Abstract
Purpose:
Gestational diabetes mellitus (GDM) and preeclampsia are leading causes of mortality and morbidity in mothers and children. High childhood body mass index (BMI) is among their myriad of negative outcomes. However, little is known about the trajectory of the child BMI exposed to GDM and co-occurring preeclampsia from early to mid-childhood. This study examined the independent and joint impact of GDM and preeclampsia on childhood BMI trajectory.
Methods:
A population-based sample of 356 mothers were recruited from OB/GYN clinics in New York. Their children were then followed annually from 18 to 72 months. Maternal GDM and preeclampsia status were obtained from medical records. Child BMI was calculated based on their height and weight at annual visits.
Results:
Hierarchical Linear Modeling was used to evaluate the trajectories of child BMI exposed to GDM and preeclampsia. BMI trajectory by GDM decreased (t-ratio = −2.24, β=.45, 95% CI=−.05-.95, p = .07), but the trajectory by preeclampsia increased over time (t-ratio = 3.153, β=.65, 95% CI=.11-1.18, p = .002). Moreover, there was a significant interaction between the two (t-ratio = −2.24, β=−1.244, 95% CI=.15-2.33, p = .02), such that the BMI of children born to mothers with both GDM and preeclampsia showed consistent increases over time.
Conclusions:
GDM and preeclampsia could be used as a marker for childhood obesity risk and the identification of a high-risk group, providing potential early intervention. These findings highlight the importance of managing obstetric complications, as an effective method of child obesity prevention.
Keywords: gestational diabetes mellitus, preeclampsia, childhood obesity, body mass index, growth trajectory, prenatal origin of childhood obesity
Childhood obesity, defined as body mass index (BMI) at or above the 95th percentile, is a growing problem; the United States has observed significant trend increases from 1999-2000 through 2015-2016 [1]. Recent estimates of its prevalence are approximately 17-19%, affecting approximately 13.7 million youth [1]. Risk factors for childhood obesity include early life BMI and lifestyle factors (e.g., sleep duration, lack of exercise, and poor diet) [2]. Less is known about the implication of prenatal maternal factors such as gestational diabetes mellitus (GDM) and preeclampsia, which are often observed in women with greater pre-pregnancy BMI [3].
GDM and preeclampsia are serious and pervasive obstetric complications. Recently, prevalence rates for GDM and preeclampsia have risen due to lifestyle changes [4], including later pregnancy age, sedentary lifestyle, and increased fast food consumption. GDM is a condition in pregnancy characterized by carbohydrate intolerance [5]. GDM affects an estimated 4.6 to 9.2% of pregnancies in the United States [6] with some reports showing a higher prevalence in minority women [7]. Preeclampsia is another serious disorder in pregnancy, accompanied by new-onset of hypertension and proteinuria after the 20th week of gestation or near term [8]. Given that the two conditions often co-occur, some studies have consider GDM as a risk factor for preeclampsia [9].
Consequences of GDM and preeclampsia on child health have been well documented. GDM increases the risk for spontaneous abortion, fetal death [10], abnormal birthweight, and malformations [11]. Recent work, however, has revealed that the effects of GDM are not only limited to the pre- and neo-natal period, but have a myriad of lasting impacts that persist into later childhood, including neurodevelopmental deficits [12], neuropsychiatric morbidities [12–13], physical health outcomes, including metabolic syndrome [14], type 2 diabetes (T2DM), and obesity [12, 15].
Metabolic syndrome and its sequelae is one of the most notable consequences of GDM [16]. Metabolic syndrome – which predisposes an individual to cardiac disease and T2DM – refers to an array of conditions including hypertension, hyperglycemia, large waist circumference, and low HDL cholesterol [17]. Mechanistically, GDM is believed to impact metabolic imprinting, such that it alters the metabolic milieu and escalates the risk for T2DM among the offspring and for obesity in childhood and in adolescence [18–19]. Moreover, pregnancies complicated by GDM may result in excess glucose that goes in the fetal circulation, leading to macrosomia [20]. As such, the putative fate of offspring born to mothers with GDM is thought to be high BMI and a greater chance of developing metabolic syndrome. Hyperglycemia due to GDM has been reported to increase risk for obesity in children at age 5-7 years-old, although treatment greatly attenuated the risk [18]. To date, studies examining maternal GDM’s effect on offspring growth trajectory have been sparse and largely inconsistent. Nevertheless, it is notable that infants born to mothers with GDM can either be small for gestational age, normal birth weight, or macrosomic [21–23] depending on the degree of glycemic control [24], medical comorbidity, and maternal pre-pregnancy weight. Given these findings, GDM may not be the sole determinant to the increased risk of macrosomia and subsequent high BMI or obesity; other neonatal complications that are present in the pregnancy can also play a role [10].
Similarly, severe preeclampsia is associated with multitudinous biomedical problems, including hypertension, proteinuria, eclampsia, neurocognitive dysfunction, liver damage, pulmonary edema, and diabetes mellitus [24]. Fatalities resulting from these symptoms are not limited to mothers, but may extend to the child/fetus [25–26]. The primary consequence of preeclampsia on the fetus is malnourishment via utero-placental vascular insufficiency hypoxia, which restricts nutrient and oxygen supplies from the placenta to the fetus [27]. Subsequently, this leads to various perinatal and neonatal problems, including fetal growth restriction (FGR) [27–29], emergency C-section [29], reduced birth weight [29], and increased acute respiratory distress syndromes postnatally [28]. Preeclampsia has historically been considered a predictor for later maternal metabolic syndrome [30], but recent evidence shows that its effects extend to the offspring, as individuals born to mothers with preeclampsia exhibit increases in blood pressure [31–33]. Although the long-term health and developmental consequences of exposure to maternal preeclampsia for the surviving child are relatively unexplored, there is evidence for suboptimal neurocognitive development in addition to FGR, an increase in BMI [34], and childhood obesity [35–36] among infants of mothers with preeclampsia.
Despite the growing frequency of comorbid GDM and preeclampsia [37–38], to date, little research has examined the consequences of GDM and preeclampsia on child health simultaneously, especially with obesity. Among the limited existing work, Kvehaugen and colleagues reported that pregnancies complicated by both GDM and preeclampsia compared to uncomplicated pregnancies resulted in a higher proportion of offspring that were overweight at ages 5–8, but group differences did not reach significance [39].
Because the increased prevalence of comorbidity for GDM and preeclampsia coincides with the greater occurrence of childhood obesity in recent years, it becomes increasingly important to examine the growth trajectory of infants exposed to GDM and preeclampsia solely as well as jointly throughout development for early detection and prevention. Yet, there is a conspicuous paucity of work in this area, with most studies being cross-sectional or had follow-up periods without including early and mid-childhood. As both GDM and preeclampsia are known risk factors for suboptimal child development, it is valuable to evaluate the degree to which those conditions collectively influence BMI developmental trajectory among children of mothers with the two conditions. As such, the goals of the study are: 1) to investigate the major effect of GDM and preeclampsia on the trajectory of child BMI between ages 18 and 72 months, and 2) to further evaluate whether the trajectory of BMI by GDM is moderated by preeclampsia. It was hypothesized that a) GDM status would influence child BMI, such that children born to mothers with GDM would have higher BMI as they grow than their counterpart, and b) there would be a substantially steeper trajectory of linear increase in BMI among offspring of mothers with both GDM and preeclampsia.
Method
The current longitudinal investigation was based on 356 mother-child dyads contacted for annual follow-up. Mothers were originally recruited from prenatal clinics in metropolitan New York. Exclusion criteria included multiple pregnancy, significant congenital anomalies, neurological dysfunction, fetal chromosomal anomalies, and HIV positivity. Their children were then invited to the lab for annual assessments. Details of the full cohort can be found elsewhere [40]. From the total sample, 302 (52.3% boys; 47.7% girls) had information on both obstetric complications including GDM (n=26), preeclampsia (n=24) and multiple assessments. BMI data was assessed at a maximum of 6 time points (18, 24, 36, 48, 60, and 72 months). Because participants came in for their assessments as they aged, sample sizes for each assessment time differed: there were 76 children at 18 months, 218 at 24 months, 162 at 36 months, 121 at 48 months, 50 at 60 months, and 20 at 72 months.
Measures
Child Growth Measures
Height and weight were measured during each assessment by a research staff member without knowledge of the mother’s obstetric complication status. For height, the child was asked to stand in front of the growth chart with his/her back straight and feet against the wall. Height was collected by measuring the line that the child’s head reached and was recorded in centimeters (cm). For weight, the child was asked to step on the scale barefoot facing outwardly, and weight was collected and recorded in kilogram (kg). BMI was then calculated using the following formula:
Gestational Diabetes Mellitus (GDM) status
GDM was defined as glucose intolerance with the first onset during pregnancy, determined by a glucose tolerance test through the woman’s medical practitioner, and ascertained through medical record review throughout pregnancy (no=0, yes=1).
Preeclampsia status
Preeclampsia was determined from the obstetric record via participant medical chart review prospectively during pregnancy (no=0, yes=1). Defined as having high blood pressure (140/90mm Hg) and proteinuria (>300 mg via 24-hour urine collection) after the 20th week of pregnancy.
Demographics/covariates
Maternal demographic information including age, education, and parity, were collected via self-administered interview. Information on sex, birthweight (BW), gestational age (GA), and body length in centimeter of the child was collected by a nurse at delivery. Ponderal index was calculated using birthweight and body length at birth [(birthweight x 100) ÷ (birth length)3]. Demographics of the sample can be found in Table 1.
Table 1R.
Maternal and child demographics and obstetric characteristics, and body mass index (BMI) in participants (N=302)
| Maternal characteristics | Mean (SD) |
|---|---|
| Age at child’s birth (years) | 27.74 (6.07) |
| Pre-pregnancy BMI | 26.13 (6.13) |
| Educational attainment, N (%) | |
| Elementary school | 8 (2.6) |
| Some high school | 36 (11.9) |
| High school diploma/ GED | 65 (21.5) |
| Some college | 81 (26.8) |
| Associate degree | 34 (11.3) |
| Bachelor’s degree | 44 (14.6) |
| Graduate degree | 34 (11.3) |
| Marital status, N (%) | |
| Married | 124 (41.0) |
| Common law marriage | 16 (5.3) |
| Single | 160 (53.0) |
| Divorced/Separated | 2 (0.7) |
| Race, N (%) | |
| White | 53 (17.5) |
| Black | 66 (21.9) |
| Hispanic | 153 (50.7) |
| Asian | 25 (8.3) |
| Others | 5 (1.7) |
| Substance use during pregnancy, N (%) | |
| Cigarette | 34 (11.3) |
| Cannabis | 20 (6.6) |
| Alcohol | 19 (6.3) |
| Other substances | 15 (5.0) |
| Biomedical illness, N (%) | |
| Gestational diabetes myelitis | 26 (8.6) |
| Preeclampsia | 24 (7.9) |
| Child characteristics | Mean (SD) |
| Birth outcomes | |
| Birthweight (grams) | 3,224.68 (607.38) |
| Gestational age (weeks) | 38.78 (2.18) |
| Ponderal index | 25.89 (9.23) |
| Fetal growth, N (%) | |
| Small for gestational age | 24 (8.9) |
| Normal for gestational age | 224 (82.6) |
| Large for gestational age | 23 (8.5) |
| NICU admission, N (%) | 40 (13.24) |
| Gender, N (%) | |
| Male | 158 (52.3) |
| Female | 144 (47.7) |
| Body Mass Index (BMI) | Mean (SD) |
| 18 months | 18.33 (2.14) |
| 24 months | 18.01 (2.16) |
| 36 months | 16.63 (1.55) |
| 48 months | 16.43 (1.99) |
| 60 months | 16.21 (2.13) |
| 72 months | 15.63 (1.71) |
NB: N may vary due to missing values
Statistical Analyses
Hierarchical linear modeling (HLM) was selected to assess how GDM and preeclampsia influenced changes in child BMI and their trajectories. This was followed by the model with GDM, preeclampsia, and interaction of the two. Age was centered at 18 months, meaning that the intercept represented the average BMI when children were 18 months-old. The Level-1 Model was designed to characterize the trajectories (both linear and quadratic) of BMI changes across six time points ranging from 18 to 72 months. All models in the analysis were corrected for non-normal distributions of level 2 residuals by applying the full maximum likelihood estimation with robust standard errors [41].
Model 1: Change in BMI over time without predictors
Model 1 was designed to characterize the trajectories of BMI across 6 time points. We first tested a model of linear change (a). As BMI may not display a linear change, we tested for curvilinearity in the linear trajectory for BMI by adding a quadratic term for age to the model (b). Furthermore, test of relative model fit was computed by comparing the deviance statistics of both the linear and quadratic models (Table 2). The quadratic model was retained if it yielded a significant reduction in deviances according to the Chi-square difference test. In Model 1a, BMI is a function of an intercept plus a linear effect for age. In Model 1b, BMI is a function of an intercept plus a linear and curvilinear effects for age. The model equations are as follows:
Table 2.
Model comparisons with X2 deviance score in the model with degrees of freedom and associated p-value
| Linear model (a) X2 deviance (df) | Quadratic model (b) X2 deviance (df) | ΔX2 (Δdf), p-value (within Models 1 or 2) | |
|---|---|---|---|
| Model 1 | 1945.56 (6) | 1927.81 (10) | 17.75 (4), p = .0013 |
| Model 2 | 1891.98 (25) | 1869.79 (39) | 22.19 (14), p = .075 |
| ΔX2 (Δdf), p-value (Models 1 vs 2) | 53.58 (19), p < .0001 | 58.02 (29), p = .001 | |
NB: ΔX2 (Δdf), p-value for Model 1b vs Model 2a was X2(15) = 35.83, p =.002. Model 2a was selected as the best model.
Linear Model (Model 1a):
Level-1
Level-2
Quadratic Model (Model 1b):
Level-1
Level-2
Model 2: Predictors of intercepts and slopes
We examined whether GDM and preeclampsia, and their interaction explained significant variance in mean intercept or slope of child BMI. If BMI displayed neither linear nor quadratic change over time, predictors were added to calculate the main effects only models. Child sex, BW, GA, marital status, maternal age, maternal education, and parity were included as covariates in modeling the predictors of change in BMI.
Linear Model (Model 2a):
Level 1
Level 2
Quadratic Model (Model 2b):
Level 1
Level 2
Missing data
HLM provided a robust method of dealing with the missing data and yields parameter estimates for missing time points for dependent variable data (BMI) at level 1 (i.e., within subject variability) but not for predictor variables at level 2 (i.e., between subject variability). Rather than removing a portion of the sample by using repeated-measures analysis, we leveraged this central methodological strength of HLM and generated estimates for missing data at certain time points. There were no missing data at level 2.
Results
Model selection
We modeled BMI as a function of the intercept with the linear and quadratic effect of age to explore whether the mean intercepts (BMI at 18 months) or slopes (rate/direction of change of BMI over time) differ between offspring of mothers with the obstetric risks (GDM and preeclampsia) and without them. We built four models and chose our best fitted model. Changes indices for model fit for the two models (Model 1 and Model 2) in two growth trajectories (linear and quadratic) are listed in Table 2.
We first tested our intercept only model (Model 1) with a linear (a) vs. quadratic (b) slope. Model 1a predicted a β1 of −.57 (95% CI −.73, −.41, p <.001, t-ratio = −6.68) with an X2 deviance score of 1945.56 with a degree of freedom of 6. Model 1b predicted a β1 (linear slope) of −1.20 (95% CI −1.60, −.80, p <.001, t-ratio=−5.99) and a β2 (quadratic slope) of .04 (95% CI .10, .26, p<.001, t-ratio=3.96) with a X2 deviance score of 1927.81 with a degree of freedom of 10. As seen in Table 2, this indicates that the model with a quadratic term to predict BMI is significantly better than the model with only a linear term) [X2(4) = 17.75, p=.001]. Figure 1 shows our preferred model (Model 1b).
Fig 1.
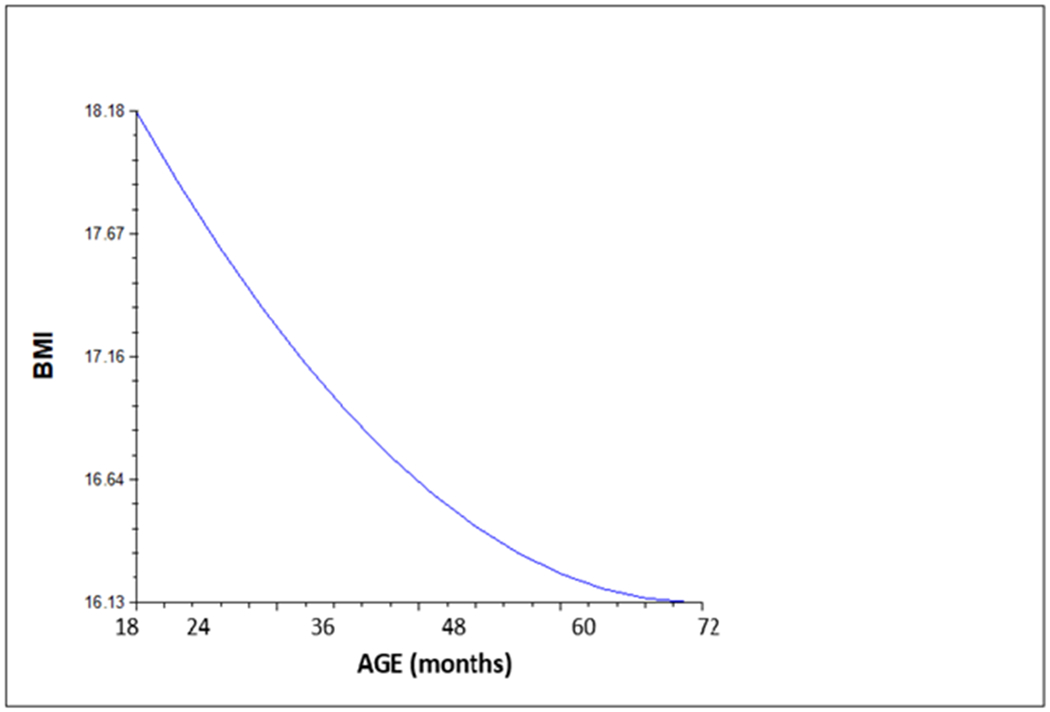
Growth trajectory of child BMI between 18 and 72 months – Intercept only model with curvilinear growth (Model 1b)
Following Model 1, we tested Model 2 with intercept and predictors (GDM, preeclampsia, and the interaction) in the linear model (Model 2a) and quadratic model (Model 2b). Model 2a predicted a X2 deviance score of 1891.98 with a degree of freedom of 25, whereas the quadratic model predicted a X2 deviance score of 1869.79 with a degree of freedom of 39. Since Model 2b was found to be only marginally [X2(14)=22.19, p=.075] better than Model 2a, we chose Model 2a as a better model, presented in Figure 2. Finally, between Model 1b and Model 2a, Model 2a was selected as the final model because it was significantly better fitted [X2(15)=35.83, p=.002].
Fig 2.
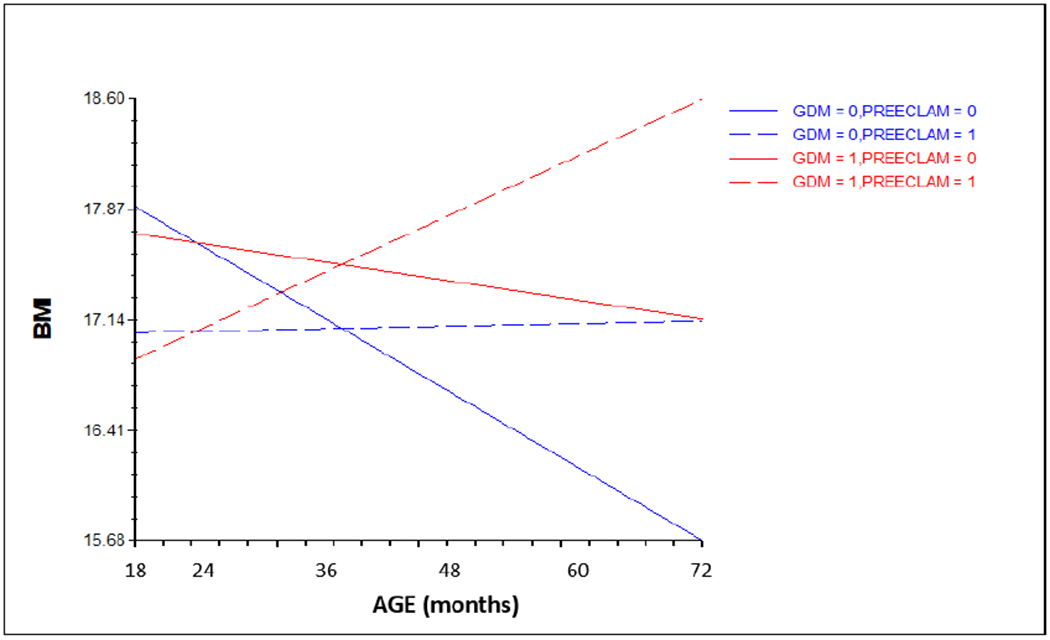
Growth trajectory of child BMI between 18 and 72 months of age – Intercept and predictors (GDM, preeclampsia, and the interaction of the two) (Model 2a – linear model)
NB: BMI = body mass index
0 = absence; 1 = presence
Trajectories of BMI predicted by GDM, preeclampsia, and the interaction in our final model Our final model (Model 2a) with an intercept and predictors (GDM, preeclampsia, and the interaction) shows that there were no significant effects of GDM (β=−.014, 95% CI −1.33, 1.30, p=.75, t-ratio=−.31), preeclampsia (β=−.84, 95% CI −1.94, .17, p=.14, t-ratio=−1.47), and the interaction of the two (β=.56, 95% CI −2.87, 3.79, p=.74, t-ratio=.54) in predicting intercept for BMI. However, preeclampsia (β=.65, 95% CI .11, 1.19, p=.02, t-ratio=3.15) and the interaction of the two (β=−1.24, 95% CI −2.33, −.15, p=.02, t-ratio=−2.24) were significant and GDM (β=.45, 95% CI −.05, .95, p=.07, t-ratio = 1.79) was marginally significant in the linear model. Figure 2 shows the significant interaction between GDM and preeclampsia, where BMI of children born to mothers with both GDM and preeclampsia steadily increased over time whereas BMI of children with only GDM and only preeclampsia slowly decreased over time, and BMI of children with neither GDM nor preeclampsia decreased more over time.
Discussion
The current study has two main findings: First, children from pregnancies complicated by preeclampsia are more likely to have significantly greater childhood BMI. The pattern is the same with GDM, but it was only marginally significant. Second, comorbid of GDM and preeclampsia had the greater chance and upward trajectory of having greater BMI as they grow. Overall, our findings were consistent with prior reports demonstrating associations between GDM and an increased risk for childhood obesity later in childhood [18]. The study also extended our knowledge by providing initial evidence that children of mothers with both GDM and preeclampsia had a greater propensity of obesity as evidenced by a significant and upward BMI trajectory. Interestingly, children born to mothers with preeclampsia only had relatively stable BMI across the examined time period, albeit significantly higher than children born from healthy mothers. Fetuses of mothers with preeclampsia may have had to develop in the womb with less blood flow, potentially meaning their bodies would have to do more with less means. As they grow up, their bodies may be used to not having as much, and thus hold onto extra weight more efficiently. Alternatively, the effects of increasing trajectory in preeclampsia only may not emerge until later ages when adiposity rebound occurs. While the BMI we have observed during this period did not reach the alarming level of childhood obesity, it is important to see the longer term patterns of BMI changes among children whose mothers had biomedical complications such as GDM and preeclampsia, which are known to influence endocrine and adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis functioning [45].
Prior studies have looked at both obstetric risks independently, but to the best of our knowledge, this is the first study to examine the combination of both GDM and preeclampsia on child BMI, which are often co-occurring obstetric conditions. Indeed, the presence of either complication has impacts on child health, but we illustrate that their co-occurrence substantially increases child BMI trajectory. Moreover, we covered a longer period of growth trajectory (e.g., 18-72 months). Based on our results, having GDM or preeclampsia does affect child BMI trajectory to some extent, but the combination of the two is especially effectual in driving higher child BMI. The present findings have important implications for maternal health in pregnancy and later childhood health outcomes.
The current study also has limitations. First, the study has a relatively small sample size. As prevalence for GDM and preeclampsia was 12% and 18% respectively, with 7 cases having both diagnoses, cases with positive diagnoses were small. Thus, our results should would be interpreted with caution. However, it is known that statistical strategy with repeated measures increases statistical power. While preliminary, our findings provide guidance for future studies with a larger sample size. Second, there was no information on GDM such as the level of glycemic control (e.g., A1C levels) and preeclampsia (type and severity) during pregnancy, as well as information on whether or not mothers with the condition underwent treatment or intervention. Evidence suggests that glycemic control can impact offspring weight [43]. Third, there was no data on child diet and physical activity. Dietary intake and physical activity level play a role in weight changes during childhood and adolescence [44]. Even as early as infancy, intensive breastfeeding from birth to 12 months has been found to be associated with lower weight gain and slower ponderal growth in children born to mothers with GDM [45]. Fourth, BMI measurements in our study were based on height and weight measured by the same equipment by two research staff in order to avoid errors due to the measurements by different equipment. However no other measurement methods (e.g., calipers or 3D body imaging) were used to increase the validity of the BMI measure. Relying on one method may have reduced the validity of the BMI scores. Taken together, future work would benefit with obtaining information on those factors, including the influence of glycemic control, management and treatment of obstetric complications, child diet or activity level, and collect height and weight measures with a minimum of two types of equipment.
Despite these caveats, the present findings from this research help us better understand the effect of maternal GDM and/or preeclampsia on subsequent child BMI. This is the first longitudinal investigation that has examined the role of both GDM and preeclampsia on child BMI simultaneously at multiple follow-up assessments. When possible, future studies should opt to design longitudinal investigations to replicate our longitudinal findings to help researchers confirm at what age the effects of obstetric complications emerge in children and their developmental trajectory. Given our conclusion that GDM and preeclampsia could be used as a marker for childhood weight problems (overweight and obesity) and the identification of high-risk children, expectant mothers and health professionals should monitor patients and their offspring more closely for a longer period of time even after the birth, if their pregnancies are complicated by these two conditions. For example, prescription Aspirin of 150 milligrams daily from 11 up till 36 weeks gestation substantially decreases the risk of child obesity up until 72 months of age [46]. Because GDM and preeclampsia are common and manageable obstetric risks, it is hoped that gaining more knowledge on its long-term impact can inform and encourage individuals to acknowledge the importance of their management and treatment during pregnancy as one of the most cost-effective methods of childhood obesity prevention.
Acknowledgements:
The authors would like to the families for their participation, the Stress in Pregnancy laboratory staff at Queens College CUNY (especially Jackie Finik, Westar Zong, and Victoria Kuo), and the staff at the prenatal clinics and OB/GYN departments of Icahn School of Medicine at Mount Sinai and New York Presbyterian-Queens Hospital.
This research work was supported by the National Institute of Mental Health under award number R01MH102729 and the Professional Staff Congress City University of New York grant to Y Nomura. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This study was funded by the National Institute of Mental Health under award number R01MH102729 and the Professional Staff Congress City University of New York grant to Y Nomura.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research board committee of the City University of New York and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Hales CM, Carroll MD, Fryar CD et al. (2017) Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288:1–8. [PubMed] [Google Scholar]
- 2.Reilly JJ, Armstrong J, Dorosty AR et al. (2005) Early life risk factors for obesity in childhood: cohort study. Bmj 330(7504):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer-Graf UM, Pawliczak J, Passow D et al. (2005) Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 28(7):1745–1750. [DOI] [PubMed] [Google Scholar]
- 4.Zhou T, Sun D, Li , et al. (2018) Prevalence and Trends in Gestational Diabetes Mellitus among Women in the United States, 2006–2016. Diabetes 67(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ACOG Committee on Obstetric Practice (2018) ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 131(2):e49–64. [DOI] [PubMed] [Google Scholar]
- 6.Dornhorst A, Paterson CM, Nicholls JSD et al. (1992) High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med 9(9):820–825. [DOI] [PubMed] [Google Scholar]
- 7.Williams D (2011) Long-term complications of preeclampsia In: Seminars in Nephrology, vol 31, no 1 WB Saunders, Philadelphia, pp 111–122. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee on Obstetric Practice (2019) ACOG Practice bulletin No. 202: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 133(1):e1–25.30575675 [Google Scholar]
- 9.Ornoy A (2011) Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32(2):205–212. [DOI] [PubMed] [Google Scholar]
- 10.Mitanchez D (2010) Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 36(6):617–62. [DOI] [PubMed] [Google Scholar]
- 11.Farahvar S, Walfisch A, Sheiner E (2019) Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab 14(1):63–74. [DOI] [PubMed] [Google Scholar]
- 12.Nomura Y, Marks DJ, Grossman B et al. (2012) Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention- deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med 166(4):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clausen TD, Mathiesen ER, Hansen T et al. (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31(2):340–346. [DOI] [PubMed] [Google Scholar]
- 14.Abokaf H, Shoham-Vardi I, Sergienko R et al. (2018) In utero exposure to gestational diabetes mellitus and long term endocrine morbidity of the offspring. Diabetes Res Clin Prac 144:231–5. [DOI] [PubMed] [Google Scholar]
- 15.Reece EA, Leguizamón G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373(9677):1789–1797. [DOI] [PubMed] [Google Scholar]
- 16.Cornier MA, Dabelea D, Hernandez TL et al. (2008) The metabolic syndrome. Endocr Rev 29(7):777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillman MW, Rifas-Shiman S, Berkey CS et al. (2003) Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111(3): e221–e226. [DOI] [PubMed] [Google Scholar]
- 18.Hillier TA, Pedula KL, Schmidt MM et al. (2007) Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 30(9):2287–2292. [DOI] [PubMed] [Google Scholar]
- 19.Kamana KC, Shakya S, Zhang H (2015) Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66(Suppl. 2):14–20. [DOI] [PubMed] [Google Scholar]
- 20.Ergaz Z, Avgil M, Ornoy A (2005) Intrauterine growth restriction—etiology and consequences: what do we know about the human situation and experimental animal models? Reprod Toxicol 20(3):301–322. [DOI] [PubMed] [Google Scholar]
- 21.Barker DJ, Hales CN, Fall CHD et al. (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36(1):62–67. [DOI] [PubMed] [Google Scholar]
- 22.Hod M, Diamant YZ (1992) The offspring of a diabetic mother--short-and long-range implications. Isr J Med Sci 28(2):81–86. [PubMed] [Google Scholar]
- 23.Ghulmiyyah L, Sibai B (2012) Maternal mortality from preeclampsia/eclampsia. Semin in Perinatol 36(1):56–59. [DOI] [PubMed] [Google Scholar]
- 24.Habli M, Levine RJ, Qian C et al. (2007) Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. AJOG 197(4):406 e1–7. [DOI] [PubMed] [Google Scholar]
- 25.Masoura S, Kalogiannidis I, Margioula-Siarkou C et al. (2012) Neonatal outcomes of late preterm deliveries with pre-eclampsia. Minerva Ginecologica 64(2):109–115. [PubMed] [Google Scholar]
- 26.Powe CE, Ecker J, Rana S et al. (2011) Preeclampsia and the risk of large-for-gestational-age infants. AJOG 204(5):425 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramham K, Briley AL, Seed P et al. (2011) Adverse maternal and perinatal outcomes in women with previous preeclampsia: a prospective study. AJOG 204(6):512 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saadat M, Nejad SM, Habibi G et al. (2007) Maternal and neonatal outcomes in women with preeclampsia. Taiwan J of Obstet & Gynecol 46(3):255–9. [DOI] [PubMed] [Google Scholar]
- 29.Forest JC, Girouard J, Massé J (2005) Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet & Gynecol 105(6):1373–1380. [DOI] [PubMed] [Google Scholar]
- 30.Palti H, Rothschild E (1989) Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev 19(4):263–269. [DOI] [PubMed] [Google Scholar]
- 31.Seidman DS, Laor A, Gale R et al. (1991) Pre‐eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17‐years‐of‐age. BJOG 98(10):1009–1014. [DOI] [PubMed] [Google Scholar]
- 32.Tenhola S, Rahiala E, Martikainen A et al. (2003) Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. The J of Clin Endocrinol & Metab 88(3):1217–1222. [DOI] [PubMed] [Google Scholar]
- 33.Davis EF, Lazdam M, Lewandowski AJ et al. (2012) Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129(6):e1552–e1561. [DOI] [PubMed] [Google Scholar]
- 34.Bos AF, Einspieler C, Prechtl HF (2001) Intrauterine growth retardation, general movements, and neurodevelopmental outcome: a review. Developmental Medicine & Child Neurology 43(1):61–68. [DOI] [PubMed] [Google Scholar]
- 35.Tolsa CB, Zimine S, Warfield SK et al. (2004) Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56(1):132–138. [DOI] [PubMed] [Google Scholar]
- 36.Xiong X, Saunders LD, Wang FL et al. (2001) Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J of Gynecol & Obstet 75(3):221–8. [DOI] [PubMed] [Google Scholar]
- 37.Li LJ, Aris IM, Su LL et al. (2018) Effect of gestational diabetes and [Google Scholar]; hypertensive disorders of pregnancy on postpartum cardiometabolic risk. Endocrin Connect 7(3):433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kvehaugen AS, Andersen LF, Staff AC (2010) Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstetricia et Gynecologica Scandinavica 89(11):1478–1485. [DOI] [PubMed] [Google Scholar]
- 39.Finik J, Nomura Y (2017) Cohort profile: stress in pregnancy (SIP) study. Int J of Epidemiol 46(5):1388–1388k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maas CJ, Hox JJ (2004) The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Comput Stat & Data Anal 15;46(3):427–40. [Google Scholar]
- 41.Rolland-Cachera MF, Deheeger M, Bellisle F et al. (1984) Adiposity rebound in children: a simple indicator for predicting obesity. Am J of Clin Nutr 39:129–135. [DOI] [PubMed] [Google Scholar]
- 42.Langer O, Levy J, Brustman L et al. (1989) Glycemic control in gestational diabetes mellitus-how tight is tight enough: small for gestational age versus large for gestational age? AJOG 161(3):646–653. [DOI] [PubMed] [Google Scholar]
- 43.Berkey CS, Rockett HR, Field AE et al. (2000) Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 105(4):e56–e56. [DOI] [PubMed] [Google Scholar]
- 44.Gunderson EP, Greenspan LC, Faith MS et al. (2018) SWIFT Offspring Study Investigators. Breastfeeding and growth during infancy among offspring of mothers with gestational diabetes mellitus: a prospective cohort study. Pediatr Obes 13(8):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miehle K, Stepan H, Fasshauer M (2012) Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre‐eclampsia. Clin Endocrinol 76(1):2–11. [DOI] [PubMed] [Google Scholar]
- 46.O’Gorman N, Wright D, Rolnik DL et al. (2016) Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE). BMJ open.6(6):e011801. [DOI] [PMC free article] [PubMed] [Google Scholar]


