Abstract
Background
To estimate the strength of the cross‐sectional and longitudinal association between arterial stiffness, measured by pulse‐wave velocity, and cognitive function, distinguishing between global cognition, executive functions, and memory and to examine the influence of demographic, clinical, and assessment characteristics on this relationship.
Methods and Results
Systematic review of MEDLINE (via PubMed), Scopus, and WOS databases from their inception to March 2019, to identify cross‐sectional and longitudinal studies on the association between pulse‐wave velocity and cognitive domains (ie, global cognition, executive functions, and memory) among adult population. A total of 29 cross‐sectional and 9 longitudinal studies support the negative relationship between arterial stiffness and cognitive function, including global cognition, executive function, and memory. Demographic, clinical, and assessment characteristics did not substantially modify the strength of this association.
Conclusions
Evidence reveals a negative association between arterial stiffness, measured using pulse‐wave velocity, and cognition, specifically executive function, memory, and global cognition. This association seems to be independent of demographic, clinical, and assessment characteristics. These results accumulate evidence supporting that pulse‐wave velocity assessment could be a useful tool to identify individuals at high risk of cognitive decline or early stages of cognitive decline, to implement interventions aimed at slowing the progression to dementia.
Keywords: cognitive impairment, executive function, global cognition, memory, pulse‐wave velocity
Subject Categories: Aging, Epidemiology, Mental Health
Clinical Perspective
What Is New?
This systematic review and meta‐analysis synthesizes the cross‐sectional and longitudinal association between arterial stiffness, measured by pulse‐wave velocity, and global cognition, executive functions, and memory.
Our data confirm a negative cross‐sectional and longitudinal association between pulse‐wave velocity and executive function, memory, and global cognition, regardless of demographic, clinical, and assessment characteristics.
What Are the Clinical Implications?
Our results claim for the usefulness of pulse‐wave velocity assessment in the identification of individuals at high risk of cognitive decline or early stages of cognitive decline.
Introduction
Cognitive impairment is becoming an important health concern as the older population continuously grows worldwide.1 The World Health Organization estimates that by 2050, 2 billion people will be aged >60 years and the number of people living with dementia will be 115.4 million.2 As such, cognitive impairment is one of the major causes of disability among older people, deteriorating quality of life and producing physical, cognitive, and social disabilities.3
Some cardiovascular risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia, smoking status, and adiposity, have been traditionally recognized as playing a primary role in the vascular pathogenesis of cognitive impairment and dementia.4 In addition, previous research suggests that cerebral small‐vessel disease is involved in the pathophysiological characteristics of cognitive decline, vascular dementia, and Alzheimer disease.5 The cross talk between large and small arterial vessels produces a vicious retrofeeding cycle through which the action of mechanic, inflammatory, metabolic, epigenetic, and hemodynamic factors determines arterial dysfunction and decreases arterial distensibility.6 Therefore, arterial stiffness could be considered as an indirect measure of small‐vessel damage that serves to evaluate not only the quality of brain microcirculation but also the influence that systemic changes in large arteries can produce in microcirculation; thus, arterial stiffness could be the link between vascular health and cognitive decline.7
Pulse‐wave velocity (PWV) is generally accepted as the most simple, noninvasive, robust, and reproducible method to quantify arterial stiffness.7, 8 PWV is an index closely related to vascular aging that when increased has been negatively associated with global cognition independently of traditional cardiovascular risk factors.8, 9, 10 Although less studied, this association has also been observed for different cognitive function domains, such as executive functions and memory.11, 12, 13
Previous systematic reviews and meta‐analyses9, 14, 15 have examined the association between arterial stiffness and cognitive decline. However, the association between arterial stiffness and clinically relevant cognitive domains as well as the potential moderating effect of some variables on this relationship remain unclear. Thus, the aims of this systematic review and meta‐analysis were to: (1) provide a pooled estimate of the strength of the cross‐sectional association between arterial stiffness, measured by PWV, and cognitive function, distinguishing between global cognition, executive functions, and memory; (2) examine whether this association is confirmed by longitudinal studies; and (3) examine the influence of demographic (ie, age, sex, and body mass index [BMI]), clinical (ie, systolic blood pressure [SBP] and diastolic blood pressure [DBP]), and PWV characteristics (ie, type of measure and devices used to measure PWV) on the relationship between arterial stiffness and cognitive function.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This systematic review and meta‐analysis was conducted following the Preferred Reporting Items for Systematic Reviews16 and Meta‐Analysis of Observational Studies in Epidemiology17 statements and the Cochrane Collaboration Handbook.18 The protocol for this systematic review and meta‐analysis has been previously registered on PROSPERO: CRD42019121426. The authors declare that all supporting data are available within this article and that institutional review board approval and informed consent of patients were not required as the data used for this work have exclusively been extracted from published studies. In addition, all the included trials complied with the current ethical standards and the Declaration of Helsinki.
Data Sources and Searches
A literature search was performed on Medline (via PubMed), Web of Science, and Scopus to identify studies on the association between arterial stiffness, measured using PWV, and cognitive function among adult people, to March 25, 2019. The search strategy included the following terms: “central blood pressure,” “arterial stiffness,” “pulse‐wave velocity,” “PWV,” “endothelial function,” “cognition,” “executive,” “executive function,” “cognitive control,” “memory,” “attention,” “metacognition,” “life skills,” “goal setting,” “problem solving,” “self‐regulation,” “brain development,” “brain health,” “neural,” “neuroelectric,” “neurotrophic,” “neurotrophin,” and “BDNF.” In addition, the reference lists of included studies were reviewed for any relevant study.
Study Selection
This systematic review includes studies on the relationship between arterial stiffness, as measured using PWV, and cognitive function among adults. Inclusion criteria were as follows: (1) participants: adults; (2) exposure: arterial stiffness measured through PWV; (3) outcome: cognitive function, including global cognition, executive function, and memory, measured using standardized tests; and (4) study design: cross‐sectional and longitudinal studies including at least 100 participants.
Studies were excluded when: (1) they were focused on children or adolescents, (2) arterial stiffness was measured using indicators other than PWV, or (3) they were written in languages other than English, French, Portuguese, or Spanish.
Data Extraction and Quality Assessment
The main characteristics of the included studies were summarized in tables, including information on: (1) subject characteristics (ie, sample size; percentage of women; mean age, BMI, SBP, and DBP; and type of sample), (2) exposure (ie, type of PWV measured [carotid‐femoral PWV {cfPWV}, brachial‐ankle PWV {baPWV} or aortic PWV]), device used to measure PWV, and mean PWV, and (3) outcome information (ie, test used to measure cognitive function and cognitive domain measured).
The Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies was used to evaluate the risk of bias.19 This tool evaluates 14 criteria for longitudinal studies; for cross‐sectional designs only, 11 were applied. Each criterion could be scored as “yes” when the study achieves the criterion or “no” when the study does not achieve the criterion. Criteria could be also scored as “not reported” when studies did not clearly report the required information.
Literature search, data extraction, and risk of bias assessment were independently performed by 2 researchers (C.A.‐B. and I.C.‐R.), and disagreements were solved by consensus or involving a third researcher (V.M.‐V.).
Data Synthesis and Statistical Analysis
To perform the meta‐analysis, measures of association between PWV and cognitive function were included in the analysis. Three cognitive domains were considered for the statistical analysis: (1) global cognition, (2) executive functions, and (3) memory. Separate analyses for unadjusted cross‐sectional, adjusted cross‐sectional, and longitudinal associations were conducted. Finally, data from studies reporting odds ratio or relative risk were narratively summarized.
Effect sizes (ESs) and 95% CIs were calculated for each observed correlation using Cohen's d index. A pooled ES was estimated for each cognitive domain using a random‐effects model based on the Der Simonian and Laird method.20 Fixed effects models were used when heterogeneity was not excessive.21 Heterogeneity across studies was assessed using the I2 statistic,22 whose values were considered as follows: not important (0%–40%), moderate (30%–60%), substantial (50%–90%), and considerable (75%–100%). Moreover, the corresponding P values were also taken into account.18 Finally, the Cochran's test was also used to evaluate the heterogeneity, being significative when P<0.1.18
Following similar procedures for longitudinal reports, we estimated the pooled ES for the association between the baseline PWV and the pre‐post change in cognitive domains. In addition, when studies reported baseline associations between PWV and cognitive function, these reports were included in the cross‐sectional pooled ES estimates.
Some methodological issues should be pointed out. When studies provided ≥2 measurements for the same cognitive domain, these measurements were combined to calculate a single pooled ES for the corresponding domain. For longitudinal and adjusted cross‐sectional analyses, those including the largest number of covariates were considered. Finally, when studies reported mean value trends by groups or associations using regression models or correlation coefficients, ES values were calculated.
Sensitivity analyses were performed excluding studies one by one from the pooled estimates, to evaluate whether any particular study modified the original summary estimate. Meta‐regressions were calculated on the basis of sample characteristics: percentage of women and mean age, BMI, SBP, and DBP.
Subgroup analyses were performed by: (1) type of sample identified, considering general population or specific disease group; (2) type of PWV measured (baPWV or cfPWV), and (3) device used to measure PWV, distinguishing between SphygmoCor, Complior, and others (including Pulse Trace 6000 Micro Medical, model 810‐a, Mobil‐O‐Graph, PulsePen, NIHem WF, VaSera VS‐1000, plethysmographic device, SPT‐301, and Doppler‐recorded model 810A). Finally, publication bias was estimated using Egger′s test.
Results
Systematic Review
The search retrieved 3957 studies, from which 29 cross‐sectional studies* and 9 longitudinal studies† reported data on the association between arterial stiffness and cognition (Figure 1). Studies involved 43 115 participants (Tables 1 and 2). The list of the excluded studies is available in Data S1.
Figure 1.
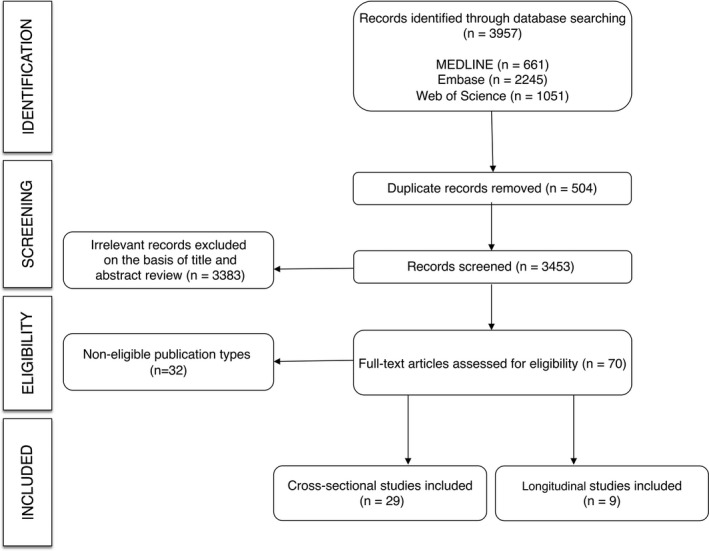
Preferred Reporting Items for Systematic Reviews flowchart.
Table 1.
Characteristics of the Studies Included in the Systematic Review and Meta‐Analysis on the Association Between Cognition Parameters and PWV
| References | Subjects Characteristics | Exposure | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, n (%) | Age, y | BMI, kg/m2 | SBP, mm Hg | DBP, mm Hg | Type of Sample | Type of PWV | PWV Device | PWV Average, m/s | Cognitive Measurement | Cognitive Construct | |
| Abbatecola et al, 200823 | 140 (NR) |
Normoalbuminuric: 78.0 (5.0) Microalbuminuric: 78.0 (4.0) |
Normoalbuminuric: 27.4 (2.4) Microalbuminuric: 27.8 (2.2) | Normoalbuminuric: 135.0 (19.0) Microalbuminuric: 155.0 (20.0) | Normoalbuminuric: 83.0 (8.0) Microalbuminuric: 88.0 (9.0) | Impaired glucose tolerance | cfPWV | Pulse trace 6000 Micro Medical |
Normoalbuminuric: 11.4 (2.1) Microalbuminuric: 13.7 (3.1) |
MMSE Trail Making Tests (A and B) Verbal Fluency Test Wechsler Adult Intelligence Scale‐Revised Digit Span |
Global cognitive function Executive and attention function Memory Mental tracking |
| Al Hazzouri et al, 201524 | 2488 (52.3) | 74.2 (2.9) | 27.4 | NR | NR | General population | cfPWV | Model 810‐a | NR | 3MS | Global cognitive function |
| Angermann et al, 201725 | 201 (29.9) | 64.5 (15.1) | NR | 123.8 (16.6) | 74.7 (12.5) | Patients undergoing hemodialysis | cfPWV | Mobil‐O‐Graph | 9.4 (2.2) | Montreal Cognitive Assessment | Global cognitive function |
| Benetos et al, 201226 | 873 (79.0) | 88.0 (5.0) | 25.8 (4.5) | 138.0 (17.0) | 73.0 (9.0) | General population | cfPWV | PulsePen | 14.4 (5.0) | MMSE | Global cognitive function |
| Cooper et al, 201611 | 1820 (60.0) | 80.0 (5.0) | 26.5 (3.9) | 144.0 (22.0) | 64.0 (10.0) | General population | cfPWV | NIHem WF | 13.6 (4.6) |
California Verbal Learning Test Digit Symbol Substitution Test, Figure Comparison, and Stroop Test (parts I and II) Digits Backward and the Stroop Test (part III) |
Immediate and delayed recall Processing speed Executive function |
| Elias et al, 200927 | 409 (62.3) | 61.3 (12.8) | 29.3 (6.0) | 128.9 (19.7) | 77.5 (10.1) | General population | cfPWV | SphygmoCor | 10.2 (2.8) |
Block Design, Object Assembly, Visual Reproductions Immediate and Delayed, Hooper Visual Organization Test, Matrix Reasoning Trail Making Tests (A and B), Digit Symbol Substitution, Symbol Search Logical Memory Immediate and Delayed, Hopkins Verbal Learning Test Digit Span Forward and Backward, Letter‐Number Sequence, Controlled Oral Word Associations |
Visual‐spatial organization and memory Scanning and tracking Verbal episodic memory Working memory |
| Fukuhara et al, 200628 | 203 (42.9) | 85.0 | 22.7 (0.2) | 144.3 (1.7) | 78.8 (1.0) | General population | baPWV | VaSera VS‐1000 | 23.7 (0.4) | MMSE | Global cognitive function |
| Geijselaers et al, 201629 | 396 (54.6) | 60 (8) | 27.2 (4.4) | 128 (14) | 76 (7) | General population | cfPWV | SphygmoCor | 8.9 (2.1) |
Verbal Learning Test Stroop Color Word Test (parts I and II), the Concept Shifting Test Part A and B, and the Letter‐Digit Substitution Test Stroop Color Word Test (part III) and the Concept Shifting Test Part C, Letter‐Digit Substitution test |
Free recall memory Processing speed Executive function and attention |
| Hajjar et al, 201612 | 591 (68.0) | 48.8 (9.7) | 28.0 (6.6) | 121.0 (24.3) | 77.0 (12.2) | General population | cfPWV | SphygmoCor | 7.2 (1.5) |
Mental flexibility SPOTING the symbol Digit Symbol Substitution Test Digit Span Forward Executive Function Test Focused Attention Sustained Attention Delayed Memory Recall Visual Spatial Memory Visual Spatial Short‐Term Recall Digit Span Backwards |
Executive function Memory Working memory |
| Hanon et al, 200530 | 308 (64.3) |
NCF: 75.0 (8.0) MCI: 77.0 (8.0) AD: 80.0 (7.0) VaD: 81.0 (7.0) |
NCF: 24.4 (4.0) MCI: 25.0 (4.0) AD: 24.0 (4.0) VaD: 24.0 (4.0) |
NCF: 139.0 (18.0) MCI: 142.0 (17.0) AD: 145.0 (20.0) VaD: 159.0 (21.0) |
NCF: 79.0 (11.0) MCI: 80.0 (9.0) AD: 81.0 (12.0) VaD: 82.0 (13.0) |
Subjects with complaint of memory loss | cfPWV | Complior |
NCF: 11.5 (2.0) MCI: 12.6 (2.6) AD: 13.3 (2.9) VaD: 15.2 (3.9) |
MMSE Cognitive Efficiency Profile |
Global cognitive function Cognitive efficiency profile |
| Karasavvidou et al, 201831 | 151 (33.6) | 57.08 (13.7) | 28.2 (5.1) | 137.2 (18.1)–142.8 (12.8) | 77.4 (11.3)–84.7 (9.8) | Patients with kidney disease | cfPWV | SphygmoCor | 6.1 (1.9)–6.9 (2.3) | MMSE | Global cognitive function |
| Kim et al, 200932 | 370 (51.6) | 55.2 (7.3) | 24.4 (5.1) | 130.8 (16.4) | 80.4 (9.3) | General population | baPWV | Plethysmographic device | 15.3 (2.9) | Korean version of the mini‐mental state examination (K‐MMSE) | Global cognitive function |
| Kim et al, 201733 | 333 (42.0) | 55.0 (13.0) | NR | NR | NR | Patients undergoing hemodialysis | cfPWV | SphygmoCor | 10.0 (7.9–12.5) |
Trail Making Tests (A and B) 3MS |
Executive function Global cognitive function |
| Lamballais et al, 201834 | 5187 (42.9) | 58.8 (7.3)–63.6 (5.7) | 26.8 (3.8)–27.4 (4.3) | 130 (18)–150 (20) | 80 (10)–86 (10) | General population | cfPWV | Complior | 9.1 (1.6)–13.0 (2.8) |
Color‐Word Interference Stroop Task Letter Digit Substitution Test Verbal Fluency Test Delayed Recall Purdue Pegboard Test |
G‐factor |
| Lee et al, 201435 | 102 (29.0) | 61.0 (9.0) | 24.0 (4.0) | 124.0 (13.0) | 77.0 (9.0) | Stroke patients | cfPWV | SphygmoCor | 10.0 (2.0) | K‐MMSE | Global cognitive function |
| Lim et al, 201613 | 463 (43.2) |
MMSE participants: 63.0 (6.1) Neurocognitive domain test participants: 64.2 (6.4) |
MMSE participants: 25.0 (4.1) Neurocognitive domain test participants: 24.6 (3.5) |
NR | NR | General population | cfPWV | SphygmoCor |
MMSE participants: 5.0 (2.6–14.1) Neurocognitive domain test participants: 4.9 (3.0–13.0) |
MMSE Digit Span‐Forward Color Trails Test 1 Rey Auditory Verbal Learning Test, Story Memory and Recall Boston Naming Test Brief Visuospatial Memory Test‐Revised Digit Span‐ Backward Block Design, Color Trails Test 2 Categorical Verbal Fluency |
Global cognitive function Attention Verbal memory Language function Visuospatial ability Executive function |
| Mitchell et al, 201136 | 668 (56.6) |
Women: 75.0 (4.0) Men: 76.0 (4.0) |
Women: 27.0 (4.0) Men: 27.0 (4.0) |
Women: 141.0 (20.0) Men: 137.0 (18.0) |
Women: 67.0 (9.0) Men: 67.0 (10.0) |
General population | cfPWV | NIHem WF |
Women: 12.2 (3.7) Men: 13.4 (4.4) |
California Verbal Learning Test Digits Forward Digit Symbol Substitution Test Figure Comparison Stroop Test (parts I and II) Digits Backwards Cambridge Neuropsychological Test Automated Battery Spatial Working Memory Stroop Test (part III) MMSE |
Memory Processing speed Executive function Global cognitive function |
| Muela et al, 201837 | 211 (55.0) |
Normotension: 52.2 (13.9) Hypertension stage 1: 52.1 (13.0) Hypertension stage 2: 52.3 (10.1) |
Normotension: 26.7 (4.2) Hypertension stage 1: 28.5 (4.6) Hypertension stage 2: 30.1 (4.6) |
Normotension: 121.9 (8.3) Hypertension stage 1: 135.0 (13.5) Hypertension stage 2: 147.5 (26.1) |
Normotension: 76.5 (6.9) Hypertension stage 1: 83.1 (9.9) Hypertension stage 2: 90.3 (14.5) |
Patients with hypertension | cfPWV | Complior |
Normotension: 7.5 (1.4) Hypertension stage 1: 7.9 (1.2) Hypertension stage 2: 7.9 (1.2) |
MMSE Montreal Cognitive Assessment Boston Naming Test Rey‐Osterrieth Complex Delayed Recall Semantic Verbal Fluency animal category Backward Digit Span Test Phonological Verbal Fluency Trail Making Test B Forward Digit Span Test Trail Making Test A Clock Drawing Test Rey Auditory Verbal Learning Test Digit Symbols Substitution Test |
Global cognitive function Language function Episodic memory Executive function Attention Visuospatial abilities Processing speed |
| Muller et al, 200738 | 396 (0.0) |
No CVD: 54.5 (10.3) Subclinical CVD: 66.8 (8.1) Prevalent CVD: 67.7 (8.8) |
No CVD: 25.9 (0.3) Subclinical CVD: 26.5 (0.3) Prevalent CVD: 27.3 (0.5) |
No CVD: 134.2 (1.3) Subclinical CVD: 145.5 (1.7) Prevalent CVD: 140.2 (2.5) |
NR | General population | cfPWV |
SphygmoCor Acuson Aspen |
No CVD: 8.5 (0.2) Subclinical CVD: 10.7 (0.2) Prevalent CVD: 10.2 (0.3) |
MMSE Rey Auditory Verbal Learning Test Doors Test Digit Span Test List of nouns Digit Symbol Substitution Test Trail Making Test (A and B) Dutch Adult Reading Test |
Global cognitive function Verbal episodic memory Memory Visual memory Short‐term memory and working memory Verbal fluency Cognitive and perceptual speed Attention and mental flexibility IQ |
| Nilsson et al, 201439 | 2637 (60.8) | 72.1 (5.6) | NR | 135.6 (17.1) | 75.6 (8.7) | General population | cfPWV | SphygmoCor | 10.5 (2.5) |
MMSE Quick test of cognitive speed (AQT) |
Global cognitive function Perceptual and cognitive speed |
| Palta et al, 201940 | 3703 (59.3) | 75.2.(5.0) | 27.8 (4.4) | 129.9 (17.2) | NR | General population | cfPWV | VP‐1000 Plus | NR |
Delayed word recall Logical memory Incidental learning Digit Symbol Substitution Test Trail Making Test Digit Span Backwards Semantic and phonemic fluency Boston Naming Test |
Memory Executive function/processing speed Language function |
| Pase et al, 201641 | 3207 (53.1) | 46.0 (9.0) | NR | 116.0 (14.0) | 74.0 (9.0) | General population | cfPWV | NIHem WF | 6.8 (6.1–7.7) |
Trail Making Test (A and B) Victoria Stroop interference task Logical Memory delayed Visual Reproductions delayed Hooper visual organization test (VOT) Digit Span Forward and Backward |
Processing speed and executive function Long‐term storage and retrieval Visual processing Working memory |
| Poels et al, 200742 | 3714 (57.7) | 72.0 (6.7) | 26.8 (4.0) | NR | NR | General population | cfPWV | Complior | 13.5 (3.0) |
MMSE Letter‐Digit Substitution Task Stroop Test Word Fluency Test |
Global cognitive function Executive function |
| Ryu et al, 201743 | 123 (70.7) |
PD‐NC: 67.0 (9.6) PD‐MCI: 70.1 (6.9) PD‐D: 73.9 (8.8) DLB: 77.4 (4.9) AD: 76.2 (9.2) |
NR | NR | NR | Patients with Parkinson disease and Lewy body disorders | baPWV | VP 1000 |
PD‐NC: 15.3 (3.0) PD‐MCI: 18.7 (4.7) PD‐D: 21.4 (4.1) DLB: 21.2 (7.0) AD: 20.4 (5.1) |
MMSE Seoul Neuropsychological Screening Battery: Korean‐Boston Naming Test and Digit Span Test Rey Complex Figure Test Calculation test Seoul Verbal Learning Test Control Oral Word Association Test |
Global cognitive function Language function Calculation Visuospatial function and memory Memory |
| Scuteri et al, 200744 | 102 (70.2) | 79.0 (6.0) | 25.7 (4.1) | 135.9 (19.2) | 78.5 (11.9) | Patients with complaints of memory loss | cfPWV | Complior | 13.5 (2.2) | MMSE | Global cognitive function |
| Singer et al, 201345 | 319 (51.7) | 79.6 (4.2) | 26.7 (4.1) | 140.9 (19.3) | NR | General population | cfPWV | SphygmoCor | 11.2 (2.4) |
Digit Symbol Coding and Trail Making Test A Logical Memory Story A (delayed) Rey Auditory Visual Verbal Learning Test Benton Visual Retention Test Animal Naming and the 30‐item Boston Naming Test Phonemic Fluency (FAS) Trail Making Test B Stroop Test Block Design |
Processing speed Memory Language function Executive function Visuospatial ability |
| Triantafyllidi et al, 200946 | 110 (47.0) | 56.1 (10.0) | 29.7 (4.0) | 147.0 (17.0) | 88.0 (10.0) | Patients with essential hypertension | cfPWV | Complior SP | 10.1 (8.8, 11.2) | MMSE | Global cognitive function |
| Tsao et al, 201347 | 1587 (55.0) | 61.0 (9.0) | NR | 126.0 (19.0) | 74.0 (10.0) | General population | cfPWV | SPT‐301 | 9.0 (7.6, 11.0) |
Logical memory delayed Trail Making Test (A and B) |
Memory Executive function |
| Tsao et al, 201648 | 1223 (56.0) | 62.0 (9.0) | NR | 125.0 (18.0) | NR | General population | cfPWV | SPT‐301 | 9.0 (7.6, 10.9) |
Trail Making Test (A and B) Similarities test |
Executive function Abstract reasoning |
| Watson et al, 201149 | 552 (52.5) | 73.1 (2.7) | 27.0 (4.6) | NR | NR | General population | aPWV | Doppler‐recorded model 810A | 8.9 (3.9) |
3MS Buschke Selective Reminding Test Boxes and Digit Copying Pattern and Letter Comparison |
Global cognitive function Verbal learning and memory Psychomotor speed Perceptual speed |
| Zhong et al, 201450 | 1394 (57.2) |
No cfPWV >12 m/s: 73.3 (6.4) cfPWV >12 m/s: 78.4 (7.5) |
No cfPWV >12 m/s: 30.7 (5.7) cfPWV >12 m/s: 30.0 (5.5) |
NR | NR | General population | cfPWV | Complior SP | 11 (3.6) |
MMSE Trail Making Test (A and B) Digit Symbol Substitution Test Rey Auditory Verbal Learning Test Verbal Fluency Test |
Global cognitive function Executive function, attention, and speed Psychomotor speed and sustained attention Memory Language |
3MS indicates modified MMSE; aPWV, aortic PWV; AD, Alzheimer disease; baPWV, brachial‐ankle PWV; BMI, body mass index; cfPWV, carotid‐femoral PWV; CVD, cardiovascular disease; DBP, diastolic blood pressure; DLB, dementia with Lewy bodies; IQ, intelligence quotient; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; NCF, normal cognitive function; NR, not reported; PD‐D, Parkinson disease with dementia; PD‐MCI, Parkinson disease with MCI; PD‐NC, Parkinson disease with normal cognition; PWV, pulse‐wave velocity; SBP, systolic blood pressure; VaD, vascular dementia.
Table 2.
Characteristics of the Studies Included in the Systematic Review and Meta‐Analysis on the Association Between Cognition Parameters and PWV for Studies Reporting OR and RR
| References | Subjects Characteristics | Exposure | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women, n (%) | Age, y | BMI, kg/m2 | SBP, mm Hg | DBP, mm Hg | Type of Sample | Type of PWV | PWV Device | PWV Average | Cognitive Measurement | Cognitive Construct | |
| Fujiwara et al, 200551 | 352 (61.1) |
MMSE <24: 75.0 (4.6) MMSE >24: 76.9 (5.6) |
MMSE <24: 22.8 (3.3) MMSE >24: 23.2 (3.2) |
MMSE <24: 155.2 (20.3) MMSE >24: 147.2 (22.0) |
MMSE <24: 84.8 (10.1) MMSE >24: 84.5 (11.0) |
General population | baPWV | AT‐Form | NR | MMSE | Global cognitive function |
| Kearney‐Schwartz et al, 200952 | 198 (52.0) | 69.3 (6.2) | 27.8 (4.3) | 129.0 (12.0) | 75.0 (9.0) | Hypertensive patients with subjective memory complains | cfPWV |
Complior IOTEC |
NR |
Cognitive Difficulties Scale of McNair MMSE Grober‐Buschke Test Benton Visual Retention Test Praxies scale Verbal Fluency Test |
Global cognitive function Immediate and delayed memory and language Visuoperceptual and visuospatial Praxies Executive function and long‐term verbal memory |
| Meyer et al, 201753 | 4461 (58.8) |
White normal: 75.2 (4.9) White MCI: 76.8 (5.2) White dementia: 78.7 (5.1) Black normal: 74.0 (4.7) Black MCI: 75.8 (5.1) Black dementia: 79.4 (4.5) |
White normal: 27.7 (4.4) White MCI: 27.6 (4.4) White dementia: 26.6 (4.3) Black normal: 29.4 (4.8) Black MCI: 29.1 (4.8) Black dementia: 26.4 (4.8) |
White normal: 128.5 (17.0) White MCI: 130.6 (18.3) White dementia: 133.3 (17.4) Black normal: 133.3 (18.0) Black MCI: 135.2 (18.8) Black dementia: 135.6 (19.1) |
White normal: 65.7 (10.1) White MCI: 65.1 (10.7) White dementia: 65.1 (9.6) Black normal: 70.0 (10.1) Black MCI: 69.2 (10.9) Black dementia: 68.7 (10.7) |
General population | cfPWV | VP‐1000 Plus | NR |
Digit Symbol Substitution Test Word recall task Word Fluency Test scores |
Global cognitive function memory |
| Nilsson et al, 201754 | 3056 (60.5) |
No dementia: 71.8 (5.5) Prevalent dementia: 76.3 (4.7) Incident dementia: 75.8 (4.7) |
NR |
No dementia: 135.6 (17.1) Prevalent dementia: 136.5 (18.8) Incident dementia: 137.8 (17.9) |
No dementia: 75.7 (8.7) Prevalent dementia: 75.6 (12.3) Incident dementia: 74.6 (8.6) |
General population | cfPWV | SphygmoCor |
No dementia: 10.5 (2.4) Prevalent dementia: 11.2 (2.6) Incident dementia: 11.3 (2.7) |
MMSE AQT Color‐Form |
Global cognitive function |
| Sugawara et al, 201055 | 388 (64.2) |
Poor cognition: 70.1 (4.9) Control: 68.3 (5.6) |
Poor cognition: 23.5 (3.3) Control: 23.3 (2.9) |
Poor cognition: 137.6 (17.8) Control: 136.8 (17.3) |
NR | General population | baPWV | Form PWV/ABI |
Poor cognition: 18.4 (4.1) Control: 17.4 (3.0) |
MMSE | Global cognitive function |
| Taniguchi et al, 201556 | 526 (57.8) | 71.7 (5.6) |
Cognitive decline: 23.3 (2.9) No cognitive decline: 23.3 (3.3) |
Cognitive decline: 133.0 (20.0) No cognitive decline: 128.0 (18.0) |
Cognitive decline: 77.0 (11.0) No cognitive decline: 75.0 (11.0) |
General population | baPWV | BP‐203 RPE III |
Cognitive decline: 19.3 (3.8) No cognitive decline: 17.5 (3.5) |
MMSE | Global cognitive function |
| Tuttolomondo et al, 201757 | 153 (42.5) |
Subjects with diabetic foot: 61.6 (10.1) Diabetic subjects without diabetic foot: 60.6 (12.5) Healthy controls: 63.0 (13.9) |
Subjects with diabetic foot: 30.2 (6.4) Diabetic subjects without diabetic foot: 29.9 (4.5) Healthy controls: 25.1 (4.3) |
Subjects with diabetic foot: 135.0 (21.8) Diabetic subjects without diabetic foot: 124.5 (16.8) Healthy controls: 116.3 (13.4) |
Subjects with diabetic foot: 67.9 (10.7) Diabetic subjects without diabetic foot: 70.9 (11.2) Healthy controls: 71.3 (12.7) |
Patients with type 2 diabetes mellitus | cfPWV | SphygmoCor |
Subjects with diabetic foot: 14.3 (3.8) Diabetic subjects without diabetic foot: 11.9 (2.6) Healthy controls: 9.2 (1.9) |
MMSE | Global cognitive function |
baPWV indicates brachial‐ankle PWV; BMI, body mass index; cfPWV, carotid‐femoral PWV; DBP, diastolic blood pressure; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; NR, not reported; OR, odds ratio; RR, relative risk; PWV, pulse‐wave velocity; SBP, systolic blood pressure.
For participants’ characteristics: (1) 12 studies reported data from specific disease populations; (2) mean age ranged from 46.0 to 85.0 years; (3) mean BMI ranged from 22.7 to 30.2 kg/m2; (4) mean SBP ranged from 116.0 to 159.0 mm Hg; and (5) mean DBP ranged from 64.0 to 90.3 mm Hg.
PWV was measured using cfPWV procedures in all studies, but 4 that used baPWV and 1 that used aortic PWV. The reported mean PWV ranged from 4.9 to 6.9 m/s for cfPWV and from 15.3 to 23.7 for baPWV. The devices used to measure PWV varied across studies, although SphygmoCor and Complior were the most widely used devices.
The tests used to measure cognitive function aimed to measure global cognition, executive function, memory, language, attention, processing speed, and visuospatial ability.
Meta‐Analysis
The unadjusted pooled ES values for the cross‐sectional associations were −0.53 (95% CI, −0.67 to −0.39) for global cognition, −0.35 (95% CI, −0.50 to −0.19) for executive function, and −0.39 (95% CI, −0.70 to −0.09) for memory. The adjusted pooled ES values were −0.21 (95% CI, −0.30 to −0.11) for global cognition, −0.08 (95% CI, −0.14 to −0.03) for executive function, and −0.13 (95% CI, −0.20 to −0.05) for memory (Figures 2 and 3).
Figure 2.
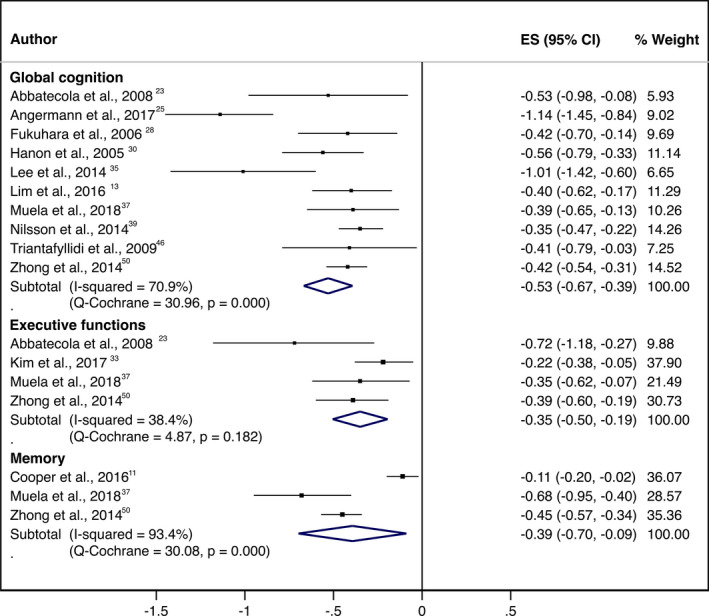
Forest plot for the unadjusted cross‐sectional association between arterial stiffness, measured by pulse‐wave velocity, and cognitive function domains. ES indicates effect size.
Figure 3.

Forest plot for the adjusted cross‐sectional association between arterial stiffness, measured by pulse‐wave velocity, and cognitive function domains. ES indicates effect size.
The pooled ES values for the longitudinal association of PWV and global cognition, executive function, and memory were −0.21 (95% CI, −0.36 to −0.06), −0.12 (95% CI, −0.22 to −0.02), and −0.05 (95% CI, −0.12 to 0.03), respectively (Figure 4).
Figure 4.

Forest plot for the longitudinal association between arterial stiffness, measured by pulse‐wave velocity, and cognitive function domains. ES indicates effect size.
Sensitivity analysis
Sensitivity analysis showed that: (1) for the unadjusted analysis, pooled ES for memory was modified after excluding Muela et al37 study; (2) for the adjusted analysis, pooled ES for memory was modified after excluding Palta et al40 study; and (3) the longitudinal pooled ES for executive functions was modified after removing 2 studies (Hajjar et al12 and Tsao et al48) and for memory after removing the 3 studies included (Hajjar et al,12 Kim et al,33 and Poels et al42) (Tables S1 through S3).
Subgroup analyses and meta‐regressions
Subgroup analyses by type of sample, type of PWV (ie, cfPWV, baPWV, and aortic PWV), and type of device (ie, SphygmoCor, Complior, and others) are displayed in Table S4. Pooled ES values were not substantially different in any of the subgroup analyses.
Meta‐regressions with longitudinal, unadjusted, and adjusted cross‐sectional analyses showed that none any of the considered variables (ie, percentage of women and mean age, BMI, SBP, and DBP) influences the relationship between arterial stiffness and cognitive function (Table S5).
Publication Bias
Publication bias, evaluated by Egger′s test and funnel plot asymmetry, was found in the unadjusted cross‐sectional analysis for global cognition (P=0.097) and in the adjusted cross‐sectional analysis for memory (P=0.035).
Risk of Bias
Cross‐sectional studies scored between 4 and 9 points, and longitudinal studies scored between 8 and 12 points. The 4 criteria in which most articles lacked information were: (1) sample size justification, power description, or variance; (2) whether the measurement of the exposure of interest precedes that of the outcome; (3) whether the outcome assessors were blinded to the exposure status of participants; and (4) whether the participation rate of eligible people was at least 50% (Table S6).
Discussion
The relationship between arterial stiffness and cognition has been repeatedly reported, but mostly always has analyzed cognition as a dimensionless construct. To our knowledge, this is the first meta‐synthesis elucidating this relationship, distinguishing between the several domains that integrate the cognitive function construct. Our results support the negative relationship between arterial stiffness with each cognitive domain, including global cognition, executive function, and memory. Furthermore, analyses of longitudinal studies confirm this negative association. Finally, demographic (age, sex, and type of sample), clinical (BMI, SBP, DBP, or PWV), and assessment characteristics (type of measure and type of device) did not substantially modify the strength of this association.
Executive function has been defined as one of the cognitive domains primarily affected by vascular aging.39 In addition, global cognition and memory are closely related to both vascular aging and arterial stiffness, and it is clinically relevant to measure cognitive decline and memory loss.32, 58 Although some tests, such as the Mini‐Mental State Examination, lack sensitivity to reflect small cognitive changes, the results of our cross‐sectional meta‐analyses are consistent with previous findings, and confirm global cognition and memory as specific cognitive functions negatively associated with arterial stiffness.9, 14
Despite the scarcity of longitudinal studies included in each specific cognitive function, the general observed effect suggests that arterial stiffness contributes to deteriorate global cognition and executive function. Thus, these findings indicate that interventions aimed to reduce arterial stiffness could help to delay or prevent cognitive impairment.59 Loss of memory is one of the most important reasons for consultations among people experiencing cognitive decline.60 However, more longitudinal research is needed to further elucidate on the potential effects and mechanisms of arterial stiffness on memory.
The negative association between arterial stiffness and cognitive function was maintained after controlling for covariates, such as age, sex, educational level, depression scale score, or cardiovascular risk factors, related to cognitive decline and vascular aging. Moreover, the consistency of these associations was strengthened by the findings from longitudinal studies, regardless of the duration of follow‐up.61 Cardiovascular risk factors, such as diabetes mellitus, hypertension, or smoking, that influence the relationship between cognitive function and arterial stiffness were also considered in some included studies.14 Finally, some studies accounted for additional factors not usually studied, such as apolipoprotein E 4 status, intracranial volume, estimated glomerular filtration rate, or minutes of leisure‐time physical activity. Our findings indicate that the association between arterial stiffness and cognitive function is not confounded by these covariates. However, individual subclinical cardiovascular health factors could partially explain the present results.62
Arterial stiffness has been associated with brain damage and cognitive decline through several mechanisms. First, it has been proposed that cerebral small vessels offer low resistance to the high‐pressure fluctuations from large arteries, and this flow transmission could damage small vessels, resulting in cognitive function decline.63 Second, small vessels tend to progressively reduce their diameter to counteract changes in pulse pressure. This strategy increases microvascular resistance and, therefore, may result in cognitive damage.64 Finally, some genetic factors, such as increased b‐amyloid levels, mediated by the presence of the apolipoprotein E ε4 allele may induce vascular damage and cognitive decline.65, 66
The results from this study confirm that arterial stiffness, measured by PWV, is a predictor of cognitive decline. Furthermore, this study shows that this association is independent of specific demographic and PWV characteristics. PWV is a low‐cost, accurate, and easy method to determine arterial stiffness and, therefore, vascular aging.14, 62, 67 Tools for early cognitive decline detection may be relevant from a global and public health perspective, given that the onset of cognitive decline at early ages is associated with higher rates of progression to dementia.59, 68 Thus, PWV assessment could be included as a routine examination in adults at high risk for cognitive decline. Therefore, hemodynamic measurements, such as PWV, should be included in the prevention and control indexes for healthy adults at risk of cardiovascular outcomes and cognitive decline. However, further studies using a neuroimaging approach are needed to overcome the limitations of the research published until now, such as small sample sizes, different covariates adjusted in the analysis, and short follow‐up times.
Some limitations of this systematic review and meta‐analysis may make us consider these findings with caution. First, there are limitations from meta‐analysis design, such as publication bias and selection bias. Additional sources of bias could be: (1) the pooled ES was not estimated using the original data, but those reported in the included articles, (2) the methods and tools used to measure cognitive function widely varied across the included studies, (3) substantial heterogeneity was found among the included studies, (4) publication bias was found for some of the observed outcomes, (5) a cause‐effect could not be inferred from the cross‐sectional analyses, and (6) language restrictions may have limited the number of included studies. Finally, to include a sample as large as possible, populations included in this meta‐analysis come from different settings and vary across studies, but the data of our meta‐analysis corroborate findings of the FHS (Framingham Heart Study)11 and the SLAS (Singapore Longitudinal Ageing Studies),13 precluding an enlarged (transcontinental) external validity of results.
Conclusions
In conclusion, this systematic review and meta‐analysis reveals a negative association between arterial stiffness, measured using PWV, and cognition, specifically executive function, memory, and global cognition. This association seems to be independent of sex, age, blood pressure levels, and PWV measurement characteristics. Separate analyses of longitudinal studies support the negative association between arterial stiffness and cognitive function found in cross‐sectional studies. Our results accumulate evidence supporting that PWV assessment could be a useful tool to identify individuals at high risk of cognitive decline or early stages of cognitive decline, to implement interventions aimed at slowing the progression to dementia.
Sources of Funding
This study was funded by Apadrina la Ciencia funds.
Disclosures
None.
Supporting information
Data S1. References excluded from the meta‐analysis.
Table S1. Sensitivity Analyses by Removing Studies One by One for Unadjusted Cross‐Sectional Analysis
Table S2. Sensitivity Analyses by Removing Studies One by One for Adjusted Cross‐Sectional Analysis
Table S3. Sensitivity Analyses by Removing Studies One by One for Longitudinal Analysis
Table S4. Subgroup Analyses for the Association Between PWv and Cognition Domains by Type of Sample, PWv Measured and Device Used
Table S5. Meta‐Regression of PWV and Cognition Domains by Percentage of Females and Mean Age, BMI, SBP and DBP of Included Studies
Table S6. Risk of Bias of Cross‐Sectional and Longitudinal Included Studies
(J Am Heart Assoc. 2020;9:e014621 DOI: 10.1161/JAHA.119.014621.)
Footnotes
References
- 1. The World Health Report: Primary Health Care Now More Than Ever. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2. Mavrodaris A, Powell J, Thorogood M. Prevalences of dementia and cognitive impairment among older people in sub‐Saharan Africa: a systematic review. Bull World Health Organ. 2013;91:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barberger‐Gateau P, Fabrigoule C. Disability and cognitive impairment in the elderly. Disabil Rehabil. 1997;19:175–193. [DOI] [PubMed] [Google Scholar]
- 4. Dao E, Hsiung GYR, Liu‐Ambrose T. The role of exercise in mitigating subcortical ischemic vascular cognitive impairment. J Neurochem. 2018;144:582–594. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg GA, Wallin A, Wardlaw JM, Markus HS, Montaner J, Wolfson L, Iadecola C, Zlokovic BV, Joutel A, Dichgans M, Duering M, Schmidt R, Korczyn AD, Grinberg LT, Chui HC, Hachinski V. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36:6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 2018;114:513–528. [DOI] [PubMed] [Google Scholar]
- 7. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; on behalf of the European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 8. Scuteri A, Wang H. Pulse wave velocity as a marker of cognitive impairment in the elderly. J Alzheimers Dis. 2014;42:S401–S410. [DOI] [PubMed] [Google Scholar]
- 9. Pase MP, Herbert A, Grima NA, Pipingas A, O'rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta‐analysis. Intern Med J. 2012;42:808–815. [DOI] [PubMed] [Google Scholar]
- 10. Rabkin SW, Jarvie G. Comparison of vascular stiffness in vascular dementia, Alzheimer dementia and cognitive impairment. Blood Press. 2011;20:274–283. [DOI] [PubMed] [Google Scholar]
- 11. Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Gary F, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension. 2016;67:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension‐associated cognitive decline in healthy adults. Hypertension. 2016;67:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim SL, Gao Q, Nyunt MSZ, Gong L, Lunaria JB, Lim ML, Lingb A, Su‐Ping Lama C, Richardsc AM, Linga LH, Ng TP. Vascular health indices and cognitive domain function: Singapore longitudinal ageing studies. J Alzheimers Dis. 2016;50:27–40. [DOI] [PubMed] [Google Scholar]
- 14. Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev. 2014;15:16–27. [DOI] [PubMed] [Google Scholar]
- 15. van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2015;53:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thecker SB; for the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2008;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://www.handbook.cochrane.org.
- 19. National Heart, Lung, and Blood Institute . Quality assessment tool for observational cohort and cross‐sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed January 31, 2018.
- 20. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 21. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 22. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 23. Abbatecola AM, Barbieri M, Rizzo MR, Grella R, Laieta MT, Quaranta E, Molinari AM, Cioffi M, Fioretto P, Paolisso G. Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. J Gerontol A Biol Sci Med Sci. 2008;63:991–996. [DOI] [PubMed] [Google Scholar]
- 24. Al Hazzouri AZ, Vittinghoff E, Sidney S, Reis JP, Jacobs DR Jr, Yaffe K. Intima‐media thickness and cognitive function in stroke‐free middle‐aged adults: findings from the Coronary Artery Risk Development in Young Adults Study. Stroke. 2015;46:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Angermann S, Baumann M, Wassertheurer S, Mayer CC, Steubl D, Hauser C, Suttmann Y, Reichelt AL, Satanovskij R, Lorenz G, Lukas M, Haller B, Heemann U, Grimmer T, Schmaderer C. Pulse‐wave velocity is associated with cognitive impairment in hemodialysis patients. Clin Sci. 2017;131:1483–1493. [DOI] [PubMed] [Google Scholar]
- 26. Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, Manckoundia P, Agnoletti D, Labat C, Gautier S; PARTAGE Study Investigators . Pulse wave velocity is associated with 1‐year cognitive decline in the elderly older than 80 years: the PARTAGE study. J Am Med Dir Assoc. 2012;13:239–243. [DOI] [PubMed] [Google Scholar]
- 27. Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukuhara M, Matsumura K, Ansai T, Takata Y, Sonoki K, Akifusa S, Wakisaka M, Hamasaki T, Fujisawa K, Yoshida A, Fujii K, Iida M, Takehara T. Prediction of cognitive function by arterial stiffness in the very elderly. Circ J. 2006;70:756–761. [DOI] [PubMed] [Google Scholar]
- 29. Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Henry RM, Reesink KD, Kroona AA, Koster A, Schapera NC, Dagnelie PC, van der Kallena CJH, Biessels GJ, Stehouwer CDA. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes: the Maastricht Study. Atherosclerosis. 2016;253:186–193. [DOI] [PubMed] [Google Scholar]
- 30. Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. [DOI] [PubMed] [Google Scholar]
- 31. Karasavvidou D, Boutouyrie P, Kalaitzidis R, Kettab H, Pappas K, Stagikas D, Antonakis N, Tsalikakis D, Elisaf M, Laurent S. Arterial damage and cognitive decline in chronic kidney disease patients. J Clin Hypertens. 2018;20:1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YS, Kim DH, Choi BH, Sohn EH, Lee AY. Relationship between brachial‐ankle pulse wave velocity and cognitive function in an elderly community‐dwelling population with metabolic syndrome. Arch Gerontol Geriatr. 2009;49:176–179. [DOI] [PubMed] [Google Scholar]
- 33. Kim ED, Meoni LA, Jaar BG, Shafi T, Kao WHL, Estrella MM, Parekh R, Sozio SM. Association of arterial stiffness and central pressure with cognitive function in incident hemodialysis patients: the PACE Study. Kidney Int Rep. 2017;2:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lamballais S, Sajjad A, Leening MJ, Gaillard R, Franco OH, Mattace‐Raso FU, Jaddoe VWV, Roza SJ, Tiemeier H, Ikram MA. Association of blood pressure and arterial stiffness with cognition in 2 population‐based child and adult cohorts. J Am Heart Assoc. 2018;7:e009847 DOI: 10.1161/JAHA.118.009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee YH, Yoon ES, Park SH, Heffernan KS, Lee C, Jae SY. Associations of arterial stiffness and cognitive function with physical fitness in patients with chronic stroke. J Rehabil Med. 2014;46:413–417. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility‐Reykjavik study. Brain. 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muela HC, Costa‐Hong VA, Yassuda MS, Moraes NC, Memória CM, Machado MF, Bor‐Seng‐Shu E, Nogueira RC, Mansur AJ, Massaro AR, Nitrini R, Macedo TA, Bortolotto LA. Higher arterial stiffness is associated with lower cognitive performance in patients with hypertension. J Clin Hypertens. 2018;20:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muller M, Grobbee DE, Aleman A, Bots M, Van der Schouw YT. Cardiovascular disease and cognitive performance in middle‐aged and elderly men. Atherosclerosis. 2007;190:143–149. [DOI] [PubMed] [Google Scholar]
- 39. Nilsson ED, Elmståhl S, Minthon L, Nilsson PM, Pihlsgård M, Tufvesson E, Nägga K. Non‐linear association between pulse wave velocity and cognitive function: a population‐based study. J Hypertens. 2014;32:2152–2157. [DOI] [PubMed] [Google Scholar]
- 40. Palta P, Sharrett AR, Wei J, Meyer ML, Kucharska‐Newton A, Power MC, Deal JA, Jack CR, Knopman D, Wright J, Griswold M, Tanaka H, Mosley TH, Heiss G. Central arterial stiffness is associated with structural brain damage and poorer cognitive performance: the ARIC study. J Am Heart Assoc. 2019;8:e011045 DOI: 10.1161/JAHA.118.011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle‐aged adults: the Framingham Third Generation Cohort Study. Hypertension. 2016;67:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poels MM, van Oijen M, Mattace‐Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MMB. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007;38:888–892. [DOI] [PubMed] [Google Scholar]
- 43. Ryu DW, Kim JS, Lee JE, Park JW, Oh YS, An JY, Lee KS. Association of arterial stiffness with cognition in patients with Lewy body disorder. Neurol Sci. 2017;38:1307–1313. [DOI] [PubMed] [Google Scholar]
- 44. Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–1040. [DOI] [PubMed] [Google Scholar]
- 45. Singer J, Trollor JN, Crawford J, O'Rourke MF, Baune BT, Brodaty H, Samaras K, Kochan NA, Campbell L, Sachdev PS, Smith E. The association between pulse wave velocity and cognitive function: the Sydney Memory and Ageing Study. PLoS One. 2013;8:e61855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never‐treated essential hypertension. Am J Hypertens. 2009;22:525–530. [DOI] [PubMed] [Google Scholar]
- 47. Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Watson NL, Sutton‐Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, Najjar SS, Launer LJ, Yaffe K, Atkinson HH, Satterfield S, Newman AB. Arterial stiffness and cognitive decline in well‐functioning older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhong W, Cruickshanks KJ, Schubert CR, Carlsson CM, Chappell RJ, Klein BE, Acher CW. Pulse wave velocity and cognitive function in older adults. Alzheimer Dis Assoc Disord. 2014;28:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fujiwara Y, Chaves PH, Takahashi R, Amano H, Yoshida H, Kumagai S, Fujita K, Wang DG, Shinkai S. Arterial pulse wave velocity as a marker of poor cognitive function in an elderly community‐dwelling population. J Gerontol A Biol Sci Med Sci. 2005;60:607–612. [DOI] [PubMed] [Google Scholar]
- 52. Kearney‐Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–1236. [DOI] [PubMed] [Google Scholar]
- 53. Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH, Heiss G. Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the Atherosclerosis Risk in Communities Study‐Neurocognitive Study (ARIC‐NCS). J Alzheimers Dis. 2017;57:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nilsson ED, Elmståhl S, Minthon L, Pihlsgård M, Nilsson PM, Hansson O, Nägga K. No independent association between pulse wave velocity and dementia: a population‐based, prospective study. J Hypertens. 2017;35:2462–2467. [DOI] [PubMed] [Google Scholar]
- 55. Sugawara N, Yasui‐Furukori N, Umeda T, Kaneda A, Sato Y, Takahashi I, Matsuzaka M, Danjo K, Nakaji S, Kaneko S. Comparison of ankle‐brachial pressure index and pulse wave velocity as markers of cognitive function in a community‐dwelling population. BMC Psychiatry. 2010;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taniguchi Y, Fujiwara Y, Nofuji Y, Nishi M, Murayama H, Seino S, Tajima R, Matsuyama Y, Shinkai S. Prospective study of arterial stiffness and subsequent cognitive decline among community‐dwelling older Japanese. J Epidemiol. 2015;25:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tuttolomondo A, Casuccio A, Guercio G, Maida C, Del Cuore A, Di Raimondo D, Simonetta I, Di Bona D, Pecoraro R, Della Corte V, Gulotta E, Gulotta G, Pinto A. Arterial stiffness, endothelial and cognitive function in subjects with type 2 diabetes in accordance with absence or presence of diabetic foot syndrome. Cardiovasc Diabetol. 2017;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scuteri A, Tesauro M, Guglini L, Lauro D, Fini M, Di Daniele N. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol. 2013;169:371–377. [DOI] [PubMed] [Google Scholar]
- 59. Blondell SJ, Hammersley‐Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta‐analysis of longitudinal studies. BMC Public Health. 2014;14:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adams ML, Deokar AJ, Anderson LA, Edwards VJ. Self‐reported increased confusion or memory loss and associated functional difficulties among adults aged≥ 60 years—21 states, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:345–350. [PubMed] [Google Scholar]
- 61. Sun X, Rundek T. Does increased arterial stiffness herald cognitive impairment? Stroke. 2016;47:2171–2172. [DOI] [PubMed] [Google Scholar]
- 62. Pase MP. Modifiable vascular markers for cognitive decline and dementia: the importance of arterial aging and hemodynamic factors. J Alzheimers Dis. 2012;32:653–663. [DOI] [PubMed] [Google Scholar]
- 63. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 64. Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. [DOI] [PubMed] [Google Scholar]
- 65. Panza F, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Capurso SA, Caselli RJ, Pilotto A, Scafato E, Capurso A, Solfrizzi V. Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27:933–940. [DOI] [PubMed] [Google Scholar]
- 66. Cambronero FE, Liu D, Neal JE, Moore EE, Gifford KA, Terry JG, Nair S, Pechman KR, Osborn KE, Hohman TJ, Bell SP, Sweatt JD, Wang TJ, Beckman JA, Carr JJ, Jefferson AL. APOE genotype modifies the association between central arterial stiffening and cognition in older adults. Neurobiol Aging. 2018;67:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takeda JRT, Matos TM, Souza‐Talarico JND. Cardiovascular risk factors and cognitive performance in aging. Dement Neuropsychol. 2017;11:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scuteri A, Volpe M, Asmar R. Arterial stiffness and cognitive impairment in the elderly. High Blood Press Cardiovasc Prev. 2007;14:33–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. References excluded from the meta‐analysis.
Table S1. Sensitivity Analyses by Removing Studies One by One for Unadjusted Cross‐Sectional Analysis
Table S2. Sensitivity Analyses by Removing Studies One by One for Adjusted Cross‐Sectional Analysis
Table S3. Sensitivity Analyses by Removing Studies One by One for Longitudinal Analysis
Table S4. Subgroup Analyses for the Association Between PWv and Cognition Domains by Type of Sample, PWv Measured and Device Used
Table S5. Meta‐Regression of PWV and Cognition Domains by Percentage of Females and Mean Age, BMI, SBP and DBP of Included Studies
Table S6. Risk of Bias of Cross‐Sectional and Longitudinal Included Studies


