Abstract
Introduction
Celiac disease (CD) has been linked to portal hypertension (PHT) of varied etiology, but the causality association has never been proved. We aim to study the prevalence of CD in patients of PHT of different etiology.
Methods
A prospective observational study was conducted from June 2017 to December 2018 involving all the cases of PHT of varied etiology. Consecutive patients of PHT with chronic liver disease (CLD) of defined etiology like ethanol, viral hepatitis (B or C), Budd–Chiari syndrome (BCS), autoimmune-related cirrhosis, and cryptogenic CLD (cCLD) (group A) and those with noncirrhotic PHT (NCPHT), which included noncirrhotic portal fibrosis (NCPF) and extrahepatic portal vein obstruction (EHPVO) (group B), were screened for CD by IgA anti-tTG antibody followed by duodenal biopsy in serology-positive patients.
Results
Out of a total of 464 patients, group A constituted 382 patients, CLD related to ethanol (155), cCLD (147), hepatitis B (42), hepatitis C (21), autoimmune (10), and BCS (7), whereas 82 patients were in group B with NCPF (64) and EHPVO (18). Total 29 patients were diagnosed with CD in both groups, 17 in group A (4.5%) and 12 in group B (14.6%). In group A, 13 patients with cCLD, two with HBV-related CLD, one with BCS, and one with autoimmune-related CLD were concomitantly diagnosed as CD. In group B, CD was diagnosed in 12 patients of NCPF (11) and EHPVO (1). Liver histology showed chronic hepatitis in two patients and was normal in three patients.
Conclusion
CD is common in PHT of different etiology, especially in cCLD, NCPH and autoimmune hepatitis; however, the etiological basis for this association is still to be defined. The likelihood of CD is higher in liver disease than the general population, and these patients should be screened for CD.
Keywords: celiac disease, portal hypertension, chronic liver disease, noncirrhotic portal hypertension
Abbreviations: AIH, autoimmune hepatitis; ANA, anti-nuclear antibody; Anti LKM, anti-liver kidney microsome antibody; ASMA, anti-smooth muscle antibody; BCS, Budd–Chiari syndrome; c CLD, cryptogenic chronic liver disease; CD, celiac disease; CLD, chronic liver disease; EHPVO, extrahepatic portal vein obstruction; HBs Ag, hepatitis B surface antigen; HBV, hepatitis B virus; HLA, human leukocyte antigen; Ig G, immunoglobulin G; NCIPH, noncirrhotic idiopathic portal hypertension; NCPF, noncirrhotic portal fibrosis; NCPH, noncirrhotic portal hypertension; PHT, portal hypertension; tTG antibody, tissue transglutaminase antibody
Celiac disease (CD) is an autoimmune enteropathy affecting genetically susceptible individuals on exposure to prolamine fraction of gluten.1 Essential components for development of CD include the presence of susceptible human leukocyte antigen (HLA) and exposure to gluten. The major predisposing heterodimers are HLA-DQ2 and DQ8, found in nearly 98% of CD patients.2 CD is prevalent in 1% of general population in India, which is similar to that reported from other parts of the world.3 Gluten-induced immune effects are not limited to intestine alone, but other organs such as the skin, brain, and bones are also affected.4 Hepatobiliary involvement in CD has been studied for last three decades. The liver is also involved commonly in patients with CD.5 Liver dysfunctions in CD include asymptomatic transaminitis, autoimmune hepatitis, nodular regeneration of the liver, and cirrhosis.6, 7, 8, 9 However, with a surge in interest and research on CD, patients with idiopathic portal hypertension were being found to have positive serology and diagnostic duodenal biopsy changes for CD.10 A study from Sweden reported 15 times higher prevalence of CD in patients with cryptogenic chronic liver disease (cCLD) than general population.11 In a study from India, portal hypertension was present in about 10% of the patients with CD.12 In another study, 10% of noncirrhotic idiopathic portal hypertension (NCIPH) had positive serology and duodenal biopsy-proven CD.13 So, this study aimed to investigate the coexistence and prevalence of CD in patients with portal hypertension due to varied etiology and clinical profile of patients of CD associated with portal hypertension.
Materials and methods
This is a prospective observational study conducted at the department of gastroenterology, SMS hospital in Jaipur, Rajasthan. Consecutive patients with portal hypertension with chronic liver disease (CLD) of defined etiology (ethanol, hepatitis B or C, Budd–Chiari syndrome [BCS], autoimmune-related cirrhosis, and cCLD) constituted group A, while those with NCPH, which included noncirrhotic portal fibrosis (NCPF) and extrahepatic portal vein obstruction (EHPVO) (group B), were enrolled in the study between June 2017 and December 2018. Cirrhosis was diagnosed on the basis of clinical, biochemical, and imaging features. Portal hypertension was defined as the presence of gastroesophageal varices (GEV) and/or high gradient ascites. For etiology of portal hypertension, patients were screened for history of alcohol intake, HBsAg, anti-HCV antibody, ultrasound abdomen with Doppler of portal vein and hepatic veins, multiphase contrast-enhanced computed tomography abdomen, IgA-tTG, autoantibodies (AMA, ASMA, LKM, ANA, IgG), and serum ceruloplasmin as per clinical evaluation. Patients of CD with portal hypertension (PHT) were also subjected to ultrasound-guided percutaneous liver biopsy in absence of absolute contraindications. Patients of cirrhosis with PHT and negative evaluation for cause of liver disease were defined as cCLD. NCPF was defined as the presence of PHT, patient hepatic and portal veins on Doppler, no identifiable etiology for liver disease, and absence of cirrhosis.14 EHPVO was diagnosed in the presence of PHT with obstruction of the extrahepatic portal vein with or without involvement of intrahepatic portal vein radicles or splenic or superior mesenteric veins with the presence of portal cavernoma and absence of cirrhosis.15,16 Autoimmune hepatitis (AIH) was diagnosed by a simplified scoring system: probable AIH when pretreatment aggregate score ≥6, and definite AIH with score ≥7.17 Serum IgA anti-tissue transglutaminase (tTG) antibody determination was done in all study patients at baseline by a commercially available solid-phase enzyme immunoassay kit, CHORUS tTg-A (DIESSE Diagnostica Senese), which uses a human recombinant antigen and interpreted as positive when >18 AU/ml, negative <12, and borderline between 12 and 18. For duodenal biopsy, at least four specimens were obtained from the second part of the duodenum in all tTG antibody-positive patients. Duodenal histology was assessed according to the modified Marsh classification.18,19
Results
Total 464 patients with PHT were enrolled in the study. Group A constituted 382 patients with parenchymal liver disease related to ethanol (155), cCLD (147), hepatitis B,42 hepatitis C,21 BCS,7 and autoimmune,10 whereas group B constituted 82 patients with NCPF (64) and EHPVO.18 Demographics and baseline investigations in the groups are depicted in Table 1. Total 29 patients were diagnosed with CD in both groups, 17 in group A and 12 in group B. In group A, 17 (4.5%) patients were diagnosed with CD, 13 patients with cCLD, two with HBV-related CLD, one with BCS and 1 in Autoimmune related CLD. Twelve patients in ethanol related CLD were borderline positive for IgA-tTG (IgA anti-tTG titer = 12–18) but histology was negative for CD. In group B, 12 (14.6%) patients were diagnosed with CD, NCPF,11 and EHPVO.1
Table 1.
Demographics and Baseline Investigations.
| Parameter | Group A (n = 382). Median (Range) |
Group B (n = 82) Median (Range) |
|---|---|---|
| ETIOLOGY | Ethanol [n = 155] | NCPF [n = 64] |
| cCLD [n = 147] | EHPVO [n = 18] | |
| HBV [n = 42] | ||
| HCV [n = 21] | ||
| AIH [n = 10] | ||
| BCS [n = 7] | ||
| AGE (years) | 39 (26–68) | 26 (13–44) |
| SEX | M (n = 249 [65%]) F (n = 133 [35%]) |
M (n = 25 [31%]) F (n = 57 [69%]) |
| Hb (g/dl) | 9.3 (4.9–14) | 7.2 (3.6–11.8) |
| TLC (cumm) | 7210 (2350–3,2000) | 4590 (1020–7410) |
| Platelets (lakhs/ml) | 0.84 (0.26–1.64) | 0.68 (0.13–1.34) |
| INR | 1.98 (1.2–4.6) | 1.1 (1.04–1.3) |
| Bilirubin (mg/dl) | 3.2 (0.6–28) | 1.2 (0.8–1.5) |
| SGOT (U/L) | 99 (19–218) | 29 (19–44) |
| SGPT (U/L) | 55 (15–160) | 26 (10–39) |
| ALP (U/L) | 156 (24–433) | 109 (24–146) |
| TOTAL PROTEIN (g/dl) | 5.1 (4.5–8.6) | 6.2 (5.8–7.6) |
| GLOBULIN (g/dl) | 3.1 (2–4.9) | 2.8 (2.6–3.2) |
| ALBUMIN (g/dl) | 2.3 (1.6–3.9) | 3.7 (3.4–4.1) |
| CREATININE (mg/dl) | 1.32 (0.6–5.1) | 0.9 (0.7–1.3) |
| Disease severity- | CTP-A = 12 (3.1%) CTP-B = 89 (23.3%) CTP-C = 281 (73.6) |
Clinical profile of celiac patients
Demographic Profile
Out of the total 29 patients diagnosed with CD, 23 were female and six were male, with the median age of 27.6 years (range: 14–65). The hematological and biochemical profiles of these patients are depicted in Table 2.
Table 2.
Demographics and Baseline Investigations of CD Patients with PHT (n = 29).
| Parameter | Group A (n = 17). Median (Range) |
Group B (n = 12) Median (Range) |
|---|---|---|
| ETIOLOGY | cCLD [n = 13] | NCPF [n = 11] |
| HBV (n = 2) | EHPVO (n = 1) | |
| AIH (n = 1) | ||
| BCS (n = 1) | ||
| AGE (years) | 33.5 (27–65) | 24.5 (15–42) |
| SEX | M (n = 424) F (n = 13 [76%]) |
M (n = 2 [17%]) F (n = 10 [83%]) |
| Hb (g/dl) | 8.1 (5.1–11.2) | 6.9 (3.8–9.2) |
| TLC (cumm) | 6650 (3400–9020) | 4590 (1400–6810) |
| Platelets (lacs/ml) | 0.76 (0.35–1.59) | 0.68 (0.33–1.32) |
| INR | 1.87 (1.4–3.9) | 1.2 (1.09–1.3) |
| Bilirubin (mg/dl) | 2.9 (1.9–10) | 1.1 (0.9–1.4) |
| SGOT (U/L) | 97 (26–212) | 39.5 (19–44) |
| SGPT (U/L) | 61 (39–102) | 35 (25–38) |
| ALP (U/L) | 166 (45–233) | 112 (36–145) |
| TOTAL PROTEIN (g/dl) | 5.2 (4.5–7.6) | 6.6 (5.9–7.1) |
| GLOBULIN (g/dl) | 3.1 (2.2–4.3) | 2.9 (2.6–3.1) |
| ALBUMIN (g/dl) | 2.4 (1.7–3.8) | 3.7 (3.6–4.1) |
| CREATININE (mg/dl) | 1.4 (0.6–2.3) | 0.8 (0.7–1.1) |
| Ig-A anti-tTG titre- | 2 folds (n = 2) | 2 folds (n = 0) |
| 3–5 folds (n = 1) | 3–5 folds (n = 3) | |
| 6–10 folds (n = 4) | 6–10 folds (n = 5) | |
| >10 folds (n = 10) | >10 folds (n = 4) |
Spectrum of PHT in Celiac Patients
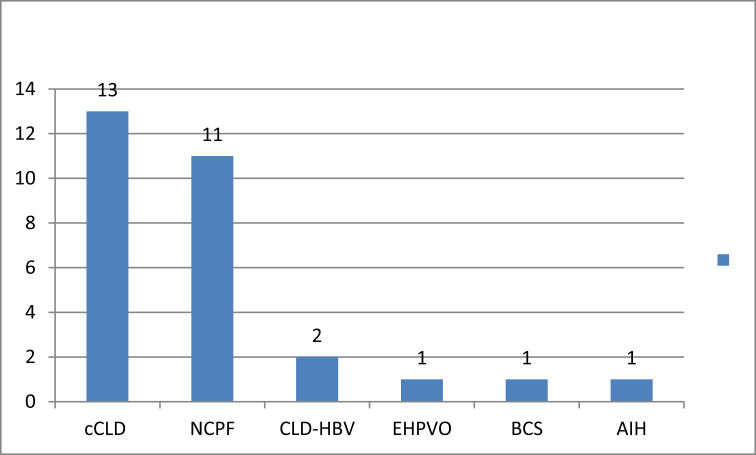
Out of 29 patients, 13 (49%) had cCLD, 11 (38%) NCPF, two (7%) CLD-HBV, one (3.5%) EHPVO, one (3.5%) AIH, and one (3.5%) chronic BCS (Figure 1).
Figure 1.
Spectrum of PHT in CD patients.
Clinical Presentation
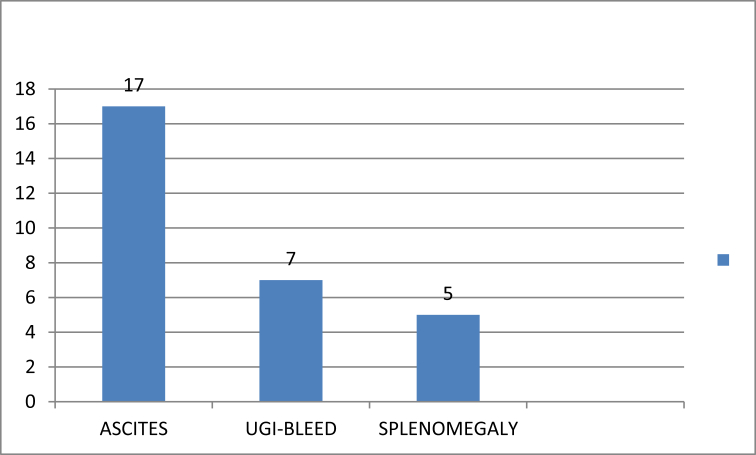
In total, 17 (59%) patients presented with ascites, seven (24%) with variceal bleeding, and five (17%) with splenomegaly (Figure 2).
Figure 2.
Clinical presentation of CD patients.
Endoscopic Findings
Out of 29 patients, 25 (86%) had GEV and four (14%) had portal hypertensive gastropathy. Among the 25 patients of GEV, 23 patients had esophageal varices (grade I in 5 [21%], grade II in 6 [25%], grade III in 10 [42%], grade IV in 2 [8%]), one patient had GEV (GOV2), and one had isolated gastric varices (IGV1). Of the 29 patients, 19 patients had scalloped duodenal folds, 10 had decreased duodenal folds height, and five patients had normal duodenum.
Duodenal Biopsy Histological Features
According to the modified Marsh grade, eight (28%) patients had Marsh 3a, nine (31%) Marsh grade 3b, and 12 (41%) Marsh grade 3c histological feature.
Liver Biopsy Findings
Five patients of CD with PHT with underlying etiology as NCPF3 and cCLD2 (after control of ascites) underwent percutaneous liver biopsy. Histology showed chronic hepatitis in two patients of cCLD and was normal in three patients of NCPF.
Associated Disease
Two patients had hypothyroidism (TSH >10), one patient had Down syndrome coexisting with CD in cCLD group, and one patient had short stature in NCPF group. Dual-energy X-ray absorptiometry scan was not done for bone mineral density (BMD). No patient reported with infertility.
Diagnosis of CD in Relation to Diagnosis of PHT of Varied Etiology
The diagnosis of CD preceded the diagnosis of PHT in five (17%) patients, since childhood in three and two years before PHT diagnosis in two patients. All five had history of diarrhea and anemia. Further, 17 (59%) patients were diagnosed simultaneously and seven (24%) patients were diagnosed after diagnosis of PHT (mean time six months).
Discussion
In our study, we found CD in 13 of the 147 (8.8%) patients in cCLD. A study from Sweden reported 15 times higher prevalence of CD in patients with cCLD than general population.11 In an Indian study, PHT was present in about 10% of the patients with CD.13 Another studies showed the prevalence of CD in cCLD as 13.1% in pediatric patients20 and 4.3% of adult CLD patients21 Prevalence of CD in cCLD in our study is comparable to other studies in adults, but less prevalent as compared to pediatric population, which might be explained by the fact that CD presentation is more common in pediatric patients. In this study, we found CD in 11 of the 64 (17%) patients in NCPF. In a study by Maiwall et al, 14% of NCIPH had positive serology and duodenal biopsy-proven CD.13 The association of CD with NCPF has been recently reported in the literature.22,23 The coexistence of CD and NCPF suggests that there may be an immunological link between these two conditions.23
CD is associated with many autoimmune diseases like type 1 diabetes mellitus, autoimmune thyroiditis, psoriasis, AIH, inflammatory bowel disease, and vitiligo.24 In our study, we found CD in only one (10%) out of 10 patients in AIH, which reflects the higher prevalence than that found in the general population, with two studies reporting the prevalence as 6.4% and 4%.25,26 This can be explained by the fact that our study was not primarily designed to see the prevalence of CD in AIH. However, a study from India also found prevalence of CD in AIH as 8%.27 AIH and CD share a common immunological basis. HLA-DR3 expressed in AIH has a strong linkage with HLA-DQ2 and may account for the association between these diseases.28 Two patients of CD with cCLD also had hypothyroidism, which might explain the autoimmune basis of these diseases. We also found CD in 1 (14%) of the seven patients of BCS and one (5.6%) out of the 18 of EHPVO. The association of CD with BCS and EHPVO is not so uncommon as previously thought. There are many case reports of CD associated with BCS.29, 30, 31, 32 In this study, we found CD in two of the 42 patients of HBV-related CLD. In a study done by Sima H et al in Iran, the prevalence of celiac autoantibodies in chronic hepatitis B (11.3%) is relatively high compared with the general population.33 Celiac antibodies have been described in patients with viral hepatitis, but the causal association has never been proved.34 However, if the patient has to undergo interferon-based therapy, screening is recommended, and if celiac antibodies are present, a gluten-free diet (GFD) should be established before initiating treatment to reduce the risk of triggering overt CD that can lead to discontinuation of interferon.35 Additionally, CD patients are genetically predisposed to hepatitis B vaccine failure. Indeed, 54% and 68% of children and adults with CD do not show a response to standard vaccination regimens for hepatitis B virus, which seems to be linked to the HLA DQ2 haplotype.36,37 Anti-tTG antibody can be false positive in CLD patients, In one study, prevalence of anti-tTG antibodies was 25.2% in CLD patients, but only 2.4% of these patients with CLD were affected with CD.38 In our study, 12 patients in ethanol-related CLD were borderline positive for IgA-tTG (IgA anti-tTG titer = 12–18), but histology negative for CD. High anti-tTG with normal histology common in CLD patients may be falsely elevated. In our study, liver biopsy was done in five patients of CD with PHT who had no contraindication to percutaneous liver biopsy. Histology showed chronic hepatitis in two patients and normal histology in three patients. This may be explained due to sampling error or undefined natural history of liver disease in CD. The possible mechanisms of liver injury include increased intestinal permeability,39 systemic autoimmunity,40 mucosal damage with inflammation,41 malnutrition,42 and intestinal bacterial overgrowth.43
The limitations of our study were 1) liver biopsy was not done in each CD patient, 2) follow-up was not done to evaluate the effect of GFD on clinical and biochemical profile, 3) in celiac serology, only IgA anti-tTG antibody was tested, and in some patients, IgA-tTG was borderline elevated, but biopsy was negative for CD but HLA DQ2, antiendomysial antibody, and IgA level were not done in these patients.
CD is common in PHT of varied etiology, especially in cCLD, NCPH, and AIH. Serological evaluations for CD should be part of the general workup of patients with PHT, especially when other causes of liver disease have been ruled out. Does CD actually lead to development of PHT or is it just an association? At present, there is no definite answer. A large sample study with long follow-up of CD patients developing PHT with histological evidence of liver disease could answer this enigma. In our knowledge, this is largest study till date from India. Further studies are needed to explore the pathogenesis of PHT and CLD in CD. However, in patients with CLD, attention should be paid to the risk of false-positive results of serum anti-tTG, due to the different degree of specificity of the methods used and to the high immunoglobulin concentration.
Conflicts of interest
The authors have none to declare.
References
- 1.Catassi C., Fabiani E., Ratsch J.M. The coeliac disease in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Pediatr. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 2.Sollid L.M., Throsby E. HLA Susceptibility genes in celiac disease: genetic mapping and role of pathogenesis. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 3.Makharia G.K., Verma A.K., Amarchand R. Prevalence of celiac disease in northern part of India: a community based study. J Gastroenterol Hepatol. 2011;26:894–900. doi: 10.1111/j.1440-1746.2010.06606.x. [DOI] [PubMed] [Google Scholar]
- 4.Makharia G.K., Baba C.S., Khadgawat R. Celiac disease: variations of presentations in adults. Indian J Gastroenterol. 2007;26:162–166. [PubMed] [Google Scholar]
- 5.Mounajjed T., Oxentenko A., Shmidt E., Smyrk T. The liver in celiac disease: clinical manifestations, histologic features, and response to gluten-free diet in 30 patients. Am J Clin Pathol. 2011;136:128–137. doi: 10.1309/AJCPDOMY5RI5TPMN. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen M.B., Fausa O., Elgjo K., Schrumpf E. Hepatic lesions in adult coeliac disease. Scand J Gastroenterol. 1990;25:656–662. doi: 10.3109/00365529008997589. [DOI] [PubMed] [Google Scholar]
- 7.Bardella M.T., Fraquelli M., Quatrini M., Molteni N., Bianchi P., Conte D. Prevalence of hypertransaminasemia in adult celiac. Hepatology. 1995;22:833–836. [PubMed] [Google Scholar]
- 8.Kaukinen K., Halme L., Collin P. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterol. 2002;122:881–888. doi: 10.1053/gast.2002.32416. [DOI] [PubMed] [Google Scholar]
- 9.Logan R.F., Ferguson A., Finlayson N.D., Weir D.G. Primary biliary cirrhosis and coeliac disease: an association? Lancet. 1978;1:230–233. doi: 10.1016/s0140-6736(78)90480-4. [DOI] [PubMed] [Google Scholar]
- 10.Goel A., Ramakrishna B., Madhu K. Idiopathic noncirrhotic intrahepatic portal hypertension is an ongoing problem in India. Hepatology. 2011;54:2275–2276. doi: 10.1002/hep.24750. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren S., Sjoberg K., Eriksson S. Unsuspected celiac disease in chronic “Cryptogenic” liver disease. Scand J Gastroenterol. 1994;29:661–664. doi: 10.3109/00365529409092489. [DOI] [PubMed] [Google Scholar]
- 12.Nijhawan S., Katiyar P., Nagaich N. Prevalence of associated disorder in Indian patients with Celiac Disease. Indian J Gastroenterol. 2013;32:330–334. doi: 10.1007/s12664-013-0345-y. [DOI] [PubMed] [Google Scholar]
- 13.Maiwall R., Goel A., Pulimood A.B. Investigating into celiac disease in Indian patients with portal hypertension. Indian J Gastroenterol. 2014;33:517–523. doi: 10.1007/s12664-014-0501-z. [DOI] [PubMed] [Google Scholar]
- 14.Hillaire S., Bonte E., Denninger M.H. Idiopathic non-cirrhotic intrahepatic portal hypertension in the west: a re-evaluation in 28 patients. Gut. 2002;51:275–280. doi: 10.1136/gut.51.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarin S.K., Sollano J.D., Chawla Y.K. Members of the APASL working party on portal hypertension. Consensus on extra-hepatic portal vein obstruction. Liver Int. 2006;26:512–519. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 16.de Franchis R., Burroughs A.K., Bosch J. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Hennes E.M., Zeniya M., Czaja A.J. Simplified diagnostic criteria for autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 18.Marsh M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 19.Oberhuber G., Granditsch G., Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Joshi A., Falodia S., Kumar N., Gupta P., Khatri P.C. Prevalence of celiac disease among pediatric patients with cryptogenic cirrhosis and effect of gluten-free-diet. Indian J Gastroenterol. 2018;37:243–247. doi: 10.1007/s12664-018-0857-6. [DOI] [PubMed] [Google Scholar]
- 21.Kochhar R., Dutta U., Miglani A. Celiac disease suspected at endoscopy in patients with chronic liver disease. Indian J Gastroenterol. 2011 Jul;30:166–169. doi: 10.1007/s12664-011-0106-8. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B.C., Bhasin D.K., Nada R. Association of celiac disease with non-cirrhotic portal fibrosis. J Gastroenterol Hepatol. 2006;21:332–334. doi: 10.1111/j.1440-1746.2006.03296.x. [DOI] [PubMed] [Google Scholar]
- 23.Zamani F., Amiri A., Shakeri R., Zare A., Mohamadnejad M. Celiac disease as a potential cause of idiopathic portal hypertension: a case report. J Med Case Rep. 2009;3:68. doi: 10.1186/1752-1947-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosnes J., Cellier C., Viola S. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol. 2008;6:753–758. doi: 10.1016/j.cgh.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Villalta D., Girolami D., Bidoli E. High prevalence of celiac disease in autoimmune hepatitis detected by anti-tissue tranglutaminase autoantibodies. J Clin Lab Anal. 2005;19:6–10. doi: 10.1002/jcla.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volta U., De Franceschi L., Molinaro N. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci. 1998;43:2190–2195. doi: 10.1023/a:1026650118759. [DOI] [PubMed] [Google Scholar]
- 27.Kapil S., Duseja A., Lal S., Das A., Dhiman R.K., Chawla Y.K. Celiac disease screening in patients with autoimmune liver disease, cryptogenic cirrhosis and non-alcoholic fatty liver disease. JCEH. 2011;1:46. [Google Scholar]
- 28.Zali M.R., Rostami Nejad M., Rostami K., Alavian S.M. Liver complications in celiac disease. Hepat Mon. 2011;11:333–341. [PMC free article] [PubMed] [Google Scholar]
- 29.Kochhar R., Masoodi I., Dutta U. Celiac disease and Budd Chiari syndrome: report of a case with review of literature. Eur J Gastroenterol Hepatol. 2009;21:1092–1094. doi: 10.1097/MEG.0b013e328328f47f. [DOI] [PubMed] [Google Scholar]
- 30.Martinez F., Berenguer M., Prieto M., Montes H., Rayon M., Berenguer J. Budd-Chiari syndrome caused by membranous obstruction of the inferior vena cava associated with coeliac disease. Dig Liver Dis. 2004;36:157–162. doi: 10.1016/j.dld.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Gelsi E., Ruitord F., Saint-Paul M.-C., Filippi J., Arab K., H´ebuterne X. Association of Budd-Chiari syndrome with a coeliac disease in patient native from North Africa. Gastroenterol Clin Biol. 2004;28:903–905. doi: 10.1016/s0399-8320(04)95155-x. [DOI] [PubMed] [Google Scholar]
- 32.Afredj N., Metatla S., Faraoun S.A. Association of Budd-Chiari syndrome and celiac disease. Gastroenterol Clin Biol. 2010;34:621–624. doi: 10.1016/j.gcb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Sima H., Hekmatdoost A., Ghaziani T., Alavian S.M., Mashayekh A., Zali M.R. The prevalence of celiac autoantibodies in hepatitis patients. Iran J Allergy, Asthma Immunol. 2010;9:157–162. [PubMed] [Google Scholar]
- 34.Silano M., Volta U., Vincentini O., Vincenzi M.D. Clinical features of chronic c virus hepatitis in patients with celiac disease. Eur J Clin Microbiol Infect Dis : off pub Eur Soc Clin Microbiol. 2009;28:1267–1269. doi: 10.1007/s10096-009-0769-6. [DOI] [PubMed] [Google Scholar]
- 35.Durante-Mangoni E., Iardino P., Resse M. Silent celiac disease in chronic hepatitis C: impact of interferon treatment on the disease onset and clinical outcome. J Clin Gastroenterol. 2004;38:901–905. doi: 10.1097/00004836-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Noh K.W., Poland G.A., Murray J.A. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol. 2003;98:2289–2292. doi: 10.1111/j.1572-0241.2003.07701.x. [DOI] [PubMed] [Google Scholar]
- 37.Park S.-D., Markowitz J., Pettei M. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;44:431–435. doi: 10.1097/MPG.0b013e3180320654. [DOI] [PubMed] [Google Scholar]
- 38.Sood A., Khurana M.S., Mahajan R. Prevalence and clinical significance of IgA anti-tissue transglutaminase antibodies with chronic liver disease. J Gastroenterol Hepatol. 2017;32:446–450. doi: 10.1111/jgh.13474. [DOI] [PubMed] [Google Scholar]
- 39.Novacek G., Miehsler W., Wrba F. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11:283–288. doi: 10.1097/00042737-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Korponay-Szabó I.R., Halttunen T., Szalai Z. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindberg T., Berg N.O., Borulf S. Liver damage in coeliac disease or other food intolerance in childhood. Lancet. 1978;1:390–391. doi: 10.1016/s0140-6736(78)91115-7. [DOI] [PubMed] [Google Scholar]
- 42.Davison S. Coeliac disease and liver dysfunction. Arch Dis Child. 2002;87:293–296. doi: 10.1136/adc.87.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunes D.P., Nolan N.P.M., O'Connor M.P. Fasting breath hydrogen, small bacterial overgrowth and intestinal transit in coeliac disease. Eur J Gastroenterol Hepatol. 1991;3:313–319. [Google Scholar]