Abstract
Models of anorexia nervosa (AN) posit that symptoms are maintained through deficient reward and enhanced punishment processing. However, theoretical and empirical inconsistencies highlight the need for a more nuanced conceptualization of this literature. Our goal was to comprehensively review the research on reward and punishment responding in AN from a cue-specific lens to determine which stimuli evoke or discourage reward and punishment responses in this population, and, ultimately, what properties these rewarding and punishing cues might share. A systematic review interrogating reward and punishment responses to specific cues yielded articles (n = 92) that examined responses to disorder relevant (e.g., food) and irrelevant (e.g., money) stimuli across self-report, behavioral, and biological indices. Overall, in most studies individuals with AN exhibited aversive responses to cues signaling higher body weights, social contexts, and monetary losses, and appetitive responses to cues for weight loss behaviors and thinness. Findings were more mixed on responses to palatable food and monetary gains. Results highlight that reward and punishment responding in AN are context specific and may be affected by varied stimulus qualities (e.g., predictability, controllability, delay, effort). Increasing specificity in future research on reward and punishment mechanisms in AN will better inform development of precisely-targeted interventions.
Keywords: anorexia nervosa, eating disorders, reward, punishment, appetitive, aversive
Anorexia nervosa (AN) is a serious eating disorder characterized by elevated physical and psychological morbidity (Braun, Sunday, & Halmi, 1994; Mitchell & Crow, 2006) and a premature mortality rate as high as any other psychiatric disorder (Crow et al., 2009). Although family-based therapy has strong empirical support for treating adolescents with AN, less than half receiving this approach achieve remission (Lock & Le Grange, 2019). Further, no specific treatment has been identified as superior for adults with AN and treatment outcomes are often poor in this population (Murray, Quintana, Loeb, Griffiths, & Le Grange, 2019). As a result, AN recovery rates are low (Ackard, Cronemeyer, Richter, & Egan, 2015) and even when healthy weight restoration is achieved, psychological symptoms often endure (Murray et al., 2019).
Many theoretical models have been proposed to account for the complexity and persistence of AN (Pennesi & Wade, 2016). However, these models have not resulted in the development of treatments that achieve greater rates of long-term remission. This may be due to a lack of precision in the field’s knowledge of the basic psychological and neurobiological mechanisms implicated in this disorder (Walsh, 2013), which is an area of research that is rapidly expanding (Frank, Shott, & DeGuzman, 2019). In order to improve upon existing theoretical models and ultimately enhance treatment outcomes, there is a need to better characterize the basic mechanistic processes that contribute to clinical symptoms in AN.
One mechanistic domain that has received increasing attention in relation to AN is reward and punishment processing. Prominent models suggest that AN is characterized by deficits in reward valuation (the ability to identify desirable outcomes warranting approach) coupled with excesses in reward inhibition (the ability to exert cognitive and behavioral control to temper reward pursuit) (Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013; Wierenga et al., 2014). This imbalance is hypothesized to maintain clinical symptoms by simultaneously reducing orientation towards food rewards and allowing strict control over intake. In contrast, punishment processes are relatively under-investigated in AN; however, existing theories posit that enhanced punishment sensitivity maintains AN symptoms through avoidance of the perceived aversive properties of food and weight gain (Harrison, O’Brien, Lopez, & Treasure, 2010). These more generalized perspectives on reward and punishment in AN have been helpful in promoting research in this area, but fail to account for the potential heterogeneity in responding based on specific features of rewarding or punishing cues.
The word “cue” or “stimulus” is generally used to refer to any object or experience that can serve as a response probe by signaling the availability of reward or punishment (e.g., food cues can signal the potential for reward through satiety or punishment through weight gain). Although the outcome, rather than the cue itself can be considered the reward or punishment, there is evidence that antecedent conditions signaling reward or punishment can develop an appetitive or aversive value themselves over time (Oleson, Gentry, Chioma, & Cheer, 2012; Schultz, Dayan, & Montague, 1997); therefore, the stimulus itself can develop reward value over time. Notably, investigations of generalized reward deficits or punishment surpluses in the AN literature without explicit consideration of the cues used to evoke these responses may contribute to an incomplete and inconsistent body of literature for several reasons.
First, from a behavioral perspective, reward (used here synonymously with reinforcement) is the process by which any behavior is perpetuated, and punishment and/or non-reward (e.g., extinction) are the processes by which a behavior is diminished (Skinner, 1976). Models of psychopathology predicated upon widespread deficits in reward and surpluses in punishment responding implicate a near absence of all behavior (such as is often seen in depressive episodes), given that punishment results in behavioral dissolution and the ability to pursue reward is necessary for the initiation and continuation of any behavior. However, AN is associated with certain extremely motivated behavioral excesses (e.g., driven behavior towards weight loss) that are insensitive to consequences most would consider punishing, such as hunger and fatigue (Guarda, Schreyer, Boersma, Tamashiro, & Moran, 2015). There is also a robust link between AN and perfectionism, which promotes persistence in goal-directed activities not directly related to the eating disorder (Lloyd, Yiend, Schmidt, & Tchanturia, 2014). This suggests that individuals with AN may be sensitive to rewards associated with certain actions (e.g., weight loss, perfectionistic behaviors). Thus, reward and punishment processes in AN may reflect nuanced patterns that many studies in this area have not been designed to examine.
Second, it is well documented that there are individual differences in the reward or punishment value of any stimulus, such that experiences that outwardly seem aversive (e.g., physical pain) can be experienced as rewarding (e.g., through alleviation of tension; Iwata, Dorsey, Slifer, Bauman, & Richman, 1994) and, likewise, experiences that are ostensibly positive (e.g., praise) can be experienced as punishing (e.g., due to discomfort with directed attention; Dozier, Iwata, Thomason-Sassi, Worsdell, & Wilson, 2012). However, many of the standard paradigms used to probe reward and punishment responsivity implicitly assume a universal value to certain cues (e.g., food, money), despite evidence that myriad factors alter their desirability. This assumption of fixed stimulus value can at times yield incorrect or imprecise conclusions about general reward and punishment processing capabilities. For instance, monetary delay discounting tasks are frequently used to infer heightened impulsivity surrounding reward pursuit (Lempert, Steinglass, Pinto, Kable, & Simpson, 2018). However, there is evidence that this measure is sensitive to factors such as income, as immediate receipt of money is more valuable to individuals in greater financial need. This quality has sometimes led to incorrect conclusions about reward pursuit between economically disparate groups (da Matta, Gonçalves, & Bizarro, 2012). For this reason, there is a need for careful examination of which cues have been utilized to infer underlying reward and punishment processes in AN to determine whether the cue features or underlying reward or punishment processing drive observed effects.
Third, models of reward and punishment focusing on overarching deficits and surpluses may contribute to an inconsistent body of results due to overlooking heterogeneity of context and, thereby, direct investigation away from important lines of inquiry. However, such inconsistencies also could be usefully leveraged to identify qualitative similarities in the cues that are or are not associated with reward or punishment responses in AN. This approach would promote an empirical shift from investigating whether there are problems with reward or punishment responding in AN to examining what signals reward or punishment, and ultimately why or how certain stimuli are associated with reward or punishment expectancies in AN. Such a perspective is especially important when considering the maintaining factors associated with AN; whereas, more global processes can be useful in identifying and modifying risk factors in a broad population, targeting causal factors demands greater mechanistic precision.
For these reasons, in this paper we review the reward and punishment literatures in AN through the lens of identifying cue-specific (versus generalized) differences in these processes, and begin to posit the stimulus qualities that might distinguish reward versus punishment responses to particular cues in AN. In line with these aims, the manuscript includes a: (1) brief overview of the research on generalized reward and punishment abnormalities in AN; (2) systematic review of the cue-specific literature on reward and punishment abnormalities in AN, focusing on specific stimuli associated with punishment or reward responses; and (3) discussion of the shared qualities that may unite the cues generating reward or punishment responses in AN, with suggestions for how these qualities may inform future research.
Overview of Research on Generalized Reward and Punishment Abnormalities in AN
Models of general reward deficits and punishment excesses in AN have been reviewed previously (e.g., Kaye et al., 2013; Wierenga et al., 2014), and it is beyond the scope of this manuscript to thoroughly detail that literature. However, we provide an abbreviated overview as a foundation for interpreting cue-based findings on reward and punishment in AN.
Self-report Personality/Temperament Assessments
On self-report measures, individuals with AN have consistently endorsed higher harm avoidance or punishment sensitivity, indicating increased awareness of and desire to avoid aversive experiences, compared to non-eating disorder comparison groups (NCs) and, in some cases, those with other eating disorders (Atiye, Miettunen, & Raevuori-Helkamaa, 2015; Glashouwer, Bloot, Veenstra, Franken, & de Jong, 2014). Although the findings related to punishment orientation in AN are consistent, evidence supporting lower self-reported reward sensitivity and responsivity is mixed. Several studies suggest that individuals with AN, especially restricting subtype (AN-R), endorse less responsivity to rewards compared to NCs and those with other eating disorders, at least during acute illness (Atiye et al., 2015). Individuals with AN have often scored lower on measures of sensation-seeking, which capture reactions to immediate novel rewards, compared to NCs and those with bulimia nervosa (BN) or binge eating disorder (BED; Matton, Goossens, Vervaet, & Braet, 2015; Rotella et al., 2018). However, in discrepant research, individuals with AN have scored higher on trait reward measures, indicating increased sensitivity and responsivity to rewards, especially long-term, social, or appearance rewards, compared to NCs and those with other eating disorders (Glashouwer et al., 2014; Jappe et al., 2011; Rotella et al., 2018). These inconsistencies have yet to be reconciled.
Structural and Resting Functional Brain Abnormalities
Neuroimaging data frequently have been referenced to provide support for generalized differences in reward and punishment processing in AN. The brain regions and circuits involved in the processing of reward and punishment are complex and often overlapping (Haber, 2016). However, the structures most commonly cited as involved in the processing of reward salience and learning include the basal ganglia (e.g., ventral and dorsal striatum) and areas of the frontal cortex, such as the orbitofrontal cortex (OFC) (Haber, 2016). Those most frequently implicated in aversive processing include limbic regions (e.g., amygdala, insula), corresponding with certain parts of the frontal cortex (e.g., anterior cingulate cortex [ACC]) (Hartley & Phelps, 2012). Compared to NCs, individuals with AN in different illness stages have been shown to exhibit decreased grey matter volume in reward-related areas, such as the striatum and OFC (Frank, Shott, Hagman, & Mittal, 2013; Titova, Hjorth, Schiöth, & Brooks, 2013) and, in one study, greater grey matter volume in the anterior insula (Frank et al., 2013). However, evidence suggests that grey matter volume normalizes with weight restoration and, therefore, may represent a consequence, rather than cause, of AN symptoms (Bernardoni et al., 2016). Structural alterations in white matter connectivity between reward regions (e.g., striatum and OFC) also have been reported in AN across various illness stages (Cha et al., 2016; Via et al., 2014).
Studies of resting functional connectivity between brain regions have indicated disorganization of neural networks associated with reward in AN, although the direction of the findings has been inconsistent: two studies reported reduced connectivity (Haynos et al., 2018; Kullmann et al., 2014b) and one found increased connectivity between the striatum and prefrontal cortex (PFC; Cha et al., 2016). Other studies have detected aberrant functional connectivity of limbic regions associated with punishment, although the precise nature of these patterns has varied by analysis (Gaudio, Wiemerslage, Brooks, & Schiöth, 2016). Thus, initial data suggest altered organization of brain regions associated with reward and punishment in AN. However, this research is nascent and some findings are mixed. Additionally, both brain structure and function are sensitive to nutritional status independent of the psychopathology of AN (Yoncheva, Castellanos, Pizinger, Kovtun, & St-Onge, 2016; Zamroziewicz, Talukdar, Zwilling, & Barbey, 2017), which presents a common confound to this research.
Neurotransmitter Abnormalities
The role of dopamine in reward salience and learning is well recognized (Wise, 2004) and evidence suggests that the dopamine production may be altered in AN. Several studies have found that weight-restored individuals with AN have more dopamine receptor availability in the ventral striatum (Frank et al., 2005) and reduced production of homovanillic acid, a dopamine metabolite (Kaye, Frank, & McConaha, 1999), compared to NCs. Further, alterations in the serotonergic system, which is involved in mediating punishment-related learning (Cools, Roberts, & Robbins, 2008), have been implicated in AN. Some data suggest that tryptophan, a precursor to serotonin, and 5-Hydroxyindoleacetic acid, a metabolite of serotonin, are reduced in acute AN (Attia, Wolk, Cooper, Glasofer, & Walsh, 2005), but elevated following weight restoration (Kaye et al., 1988). Tryptophan depletion also has been found to reduce anxiety in acute and weight-restored AN (Kaye et al., 2003). It has been hypothesized that individuals with AN restrict food intake to regulate an over-active serotonergic system and its associated aversive subjective experience (Bailer & Kaye, 2011). Starvation may simultaneously inhibit dopamine metabolism, reducing reward sensitivity (Kaye et al., 2013). However, other findings run contrary to this hypothesis. Some studies find that tryptophan levels remain low (Gauthier et al., 2014) or are no different than NCs (Attia et al., 2005) after weight restoration, and other studies have failed to replicate evidence of reduced dopamine production (e.g., Broft et al., 2015).
Summary
Data derived from self-report and neurobiological metrics have informed models emphasizing general anomalies in reward and punishment processes in AN. This literature suggests an overall tendency in AN towards higher punishment sensitivity and away from immediate high-intensity rewards. Differences in brain structure and function (e.g., fronto-striatal and limbic circuit, dopamine, and serotonin irregularities) have been found that may contribute to these propensities. Though this literature provides a broad view of the reward and punishment processes that may characterize AN, certain incongruities remain unexplained. Analysis of the specific cues used to probe reward and punishment processes, and their shared features, may enhance understanding of how response tendencies interact with context in AN.
Systematic Review of Cue-Specific Reward and Punishment Abnormalities in AN Search Criteria and Results
To examine cue features associated with reward and punishment in AN, a systematic literature review of PubMed and PsycINFO was conducted according to PRISMA guidelines (Moher et al., 2009), which included search terms “reward” and “punishment” crossed with “anorexia nervosa.” A meta-analysis was not conducted due to the extensive heterogeneity of the methodology in this area. All published studies through July 1, 2019 were interrogated, including those published online. Inclusion criteria required that papers: (1) be empirical; (2) be available in English; (3) include human participants; (4) include individuals with a diagnosis of AN; (5) involve exposure to a specific cue; and (6) examine a domain of reward or punishment responsivity (e.g., self-report, physiological, neurobiological). Because this review focused on mechanism, treatment studies were excluded. Reference lists of retrieved studies were examined to identify other appropriate manuscripts. Study selection was determined by title and abstract review for initial selection, followed by full-text review of potentially eligible studies to determine final inclusion. Data were extracted pertaining to sample characteristics and size, cue category, unit of analysis measured as reward or punishment response, and primary relevant findings (see Supplemental Table 1). When possible, differences between restricting (AN-R) and binge eating/purging (AN-BP) subtypes were highlighted; however, many studies combined subtypes or investigated only one subtype. The initial search resulted in 355 non-duplicate studies, 92 of which were included in the review (see details in Figure 1). Studies not included due to lacking cue exposure examined temperament, genetics, and resting biology. Studies excluded for not focusing on reward examined topics not directly relevant to this investigation (e.g., taste perception). Below, we review the search results organized according to cues associated with (a) non-reward/punishment; or (b) reward.
Figure 1.
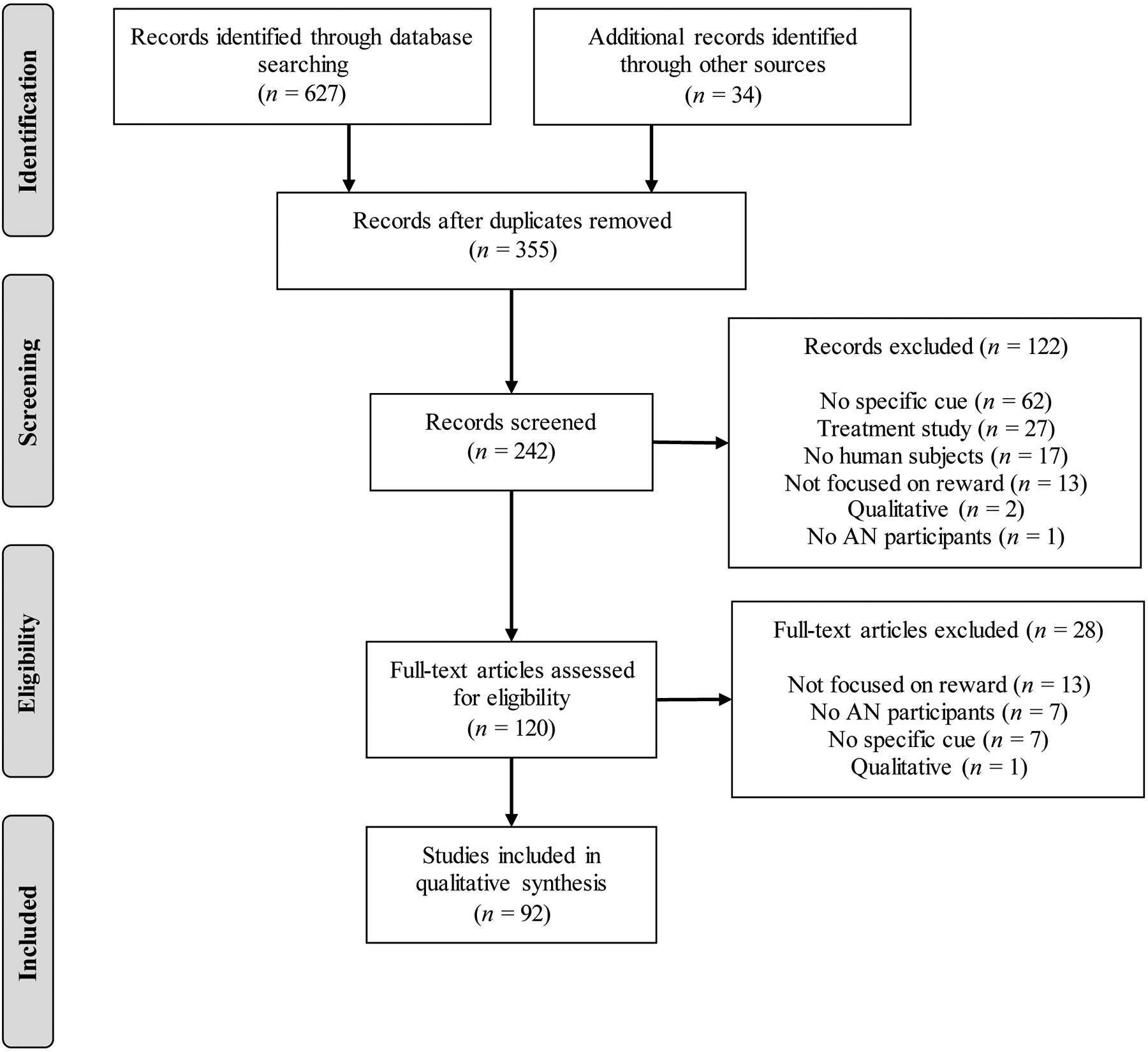
PRISMA (Moher et al., 2009) Flow Diagram for Systematic Review
Note: AN = anorexia nervosa
Stimuli Associated with Non-Reward and/or Punishment in AN
This section reviews the studies reporting results consistent with reward deficits or punishment excesses in relation to specific cues in AN. Data on non-reward and punishment are reviewed together because most studies do not clearly distinguish between these processes and there is limited information on whether there are distinct biobehavioral profiles associated with absence of reward versus punishment (Kim, Shimojo, & O’Doherty, 2006). Based on theory, results reflecting negative reactions are referred to as punishment, whereas reactions that indicate the absence or reduction of appetitive cue responding are considered non-reward.
Palatable food.
Our review revealed much evidence suggesting that individuals with AN demonstrate non-reward or punishment responses to palatable food cues. Across several studies, ill and recovered individuals with AN have rated visual, olfactory, and taste cues for high-fat and high-calorie foods as less pleasant and have described “liking” (i.e., experiencing pleasure from) and “wanting” (i.e., desiring) these foods less than NCs and individuals with other eating disorders (Cowdrey, Finlayson, & Park, 2013; Godier, Scaife, Braeutigam, & Park, 2016; Horndasch et al., 2018; Jiang, Soussignan, Carrier, & Royet, 2019; Jiang, Soussignan, Rigaud, & Schaal, 2010; Neimeijer, Roefs, Glashouwer, Jonker, & de Jong, 2019; Soussignan, Jiang, Rigaud, Royet, & Schaal, 2010; Stoner, Fedoroff, Andersen, & Rolls, 1996; Uher et al., 2004). In one study with a small AN sample (n = 6), participants with AN reported increased negative and decreased positive affect after normal meals (Anderson, Crow, & Peterson, 2014), which could indicate that this experience was aversive. A reverse pattern has been shown for loss of control eating, with decreased negative affect and increased positive affect following the behavior (Engel et al., 2013). This suggests that binge eating might function differently than other types of eating. Indeed, individuals with AN-BP have been found to engage in more effort to access certain food rewards than individuals with BN and NCs (Schebendach et al., 2017).
Similar findings have emerged from studies examining physiological correlates of reward and punishment response to palatable food cues. High-calorie food cues have been found to be more emotionally salient in AN. For instance, a study using magnetoencephalography found enhanced early neural responses to high-calorie food cues, suggesting increased attention (which could be positively or negatively valanced), among currently ill individuals with AN-R versus NCs or those recovered from AN (Godier et al., 2016). Similar findings have emerged from research on event related potentials from electroencephalogram (EEG) to high-calorie foods (Blechert, Feige, Joos, Zeeck, & Tuschen-Caffier, 2011). Further, in response to high-calorie food cues, individuals with AN have displayed marked lower zygomatic (Soussignan, Schaal, Rigaud, Royet, & Jiang, 2011) and higher corrugator (Soussignan et al., 2010) activity, indicating less positive and more negative facial expression, compared to NCs. These findings correspond with experimenter ratings of observed facial affect, which have demonstrated that individuals with AN smile less in response to palatable food cues (Soussignan et al., 2010). Behavioral tasks to measure implicit food attitudes and motivations have revealed similar findings reflecting non-appetitive or aversive implicit responses to palatable foods relative to NCs (Cowdrey et al., 2013; Neimeijer, de Jong, & Roefs, 2015; Neimeijer et al., 2019; Payne, Cheng, Govorun, & Stewart, 2005; Spring & Bulik, 2014; Veenstra & de Jong, 2011).
The neuroimaging findings on reward responses to palatable food cues are more mixed. Findings from several functional magnetic resonance imaging (fMRI) studies have been interpreted as highlighting deficient reward responding and enhanced punishment responding to food cues. For instance, in some studies, individuals with AN have exhibited less activation in reward-related regions (e.g., ventral tegmental area, dorsal striatum) in response to palatable food cues compared to those with BN (Brooks et al., 2011; Jiang et al., 2019). Additional research has identified reduced responding in regions such as the dorsal striatum, insula, amygdala, ACC, and OFC in response to palatable food tastes, scents, and images in ill and recovered individuals with AN compared to NCs (Holsen et al., 2012; Jiang et al., 2019; McFadden, Tregellas, Shott, & Frank, 2014; Oberndorfer et al., 2013a), which has varied depending on hunger and satiety level. The hypoactivity patterns identified in these studies were interpreted as evidence of reward disturbance, although several of these identified regions (e.g., amygdala, insula) have been implicated in aversive responding (Hartley & Phelps, 2012). Indeed, other studies have cited hyperactivity of these regions to anticipation and receipt of palatable food cues in various stages of AN as evidence of heightened punishment responding to these stimuli (Cowdrey, Park, Harmer, & McCabe, 2011; Ellison et al., 1998; Joos et al., 2011; Oberndorfer et al., 2013b; Vocks, Herpertz, Rosenberger, Senf, & Gizewski, 2011). In addition, one study found that reward- and punishment-related circuitry responses depended on internal state. When experiencing greater stomach sensations, individuals with AN showed less activity in the ventral tegmental area and more activity in the amygdala and ACC in response to palatable versus less palatable food cues (Kerr, Moseman, Avery, Bodurka, & Simmons, 2017).
A parallel line of evidence has highlighted enhanced activation of frontal regions associated with reward inhibition (e.g., medial PFC; ACC) in response to high-calorie food cues among acute and recovered individuals with AN (Kerr et al., 2017; Uher et al., 2003; 2004). Further, during food prediction error (reflecting the difference between anticipated and actual receipt of a sweet versus neutral taste), functional connectivity between the striatum and hypothalamus has been associated with higher OFC and insula responding for individuals with AN (Frank et al., 2018), which could also signal enhanced cognitive control over food reward. However, this study examined absolute prediction error, not response to unexpected food receipt specifically; thus, it cannot be determined if these effects reflect response patterns to all unanticipated events, or just unexpected sweet taste delivery. Finally, one study of individuals with AN reported hypoactivity in the putamen during a food-related go/no-go inhibition task in which they were expected to inhibit prepotent responses to food stimuli, suggesting that they required less engagement of inhibitory networks to restrain food-related responses (Kullmann et al., 2014a). However, this pattern of neural responding was also found for a go/no-go task not related to food. Together these data suggest that responses to palatable food cues may be characterized by less reward, more aversion, and inhibition in AN.
In contrast, other research has identified enhanced activation in reward circuitry in response to palatable food cues in AN. Studies have found increased brain activity in reward-related regions, such as the ventral and dorsal striatum, OFC, and dorsolateral PFC to the sight and taste of palatable food among individuals with AN in different illness stages compared to NCs (Cowdrey et al., 2011; Frank et al., 2012; Monteleone et al., 2017; Rothemund et al., 2011; Sanders et al., 2015). One group found that individuals with AN exhibited greater activity in the striatum and insula corresponding with sweet taste absolute prediction error compared to NCs (Frank et al., 2018) and enhanced ACC activation associated with the value of an expected food reward compared to NC and BN groups (Olsavsky, Shott, DeGuzman, & Frank, 2018). This suggests that individuals with AN may closely track food contingencies. Similar findings have emerged from studies of individuals recovered from AN, including one that found increased activation relative to NCs in the ventral striatum to a chocolate taste (Cowdrey et al., 2013). Another identified elevated insula activity after omission of an expected food reward, potentially reflecting aversion to food omission (Frank, Collier, Shott, & O’Reilly, 2016).
In sum, self-report and implicit measures have captured reduced reward and enhanced punishment responses to palatable food cues in AN, except in the case of binge eating. Several neuroimaging studies similarly suggest reduced reward and enhanced aversion and inhibition responses to palatable food cues in AN, but there are also a number of studies reporting an opposite pattern in which reward response to food cues is enhanced in AN.
Normal- or higher- weight bodies.
Contrasting the literature on responses to palatable food in AN, research on reactions to cues of normal or higher body mass have more consistently demonstrated an aversive reaction to these cues among individuals with AN. Ill and weight-restored individuals with AN have been found to rate visual cues of normal and overweight bodies more negatively than NCs (Cserjési et al., 2010; Horndasch et al., 2018; Spring & Bulik, 2014; Sweitzer et al., 2018). Implicit measures have also found individuals with AN to display stronger automatic attentional biases (Redgrave et al., 2008) and implicit negative biases (Cserjési et al., 2010; Spring & Bulik, 2014) to overweight cues. Additionally, relative to NCs or people with BN, ill and weight-restored individuals with AN have demonstrated elevated amygdala response to body-focused visual stimuli, such as pictures of their body distorted to be larger, pictures of normal weight women, or negative body image words (Miyake, Okamoto, Onoda, Kurosaki, et al., 2010; Miyake, Okamoto, Onoda, Shirao, et al., 2010; Pruis, Keel, & Janowsky, 2012; Seeger, Braus, Ruf, Goldberger, & Schmidt, 2002; Vocks et al., 2010). Individuals with current or recent AN also have shown lower striatal activation relative to NCs to normal or higher weight body cues (Fladung, Schulze, Schöll, Bauer, & Grön, 2013; Sweitzer et al., 2018). Thus, across measures, stimuli of normal and higher weight bodies have been associated with non-reward and punishment responses in AN.
Social contexts.
Social stimuli have also been found to elicit reduced reward responding and evidence of punishment in AN. Compared to NCs, individuals with current or lifetime AN have been found to display greater attentional biases towards angry or rejecting social cues (Cardi, Di Matteo, Corfield, & Treasure, 2013; Cserjési, Vermeulen, Lénárd, & Luminet, 2011), more accurate recall of rejection experiences (Via et al., 2015), and hyperactivation of attentional networks during social rejection (Via et al., 2015). These findings suggest that negative social experiences may be especially salient for individuals with AN. Studies have also found individuals with AN to report less positive ratings and display more negative affect (Davies, Schmidt, & Tchanturia, 2013) and avoidance (Davies, Schmidt, Stahl, & Tchanturia, 2011) related to aversive social stimuli. Further, individuals with AN have demonstrated elevated insula activation relative to NCs when exposed to pictures of infants displaying negative affect (Leppanen et al., 2017b). Thus, negative social stimuli tend to yield punishment-consistent responses for those with AN.
A less expected set of findings are those that have indicated less reward responding and, in fact, more punishment responding to positive social stimuli for individuals with AN. Studies have found individuals with AN to rate positive social cues, such as accepting faces and social touch, as less pleasant than NCs (Cardi et al., 2015; Crucianelli, Cardi, Treasure, Jenkinson, & Fotopoulou, 2016; Davies et al., 2013). Additionally, individuals in different stages of AN have been shown to display less positive and more negative facial affect in response to positive social cues (Cardi et al., 2015; Davies et al., 2013) and are more avoidant of these cues (Cardi et al., 2013; Watson, Werling, Zucker, & Platt, 2010) compared to NCs. Consistent with this literature, individuals with AN have been found to exhibit elevated amygdala and insula reactivity to faces displaying positive affect (Leppanen et al., 2017b, 2017a). Individuals with AN also have shown aberrant activity of brain regions associated with cognitive control in response to positive social stimuli, though the direction of these findings is mixed (Boehm et al., 2018; Leppanen et al., 2017b; Via et al., 2015). Additionally, one study did not find any difference in neural activity between individuals with AN and NCs in response to social cues (Sweitzer et al., 2018). This literature also contrasts with data suggesting self-reported sensitivity to social rewards in AN (Glashouwer et al., 2014), which may indicate that responses to social cues may vary according to stage of illness (e.g., risk versus maintenance) or the quality or intensity of the social stimuli. However, the clinical presentation of AN is often associated with social anhedonia, submission, and avoidance (Hartmann, Zeeck, & Barrett, 2010; Tchanturia et al., 2012) and elevated threat and reduced reward responses to social cues may underlie these interpersonal difficulties.
Monetary gains.
A notable literature has suggested that individuals with AN (especially AN-R) orient less towards immediate monetary gain compared to NCs (Decker, Figner, & Steinglass, 2015; Steinglass et al., 2017a) or individuals with BN or BED (Bartholdy et al., 2017; Chan et al., 2014; Steward et al., 2017), preferring instead to opt for greater monetary gains at a later time. Additionally, individuals with AN have been shown to under-value immediate receipt of monetary rewards relative to interpersonal processes such as fairness in money-sharing games (Isobe et al., 2018). In acute and recovered stages of AN, these monetary choice preferences have corresponded with different patterns of responding in reward- and control- related brain regions. For instance, relative to NCs, individuals with AN have demonstrated less activation in the striatum and ACC when delaying monetary receipt (Decker et al., 2015), as well as poorer differentiation between monetary wins and losses in the ventral striatum (Wagner et al., 2007). Further, whereas NCs have demonstrated greater positive affect and reward circuit activation following immediate monetary gains when hungry and more cognitive control circuit activation in response to monetary decision-making when full, positive affect and reward and control circuit activation has been found to be unrelated to hunger in different stages of AN (Piccolo et al., 2019; Wierenga et al., 2015). Other studies have found PFC function to differ for individuals with a history of AN during decision-making and anticipation regarding monetary gains, though the direction of these findings has varied (Ehrlich et al., 2015; King et al., 2016). These studies provide evidence that immediate monetary gain may be less salient for individuals with AN.
However, a discrepant literature suggests that reward responding to immediate monetary gain may be the same or enhanced in AN relative to NCs. Some research has found no difference in preferences for immediate receipt of money between individuals with current or past AN and NCs (King et al., 2016; Ritschel et al., 2017). Similarly, several studies suggest that positive affect and reward circuit activation to anticipation or receipt of monetary gains are no different (Bernardoni et al., 2018; Bischoff-Grethe et al., 2013; Murao et al., 2017; Piccolo et al., 2019) or elevated (DeGuzman, Shott, Yang, Riederer, & Frank, 2017) in AN versus NC participants. Additionally, weight-restored groups with AN have displayed greater activation of the striatum, ACC, and dorsolateral PFC during monetary choice and gain compared to NCs, especially when sated (Decker et al., 2015; Wagner et al., 2007; Wierenga et al., 2015). Thus, data on response to monetary gains in AN are mixed and potentially affected by other factors (e.g., hunger, weight).
Monetary losses.
Findings demonstrating that monetary losses yield punishment responses for individuals with AN are less equivocal. On behavioral tasks, individuals with AN at various stages of illness have been found to direct greater attention to monetary losses (Giannunzio et al., 2018), learn contingencies more readily after losses (Bernardoni et al., 2018), and shift away from decisions that lead to losses (Geisler et al., 2017; Ritschel et al., 2017) compared to NCs. In contrast, two studies suggested that individuals with AN demonstrated less aversion to monetary losses than NCs and individuals with BN (Chan et al., 2014; Verharen et al., 2019). In neuroimaging studies, individuals with AN have exhibited elevated ACC activation prior to making behavioral changes after monetary loss (Geisler et al., 2017) and elevated ACC and insula response to anticipated or actual monetary loss (Bischoff-Grethe et al., 2013; Murao et al., 2017). Further, compared to NCs, individuals acutely ill with or recovered from AN also have exhibited elevated response to monetary loss or omission in brain regions such as the medial frontal cortex and OFC (Bernardoni et al., 2018; Bischoff-Grethe et al., 2013; DeGuzman et al., 2017; Ritschel et al., 2017; Wagner et al., 2007), suggesting that monetary loss may engage circuitry that assists in learning from the aversive experience.
Summary.
Several studies utilizing self-report, implicit measures, and neuroimaging methods have suggested deficits in reward responding to palatable food cues in AN. However, a discrepant neuroimaging literature has suggested potentially enhanced reward responding to palatable food cues among individuals with AN. A growing literature using monetary cues to assess deficits in reward and punishment responsivity has generally identified monetary loss as aversive to individuals with AN, but is mixed in terms of responding to monetary gains. The literatures on reactions to social cues and normal- and higher-weight stimuli are less developed, but both consistently suggest that these cues are associated with punishment in AN.
Stimuli Associated with Reward and/or Non-Punishment
The following section reviews studies indicating cues for which there is evidence of subsequent enhanced reward response or decreased aversive response in AN. These data are not yet reconciled within theories of global reward and punishment processing abnormalities in AN.
Restrictive eating.
Restrictive eating precedes the development of AN (Affenito, Dohm, Crawford, Daniels, & Striegel-Moore, 2002), persists following treatment (Mayer, Schebendach, Bodell, Shingleton, & Walsh, 2012), and predicts relapse (Schebendach et al., 2011). Thus, it can be hypothesized that restrictive eating cues would be associated with enhanced reward responding in AN. This idea is supported by initial data from ecological momentary assessment (EMA) studies examining real-time affective patterns relative to disordered eating. EMA studies in AN have found: (1) positive affect to be higher and negative affect to be lower before, during, and after restrictive eating episodes compared to normal meals (Fitzsimmons-Craft et al., 2015), (2) positive affect lability to be associated with greater restrictive eating (Selby et al., 2015), and (3) restrictive eating to be associated with subsequent decreases guilt in AN and increases in self-assurance for individuals specifically with AN-R (Haynos et al., 2017). These studies suggest that restrictive eating is associated with seemingly desirable emotional outcomes in AN.
Studies examining reactions to low-calorie food items as a proxy for restrictive eating also have yielded data consistent with greater reward responding to these cues. Individuals with AN have been found to show greater attentional bias to low-calorie foods compared to NCs (Blechert et al., 2011; Novosel et al., 2014). Further, relative to NCs, individuals with AN in different illness stages have rated healthier foods as tastier (Foerde, Steinglass, Shohamy, & Walsh, 2015) and displayed more explicit (Cowdrey et al., 2013; Scaife et al., 2016; Stoner et al., 1996) and implicit (Cowdrey et al., 2013; Neimeijer et al., 2015) “liking” and “wanting” of low-calorie foods (one study found less liking of low-calorie foods; Veenstra & de Jong, 2011).
Findings from neuroimaging studies also have indicated that exposure to low-calorie food items is associated with reactions consistent with reward. One study found that compared to NCs, individuals with AN exhibited greater functional connectivity between the dorsal striatum and dorsolateral PFC in response to food choices, which correlated with lower test meal caloric intake (Foerde et al., 2015). Another found a significant positive association between OFC activation in response to low-calorie food cues and fasting acylated ghrelin for individuals with AN-R, which was interpreted as hunger enhancing the value of low-calorie foods (Holsen et al., 2014). Additional research also demonstrated decreased activation of the frontal pole in response to low-calorie food images, which may imply less engagement of brain regions involved with cognitive inhibition (Scaife et al., 2016). The sum of these findings suggest that low-calorie foods may be associated with reward salience and value in AN.
Thinness.
Another characteristic of AN is a drive towards thinness (APA, 2013). Thus, thinness cues would be expected to prompt reward responses in AN. Findings have been mixed on explicit liking of thin bodies; in some studies individuals across various stages of AN have rated thin bodies more positively (Fladung et al., 2010; Horndasch et al., 2018) and in others no differently (Cserjési et al., 2010; Horndasch, Heinrich, Kratz, & Moll, 2012; Spring & Bulik, 2014; Watson et al., 2010) than NCs. However, when examining implicit indicators, a consistent picture has emerged supporting thinness cues as signaling reward in AN. In one study, conducted on a small sample (n = 12), individuals with AN showed greater attentional bias to thinness cues compared to NCs (Redgrave et al., 2008). In another study utilizing eye tracking data, weight-restored individuals with AN, when shown a picture of a slender female, looked longer at her body, whereas NCs looked longer at her face (Watson et al., 2010). Individuals with AN also may be motivated by their own thinness; weighing and body checking have been associated with negative and positive affect lability in EMA research (Engel et al., 2013; Selby et al., 2015).
There is also behavioral evidence that thin bodies generate appetitive responses among individuals with AN. In a monetary choice task, in which subjects could sacrifice money to view images, individuals weight-restored from AN sacrificed monetary gain to a greater degree to view thinner bodies (Watson et al., 2010), which suggests greater reward value of thinness over monetary gain. Psychophysiological research yields similar data. Studies have found elevated skin conductance, decreased eyeblink startle, and increased EEG late positive potential to underweight stimuli among individuals with current or lifetime AN, indicative of increased salience and appetitive responding to these cues (Clarke, Ramoz, Fladung, & Gorwood, 2016; Horndasch et al., 2018; Horndasch et al., 2012; O’Hara, Keyes, Renwick, Giel, et al., 2016a). Further, viewing underweight bodies has been associated with greater ventral striatum activation compared to viewing normal weight bodies for individuals with AN (Fladung et al., 2013; Fladung et al., 2010). Another study found that viewing words depicting thinness was associated with hyperactivation in the insula and frontal lobes, which was interpreted as reflecting reward responsivity (Redgrave et al., 2008). Thus, across methods, there has been evidence that thinness cues are associated with appetitive responding in AN.
Physical activity and compensatory behavior.
Many individuals with AN engage in excessive exercise and/or compensatory behavior, such as purging (APA, 2013). Accordingly, there is also evidence that physical activity and purging are associated with reward responding in AN. Across several studies, individuals with AN have exhibited greater attentional bias to physical activity cues (Giel et al., 2013; O’Hara et al., 2016a), rated these cues as more pleasant (Giel et al., 2013), and reported greater positive affect lability on days with more physical activity (Selby et al., 2015). Further, ill and recovered participants with AN have been found to exert more effort to engage in physical activity compared to NCs (Klein et al., 2010; O’Hara, Keyes, Renwick, Leyton, et al., 2016b), although this tendency has been shown to be more pronounced when underweight (Gianini et al., 2016). For compensatory behavior, EMAmeasured negative affect has been found to decrease after vomiting, suggesting that this behavior is also associated with desirable affective consequences (Engel et al., 2013).
Neuroimaging evidence suggests that physical activity may be more difficult for individuals with AN to inhibit. In one study, individuals with AN displayed increased PFC activation during a go/no-go task with physical activity cues compared to NCs, suggesting more cognitive effort to resist responding to these cues (Kullmann et al., 2014a). The same group found an association between elevated physical activity and reduced functional connectivity between frontal brain regions involved in motor inhibition for individuals with AN (Kullmann et al., 2014a). In the context of the broader literature, these data suggest that reward derived from exercise may make inhibition of such behavior more challenging among those with AN.
Physical pain.
Several studies have suggested that individuals with AN display elevated tolerance to thermally and mechanically induced pain compared to NCs (e.g., Bär, Berger, Schwier, Wutzler, & Beissner, 2013; Lautenbacher, Pauls, Strian, Pirke, & Krieg, 1990, 1991). Although there has been little examination of whether this heightened pain tolerance reflects lesser punishment from pain or greater distress tolerance, one study found that participants with AN had less insula activation compared to NCs during exposure to thermally-induced pain, which could indicate that physical pain leads to less aversive responding in AN (Bär et al., 2013). Further investigation is needed to better understand the function of physical pain in AN.
Summary.
Self-report, behavioral, psychophysiological, and neuroimaging data converge to suggest that several disorder-relevant stimuli, including cues for restrictive eating, thinness, physical activity, and purging are associated with enhanced reward and decreased punishment response in AN. It is unclear whether there are stimuli unrelated to the disorder associated with enhanced reward or reduced punishment for individuals with AN relative to NCs. Emerging data suggest that this could be the case for physical pain, but more research is needed.
Discussion
In this systematic review, we investigated the literature on reward and punishment responding using a cue-specific lens to determine: (1) whether there were studies that ran counter to prevailing theories suggesting that individuals with AN are globally over-responsive to punishment and under-responsive to reward and; (2) if so, whether information could be gleaned from these discrepancies to better understand the reward and punishment properties salient to this population. Many studies were identified that demonstrated heightened punishment and decreased reward responding in AN; however, there were also many demonstrating an opposite pattern. Importantly, this indicates that individuals with AN do have the capacity for heightened reward and decreased punishment responding under certain conditions. With a few exceptions, these patterns of responding were organized according to the specific cues presented to participants. Considered in the context of the temperamental and neurobiological data demonstrating more widespread patterns of punishment versus reward sensitivity in AN, the findings of this review suggest that, while individuals with AN might tend towards prioritizing safety over pursuit of novelty, these tendencies do not apply to all contexts. Indeed, given these pre-existing response patterns, the situations that elicit reward responding and/or decrease aversive responding in this population can be considered to be particularly potent reinforcers.
One anticipated finding of this review was that reward and punishment responding were consistent with disorder-specific patterns reflecting the phenomenology of AN. Normal- and higher- weight cues reliably evoked punishment responses, whereas restrictive eating, exercise, purging, and thinness contexts consistently prompted reward responses. Reactions to palatable food cues were highly variable, which corresponds with the ambivalence with which individuals with AN interact with food in a way that results in a paradoxical combination of avoidance (e.g., restrictive eating) and approach (e.g., preoccupation, binge eating) (Murray & Strigo, 2018). In contrast, fewer studies examined responses to cues not directly related to core eating disorder symptoms, such as social stimuli and monetary rewards. Although studying responses to disorder-specific cues has provided important data about experiences surrounding symptom engagement, this approach cannot yield information about the more generalized functioning of response systems, which could represent shared mechanisms with other psychiatric illnesses.
While there was variability in the literature across different cues, the findings relating to punishment responding emerged as more consistent than those related to reward responding. To our knowledge, all studies investigating self-reported temperament have identified elevated punishment sensitivity in AN (Atiye et al., 2015; Harrison et al., 2010). Additionally, cues typically considered aversive (e.g., social threat, monetary loss) were reliably met with responses consistent with punishment in AN. One potential exception may be that of physical pain, which some evidence suggested might be less punishing for individuals with AN (Bär et al., 2013), although more research is needed in this area. In contrast, the literature on reward responding was far more mixed with multiple exemplars in which reward response was heightened in AN. These findings indicate that the tendency to excessively respond to punishment may be more generalized than the tendency to orient away from reward in this population. However, punishment responding is heightened in other psychiatric disorders (Miettunen & Raevuori, 2012), potentially signaling a non-specific distress marker versus a unique mechanism of AN.
Shared Properties of Rewarding and Punishing Cues in AN
A primary goal of our systematic review was to closely examine the specific variables associated with reward and punishment experiences in AN to determine if there were properties shared among these cues that could inform why or how certain experiences become rewarding or punishing in this population. Therefore, below we present suggestions about several qualities that may unite the cues evoking reward or punishment responding for individuals with AN.
Predictability versus unpredictability.
Intolerance of uncertainty, reflecting a negative reaction to unpredictable contexts, has been noted as being salient for individuals with AN (Brown et al., 2017). Human and animal studies suggest that, for most organisms, unpredictable punishment is more aversive than predictable punishment, but that this tendency is enhanced in certain psychiatric disorders (Grillon et al., 2017). In contrast, unpredictable rewards are usually associated with greater appetitive responding than predictable rewards (Berns, McClure, Pagnoni, & Montague, 2001), resulting from elevated short-term phasic dopamine response (Hernandez et al., 2007). However, there is evidence that rapid dopamine release may be anxiogenic, rather than pleasurable, in AN (Bailer et al., 2012), making unpredictable reward potentially less positive for this population. Furthermore, there is evidence suggesting that individuals in different stages of AN make slower (Piccolo et al., 2019) and poorer (Giannunzio et al., 2018) decisions than NCs in uncertain or ambiguous contexts. Studies have also found enhanced insula responses to unexpected punishment and reward in AN (DeGuzman et al., 2017; Frank et al., 2016), potentially signaling an aversive reaction to both such consequences.
For individuals with AN, pursuit of weight loss may constitute a process associated with relatively predictable outcomes. There is evidence that AN is associated with genetically determined metabolic tendencies towards leanness (Watson et al., 2019); therefore, weight loss behaviors are likely to reliably lead to the valued state of thinness in this group. Similarly, the immediate aversive consequences (e.g., hunger, fatigue) of weight loss behaviors are expected physiological outcomes. This matches the framework of studies investigating pain tolerance in AN, in which participants were predictably alerted to impending pain administration. Thus, one quality that restrictive eating, thinness, exercise, and physical pain cues may share is that they signal predictable, rather than unpredictable, expectancies of short-term reward and punishment. Supporting this idea, activity in the dorsal striatum, which has been shown to be especially reactive to food cues in AN (Foerde et al., 2015; Sanders et al., 2015; Zhu et al., 2012), tends to be more active in situations signaling predictable versus unpredictable rewards (Tanaka et al., 2006). In contrast, social and eating situations are often associated with uncertainty due to multifaceted factors producing fluctuations in human behavior and momentary weight, perhaps contributing to their punishing and/or non-rewarding qualities in AN.
Controllability versus uncontrollability.
Related to predictability is the construct of controllability, with the latter representing a state in which consequences are not only expected, but also under one’s volition. Substantial data suggest that humans and other animals prefer controllable over uncontrollable punishments (Rigoli, Pavone, & Pezzulo, 2012). Similarly, most individuals prefer controllable rewards (Miura, Tanabe, Sasaki, Harada, & Sadato, 2017) and perform better on tasks that provide a sense of outcome controllability (Murayama et al., 2015).
This preference may be enhanced in AN. Illness narratives of AN often indicate that eating disorder symptoms provide a sense of control when other aspects of life are uncontrollable (Espíndola & Blay, 2009). Because adolescents and adults are mostly responsible for their own eating and exercise habits, and there is evidence that individuals with AN have an enhanced capacity for leanness (Watson et al., 2019), weight loss and associated behaviors may represent situations in which the consequences are perceived as controllable. An orientation towards controllable outcomes could also explain the high academic achievement that has been reported in AN (Sundquist, Ohlsson, Winkleby, Sundquist, & Crump, 2016). Academic contexts that reward effort in addition to ability represent situations in which outcomes can be under one’s control. This may also contribute to initial findings of heightened pain tolerance in AN, given that most pain studies allow participants to control when painful stimuli are terminated. This preference for controllability could also explain the aversive nature of social situations for those with AN, as social outcomes are often out of one’s control. Indeed, individuals with AN express a reduced sense of control over social situations (Soh, Surgenor, Touyz, & Walter, 2007).
Immediacy versus delay.
Individuals with AN pursue weight loss efforts despite the initially distal nature of the rewarding outcome (Walsh, 2013). One explanation for this pattern is that individuals with AN are drawn to long-term rewards. Most organisms devalue rewards delivered later over those delivered immediately (da Matta et al., 2012) and this tendency is heightened in most externalizing psychiatric disorders (Lempert et al., 2018). However, as previously noted, there is evidence that individuals with AN may show enhanced preference for delayed over immediate monetary gains compared to NCs and other eating disorder and psychiatric groups (Lempert et al., 2018). On delay discounting tasks, individuals with AN have been shown to make quicker decisions (Ritschel et al., 2015), especially when accepting delayed rewards (Decker et al., 2015), suggesting an automaticity of this response. Although a common interpretation has been that individuals with AN are engaging enhanced self-control to inhibit short-term reward pursuit (Steward et al., 2017), an alternative explanation is that individuals with AN experience longer-term rewards as more gratifying than short-term rewards. Valuable delayed rewards activate reward circuitry (Prévost, Pessiglione, Météreau, Cléry-Melin, & Dreher, 2010), suggesting that these neural systems can facilitate desire for long-term rewards. Less is known about preferences for immediate versus delayed punishments; however, some research suggests that most people prefer immediate punishment to mitigate dread (Story et al., 2013). Although this has not been directly tested in AN, it is possible that this tendency is enhanced among this group, which has been shown to exhibit enhanced insula activation to punishment anticipation (Frank et al., 2016; Murao et al., 2017).
The pursuit of weight loss often involves immediate discomfort in exchange for a long-term desired outcome. In contrast, food reward is relatively immediate and negative health consequences from persistent over-consumption are delayed. Thus, preference for immediate punishment in the service of delayed rewards may contribute to symptoms of AN. This tendency may be enhanced if weight loss yields positive outcomes, as data suggest that preference for a delay cue is enhanced with positive conditioning to the delayed outcome (Fobbs & Mizumori, 2017). Further, willingness to tolerate short-term discomfort has been found to be enhanced when long-term outcomes are highly valued (da Matta et al., 2012). Thus, an alternate possibility is that individuals with AN are not more inclined towards long-term rewards, but rather certain long-term rewards (e.g., thinness) are so compelling that they override automatic tendencies to over-emphasize short-term outcomes. More research is needed to investigate these hypotheses.
Effortful versus non-effortful.
For most organisms, mental and physical effort is considered aversive and actively avoided. High effort activities have been associated with physiological (Van Boxtel & Jessurun, 1993) and neurobiological (Massar, Libedinsky, Weiyan, Huettel, & Chee, 2015) responses consistent with aversion. As such, humans and non-human animals have been shown to typically select low effort work options when available and to forgo rewards to avoid effort (Inzlicht, Shenhav, & Olivola, 2018). Thus, most theories conceptualize effort as a punishing experience. However, a contrasting literature suggests that effort can be rewarding. Outcomes following high effort have been associated with greater subjective value (Inzlicht et al., 2018) and activation in reward circuitry (Dobryakova, Jessup, & Tricomi, 2017). Consequently, effortful behavior itself can be conditioned to become rewarding (Eisenberger, 1992). Individual differences in the desire to engage in effortful actions have been found, as some people more readily seek mental and physical challenges than others (Chevalier, 2018).
As noted, individuals with AN have been found to demonstrate an elevated propensity towards persistence (e.g., Lloyd et al., 2014). Thus, it is possible that individuals with AN tend towards experiencing appetitive, rather than aversive effects from sustained effort. This preference could account for why behaviors such as restrictive eating and physical activity have been shown to generate reward responding, as each require sustained effort to obtain the desired outcome. This corresponds with the finding from some research that individuals with AN will work harder to exercise than NCs (Klein et al., 2010). In contrast, other stimuli that have not been shown to be as clearly rewarding to individuals with AN either typically do not require sustained effort to obtain (e.g., food, immediate monetary reward), or else do not benefit from effortful action (e.g., effort does not necessarily lead to positive social interactions).
Summary.
We posit that cue properties may contribute an experience being rewarding or punishing for individuals with AN. Based on the prior research, we expect that individuals with AN may be drawn to reward cues that are predictable, controllable, delayed, and/or effortful and may avoid punishment cues that are unexpected, uncontrollable, and/or delayed.
Limitations
There are several considerations to be noted regarding the reviewed research. A first major limitation of the research on reward and punishment responding in AN is that many interpretations have been predicated on reverse inference derived from implicit or biological metrics. Although the accumulation of neuroimaging and psychophysiological data on reward- and punishment- associated processes is a relative strength of this literature, caution is needed when extrapolating psychological experience or intention from biology alone. Interpretation based solely on biological indices can often lead to circular logic (e.g., food cues evoke different striatal responses between individuals with AN and NCs, thus reward responding must be different because it is known that food is a reward based on striatal responses from NCs). Further, there is evidence of significant functional overlap between the neural and physiological responses associated with reward and punishment (Haber, 2016), which has led to inconsistencies in the interpretation of which biological patterns constitute reward versus punishment and complicated the synthesis of results across studies. Activation in the same regions (e.g., insula, amygdala) has at times been interpreted as reflecting reward responding (Holsen et al., 2012; Oberndorfer et al., 2013a), and at others as demonstrating punishment responding (Ellison et al., 1998; Joos et al., 2011; Vocks et al., 2011). Similarly, many implicit (e.g., attention bias) and psychophysiological (e.g., heart rate) measures capture salience, not valence, and therefore have been alternatively suggested to highlight appetitive or aversive interest (e.g., Redgrave et al., 2008). Indeed, often biological differences have been detected without evidence of different behavior between groups, leading to confusion in how to interpret findings. For parallel reasons, little attention has been paid to untangling punishment from non-reward, or positive from negative reinforcement (Kim et al., 2006), limiting the specificity with which the mechanisms promoting eating disorder behavior are understood. This is particularly confusing in the case of behavioral patterns like restrictive eating, which could constitute states motivated by reward (i.e., approaching thinness) or punishment (i.e., avoiding weight gain).
Additionally, studies in this area, like many in the eating disorders field, have enrolled small samples. The median size for AN samples across reviewed studies was n = 20 (range: n = 3 to 121), with over 70% of papers included fewer than 30 participants with AN. Studies utilizing fMRI in particular tended to include smaller AN samples (median: n = 16). As a result, a substantial amount of work in this area is likely to be underpowered and non-conclusive. The samples have also been homogeneous (mostly enrolling Caucasian females) and potentially not generalizable to the broader population with AN. Further, inconsistencies in stimulus sets, even in the same cue category (e.g., palatable foods), have introduced variability limiting the ability to compare across studies. For example, most studies finding an enhanced reward response to food cues used sweet stimuli (e.g., Frank et al., 2018, 2012; Olsavsky et al., 2018), and studies finding reduced reward responding to food used high-fat stimuli (e.g., Brooks et al., 2011; Cowdrey et al., 2013; Godier et al., 2016). Given that restrictive eating in AN focuses on eliminating foods higher in fat, not carbohydrate content (Mayer et al., 2012), such differences can impact findings.
Other factors may contribute to the variability in findings on reward and punishment responding in AN. This literature spans across different versions of diagnostic classification systems; therefore, the criteria used for AN diagnosis were not consistent. Additionally, diagnostic subtype received variable treatment across studies: some reported separate results for subtypes, many combined subtypes, and others focused only on AN-R. Most research also did not control potential confounding variables (e.g., food intake, menstrual status, medication use, co-morbidities), despite evidence that these variables alter bio-behavioral responding (Frank, Favaro, Marsh, Ehrlich, & Lawson, 2018). Further, few studies considered the relationship of duration of illness in reward and punishment responding, despite models suggesting that contingency patterns shift over stages of illness (Walsh, 2013). Similarly, neurodevelopmental stage impacts reward- and punishment-based decision-making (Braams, van Duijvenvoorde, Peper, & Crone, 2015), yet few studies compared response patterns in adolescents versus adults with AN. Finally, our review may have been limited by considering only published studies available in English, as well as by not requiring a comparison group or control condition, which yielded a more comprehensive survey of the literature in this area, but also may have allowed the inclusion of certain studies of comparatively lesser quality in the review.
Future Directions
Based on this review, it is clear that contextual variables, both those that pertain to sample characteristics (e.g., age, chronicity, hunger) and those that reflect stimulus properties (e.g., type of reward/punishment cue, including qualities of when and how these outcomes occur), warrant further attention in the research on reward and punishment processes in AN. In future investigations of reward and punishment, contextual variables should be either carefully considered when drawing conclusions to avoid overgeneralization or manipulated to investigate their specific influence in AN. Theories on reward and punishment have been more fully specified to account for a range of relevant environmental and biological variables in other areas of the behavioral sciences, such as addictive and mood disorders (e.g., Berridge & Robinson, 2016; Dygdon & Dienes, 2013). However, more research informed by these and other models is needed to produce a fine-grained analysis of the reward and punishment mechanisms in AN that considers the influence of cue qualities. Several recommendations may support this goal.
First, our review suggested that most research in this area (nearly two-thirds of the reviewed studies) has focused on disorder-relevant cues (e.g., food). Even personality or temperament measures, which proport to assess generalized reward and punishment tendencies, include disorder-relevant items (e.g., pertaining to appearance or eating) that limit their ability to determine broader psychological functioning in AN (Glashouwer et al., 2014). To better understand basic learning processes that contribute to AN, it will be essential to examine responses to cues or prompts not directly related to eating or weight (e.g., pleasant videos) and to compare responses between disorder-relevant versus irrelevant stimuli. It will also be crucial to include designs that allow researchers to parse whether any motivational differences to varied stimuli constitute risk factors, maintenance factors, symptoms, or consequences of AN. Further, because appetitive and aversive processes are complex and interconnected, multimodal assessments integrating biological, behavioral, and subjective inputs, as well as more precise and informative experimental designs, are encouraged to develop more accurate interpretations of the findings in this area. Along these lines, it will be essential to take subjective value of a reward under consideration when interpreting behavioral, physiological, neurobiological findings to distinguish differential functioning of appetitive and aversive systems from typical functioning of these response systems under matched stimulus value (e.g., lower activity of reward-related neurobiology to food cues could mean that individuals with AN are less biologically disposed to reward reactivity than NCs, or equally reward-oriented, but food is less valued).
Additionally, research capitalizing on emerging computational methods to parse relations between specific reward and punishment qualities (e.g., predictability, effort) and eating disorder outcomes holds promise for advancing this literature. Computational approaches can identify distinct decision-making disruptions in AN by creating mathematical models of aberrant behavior patterns in response to contextual manipulations (e.g., reward probability) and comparing them to observed behavior (Ferrante et al., 2019). Research using these approaches to examine psychiatric mechanisms is emerging, but very few such investigations have been conducted in AN (Steinglass, Dalack, & Foerde, 2019). Small sample sizes have been a particular limitation of studies in this area, and in order to enhance the future literature, larger and well-powered multisite studies will be needed to develop a better understanding of the neurobiological correlates of reward and punishment in AN.
Finally, expanding beyond generalized aberrations to a more nuanced understanding of reward and punishment processing in AN can increase the potential for more precisely targeted interventions. Most treatments for adults with AN have been derived from models adapted from mood and anxiety disorders that target heightened punishment responding (Pennesi & Wade, 2016). There is some emerging work to develop treatments accounting for AN-specific reward processes (Wierenga et al., 2018); however, in general, clinical approaches that target reward processes by enhancing alternative non-destructive rewards with functional equivalence to eating disorder behaviors (e.g., Craske, Meuret, Ritz, Treanor, & Dour, 2016) have not been tested in AN. Further, with more information about the reward and punishment value of varied types of experiences that maintain symptoms of AN, treatments can be developed to directly modify the expected or experienced appetitive or aversive properties of these contexts. Thus, more fully specified data on cue features that may be rewarding and punishing for those with AN can aid in optimizing interventions to account for specific learning processes that promote this illness.
Conclusions
Although there is some evidence of biological and behavioral patterns reflecting enhanced punishment and decreased reward sensitivity in AN, upon closer examination it is apparent that this population demonstrates elevated reward and diminished punishment responding in certain contexts. Several factors, such as level of predictability, controllability, immediacy, and effort may modify the rewarding and punishing qualities of various experiences, and further research is needed to specify the relationship of these factors to symptom provocation in AN. Continued research building on existing reward and punishment models using finer-grained analyses of mechanism will move the field towards a deeper understanding of the properties salient to the behavior that become entrenched and life threatening in AN.
Supplementary Material
Highlights.
Theories suggest low reward and high punishment responding in anorexia nervosa (AN)
A systematic review identified cues associated with reward and punishment in AN
Overweight, social, and money loss cues are punishing and weight loss cues rewarding
Evidence is mixed for whether food, monetary gain, and pain are rewarding in AN
Reward and punishment in AN may be affected by context-specific stimulus qualities
Acknowledgements
The authors would like to thank Dr. Stephen Wonderlich and Dr. Kelly Berg who informed the concept of this manuscript.
Declarations of Interest/Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health (award numbers T32MH082761, K23MH112867, and K23MH101342), Klarman Family Foundation, and Hilda and Preston Davis Foundation. This research did not receive funding from agencies in the public, commercial, or not-for-profit sectors. The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the National Institute of Health, Uniformed Services University, or the Department of Defense. We thank Dr. Stephen Wonderlich and Dr. Kelly Berg for their contributions to informing the concept of this manuscript.
Role of Funding Sources
This work was supported by the National Institute of Mental Health of the National Institutes of Health (award numbers T32MH082761, K23MH112867, and K23MH101342), Klarman Family Foundation, and Hilda and Preston Davis Foundation. The National Institutes of Health, Klarman Family Foundation, and Hilda and Preston Davis Foundation had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Author Biographies
Ann F. Haynos, Ph.D. is an Assistant Professor in the Department of Psychiatry and Behavioral Sciences at the University of Minnesota. Dr. Haynos received her Ph.D. in clinical psychology from the University of Nevada, Reno and completed a predoctoral internship at Duke University Medical Center and a postdoctoral fellowship at the University of Minnesota through the NIMH-funded T32 Midwest Regional Postdoctoral Training Grant in Eating Disorders Research. Dr. Haynos’ research focuses on unifying psychology and neuroscience to elucidate and intervene upon the precise decision-making mechanisms that promote the development and maintenance of eating disorders, especially anorexia nervosa. She is also interested in using novel computational methods and analytical tools to enhance research on eating disorders.
Jason M. Lavender, Ph.D. is the Deputy Director of Research for the Military Cardiovascular Outcomes Research Program (MiCOR) at the Uniformed Services University of the Health Sciences. He received his Ph.D. in clinical psychology from the University at Albany, State University of New York and completed a postdoctoral research fellowship at the Neuropsychiatric Research Institute through the NIMH-funded T32 Midwest Regional Postdoctoral Training Grant in Eating Disorders Research. Dr. Lavender’s research focuses on dispositional and momentary factors contributing to the onset and maintenance of dysregulated eating among individuals across the weight spectrum. His interests also include multi-method study designs, with an emphasis on linking laboratory-based methods and real-world, real-time data collection.
Jillian D. Nelson, B.S. is a second-year graduate student in the clinical psychology doctoral program at George Mason University. Jillian received her Bachelor of Science in neuroscience from the University of Minnesota. Her current research involves the use of network analysis to explore psychiatric comorbidity in individuals with eating disorders.
Scott Crow, M.D. is Professor and Vice Chair in the Department of Psychiatry and Behavioral Sciences at the University of Minnesota and Director of Research at The Emily Program (a national eating disorders treatment program). He received his M.D. from and completed his Psychiatry residency at the University of Minnesota. Dr. Crow is Director of the Midwest Regional Postdoctoral Training Program in Eating Disorders Research (T32MH082761). His research focuses on the course, complications, causes, and treatment of eating disorders.
Carol B. Peterson, Ph.D. is an Associate Professor in the Department of Psychiatry and Behavioral Sciences at the University of Minnesota and the Chief Training Officer of The Emily Program. She received her undergraduate degree from Yale University and her doctorate in clinical psychology from the University of Minnesota. The recipient of federal and foundation grants, Dr. Peterson has co-authored over 175 peer-reviewed articles on the topics of eating disorders treatment, assessment, and maintenance mechanisms. She is a Fellow of the Academy for Eating Disorders and a member of the Editorial Board of the International Journal of Eating Disorders, as well as a clinician and a clinical supervisor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Ackard DM, Cronemeyer CL, Richter S, & Egan A (2015). Do symptom-specific stages of change predict eating disorder treatment outcome? Eating and Weight Disorders, 20, 49–62. [DOI] [PubMed] [Google Scholar]
- Affenito SG, Dohm FA, Crawford PB, Daniels SR, & Striegel-Moore RH (2002). Macronutrient intake in anorexia nervosa: The National Heart, Lung, and Blood Institute Growth and Health Study. The Journal of Pediatrics, 141, 701–705. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition Washington, D.C: American Psychiatric Publishing. [Google Scholar]
- Anderson LM, Crow SJ, & Peterson CB (2014). The impact of meal consumption on emotion among individuals with eating disorders. Eating and Weight Disorders, 19, 347–354. [DOI] [PubMed] [Google Scholar]
- Atiye M, Miettunen J, & Raevuori-Helkamaa A (2015). A meta-analysis of temperament in eating disorders. European Eating Disorders Review, 23, 89–99. [DOI] [PubMed] [Google Scholar]
- Attia E, Wolk S, Cooper T, Glasofer D, & Walsh BT (2005). Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biological Psychiatry, 57, 674–678. [DOI] [PubMed] [Google Scholar]
- Bailer UF, & Kaye WH (2011). Serotonin: Imaging findings in eating disorders. Current Topics in Behavioral Neurosciences, 6, 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer UF, Narendran R, Frankle WG, Himes ML, Duvvuri V, Mathis CA, & Kaye WH (2012). Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. International Journal of Eating Disorders, 45, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär K-J, Berger S, Schwier C, Wutzler U, & Beissner F (2013). Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatrica Scandinavica, 127, 269–278. [DOI] [PubMed] [Google Scholar]
- Bartholdy S, Rennalls S, Danby H, Jacques C, Campbell IC, Schmidt U, & O’Daly OG (2017). Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. European Eating Disorders Review, 25, 344–350. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardoni F, Geisler D, King JA, Javadi A-H, Ritschel F, Murr J, … Ehrlich S (2018). Altered medial frontal feedback learning signals in anorexia nervosa. Biological Psychiatry, 83, 235–243. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, & Montague PR (2001). Predictability modulates human brain response to reward. The Journal of Neuroscience, 21, 2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E, Irvine LEZ, Wagner A, Yau W-YW, … Kaye WH (2013). Altered brain response to reward and punishment in adolescents with Anorexia nervosa. Psychiatry Research, 214, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Feige B, Joos A, Zeeck A, & Tuschen-Caffier B (2011). Electrocortical processing of food and emotional pictures in anorexia nervosa and bulimia nervosa. Psychosomatic Medicine, 73, 415–421. [DOI] [PubMed] [Google Scholar]
- Boehm I, King JA, Bernardoni F, Geisler D, Seidel M, Ritschel F, … Ehrlich S (2018). Subliminal and supraliminal processing of reward-related stimuli in anorexia nervosa. Psychological Medicine, 48, 790–800. [DOI] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, & Crone EA (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DL, Sunday SR, & Halmi KA (1994). Psychiatric comorbidity in patients with eating disorders. Psychological Medicine, 24, 859–867. [DOI] [PubMed] [Google Scholar]
- Broft A, Slifstein M, Osborne J, Kothari P, Morim S, Shingleton R, … Walsh BT (2015). Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Research, 233, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, O’Daly OG, Uher R, Friederich H-C, Giampietro V, Brammer M, … Campbell IC (2011). Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS ONE, 6, e22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Robinson L, Campione GC, Wuensch K, Hildebrandt T, & Micali N (2017). Intolerance of uncertainty in eating disorders: A systematic review and meta-analysis. European Eating Disorders Review, 25, 329–343. [DOI] [PubMed] [Google Scholar]
- Cardi V, Corfield F, Leppanen J, Rhind C, Deriziotis S, Hadjimichalis A, … Treasure J (2015). Emotional processing, recognition, empathy and evoked facial expression in eating disorders: An experimental study to map deficits in social cognition. PLoS ONE, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardi V, Di Matteo R, Corfield F, & Treasure J (2013). Social reward and rejection sensitivity in eating disorders: An investigation of attentional bias and early experiences. The World Journal of Biological Psychiatry, 14, 622–633. [DOI] [PubMed] [Google Scholar]
- Cha J, Ide JS, Bowman FD, Simpson HB, Posner J, & Steinglass JE (2016). Abnormal reward circuitry in anorexia nervosa: A longitudinal, multimodal MRI study. Human Brain Mapping, 37, 3835–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TWS, Ahn W-Y, Bates JE, Busemeyer JR, Guillaume S, Redgrave GW, … Courtet P (2014). Differential impairments underlying decision making in anorexia nervosa and bulimia nervosa: A cognitive modeling analysis. International Journal of Eating Disorders, 47, 157–167. [DOI] [PubMed] [Google Scholar]
- Chevalier N (2018). Willing to think hard? The subjective value of cognitive effort in children. Child Development, 89, 1283–1295. [DOI] [PubMed] [Google Scholar]
- Chowdhury M, & Benson BA (2011). Use of differential reinforcement to reduce behavior problems in adults with intellectual disabilities: A methodological review. Research in Developmental Disabilities, 32, 383–394. [DOI] [PubMed] [Google Scholar]
- Clarke J, Ramoz N, Fladung A-K, & Gorwood P (2016). Higher reward value of starvation imagery in anorexia nervosa and association with the Val66Met BDNF polymorphism. Translational Psychiatry, 6, e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Roberts AC, & Robbins TW (2008). Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences, 12, 31–40. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Finlayson G, & Park RJ (2013). Liking compared with wanting for high- and low-calorie foods in anorexia nervosa: Aberrant food reward even after weight restoration. The American Journal of Clinical Nutrition, 97, 463–470. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, & McCabe C (2011). Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biological Psychiatry, 70, 736–743. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for anhedonia: A neuroscience driven approach. Depression and Anxiety, 33, 927–938. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Peterson CB, Swanson SA, Raymond NC, Specker S, Eckert ED, & Mitchell JE (2009). Increased mortality in bulimia nervosa and other eating disorders. The American Journal of Psychiatry, 166, 1342–1346. [DOI] [PubMed] [Google Scholar]
- Crucianelli L, Cardi V, Treasure J, Jenkinson PM, & Fotopoulou A (2016). The perception of affective touch in anorexia nervosa. Psychiatry Research, 239, 72–78. [DOI] [PubMed] [Google Scholar]
- Cserjési R, Vermeulen N, Lénárd L, & Luminet O (2011). Reduced capacity in automatic processing of facial expression in restrictive anorexia nervosa and obesity. Psychiatry Research, 188, 253–257. [DOI] [PubMed] [Google Scholar]
- Cserjési R, Vermeulen N, Luminet O, Marechal C, Nef F, Simon Y, & Lénárd L (2010). Explicit vs. implicit body image evaluation in restrictive anorexia nervosa. Psychiatry Research, 175, 148–153. [DOI] [PubMed] [Google Scholar]
- da Matta A, Gonçalves FL, & Bizarro L (2012). Delay discounting: concepts and measures. Psychology and Neuroscience, 5, 135–146. [Google Scholar]
- Davies H, Schmidt U, Stahl D, & Tchanturia K (2011). Evoked facial emotional expression and emotional experience in people with anorexia nervosa. International Journal of Eating Disorders, 44, 531–539. [DOI] [PubMed] [Google Scholar]
- Davies H, Schmidt U, & Tchanturia K (2013). Emotional facial expression in women recovered from anorexia nervosa. BMC Psychiatry, 13, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JH, Figner B, & Steinglass JE (2015). On weight and waiting: Delay discounting in anorexia nervosa pretreatment and posttreatment. Biological Psychiatry, 78, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGuzman M, Shott ME, Yang TT, Riederer J, & Frank GKW (2017). Association of elevated reward prediction error response with weight gain in adolescent anorexia nervosa. The American Journal of Psychiatry, 174, 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E, Jessup RK, & Tricomi E (2017). Modulation of ventral striatal activity by cognitive effort. NeuroImage, 147, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier CL, Iwata BA, Thomason-Sassi J, Worsdell AS, & Wilson DM (2012). A comparison of two pairing procedures to establish praise as a reinforcer. Journal of Applied Behavior Analysis, 45, 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dygdon JA, & Dienes KA (2013). Behavioral excesses in depression: a learning theory hypothesis. Depression and Anxiety, 30, 598–605. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Geisler D, Ritschel F, King JA, Seidel M, Boehm I, … Kroemer NB (2015). Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. Journal of Psychiatry & Neuroscience, 40, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger R (1992). Learned industriousness. Psychological Review, 99, 248–267. [DOI] [PubMed] [Google Scholar]
- Ellison Z, Foong J, Howard R, Bullmore E, Williams S, & Treasure J (1998). Functional anatomy of calorie fear in anorexia nervosa. Lancet, 352, 1192. [DOI] [PubMed] [Google Scholar]
- Engel SG, Wonderlich SA, Crosby RD, Mitchell JE, Crow S, Peterson CB, … Gordon KH (2013). The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. Journal of Abnormal Psychology, 122, 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola CR, & Blay SL (2009). Anorexia nervosa’s meaning to patients: A qualitative synthesis. Psychopathology, 42, 69–80. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Redish AD, Oquendo MA, Averbeck BB, Kinnane ME, & Gordon JA (2019). Computational psychiatry: a report from the 2017 NIMH workshop on opportunities and challenges. Molecular Psychiatry, 24, 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons-Craft EE, Accurso EC, Ciao AC, Crosby RD, Cao L, Pisetsky EM, … Wonderlich SA (2015). Restrictive eating in anorexia nervosa: Examining maintenance and consequences in the natural environment. International Journal of Eating Disorders, 48, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladung A-K, Schulze UME, Schöll F, Bauer K, & Grön G (2013). Role of the ventral striatum in developing anorexia nervosa. Translational Psychiatry, 3, e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladung Anne-Katharina, Grön G, Grammer K, Herrnberger B, Schilly E, Grasteit S, … von Wietersheim J (2010). A neural signature of anorexia nervosa in the ventral striatal reward system. The American Journal of Psychiatry, 167, 206–212. [DOI] [PubMed] [Google Scholar]
- Fobbs WC, & Mizumori SJY (2017). A framework for understanding and advancing intertemporal choice research using rodent models. Neurobiology of Learning and Memory, 139, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Steinglass JE, Shohamy D, & Walsh BT (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 18, 1571–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, … Kaye WH (2005). Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biological Psychiatry, 58, 908–912. [DOI] [PubMed] [Google Scholar]
- Frank GKW, Favaro A, Marsh R, Ehrlich S, & Lawson EA (2018). Toward valid and reliable brain imaging results in eating disorders. International Journal of Eating Disorders, 51, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, & Mittal VA (2013). Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. The American Journal of Psychiatry, 170, 1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Collier S, Shott ME, & O’Reilly RC (2016). Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. Journal of Psychiatry & Neuroscience, 41, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, DeGuzman MC, Shott ME, Laudenslager ML, Rossi B, & Pryor T (2018). Association of brain reward learning response with harm avoidance, weight gain, and hypothalamic effective connectivity in adolescent anorexia nervosa. JAMA Psychiatry, 75, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, & O’Reilly RC (2012). Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology, 37, 2031–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Shott ME, & DeGuzman MC (2019). Recent advances in understanding anorexia nervosa. F1000Research, 8 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio S, Wiemerslage L, Brooks SJ, & Schiöth HB (2016). A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neuroscience and Biobehavioral Reviews, 71, 578–589. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Hassler C, Mattar L, Launay J-M, Callebert J, Steiger H, … Godart N (2014). Symptoms of depression and anxiety in anorexia nervosa: Links with plasma tryptophan and serotonin metabolism. Psychoneuroendocrinology, 39, 170–178. [DOI] [PubMed] [Google Scholar]
- Geisler D, Ritschel F, King JA, Bernardoni F, Seidel M, Boehm I, … Ehrlich S (2017). Increased anterior cingulate cortex response precedes behavioural adaptation in anorexia nervosa. Scientific Reports, 7, 42066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannunzio V, Degortes D, Tenconi E, Collantoni E, Solmi M, Santonastaso P, & Favaro A (2018). Decision-making impairment in anorexia nervosa: New insights into the role of age and decision-making style. European Eating Disorders Review, 26, 302–314. [DOI] [PubMed] [Google Scholar]
- Giel KE, Kullmann S, Preißl H, Bischoff SC, Thiel A, Schmidt U, … Teufel M (2013). Understanding the reward system functioning in anorexia nervosa: Crucial role of physical activity. Biological Psychology, 94, 575–581. [DOI] [PubMed] [Google Scholar]
- Glashouwer KA, Bloot L, Veenstra EM, Franken IH, & de Jong PJ (2014). Heightened sensitivity to punishment and reward in anorexia nervosa. Appetite, 75, 97–102. [DOI] [PubMed] [Google Scholar]
- Godier LR, Scaife JC, Braeutigam S, & Park RJ (2016). Enhanced early neuronal processing of food pictures in anorexia nervosa: A magnetoencephalography study. Psychiatry Journal, 2016, 1795901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, O’Connell K, Lieberman L, Alvarez G, Geraci M, Pine DS, & Ernst M (2017). Distinct responses to predictable and unpredictable threat in anxiety pathologies: Effect of panic attack. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 2, 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, & Moran TH (2015). Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiology & Behavior, 152, 466–472. [DOI] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, O’Brien N, Lopez C, & Treasure J (2010). Sensitivity to reward and punishment in eating disorders. Psychiatry Research, 177, 1–11. [DOI] [PubMed] [Google Scholar]
- Hartley CA, & Phelps EA (2012). Anxiety and decision-making. Biological Psychiatry, 72, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Zeeck A, & Barrett MS (2010). Interpersonal problems in eating disorders. International Journal of Eating Disorders, 43, 619–627. [DOI] [PubMed] [Google Scholar]
- Haynos AF, Berg KC, Cao L, Crosby RD, Lavender JM, Utzinger LM, … Crow SJ (2017). Trajectories of higher- and lower-order dimensions of negative and positive affect relative to restrictive eating in anorexia nervosa. Journal of Abnormal Psychology, 126, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynos AF, Hall LMJ, Lavender JM, Peterson CB, Crow SJ, Klimes-Dougan B, … Camchong J (2018). Resting state functional connectivity of networks associated with reward and habit in anorexia nervosa. Human Brain Mapping, 40, 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Haines E, Rajabi H, Stewart J, Arvanitogiannis A, & Shizgal P (2007). Predictable and unpredictable rewards produce similar changes in dopamine tone. Behavioral Neuroscience, 121, 887–895. [DOI] [PubMed] [Google Scholar]
- Holsen L, Lawson E, Blum J, Ko E, Makris N, Fazeli P, … Goldstein J (2012). Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 37, 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lawson EA, Christensen K, Klibanski A, & Goldstein JM (2014). Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Research, 223, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horndasch S, Kratz O, Van Doren J, Graap H, Kramer R, Moll GH, & Heinrich H (2018). Cue reactivity towards bodies in anorexia nervosa - common and differential effects in adolescents and adults. Psychological Medicine, 48, 508–518. [DOI] [PubMed] [Google Scholar]
- Horndasch S, Heinrich H, Kratz O, & Moll GH (2012). The late positive potential as a marker of motivated attention to underweight bodies in girls with anorexia nervosa. Journal of Psychosomatic Research, 73, 443–447. [DOI] [PubMed] [Google Scholar]
- Horndasch Stefanie, Roesch J, Forster C, Dörfler A, Lindsiepe S, Heinrich H, … Kratz O (2018). Neural processing of food and emotional stimuli in adolescent and adult anorexia nervosa patients. PloS One, 13, e0191059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Shenhav A, & Olivola CY (2018). The effort paradox: Effort is both costly and valued. Trends in Cognitive Sciences, 22, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M, Kawabata M, Murao E, Noda T, Matsukawa N, Kawada R, … Takahashi H (2018). Exaggerated envy and guilt measured by economic games in Japanese women with anorexia nervosa. BioPsychoSocial Medicine, 12, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata BA, Dorsey MF, Slifer KJ, Bauman KE, & Richman GS (1994). Toward a functional analysis of self-injury. Journal of Applied Behavior Analysis, 27, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jappe LM, Frank GKW, Shott ME, Rollin MDH, Pryor T, Hagman JO, … Davis E (2011). Heightened sensitivity to reward and punishment in anorexia nervosa. International Journal of Eating Disorders, 44, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Soussignan R, Carrier E, & Royet J-P (2019). Dysfunction of the mesolimbic circuit to food odors in women with anorexia and bulimia nervosa: A fMRI Study. Frontiers in Human Neuroscience, 13, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Soussignan R, Rigaud D, & Schaal B (2010). Pleasure for visual and olfactory stimuli evoking energy-dense foods is decreased in anorexia nervosa. Psychiatry Research, 180, 42–47. [DOI] [PubMed] [Google Scholar]
- Joos AAB, Saum B, van Elst LT, Perlov E, Glauche V, Hartmann A, … Zeeck A (2011). Amygdala hyperreactivity in restrictive anorexia nervosa. Psychiatry Research, 191, 189–195. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, & McConaha C (1999). Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology, 21, 503–506. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, George DT, Jimerson DC, & Ebert MH (1988). CSF 5-HIAA concentrations in anorexia nervosa: Reduced values in underweight subjects normalize after weight gain. Biological Psychiatry, 23, 102–105. [DOI] [PubMed] [Google Scholar]
- Kaye Walter H., Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, … Kishore A (2003). Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. International Journal of Eating Disorders, 33, 257–267. [DOI] [PubMed] [Google Scholar]
- Kaye Walter H., Wierenga CE, Bailer UF, Simmons AN, & Bischoff-Grethe A (2013). Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends in Neurosciences, 36(2), 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, & Simmons WK (2017). Influence of visceral interoceptive experience on the brain’s response to food images in anorexia nervosa. Psychosomatic Medicine, 79, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, & O’Doherty JP (2006). Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biology, 4, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Geisler D, Bernardoni F, Ritschel F, Böhm I, Seidel M, … Ehrlich S (2016). Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 972–979. [DOI] [PubMed] [Google Scholar]
- Klein DA, Schebendach JE, Gershkovich M, Bodell LP, Foltinb RW, & Walsh BT (2010). Behavioral assessment of the reinforcing effect of exercise in women with anorexia nervosa: Further paradigm development and data. International Journal of Eating Disorders, 43, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Giel KE, Hu X, Bischoff SC, Teufel M, Thiel A, … Preissl H (2014). Impaired inhibitory control in anorexia nervosa elicited by physical activity stimuli. Social Cognitive and Affective Neuroscience, 9, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Giel KE, Teufel M, Thiel A, Zipfel S, & Preissl H (2014). Aberrant network integrity of the inferior frontal cortex in women with anorexia nervosa. NeuroImage: Clinical, 4, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbacher S, Pauls AM, Strian F, Pirke KM, & Krieg JC (1990). Pain perception in patients with eating disorders. Psychosomatic Medicine, 52, 673–682. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Pauls AM, Strian F, Pirke KM, & Krieg JC (1991). Pain sensitivity in anorexia nervosa and bulimia nervosa. Biological Psychiatry, 29, 1073–1078. [DOI] [PubMed] [Google Scholar]
- Lempert KM, Steinglass JE, Pinto A, Kable JW, & Simpson HB (2018). Can delay discounting deliver on the promise of RDoC? Psychological Medicine, 1–10. [DOI] [PubMed] [Google Scholar]
- Leppanen J, Cardi V, Paloyelis Y, Simmons A, Tchanturia K, & Treasure J (2017a). Blunted neural response to implicit negative facial affect in anorexia nervosa. Biological Psychology, 128, 105–111. [DOI] [PubMed] [Google Scholar]
- Leppanen J, Cardi V, Paloyelis Y, Simmons A, Tchanturia K, & Treasure J (2017b). FMRI study of neural responses to implicit infant emotion in anorexia nervosa. Frontiers in Psychology, 8, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Yiend J, Schmidt U, & Tchanturia K (2014). Perfectionism in anorexia nervosa: Novel performance based evidence. PloS One, 9, e111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, & Le Grange D (2019). Family-based treatment: Where are we and where should we be going to improve recovery in child and adolescent eating disorders. International Journal of Eating Disorders, 52, 481–487. [DOI] [PubMed] [Google Scholar]
- Massar SAA, Libedinsky C, Weiyan C, Huettel SA, & Chee MWL (2015). Separate and overlapping brain areas encode subjective value during delay and effort discounting. NeuroImage, 120, 104–113. [DOI] [PubMed] [Google Scholar]
- Matton A, Goossens L, Vervaet M, & Braet C (2015). Temperamental differences between adolescents and young adults with or without an eating disorder. Comprehensive Psychiatry, 56, 229–238. [DOI] [PubMed] [Google Scholar]
- Mayer LES, Schebendach J, Bodell LP, Shingleton RM, & Walsh BT (2012). Eating behavior in anorexia nervosa: Before and after treatment. International Journal of Eating Disorders, 45, 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Tregellas JR, Shott ME, & Frank GKW (2014). Reduced salience and default mode network activity in women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 39, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettunen J, & Raevuori A (2012). A meta-analysis of temperament in axis I psychiatric disorders. Comprehensive Psychiatry, 53, 152–166. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, & Crow S (2006). Medical complications of anorexia nervosa and bulimia nervosa. Current Opinion in Psychiatry, 19, 438–443. [DOI] [PubMed] [Google Scholar]
- Miura N, Tanabe HC, Sasaki AT, Harada T, & Sadato N (2017). Neural evidence for the intrinsic value of action as motivation for behavior. Neuroscience, 352, 190–203. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Okamoto Y, Onoda K, Kurosaki M, Shirao N, Okamoto Y, & Yamawaki S (2010). Brain activation during the perception of distorted body images in eating disorders. Psychiatry Research, 181, 183–192. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Okamoto Y, Onoda K, Shirao N, Okamoto Y, Otagaki Y, & Yamawaki S (2010). Neural processing of negative word stimuli concerning body image in patients with eating disorders: An fMRI study. NeuroImage, 50, 1333–1339. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Monteleone P, Esposito F, Prinster A, Volpe U, Cantone E, … Maj M (2017). Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: An fMRI study. Journal of Psychiatric Research, 90, 94–101. [DOI] [PubMed] [Google Scholar]
- Murao E, Sugihara G, Isobe M, Noda T, Kawabata M, Matsukawa N, … Noma S (2017). Differences in neural responses to reward and punishment processing between anorexia nervosa subtypes: An fMRI study. Psychiatry and Clinical Neurosciences, 71, 647–658. [DOI] [PubMed] [Google Scholar]
- Murayama K, Matsumoto M, Izuma K, Sugiura A, Ryan RM, Deci EL, & Matsumoto K (2015). How self-determined choice facilitates performance: A key role of the ventromedial prefrontal cortex. Cerebral Cortex, 25, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Murray SB, Quintana DS, Loeb KL, Griffiths S, & Le Grange D (2019). Treatment outcomes for anorexia nervosa: A systematic review and meta-analysis of randomized controlled trials. Psychological Medicine, 49, 535–544. [DOI] [PubMed] [Google Scholar]
- Murray SB, & Strigo IA (2018). Anorexia nervosa, neuroimaging research, and the contextual salience of food cues: The food approach-avoidance conundrum. International Journal of Eating Disorders, 51, 822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimeijer RA, de Jong PJ, & Roefs A (2015). Automatic approach/avoidance tendencies towards food and the course of anorexia nervosa. Appetite, 91, 28–34. [DOI] [PubMed] [Google Scholar]
- Neimeijer RAM, Roefs A, Glashouwer KA, Jonker NC, & de Jong PJ (2019). Reduced automatic approach tendencies towards task-relevant and task-irrelevant food pictures in anorexia nervosa. Journal of Behavioral Therapy and Experimental Psychiatry, 65, 101496. [DOI] [PubMed] [Google Scholar]
- Novosel A, Lackner N, Unterrainer H-F, Dunitz-Scheer M, Scheer PJZ, Wallner-Liebmann SJ, & Neuper C (2014). Motivational processing of food cues in anorexia nervosa: A pilot study. Eating and Weight Disorders, 19, 169–175. [DOI] [PubMed] [Google Scholar]
- Oberndorfer TA, Frank GKW, Simmons AN, Wagner A, McCurdy D, Fudge JL, … Kaye WH (2013). Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. American Journal of Psychiatry, 170, 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, … Kaye W (2013). Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Research, 214, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara CB, Keyes A, Renwick B, Giel KE, Campbell IC, & Schmidt U (2016). Evidence that illness-compatible cues are rewarding in women recovered from anorexia nervosa: A study of the effects of dopamine depletion on eye-blink startle responses. PloS One, 11, e0165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara CB, Keyes A, Renwick B, Leyton M, Campbell IC, & Schmidt U (2016). The effects of acute dopamine precursor depletion on the reinforcing value of exercise in anorexia nervosa. PloS One, 11, e0145894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, & Cheer JF (2012). Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. Journal of Neuroscience, 32, 14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsavsky AK, Shott ME, DeGuzman MC, & Frank GKW (2018). Neural correlates of taste reward value across eating disorders. Psychiatry Research. Neuroimaging [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BK, Cheng CM, Govorun O, & Stewart BD (2005). An inkblot for attitudes: Affect misattribution as implicit measurement. Journal of Personality and Social Psychology, 89, 277–293. [DOI] [PubMed] [Google Scholar]
- Pennesi JL, & Wade TD (2016). A systematic review of the existing models of disordered eating: Do they inform the development of effective interventions? Clinical Psychology Review, 43, 175–192. [DOI] [PubMed] [Google Scholar]
- Piccolo M, Milos G, Bluemel S, Schumacher S, Müller-Pfeiffer C, Fried M, … Martin-Soelch C (2019). Food vs money? Effects of hunger on mood and behavioral reactivity to reward in anorexia nervosa. Appetite, 134, 26–33. [DOI] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin M-L, & Dreher J-C (2010). Separate valuation subsystems for delay and effort decision costs. The Journal of Neuroscience, 30, 14080–14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruis TA, Keel PK, & Janowsky JS (2012). Recovery from anorexia nervosa includes neural compensation for negative body image. International Journal of Eating Disorders, 45, 919–931. [DOI] [PubMed] [Google Scholar]
- Redgrave GW, Bakker A, Bello NT, Caffo BS, Coughlin JW, Guarda AS, … Moran TH (2008). Differential brain activation in anorexia nervosa to fat and thin words during a stroop task. Neuroreport, 19, 1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoli F, Pavone EF, & Pezzulo G (2012). Aversive pavlovian responses affect human instrumental motor performance. Frontiers in Neuroscience, 6, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritschel F, King JA, Geisler D, Flohr L, Neidel F, Boehm I, … Ehrlich S (2015). Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychological Medicine, 45, 1229–1239. [DOI] [PubMed] [Google Scholar]
- Ritschel F, Geisler D, King JA, Bernardoni F, Seidel M, Boehm I, … Ehrlich S (2017). Neural correlates of altered feedback learning in women recovered from anorexia nervosa. Scientific Reports, 7, 5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotella F, Mannucci E, Gemignani S, Lazzeretti L, Fioravanti G, & Ricca V (2018). Emotional eating and temperamental traits in eating disorders: A dimensional approach. Psychiatry Research, 264, 1–8. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Buchwald C, Georgiewa P, Bohner G, Bauknecht H-C, Ballmaier M, … Klingebiel R (2011). Compulsivity predicts fronto striatal activation in severely anorectic individuals. Neuroscience, 197, 242–250. [DOI] [PubMed] [Google Scholar]
- Sanders N, Smeets PAM, van Elburg AA, Danner UN, van Meer F, Hoek HW, & Adan RAH (2015). Altered food-cue processing in chronically ill and recovered women with anorexia nervosa. Frontiers in Behavioral Neuroscience, 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife JC, Godier LR, Reinecke A, Harmer CJ, & Park RJ (2016). Differential activation of the frontal pole to high vs low calorie foods: The neural basis of food preference in Anorexia Nervosa? Psychiatry Research. Neuroimaging, 258, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, & Walsh BT (2011). Food choice and diet variety in weight-restored patients with anorexia nervosa. Journal of the American Dietetic Association, 111, 732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebendach J, Klein DA, Mayer LES, Attia E, Devlin MJ, Foltin RW, & Walsh BT (2017). Assessment of the motivation to use artificial sweetener among individuals with an eating disorder. Appetite, 109, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Seeger G, Braus DF, Ruf M, Goldberger U, & Schmidt MH (2002). Body image distortion reveals amygdala activation in patients with anorexia nervosa -- a functional magnetic resonance imaging study. Neuroscience Letters, 326, 25–28. [DOI] [PubMed] [Google Scholar]
- Selby EA, Cornelius T, Fehling KB, Kranzler A, Panza EA, Lavender JM, … Le Grange D (2015). A perfect storm: Examining the synergistic effects of negative and positive emotional instability on promoting weight loss activities in anorexia nervosa. Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1976). About Behaviorism. New York: Vintage. [Google Scholar]
- Soh N, Surgenor LJ, Touyz S, & Walter G (2007). Eating disorders across two cultures: Does the expression of psychological control vary? The Australian and New Zealand Journal of Psychiatry, 41, 351–358. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Jiang T, Rigaud D, Royet JP, & Schaal B (2010). Subliminal fear priming potentiates negative facial reactions to food pictures in women with anorexia nervosa. Psychological Medicine, 40, 503–514. [DOI] [PubMed] [Google Scholar]
- Soussignan R Schaal B, Rigaud D, Royet J-P, & Jiang T (2011). Hedonic reactivity to visual and olfactory cues: Rapid facial electromyographic reactions are altered in anorexia nervosa. Biological Psychology, 86, 265–272. [DOI] [PubMed] [Google Scholar]
- Spring VL, & Bulik CM (2014). Implicit and explicit affect toward food and weight stimuli in anorexia nervosa. Eating Behaviors, 15, 91–94. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Dalack M, & Foerde K (2019). The promise of neurobiological research in anorexia nervosa. Current Opinions in Psychiatry. doi: 10.1097/YCO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinglass JE, Lempert KM, Choo T-H, Kimeldorf MB, Wall M, Walsh BT, … Simpson HB (2017). Temporal discounting across three psychiatric disorders: Anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depression and Anxiety, 34, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T, Mestre-Bach G, Vintró-Alcaraz C, Agüera Z, Jiménez-Murcia S, Granero R, & Fernández-Aranda F (2017). Delay discounting of reward and impulsivity in eating disorders: From anorexia nervosa to binge eating disorder. European Eating Disorders Review, 25, 601–606. [DOI] [PubMed] [Google Scholar]
- Stoner SA, Fedoroff IC, Andersen AE, & Rolls BJ (1996). Food preferences and desire to eat in anorexia and bulimia nervosa. International Journal of Eating Disorders, 19, 13–22. [DOI] [PubMed] [Google Scholar]
- Story GW, Vlaev I, Seymour B, Winston JS, Darzi A, & Dolan RJ (2013). Dread and the disvalue of future pain. PLoS Computational Biology, 9, e1003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist J, Ohlsson H, Winkleby MA, Sundquist K, & Crump C (2016). School achievement and risk of eating disorders in a swedish national cohort. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 41–46.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Watson KK, Erwin SR, Winecoff AA, Datta N, Huettel S, … Zucker NL (2018). Neurobiology of social reward valuation in adults with a history of anorexia nervosa. PloS One, 13, e0205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Samejima K, Okada G, Ueda K, Okamoto Y, Yamawaki S, & Doya K (2006). Brain mechanism of reward prediction under predictable and unpredictable environmental dynamics. Neural Networks, 19, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Harrison A, Fox JRE, Treasure J, & Schmidt U (2012). Altered social hedonic processing in eating disorders. International Journal of Eating Disorders, 45, 962–969. [DOI] [PubMed] [Google Scholar]
- Titova OE, Hjorth OC, Schiöth HB, & Brooks SJ (2013). Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta-analysis of VBM studies. BMC Psychiatry, 13, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SCR, & Treasure J (2003). Recovery and chronicity in anorexia nervosa: Brain activity associated with differential outcomes. Biological Psychiatry, 54, 934–942. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, … Treasure J (2004). Medial prefrontal cortex activity associated with symptom provocation in eating disorders. American Journal of Psychiatry, 161, 1238–1246. [DOI] [PubMed] [Google Scholar]
- Van Boxtel A, & Jessurun M (1993). Amplitude and bilateral coherency of facial and jaw-elevator EMG activity as an index of effort during a two-choice serial reaction task. Psychophysiology, 30, 589–604. [DOI] [PubMed] [Google Scholar]
- Veenstra EM, de Jong PJ (2011). Reduced automatic motivational orientation towards food in restricting anorexia nervosa. Journal of Abnormal Psychology, 120, 708–718. [DOI] [PubMed] [Google Scholar]
- Verharen JPH, Danner UN, Schröder S, Aarts E, van Elburg AA, Adan RAH (2019). Insensitivity to losses: A core feature in patients with anorexia nervosa? Biological Psychiatry Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Via E, Soriano-Mas C, Sánchez I, Forcano L, Harrison BJ, Davey CG, … Cardoner N (2015). Abnormal social reward responses in anorexia nervosa: An fMRI study. PLOS ONE, 10, e0133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E, Zalesky A, Sánchez I, Forcano L, Harrison BJ, Pujol J, … Fornito A (2014). Disruption of brain white matter microstructure in women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 39, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, & Suchan B (2010). Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: An fMRI study. Journal of Psychiatry & Neuroscience, 35, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S, Herpertz S, Rosenberger C, Senf W, & Gizewski ER (2011). Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: An fMRI study. Journal of Psychiatric Research, 45, 395–403. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, … Kaye WH (2007). Altered reward processing in women recovered from anorexia nervosa. The American Journal of Psychiatry, 164, 1842–1849. [DOI] [PubMed] [Google Scholar]
- Walsh BT (2013). The enigmatic persistence of anorexia nervosa. The American Journal of Psychiatry, 170(5), 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, … Rockert W (2006). Fluoxetine after weight restoration in anorexia nervosa: A randomized controlled trial. JAMA, 295, 2605–2612. [DOI] [PubMed] [Google Scholar]
- Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, … Bulik CM (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics, 51, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Werling DM, Zucker NL, & Platt ML (2010). Altered social reward and attention in anorexia nervosa. Frontiers in Psychology, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Bischoff-Grethe A, Melrose AJ, Irvine Z, Torres L, Bailer UF, … Kaye WH (2015). Hunger does not motivate reward in women remitted from anorexia nervosa. Biological Psychiatry, 77, 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Ely A, Bischoff-Grethe A, Bailer UF, Simmons AN, & Kaye WH (2014). Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Frontiers in Behavioral Neuroscience, 8, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hill L, Knatz Peck S, McCray J, Greathouse L, Peterson D, … Kaye WH (2018). The acceptability, feasibility, and possible benefits of a neurobiologically-informed 5-day multifamily treatment for adults with anorexia nervosa. International Journal of Eating Disorders, 51, 863–869. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004). Dopamine, learning and motivation. Nature Reviews Neuroscience, 5, 483–494. [DOI] [PubMed] [Google Scholar]
- Yoncheva YN, Castellanos FX, Pizinger T, Kovtun K, St-Onge MP (2016). Sleep and meal-time misalignment alters functional connectivity: a pilot resting-state study. International Journal of Obesity, 40, 1813–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamroziewicz MK, Talukdar MT, Zwilling CE, & Barbey AK (2017). Nutritional status, brain network organization, and general intelligence. Neuroimage, 161, 241–250. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hu X, Wang J, Chen J, Guo Q, Li C, & Enck P (2012). Processing of food, body and emotional stimuli in anorexia nervosa: A systematic review and meta-analysis of functional magnetic resonance imaging studies. European Eating Disorders Review 20, 439–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


