%DS] by quantitative coronary angiography), to be detected in a diagnostic index angiography with intention to perform either a staged percutaneous coronary intervention or coronary artery bypass graft surgery. Further inclusion criteria were that CMR scanning could be performed before completion of revascularization, and also that there was a nonobstructed coronary vascular territory subtending at least 2 adjacent segments (according to the American Heart Association 17‐segment model), in order to serve as intraindividual control tissue. All participants were required to be aged >18 years, capable of providing written informed consent, not be pregnant, or with the presence any standard magnetic resonance imaging contraindications. Patients with acute myocardial infarction within <4 weeks preceding the magnetic resonance imaging exam, with pre‐existent coronary bypass grafts, or with severe pulmonary disease were excluded.
Healthy control participants were recruited by public advertisement, were required to be aged <35 years, nonsmokers for the past 6 months, free of any medication that would affect the cardiac or circulatory system and with a medical history free of cardiac or pulmonary disorders, or other disorders known to affect the microvasculature. All participants were asked to refrain from consuming caffeine within 12 hours before the exam.
Quantitative Coronary Angiography
Quantitative coronary angiography was performed from the angiography images (assessed following routine nitroglycerin administration) obtained in the index angiography. The %DS of all coronary arteries ≥1.5 mm was determined by the Bern University CoreLab with analysts concealed for the magnetic resonance imaging analysis (QangioXA version 7.3; Medis Medical Imaging Systems, Leiden, The Netherlands). To allow a comparison between results from angiography and CMR, this reader coded each myocardial segment as either (1) remote, perfused by a nondiseased coronary branch; (2) affected by a relevant stenosis (%DS >50%); or (3) reperfused by percutaneous coronary intervention with a stent during index angiography.
CMR Protocol
CMR imaging was performed using a clinical 3 Tesla magnetic resonance imaging system (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany).
The CMR included native pixel‐wise parametric T1 and T2 mapping in a basal and mid‐ventricular short‐axis slice, while participants breathed room air (RA), with normoxemia confirmed by pulse oximetry (SpO2 ≥94%). The 5(3)3‐modified Look–Locker sequence was used for T1 mapping (repetition time/echo time 281/1.12 ms, flip angle 35 degrees, voxel size 1.4×1.4×8.0 mm, and bandwidth 1085 Hz/Px). T2 maps were generated after acquiring 3 single‐shot gradient echo images (repetition time/echo time 207/1.32 ms, flip angle=12 degrees, voxel size 1.9×1.9×8.0 mm, and bandwidth 1184 Hz/Px) at different T2 preparation times of 0, 30, and 55 ms.
Standard cine acquisition for ventricular function analysis of 7 to 10 short‐axis slices and OS‐CMR12 in a basal and a mid‐ventricular short‐axis slice were acquired, first, during normoxemia and, second, during hyperoxia after breathing 100% oxygen with a clinical oxygen mask with reservoir bag at 10 L/min for 5 minutes. For function imaging, a standard gated balanced steady‐state free precession cine sequence was used (repetition time/echo time 3.3/1.43 ms, temporal resolution 44.8 ms, flip angle 65 degrees, voxel size 1.6×1.6×6.0 mm, matrix 192×120, and bandwidth 962 Hz/Px). OS‐CMR was imaged using a triggered balanced steady‐state free precession sequence (repetition time/echo time 3.4/1.70 ms, flip angle 35 degrees, voxel size 2.0×2.0×10.0 mm, matrix 192×120, and bandwidth 1302 Hz/Px).12 Noninvasive blood pressure, heart rate, and standard pulse oximetry were monitored and recorded throughout the exam. Occurrence of any adverse effects was documented.
CMR Image Blinding and Analysis
CMR images were recoded and the reader was blinded for the group (patient versus control) or sequence allocation (breathing RA or 100% oxygen), the angiography, and pulse oximetry data. Changes in myocardial oxygenation are expressed as changes in percent signal intensity between OS‐CMR images acquired under normoxemic and hyperoxic conditions, with manual tracing of epi‐ and endocardial contours using cvi42 (Circle Cardiovascular Imaging, Calgary, AB, Canada) by an experienced reader.
Standard cardiac function parameters were calculated, including LVEF, cardiac index (CI), end‐diastolic and end‐systolic volumes, and myocardial mass, each indexed to body surface area. Using feature‐tracking software, strain parameters were calculated for the left ventricle from cine images (excluding slices containing outflow tract and mitral valve planes). Peak strain (PS), time to PS, systolic strain rate, and early diastolic strain rate (e‐dSR) were obtained in both radial and circumferential orientation. Measures of peak displacement, time to peak displacement, and systolic and early diastolic velocity were reported for radial orientation. Measurements from strain analysis and OS‐CMR are reported as global values and also for myocardium subtended to the stenosed vessel (poststenotic territory, based on angiography results). Regional myocardial oxygenation response to hyperoxia was further categorized as concordant or discordant. Discordance was assumed when oxygenation response in poststenotic myocardium decreased more than in myocardium perfused by a patent coronary vessel (remote territory).
Statistical Analysis
Continuous data are reported as mean and SD, and categorical data as frequency and percentage. Radial and circumferential feature‐tracking results are denoted with letters R or C. Measurements obtained at hyperoxia were compared with normoxemic measurements using paired t testing for continuous variables or chi‐square and Fisher's exact tests for categorical variables. For testing the effects of T1 prolongation on global and regional hyperoxic response, patients were grouped according to a global T1 <1220 or ≥1220 ms (using our local cutoff for normal T1). Linear association with quantitative coronary angiography results was explored with Spearman's correlation test. Univariate linear regression was used to identify potential markers at normoxemia associated with hyperoxia‐induced oxygenation discordance. Multivariate analysis was not performed owing to the small sample size. Nonparametric equivalents were used, if appropriate. Tests were performed with GraphPad Prism (version 8.0; GraphPad Software Inc, La Jolla, CA) and SPSS software (version 26; SPSS IBM, Armonk, NY). Results were considered statistically significant at a 2‐tailed P<0.05.
Results
Participant Characteristics
Participant characteristics and indications for the index angiography are shown in Table 1. All patients had at least 1 vessel with a relevant coronary artery stenosis, defined as a %DS >50% (mean, 63±15%; chronic total occlusion, n=3). Twenty‐two patients (88%) had a percutaneous coronary intervention performed on 1 vessel during the initial visit with a good result; 3 patients had diagnostic angiography and were subsequently scheduled for percutaneous coronary intervention (n=1) or coronary artery bypass graft surgery (n=2). Hyperoxia did not induce changes of heart rate or systolic blood pressure in patients (Table 2), but healthy controls experienced an increase in diastolic blood pressure from 54±6 to 60±7 mm Hg (P=0.002). The change from breathing RA to hyperoxia by O2 insufflation increased SpO2 from 96±1% (CAD patients) and 97±1% (healthy controls), respectively, to above 99±1% in both groups (P<0.001).
Table 1.
Baseline Characteristics
| Controls (n=10) | Patients With CAD (n=25) | |
|---|---|---|
| Age, years | 27±4 | 64±11 |
| Sex, female, n (%) | 4 (40) | 3 (12) |
| BMI, kg/m2 | 23.9±2.3 | 27.4±4.5 |
| T1, ms | 1196±36 | 1244±48 |
| T2, ms | 38.4±1.7 | 40.2±2.9 |
| QCA (%DS) | 63±15 | |
| Days since index angiography | 37±10 | |
| Indication for index angiography | ||
| STEMI | 5 (20) | |
| NSTEMI | 13 (52) | |
| Stable angina | 7 (28) | |
Mean±SD or n (%) are displayed. BMI indicates body mass index; CAD, coronary artery disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; QCA (%DS), quantitative coronary angiography as percent diameter stenosis; STEMI, ST‐segment–elevation myocardial infarction.
Table 2.
Hemodynamics, Ventricular Function, and FT‐CMR at Normoxia and Hyperoxia
| Controls | Patients With CAD | |||||
|---|---|---|---|---|---|---|
| Room Air | O2 | P Value | Room Air | O2 | P Value | |
| Hemodynamics | ||||||
| Heart rate, bpm | 66±10 | 64±13 | 0.472 | 62±12 | 61±10 | 0.332 |
| Systolic BP, mm Hg | 107±10 | 111±15 | 0.230 | 130±16 | 132±17 | 0.312 |
| Diastolic BP, mm Hg | 54±6 | 60±7 | 0.002* | 75±8 | 76±9 | 0.594 |
| SpO2, % | 96.6±1.2 | 99.4±0.7 | <0.001* | 96.3±1.3 | 99.2±0.8 | <0.001* |
| LV function | ||||||
| Ejection fraction, % | 68.4±7.1 | 69.3±4.9 | 0.161 | 60.0±11.6 | 58.3±11.8 | 0.127 |
| End‐diastolic volume index, mL/m2 | 85±16 | 90±18 | 0.952 | 75±15 | 76±14 | 0.452 |
| End‐systolic volume index, mL/m2 | 26±5 | 27±6 | 0.360 | 30±12 | 31±12 | 0.186 |
| Stroke volume index, mL/m2 | 58±15 | 62±14 | 0.609 | 44±10 | 44±11 | 0.857 |
| Cardiac index, L/min/m2 | 4.06±1.10 | 3.87±1.23 | 0.051 | 2.89±0.84 | 2.75±0.79 | 0.040* |
| Mass index, g/m2 | 63±15 | 68±11 | ||||
| FT‐CMR | ||||||
| Circumferential | ||||||
| Peak strain, % | −22.0±2.0 | −21.0±1.7 | 0.149 | −19.7±4.1 | −19.4±4.1 | 0.219 |
| Time to peak strain, ms | 291±25 | 311±35 | 0.010* | 328±51 | 346±64 | 0.017* |
| Systolic strain rate, /s | −1.25±0.38 | −1.17±0.24 | 0.608 | −0.99±0.29 | −0.96±0.25 | 0.152 |
| Diastolic strain rate, /s | 1.78±0.31 | 1.63±0.38 | 0.316 | 0.94±0.44 | 0.80±0.32 | 0.048* |
| Radial | ||||||
| Peak strain, % | 42.6±6.3 | 39.3±6.0 | 0.090 | 37.1±11.3 | 36.4±11.2 | 0.411 |
| Time to peak strain, ms | 294±26 | 318±30 | 0.010* | 326±49 | 339±56 | 0.193 |
| Systolic strain rate, /s | 2.47±0.61 | 2.11±0.58 | 0.005* | 1.95±0.81 | 1.80±0.62 | 0.072 |
| Diastolic strain rate, /s | −3.22±0.64 | −2.83±0.52 | 0.055 | −1.89±0.92 | −1.71±0.66 | 0.158 |
| Displacement, mm | 7.02±0.93 | 6.58±0.84 | 0.032* | 6.55±1.47 | 6.51±1.40 | 0.589 |
| Time to displacement, ms | 294±26 | 312±48 | 0.176 | 333±54 | 344±71 | <0.001* |
| Systolic velocity, mm/s | 38.37±8.73 | 35.66±9.23 | 0.011* | 32.29±9.48 | 31.31±7.90 | 0.304 |
| Diastolic velocity, mm/s | −55.48±14.23 | −49.42±8.87 | 0.125 | −33.40±17.40 | −30.68±16.10 | 0.089 |
Global measurements are displayed as mean±SD. BP indicates blood pressure; bpm, beats per minute; CAD, coronary artery disease; FT‐CMR indicates feature tracking cardiovascular magnetic resonance; LV, left ventricular; SpO2, peripheral oxygen saturation. *P<0.05 indicates a significant change from normoxemia (room air) to hyperoxia.
Hyperoxic Response of Myocardial Oxygenation
The global change in myocardial signal intensity was comparable between healthy participants (−1.4±3.5%) and patients (−0.6±4.0%; P=0.572). In poststenotic territories of patients, this response was −0.9±3.7% with a maximum decrease of −7.4%. Degree of deoxygenation correlated significantly with an increasing %DS of the corresponding coronary artery (r=−0.434; P=0.033). In poststenotic myocardium, no relationship between hyperoxic oxygenation response and changes in ventricular function was observed.
Hyperoxic Response of Ventricular Function and Strain Parameters
In healthy participants, hyperoxia did not significantly affect global ventricular function or CI (Table 2). In the CAD population, CI decreased significantly with hyperoxia, from 2.89±0.84 to 2.75±0.79 L/min/m2 (P=0.040).
In feature‐tracking analysis, both healthy controls and CAD patients showed global reduction of systolic parameters like systolic strain rate and velocities when exposed to hyperoxia. In the CAD group, however, there was greater variability and baseline measurements on room air already indicated some functional impairment. In both groups, hyperoxia significantly prolonged circumferential time to peak strain (controls, from 291±25 to 311±35 ms [P=0.010]; CAD, from 328±58 to 346±64 ms [P=0.017]). Only CAD patients demonstrated significant hyperoxia‐induced deterioration of global e‐dSR (0.94±0.44/s at normoxemia versus 0.80±0.32/s at hyperoxia; P=0.048).
In myocardium distal to the stenosis in CAD patients, hyperoxia resulted in slowing of diastolic relaxation in both radial and circumferential orientation (radial e‐dSR: −3.17±1.46 versus −2.76±1.56; P=0.038; circumferential e‐dSR: 1.39±0.57 versus 1.18±0.65; P=0.045). Specifically, the poststenotic myocardium of patients exhibited deterioration of radial systolic strain rate and velocity (radial systolic strain rate: −2.56±1.39 versus 2.32±0.97; P=0.056; radial systolic velocity: 37.40±17.22 versus 32.88±13.58; P=0.038), whereas this was not observed in remote territories. Figures 1 and 2 show exemplary patients with discordant hyperoxia‐induced oxygenation response and changes in systolic and diastolic function parameters.
Figure 1.
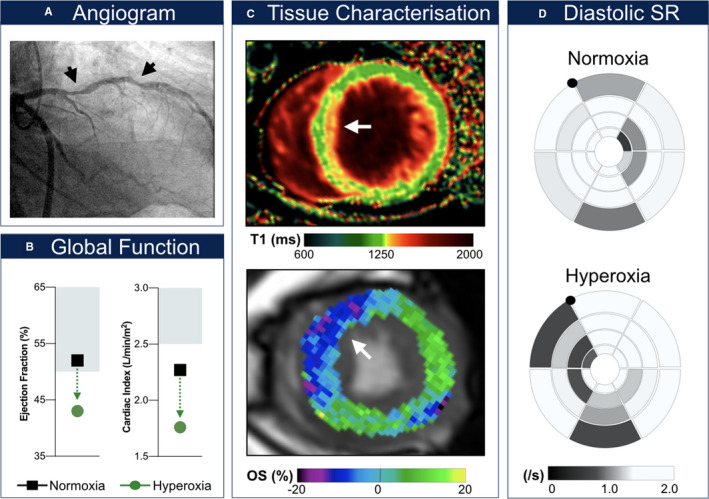
Exemplary coronary artery disease patient with hyperoxia‐induced decrease of global systolic and diastolic function. This patient had a maximal left anterior descending coronary artery (LAD) diameter stenosis of 61% (A) at CMR (cardiovascular magnetic resonance). The left circumflex coronary artery with a left‐dominant pattern was reperfused (stented) after presenting with a non–ST‐segment–elevation myocardial infarction 44 days before the staged percutaneous coronary intervention and CMR. Left ventricular ejection fraction decreased below 50% and cardiac index below 2.5 L/min/m2 during hyperoxia (B). The T1 mapping image (C, top) shows myocardial injury in the subendocardial layer of the anteroseptal segment (orange). This matches a hyperoxia‐induced oxygenation deficit (blue; C, bottom) observed in the LAD territory. This further corresponded to a regional decrease in early diastolic circumferential peak strain rate (D). Diastolic SR indicates diastolic strain rate; OS (%), % change in oxygenation‐sensitive signal intensity.
Figure 2.
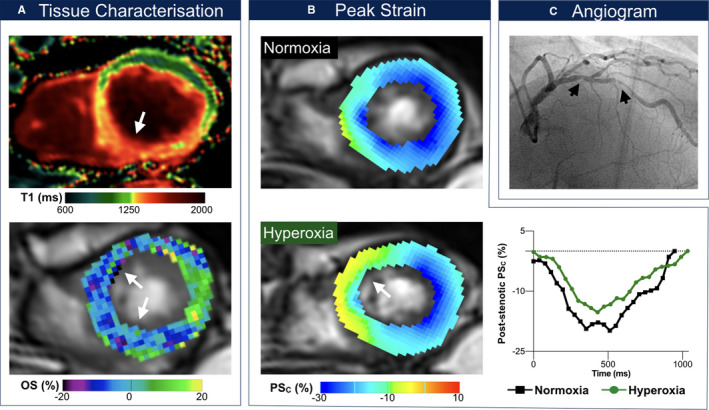
Exemplary patient with systolic dysfunction. Findings in an 80‐year‐old male patient with serial left anterior descending artery stenoses (C, maximal degree: 63% diameter stenosis). This patient had a previous percutaneous coronary intervention of serial right coronary artery stenoses following presentation with typical stable angina and inconclusive ergometry. In this patient, left ventricular ejection fraction dropped from 64% to 57% and cardiac index from 2.77 to 2.37 L/min/m2 after breathing oxygen, respectively. The T1 mapping image (A, top) shows myocardial injury in the septum and inferior wall, which is colocalized with both the oxygenation deficit (A, bottom) and circumferential strain (PSC) abnormalities induced with hyperoxia. At rest, the septum exhibits subnormal peak circumferential strain at end‐systole (B), which is exacerbated further at hyperoxia (yellow region). The graph shows that peak circumferential strain in poststenotic segments is attenuated across the entire cardiac cycle at hyperoxia (green) in comparison with normoxemia at room air (black). OS (%) indicates % change in oxygenation‐sensitive signal intensity.
Discord of Hyperoxia‐Induced Changes of Myocardial Oxygenation and Function
Discordance of hyperoxia‐induced myocardial oxygenation responses and its relationship to myocardial function parameters were explored. In the CAD group, a regionally discordant myocardial oxygenation response to hyperoxia was shown in 56%, that is, with attenuated response in poststenotic when compared with that of remote territories. Presence of regional oxygenation discordance was associated with hyperoxia‐induced reduction of LVEF (CAD with discordance: −4.0±5.3%, versus CAD with concordance: +0.7±4.0%; P=0.026).
Association Between Parameters of Myocardial Injury and Systolic Function
In comparison with healthy controls (1196±36 ms), CAD patients showed prolonged T1 (1244±48 ms; P=0.008; Table S1). Patient response to hyperoxia was dichotomized into 2 categories based on a baseline T1 mapping value of <1220 ms (normal reference range) or 1220 ms and longer (prolonged, signifying myocardial injury). Patients with prolonged normoxemic baseline T1 showed significantly reduced systolic function parameters LVEF and radial and circumferential PS during hyperoxia, which was in contrast to CAD patients with a baseline T1 of <1220 ms (Figure 3).
Figure 3.
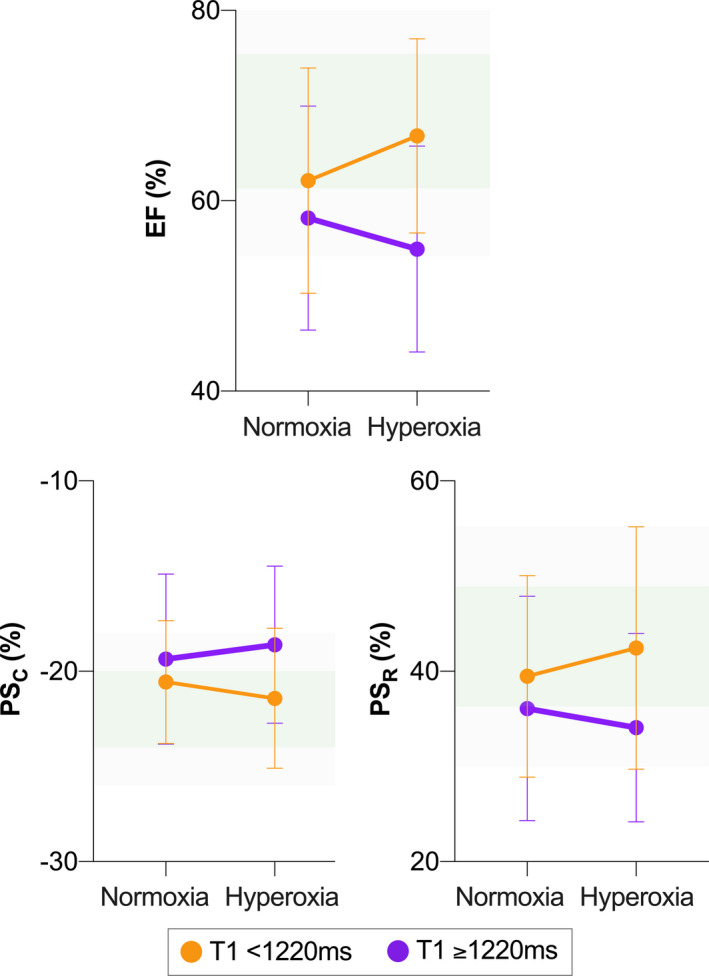
Association between parameters of myocardial injury and hyperoxic ventricular function. Patients with indicators of myocardial injury (T1 ≥1220 ms, purple) demonstrate reduced ejection fraction (EF) and peak strain in both circumferential (PSC) and radial (PSR) orientation, all of which significantly differ from those in patients with T1 <1220 ms (orange, P=0.017, 0.025, and 0.047, respectively). Shaded green areas represent reference ranges, with the darker shade indicating mean±1 SD of healthy control measurements at normoxemia (room air) and the lighter shade representing the ±2 SD cutoff.
Functional Deterioration in Patients With Myocardial Oxygenation Discordance in Hyperoxia
In 5 patients (25%), induction of hyperoxia reduced global LVEF to below 50%. In every one of these cases, mapping of myocardial oxygenation revealed regionally discordant responses (P=0.034). Also, in each of the 7 patients (28%) whose CI decreased to <2.5 L/min/m2 at hyperoxia, regional myocardial oxygenation was discordant (P=0.008).
Indicators of Hyperoxia‐Induced Myocardial Oxygenation Discordance
Univariate analysis was performed to assess which parameters at normoxemia could indicate regionally discordant oxygenation of the myocardium at hyperoxia (Figure 4). Of the clinical measurements, a low stroke volume index and subsequent low CI were associated with discordance, but not ejection fraction. All markers from feature‐tracking except for time to peak were associated with discordance, with the highest correlation coming from peak displacement and diastolic strain rate and velocities in the radial orientation. Presence of a more‐recent myocardial injury indicated by an elevated T2 showed a relationship to a hyperoxia‐induced oxygenation mismatch, whereas a nonsignificant trend was observed with T1 (P=0.056).
Figure 4.
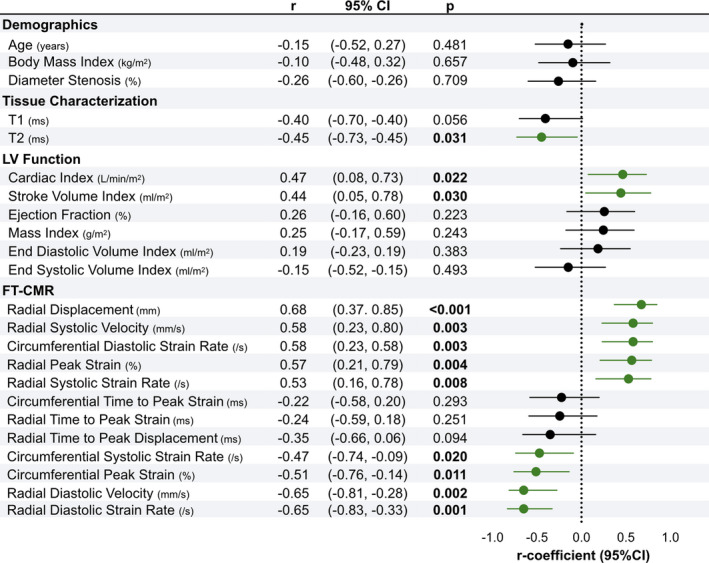
Indicators of regional myocardial oxygenation discordance. The plot shows the correlation coefficient (r) with 95% CI demonstrating the association between measurements obtained at normoxemia and hyperoxia‐induced myocardial oxygenation discordance (56% of patients). Green indicates P<0.05. Increasingly positive values are considered abnormal for circumferential peak strain and systolic strain rate, as well as for radial diastolic velocity and strain rate. FT‐CMR indicates feature tracking cardiovascular magnetic resonance; LV, left ventricular.
Discussion
The main finding of this observational study is that more than half (56%) of the CAD patients in our cohort experienced regional myocardial deoxygenation in response to inhalational hyperoxia. This was not observed in any of the healthy control subjects. Myocardial deoxygenation in poststenotic segments during hyperoxia was correlated with diameter of the respective stenosis. Poststenotic segments also exhibited a significant reduction of e‐dSR. The latter is an early marker of myocardial ischemia, whereas systolic dysfunction is observed later in the ischemic cascade, after diastolic function is already impaired.13 Also, in poststenotic segments, a reduction of systolic velocity and a trend to decreased systolic strain rate were apparent.
A subset of CAD patients responded more severely to hyperoxia, experiencing a global decrease of LVEF to <50% (25% of patients) and a decrease of CI to <2.5 L/min/m2 (28% of patients). These were exclusively patients with a hyperoxia‐induced oxygenation discordance (Figure 1).
Patients with globally prolonged native T1 appeared prone to hyperoxia‐induced deterioration of PS and LVEF. In patients with normal T1, these parameters were within 1 SD of PS and LVEF of healthy participants during both normoxemia and hyperoxia (Figure 3). In contrast, patients with prolonged native T1 had PS and LVEF at RA, which were already outside the 1‐SD range of healthy controls, and this deteriorated further during hyperoxia. Prolonged native T1 is a marker of abnormal tissue composition.14 Mild prolongation is an indicator of fibrosis, whereas myocardial edema induces a more‐marked T1 prolongation. Discrimination of these 2 entities requires contrast‐enhanced T1 studies or addition of, and cross‐referencing with, water content‐specific T2 maps.15 Presence of elevated T1 alone could be a marker of potential aggravating effects of hyperoxia in CAD patients. The findings of myocardial edema in poststenotic territories of some patients may indicate a more‐vulnerable myocardium prone to repetitive ischemia given that our data show an association between the global increase of myocardial T2 and development of a hyperoxia‐induced regional oxygenation discordance.
In our series, patients with globally impaired diastolic and systolic function at normoxemia, as demonstrated by reduction of strain parameters, stroke volume index, and myocardial edema (globally prolonged T2), were prone to hyperoxia‐induced regional oxygenation discordance. Only patients with regional oxygenation discordance showed severe impairment of LVEF and CI, whereas low LVEF at RA did not predict hyperoxia‐induced deterioration of myocardial function. This suggests a potential utility of CMR‐based strain analysis and tissue characterization for identifying CAD patients who will not tolerate hyperoxia well. In the future, larger studies are needed to demonstrate whether CMR markers of tissue injury, inducible regional oxygenation discordance, and hemodynamic depression are indeed predictors of clinical outcomes in patients receiving supplemental hyperoxic respiratory gas mixtures.
Clinical Implications
There is increasing recognition that routine generation of supraphysiological oxygen tensions may be harmful. Retrospective and prospective studies suggest detrimental effects of excessive oxygen administration in patients with acute myocardial ischemia or in patients after cardiac arrest.4, 16, 17 However, these results are matched by studies that report no harm.18, 19, 20, 21 Publications intermittently emerge that hold a case for or against hyperoxia versus normoxemia. Nevertheless, the European Society of Cardiology has reinforced its recommendations in favor of a more‐restrictive oxygen therapy in the guidelines of 2015 and 2017 for management of acute coronary syndrome and ST‐segment–elevation myocardial infarction, respectively, and now advises treatment with supplemental oxygen only below an SpO2 value <90% or in respiratory distress.5, 6
Apart from acute cardiac care, supraphysiological oxygen tensions are administered to stable CAD patients in many other clinical situations (ie, in anesthesia, critical care, and emergency medicine). Robust data on the value of supraphysiological oxygen supplementation to this patient group without ongoing ischemia are even scarcer and the issue remains even more controversial. A systematic review and meta‐analysis has investigated morbidity and mortality in critically ill adults treated with liberal (SpO2 >94%) versus conservative oxygen therapy, and found evidence of high quality that liberal oxygen therapy may increase mortality without improving other outcomes.3
The number of patients with CAD or other cardiovascular comorbidity undergoing major cardiac and noncardiac surgery is increasing, amounting in Europe to ≈5.7 million procedures each year.22 Up to 42% of complications occurring with noncardiac surgery are attributed to cardiovascular causes, adding up to 167 000 cardiac complications annually in Europe, of which 19 000 are life‐threatening.22, 23 A significant contributor may be myocardial injury after noncardiac surgery (MINS), which has recently gained more attention. MINS is reported to occur in up to 41% of patients undergoing noncardiac surgery with diagnosed or risk for CAD. This results in an increase in cardiac troponin levels, and depending on the cut‐off values, this is an independent predictor of 30‐day mortality of up to 17%.23, 24 The cause for MINS is likely to be multifactorial; however, some research groups have ventured to try and identify triggers for MINS. It has long been thought that volatile anesthetics exert cardioprotective effects; however, the study by Lurati Buse et al showed no difference between volatile and total intravenous anesthesia in regard to incidence of MINS.25 More recently, it has also been shown that there is no difference in outcome between volatile anesthesia versus total intravenous anesthesia in patients undergoing cardiac surgery.26 Another group currently investigates the effect of a protocolized blood pressure regime (NCT02533128) and the effect of incidence of MINS. Our results suggest that hyperoxia in CAD patients may account for some of the MINS cases, which should be elaborated on in future studies. Interestingly, iatrogenic perioperative hyperoxia may be a potential contributor to adverse outcome. The Danish PROXI (Perioperative oxygen fraction—effect on surgical site infection and pulmonary complications after abdominal surgery) trial, comparing 80% versus 30% oxygen at laparotomies, found an increase in long‐term mortality after exposure to perioperative hyperoxia.27 In addition, the post hoc analysis described an increased incidence of new acute coronary syndrome (2.5% in the 80% oxygen group versus 1.3% in the 30% oxygen group; hazard ratio, 2.15 [95% CI, 0.96–4.84]).28 Long‐term risk of myocardial infarction was also significantly increased in the 80% oxygen group (hazard ratio, 2.86 [95% CI, 1.10–7.44]; P=0.03), suggesting that perioperative hyperoxia may adversely affect cardiovascular health even over prolonged periods of time.
The findings of this study support our working hypothesis that in a subset of patients with stable multivessel CAD, hyperoxia may trigger potentially adverse effects. Nevertheless, there appear to be patients who benefit from hyperoxia, whereas others do not. So far, there is no technique to distinguish CAD patients who may benefit from hyperoxia from those to whom hyperoxia may be harmful. More insight into the cardiovascular pathophysiology of hyperoxia by using advanced imaging technology and an improved ability to discriminate between patients who benefit from or deteriorate under hyperoxic conditions would facilitate targeted and individualized oxygen therapies in the future.
Limitations
This study recruited patients with stable multivessel CAD, of whom a majority had had a previous revascularization procedure. This is a confounder given that reperfused segments may be affected by edema, fibrosis, and scar, as well as pre‐existing wall motion abnormalities. Nevertheless, this setting is very representative for the majority of CAD patients. We assessed the severity of coronary artery stenosis by quantitative coronary angiography measurements rather than fractional flow reserve (ie, the invasive gold standard). Also, microvascular disease was not quantified. In addition, we did not assess scar or myocardial fibrosis by using contrast‐enhanced T1 mapping and calculation of extracellular volume maps. OS images are susceptible to artifacts within regions of interest, which can degrade image quality and lead to exclusion of segments.
When designing the study, we chose to recruit younger participants in good general health given that they were most likely to have minimal microvascular or other cardiovascular dysfunction. An age‐matched control group would have been more appropriate, but would have introduced the confounding effect of increased fibrotic load attributable to remodeling with age. Of note, oxygenation response of this control group was similar to that of an older healthy cohort in our previous publication.12, 29 Further research should also study why healthy controls showed some impairment of systolic function parameters as well, even in the absence of cardiovascular disease, and whether this is of any consequence considering that these measurements remained within normal ranges. This direction could also be expanded to investigate the magnitude of changes between patients and healthy controls, whereas our work focused on the impact of hyperoxia within the individual groups.
Conclusions
Patients with CAD and pre‐existent myocardial injury who respond to hyperoxic challenge with strain abnormalities appear susceptible for hyperoxia‐induced regional deoxygenation and deterioration of myocardial function. Further research in larger cohorts is warranted to confirm these findings and validate risk prediction for adverse outcomes from nontherapeutic hyperoxia. In the future, this may lead to a more‐individualized oxygen therapy in acute coronary patient care.
Sources of Funding
The work presented was supported by Institutional Research Funds of the Department of Anaesthesiology and Pain Medicine at Inselspital/University Hospital (Bern, Switzerland).
Disclosures
None.
Supporting information
Table S1. Impact of Myocardial Injury on the Ventricular Response to Hyperoxia
Acknowledgments
This study was only made possible by the professional work of the study nurses, Loreen Errass, Monika Stucki, and Christine Riggenbach, and by imaging technicians Carina Bertschinger and Leonie Scheuner.
(J Am Heart Assoc. 2020;9:e014739 DOI: 10.1161/JAHA.119.014739.)
References
- 1. Moradkhan R, Sinoway LI. Revisiting the role of oxygen therapy in cardiac patients. J Am Coll Cardiol. 2010;56:1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Jonge S, Egger M, Latif A, Loke YK, Berenholtz S, Boermeester M, Allegranzi B, Solomkin J. Effectiveness of 80% vs 30‐35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta‐analysis. Br J Anaesth. 2019;122:325–334. [DOI] [PubMed] [Google Scholar]
- 3. Chu DK, Kim LH‐Y, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schünemann HJ, Neary JD, Alhazzani W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta‐analysis. Lancet. 2018;391:1693–1705. [DOI] [PubMed] [Google Scholar]
- 4. Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM; AVOID Investigators . Air versus oxygen in ST‐segment‐elevation myocardial infarction. Circulation. 2015;131:2143–2150. [DOI] [PubMed] [Google Scholar]
- 5. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 7. Wenk M, Van Aken H, Zarbock A. The new World Health Organization recommendations on perioperative administration of oxygen to prevent surgical site infections: a dangerous reductionist approach? Anesth Analg. 2017;125:682–687. [DOI] [PubMed] [Google Scholar]
- 8. McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, Chambers CE, Demers LM, Sinoway LI. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. [DOI] [PubMed] [Google Scholar]
- 9. Smit B, Smulders YM, van der Wouden JC, Oudemans‐van Straaten HM, Spoelstra‐de Man AME. Hemodynamic effects of acute hyperoxia: systematic review and meta‐analysis. Crit Care. 2018;22:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guensch DP, Fischer K, Shie N, Lebel J, Friedrich MG. Hyperoxia exacerbates myocardial ischemia in the presence of acute coronary artery stenosis in swine. Circ Cardiovasc Interv. 2015;8:e002928. [DOI] [PubMed] [Google Scholar]
- 11. Fischer K, Yamaji K, Luescher S, Ueki Y, Jung B, von Tengg‐Kobligk H, Windecker S, Friedrich MG, Eberle B, Guensch DP. Feasibility of cardiovascular magnetic resonance to detect oxygenation deficits in patients with multi‐vessel coronary artery disease triggered by breathing maneuvers. J Cardiovasc Magn Reson. 2018;20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischer K, Guensch DP, Friedrich MG. Response of myocardial oxygenation to breathing manoeuvres and adenosine infusion. Eur Heart J Cardiovasc Imaging. 2015;16:395–401. [DOI] [PubMed] [Google Scholar]
- 13. Stillman AE, Oudkerk M, Bluemke DA, de Boer MJ, Bremerich J, Garcia EV, Gutberlet M, van der Harst P, Hundley WG, Jerosch‐Herold M, Kuijpers D, Kwong RY, Nagel E, Lerakis S, Oshinski J, Paul JF, Slart RHJA, Thourani V, Vliegenthart R, Wintersperger BJ. Imaging the myocardial ischemic cascade. Int J Cardiovasc Imaging. 2018;34:1249–1263. [DOI] [PubMed] [Google Scholar]
- 14. Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease. Circ Res. 2016;119:277–299. [DOI] [PubMed] [Google Scholar]
- 15. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caldeira D, Vaz‐Carneiro A, Costa J. Cochrane Corner: what is the clinical impact of oxygen therapy for acute myocardial infarction? Evaluation of a Cochrane systematic review. Rev Port Cardiol. 2014;33:641–643. [DOI] [PubMed] [Google Scholar]
- 17. Khoshnood A, Akbarzadeh M, Carlsson M, Sparv D, Bhiladvala P, Mokhtari A, Erlinge D, Ekelund U. Effect of oxygen therapy on chest pain in patients with ST elevation myocardial infarction: results from the randomized SOCCER trial. Scand Cardiovasc J. 2018;52:69–73. [DOI] [PubMed] [Google Scholar]
- 18. Abuzaid A, Fabrizio C, Felpel K, Al Ashry HS, Ranjan P, Elbadawi A, Mohamed AH, Barssoum K, Elgendy IY. Oxygen therapy in patients with acute myocardial infarction: a systemic review and meta‐analysis. Am J Med. 2018;131:693–701. [DOI] [PubMed] [Google Scholar]
- 19. Sepehrvand N, James SK, Stub D, Khoshnood A, Ezekowitz JA, Hofmann R. Effects of supplemental oxygen therapy in patients with suspected acute myocardial infarction: a meta‐analysis of randomised clinical trials. Heart. 2018;104:1691–1698. [DOI] [PubMed] [Google Scholar]
- 20. Andell P, James S, Östlund O, Yndigegn T, Sparv D, Pernow J, Jernberg T, Lindahl B, Herlitz J, Erlinge D, Hofmann R. Oxygen therapy in suspected acute myocardial infarction and concurrent normoxemic chronic obstructive pulmonary disease: a prespecified subgroup analysis from the DETO2X‐AMI trial. Eur Heart J Acute Cardiovasc Care. 2019. DOI: 10.1177/2048872619848978. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Sparv D, Hofmann R, Gunnarsson A, James S, Hedberg C, Lauermann J, Torild P, Omerovic E, Bergström K, Haugen E, Bergström C, Linder R, Borg P, Haaga U, Olsson A, Böving E, Östlund O, Rylance R, Witt N, Erlinge D; DETO2X‐SWEDEHEART Investigators . The analgesic effect of oxygen in suspected acute myocardial infarction: a substudy of the DETO2X‐AMI trial. JACC Cardiovasc Interv. 2018;11:1590–1597. [DOI] [PubMed] [Google Scholar]
- 22. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, De Hert S, Ford I, Gonzalez Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Luescher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Uva MS, Voudris V, Funck‐Brentano C; Authors/Task Force Members . 2014 ESC/ESA guidelines on non‐cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol. 2014;31:517–573. [DOI] [PubMed] [Google Scholar]
- 23. Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators , Devereaux PJ, Chan MTV, Alonso‐Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels‐Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30‐day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. [DOI] [PubMed] [Google Scholar]
- 24. Writing Committee for the VISION Study Investigators , Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, Walsh M, Abraham V, Pearse R, Wang CY, Sessler DI, Kurz A, Szczeklik W, Berwanger O, Villar JC, Malaga G, Garg AX, Chow CK, Ackland G, Patel A, Borges FK, Belley‐Cote EP, Duceppe E, Spence J, Tandon V, Williams C, Sapsford RJ, Polanczyk CA, Tiboni M, Alonso‐Coello P, Faruqui A, Heels‐Ansdell D, Lamy A, Whitlock R, LeManach Y, Roshanov PS, McGillion M, Kavsak P, McQueen MJ, Thabane L, Rodseth RN, Buse GAL, Bhandari M, Garutti I, Jacka MJ, Schünemann HJ, Cortes OL, Coriat P, Dvirnik N, Botto F, Pettit S, Jaffe AS, Guyatt GH. Association of postoperative high‐sensitivity troponin levels with myocardial injury and 30‐day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642–1651. [DOI] [PubMed] [Google Scholar]
- 25. Lurati Buse GAL, Schumacher P, Seeberger E, Studer W, Schuman RM, Fassl J, Kasper J, Filipovic M, Bolliger D, Seeberger MD. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation. 2012;126:2696–2704. [DOI] [PubMed] [Google Scholar]
- 26. Landoni G, Lomivorotov VV, Nigro Neto C, Monaco F, Pasyuga VV, Bradic N, Lembo R, Gazivoda G, Likhvantsev VV, Lei C, Lozovskiy A, Di Tomasso N, Bukamal NAR, Silva FS, Bautin AE, Ma J, Crivellari M, Farag AMGA, Uvaliev NS, Carollo C, Pieri M, Kunstýř J, Wang CY, Belletti A, Hajjar LA, Grigoryev EV, Agrò FE, Riha H, El‐Tahan MR, Scandroglio AM, Elnakera AM, Baiocchi M, Navalesi P, Shmyrev VA, Severi L, Hegazy MA, Crescenzi G, Ponomarev DN, Brazzi L, Arnoni R, Tarasov DG, Jovic M, Calabrò MG, Bove T, Bellomo R, Zangrillo A. Volatile anesthetics versus total intravenous anesthesia for cardiac surgery. N Engl J Med. 2019;380:1214–1225. [DOI] [PubMed] [Google Scholar]
- 27. Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS; PROXI Trial Group . Increased long‐term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow‐up of a randomized clinical trial. Anesth Analg. 2012;115:849–854. [DOI] [PubMed] [Google Scholar]
- 28. Fonnes S, Gögenur I, Søndergaard ES, Siersma VD, Jorgensen LN, Wetterslev J, Meyhoff CS. Perioperative hyperoxia—long‐term impact on cardiovascular complications after abdominal surgery, a post hoc analysis of the PROXI trial. Int J Cardiol. 2016;215:238–243. [DOI] [PubMed] [Google Scholar]
- 29. Roubille F, Fischer K, Guensch DP, Tardif JC, Friedrich MG. Impact of hyperventilation and apnea on myocardial oxygenation in patients with obstructive sleep apnea—an oxygenation‐sensitive CMR study. J Cardiol. 2017;69:489–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Impact of Myocardial Injury on the Ventricular Response to Hyperoxia


