Abstract
Background
The inflammatory biomarker YKL‐40 has previously been studied as a potential risk marker in cardiovascular disease. We aimed to assess the prognostic reclassification potential of serum YKL‐40 in patients with stable coronary artery disease.
Methods and Results
The main study population was the placebo group of the CLARICOR (Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease) trial. The primary outcome was a composite of acute myocardial infarction, unstable angina pectoris, cerebrovascular disease, and all‐cause mortality. We used Cox proportional hazards regression models adjusted for C‐reactive protein level and baseline cardiovascular risk factors. Improvement in prediction by adding serum YKL‐40 to the risk factors was calculated using the Cox‐Breslow method and c‐statistic. A total of 2200 patients were randomized to placebo, with a follow‐up duration of 10 years. YKL‐40 was associated with an increased risk of the composite outcome (hazard ratio per unit increase in (YKL‐40) 1.13, 95% CI 1.03–1.24, P=0.013) and all‐cause mortality (hazard ratio 1.32, 95% CI 1.17–1.49, P<0.0001). Considering whether a composite‐outcome event was more likely to have, or not have, occurred to date, we found 68.4% of such predictions to be correct when based on the standard predictors, and 68.5% when serum YKL‐40 was added as a predictor. Equivalent results were obtained with c‐statistics.
Conclusions
Higher serum YKL‐40 was independently associated with an increased risk of adverse cardiovascular outcomes and mortality. Addition of YKL‐40 did not improve risk prediction in patients with stable coronary artery disease.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00121550.
Keywords: CHI3L1, cohort study, coronary atherosclerosis, YKL‐40
Subject Categories: Biomarkers, Coronary Artery Disease, Risk Factors, Quality and Outcomes
Clinical Perspective
What Is New?
In patients with stable coronary artery disease, higher YKL‐40 is significantly related to cardiovascular events and mortality over 10 years of follow‐up after adjustment for standard predictors and high‐sensitivity C‐reactive protein, possibly reflecting high‐risk atherosclerotic progression and inflammation.
What Are the Clinical Implications?
In reclassification analysis YKL‐40 did not improve prognostic accuracy compared with predictions based only on standard predictors, and the value of measuring YKL‐40 to support clinical decision making appears limited.
Introduction
The desire to better assess prognosis in patients with cardiovascular disease (CVD) and further advance the understanding of the underlying atherosclerotic process has prompted the emergence of numerous potential CVD biomarkers. Many candidates have demonstrated consistent association with standard CVD outcome measures. Nonetheless, auxiliary predictive potential in addition to that offered by standard available clinical characteristics is in many cases absent or unclear, hindering translation of results into specific changes in patient management strategies.1, 2, 3 However, in addition to possible utility in risk prediction, biomarker studies may unravel new key factors in CVD pathogenesis such as proinflammatory effectors, and ultimately help identify new potential upstream treatment targets.4, 5
YKL‐40, also known as chitinase‐3‐like‐1, is an inflammatory biomarker which has gathered increasing evidence during the past 2 decades.6 It is considered an acute‐phase protein and has been specifically linked to the inflammatory process in the atherosclerotic plaque,7 diseases of extracellular remodeling and fibrosis,8 angiogenesis and regulation of proinflammatory cascades.9, 10 YKL‐40 has recently been associated with development of hyperlipidemia and ischemic stroke in a study of almost 100 000 individuals from the general population,11 and it has been studied as a risk marker in both atherosclerotic development and CVD outcome prediction in patients with acute coronary syndrome and stable coronary artery disease (CAD).12, 13, 14
In the present study we assess the power of YKL‐40 as a predictor of cardiovascular outcomes and death in a cohort of stable CAD patients using 10‐year follow‐up data obtained from the CLARICOR (Effect of Clarithromycin on Mortality and Morbidity in Patients with Ischemic Heart Disease) trial.15 This study adds to prior reports on YKL‐40 by extending follow‐up duration to 10 years, and appraising the potential clinical translation of YKL‐40 measurement using reclassification analysis.16, 17, 18 All analyses were performed according to a previously published statistical analyses plan.19, 20
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial Design and Patients
The CLARICOR trial was an investigator‐initiated, randomized, placebo‐controlled, multicenter superiority trial including patients with stable CAD. A total of 13 702 patients aged 18 to 85 years who had a discharge diagnosis of myocardial infarction, angina pectoris, percutaneous transluminal coronary angioplasty, or coronary artery bypass surgery during 1993 to 1999 and were alive in August 1999 were invited by letter for a 14‐day trial of clarithromycin versus placebo.15 Of these, 6116 accepted the invitation: 177 refused to participate, and 1567 were excluded while 4372 were randomized by a central algorithm in a 1:1 ratio to clarithromycin versus placebo. Patients were non‐hospitalized and stable at trial inclusion.
Baseline data were obtained from the hospital files and patient interviews at 6 cardiology clinics covering the Copenhagen area, and participants were followed up through Danish population registries. Exclusion criteria were presence of the following conditions: (1) acute myocardial infarction or unstable angina pectoris within the previous 3 months; (2) percutaneous transluminal coronary angioplasty and coronary bypass surgery within the previous 6 months; (3) impaired renal or (4) hepatic function; (5) congestive heart failure (New York Heart Association IV classification of heart failure); (6) active malignancy; (7) absence of capacity to manage own affairs, and (8) breastfeeding or possible pregnancy.
Participants were followed up for 10 years on average from the end of treatment in October 1999 to April 2000 until December 31, 2009. The follow‐up data set was solely based on register excerpts.
The main result of the CLARICOR trial was that clarithromycin increased the risk of cardiovascular and all‐cause death.15, 21, 22, 23 The present study is a prognostic analysis focused on the placebo group which may be considered a representative sample of stable CAD patients, ie, not treated with clarithromycin. For corroboration and discovery, results in the clarithromycin‐treated patient group are similarly summarized.
Baseline Data and Predictors
Enrollment interviews at baseline provided smoking status, current medication, presence of hypertension and diabetes mellitus, while further data about sex, age, and cardiovascular history (presence of previous acute myocardial infarction) were obtained through local hospital files. Blood samples were obtained just before randomization and immediately frozen at −80°C. Biochemical measurements on plasma included lipoproteins, high‐sensitivity C‐reactive protein (hs‐CRP, mg/L)24 and estimated glomerular filtration rate (mL/min) using creatinine.25
The above‐mentioned baseline variables and quantities are collectively referred to as “standard predictors,” see Table 1 and Statistical Analyses section below.
Table 1.
The Distribution of Standard Predictors in the Placebo Group and in the Clarithromycin Group of the CLARICOR Trial
| Standard Predictors | Placebo Patients (n=2200) | Clarithromycin Patients (n=2172) |
|---|---|---|
| Demographics and history | ||
| Sex (male), n (%) | 1518 (69.0) | 1514 (69.7) |
| Age/y, mean (SD) | 65.2 (10.4) | 65.4 (10.3) |
| Smoking status (smoking, ex‐smoker, never smoked), n (%) | Smokers, 753 (34.2) | Smokers, 819 (37.6) |
| Ex‐smokers, 1011 (46.0) | Ex‐smokers, 982 (45.2) | |
| Never smoked, 435 (19.8) | Never smoked, 371 (17.1) | |
| Hypertension, n (%) | 883 (40.2) | 878 (40.4) |
| Diabetes mellitus, n (%) | 337 (15.3) | 341 (15.7) |
| Previous AMI, n (%) | 1494 (67.9) | 1470 (67.7) |
| Medication at randomization | ||
| Aspirin, n (%) | 1937 (88.1) | 1902 (87.6) |
| Beta‐blocker, n (%) | 681 (31.0) | 653 (30.1) |
| Calcium antagonist, n (%) | 772 (35.1) | 755 (34.8) |
| ACE inhibitor, n (%) | 577 (26.3) | 604 (27.8) |
| Long‐lasting nitrate, n (%) | 457 (20.8) | 453 (20.9) |
| Diuretics, n (%) | 773 (35.2) | 762 (35.1) |
| Digoxin, n (%) | 126 (5.7) | 154 (7.1) |
| Statins, n (%) | 904 (41.1) | 896 (41.3) |
| Antiarrhythmic drugs, n (%) | 51 (2.3) | 55 (2.5) |
| Biochemical predictors | ||
| CRP mg/L mean (SD), na | 2.80 (3.06) 2159 | 2.91 (3.16) 2128 |
| ApoA1 mg/dL mean (SD), n | 1.70 (0.34) 2076 | 1.70 (0.36) 2041 |
| Apolipoprotein B mg/dL mean (SD), n | 1.17 (1.31) 2075 | 1.16 (1.31) 2040 |
| Cholesterol‐HDL mg/dL mean (SD), n | 39.44 (12.37) 2074 | 39.44 (12.37) 2039 |
| Cholesterol‐LDL mg/dL mean (SD), n | 99 (27.84) 2079 | 98.6 (28.23) 2044 |
| Cholesterol mg/dL mean (SD), n | 218.1 (47.18) 2075 | 215.78 (47.18) 2039 |
| Triglyceride mg/dL mean (SD), n | 184.23 (150.58) 2078 | 179.8 (148.8) 2040 |
| Glomerular filtration rate (mL per min) mean (SD), n | 71.8 (19.2) 2079 | 72.1 (19.2) 2044 |
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease; CRP, C‐reactive protein; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
The value of n varies because the laboratory tests have missing values.
YKL‐40 Analysis
In 4298 (98%) of the included patients’ serum was available for YKL‐40 determination, divided between 2163 in the placebo group and 2135 in the clarithromycin group. Serum concentrations were determined in duplicates by a commercial ELISA (Quidel, San Diego, CA, USA) using streptavidin‐coated microplate wells, a biotinylated‐Fab monoclonal capture antibody, and an alkaline phosphatase‐labeled polyclonal detection antibody. The recovery is 102%, detection limit 20 mg/L, intra‐assay coefficient of variation 5.0%, and inter‐assay coefficients of variation are <5.4% (low control) and <6.7% (high control) in the 127 ELISA kits used for the analysis of the 4298 samples.
Outcomes
Information about the underlying cause of death was obtained from the National Register of Causes of Death26; The Danish National Patient Register covering all somatic hospital admissions provided hospitalization data.27 The Danish 10‐digit central person registration number is used at all contacts with the healthcare system, and somatic hospital contact cannot be completed without a diagnosis based upon the International Classification of Diseases, Tenth Revision (ICD‐10) and subsequent notification of the National Patient Register. All registers have coverage close to 100%.
For each recorded main diagnosis and for each underlying cause of death, we classified the outcome into disjoint and exhaustive categories28, 29: Acute myocardial infarction (ICD codes I21.0–I23.9), unstable angina pectoris (ICD codes I20.0 and I24.8–I24.9), both ischemic and hemorrhagic cerebrovascular disease (ICD codes I60.0–I64.9 and G45.0–G46.8), other cardiovascular diseases (ICD codes I00.0–I99.9, unless already covered), and non‐cardiovascular disease (ICD codes A00.0–T98.3, unless already covered).
We constructed a composite outcome composed of acute myocardial infarction, unstable angina pectoris, cerebrovascular disease, or all‐cause death during follow‐up.
Statistical Analyses
A detailed description of the predefined statistical analysis plan for selected cardiovascular biomarkers based on the CLARICOR trial cohort, the Predictors for major cardiovascular outcomes in stable ischemic heart disease (PREMAC) protocol, has formerly been published.19 All primary analyses were performed on placebo group data because it was previously shown that clarithromycin had a significant and harmful effect on patient prognosis21; however, all analyses have additionally been performed in the clarithromycin group.
To fulfill linearity assumption YKL‐40/μg per L was log‐transformed. We examined the adjusted hazard ratio (HR) per unit increase in ln(YKL40/μg per L).
We used Cox proportional hazards regression models supplemented by Breslow estimation of the baseline hazard to study the association between all the above‐mentioned standard predictors and YKL‐40 as covariates, and the composite outcome. The associations were examined in univariate analysis, and in analysis adjusted for all standard predictors. Model assumptions were tested using Bonferroni adjusted thresholds for significance, and all covariate analyses were stratified by center. In exploratory sub‐analyses, the association with the individual outcomes was also investigated.
Entry information in the CLARICOR trial had virtually no missing data, while 9.4% of patients had ≥1 laboratory tests missing. The Little test was applied to decide whether multiple imputation or complete case analyses should be used, and after obtaining P=0.93 complete case analysis was used.
To examine the predictive improvement obtainable with YKL‐40, we applied 2 different reclassification analysis approaches. In the first analysis we read off the predicted Cox‐Breslow survival curve of each patient at 3 years of follow‐up and checked the likely outcome against the patient's actual course. If the patient's actual status (still event‐free or not) was also the status predicted to be more likely, the prediction was classified as “true favorable” (or “true unfavorable”). This analysis, first based on the standard predictors, was then repeated with YKL‐40 included as a predictor. Analogous results based on raw Kaplan‐Meier curves (no individual predictors) are reported for comparison. The 6‐ and 9‐year status was similarly analyzed. Secondly, we present a receiver operating characteristic (ROC) analysis of the Cox‐Breslow risks applied to the same time window 0‐to‐9 years. Conventional “observed” ROCs are plots of cumulative events against the cumulative event‐free contingency, with cumulation from large to small estimated risks. The corresponding “predicted” ROC is based on cumulating the predicted risks. The area under the curves (or c‐index) estimates the pairwise concordance rate between risks and outcomes. To reward correct prediction of time of event, we further determined a “dynamic” c‐index, alias risk concordance within any pair of participants whose event order was deducible from the 9‐year data window.
All analyses were performed using SAS V.9.4
Ethics
The study was performed in accordance with the Helsinki Declaration and was approved by the local ethics committee (KF 01‐076/99), the Danish Medicines Agency (2612‐975), and the Danish Data Protection Agency (1999 1200‐174). All participants gave written informed consent, after receiving oral and written information about the study.
Results
Study Population and Prognostic Importance of Standard Predictors
Patients randomized to placebo and clarithromycin amounted to 2200 and 2172, respectively.
The median serum concentration of YKL‐40 in all patients was 110 μg/L (25th/75th percentiles 75/166 μg per L).
Table 1 presents baseline characteristics of the placebo and clarithromycin groups, illustrating that randomization was apparently successful with equal distribution of the studied quantities.
At 3 years follow‐up in the placebo group 1826 (83.0%) patients had not experienced a composite outcome, and 2073 (94.3%) were still alive. At 6 years the respective numbers were 1261 (57.3%) free of the composite outcome and 1758 (79.9%) still alive, and at 9 years the numbers were 969 (44.9%) and 1487 (67.6%). See Table S1 for cumulative events specified by subtype.
When all standard predictors were included in the model, smoking status, diabetes mellitus, long‐lasting nitrate medication, digoxin, and interaction between age and time were associated with a higher risk of the composite outcome, while a higher glomerular filtration rate was associated with a lower HR (Table S2).
Follow‐Up Results
Table 2 shows the HRs for YKL‐40 for each specified outcome, the composite outcome, and all‐cause death per unit increase in ln(YKL‐40/μg per L). YKL‐40 was significantly associated with the composite outcome and all‐cause mortality when used alone, and in combination with all standard predictors.
Table 2.
Hazard Ratios of YKL‐40 When Used Alone and Adjusted for the Rest of the Standard Predictors in the Placebo Group of the CLARICOR Trial
| Outcome | YKL‐40 Used Alone | YKL‐40 Adjusted for Standard Predictors | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI of HR | P Values | HR | 95% CI of HR | P Values | |
| Unstable angina pectoris | 1.09 | 0.93 to 1.29 | 0.28 | 0.99 | 0.83 to 1.19 | 0.94 |
| Acute myocardial infarction | 1.36 | 1.18 to 1.56 | <0.0001 | 1.19 | 1.02 to 1.40 | 0.028 |
| Cerebrovascular disease | 1.18 | 0.99 to 1.40 | 0.06 | 0.95 | 0.78 to 1.16 | 0.64 |
| Cardiovascular death | 1.67 | 1.44 to 1.93 | <0.0001 | 1.23 | 1.03 to 1.47 | 0.025 |
| Composite outcome | 1.35 | 1.24 to 1.47 | <0.0001 | 1.13 | 1.03 to 1.24 | 0.013 |
| All‐cause mortality | 1.76 | 1.59 to 1.95 | <0.0001 | 1.32 | 1.17 to 1.49 | <0.0001 |
Composite outcome including acute myocardial infarction, unstable angina pectoris, cerebrovascular disease, or all‐cause mortality. HR indicates hazard ratio; CLARICOR, Effect of Clarithromycin on Mortality and Morbidity in Patients With Ischemic Heart Disease.
Prognostic Improvement
Table 3 shows the true favorable and unfavorable predictions obtained when using the Cox model for both the composite outcome and all‐cause mortality. Percentages are correct predictions out of total number of predictions (total predictions=5970, 3 per patient unless dropout). Without any individual data being considered, 63.2% of predictions were correct for the composite outcome, and with addition of standard predictors 68.4% of predictions were correct. With the further addition of YKL‐40 68.5% of predictions were correct. Turning to all‐cause mortality (lower part of the table), we see a similar pattern with no visible improvement ascribable to YKL‐40.
Table 3.
The Types and Numbers of Correct Predictions Obtained From the Cox Proportional Hazards Model
| Prediction Type | No Covariates | SP Included | SP Plus YKL‐40 Included |
|---|---|---|---|
| Composite outcome | |||
| True favorable predictions, n (%) | 2658 (44.5) | 2910 (48.7) | 2909 (48.7) |
| True unfavorable predictions n, (%) | 1115 (18.7) | 1174 (19.7) | 1179 (19.7) |
| Total number of true predictions, n (%) | 3773 (63.2) | 4084 (68.4) | 4088 (68.5) |
| All‐cause mortality | |||
| True favorable predictions, n (%) | 4768 (79.9) | 4585 (76.8) | 4582 (76.7) |
| True unfavorable predictions, n (%) | 0 (0)a | 392 (6.57) | 398 (6.67) |
| Total number of true predictions, n (%) | 4768 (79.9) | 4977 (83.4) | 4980 (83.4) |
For comparison results without covariates, with standard predictors included, and with YKL‐40 included, are shown. For each patient at time (T) equal to 3, 6, and 9 years and using the patient's individual survival curve the predicted outcome (patient “alive at T” (favorable outcome) compared with patient “not alive at T” [unfavorable outcome]) was read off the survival curve and the results compared with the observed outcome. When no covariates were included in the model, this coincides with the Kaplan‐Meier survival curve which was used to calculate the predictions. To allow for the fact that age violated the proportional hazard assumption we stratified by age categories in addition to center and excluded age from the covariates. Total predictions=5970, n (%)=true favorable predictions/total predictions. SP indicates standard predictors.
As total mortality is <50%, all predictions are automatically favorable (and 79.9% of them pertain to a so far survivor).
Table 4 and Figure summarize the ROC analysis. For prediction of a composite outcome, the area under the ROC increases from 0.711 to 0.713 when log(YKL40) is added to the “standard predictors.” In the figure the 2 observed ROCs practically coincide with each other, as well as their predicted counterparts (omitted). The “dynamic” c‐index values are smaller as prediction of event times is more difficult, but the gains of introducing YKL‐40 are similar.
Table 4.
c Index of 9‐Year Outcomes
| Binary c (AUC), Observed (Predicted) | Dynamic c, Observed | |
|---|---|---|
| Composite outcome (1115 events) | ||
| YKL40 included | 0.713 (0.708) | 0.641 |
| Standard predictors only | 0.711 (0.707) | 0.640 |
| All‐cause death (644 deaths) | ||
| YKL40 included | 0.795 (0.796) | 0.741 |
| Standard predictors only | 0.792 (0.793) | 0.737 |
Composite outcome including acute myocardial infarction, unstable angina pectoris, cerebrovascular disease or all‐cause mortality. AUC indicates area under curve.
Figure 1.
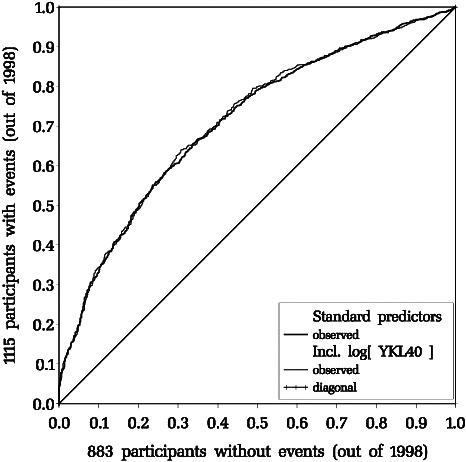
Receiver operating characteristics pertaining to the composite outcome within the 9‐year data window. Standard predictors only: fat curve; ln(YKL40) added: thin curve. For area under curve see Table 4.
Results in the Clarithromycin‐Treated Group
Both the direction and magnitude of relations between the standard predictors and YKL‐40 and the investigated outcomes in the clarithromycin group were largely equivalent to those obtained in the placebo group outlined above (Table S3).
Similarly, the net increase in true prediction percentages when adding YKL‐40 to the standard predictors was from 69.1% to 69.5% for the composite outcome, and from 83.0% to 83.1% for all‐cause death (Table S4).
Discussion
Main Findings
In the placebo group of the CLARICOR trial in patients with stable CAD, elevated levels of YKL‐40 were significantly associated with increased risk of cardiovascular outcomes and all‐cause mortality over 10 years of follow‐up in both univariate and multiple adjusted survival analyses. The ability to predict future CVD outcomes and mortality was not improved following addition of YKL‐40 to the standard model. Results were similar in the clarithromycin‐treated patient group.
Study Strengths and Limitations
The principal strengths of the present study are the patient population size, the 10‐year long follow‐up in registers with no loss to follow‐up of events and the detailed characterization of patients. In addition to reporting HRs, the study provides insight into the potential clinical implementation of YKL‐40 as a prognostic biomarker by evaluating obtained prediction improvement when adding YKL‐40 to standard predictors.
Limitations include the lack of certain baseline characteristics, eg, body mass index and systolic blood pressure. Data on previous peripheral artery disease or ischemic stroke were not available for analysis. As a consequence, we did not have data on all variables included in contemporary risk algorithms for secondary prevention such as American College of Cardiology/American Heart Assocation 2018 guidelines or the TIMI Risk Score for Secondary Prevention.30, 31 Further, we did not have access to longitudinal monitoring of the biomarker.
Atherosclerosis, Inflammation, and YKL‐40
In the CLARICOR cohort, we have previously reported that YKL‐40 levels were significantly higher in patients with age ≥60 years, hypertension or diabetes mellitus, and in patients who used calcium antagonists, angiotensin‐converting enzyme inhibitors, long‐lasting nitrates, diuretics, or digoxin.18 The present study shows that most of these risk factors were also associated with the composite outcome in univariate analyses (Table S2). Further, the association between YKL‐40 and the composite outcome was attenuated after multivariate adjustment for the baseline standard predictors (Table 2). Our results suggest that the presence of risk factors, and comorbidities requiring medical therapy, may initiate a progression in the atherosclerotic process ultimately leading to CVD outcomes, and concomitant or co‐incident YKL‐40 stimulation. YKL‐40 may therefore be an inflammatory biomarker which reflects plaque development primarily elicited by risk factor presence in itself.4, 32
Activated macrophages and neutrophils secrete YKL‐40 in various disease tissues characterized by inflammation, extracellular remodeling, and fibrosis.33 It is an angiogenic stimulator, and has been shown to maintain connective tissue structure and attenuate the inflammatory response in the presence of tumor necrosis factor‐alpha stimulation.34 Macrophages located deep in the developing atherosclerotic plaque exhibit the highest expression of YKL‐40.7
Mendelian randomization studies have suggested that genetically elevated YKL‐40 levels do not play a causative role in the development of CVD,11, 35 but this finding does not rule out the possibility that YKL‐40 could play a role in disease progression after diagnosis, and whether alteration of YKL‐40 function might improve outcomes in atherosclerotic disease has not yet been determined. An experimental study has shown that YKL‐40 attenuates inflammasome activation, thereby acting as an anti‐inflammatory effector, while YKL‐40 inhibition with chitosan led to increased inflammasome activity.10
Comparison With Other Cardiovascular Disease Studies
Previous studies in varied CVD patient groups and the general population have evaluated the possible association between YKL‐40 levels, diagnostic tests, and prognosis.
In acute coronary syndrome patients, several studies have demonstrated a significant increase in YKL‐40 level immediately following acute coronary events which persists regardless of revascularization. YKL‐40 was associated with a detrimental prognosis and persisting left ventricular dysfunction.13, 36, 37 In addition, a recent study reported an overall association between degree of angiographic atherosclerosis severity and YKL‐40 level.38 These results suggest a possible increase in plasma YKL‐40 synchronous with atherosclerotic progression and coronary events, which persists and may predict a detrimental outcome in acute coronary syndrome patients.
In stable CAD patients, YKL‐40 level was significantly associated with number of diseased coronary vessels with >50% stenosis in 1 study,39 and has been linked to both higher SYNTAX score, poor development of coronary collaterals and myocardial perfusion defects.14, 16, 40, 41 In 313 patients with stable CAD, YKL‐40 performed slightly better than hs‐CRP in predicting re‐stenosis after percutaneous transluminal coronary angioplasty.42 Our group has published a large prognostic study in stable CAD patients from the entire CLARICOR cohort which found that YKL‐40 level was associated with all‐cause and cardiovascular mortality, and this association remained significant after adjustment for hs‐CRP and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels.17, 18
In healthy individuals, Ridker et al43 performed a nested case‐control study in a cohort of >20,000 women and found that YKL‐40 predicted thromboembolic stroke with a 40% increase in odds ratio per quartile. Another large observational study investigating YKL‐40 in almost 100,000 individuals from the Danish general population found that individuals in the 91% to 100% highest YKL‐40 percentile had a significantly increased risk for ischemic cerebrovascular disease and ischemic heart disease compared with the 0% to 33% percentile over a 36‐year follow‐up period.11
Clinical Implications
Although the main results in the present study underline a connection between YKL‐40 and CVD severity and outcomes, we also found that addition of YKL‐40 to standard predictors did not alter outcome prediction ability to a noteworthy degree. Morrow et al1 have proposed criteria for the potential translation of a biomarker to clinical implementation, ie, (1) can the clinician measure it, (2) does it add new information, and (3) does it help the clinician manage patients? For YKL‐40, existing evidence does not fulfill the last 2 of the 3 criteria at present.2, 44 Direct comparisons with established prediction models when evaluating predictive benefit of a biomarker would appear especially relevant in ischemic heart disease, where several clinical scores such as Framingham Risk Score45 and GRACE (Global Registry of Acute Coronary Events) Score46 are already used in the general population and acute coronary syndrome patients, respectively. Our results suggest that YKL‐40 is not an important prognostic biomarker when taking baseline risk factors into account.
Candidate protein biomarkers in CVD research are numerous, and as a result several attempts have been made to develop stringent methodological approaches to biomarker evaluation.47, 48 Significant association between the biomarker and the outcome of interest is a necessary requirement, but biomarker usefulness should also be evaluated with statistical methods which directly convey the absolute changes in patient categorization. Proposed statistical indices of valuable reclassification include the ROC and more recently the net reclassification index (NRI). NRI may however be more unreliable and misleading than initially thought with the risk of yielding false‐positive NRI statistics.49, 50
The majority of candidate CVD biomarkers lack sufficient reclassification assessment, and even some of the most thoroughly investigated candidate biomarkers have failed to exhibit clinically meaningful reclassification potential.51 As an example, value of hs‐CRP measurement has been evaluated against Framingham Risk Score: The increases in c‐statistic and NRI were diminutive and clinically irrelevant,51 and in a large pooled analysis of 52 prospective studies, the addition of hs‐CRP to standard risk factors failed to increase the c‐index or NRI.52 A White Paper evaluating the role of hs‐CRP in CVD did not find that current evidence supported the use of hs‐CRP in risk prediction, and this recommendation was specifically based on a lack of reclassification improvement.53 The findings for hs‐CRP resemble our findings for YKL‐40, ie, a multivariate‐adjusted significant HR for cardiovascular outcomes which simultaneously demonstrates little benefit.
CLARICOR Main Findings and YKL‐40
The CLARICOR study main finding of an increased risk of CVD outcomes in patients treated with clarithromycin was unexpected.15 In the present YKL‐40 study, HRs and reclassification results in the placebo group versus the clarithromycin group were similar. This study does not contribute new information which may help explain the different event rates observed in the CLARICOR trial.
Conclusions
We have shown that YKL‐40 is associated with adverse CVD outcomes and death independently of established risk factors in patients with stable CAD. Improvement in reclassification after addition of YKL‐40 to standard predictors was negligible. Elevated YKL‐40 levels may signify high‐risk atherosclerosis and ongoing inflammation in the atherosclerotic plaque in stable CAD patients with cardiovascular risk factors. Whether targeted anti‐inflammatory therapy could carry a prognostic benefit in patients with elevated YKL‐40 levels remains to be investigated.
Sources of Funding
This study was supported by the Copenhagen Trial Unit. The CLARICOR (Effect of Clarithromycin on Mortality and Morbidity in Patients with Ischemic Heart Disease) trial is investigator initiated and controlled. This trial was supported by grants from nonprofit funds (the Danish Heart Foundation, the Copenhagen Hospital Corporation, the Danish Research Council, and the 1991 Pharmacy Foundation) as well as from the Copenhagen Trial Unit. Abbott Laboratories, IDC, Queensborough, UK, supplied the clarithromycin and placebo tablets free of charge. Those supporting the trial had no role in design, data collection, data analyses, data interpretation, or writing the report. The funding sources did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Disclosures
None.
Supporting information
Table S1. Cumulative Number of Outcomes, by Subtype
Table S2. Composite Outcome Hazard Ratios of Standard Predictors When Used Alone and Adjusted for the Rest of the Standard Predictors in the Placebo Group of the CLARICOR Trial
Table S3. Hazard Ratios of YKL‐40 When Used Alone and When Adjusted for Standard Predictors in the Clarithromycin Group of the CLARICOR Trial
Table S4. The Types and Numbers of Correct Predictions Obtained From the Cox Proportional Hazards Model in the Clarithromycin Group of the ClARICOR Trial
Acknowledgments
We thank the CLARICOR trial participants. We thank the investigators and other staff involved in the first phases of the CLARICOR trial (for full list of names please see references 6, 15, 19, and 20). We also thank The Copenhagen Trial Unit, Centre for Clinical Intervention Research for providing monetary support for part of biochemical analyses for the PREMAC study as well as wages for Per Winkel, Janus C. Jakobsen, and Christian Gluud.
(J Am Heart Assoc. 2020;9:e014634 DOI: 10.1161/JAHA.119.014634.)
References
- 1. Morrow DA, de Lemos JA. Benchmarks for the Assessment of Novel Cardiovascular Biomarkers. Circulation. 2007;115:949–952. [DOI] [PubMed] [Google Scholar]
- 2. Macnamara J, Eapen DJ, Quyyumi A, Sperling L. Novel biomarkers for cardiovascular risk assessment: current status and future directions. Future Cardiol. 2015;11:597–613. [DOI] [PubMed] [Google Scholar]
- 3. Libby P, King K. Biomarkers: a Challenging Conundrum in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2015;35:2491–2495. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Lüscher TF. Anti‐inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 6. Ziatabar S, Zepf J, Rich S, Danielson BT, Bollyky PI, Stern R. Chitin, chitinases, and chitin lectins: emerging roles in human pathophysiology. Pathophysiology. 2018;25:253–262. [DOI] [PubMed] [Google Scholar]
- 7. Boot RG, Van Achterberg TAE, Van Aken BE, Renkema GH, Jacobs MJHM, Aerts JMFG, De Vries CJM. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp‐39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–694. [DOI] [PubMed] [Google Scholar]
- 8. Johansen JS. Studies on serum YKL‐40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 9. Nishikawa KC, Millis AJT. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003;287:79–87. [DOI] [PubMed] [Google Scholar]
- 10. Gudmundsdottir S, Lieder R, Sigurjonsson OE, Petersen PH. Chitosan leads to downregulation of YKL‐40 and inflammasome activation in human macrophages. J Biomed Mater Res ‐ Part A. 2015;103:2778–2785. [DOI] [PubMed] [Google Scholar]
- 11. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Elevated plasma YKL‐40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke. 2015;46:329–335. [DOI] [PubMed] [Google Scholar]
- 12. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Role of inflammatory marker YKL‐40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit Rev Clin Lab Sci. 2016;53:396–408. [DOI] [PubMed] [Google Scholar]
- 13. Nøjgaard C, Høst NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, Johansen JS. Serum levels of YKL‐40 increases in patients with acute myocardial infarction. Coron Artery Dis. 2008;19:257–263. [DOI] [PubMed] [Google Scholar]
- 14. Akboga MK, Yalcin R, Sahinarslan A, Yilmaz Demirtas C, Abaci A. Effect of serum YKL‐40 on coronary collateral development and SYNTAX score in stable coronary artery disease. Int J Cardiol. 2016;224:323–327. [DOI] [PubMed] [Google Scholar]
- 15. Jespersen CM. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. Br Med J. 2006;332:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathiasen AB, Harutyunyan MJ, Jrgensen E, Helqvist S, Ripa R, Gtze JP, Johansen JS, Kastrup J. Plasma YKL‐40 in relation to the degree of coronary artery disease in patients with stable ischemic heart disease. Scand J Clin Lab Invest. 2011;71:439–447. [DOI] [PubMed] [Google Scholar]
- 17. Harutyunyan M, Gøtze JP, Winkel P, Johansen JS, Hansen JF, Jensen GB, Hilden J, Kjøller E, Kolmos HJ, Gluud C, Kastrup J. Serum YKL‐40 predicts long‐term mortality in patients with stable coronary disease: a prognostic study within the CLARICOR trial. Immunobiology. 2013;218:945–951. [DOI] [PubMed] [Google Scholar]
- 18. Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, Jespersen CM, Kjøller E, Kolmos HJ, Lind I, Nielsen H, Gluud C. High serum YKL‐40 concentration is associated with cardiovascular and all‐cause mortality in patients with stable coronary artery disease. Eur Heart J. 2009;30:1066–1072. [DOI] [PubMed] [Google Scholar]
- 19. Winkel P, Jakobsen JC, Hilden J, Lange T, Jensen GB, Kjøller E, Sajadieh A, Kastrup J, Kolmos HJ, Larsson A, Ärnlöv J, Gluud C. Predictors for major cardiovascular outcomes in stable ischaemic heart disease (PREMAC): statistical analysis plan for data originating from the CLARICOR (clarithromycin for patients with stable coronary heart disease) trial. Diagnostic Progn Res. 2017;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winkel P, Jakobsen JC, Hilden J, Jensen G, Kjøller E, Sajadieh A, Kastrup J, Kolmos HJ, Larsson A, Ärnlöv J, Gluud C. Prognostic value of routinely available data in patients with stable coronary heart disease. A 10‐year follow‐up of patients sampled at random times during their disease course. Open Hear. 2018;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjøller E, Jensen GB, Skoog M, Lindschou J, Gluud C. Clarithromycin for stable coronary heart disease increases all‐cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol. 2015;182:459–465. [DOI] [PubMed] [Google Scholar]
- 22. Gluud C, Als‐Nielsen B, Damgaard M, Fischer Hansen J, Hansen S, Helø OH, Hildebrandt P, Hilden J, Jensen GB, Kastrup J, Kolmos HJ, Kjøller E, Lind I, Nielsen H, Petersen L, Jespersen CM. Clarithromycin for 2 weeks for stable coronary heart disease: 6‐Year follow‐up of the CLARICOR randomized trial and updated meta‐analysis of antibiotics for coronary heart disease. Cardiology. 2008;111:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winkel P, Hilden J, Fischer Hansen J, Hildebrandt P, Kastrup J, Kolmos HJ, Kjøller E, Jespersen CM, Gluud C, Jensen GB. Excess sudden cardiac deaths after short‐term clarithromycin administration in the CLARICOR trial: why is this so, and why are statins protective? Cardiology. 2011;118:63–67. [DOI] [PubMed] [Google Scholar]
- 24. Harutyunyan MJ, Mathiasen AB, Winkel P, Gøtze JP, Hansen JF, Hildebrandt P, Jensen GB, Hilden J, Jespersen CM, Kjøller E, Kolmos HJ, Gluud C, Kastrup J. High‐sensitivity C‐reactive protein and N‐terminal pro‐B‐type natriuretic peptide in patients with stable coronary artery disease: a prognostic study within the CLARICOR Trial. Scand J Clin Lab Invest. 2010;71:52–62. [DOI] [PubMed] [Google Scholar]
- 25. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. CKD‐EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helweg‐Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. [DOI] [PubMed] [Google Scholar]
- 27. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 28. Kjøller E, Hilden J, Winkel P, Galatius S, Frandsen NJ, Jensen GB, Fischer Hansen J, Kastrup J, Jespersen CM, Hildebrandt P, Kolmos HJ, Gluud C. Agreement between public register and adjudication committee outcome in a cardiovascular randomized clinical trial. Am Heart J. 2014;168. [DOI] [PubMed] [Google Scholar]
- 29. Kjoller E, Hilden J, Winkel P, Frandsen NJ, Galatius S, Jensen G, Kastrup J, Hansen JF, Kolmos HJ, Jespersen CM, Hildebrandt P, Gluud C. Good interobserver agreement was attainable on outcome adjudication in patients with stable coronary heart disease. J Clin Epidemiol. 2012;65:444–453. [DOI] [PubMed] [Google Scholar]
- 30. Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, He P, Lewis BS, Merlini PA, Murphy SA, Sabatine MS, Scirica BM, Morrow DA. Atherothrombotic Risk Stratification and the Efficacy and Safety of Vorapaxar in Patients with Stable Ischemic Heart Disease and Previous Myocardial Infarction. Circulation. 2016;134:304–313. [DOI] [PubMed] [Google Scholar]
- 31. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:E1082–E1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ridker PM. From C‐Reactive Protein to Interleukin‐6 to Interleukin‐1: moving Upstream to Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase‐like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 34. Ling H, Recklies AD. The chitinase 3‐like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin‐1 and tumour necrosis factor‐alpha. Biochem J. 2004;380:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Observationally and genetically high YKL‐40 and risk of venous thromboembolism in the general population: cohort and mendelian randomization studies. Arterioscler Thromb Vasc Biol. 2016;36:1030–1036. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbrüchel DA, Friis T, Bindslev L, Haack‐Sørensen M, Jørgensen E, Kastrup J. YKL‐40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J. 2008;42:295–302. [DOI] [PubMed] [Google Scholar]
- 37. Hedegaard A, Sejersten Ripa R, Johansen JS, Jørgensen E, Kastrup J. Plasma YKL‐40 and recovery of left ventricular function after acute myocardial infarction. Scand J Clin Lab Invest. 2010;70:80–86. [DOI] [PubMed] [Google Scholar]
- 38. Sciborski K, Kuliczkowski W, Karolko B, Bednarczyk D, Protasiewicz M, Mysiak A, Negrusz‐Kawecka M. Plasma YKL‐40 levels correlate with the severity of coronary atherosclerosis assessed with the SYNTAX score. Polish Arch Intern Med. 2018;128:644–648. [DOI] [PubMed] [Google Scholar]
- 39. Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL‐40 levels in patients with coronary artery disease. Coron Artery Dis. 2007;18:391–396. [DOI] [PubMed] [Google Scholar]
- 40. Xu Y, Meng HL, Su YM, Chen C, Huang YH, Li XF, Fan MK, Yan YJ, Wu J, Jiang MH, Pan M. Serum YKL‐40 is increased in patients with slow coronary flow. Coron Artery Dis. 2015;26:121–125. [DOI] [PubMed] [Google Scholar]
- 41. Rathcke CN, Kjøller E, Fogh‐Andersen N, Zerahn B, Vestergaard H. NT‐proBNP and circulating inflammation markers in prediction of a normal myocardial scintigraphy in patients with symptoms of coronary artery disease. PLoS ONE. 2010;5:e14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng JL, Lu L, Hu J, Zhang RY, Zhang Q, Chen QJ, Shen WF. Increased serum YKL‐40 and C‐reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis. 2010;210:590–595. [DOI] [PubMed] [Google Scholar]
- 43. Ridker PM, Chasman DI, Rose L, Loscalzo J, Elias JA. Plasma levels of the proinflammatory chitin‐binding glycoprotein YKL‐40, variation in the chitinase 3‐like 1 gene (CHI3 l1), and incident cardiovascular events. J Am Heart Assoc. 2014;3:e000897 DOI: 10.1161/JAHA.114.000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS, Kerr K. Net reclassification indices for evaluating risk‐prediction instruments: a critical review NIH Public Access. Epidemiology. 2014;25:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 46. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum Á, Flather MD, Fox KAA; for the GRACE Investigators . Validated prediction model for all forms of acute coronary syndrome. JAMA. 2004;291:2727. [DOI] [PubMed] [Google Scholar]
- 47. Mallikethi‐Reddy S, Briasoulis A, Akintoye E, Afonso L. Novel biomarkers with potential for cardiovascular risk reclassification. Biomarkers. 2017;22:189–199. [DOI] [PubMed] [Google Scholar]
- 48. Rodríguez H, Fisher E, Schuurbiers D. Integrating science and society in European Framework Programmes: trends in project‐level solicitations. Res Policy. 2013;42:1126–1137. [Google Scholar]
- 49. Pepe MS, Fan J, Feng Z, Gerds T, Hilden J. The net reclassification index (NRI): a misleading measure of prediction improvement even with independent test data sets. Stat Biosci. 2015;7:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pepe MS, Janes H, Li CI. Net risk reclassification P values: valid or misleading? J Natl Cancer Inst. 2014;106:dju041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaptoge S, Di Angelantonio E. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High‐sensitivity C‐reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cumulative Number of Outcomes, by Subtype
Table S2. Composite Outcome Hazard Ratios of Standard Predictors When Used Alone and Adjusted for the Rest of the Standard Predictors in the Placebo Group of the CLARICOR Trial
Table S3. Hazard Ratios of YKL‐40 When Used Alone and When Adjusted for Standard Predictors in the Clarithromycin Group of the CLARICOR Trial
Table S4. The Types and Numbers of Correct Predictions Obtained From the Cox Proportional Hazards Model in the Clarithromycin Group of the ClARICOR Trial


