Abstract
On March 11, 2020, the World Health Organization declared coronavirus disease (COVID-19), caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a pandemic. During the COVID-19 pandemic, an age-associated vulnerability in the burden of disease has been uncovered. Understanding the spectrum of illness and the pathogenic mechanism of the disease in a vulnerable population is critical, especially during the pandemic. Herein, we reviewed published COVID-19 epidemiology data from several countries to identify any consistent trends in the relationship between age and COVID-19-associated morbidity or mortality. We also reviewed the literature for studies explaining the difference in the host response to SARS-CoV-2 infection according to age. The insights from these data will be useful in determining the treatment policies and preventive measures of COVID-19.
Keywords: COVID-19, Pandemic, Age, Morbidity, Mortality
INTRODUCTION
Since the first reported case of coronavirus disease (COVID-19) in China on December 31, 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected 5,592,890 people and killed 353,334 people worldwide as of May 28, 2020, according to the World Health Organization (WHO) [1]. In the last 3 – 4 months, there has been an overflow of scientific and non-scientific information on SARS-CoV-2 and COVID-19. The relationship between age and COVID-19-associated morbidity or mortality has also been addressed among health professionals and the general population. The surge of infected patients beyond the limits of medical systems has raised social concerns on whether age should be considered in determining treatment intensity [2]. The perception that young people's morbid condition is relatively less severe also lowered their awareness of preventing infection in some regions.
One of the most critical issues dealt by clinical and public health professionals during the pandemic is the spectrum of illness severity. This affects the triage, diagnostic and therapeutic decision making, and prognostic expectations; therefore, understanding COVID-19-associated morbidity and mortality according to age is important. However, the data published in each country are inconsistent because of multiple reasons such as different populations, extent of available laboratory tests, and medical systems. Hence, we reviewed COVID-19 epidemiology data published from several countries to identify any consistent trends in the relationship between age and COVID-19-associated morbidity or mortality. We also reviewed the literature for studies explaining the difference in the host response to SARS-CoV-2 infection according to age.
COVID-19-associated morbidity and mortality according to age
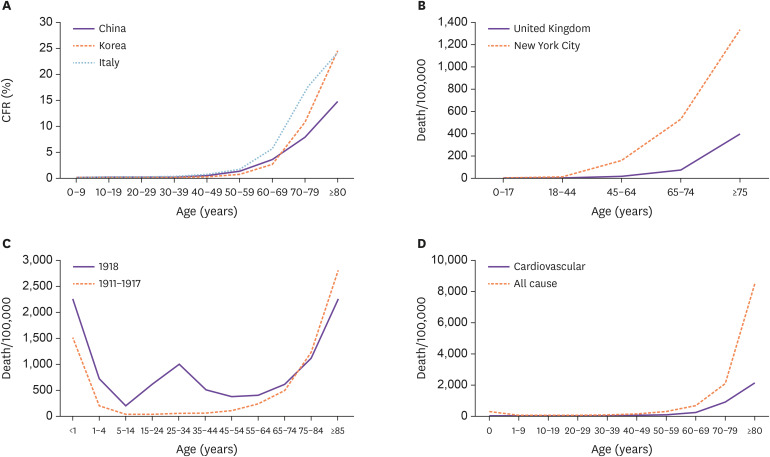
COVID-19 fatality rates vary significantly depending on the country; it was 0.06% in Qatar and 16.25% in Belgium as of May 26, 2020 [3]. This variation in fatality rates may be due to differences in healthcare adequacy and/or epidemiological characteristics of patients; the frequency of diagnostic screening in asymptomatic or mildly symptomatic patients may also influence the rate [4]. However, there is a consistent and clear pattern of an age-based exponential increase in fatality rate, regardless of the geographic region. According to the Korea Centers for Disease Control and Prevention, the overall case fatality rate (CFR) was 2.37% in 11,344 patients with confirmed cases on May 28, 2020, but it was much higher in the elderly (10.9% in patients aged 70 – 79 years and 26.6% in patients ≥80 years) [5]. In another analysis of 44,672 cases in China diagnosed as of February 11, 2020, the overall CFR was 2.3%. However, the CFR was 8.0% in patients aged 70 – 79 years and 14.8% in patients aged ≥80 years [6]. In the report released by the Higher Institute of Health of Italy, the overall CFR on March 26, 2020, was 9.2%, which was four times higher than that in Korea or China; however, the pattern of increasing fatality with age was similar to that in Korea and China. The CFR was <1% in the age group of <50 years and rapidly increased in the age group of ≥60 years, reaching 16.9% and 24.4% in the age group of 70 – 79 years and ≥80 years, respectively (Fig. 1A) [7]. In terms of death per 100,000 individuals in the population (as of May 11, 2020, data from the New York City Department of Health and Mental Hygiene, and as of May 8, 2020, data from the Office for National Statistics of United Kingdom), clear patterns of age-based exponential increase in fatality has been observed (Fig. 1B) [8,9].
Figure 1. Age-related risk of death from coronavirus disease (COVID-19), influenza, and overall causes. (A) Case fatality rate (CFR, death/laboratory confirmed cases) of COVID-19 in China, Korea (accumulated data until May 10, 2020), and Italy (as of March 26, 2020). (B) Death per 100,000 from COVID-19 in New York City (as of May 11, 2020) and United Kingdom (as of May 8, 2020). (C) Death per 100,000 from influenza and pneumonia in interpandemic years (dashed line 1911 - 1917) and pandemic year (solid line 1918) in the United Sates. Adapted from Taubenberger et al [10]. (D) All-cause mortality and cardiovascular mortality (death per 100,000) according to age in Korea, 2018. Data were extracted from “Cause-of-death statistics in 2018,” released by Statistics Korea [15].
Influenza, the most commonly reported pandemic disease in human history, had a W-shaped mortality distribution during the pandemic (Fig. 1C) [10,11]. This atypical pattern of mortality with an elevated relative or absolute risk of death in middle-age groups is usually explained by differences in age-related susceptibility to infection, affected by pre-existing age-related immunity [11]. As SARS-CoV-2 is a novel virus introduced to human, this type of mortality distribution is unlikely to be expected. However, the age-related pattern of COVID-19 fatality differs from that of other respiratory viral infections, wherein the severity pattern is often described as a U-shaped curve, with morbidity and mortality concentrated at extreme age groups (younger children and the elderly). Population-based studies of seasonal influenza have typically shown this type of mortality distribution according to age (Fig. 1C) [12]. Several cohort studies have shown that the hospitalization rate of influenza was highest for the age group of 0 – 4 years compared to that in other age groups, which indicates that children under the age of 5 years could be more vulnerable to influenza and/or more severely affected [13,14]. As the hospitalization fatality rate was relatively low for the age group of 0 – 4 years [13,14], the mortality curve based on 100,000 individuals showed a much smaller peak in this age group than in the elderly age group. However, this type of mortality peak has not been observed thus far in children affected with COVID-19, which suggests that children could be less susceptible to the infection or experience less severe symptoms and have a low CFR.
The dynamics of age-specific mortality for COVID-19 is relatively familiar because they mirror other major causes of mortality, especially chronic diseases such as cardiovascular disease (Fig. 1D) [15]. Promislow et al. have shown that the mortality rate doubling time (MRDT) of all-cause mortality (9 years) in the United States was approximately the same as that of COVID-19 reported in the New York City [16]. In other words, the increasing case fatality or mortality rate with age is not a specific finding for COVID-19. However, many scientists and media have paid considerable attention to age as a risk factor for mortality in COVID-19. This might be due to the surge of infected patients within a short period; hence, the death pattern — high mortality in older people — became more apparent.
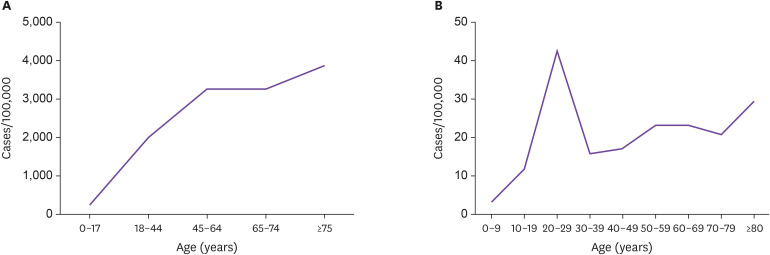
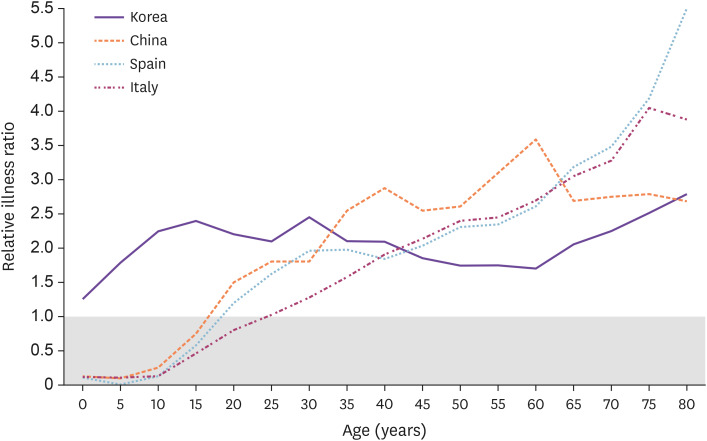
Investigating the susceptibility to infection, unlike mortality or case fatality, in the general population is more complicated because mildly symptomatic or asymptomatic patients may not have been tested and there is an uneven chance of being exposed to the source of infection depending on the groups (closure of child care facilities, preschools, and schools vs. non-closure of long-term care facilities). The case rate of COVID-19 by age has been reported to have wide regional variability. According to New York City data (as of May 11, 2020), the CFR of COVID-19 in the general population (cases per 100,000) seemed to increase with age (Fig. 2A) [8]. Contrarily, in Korea (as of May 10, 2020), where diagnostic tests were performed intensely in groups associated with certain religious gatherings, regardless of any symptoms, the CFR seemed to be highest in the 20 – 29-years age group (Fig. 2B) [5]. Natale et al. have compared the relative illness ratio (RIR) by age between different countries (Fig. 3) [17]. In their study, as of Mar 30, 2020, the RIR in Spain and Italy increased with age, while in China and Korea [18], middle or younger age groups seemed to have a higher RIR. This could be explained by more extensive testing performed in China and Korea than in other countries, which would help to better measure the morbidity among young age groups. In Italy and New York City, where there was a surge in the number of COVID-19 patients in a short period, it is likely that the viral test was performed first in individuals with more apparent or severe symptoms, while asymptomatic or mildly symptomatic patients were not tested. Overall, these findings suggest that susceptibility to symptomatic infection seems to increase with age, but susceptibility to infection is probably similar among different age groups.
Figure 2. Case rate (cases per 100,000) of COVID-19 according to age in different regions. (A) New York City (as of May 11, 2020). (B) Korea (as of May 10, 2020).
Figure 3. Relative illness ratio by 5-year age groups. Relative Illness Ratio (RIR) is a measure of morbidity calculated according to the following formula as the share of COVID-19 cases in each age group with regard to the total number of morbidity cases divided by the share of the population in the same age group. , where COVID is the estimated number of cases of COVID-19 in age group ἰ, and P is the population in the same age group. A value above 1 indicates that there is a higher proportion of cases in the age group relative to other age groups, after considering differences in the size of the population. Adapted from Natale et al. [17].
COVID-19 in the elderly
Among COVID-19 patients, elderly patients have a higher mortality rate due to high CFR and symptomatic infection rate. Approximately 80% and 90% of deaths have occurred in patients aged >70 years and ≥60 years in Korea and Italy, respectively [5,7]. A similar pattern was observed in other countries affected by COVID-19. Several studies have reported old age to be a significant risk factor for COVID-19 mortality [19,20]. Age also affects the time from hospitalization to death and viral clearance [21,22]. In an animal study, SARS-CoV-2 caused more severe interstitial pneumonia and viral replication in lung tissues of old monkeys than in those of young monkeys [23].
In immunopathology, vulnerability to an infection in the elderly is usually explained by immunosenescence [24]. Immunosenescence is quite complicated. Briefly, in old age, the production of naïve T and B cells decreases, and the function of innate immune cells is impaired; hence, cells involved in the innate immunity do not get activated efficiently during an infection, and progression to an adaptive immune response does not occur in a coordinated manner [24]. These changes reduce the effectiveness of viral clearance and increase the likelihood of triggering a dysregulated immune response in which cytokines are released extensively by activated immune cells, resulting in a cytokine storm [25]. Another well-recognized feature of aging immunity is chronic subclinical systemic inflammation, also known as inflammaging. Inflammation is a key pathogenic mechanism in COVID-19; hence, inflammaging has been estimated to contribute to the poorer outcome in elderly patients with COVID-19 [26].
Some scientists have claimed that the biologically plausible pathomechanism explaining the difference in vulnerability to SARS-CoV-2 infection involves the so-called antibody-dependent enhancement (ADE) [27,28,29,30]. ADE is a well-known cascade of events by which viruses may infect susceptible cells through interactions between virions complexed with antibodies and Fc receptors, where they are more extensively endocytosed and eventually replicated more efficiently [31]. The antibodies that bind to virions could be neutralizing or non-neutralizing antibodies, which were previously formed in response to SARS-CoV-2 or other coronaviruses with similar antigenicity to that of SARS-CoV-2. The fact that the seroprevalence of community-acquired coronaviruses among adults was very high (90 – 100%) [32] but not in pediatrics was presented as an evidence [33].
In addition to the aging immunity or ADE, there are several other factors related to aging that could be reasons for higher mortality and morbidity in the elderly. The average number of comorbid conditions steadily increased with age. According to Liu et al., elderly COVID-19 patients had a significantly higher performance score than young and middle-aged patients [34]. In addition, older adults living in long-term care facilities are at the highest risk because of their chronic illness and the impact of congregate housing [35].
COVID-19 in children
Based on published data, SARS-CoV-2 infection seems to affect children less frequently and less severely than adults. According to the data published from different regions, the proportion of children among COVID-19 patients was quite low (2.1 – 2.4% in China, 1.3% in Italy, 2.8% in Australia, and 7.0% in Korea) [5,6,7,36,37]. In the largest pediatric study to date that analyzed 2,143 children infected with SARS-CoV-2, 5.8% children showed severe and critical illness [38]. Contrary to the pediatric study, 18.5% patients were severe and critical among all age groups in an analysis of 44,672 Chinese cases with COVID-19 [6]. Among children, Dong et al. found that infants were relatively more vulnerable to SARS-CoV-2 infection, and the proportion of severe and critical cases was 10.7% [38]. However, because the study also included suspected cases (not laboratory confirmed cases, 65.9% of study population), the possibility of pathogens other than SARS-CoV-2 responsible for such clinical observations remains controversial. In other studies, even though very small number of cases was reported, a severe outcome in infants was not observed [39,40,41]. Although these findings were not similar to the generally accepted fact that children aged <5 years were more vulnerable to respiratory viral infections, there was a common aspect with the previous finding observed in SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) epidemics, which also had a children-sparing pattern [42,43,44]. Stockman et al. reported that the clinical outcome of SARS was more favorable in children aged <12 years of age than in other age groups [43]. No deaths were reported in children with SARS-CoV infection [44]. In the MERS-CoV epidemic, only two pediatric deaths were reported, both of which had serious comorbidities: infantile nephrotic syndrome and cystic fibrosis [45,46,47].
Thus far, there is no clear explanation for the children-sparing pattern of SARS-CoV-2 infection. The following hypotheses have been reported in the literature: 1) the possibility that children are less frequently exposed to infection sources due to closure of child care facilities, preschools, and schools, 2) children are less susceptible to the infection itself, and 3) children are less likely to be symptomatic or develop severe symptoms; hence, the number of cases is underestimated [48,49,50]. More evidence and studies are needed to prove the hypotheses. However, recently, Bi et al reported the result of tracing 1,286 close contacts in China; children were as likely to be infected as adults (infection rate in children aged <10 years: 7.4% vs. population average: 6.6%) but less likely to be symptomatic or develop severe symptoms [51].
A milder symptom or low CFR in children with SARS-CoV-2 infection could be explained by the interaction between host immunological response and viral pathogen mechanism [48,50]. As the differences in the distribution, maturation, and function of viral receptors in the host are frequently reported as a possible reason for the age-related difference in infection, angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-CoV, HCoV-NL63, and SARS-CoV-2, has attracted increasing attention. ACE2 plays a key role is in catalyzing the hydrolysis of angiotensin II into angiotensin (1–7), which has an antihypertensive and profibrotic effect [52,53]. Following viral entry, ACE2 expression is downregulated [54]. Downregulation of ACE2 expression leads to compensatory overproduction of angiotensin II by ACE. Angiotensin II, in turn, stimulates its type 1a receptor, which increases the lung vascular permeability and potentiates lung pathology [55,56,57]. In a study conducted by Xie et al., ACE2 expression was observed to dramatically decrease with aging in a rat model [58]. Low levels of ACE2 have been detected in patients with underlying chronic conditions, which generally do not affect the pediatric population [53,59]. These findings suggest that increased levels of ACE2 receptors in lung pneumocytes in children may have a protective effect from severe clinical manifestations of SARS-CoV-2 infection [48,50,60].
More active and rapid innate immune response to an antigen and an elevated number of B lymphocytes, T lymphocytes, and natural killer cells are also suggested as an immunological mechanism for children to resist an infection [61,62,63]. However, these general immunological characteristics of children are insufficient to explain the children-sparing pattern of SARS-CoV-2, which is a distinctive feature from other respiratory viral infections.
As it is known that many children are asymptomatic or have mild symptoms, there are increasing concerns on children's role in transmitting disease. However, the importance of children in transmitting the virus remains uncertain. The majority of the children infected with SARS-CoV-2 reported thus far have a documented household contact, often showing symptoms before infected children, suggesting the possibility that children are not an important reservoir for SARS-CoV-2.
Another emerging children-related issue is a multisystem inflammatory syndrome mentioned in a scientific brief report published by the WHO on May 15, 2020 [64]. The report describes clusters of children and adolescents requiring admission to intensive care units; these children have a multisystem inflammatory condition, with some features similar to those of Kawasaki disease and toxic shock syndrome, in Europe and North America [65,66,67]. This syndrome has not been reported in other regions thus far, but the full spectrum of the disease is still unclear. Hence, special attention should be warranted to all children with COVID-19 [68].
Conclusion
The CFR and susceptibility to symptomatic COVID-19 is higher in the elderly. As there is no definitive therapeutic drug or a vaccine, prevention is the only and the most important strategy for older adults; the added strain of social distancing on this vulnerable population should be acknowledged and managed. Furthermore, studies on immunopathology for determining the severity of SARS-CoV-2 infection are warranted to develop more effective therapeutic and preventive strategies.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: SJK, SIJ.
- Data curation: SJK, SIJ.
- Formal analysis: SJK, SIJ.
- Writing - original draft: SJK, SIJ.
- Writing - review & editing: SIJ.
References
- 1.World Health Organization (WHO) Coronavirus disease (COVID-2019) situation report -110. [Accessed 29 May 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200509covid-19-sitrep-110.pdf?sfvrsn=3b92992c_6.
- 2.Cesari M, Proietti M. COVID-19 in Italy: ageism and decision making in a pandemic. J Am Med Dir Assoc. 2020;21:576–577. doi: 10.1016/j.jamda.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Centre for Evidence-Based Medicine develops, promotes and disseminates better evidence for healthcare (CEBM) Global covid-19 case fatality rates. [Accessed 29 May 2020]. Available at: https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/
- 4.Kim DH, Choe YJ, Jeong JY. Understanding and interpretation of case fatality rate of coronavirus disease 2019. J Korean Med Sci. 2020;35:e137. doi: 10.3346/jkms.2020.35.e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention (KCDC) Status of COVID-19 in Korea. [Accessed 29 May 2020]. Available at: http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun=
- 6.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) - China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Istituto Superiore di Sanità. Epidemia COVID-19. [Accessed 10 May 2020]. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-aprile-2020.pdf.
- 8.NYC Department of Health and Mental Hygiene. Coronavirus-data. [Accessed 11 May 2020]. Available at: https://github.com/nychealth/coronavirus-data/blob/master/by-age.csv.
- 9.Office for National Statics. Deaths registered weekly in England and Wales, provisional: week ending 8 May 2020. [Accessed 8 May 2020]. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/latest.
- 10.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greer AL, Tuite A, Fisman DN. Age, influenza pandemics and disease dynamics. Epidemiol Infect. 2010;138:1542–1549. doi: 10.1017/S0950268810000579. [DOI] [PubMed] [Google Scholar]
- 12.Bouvier NM. The future of influenza vaccines: a historical and clinical perspective. Vaccines (Basel) 2018;6:58. doi: 10.3390/vaccines6030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliva J, Delgado-Sanz C, Larrauri A Spanish Influenza Surveillance System. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010-2016. Influenza Other Respi Viruses. 2018;12:161–170. doi: 10.1111/irv.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von der Beck D, Seeger W, Herold S, Günther A, Löh B. Characteristics and outcomes of a cohort hospitalized for pandemic and seasonal influenza in Germany based on nationwide inpatient data. PLoS One. 2017;12:e0180920. doi: 10.1371/journal.pone.0180920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Korea. Cause-of-death statistics in 2018. [Accessed 29 May 2020]. Available at: https://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=read&bSeq=&aSeq=377606&pageNo=18&rowNum=10&navCount=10&currPg=&searchInfo=&sTarget=title&sTxt=
- 16.Promislow DEL. A geroscience perspective on COVID-19 mortality. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa094. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natale A, Ghio D, Tarchi D, Goujon A, Conte A. COVID-19 cases and case fatality rate by age. Knowledge for policy. 2020. [Accessed 11 May 2020]. Available at: https://ec.europa.eu/knowledge4policy/publication/covid-19-cases-case-fatality-rate-age_en.
- 18.Koo BK, Bang JH, Kim SY, Kim EJ, Park SW. Glove-Wall System for Respiratory Specimen Collection and COVID-19 Mass Screening. Infect Chemother. 2020;52:219–221. doi: 10.3947/ic.2020.52.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech Ageing Dev. 2020;188:111255. doi: 10.1016/j.mad.2020.111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Xing Y, Jia J, Ni W, Liang J, Zhao D, Song X, Gao R, Jiang F. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi: 10.1016/j.scitotenv.2020.138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev Med Virol. 2020;30:e2103. doi: 10.1002/rmv.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu P, Qi F, Xu Y, Li F, Liu P, Liu J, Bao L, Deng W, Gao H, Xiang Z, Xiao C, Lv Q, Gong S, Liu J, Song Z, Qu Y, Xue J, Wei Q, Liu M, Wang G, Wang S, Yu H, Liu X, Huang B, Wang W, Zhao L, Wang H, Ye F, Zhou W, Zhen W, Han J, Wu G, Jin Q, Wang J, Tan W, Qin C. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med. 2020;3:93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 25.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F. Inflamm-aging: Why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.04.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadkhoda K. COVID-19: an immunopathological view. MSphere. 2020;5:e00344–20. doi: 10.1128/mSphere.00344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilocca B, Soggiu A, Musella V, Britti D, Sanguinetti M, Urbani A, Roncada P. Molecular basis of COVID-19 relationships in different species: a one health perspective. Microbes Infect. 2020;22:218–220. doi: 10.1016/j.micinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly. 2020;150:w20249. doi: 10.4414/smw.2020.20249. [DOI] [PubMed] [Google Scholar]
- 31.Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13:387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 32.Gorse GJ, Patel GB, Vitale JN, O'Connor TZ. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010;17:1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prill MM, Iwane MK, Edwards KM, Williams JV, Weinberg GA, Staat MA, Willby MJ, Talbot HK, Hall CB, Szilagyi PG, Griffin MR, Curns AT, Erdman DD New Vaccine Surveillance Network. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J. 2012;31:235–240. doi: 10.1097/INF.0b013e31823e07fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–8. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T. Improving preparedness for and response to coronavirus disease 19 (COVID-19) in long-term care hospitals in Korea. Infect Chemother. 2020;52:133–141. doi: 10.3947/ic.2020.52.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO) Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19), 16–24 February 2020. [Accessed 11 May 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 37.COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: epidemiology report 6 (Reporting week ending 19:00 AEDT 7 March 2020) Commun Dis Intell. 2020:44. doi: 10.33321/cdi.2020.44.21. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 39.Kam KQ, Yung CF, Cui L, Lin Tzer Pin R, Mak TM, Maiwald M, Li J, Chong CY, Nadua K, Tan NWH, Thoon KC. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa201. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, Bi Y. Novel coronavirus infection in newborn babies <28 days in China. Eur Respir J. 2020;55:2000697. doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- 43.Stockman LJ, Massoudi MS, Helfand R, Erdman D, Siwek AM, Anderson LJ, Parashar UD. Severe acute respiratory syndrome in children. Pediatr Infect Dis J. 2007;26:68–74. doi: 10.1097/01.inf.0000247136.28950.41. [DOI] [PubMed] [Google Scholar]
- 44.Leung CW, Kwan YW, Ko PW, Chiu SS, Loung PY, Fong NC, Lee LP, Hui YW, Law HK, Wong WH, Chan KH, Peiris JS, Lim WW, Lau YL, Chiu MC. Severe acute respiratory syndrome among children. Pediatrics. 2004;113:e535–43. doi: 10.1542/peds.113.6.e535. [DOI] [PubMed] [Google Scholar]
- 45.Bartenfeld M, Griese S, Uyeki T, Gerber SI, Peacock G. Middle East respiratory syndrome coronavirus and children. Clin Pediatr (Phila) 2017;56:187–189. doi: 10.1177/0009922816678820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thabet F, Chehab M, Bafaqih H, Al Mohaimeed S. Middle East respiratory syndrome coronavirus in children. Saudi Med J. 2015;36:484–486. doi: 10.15537/smj.2015.4.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Memish ZA, Al-Tawfiq JA, Assiri A, AlRabiah FA, Al Hajjar S, Albarrak A, Flemban H, Alhakeem RF, Makhdoom HQ, Alsubaie S, Al-Rabeeah AA. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904–906. doi: 10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 48.Cristiani L, Mancino E, Matera L, Nenna R, Pierangeli A, Scagnolari C, Midulla F. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55:2000749. doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, Liu X, Wei L, Truelove SA, Zhang T, Gao W, Cheng C, Tang X, Wu X, Wu Y, Sun B, Huang S, Sun Y, Zhang J, Ma T, Lessler J, Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 53.Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) Is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB, Jr, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172:147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tosato F, Bucciol G, Pantano G, Putti MC, Sanzari MC, Basso G, Plebani M. Lymphocytes subsets reference values in childhood. Cytometry A. 2015;87:81–85. doi: 10.1002/cyto.a.22520. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization (WHO) Multisystem inflammatory syndrome in children and adolescents with COVID-19. [Accessed 11 May 2020]. Available at: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 65.DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.05.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 68.Kim YJ, Park H, Choi YY, Kim YK, Yoon Y, Kim KR, Choi EH. Defining association between COVID-19 and the multisystem inflammatory syndrome in children through the pandemic. J Korean Med Sci. 2020;35:e204. doi: 10.3346/jkms.2020.35.e204. [DOI] [PMC free article] [PubMed] [Google Scholar]