Abstract
Introduction and aim
Low serum ceruloplasmin levels can occur in patients without Wilson's disease (WD) liver disorders. When present, extensive, costly, and potentially harmful additional investigations for WD may be undertaken. The purpose of this study was to document the prevalence of low serum ceruloplasmin levels in adult patients without WD and describe the features commonly associated with this finding.
Materials and methods
Serum ceruloplasmin levels were measured by an enzymatic assay in 3040 adult patients attending an urban, liver diseases outpatient clinic.
Results
A total of 122 (4.0%) patients without WD had serum ceruloplasmin levels less than the lower limit of normal documented at their initial visit. Their mean age was 44 ± 14 years, and 80 (66%) were men. The Model for End-stage Liver Disease (MELD) score was 9.0 ± 4.0. Approximately, one half (65/122, 53%) had underlying viral hepatitis (52% hepatitis B and 48% hepatitis C). When compared with 64 MELD-matched control patients with normal or elevated serum ceruloplasmin levels, there were no significant differences in liver enzyme/function tests, ferritin, creatinine values, or survival. However, the low serum ceruloplasmin cohort patients were younger (43 ± 14 versus 52 ± 13 years, p = 0.0002), less often men (66% vs. 88%, p = 0.001), and viral hepatitis was significantly more common (53% versus 27%, p = 0.0005).
Conclusion
Low serum ceruloplasmin levels were documented in 4.0% of adult patients without WD attending this urban liver diseases outpatient clinic. These patients tend to be younger, less often men, and more often have viral hepatitis as the underlying cause of their liver disease.
Keywords: ceruloplasmin, wilson's disease, liver, viral hepatitis, hepatitis
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASH, alcoholic steatohepatitis; Dx, diagnosis; GGT, gamma-glutamyl transferase; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international ratio of prothrombin times; MELD, Model for End-stage Liver Disease; NASH, nonalcoholic steatohepatitis; T.Bili, total bilirubin; WD, Wilson Disease
Ceruloplasmin is a 151-kDa glycoprotein largely responsible for copper transport within the body. It also possesses ferroxidase activity and antioxidant properties and is involved in the metabolism of polyamines, catecholamines, and phenols.1 Previous data suggest that ceruloplasmin may alter the antigenic shift and viral replicative activity and thus influence the outcome of viral infections.2, 3, 4
The liver and specifically hepatocytes within zone 1 of the liver lobule is the principal site responsible for ceruloplasmin synthesis.5,6 As an acute phase reactant, serum ceruloplasmin levels tend to be increased in conditions associated with active necroinflammatory disease, various malignancies, and during pregnancy.7,8 Levels are also increased in chronic cholestatic disorders of the hepatobiliary system such as primary biliary cirrhosis and sclerosing cholangitis.9,10 Conversely, low serum ceruloplasmin levels are a common finding in patients with Wilson's disease (WD), heterozygote carriers for WD-associated mutations, and in a limited number of patients with fulminant hepatic failure, malabsorption, protein losing enteropathies, renal failure, and aceruloplasminemia, a rare condition associated with severe iron overload.11, 12, 13
Low serum ceruloplasmins have also been described in patients without WD with chronic liver disease.12,14,15 When present, this finding will often result in extensive, costly, and potentially harmful additional investigations. Thus, the objectives of this study were to document the prevalence of low serum ceruloplasmin levels in adult patients without WD attending a large urban liver diseases outpatient program and determine whether certain clinical and/or biochemical features help to identify when non-WD hypoceruloplasminemia is more likely to be present in this patient population.
Materials and Methods
This was a retrospective analysis of adult patients referred to the Liver Diseases Outpatient Program at the Health Sciences Centre, Winnipeg, Manitoba, Canada between 1987 and 2015. A total of 3057 patients had serum ceruloplasmin levels documented during that time period as part of their routine, initial evaluation.
Patients with WD were diagnosed on the basis of their clinical presentations, low serum ceruloplasmin levels, elevated 24 h urine copper levels (>10 fold), presence of Kayser-Fleischer rings on slit-lamp examination, and/or liver histology findings including tissue copper levels being considered diagnostic for WD.12 Genetic testing for ATP7B mutations was not available. Patients without WD with low serum ceruloplasmin levels were matched by Model for End-stage Liver Disease (MELD) scores to patients without WD with normal or elevated serum ceruloplasmin levels referred to the clinic over the same time period.
Pregnant women, patients with cholestatic liver disease, and patients with more than one liver diagnosis were excluded from the analyses.
The following clinical and biochemical features were extracted from the clinic's electronic patient database: age, gender, underlying liver diagnosis, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (T.Bili), albumin, international normalized ratio for prothrombin times (INR), creatinine, ferritin, ceruloplasmin (at presentation and last follow-up visit), duration of follow-up, and survival.
The same database was used to document the prevalence of low serum ceruloplasmin levels in patients with hepatitis B or C infections, nonalcoholic steatohepatitis (NASH), and alcohol-induced liver disease, each diagnosed by standard criteria.16
All laboratory testings were performed by the Clinical Chemistry Department at the Health Sciences Centre or the Cadham Provincial Laboratory (viral loads) in Winnipeg, Manitoba, Canada. Serum ceruloplasmin levels were measured by determining serum oxidase activity in the presence and absence of ozide using p-phenylenediamine as substrate.17 The reaction product was measured at 530 nm.
The chi-square test of association was used to examine differences between categorical variables. Continuous variables were assessed using Student t-test for difference between means or Mann–Whitney U test for differences in medians (as appropriate). Survival data were analyzed by Kaplan–Meier survival analysis and the Cox proportional hazard regression model. Statistical significance was considered present when a P-value observed was less than 5% in all analyses. All statistical analyses were carried out using the SAS statistical software (version 9.3; SAS Institute, Cary, North Carolina).
The study protocol was approved by the University of Manitoba Institutional Research Ethics Board.
The University of Manitoba conjoint Ethics Committee has approved consenting patients assessed in the liver disease outpatient clinic for inclusion of anonymized data in clinical research reviews.
Results
The clinical and laboratory findings for patients with low and normal or elevated serum ceruloplasmin levels are provided in Table 1. Also included for reference purposes only are the findings for 15 patients with WD attending the same clinic over the same period of time.
Table 1.
Demographic and Clinical Characteristics of Patients with Wilson's Disease, Non-WD Low Serum Ceruloplasmin Levels, and MELD-Matched Controls at Presentation.
|
Variable (units and/or normal values) |
Wilson's disease (N = 15) Mean ± SD. |
Non-WD low ceruloplasmin (N = 122) Mean ± SD. |
Controls (N = 64) Mean ± SD. |
P-valuea |
|---|---|---|---|---|
| Age (years) | 27 ± 11 | 44 ± 14 | 52 ± 14 | 0.0002 |
| Men n (%) | 12 (80%) | 80 (66%) | 56 (88%) | 0.001 |
| MELD score | 11 ± 6 | 9 ± 4 | 9 ± 2 | 0.69 |
| Viral hepatitis Dx n (%) | 0 (0%) | 65 (53%) | 17 (27%) | 0.0005 |
| Ceruloplasmin (180–450 mg/L) | 89 ± 96 | 144 ± 39 | 321 ± 75 | 0.00000 |
| ALT (<25 U/L) | 172 ± 449 | 71 ± 123 | 104 ± 265 | 0.35 |
| AST (10–32 U/L) | 144 ± 401 | 59 ± 102 | 65 ± 130 | 0.72 |
| AP (30–120 U/L) | 186 ± 145 | 110 ± 74 | 132 ± 202 | 0.39 |
| GGT (5–29 U/L) | 163 ± 400 | 114 ± 228 | 172 ± 222 | 0.09 |
| Albumin (33–45 g/L) | 39 ± 6 | 39 ± 18 | 39 ± 6 | 0.97 |
| Total bilirubin (5–21 umol/L) | 43 ± 100 | 17 ± 26 | 18 ± 9 | 0.68 |
| INR (0.9–1.1) | 1.3 ± 0.56 | 1.11 ± 0.23 | 1.07 ± 0.09 | 0.26 |
| Mean follow-up (months) | 116 ± 84 | 99 ± 70 | 62 ± 55 | 0.0003 |
| Died during follow-up n (%) | 1 (7%) | 25 (21%) | 5 (8%) | 0.001 |
Abbreviations: MELD, Model of End-stage Liver Disease; Dx, diagnosis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; INR, International Normalized Ratio for prothrombin times; SD, standarrd deviation; WD, Wilson disease.
P-values refer to comparisons between patients without WD with low ceruloplasmin levels versus MELD-matched control patients.
In total, 122 of 3040 (4.0%) patients without WD liver disorders were documented to have low serum ceruloplasmin levels on initial evaluation. All 122 were confirmed negative for WD as outlined in Methods. These individuals without WD were matched by MELD score with 64 patients without WD with normal or elevated serum ceruloplasmin levels. The patients in the low ceruloplasmin cohort were significantly younger than those in the cohort with normal/elevated ceruloplasmin levels (44 ± 14 versus 52 ± 14 years, p = 0.0002), and a lower percent of patients were men in the low ceruloplasmin cohort (66% versus 88%), (p = 0.001).
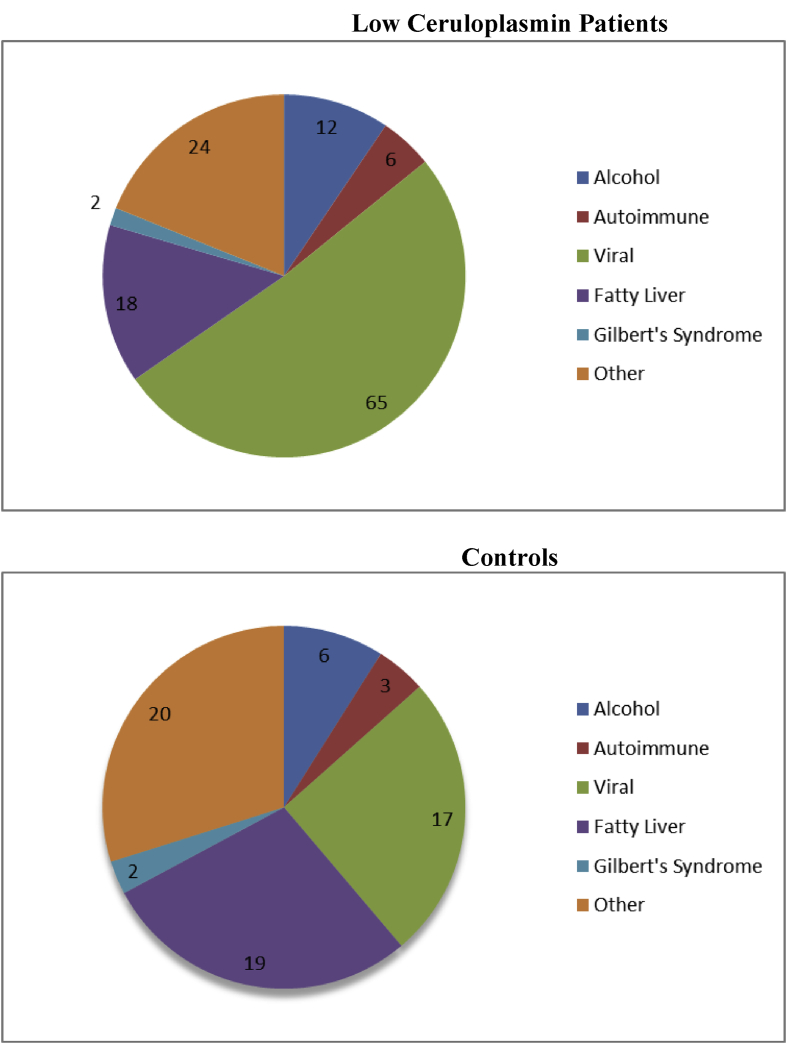
A higher percent of the patients with low ceruloplasmin had yet untreated viral hepatitis as their primary underlying liver disease (53% versus 27%, p = 0.0005) with an approximately equal distribution of hepatitis B and C infections (52% hepatitis B virus [HBV] and 48% hepatitis C virus [HCV] in the low ceruloplasmin cohort and 53% HBV and 47% HCV in the normal/elevated ceruloplasmin cohort) (Figure 1). HBV viral loads were available in 22 patients with low ceruloplasmin and in 5 normal/elevated ceruloplasmin controls. Median viral loads (log 10) were similar in the two cohorts (4.23, interquartile range (IQR): 2.83–6.57 versus 2.93, IQR: 2.22–4.41, respectively, p = 0.31). HCV viral loads were available in 7 patients with low ceruloplasmin and in 2 normal/elevated ceruloplasmin controls. Here again, median viral loads were similar in the two cohorts (6.00, IQR: 5.13–6.73 versus 6.01, respectively, p = 0.99).
Figure 1.
Underlying liver disease in 122 patients with low serum ceruloplasmin levels (upper panel) and 64 MELD-matched controls (lower panel). Viral hepatitis was significantly more common in patients with low ceruloplasmin compared with controls (53% versus 27%, p = 0.0005). Numbers in figures refer to actual patient numbers. MELD = Model of End-stage Liver Disease
As per the study protocol, serum ceruloplasmin levels were significantly lower in the low ceruloplasmin cohort (144 ± 39 versus 321 ± 75 mg/L, respectively), however, serum ALT, AST, ALP, and GGT values were similar in the two cohorts. Liver function tests as reflected by serum T.Bili, albumin, INR, and MELD values were also similar in the two cohorts as were serum ferritin and creatinine levels.
The mean follow-up was 99 ± 70 months in the low ceruloplasmin cohort and 62 ± 55 months in the normal/elevated ceruloplasmin cohort (p = 0.0003). In the low serum ceruloplasmin cohort, 35% had ceruloplasmin values that had returned to normal at their last follow-up visit. Ceruloplasmin levels were not repeated in the controls with initially normal/elevated levels.
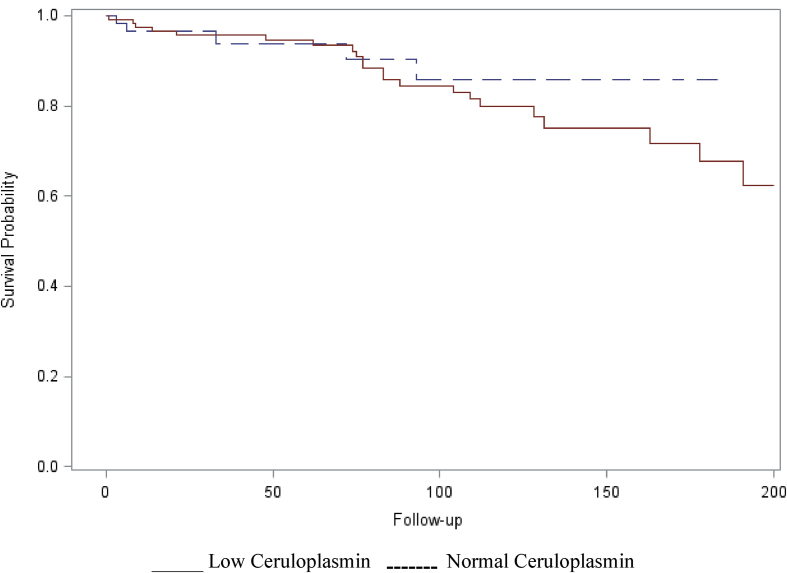
Twenty-five of 122 (21%) patients in the low ceruloplasmin cohort died during follow-up compared with 5 of 64 (7.8%) of those with normal/elevated ceruloplasmin levels (p = 0.001). However, when analyzed over the same period of follow-up (200 months), survival curves were not significantly different between the two cohorts (Figure 2). The causes of death had not been entered into the database.
Figure 2.
Kaplan–Meier survival analysis at 200-month follow-up in 122 patients with low serum ceruloplasmin levels and 64 MELD-matched controls with normal or elevated serum ceruloplasmin levels. There was no significant difference in survival. Log-rank test: Chi-square-0.55 DF-1, P = 0.46. MELD = Model of End-stage Liver Disease
To exclude the possibility that viral hepatitis was more common in patients with low serum ceruloplasmin levels because these patients were significantly younger than those with normal/elevated ceruloplasmin levels and viral hepatitis tends to occur in younger patients than in those with fatty liver disease, the prevalence of low serum ceruloplasmin levels in 1099 clinic patients with viral hepatitis was compared with 1941 age matched, fatty liver disease patients. Low serum ceruloplasmin levels were documented in 52 (4.7%) of patients with the viral hepatitis and 25 (1.3%) of those with fatty liver disease (p < 0.0001).
Discussion
The results of this study suggest that low serum ceruloplasmin levels occur but are an uncommon finding in adult patients attending an urban liver diseases outpatient program. When present, patients with this abnormality tend to be younger, more often are women, and more often have viral hepatitis as the underlying cause of their liver disease than MELD-matched controls.
Previous studies have reported various findings with respect to serum ceruloplasmin levels and the underlying etiology in patients without WD with chronic liver disease. Specifically, Cauza et al12 identified 16 of 2867 (0.56%) patients without WD attending their liver diseases outpatient clinic with hypoceruloplasminemia. Of the 10 in whom an underlying etiology had been established, 5 had acute or chronic viral hepatitis; three, drug induced liver disease, and two, decompensated alcoholic cirrhosis. Unfortunately, the age, gender, and total number of patients referred to their clinic with these conditions were not provided. Thus, perhaps the lower prevalence of low serum ceruloplasmin levels in the Cauza study reflected differences in the demographics and underlying etiologies of the two study populations. Given that low serum ceruloplasmin levels were associated with younger patients, women, and those with viral hepatitis, it is conceivable that these features were more common in our study population.
There are a number of potential explanations as to why some patients with hepatic dysfunction have low serum ceruloplasmin levels, whereas others do not. Being an acute-phase reactant, it is conceivable that those with low levels have less necroinflammatory disease activity than those with normal or elevated values. However, in a study by Le Lan et al18, there was no association between serum ceruloplasmin and c-reactive protein levels. Similarly, we did not find lower serum ALT and AST values, biochemical parameters of hepatic necroinflammatory activity, in our low ceruloplasmin cohort. Moreover, serum ferritin levels, another acute phase reactant, were similar in our two cohorts. Given that cholestasis can be associated with increased serum ceruloplasmin levels, an additional consideration would have less of a cholestatic component to the liver disease being present in the low serum ceruloplasmin cohort. Indeed, serum ALP and GGT values were somewhat lower in the low ceruloplasmin cohort but such a finding would only explain differences in the two cohorts, not why levels would be less than the normal range of values in the low serum ceruloplasmin cohort. Chronic renal disorders can be associated with low serum ceruloplasmin levels but serum creatinine values were similar in the two cohorts.19 Finally, it is quite possible that many of these patients were heterozygous for the WD ATP7B mutations and had developed superimposed chronic liver disorders. That low serum ceruloplasmin levels persisted after treatment and/or resolution of the liver disease in 65% of patients would support that possibility.
The finding that viral hepatitis was significantly more common in patients with low serum ceruloplasmin levels than those with normal or elevated values is a unique and intriguing finding that may provide an explanation for the previously reported discordant findings. Specifically, as indicated earlier, ceruloplasmin is a glycoprotein synthesized by hepatocytes in zone 1, the periportal region of the liver lobule. Chronic viral hepatitis (both HBV and HCV) predominantly affect zone 1 hepatocytes whereas NASH and alcohol-induced liver disease, two conditions that were relatively underrepresented in the low ceruloplasmin cohort, are typically zone 3 or pericentral vein disorders.5 Thus, intuitively one would predict that serum ceruloplasmin levels would be lower in patients with chronic viral hepatitis and other disorders that preferentially affect zone 1, whereas levels would be normal or elevated in disorders confined to zone 3 of the liver lobule such as NASH and alcohol-induced liver disease. Unfortunately, the number of noncholestatic, nonviral patients with additional causes of zone 1 disease was too small to test this hypothesis. Moreover, the explanation for why viral hepatitis might decrease ceruloplasmin synthesis (or shorten its half-life) in some but not all infected patients would need to be explained. Alternatively, because ceruloplasmin may interfere with viral encapsulation and release, low serum ceruloplasmin levels could result in increased viral loads and thus increase the number of viral hepatitis patients presenting with low serum ceruloplasmin levels.4
There are a number of limitations to this study that warrant emphasis. First, genetic testing for WD would have been helpful in determining whether patients without WD with low ceruloplasmin levels were heterozygous for WD mutations. Second, documentation of the extent of the inflammatory liver disease activity and thereby, induction of acute phase reactants was limited to serum ALT, AST, and ferritin levels and no effort was undertaken to exclude extrahepatic inflammatory conditions or malignancies. Third, renal dysfunction was assessed by serum creatinine rather than urinary protein levels with proteinuria being most often implicated as a renal cause of hypoceruloplasminemia.20 Fourth, the durations of follow-up were different and causes of death were not documented in either patients or controls. Fifth, it remains unclear as to whether the higher prevalence of women among the low ceruloplasmin cohort truly reflects a gender effect or an unexpectedly low prevalence of women in the MELD-matched control cohort. Finally, the database did not specify the extent to which malnutrition, malabsorption, and protein losing enteropathies might have contributed to the low ceruloplasmin levels.
In conclusion, the results of this study indicate that serum ceruloplasmin levels are low in approximately 5% of adult patients presenting to a large, urban, liver diseases outpatient clinic. These patients tend to be younger, more often women, and more often have viral hepatitis as the underlying cause of their liver disease. Additional studies are required to confirm the apparent association between low serum ceruloplasmin levels and viral hepatitis and whether this association alters the natural history of the viral infections.
Author contributions
A.G. and S.L. performed the research and collected data. J. U. analyzed data. G.Y.M. designed the research and wrote the manuscript. All authors approved the final version of the manuscript.
Authorship statement
The corresponding author (G.Y.M.) serves as the submission's guarantor.
Conflicts of interest
The authors have none to declare.
Funding
This research did not receive support from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
All authors approved the final version of the manuscript.
The authors wish to thank Ms R. Vizniak for her prompt and accurate typing of the manuscript.
References
- 1.Frieden E. Caeruloplasmin: a multi-functional metalloprotein of vertebrate plasma. Ciba Found Symp. 1980:93–124. doi: 10.1002/9780470720622.ch6. [DOI] [PubMed] [Google Scholar]
- 2.Tomas E., Toparceanu F. Considerations about the possible function of ceruloplasmin in influenza and parainfluenza virus infections. Virologie. 1986 Oct-Dec:279–287. [PubMed] [Google Scholar]
- 3.Samuel I., Tomas E. Effects and mechanism of the interaction between ceruloplasmin and some viruses or subviral components. Virologie. 1978 Apr-Jun:129–139. [PubMed] [Google Scholar]
- 4.Zhao K., Wu C., Yao Y. Ceruloplasmin inhibits the production of extracellular hepatitis B virions by targeting its middle surface protein. J Gen Virol. 2017;98:1410–1421. doi: 10.1099/jgv.0.000794. [DOI] [PubMed] [Google Scholar]
- 5.Lefkowitch J.H. Hepatobiliary pathology. Curr Opin Gastroenterol. 2006 May:198–208. doi: 10.1097/01.mog.0000218955.55688.af. [DOI] [PubMed] [Google Scholar]
- 6.Salaspuro M., Sipponen P. Demonstration of an intracellular copper-binding protein by orcein staining in long-standing cholestatic liver diseases. Gut. 1976 Oct:787–790. doi: 10.1136/gut.17.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gitlin J.D. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem. 1988:6281–6287. [PubMed] [Google Scholar]
- 8.Ramadori G., Van Damme J., Rieder H., Meyer zum Buschenfelde K.H. Interleukin 6, the third mediator of acute-phase reaction, modulated hepatic protein synthesis in human and mouse. Comparison with interleukin 1B and tumor necrosis factor alpha. Eur J Immunol. 1988:1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- 9.Ritland S., Steinnes E., Skrede S. Hepatic copper content, urinary copper excretion, and serum ceruloplasmin in liver disease. Scand J Gastroenterol. 1977:81–88. [PubMed] [Google Scholar]
- 10.Gross J.B., Jr., Ludwig J., Wiesner R.H., McCall J.T., LaRusso N.F. Abnormalities in tests of copper metabolism in primary sclerosing cholangitis. Gastroenterology. 1985 Aug:272–278. doi: 10.1016/0016-5085(85)90326-9. [DOI] [PubMed] [Google Scholar]
- 11.Czaja M.J., Weiner F.R., Schwarzenberg S.J. Molecular studies of ceruloplasmin deficiency in Wilson's disease. J Clin Investig. 1987:1200–1204. doi: 10.1172/JCI113180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauza E., Maier-Dobersberger T., Polli C., Kaserer K., Kramer L., Ferenci P. Screening for Wilson's disease in patients with liver disease by serum ceruloplasmin. J Hepatol. 1997:358–362. doi: 10.1016/s0168-8278(97)80182-1. [DOI] [PubMed] [Google Scholar]
- 13.Loreal O., Turlin B., Pigeon C. Aceruloplasminemia: new clinical, pathophysiological and therapeutic insights. J Hepatol. 2002:851–856. doi: 10.1016/s0168-8278(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 14.Dalal K., Khorate P., Dalal B. Differentially expressed serum host proteins in hepatitis B and C viral infections. Virusdisease. 2017;29:468–477. doi: 10.1007/s13337-018-0484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walshe J.M., Briggs J. Ceruloplasmin in liver disease. A diagnostic pitfall. Lancet. 1962:263–265. doi: 10.1016/s0140-6736(62)90171-x. [DOI] [PubMed] [Google Scholar]
- 16.Pratts D.S., Kaplan M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000 Apr 27:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 17.Sunderman F.W., Jr., Nomoto S. Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem. 1970 Nov:903–910. [PubMed] [Google Scholar]
- 18.Le Lan C., Ropert M., Laine F. Serum ceruloplasmin and ferroxidase activity are not decreased in hepatic failure related to alcoholic cirrhosis: clinical and pathophysiological implications. Alcohol Clin Exp Res. 2004 May:775–779. doi: 10.1097/01.alc.0000125341.42253.c2. [DOI] [PubMed] [Google Scholar]
- 19.Marecek Z., Vulterinova M., Skala I. The effect of a low-protein diet on serum levels of ceruloplasmin and transferrin patients with chronic renal failure. Clin Nephrol. 1978 Jan:38–40. [PubMed] [Google Scholar]
- 20.Mainero A., Cruz C., Pedraza-Chaverri J. Serum and urinary ceruloplasmin in experimental nephrotic syndrome. Clin Investig Med. 1992 Aug:295–300. [PubMed] [Google Scholar]