Abstract
Background/Aims
Hepatocellular carcinoma (HCC) remains the leading cause of cancer-related death among patients with type 2 diabetes mellitus (T2DM). We aimed to assess the independent role of T2DM on HCC risk among patients with different liver disease etiologies.
Methods
We analyzed the United Network for Organ Sharing database of all adults registered for liver transplantation (LT) between February 27, 2002 and December 31, 2017. For initial analyses, patients were divided into four groups: nonalcoholic steatohepatitis (NASH) and all other etiologies with or without T2DM. For additional analyses, we divided them based on underlying etiology. Logistic regression was used to evaluate the impact of T2DM with NASH and other etiologies on HCC risk.
Results
Overall, 24,149 (21.6%) of the listed patients had HCC. Of those, 23.9% had T2DM. When compared with nondiabetics, patient with NASH and T2DM had the highest risk of HCC (odds ratio [OR] 1.68; 95% confidence interval [CI] 1.52–1.86), followed by patients with other etiologies and diabetes. After adjusting for other risk factors, these associations remained unchanged. Registrants with T2DM and NASH, cryptogenic cirrhosis, hepatitis C, and alcoholic liver disease were at higher risk of HCC than those without diabetes, but in patients with chronic hepatitis B or primary biliary cholangitis, diabetes did not increase the HCC risk. Between 2004 and 2016, the annual percentage change of HCC incidence increased for all patients with NASH and hepatitis C regardless of their diabetes status. For those with hepatitis B, this trend was significant only for diabetics.
Conclusions
The additive risk of T2DM for HCC development was highest in patients with NASH. HCC risk may vary depending on the underlying etiology.
Keywords: liver cancer, fatty liver, UNOS, HCC incidence
Abbreviations: ALD, alcoholic liver disease; APC, annual percentage change; CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; NAFLD, nonalcoholic liver disease; NASH, nonalcoholic steatohepatitis; OR, odds ratio; PBC, primary biliary cholangitis; T2DM, type 2 diabetes mellitus; UNOS, United Network for Organ Sharing
In 2016, the estimated prevalence of type 2 diabetes mellitus (T2DM) was 8.6% (21 million) of the U.S. population,1 which is projected to increase to 55 millions by 2030.2 Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death among diabetics.3 Mortality rate from HCC in the USA has increased by 35% between 2002 and 2012.4
Over the last two decades, nonalcoholic fatty liver disease (NAFLD) and its aggressive form nonalcoholic steatohepatitis (NASH) has emerged as a major cause of HCC accounting for 18% of patients with HCC listed for liver transplantation (LT) in 2017 in the USA,5 10% in Europe, and up to 6% in Asia.6 In those who are transplanted for HCC, NAFLD currently ranks second after hepatitis C virus (HCV) infection and is the most rapidly growing indication for LT among waitlisted candidates with HCC in the U.S.A. with an 8-fold increase since 2002.5
Patients with T2DM are at a higher risk for NAFLD and NASH with an estimated global NAFLD prevalence of approximately 60% in this population.7 T2DM accelerates NAFLD course resulting in higher rates of advanced fibrosis, cirrhosis, and HCC.8 The presence of T2DM may also increase the risk of HCC in patients with hepatitis B virus (HBV) infection9,10 and HCV,11 but the independent role and oncogenic impact of T2DM in patients with different liver etiologies has not been previously elucidated. The aim of our study was to assess the risk of HCC in patients with NASH and other etiologies stratified by the presence or absence of T2DM using a national registry of patients listed for LT in the USA. We also estimated the annual trend of HCC in these groups, stratified by their diabetes status, between 2004 and 2016.
Methods
We used the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network registry data to identify all subjects aged 18 years or older who were registered for LT with HCC diagnosis between February 27, 2002 (the implementation of Model for End-Stage Liver Disease [MELD]) and December 31, 2017. The HCC diagnosis and indication for LT were based on the primary or secondary diagnosis (diagnostic codes: 4400, 4401). Patients with prior history of transplantation or multiorgan transplantation were excluded. Patients were classified into the following etiologies of liver disease: HCV, HBV, alcoholic liver disease (ALD), NASH, cryptogenic cirrhosis, primary biliary cholangitis (PBC), and all other etiologies. Patients with both HCV and ALD diagnostic codes were included in the HCV group.
Statistical Analyses
For initial analyses, patients listed for LT were divided into four groups: (1) NASH with T2DM; (2) NASH without T2DM; (3) Other etiologies with T2DM; (4) Other etiologies without T2DM. For additional analyses, patients were divided into the following groups: HCV, HBV, ALD, NASH, cryptogenic cirrhosis, PBC, and all other etiologies.
Descriptive statistics for characteristics of patients were presented as means and standard deviations for continuous variables and frequencies for categorical variables. Patient characteristics were compared among four groups using a chi-Squared test for categorical variables and analysis of variance for continuous variables.
Logistic regression was performed to evaluate the impact of T2DM with NAFLD and other etiologies on the risk of HCC. We started with univariate analysis, followed by multivariate analysis. Any significant variable on univariate effect was considered a candidate for initial multivariate modeling. The final model retained variables with a P-value 0.05 or less. Estimations of adjusted odds ratios and 95% confidence intervals (CI) were reported. Region was used as an adjustment in all univariate and multivariate analyses.
Poisson regression modeling approach was used to estimate the proportion of HCC change over time. Annual percentage change (APC) was used to assess the changes in HCC proportion. Rate ratio was used to describe differences between T2DM and non-T2DM groups. We also evaluated whether APC differed from no change (APC = 0). The study was exempt from institutional review board because of deidentification of the analyzed data. SAS 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Baseline Characteristics of the Cohort
A total of 111,992 patients were listed for LT over the study period. Of them, 24,149 (21.6%) had HCC. Among all patients with HCC, 5773 (23.9%) had type 2 diabetes. Patients' characteristics are shown in Table 1. Patients with diabetes were older at the time of listing. When compared with nondiabetics, HCC patients with NASH and T2DM had a lower MELD at listing and a larger proportion of them were classified as Child-Turcotte-Pugh class A. Among those with other etiologies, MELD scores at listing and at the time of transplantation were higher in nondiabetics. There was no difference in body mass index (BMI) or obesity rates in the NASH groups; whereas in the group with other etiologies, mean BMI was higher among diabetics, but obesity was more common in nondiabetics. Diabetics with other etiologies had a larger median tumor size than those without T2DM, but there were no differences in the number and diameter of the tumors.
Table 1.
Demographic and Clinical Characteristics of Liver Transplant Registrants with and without Type 2 Diabetes.
| NASH with T2DM (N = 1193) | NASH without T2DM (N = 732) | P-value | Other etiologies with T2DM (N = 4577) | Other etiologies without T2DM (N = 17,639) | P-value | |
|---|---|---|---|---|---|---|
| Age at listing, mean ± SD | 62.5 ± 6.1 | 61.7 ± 7.2 | 0.0229 | 59.4 ± 6.5 | 57.3 ± 7.6 | <0.0001 |
| Male, n (%) | 768 (64%) | 476 (65%) | 0.7715 | 3692 (81%) | 13,668 (77%) | <0.0001 |
| Ethnicity | ||||||
| White, n (%) | 889 (75%) | 567 (77%) | 0.4743 | 2631 (57%) | 11,644 (66%) | <0.0001 |
| Black, n (%) | 8 (1%) | 7 (1%) | 520 (11%) | 1750 (10%) | ||
| Hispanic, n (%) | 237 (20%) | 125 (17%) | 960 (21%) | 2693 (15%) | ||
| Asian, n (%) | 40 (3%) | 20 (3%) | 399 (9%) | 1302 (7%) | ||
| Others, n (%) | 19 (2%) | 13 (2%) | 67 (1%) | 250 (1%) | ||
| BMI at listing, mean ± SD | 32.8 ± 5.5 | 32.6 ± 5.6 | 0.5225 | 29.1 ± 5.1 | 28.3 ± 5.1 | <0.0001 |
| Obesity (BMI≥30), n (%) | 800 (67%) | 490 (67%) | 0.9574 | 1786 (39%) | 5731 (33%) | <0.0001 |
| MELD at listing, mean ± SD | 13.5 ± 5.9 | 14.5 ± 6.4 | 0.0005 | 12.5 ± 6.2 | 13.0 ± 6.3 | <0.0001 |
| MELD at transplant, mean ± SD | 17.1 ± 8.5 | 17.8 ± 8.7 | 0.1456 | 14.8 ± 8.3 | 15.5 ± (8.5) | 0.0002 |
| Creatinine at listing (mg/dL), mean ± SD | 1.1 ± 0.8 | 1.0 ± 0.6 | 0.0536 | 1.1 ± 0.8 | 1.0 ± 0.6 | <0.0001 |
| Total bilirubin at listing (mg/dL), mean ± SD | 2.3 ± 2.8 | 3.0 ± 4.2 | 0.0001 | 2.3 ± 4.0 | 2.7 ± 4.4 | <0.0001 |
| INR at listing, mean ± SD | 1.4 ± 0.4 | 1.4 ± 0.4 | 0.0032 | 1.3 ± 0.4 | 1.4 ± 0.5 | <0.0001 |
| Sodium at listing (mEq/L), mean ± SD | 137 ± 4.2 | 137 ± 4.5 | 0.4594 | 137 ± 4.0 | 137 ± 4.2 | <0.0001 |
| Albumin at listing (g/dL), mean ± SD | 3.2 ± 0.6 | 3.2 ± 0.6 | 0.0939 | 3.2 ± 0.7 | 3.2 ± 0.7 | <0.0001 |
| Child-Turcotte-Pugh | ||||||
| Class A, n (%) | 343 (29%) | 176 (24%) | 0.0034 | 1593 (35%) | 5614 (32%) | <0.0001 |
| Class B, n (%) | 566 (47%) | 334 (46%) | 2096 (46%) | 7863 (45%) | ||
| Class C, n (%) | 284 (24%) | 222 (30%) | 883 (19%) | 4150 (24%) | ||
| Encephalopathy at listing, n (%) | ||||||
| None | 581 (49%) | 340 (46%) | 0.4150 | 2483 (54%) | 9655 (55%) | 0.2921 |
| Grade 1 or 2 | 574 (48%) | 373 (51%) | 1967 (43%) | 7571 (43%) | ||
| Grade 3 or 4 | 38 (3%) | 19 (3%) | 122 (3%) | 402 (2%) | ||
| Ascites at listing, n (%) | ||||||
| None | 454 (38%) | 267 (36%) | 0.7739 | 2061 (45%) | 7377 (42%) | <0.0001 |
| Mild to moderate | 553 (46%) | 350 (48%) | 2005 (44%) | 8360 (47%) | ||
| Severe | 186 (16%) | 115 (16%) | 506 (11%) | 1891 (11%) | ||
| Dialysis at listing, n (%) | 16 (1%) | 14 (2%) | 0.3220 | 87 (2%) | 161 (1%) | <0.0001 |
| Number of tumors, mean ± SD | 2.0 ± 1.3 | 1.9 ± 1.3 | 0.5543 | 1.9 ± 1.2 | 2.0 ± 1.3 | 0.2076 |
| Median tumor size (cm), mean ± SD | 2.2 ± 1.3 | 2.5 ± 1.5 | 0.1863 | 2.4 ± 1.5 | 2.3 ± 1.4 | 0.0533 |
BMI, Body mass index; MELD, Model for End-stage Liver Disease; NASH, Nonalcoholic steatohepatitis; SD, Standard deviation; T2DM, Type 2 diabetes mellitus.
HCC Risk Among Patients with NASH and Other Etiologies with and without T2DM
Patients with NASH had a lower risk of HCC when compared with those with other etiologies regardless of the presence of diabetes. Patients with diabetes with NASH carried a higher risk of HCC (odds ratio [OR]: 1.68; 95% CI: 1.52–1.86) than those without diabetes. Similarly, those with other etiologies and T2DM had a 53% higher risk for HCC than those without diabetes (OR: 1.53; 95% CI: 1.47–1.59). After adjusting for age, gender, ethnicity, obesity, and region of listing, the associations were attenuated, but the conclusions remained similar (Table 2).
Table 2.
Unadjusted and Adjusted Risk of HCC in Patients with Nonalcoholic Steatohepatitis vs All Other Etiologies.
| OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| NASH with T2DM vs NASH without T2DM | 1.68 (1.52–1.86) | 1.50 (1.35–1.66) |
| NASH with T2DM vs other etiologies with T2DM | 0.55 (0.51–059) | 0.60 (0.56–0.65) |
| NASH with T2DM vs other etiologies without T2DM | 0.84 (0.79–0.89) | 0.73 (0.68–0.78) |
| NASH without T2DM vs other etiologies with T2DM | 0.33 (0.30–0.35) | 0.40 (0.37–0.44) |
| NASH without T2DM vs other etiologies without T2DM | 0.50 (0.46–0.54) | 0.49 (0.45–0.53) |
| Other etiologies with T2DM vs other etiologies without T2DM | 1.53 (1.47–1.59) | 1.21 (1.16–1.26) |
CI, Confidence interval; OR, Odds ratio; HCC, Hepatocellular carcinoma; T2DM, Type 2 diabetes mellitus; NASH, Nonalcoholic steatohepatitis.
HCC Risk Among Patients with Different Etiologies with and without T2DM
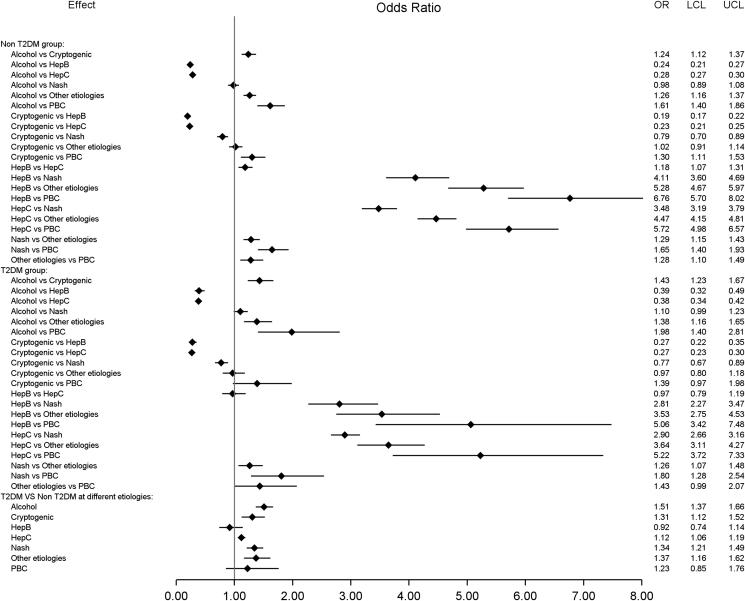
To better estimate the disease-specific risk of HCC in patients with and without T2DM, we further assessed the risk in patients with different liver disease etiologies. Patients with ALD had a higher HCC risk than those with cryptogenic cirrhosis but lower than those with HCV, HBV, and NASH, regardless of T2DM status. The risk for HCC was lower in cryptogenic cirrhosis as compared with patients with NASH (OR: 0.77; 95% CI: 0.67–0.89 in those with T2DM and OR: 0.79; 95% CI: 0.70–0.89 in those without T2DM). The presence of T2DM was associated with a higher risk of HCC in those with NASH, cryptogenic cirrhosis, ALD, and HCV, but for those with HBV or PBC, diabetes did not increase the risk of HCC (Figure 1).
Figure 1.
HCC risk based on liver disease etiology. LCL, lower control limit; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; T2DM, type 2 diabetes mellitus; UCL, upper control limit; HCC, hepatocellular carcinoma.
Trends of HCC Prevalence Among LT Registrants Between 2004 and 2016
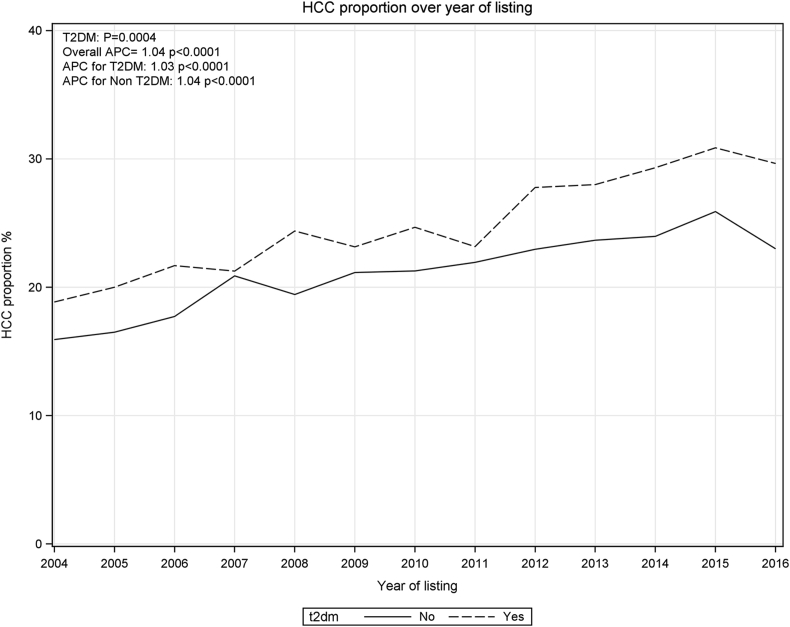
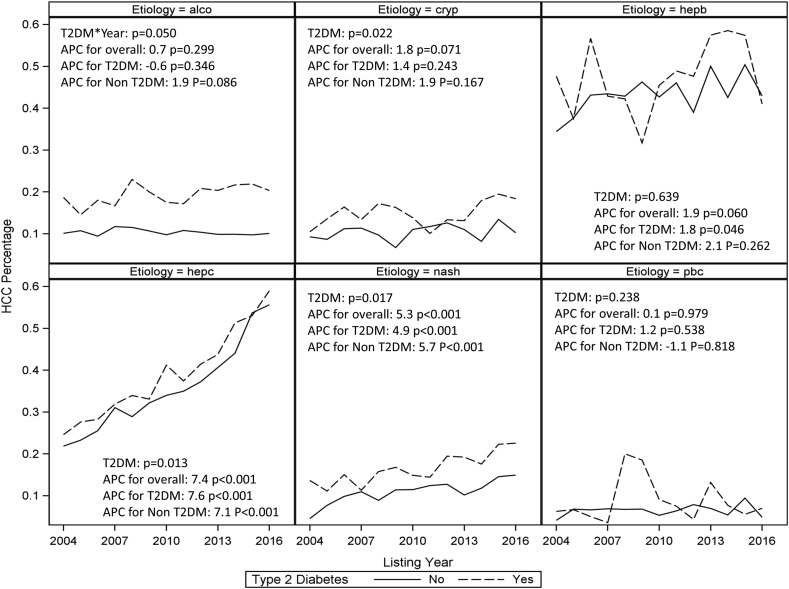
The proportion of LT registrants with HCC who had T2DM showed a continuous increase between 2004 and 2016 (Figure 2). The APC of HCC incidence and the interaction between yearly change and T2DM was also tested among patients with different etiologies. This analysis was restricted to the period between 2004 and 2016 because there were a very small number of patients in some groups before 2004. Over the study period, there was a significant difference in APC between patients with and without diabetes in the NASH group (P = 0.017), cryptogenic cirrhosis (P = 0.022), and HCV (P = 0.013) groups (Figure 3). Whereas APC of HCC incidence showed a significant increase in patients with NASH and HCV irrespective of the presence or absence of T2DM, in the group with HBV, the APC was significant only for those with T2DM. Non-diabetics patients with NASH and cryptogenic cirrhosis demonstrated a larger growth than diabetics.
Figure 2.
HCC proportion change in patients with type 2 diabetes mellitus (T2DM) and non-T2DM by listing year. The estimated annual percentage changes (APCs) are shown with their associated P-values (testing whether the APC differs from zero). P-value for rate ratio is shown to test whether APC had significant difference between T2DM and non-T2DM groups. HCC, hepatocellular carcinoma.
Figure 3.
Proportion of HCC registrants with different etiologies with and without type 2 diabetes mellitus (T2DM) change over time. The estimates annual percentage changes (APCs) are shown with their associated P-values (testing whether the APC differs from zero). P values for rate ratio are shown to test whether APC had significant difference between T2DM and non-T2DM groups. HCC, hepatocellular carcinoma.
Discussion
The findings of our study confirmed that patients with T2DM carry a higher risk of developing HCC, but the risk may depend on the underlying liver disease etiology. When compared with nondiabetics, the strongest correlation was seen among patients with NASH, and the increased risk was confirmed after adjusting for other known risk factors for HCC. Diabetics with NASH, cryptogenic cirrhosis, HCV, and ALD showed a higher risk of HCC than nondiabetics, whereas T2DM did not increase the risk of HCC among patients with HBV or PBC. Those with cryptogenic cirrhosis had a lower risk of HCC than those with NASH regardless of diabetes status. Importantly, patients with NASH and those with HCV demonstrated significant increase in APC of HCC incidence in both diabetic and nondiabetic population. The increase was faster in nondiabetics with NASH and cryptogenic cirrhosis.
Our study corroborates few other studies that had demonstrated an increase in the prevalence of HCC in those with T2DM. A meta-analysis based predominantly on an Asian population had shown that T2DM augmented the risk of HCC by 2-fold (summary relative risk 2.01; 95% CI: 1.61–2.51).12 Another systematic review that included 26 epidemiological and case-control studies with both Caucasian and Asian patients with diabetes also demonstrated a 2.5-fold increase in HCC risk.13 In 2017, NASH was noted to be the second most common etiology among patients with HCC listed for LT in the USA with a steady increase over the last 15 years.5 A recent retrospective analysis based on a large ethnically diverse cohort of patients with NAFLD reported that HCC risk was more than 7-fold higher in patients with NAFLD compared with matched controls (adjusted HR [aHR]: 7.62; 95% CI: 5.76–10.09), whereas diabetics had 3 times higher risk compared with nondiabetics (aHR: 3.03; 95% CI: 2.52–3.64).14 The aforementioned study also noted half of the HCC cases in the control group developed in those with diabetes although their risk was lower than those with NAFLD with or without T2DM. Our results are also in agreement with these findings as the risk of HCC was 1.5-fold higher among patients with NASH and diabetes compared with those without diabetes.
A population-based study that examined the population-attributable fraction of risk factors among patients with HCC diagnosed between 2000 and 2011 suggested that metabolic disorders, in particular diabetes and obesity, had the largest contribution to HCC burden (32%) and these metabolic risk factors had increased significantly over the study period.15 In contrast, risk factors such as alcohol, HBV, and genetic disorders have remained stable. The synergistic role of T2DM as a risk factor among patients with other etiologies was also seen in our analyses. We found that diabetes increased the risk of HCC in those with NASH, cryptogenic cirrhosis, ALD, and HCV. The only exception was patients with HBV and those with PBC, where presence of diabetes was not an additional risk factor, and this is an intriguing observation.
The role of T2DM in patients with chronic hepatitis B remains controversial. Two recent population-based cohort studies have demonstrated that T2DM increased HCC risk among men with HBV, independent of obesity9 and that newly diagnosed T2DM augmented HCC risk and HCC-related mortality in subjects with HBV over a long follow-up of 11 years.16 Conversely, and similar to our findings, there was no difference in association between T2DM and HCC risk among patients with HBV in a large prospective study that included over half million subjects.17 A recent meta-analysis comprising five cohort- and two case-control studies with over 20,000 patients with HBV has concluded that T2DM increased HCC incidence with a pooled HR of 1.77 (95% CI: 1.28–2.47).10 However, five of the included studies were conducted in Taiwan and overlapping of cohorts could not be excluded completely. In addition, all the aforementioned studies were based on an Asian population, used different methods to identify patients with HCC and T2DM, adjusted HCC risk for various confounders, and had different follow-up and study periods. Future large prospective studies involving geographically and ethnically diverse population are greatly needed to elucidate this controversy as well as the role of diabetes control and treatment on HCC risk.
A systematic review that included nine studies with patients with HCV reported that concomitant T2DM increased 2–3 times the risk of HCC in this population.11 Other attributable risk factors for HCC in that cohort were obesity and hepatic steatosis. Possible underlying mechanisms to explain the higher risk of HCC in HCV and T2DM are concomitant NAFLD, with reported mean prevalence of 55% among patients with HCV, as well as HCV-associated steatosis.18 Currently, with the decrease of HCV as an indication for LT, ALD has emerged as a leading indication for transplantation.19 A recent large prospective study reported an increased risk of HCC among diabetics with ALD (aHR: 2.56; 95% CI: 1.61–4.06) when compared with those with ALD without diabetes, whereas for patients with NAFLD this risk was relatively lower (aHR: 1.33; 95% CI: 1.10–1.62).17 On direct comparison between these two groups, we did not observe any difference in HCC risk among patients with ALD and NASH regardless of diabetes status. The increased risk of HCC in diabetics with ALD might be due to the independent contribution of T2DM per se or due to molecular mechanisms enhancing inflammation and fibrosis in patients with alcohol-induced steatosis.20
A recent analysis of UNOS data has shown HCC had grown as an indication for LT from 6.4% in 2002 to 23.0% in 2016.5 Although HCV has remained the main etiology for HCC over this period, NASH with HCC was the only other growing indication. We found that the APC of HCC incidence has risen from 2004 to 2016 for patients with HCC because of HCV or NASH in both diabetics and nondiabetics. Interestingly, in patients with NASH, those without T2DM have been rising faster than diabetics with NASH. One possible explanation is the increasing evidence of HCC arising in the absence of cirrhosis in those with metabolic syndrome. An HCC database study from the United Kingdom suggested that patients without underlying chronic liver disease presented with larger tumors, at more advanced stage, and were less likely to be referred for liver transplantation.21 Moreover, in their study, only 23% of patients with NAFLD-related HCC were detected by surveillance. This again highlights the need for better stratification of patients at highest risk of developing HCC and for implementation of effective preventive and surveillance programs.
We acknowledge several limitations of our study related to the inherent restrictions of large databases. One limitation is that the primary etiology of cirrhosis is based on the primary diagnosis listed in the UNOS datasets. In addition, few patients with NASH or HBV and HCC may not have underlying cirrhosis, and this could not be verified by the datasets. Similarly, the duration of T2DM and the effect of diabetic medications on HCC could not be estimated because of lack of granularity of data. However, there are several strengths including a large national dataset over a long period of time with essential demographic variables. In comparison with other studies, we also separated patients with NASH and cryptogenic cirrhosis to avoid overestimation of NASH. We have recently shown that cryptogenic cirrhosis in patients currently listed for LT could not be attributed to NASH.22
In summary, our analyses of UNOS dataset confirmed the additional risk associated with T2DM in the etiopathogenesis of HCC. Moreover, we showed the additive risk of T2DM was highest in those with NASH after adjusting for other known risk factors. We also demonstrated that contribution of T2DM in HCC development might vary depending on the underlying cause of liver disease. Future prospective studies should evaluate the benefit of better diabetic control and the role of specific diabetic medications on HCC incidence and progression.
Authors' contributions
I.D. contributed to study concept and design, interpretation of data, drafting of the manuscript, and approved the final submission; T.Z. contributed to statistical analysis and interpretation of data and approved the final submission; W.A. contributed to interpretation of data and approved the final submission; P.J.T. contributed to study concept and design, interpretation of data, critical revision of the manuscript, and approved the final submission.
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.11.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bullard K.M., Cowie C.C., Lessem S.E. Prevalence of diagnosed diabetes in adults by diabetes type – United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359–361. doi: 10.15585/mmwr.mm6712a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowley W.R., Bezold C., Arikan Y., Byrne E., Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao Kondapally Seshasai S., Kaptoge S., Thompson A. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertuccio P., Turati F., Carioli G. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z., Stepanova M., Ong J.P. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755 e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Park J.W., Chen M., Colombo M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi Z., Tacke F., Arrese M. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 9.Kim K., Choi S., Park S.M. Association of fasting serum glucose level and type 2 diabetes with hepatocellular carcinoma in men with chronic hepatitis B infection: a large cohort study. Eur J Cancer. 2018;102:103–113. doi: 10.1016/j.ejca.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y., Wei S., Zhang W., Yang J., Yang J., Yan L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. Cancer Manag Res. 2019;11:705–713. doi: 10.2147/CMAR.S188238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyal H.K., Aguilar M., Bartos G. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61:636–645. doi: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang C., Wang X., Gong G. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag H.B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Kanwal F., Kramer J.R., Mapakshi S. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–18237 e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makarova-Rusher O.V., Altekruse S.F., McNeel T.S. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyu Y.C., Huang T.S., Chien C.H., Yeh C.T., Lin C.L., Chien R.N. Diabetes poses a higher risk of hepatocellular carcinoma and mortality in patients with chronic hepatitis B: a population-based cohort study. J Viral Hepat. 2019;26:718–726. doi: 10.1111/jvh.13077. [DOI] [PubMed] [Google Scholar]
- 17.Pang Y., Kartsonaki C., Turnbull I. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adinolfi L.E., Rinaldi L., Guerrera B. NAFLD and NASH in HCV infection: prevalence and significance in hepatic and extrahepatic manifestations. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B.P., Vittinghoff E., Dodge J.L., Cullaro G., Terrault N.A. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179:340–348. doi: 10.1001/jamainternmed.2018.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goossens N., Hoshida Y. Is hepatocellular cancer the same disease in alcoholic and nonalcoholic fatty liver diseases? Gastroenterology. 2016;150:1710–1717. doi: 10.1053/j.gastro.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyson J., Jaques B., Chattopadyhay D. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Thuluvath P.J., Kantsevoy S., Thuluvath A.J., Savva Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J Hepatol. 2018;68:519–525. doi: 10.1016/j.jhep.2017.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.