Abstract
Background
Left bundle branch block (LBBB) is common after transcatheter aortic valve implantation (TAVI) and is an indicator of subsequent high‐grade atrioventricular block (HAVB). No standardized protocol is available to identify LBBB patients at risk for HAVB. The aim of the current study was to evaluate the safety and efficacy of an electrophysiology study tailored strategy in patients with LBBB after TAVI.
Methods and Results
We prospectively analyzed consecutive patients with LBBB after TAVI. An electrophysiology study was performed to measure the HV‐interval the day following TAVI. In patients with normal His‐ventricular (HV)‐interval ≤55 ms, a loop recorder was implanted (ILR‐group), whereas pacemaker implantation was performed in patients with prolonged HV‐interval >55 ms (PM‐group). The primary end point was occurrence of HAVB during a follow‐up of 12 months. Secondary end points were symptoms, hospitalizations, adverse events because of device implantation or electrophysiology study, and death. Of 373 patients screened after TAVI, 56 patients (82±6 years, 41% male) with LBBB were included. HAVB occurred in 4 of 41 patients (10%) in the ILR‐group and in 8 of 15 patients (53%) in the PM‐group (P<0.001). We did not identify other predictors for HAVB than the HV interval. The negative predictive value for the cut‐off of HV 55 ms to detect HAVB was 90%. No HAVB‐related syncope occurred in the 2 groups.
Conclusions
An electrophysiology study tailored strategy to LBBB after TAVI with a cut‐off of HV >55 ms is a feasible and safe approach to stratify patients with regard to developing HAVB during a follow‐up of 12 months.
Keywords: electrophysiology study, high‐grade AV block, left bundle branch block, transaortic valve implantation
Subject Categories: Electrophysiology, Pacemaker, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Treatment
Clinical Perspective
What Is New?
We showed that an intracardiac His ventricular‐interval measurement in patients with left bundle branch block after transaortic valve implantation stratifies patients at risk for high‐grade atrioventricular block.
With the His ventricular cut‐off of 55 ms, we safely identified patients who did not develop high‐grade atrioventricular block when presenting with a left bundle branch block after transaortic valve implantation with a negative predictive value of 90%.
What Are the Clinical Implications?
This electrophysiology‐tailored management strategy helps to identify patients with left bundle branch block who will not develop high‐grade atrioventricular block.
Consequently, it offers a potential safe and efficient way to manage patients with left bundle branch block after transaortic valve implantation.
The transcatheter aortic valve implantation (TAVI) procedure is an established therapy for patients with severe aortic stenosis with high or intermediate surgical risk.1, 2 TAVI has been shown to reduce all‐cause mortality, cardiac symptoms, and re‐hospitalization compared with medical therapy.3 However, high‐grade atrioventricular conduction disturbances (ie, second‐degree atrioventricular block Mobitz type 2 or complete atrioventricular block) requiring permanent pacemaker implantation remain a common problem after TAVI and are observed in 10% to 20% of patients.4 Even more commonly, incomplete conduction disturbance such as left bundle branch block (LBBB) develop and have been reported to occur in ≈1 quarter of patients.5 Data on the impact of LBBB after TAVI on progression to complete atrioventricular block are scarce and inconsistent.6, 7 However, patients with persistent LBBB after TAVI have been shown to have a high incidence of syncope in 16% of patients and complete atrioventricular block in 20% of the patients.8 Furthermore, a decrease in left ventricular ejection fraction and a poorer functional status was observed during 1‐year follow‐up in patients with LBBB after TAVI.8 Progression from LBBB to atrioventricular block does not necessarily occur in the peri‐interventional period, but can occur later after TAVI.9 According to current guidelines, the significance of LBBB in patients after TAVI remains unclear.4 Therefore, the treatment of patients with LBBB after TAVI is currently tailored individually (eg, based on PR‐interval and/or QRS duration) leading to marked differences in clinical management.10
Because the level of the conduction disturbance is associated with progression to atrioventricular block, electrophysiology testing can be used to characterize atrioventricular‐conduction behavior, allowing conduction disturbances to be classified as supra‐ or infranodal by measuring Atrial–His (AH) and His‐Ventricular (HV) intervals.11 The aim of the current study was to prospectively evaluate a tailored management strategy based on a simple HV‐interval measurement in patients with LBBB after TAVI. The efficacy and safety of this approach were assessed based on the prediction of progression to high‐grade atrioventricular block (HAVB) as well as symptoms, mortality, hospitalizations, and complications.
Methods
Study Design
In this prospective study, all patients undergoing TAVI were screened for LBBB. Patients with both, new‐onset LBBB after TAVI and pre‐existing LBBB before TAVI were included in the analysis. Exclusion criteria for the analysis were pre‐existing high‐grade atrioventricular block or high‐grade atrioventricular‐block directly after TAVI requiring pacemaker implantation, a previously implanted pacemaker, and a nontransfemoral implantation route.
The self‐expandable Evolut R and Evolut R Pro (Medtronic, Minneapolis, MN), Portico (St. Jude Medical, St Paul, MN), and the Acurate NEO (Boston Scientific, Natick, MA), the balloon‐expandable Sapien 3 (Edwards Life Science, Irvine, CA), or the mechanically expandable Lotus (Boston Scientific Inc., Marlborough, MA) were used. Informed consent was obtained from all patients and the study was approved by the local ethics committee for Northwest/Central Switzerland. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Transcatheter Aortic Valve Implantation
Patients with severe symptomatic aortic stenosis and an indication for valve replacement were considered for TAVI if there was an intermediate or high surgical risk. All patients underwent transthoracic echocardiography, coronary angiography, and ECG‐triggered multislice computed tomography scan of the aorta for procedural planning. Valve implantation was performed according to the individual specific manufacturer's recommendations. In general, patients underwent predilatation of the stenosed aortic valve with a balloon that was at least 2 mm smaller than the annulus size. After implantation of the TAVI prosthesis, supravalvular angiography was performed to check for paravalvular leakage. In case of intermediate or severe paravalvular leakage, postdilatation was performed with a balloon with the same size as the annular diameter measurement. During the procedure, all patients received a temporary pacemaker using a quadripolar catheter (5 F, Supreme JSN, St. Jude Medical, Minnetonka) positioned in the right ventricular apex. After the procedure, patients were transferred to the coronary care unit overnight with the temporary pacemaker left in place and programmed to VVI 30 bpm. Continuous rhythm monitoring by telemetry was performed for 72 hours. All patients were treated with clopidogrel 75 mg daily for 3 months in addition to the baseline medication.
Electrocardiographic Assessment
A 12‐lead ECG assessment was performed the day before, the day of, and the day after the TAVI procedure using a standard recorder (Schiller, Baar, Switzerland). Characterization of the ECG to identify complete LBBB was performed based on the ECG acquired the day after the procedure using the de Luna criteria.12
Electrophysiology‐Tailored Management Strategy
In patients with newly developed or pre‐existing ECG‐documented LBBB the day after TAVI, a limited electrophysiology study (EPS) was performed within 24 hours of the procedure. For the purpose of obtaining intracardiac measurements, the quadripolar diagnostic catheter used as a temporary pacemaker wire (5 F, Supreme JSN, St. Jude Medical) during TAVI was retracted from the apex and positioned at the His bundle to measure AH‐ and HV‐intervals. In addition, baseline electrophysiological parameters (PR duration, QRS duration) were assessed in a systematic fashion. Based on the mean HV‐interval of 3 measurements, patients were categorized into 2 arms: (1) In patients with a prolonged HV >55 ms, pacemaker implantation was performed immediately after the measurement (pacemaker‐group). (2) Patients with an HV‐interval ≤55 ms were monitored according to our clinical standard on telemetry for 72 hours after TAVI and if no conduction disturbance requiring pacemaker implantation was recorded during the monitoring period, an implantable loop recorder (ILR, Reveal LINQ™; Medtronic, Minneapolis) with remote monitoring capabilities was implanted (ILR‐group).
Pacemaker Implantation and Programming
In patients with a prolonged HV‐interval (>55 ms), pacemaker implantation was performed by a trained electrophysiologist in a standardized fashion using a left‐sided approach and an axillary vein puncture or cephalic vein cut‐down for vascular access. The right ventricular lead was placed in an apical position or in a septal position if the apical position showed inadequate measurements for sensing or threshold. If an atrial lead was implanted, it was positioned in the right atrial appendage. DDD‐, VDD‐, or VVI‐pacemakers were used at the physicians’ discretion. The lower rate was programmed to 40 bpm and unnecessary right ventricular pacing was avoided by programming long atrioventricular‐intervals or specific algorithms (eg, AAI‐DDD).
ILR Implantation and Programming
A validated ILR (Medtronic Reveal LINQ) was implanted by a trained cardiologist in a standardized manner in order to minimize artifacts and to maximize signal quality. The device was implanted 2 cm left of the sternum at an implantation angle of 45 at the level of the fourth or fifth intercostal space. The device pocket was created using the insertion tool. Bradycardia detection was programmed to 40 bpm. Pause detection was programmed to 4.5 s. Atrial fibrillation detection was programmed to “AF only”.
End Points
The primary end point of the study was the occurrence of HAVB (second‐degree atrioventricular‐block Mobitz type 2 or complete atrioventricular block) documented on a 12‐lead ECG and/or the implantable loop recorder (ILR‐group) or documentation of HAVB on a 12‐lead ECG and/or necessity of ventricular pacing >1% (pacemaker‐group) during a follow‐up of up to 12 months. Necessity of pacing was defined by the cumulative need for ventricular pacing extracted from the device during pacemaker interrogation at the 3‐, 6‐, and 12‐month follow‐up visits. A rhythm strip with a duration of at least 1 minute was recorded during all follow‐up visits with the device programmed to VVI mode at a rate of 30 bpm. Secondary end points were symptoms (dizziness, syncope), death (all‐cause and cardiovascular mortality), rehospitalization for cardiovascular events, and adverse events caused by device implantation or because of the electrophysiology study (pneumothorax, bleeding, perforation, mechanical atrioventricular‐block, death, or other).
Follow‐Up
Follow‐up was scheduled 3, 6, and 12 months after TAVI and included physical examination, 12‐lead ECG, transthoracic echocardiography, and pacemaker interrogation. ILR follow‐up was performed via remote monitoring using the Medtronic Carelink system. Minimal follow‐up duration was 6 months.
Statistical Analysis
Continuous variables are presented as mean±1 SD in combination with the median. For continuous variables, comparisons were made using Student t test, or Mann–Whitney U test, as appropriate. Discrete variables were compared using Fisher exact test. An intention‐to‐treat analysis was performed. Exact logistic regression to determine univariate predictors for the occurrence of HAVB was performed. Receiver‐operating characteristic curves were constructed to identify the optimal threshold (cut‐off value) aiming to identify patients with risk of HAVB with a high specificity and the area under the curve was calculated. For sensitivity analysis of each patient on the results, we performed a leave‐1‐out analysis for the primary results, the exact logistic regression, and the area under the curve. A P<0.05 was considered statistically significant. Analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 22.0; Armonk).
Sample‐size calculation was performed before the study, assuming a 1‐year incidence of the primary end point of HAVB of 33% with HV >55 ms and 5% among those with HV ≤55 ms. This resulted in a calculated sample size of 56 patients with LBBB block to have 80% power at an α level of 0.05 to show a significant between‐group difference.
Results
Of 373 TAVIs performed at our tertiary referral center, implantation was performed using a transfemoral approach in 315 patients. Excluding patients with a previously implanted pacemaker and patients with HAVB after TAVI requiring direct pacemaker implantation resulted in a set of 257 patients. Of these, 78 patients (21%) were found to have LBBB (new‐onset or pre‐existing) the day after TAVI. Of the 78 patients, 56 gave written informed consent and were included in the analysis. The flowchart of the study is shown in Figure 1.
Figure 1.
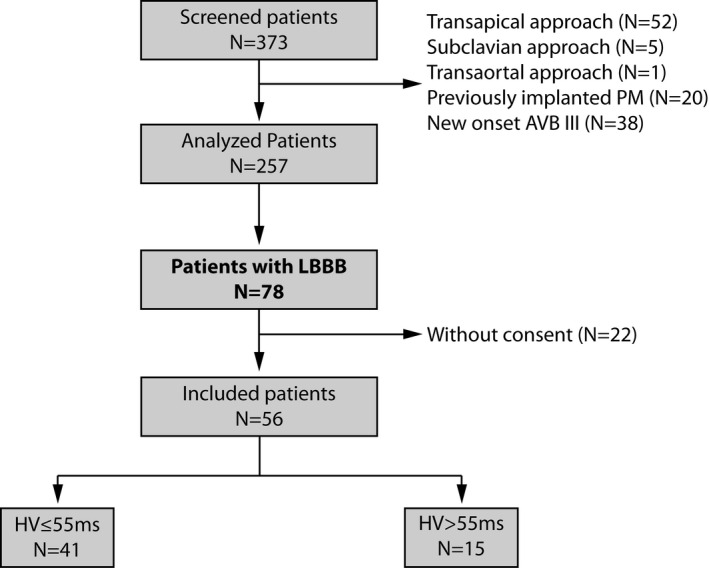
Flow chart of the total cohort. AVB indicates atrioventricular block; HV, His‐Ventricular; LBBB, left bundle branch block; PM, pacemaker.
Baseline characteristics of the 56 patients studied for this analysis are shown in Table 1. The Sapien 3, Portico, Acurate NEO, Evolut R/Pro, and Lotus valve were implanted in 16 patients (29%), 16 patients (29%), 2 patients (4%), 2 patients (4%), and 20 patients (36%), respectively. Mean follow‐up was 12±3 months. Of the 56 patients, 42 had new‐onset LBBB (75%) after TAVI and 14 patients (25%) had pre‐existing LBBB. There was no significant difference in any of the baseline parameters between patients with new‐onset and pre‐existing LBBB.
Table 1.
Baseline Data
| N=56 | |
|---|---|
| Baseline | |
| Age, y | 82±6 (83) |
| Male sex | 23 (41%) |
| Height, cm | 164±13 (165) |
| Weight, kg | 77±25 (73) |
| BMI, kg/m2 | 27±6 (26) |
| Hypertension | 35 (63%) |
| CAD | 30 (54%) |
| Dyslipidemia | 27 (48%) |
| Diabetes mellitus | 13 (23%) |
| Smoker | |
| No | 46 (82%) |
| Yes | 3 (6%) |
| Former | 7 (13%) |
| AF | 22 (39%) |
| NYHA | |
| I | 3 (5%) |
| II | 19 (34%) |
| III | 30 (54%) |
| IV | 4 (7%) |
| Preinterventional echocardiography | |
| DPmean, mm Hg | 48±15 (48) |
| Aortic valve area, mm2 | 0.7±0.2 (0.7) |
| LVEF, % | 53±11 (55) |
| Electrocardiography | |
| PR‐interval, ms | 186±35 (180) |
| QRS‐interval, ms | 102±27 (94) |
| Atrioventricular conduction | |
| AVBI | 8 (14%) |
| AVBI & LBBB | 4 (7%) |
| LBBB | 14 (25%) |
| RBBB | 0 |
Data are presented as mean±SD (median) for continuous variables and as n (%) for categorical variables. AF indicates atrial fibrillation; AVBI, AV block I; BMI, body mass index; CAD, coronary artery disease; DPmean, mean transvalvular pressure gradient; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RBBB, right bundle branch block.
During EPS, 41 patients (73% [95% CI 60–84%]) showed a normal HV‐interval ≤55 ms. One patient with a HV of 61 ms declined pacemaker implantation but remained in the pacemaker‐group according to the intention‐to‐treat analysis. In the pacemaker‐group a DDD, VDD, or VVI pacemaker was implanted in 6 (43%), 5 (36%), and 3 (21%) patients, respectively. Findings from 12‐lead ECG and intracardiac measurements of the 2 groups are shown in Table 2.
Table 2.
Electrocardiographic Characteristics After TAVI During Electrophysiology Testing
| Baseline | Overall | ILR‐Group | PM‐Group | P Value |
|---|---|---|---|---|
| Patients | 56 | 41 (73%) | 15 (27%) | |
| Diagnosed AF | 21 (38%) | 15 (38%) | 7 (44%) | 0.665 |
| RR interval | 793±155 (770) | 785±171 (769) | 821±83 (829) | 0.421 |
| AH, ms | 112±38 (108) | 116±41 (110) | 100±26 (96) | 0.213 |
| HV, ms | 53±11 (52) | 48±5 (48) | 67±8 (65) | <0.001 |
| PR, ms | 199±43 (190) | 198±46 (190) | 202±31 (192) | 0.741 |
| QRS, ms | 150±16 (150) | 150±15 (150) | 150±20 (150) | 0.824 |
Data are presented as mean±SD (median) for continuous variables and as n (%) for categorical variables. AH and PR could only be measured in n=46 patients because of AF during EPS. AF indicates atrial fibrillation; AH, Atrial His; EPS, electrophysiology study; HV, His Ventricular; ILR, implantable loop recorder; PM, pacemaker; TAVI, transcatheter aortic valve replacement.
Of note, LBBB resolved in 7 of 56 patients (13%) within 3 months of follow‐up. LBBB resolved in 6 of 41 (15%) patients in the ILR‐group and 1 of 15 (7%) patients in the pacemaker group (P=0.661 between groups).
Primary End Point
During follow‐up, HAVB occurred in 12 of 56 patients overall (21% [95% CI 12–34%]) (Table 3). In the ILR‐group, HAVB requiring subsequent pacemaker‐implantation occurred in 4 of 41 patients (10% [95% CI 3–23%]) compared with 8 of 15 patients (53% [95% CI 27–79%]) with HAVB in the pacemaker‐group (P=0.001) with 1 implantation in the ILR group within the 72 hours of telemetric monitoring. These numbers were not materially changed in the leave‐1‐out analysis. Details for the pacemaker indication of the 4 patients in the ILR‐group are summarized in Table 4.
Table 3.
Details of HAVB During Follow‐Up in Patients of the ILR‐Group
| Patient | Indication for PMI | New Onset LBBB | Time to Event After TAVI (Days) | Sex | Age (Years) | Valve Type | Cardiac Symptoms | HV (ms) | PR (ms) | QRS (ms) | Pacing During FU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AVBIII | Yes | 3 | f | 78 | Sapien 3 | Dizziness | 44 | 190 | 154 | 100% |
| 2 | Paroxysmal AVBIII | Yes | 7 | f | 75 | Portico | No | 52 | 214 | 160 | 2% |
| 3 | Paroxysmal AVBIII (single episode) | Yes | 8 | f | 90 | Lotus | No | 55 | 190 | 152 | 0% |
| 4 | Intermittent AVBII Mobitz type 2 | No | 158 | m | 76 | Sapien 3 | No | 48 | 290 | 162 | 33% |
AVBII indicates AV block II; AVBIII, AV block III; f, female; FU, follow‐up; HV, His‐ventricular; HAVB, high‐grade atrioventricular block; ILR, implantable loop recorder; LBBB, left bundle branch block; m, male; PMI, pacemaker implantation; TAVI, transcatheter aortic valve replacement.
Table 4.
Summary of Primary and Secondary End Points for the 2 Groups
| ILR‐Group (n=41) | PM‐Group (n=15) | |
|---|---|---|
| Primary | ||
| HAVB | 4 (10%) | 8 (53%) |
| Secondary | ||
| Symptoms | ||
| Syncope | 3 (8%) | 0 |
| Dizziness (noncardiac) | 6 (15%) | 1 (6%) |
| Death | 3 (8%) | 3 (19%) |
| Hospitalizations | 17 (42%) | 3 (19%) |
| HAVB | 3 | 0 |
| SND | 4 | 0 |
| Symptomatic AF | 2 | 0 |
| Dizziness or syncope (noncardiac) | 3 | 0 |
| Dizziness or syncope (cardiac) | 1 (VT) | 0 |
| Cardiac decompensation | 2 | 1 |
| Other | 2 | 2 |
Cause of death: (1) in ILR group: 1 sepsis after knee replacement, 1 intracerebral cerebral hemorrhage, 1 embolic stroke. (2) in PM‐group: 2 heart failure; 1 suspected pneumonia. HAVB indicates high‐grade AV block; ILR, implantable loop recorder; PM, pacemaker; SND, sinus node dysfunction; VT, ventricular tachycardia.
There was no statistically significant difference in any of the baseline parameters shown in Table 1 (baseline, preinterventional echocardiography, and electrocardiography) of the patients with or without postprocedural HAVB in the ILR‐group. Similarly, in the pacemaker‐group, there was no difference in any of the baseline parameters between the patients with or without postprocedural HAVB, except for age (87±4 years versus 78±4 years; P=0.001).
Secondary End Points
Symptoms such as dizziness or syncope after the implantation were observed overall in 10 patients. The symptoms were not caused by HAVB as confirmed by pacemaker and ILR interrogation.
During the follow‐up period of 12 months, all‐cause mortality was 11% (6 patients), with 3 of 40 (7.5%) patients in the ILR‐group and 3 of 16 (19%) in the pacemaker‐group (P=0.094). Three patients died of cardiovascular causes (progression of the heart failure [n=2], embolic stroke [n=1]), whereas the other 3 died from noncardiac causes. Rehospitalizations for any reason during the follow‐up period were observed in 20 of 56 (36%) patients, with 3 of 16 (19%) in the pacemaker‐group and 17 of 40 (43%) in the ILR‐group (P=0.127) (Table 4). No difference in any of the baseline or interventional parameters could be observed between patients with or without hospitalization during follow‐up. No adverse event caused by device implantation or EPS was observed in any of the patients.
Incidental Findings on ILR or Pacemaker Interrogation
During follow‐up, 4 of 41 patients (13%) in the ILR‐group were found to have symptomatic SND 1.5, 3, 3 and 5 months after TAVI, leading to pacemaker implantation. In addition, a first diagnosis of AF was made in five of 41 (12%) patients in the ILR‐group and one of 15 (7%) patients in the pacemaker‐group (P=0.66). Finally, episodes of nonsustained VT were observed in 4 patients (3 ILR‐group, 1 pacemaker‐group).
Retrospective Verification of HV‐Interval Cut‐Off
Exact logistic regression (using the baseline data) identified the HV‐interval as the parameter to predict HAVB (odds ratio=1.111 per unit increase [95% CI 1.039–1.204]; P=0.001) (Table S1). Area under the receiver‐operating characteristic curve for HAVB was 0.792 (95% CI 0.646–0.937) (Figure 2). These results were not materially changed in the leave‐one‐out analysis. With the pre‐defined cut‐off value of HV 55 ms, the sensitivity and specificity to identify HAVB was 67% (8 of 12 patients) and 84% (37 of 44 patients), respectively. The positive predictive value (PPV) and negative predictive value (NPV) was 53% (8 of 15) and 90% (37 of 41), respectively. Importantly, non‐invasive electrocardiographic parameters (PR‐interval, QRS duration) were not predictive for HAVB after TAVI. Of note, analyzing patients with new‐onset LBBB only, HV‐interval remained the only significant parameter to predict HAVB (odds ratio=1.153 [95% CI 1.046–1.271]; P=0.004) and there was no significant difference between the subgroups with new‐onset compared with pre‐existing LBBB.
Figure 2.
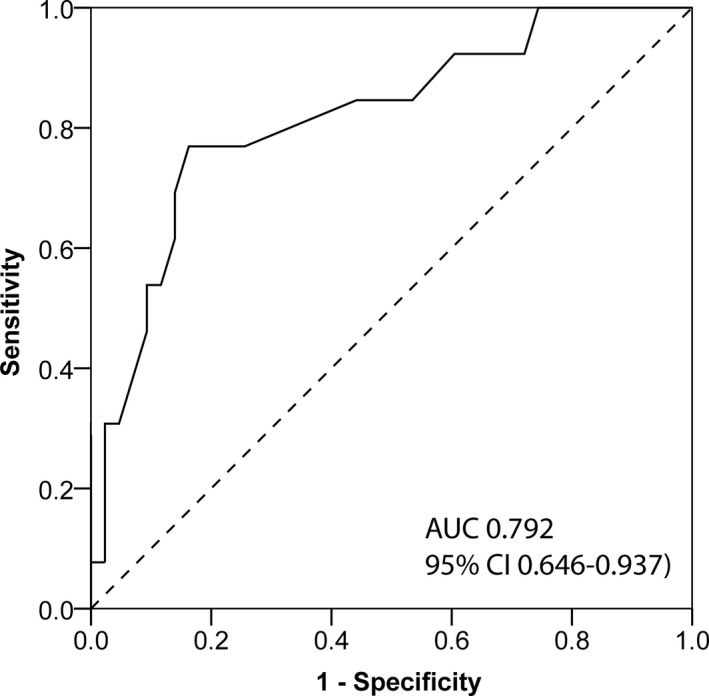
Receiver‐operating characteristics curve demonstrating the accuracy of His‐Ventricular measurement for high‐grade atrioventricular block. AUC indicates area under the curve.
Discussion
Despite being one of the most common complications of TAVI, the clinical implication and particularly the management of patients with LBBB after TAVI are still uncertain.4 To the best of our knowledge, this is the first study prospectively assessing a tailored management strategy based on electrophysiology testing for the management of LBBB after TAVI. During follow‐up, the occurrence of HAVB, reflected by the need for pacing in the pacemaker‐group and documentation of HAVB in the ILR‐group, was determined based on continuous rhythm monitoring from ILRs and PMs.
The main findings of this study are the following: (1) The presented approach using EPS with the temporary pacemaker lead (used during the TAVI procedure) to measure the HV‐interval (cut‐off for HV >55 ms) is feasible and safe. (2) HAVB was observed in 56% of patients with HV >55 ms and 10% of patients with HV ≤55 ms. (3) No syncope caused by HAVB was observed in any patient during follow‐up. (4) The cut‐off value of HV >55 ms identifies patients with subsequent HAVB with a sensitivity of 67% and specificity of 84%. (5) The important implication for clinical practice is that the negative predictive value of the HV cut‐off of 90% allows identifying patients with LBBB after TAVI who are at low risk of developing HAVB.
Current Strategies for Patients With LBBB
Because of the lack of guidelines, the management of patients with LBBB after TAVI varies greatly, and indications for pacemaker implantation are mainly based on 12‐lead ECG measurements. For periprocedural new‐onset LBBB, Auffret et al proposed a management strategy based on 24‐hour monitoring in the intensive care unit.13 If LBBB persisted more than 48 hours after TAVI, pacemaker implantation was considered if the QRS duration was ≥160 ms or in the setting of first‐degree AVB and a QRS duration between 130 and 160 ms. QRS duration and PR‐interval was also used in decision making for pacemaker implantation by Roten et al who proceeded to pacemaker implantation in case of increasing PR‐interval or increasing QRS duration in the setting of LBBB after TAVI.10 However, the PR‐interval cannot be measured in patients with AF, and AF was present in 18% of patients during EPS in our study population. In addition, in contrast to this empirical practice, our data show that neither QRS duration nor PR‐interval were predictive of HAVB in patients with LBBB after TAVI, but the measurement of the HV‐interval was.
Tovia‐Brodie et al reviewed patients undergoing EPS because of new‐onset LBBB, pre‐existing or new‐onset LBBB associated with a PR‐interval increase of >20 ms with TAVI, or pre‐existing or new‐onset LBBB and “slow” AF.14 EPS was performed at a median of 6 days after TAVI, but no TAVI‐specific protocol was in place. Indication for pacemaker implantation was the presence of severe infranodal conduction disturbance (HV‐interval ≥75 ms or occurrence of second‐degree infranodal block during incremental pacing <400 ms), which resulted in a pacemaker implantation rate of 31%. This is comparable to a recent retrospective study by Rogers et al, who reported a pacemaker implantation rate of 30% in patients undergoing EPS including a procainamide challenge and any conduction disturbance but no clear indication for pacemaker implantation.15 In our study using a standardized management strategy, we report a similar pacemaker implantation rate based on EPS of 29% in new‐onset and pre‐existing LBBB using the cut‐off of 55 ms for the HV‐interval. In addition, in an analysis of a general TAVI population not specifically studying LBBB, prolongation of the HV‐interval by more than 13 ms after TAVI was also identified as a predictor of HAVB, emphasizing the importance of the HV‐interval measurement as a predictor of HAVB.16 In our study, we used the absolute HV‐interval value as a single and simple measure determined only in patients with LBBB after TAVI.
Finally, in previous studies, only parameters associated with “the need for pacemaker implantation” were investigated. However, the “need for pacemaker implantation” because of an individual pacemaker indication is obviously not synonymous with the actual “need for pacing” because of HAVB. In addition, even if pacemaker implantation was strictly limited to patients with HAVB after TAVI, absence of “need for ventricular pacing” during follow‐up has been observed in a relevant proportion of patients.17 To address this uncertainty, we quantified the amount of ventricular pacing from pacemaker interrogation. We defined HAVB in the pacemaker group based on the documentation of HAVB on 12‐lead ECG, but also the real need for pacing during follow‐up. In addition, in order to document new‐onset rhythm disturbances after TAVI, we implanted ILRs in all patients with LBBB after TAVI and a normal HV‐interval. This allowed a detailed rhythm analysis also in patients with LBBB after TAVI but no pacemaker in addition to the systematic reporting of clinical events (eg, syncope).
Another prospective study investigating patients with LBBB after TAVI by performing EPS is being conducted using a less strict HV‐interval cut‐off value for pacemaker implantation of 70 ms, but this study is currently still ongoing.18
Definition of HAVB
HAVB is simple to diagnose in the ILR‐group but more difficult to ascertain in the pacemaker‐group. In order not to miss any HAVB, we followed 2 different strategies during pacemaker interrogation: (1) a rhythm strip was recorded for at least 1 minute with a programmed pacing rate of 30 bpm in VVI mode. Using that strategy, only persistent HAVB can be found. (2) The percentage of pacing recorded in the pacemaker over the entire follow‐up period was extracted. Of note, specific algorithms (eg, AAI‐DDD) were used to prevent unnecessary right ventricular pacing and the lower rate limit was programmed to 40 bpm. Using a cut‐off of 1% of need for pacing, the rate of HAVB might be overestimated. Nevertheless, 1% pacing corresponds to a mean of 15 minutes of pacing per day, which might be clinically relevant to prevent syncope (secondary study end point). Therefore, even when using a cut‐off of 1% pacing, some episodes of paroxysmal HAVB may be missed.
Safety
The presented approach for HV measurements using the temporary pacing lead in patients with LBBB after TAVI does not require a new vascular access and consequently does not increase the risk of additional access complications. We did not observe any periprocedural complications of this EPS‐based management strategy, neither because of the additional ILR nor because of pacemaker implantation. However, when widely adopted, the strategy may be associated with device‐related complications.4
Whereas the number of events for the primary outcome of HAVB was overall in the expected range, there were numerically more secondary end points in the ILR‐group. However, this difference was not statistically significant and mainly because of the occurrence of symptomatic sinus node disease and HAVB requiring pacemaker implantation, AF‐related symptoms, and the occurrence of syncope or dizziness because of noncardiac causes. Whereas hospitalizations because of newly developed HAVB or SND requiring pacemaker implantation obviously did not occur in the pacemaker‐group, it is conceivable that a referral bias is present because patients with a pacemaker are probably less likely to be referred when they report dizziness or syncope, or AF‐related symptoms. It is important to note that no cardiac syncope because of bradycardia was observed in any of the patients during follow‐up compared with the 16% reported by Urena et al8 in patients without pacemaker implantation.
Clinical Implication
Using the proposed management strategy of performing a simple HV‐interval measurement with the temporary pacemaker wire in patients with LBBB after TAVI, clinicians can safely identify patients with LBBB who will not develop HAVB with a negative predictive value of 90% based on the HV cut‐off of 55 ms. Therefore, HV cut‐off based pacemaker implantation offers a safe and efficient way to treat patients with LBBB after TAVI.
Limitations
This is a relatively small single‐center study with 373 patients screened, resulting in 56 patients with LBBB after TAVI being analyzed. This was addressed by dedicated statistical analysis and critical interpretation of the results. Whether the presented observations can be applied to a general TAVI population with LBBB after the procedure needs further confirmation in larger trials.
Another limitation is the primary end point definition of HAVB. The 1% cut‐off regarding necessity of ventricular pacing as a sign of HAVB is arbitrary and debatable. In the absence of evidence and recommendations, we used a conservative cut‐off. Based on the cut‐off of 1% need for pacing for the definition of HAVB in the pacemaker‐group, the occurrence of HAVB might be overestimated. On the other hand, some short paroxysmal episodes might still have been missed.
For classification of LBBB, we chose to use the de Luna criteria as opposed to the stricter Strauss criteria.12, 19 In contrast to previous studies, we included not only patients with new‐onset LBBB but also patients with pre‐existing LBBB. The rationale behind this was to evaluate a universal approach for any patient with LBBB after TAVI. This approach is justified by a study by Rivard et al, who showed that prolongation of the HV‐interval and HAVB after TAVI can be observed also in patients with pre‐existing LBBB.16 Different types of valves were included in the study, making the population on the one hand more heterogeneous, but on the other hand the results more generally admissible. We did not perform detailed electrophysiology testing including pacing maneuvers to characterize conduction behavior, but only a simple averaged HV‐interval measurement. However, in clinical practice, this simplified approach might allow application even in interventional cardiology laboratories without dedicated electrophysiology and pacing systems.
Conclusions
The proposed approach of measuring the HV‐interval in patients with LBBB after TAVI is feasible and safe. It effectively identifies patients with LBBB who will not require pacemaker implantation because of HAVB. Whether this may prevent cardiac syncope in a general TAVI population with LBBB needs further confirmation.
Disclosures
Schaer is on the Speaker's bureau of Boston Scientific and Medtronic. Jeger reports travel support from Medtronic, Abbot, and Edwards. Sticherling received fees and grants from Medtronic and Biosense Webster. Kühne received grants from the Swiss National Science Foundation, the Swiss Heart Foundation, lecture/consulting fees from Daiichi‐Sankyo, Boehringer Ingelheim, Bayer, Pfizer‐BMS, AstraZeneca, Sanofi‐Aventis, Novartis, MSD, Medtronic, Boston Scientific, St. Jude Medical, Biotronik, Sorin, Zoll and Biosense Webster. The remaining authors have no disclosures to report.
Supporting information
Table S1. Univariate Logistic Regression Analysis
Acknowledgments
We thank Aline Mühl for her contribution to this work.
(J Am Heart Assoc. 2020;9:e014446 DOI: 10.1161/JAHA.119.014446.)
References
- 1. Himbert D, Descoutures F, Al‐Attar N, Iung B, Ducrocq G, Detaint D, Brochet E, Messika‐Zeitoun D, Francis F, Ibrahim H, Nataf P, Vahanian A. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high‐risk patients with aortic stenosis. J Am Coll Cardiol. 2009;54:303–311. [DOI] [PubMed] [Google Scholar]
- 2. Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, Tapiero S, Litzler PY, Bessou JP, Babaliaros V. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid‐term follow‐up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–1223. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 4. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom‐Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 5. Franzoni I, Latib A, Maisano F, Costopoulos C, Testa L, Figini F, Giannini F, Basavarajaiah S, Mussardo M, Slavich M, Taramasso M, Cioni M, Longoni M, Ferrarello S, Radinovic A, Sala S, Ajello S, Sticchi A, Giglio M, Agricola E, Chieffo A, Montorfano M, Alfieri O, Colombo A. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. Am J Cardiol. 2013;112:554–559. [DOI] [PubMed] [Google Scholar]
- 6. Bates MG, Matthews IG, Fazal IA, Turley AJ. Postoperative permanent pacemaker implantation in patients undergoing trans‐catheter aortic valve implantation: what is the incidence and are there any predicting factors? Interact Cardiovasc Thorac Surg. 2011;12:243–253. [DOI] [PubMed] [Google Scholar]
- 7. Ando T, Takagi H. The prognostic impact of new‐onset persistent left bundle branch block following transcatheter aortic valve implantation: a meta‐analysis. Clin Cardiol. 2016;39:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Urena M, Mok M, Serra V, Dumont E, Nombela‐Franco L, DeLarochellière R, Doyle D, Igual A, Larose E, Amat‐Santos I, Côté M, Cuéllar H, Pibarot P, de Jaegere P, Philippon F, Garcia del Blanco B, Rodés‐Cabau J. Predictive factors and long‐term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon‐expandable valve. J Am Coll Cardiol. 2012;60:1743–1752. [DOI] [PubMed] [Google Scholar]
- 9. Toggweiler S, Wood DA, Rodés‐Cabau J, Kapadia S, Willson AB, Ye J, Cheung A, Leipsic J, Binder RK, Gurvitch R, Freeman M, Thompson CR, Svensson LG, Dumont E, Tuzcu EM, Webb JG. Transcatheter valve‐in‐valve implantation for failed balloon‐expandable transcatheter aortic valves. JACC Cardiovasc Interv. 2012;5:571–577. [DOI] [PubMed] [Google Scholar]
- 10. Roten L, Wenaweser P, Delacrétaz E, Hellige G, Stortecky S, Tanner H, Pilgrim T, Kadner A, Eberle B, Zwahlen M, Carrel T, Meier B, Windecker S. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473–1480. [DOI] [PubMed] [Google Scholar]
- 11. Eksik A, Gul M, Uyarel H, Surgit O, Yildirim A, Uslu N, Aksu H, Turen S, Uzun F, Satilmisoglu H, Erol MK, Bakir I. Electrophysiological evaluation of atrioventricular conduction disturbances in transcatheter aortic valve implantation with Edwards SAPIEN prosthesis. J Invasive Cardiol. 2013;25:305–309. [PubMed] [Google Scholar]
- 12. de Luna AB, Genis AB. Clinical Electrocardiography: A Textbook. John Wiley & Sons, West Sussex, UK; 2012. [Google Scholar]
- 13. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez‐Gabella T, Philippon F, Rodes‐Cabau J. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136:1049–1069. [DOI] [PubMed] [Google Scholar]
- 14. Tovia‐Brodie O, Ben‐Haim Y, Joffe E, Finkelstein A, Glick A, Rosso R, Belhassen B, Michowitz Y. The value of electrophysiologic study in decision‐making regarding the need for pacemaker implantation after TAVI. J Interv Card Electrophysiol. 2017;48:121–130. [DOI] [PubMed] [Google Scholar]
- 15. Rogers T, Devraj M, Thomaides A, Steinvil A, Lipinski MJ, Buchanan KD, Alraies MC, Koifman E, Gai J, Torguson R, Okubagzi P, Ben‐Dor I, Pichard AD, Satler LF, Waksman R. Utility of invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol. 2018;121:1351–1357. [DOI] [PubMed] [Google Scholar]
- 16. Rivard L, Schram G, Asgar A, Khairy P, Andrade JG, Bonan R, Dubuc M, Guerra PG, Ibrahim R, Macle L, Roy D, Talajic M, Dyrda K, Shohoudi A, de Waroux JBL, Thibault B. Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm. 2015;12:321–329. [DOI] [PubMed] [Google Scholar]
- 17. Ramazzina C, Knecht S, Jeger R, Kaiser C, Schaer B, Osswald S, Sticherling C, Kuhne M. Pacemaker implantation and need for ventricular pacing during follow‐up after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37:1592–1601. [DOI] [PubMed] [Google Scholar]
- 18. Massoullié G, Bordachar P, Irles D, Caussin C, Da Costa A, Defaye P, Jean F, Mechulan A, Mondoly P, Souteyrand G, Pereira B, Ploux S, Eschalier R. Prognosis assessment of persistent left bundle branch block after TAVI by an electrophysiological and remote monitoring risk‐adapted algorithm: rationale and design of the multicentre LBBB‐TAVI Study. BMJ Open. 2016;6:e010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Logistic Regression Analysis


