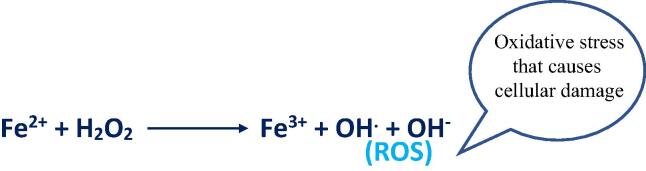Highlights
-
•
The conventional utilization of drugs is characterized by poor biodistribution, limited effectiveness, and lack of selectivity, besides undesirable side effects on multiple body systems.
-
•
Seeking a DDS with a modifiable skeleton to customize drug targeting is of extreme importance for successful therapy of many diseases.
-
•
Among the different synthesis strategies for MNPs, chemical methods are the most common, and on top of the pyramid, is the co-precipitation method.
-
•
MNPs have customizable properties, where applying a hydrophilic coating protects the particles from opsonization and human-immunity recognition, which increases their circulation time.
-
•
The route MNPs usually follow in the body starts with magnetic guidance to the target, immobilization for drug release, and finally clearance.
-
•
Interestingly, multifunctional nanocomplexes with conjugated SPIONS and PEI presented enhanced transfection while decreased PEI toxicity.
-
•
Theranostic applications of MNPs are limitless, whether it is a dual function of diagnosis and therapy simultaneously, or a multimodal imaging system.
-
•
IONPs participate in the production of oxidative stress that leads to cell damage.
-
•
Metal ferrite NPs can overcome the drawbacks of IONPs provided that the substituting metal in use is less toxic.
-
•
Metal ferrite NPs present unique properties of high saturation magnetization, enhanced encapsulation efficacy, as well as enzyme-mimetic activities.
-
•
Magnesium ferrite NPs (MFNPs) were found to exhibit greater magnetic heating capacity compared to other ferrites. MFNPs also show safe metabolism and high biocompatibility, making them a promising system for cancer applications.
Keywords: Magnetic nanoparticles, Metal ferrites, Synthesis strategies, Cancer therapy, Theranostics, Gene delivery
Abstract
In modern drug delivery, seeking a drug delivery system (DDS) with a modifiable skeleton for proper targeting of loaded actives to specific sites in the body is of extreme importance for a successful therapy. Magnetically guided nanosystems, where particles such as iron oxides are guided to specific regions using an external magnetic field, can provide magnetic resonance imaging (MRI) while delivering a therapeutic payload at the same time, which represents a breakthrough in disease therapy and make MNPs excellent candidates for several biomedical applications. In this review, magnetic nanoparticles (MNPs) along with their distinguishable properties, including pharmacokinetics and toxicity, especially in cancer therapy will be discussed. The potential perspective of using other elements within the MNP system to reduce toxicity, improve pharmacokinetics, increase the magnetization ability, improve physical targeting precision and/or widen the scope of its biomedical application will be also discussed.
1. Introduction
The conventional utilization of many marketed medicines including anti-cancer medicines is characterized by poor biodistribution, limited effectiveness, and lack of selectivity, that aside from the undesirable side effects on multiple body systems (Bhatia, 2016). For example, the general guidelines for cancer therapy circulate between chemotherapy, surgery, and radiation (Giordano et al., 2005, Mamon et al., 2005, Stenzl et al., 2011) yet despite the variability in therapeutic options, their efficacy remain time-limited and non-curative (Bhandari, 2015). Therefore, it may come as no surprise that cancer is on the rise in both developed and developing countries (Bhandari, 2015) with an estimated number of 12 million deaths in 2030, making it the leading cause of death worldwide (Alexiou et al., 2011). To overcome these health-related challenges, screening for superior and safer medicines has been a global ongoing pursuit in the last decade (Bhandari, 2015).
Nanotechnology is the engineering and manufacturing of materials at the atomic and molecular scale, around 1–100 nm in size in at least 1 dimension (Farokhzad and Langer, 2009). In recent years, nanotechnology has proved its immense benefits in drug delivery. Nanoparticles (NPs) made of different materials specifically are becoming very popular to use as nano-carriers due to many advantages such as their flexibility in accommodating different types of drugs including genes and proteins, high hydrophobicity, control drug release, high drug accumulation in target tissues, ease of preparation, tolerability, in addition to their ability to improve the PK-PD profile of different drugs (Ahmed et al., 2018, Ahmed et al., 2016, Gao et al., 2013, Kim and Lee, 2001, Tran et al., 2014). However, many of these nanosystems suffer from poor drug loading capacity, limited permeability, low targeting ability, poor physical stability, chemical problems associated with degradation and toxicity. Therefore, it becomes imperative to explore new strategies in order to produce more advanced and effective nanomedicines. The recent advancements in nanotechnology made it possible to entangle magnetic cores in nanoparticles to produce nanomagnetic systems with drug targeting ability.
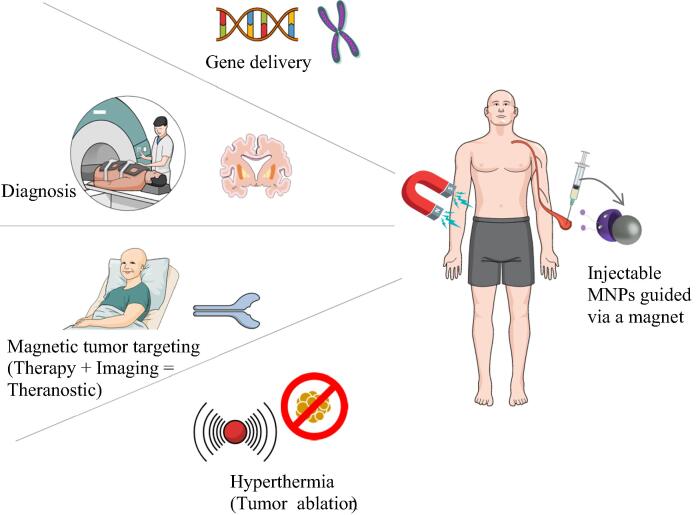
Magnetic nanoparticles (MNPs) offer added benefits compared to more conventional nanocarriers such as the ability to guide the MNPs in the body through an external or an internal magnetic field, use of an alternating magnetic field to generate local hyperthermia that can heat up the carrier to trigger drug release in the delivery site and localize actives in the selected target, and most importantly visualize and track the MNPs using Magnetic Resonance Imaging (MRI). These advantages make them unique and very attractive to use in several biomedical applications (Singamaneni et al., 2011). Fig. 1 displays a summary of the applications of MNPs.
Fig. 1.
Applications of MNPs.
Yet despite the high promises, major limitations such as poor control over the particles’ residence time in the desired organ, which can relate to the strength of the external magnetic field and variable particles’ responsiveness to the external stimulus (Arruebo et al., 2007a). These limitations can be overcome by using substituted metal ferrites to further empower the magnetization sensitivity of the drug-loaded particles to improve the diagnostic and the therapeutic outcomes and achieve multiple functions or multimodal therapeutics effects (Chaibakhsh and Moradi-Shoeili, 2019).
1.1. Properties of MNPs
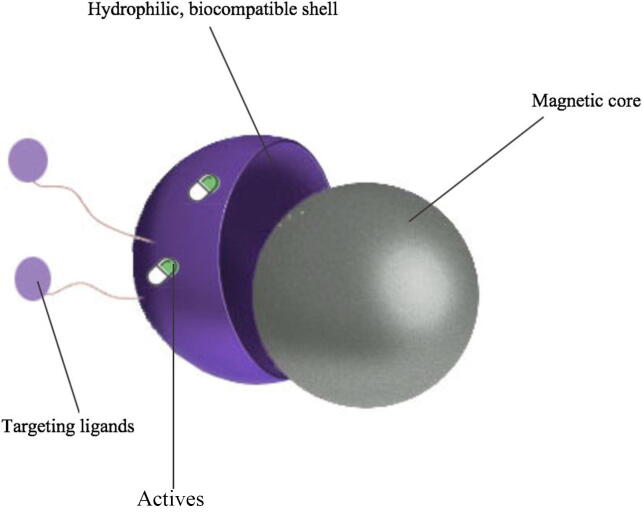
MNPs as shown in Fig. 2, are made of a magnetic core, surrounded by a biocompatible shell, and functionalized with different target molecules (Ali et al., 2016). The uniqueness of these systems lay not only in their small size, which is usually less than 100 nm, and their high magnetization values, but also in their ability to target their payloads to the intended sites with minimal side effects through surface customization with functional groups. This render MNPs very attractive to be applied in several biomedical fields such as drug delivery, hyperthermia, tumor imaging, theranostics, tissue engineering and others (Nguyen and Kim, 2014).
Fig. 2.
Components of a MNP.
1.2. MNPs synthesis strategies
The basic core of a magnetic nanocarrier approach is the synthesis of the magnet. For this reason, many strategies were developed in order to create a distinguishable magnetic core. The most widely used magnetic core is iron oxide (IO) due to its excellent super-paramagnetic property as well as its biocompatibility and biodegradability which make IO safer compared with other metals. Synthesis of MNPs is usually done by chemical methods, physical methods or microbial methods, with the chemical methods being the most commonly used. This review will mainly focus on the chemical methods used in the synthesis of MNPs, with Table 1 comparing between the aforementioned methods (Ali et al., 2016).
Table 1.
Comparison between different synthetic pathways of MNPs.
| Synthesis Method | Condition | Size Distribution | Shape Control |
|---|---|---|---|
| Co-precipitation | Simple | Relatively narrow | Poor |
| Sol gel | Simple | Narrow | Good |
| Microemulsion | Complicated | Narrow | Good |
| Hydrothermal | Complicated and inert atmosphere | Very narrow | Very good |
| Thermal decomposition | Complicated and inert atmosphere | Very narrow | Very good |
1.2.1. Co-precipitation method
The co-precipitation method is one of the very popular methodologies for MNPs synthesis, in fact, the most proper, and the most widely used. It employs safer procedures and materials than those utilized using other methods (Majidi et al., 2016). In this method, ferric and ferrous salts are used as precursors in fixed ratios. They are dissolved in deionized water, followed by the addition of ammonium hydroxide base, which works as a precipitating agent. Surfactants like oleic acid or hexanoic acid are then added to aid in the stabilization of the particles. The following equation represents the reaction happening in the solution (Petcharoen and Sirivat, 2012):
Fe+2 + 2 Fe+3 + 8 OH- → Fe(OH)2 + 2 Fe(OH)3 → Fe3O4 + 4 H2O (Majidi et al., 2016).
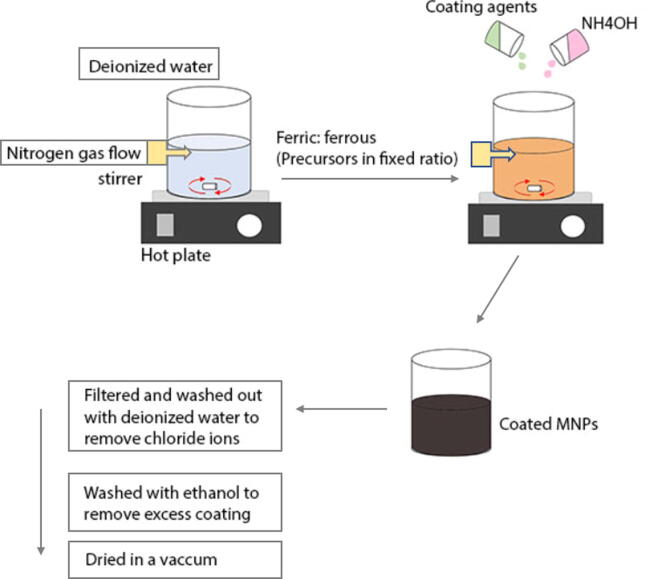
The overall system allows vigorous stirring and nitrogen gas bubbling within the solution. The nitrogen here protects the NPs against critical oxidation and also serves to reduce the particle size, as well as contributing to the formation of hydrazine. Hydrazine interacts with the dissolved oxygen in the solution to produce cationic ammonium hydroxide [NH3OH]+, which then reacts with ferrous ions (Fe+2) to produce the ferrite colloid magnetite (Fe3O4), thus increasing the yield of the iron oxide nanoparticles (IONPs). Synthesis can happen under different temperatures, though if the reaction was carried out at room temperature or less than 60 °C, it would result in amorphous hydrated oxyhydroxide that can easily convert to Fe2O3, unlike if it was done at higher temperatures > 80 °C which would form the product Fe2O3, right away (Majidi et al., 2016). The color of the solution changes from orange to black, indicating the end of the reaction and the formation of MNPs. The black solution is then filtered and washed with deionized water, to remove chloride ions of the salt. Another washing step is done with ethanol to remove excess oleic acid. The final step is using vacuum for drying (Petcharoen and Sirivat, 2012) to obtain uniform mono-dispersed MNPs with a relatively narrow size distribution (Majidi et al., 2016). Fig. 3 illustrates the summarized steps used in this method. Bare MNPs can also be coated by adding different coating agents to prevent agglomeration, avoid opsonization or provide a mean to tailor the surface properties of MNPs (Sun et al., 2008). Limitations associated with this method include particle aggregation due to small size, size and shape dis-uniformity, and poor dispensability (Majidi et al., 2016).
Fig. 3.
Detailed steps of the co-precipitation method.
1.2.2. Hydrothermal method
MNPs synthesized by the hydrothermal method are characterized by a very good shape and uniform size distribution. Reactions in this method are carried out in an autoclave with elevated temperature and pressure. An aqueous solution containing FeCl3 and FeCl2 is heated to a certain temperature, under a steady feeding of an inert gas such as argon. A reducing agent like ammonium hydroxide is then added slowly to the solution with continuous vigorous stirring. The reaction mixture is then moved into an autoclave and kept there for a specific period of time. The product is then obtained and washed with degassed water three to four times, to ensure all the salts and the excess ions are removed. Finally the product is dried to obtain the MNPs (Daou et al., 2006). The MNPs produced by this method could be synthesized with or without surfactant to reduce particle aggregation (Demirer et al., 2015).
1.2.3. Microemulsion method
The involvement of simple reaction equipment, as well as the ability to control the particle size using a variety of materials is what makes the microemulsion method a widely used method to obtain MNPs (Ali et al., 2016). Two water in oil (w/o) microemulsions are utilized, where microemulsion I contains the metal salts in the aqueous phase, and microemulsion II contains the precipitating agent in the aqueous phase. After the preparation of both microemulsions, microemulsion II, containing the precipitating agent, is added drop wise to microemulsion I, with continuous stirring and heating for a certain period of time. The formation of a black color is the indicator that MNPs have been formed. Later the precipitated MNPs is obtained by centrifugation. Washing of the product is done through precipitating and re-suspending the MNPs in a mixture of ethanol and water, then again with ethanol to remove all excess oil or surfactant (Okoli et al., 2012).
1.2.4. Sol gel method
This methodology involves the use of metal alkoxide precursors dissolved in a solvent that is usually water. Hydroxylation and condensation of the precursors and water occurs which lead to the formation of a sol. The sol then undergoes condensation to create a gel that is made of three-dimensional networks of metal oxides. Heating is used to remove the solvent, and the MNPs are then obtained (Chen et al., 2018, Reddy et al., 2012, Tavakoli et al., 2007). To stabilize the system, a surfactant can be added to the aqueous solution. This method yields MNPs of high shape control and tight size distribution (Chen et al., 2018). Several factors affect the properties of the MNPs produced by this method including the pH, temperature, nature of the solvent and the concentration of the precursors used (Chen et al., 2018, Tavakoli et al., 2007, Teja and Koh, 2009).
1.2.5. Thermal decomposition method
This method often requires special conditions including the utilization of high temperatures (around 300 – 350 °C), an inert atmosphere, as well as certain ligands and reagents. A beaker containing organic iron compound such as Iron (III) acetylacetonate dissolved in an organic solvent, in the presence of a capping agent like oleic acid is used. This mixture is heated to 110 °C for approximately an hour with continuous stirring, and then refluxed to a certain temperature for a certain amount of time. Cooling the solution to room temperature is to follow, and then the solution is introduced to ethanol. The precipitated MNPs are obtained by centrifugation and the obtained MNPs are washed again with ethanol and dried in a drying cabinet (Chen et al., 2018, Maity et al., 2008). Studies showed that increasing the reaction temperature would affect the size of the particles produced due to increase in the growth rate. The longer the time of the reaction is the bigger the particles become in size and the wider the size distribution is. The use of surfactants has been shown to provide a tight control on the size distribution of the particles (Maity et al., 2008). Accurate control of the produced MNPs has been achievable mainly in this method, but the use of toxic reagents governs its use mainly in the field of imaging (Ali et al., 2016)
1.3. Stabilization and functionalization of MNPs
MNPs if synthesized with no coating material on their surface will suffer from several problems; one of which is the agglomeration of the magnetic molecules due to the strong attractive forces that will render the MNPs unstable. Toxicity represents another problem if bare MNPs were to be used due to elevated metal level in the body (Umut, 2013). Surface coating of MNPs imparts stability to the system, and serves as a linker by providing functional groups such as amine and carboxyl groups, which in turn allow the functionalization of the surface with several active moieties (Fang and Zhang, 2009, Reddy et al., 2012).
The encapsulation of MNPs with a coating material could be accomplished during synthesis (in-situ) where the coating agent could be incorporated within the reaction solution. Coating can also be achieved post-synthesis, where the MNPs are first synthesized and stabilized with a surfactant, such as oleic acid, to create a hydrophobic surface, and then the coating agent is added and attached to the surface through hydrophobic interactions.
To incorporate the coating material onto the surface of MNPs, two approaches could be followed: end-grafting or surface encapsulation. To end graft the coating material, a capping or a functional group on the coating material is required to bind to the surface of the MNPs. Examples of functional groups include carboxyl, hydroxyl, amine, thiol and alkoxysilanes. Unfortunately, the amount of a functional group per coating material is usually limited, thus the bonds between the surface of MNPs and the coating agent could be broken down, which might lead to a decrease in the stability and the sites available for conjugation with the active moieties. And thus, to overcome the limitations of the end grafting technique, surface encapsulation has been proposed. Surface encapsulation requires the use of polymers with several functional groups that could bind to the surface of MNPs and thus create a stable system (Fang and Zhang, 2009). Several coating agents have been suggested and used for MNPs stabilization, some of which shall be discussed below.
The remarkable ability of polymers to prevent particles’ aggregation and opsonization of MNPs in the body has placed them as one of the most widely employed materials for MNPs coating (Sun et al., 2008). Polymers can be categorized according to different criteria such as their chemical structure, length or molecular weight, surface charge, conformation, the way they attach to the surface of the particle, degree of surface coverage, biodegradability and others. For instance, neutral polymers containing hydrophilic groups such as hydroxyl and ether groups may provide great protection against opsonization however they suffer from low capacity of conjugation of active molecules due to low number of functional groups on their surface. Charged polymers, on the other hand, contain an abundance of active functional groups however they are more likely to be opsonized due to electrostatic interaction with plasma proteins (Fang and Zhang, 2009). Dextran, is a good example of a neutral natural polysaccharide that is very widely used due to its good biocompatibility and stability against enzymatic degradation and modification of its surface through the introduction of silanol groups was reported to improve its capacity to conjugate active molecules (Fang and Zhang, 2009). The treatment of dextran-MNPs system with ammonia also enable the presence of primary amine bond that would be beneficial for the conjugation of several bioactive molecules (Sun et al., 2008). Polyethylene glycol (PEG) represents another excellent example of a neutral, hydrophilic, synthetic polymer, widely employed as a coating material. PEG could be incorporated with phospholipids to cover the hydrophobic surfaces of MNPs, and with the ability to manipulate the terminal group of PEG, many possible interactions might happen, and a stable functional system would be created (Fang and Zhang, 2009, Umut, 2013). PEG coated MNPs greatly affect the pharmacokinetics (PK) of the system, where PEG-MNPs circulate the blood for a longer time compared to dextran-MNPs systems (Umut, 2013). Designing novel materials for biomedical applications generally requires the use of biodegradable materials. Chitosan, a renewable natural linear amino polysaccharide, is of great interest due to its good biocompatibility and biodegradability. The polycationic nature of chitosan, due to the abundance of amino groups, has made it very popular in drug and protein delivery as well as tissue engineering (Demirer et al., 2015).
Coating agents are not limited to polymers, although they do take the majority of the share, other materials could be used for the same purpose. Good chemical stability, biocompatibility, and physical properties attracted the attention of scientists to use gold (Au) as a coating material for MNPs (Umut, 2013). Surface coating with gold enabled the production of stable Au-MNPs with high ability of surface functionalization using different bioactive molecules (Demirer et al., 2015). Another interesting coating material would be silica. The utilization of silica as a coating material has proven to be of great benefit, it protects MNPs from agglomeration, decreases toxicity and provides the opportunity for multi functionalization of MNPs surface due to the presence of silanol groups that help in the formation of strong bonds. However, the inability to produce uniform thickness of silica shell coating limits its use in several fields (Demirer et al., 2015, Umut, 2013).
1.4. MNPs characterization:
A well-established characterization is a mean to develop the exclusiveness of the MNPs figure, and since the core size and size distribution directly influence the features of the MNPs (Biehl et al., 2018), it becomes crucial to have multiple methods to investigate MNP’s properties. Table 2 provides a simplified roadmap on the in vitro physiochemical characterization tests and their functions.
Table 2.
Different in vitro tests with their specific functions.
| In Vitro Test | Specific Function |
|---|---|
| TEM, HR-TEM | Core size, shape, and size distribution |
| SEM | Morphology and size distribution |
| DLS | Hydrodynamic radius, colloidal stability, and size distribution |
| EDXD | Elemental analysis and chemical composition |
| XRD | Chrystallographic identity, phase purity, and aids in the mean particle size measurement |
| SQUID, VSM | Net magnetization |
| XPS | Surface reactions, bonding characteristics and chemical composition |
| Zetasizer | Surface charge |
| FT-IR | Organic functional groups |
1.4.1. Size and morphology
Transmission Electron Microscopy (TEM) is a routine technique used in measuring the core size of the particles; it also provides information on the shape and size distribution. However, TEM requires a large number of MNPs and needs to carry the analysis by image treatment. Similarly, High-Resolution Transmission Electron Microscopy (HR-TEM) can be used, and it has the advantage of providing the atomic rearrangement, that aids in the study of local microstructures. Scanning Electron Microscopy (SEM) is commonly used to determine the size distribution and surface morphology of the particles within the micro and nano range. SEM is good to measure the total particle size, but it is not very efficient to test particles smaller than 20 nm, not to mention the lower resolution compared to TEM. Dynamic Light Scattering (DLS) is used to obtain the hydrodynamic radius and the colloidal stability using Brownian motion in the dispersion, as well as the size distribution of the NPs through Polydispersity Index (PDI). Narrow size distribution is attained when PDI values lie between 0.1 and 0.25, anything above 0.5 is considered broad distribution, indicating aggregation and particle size changes. It is fair to mention that DLS only provides an average value, and is less accurate than TEM, therefore it is preferable to use it in combination with TEM (Biehl et al., 2018).
1.4.2. Structure and elemental analysis
Energy Dispersive X-Ray Diffraction (EDXD) is a great tool for elemental analysis, and to supply information on the chemical composition of MNPs. It provides an estimate of the ratio between MNPs elements and has the advantage of being held in a suspension. X-Ray Diffraction (XRD) is used to determine the crystallographic identity, phase purity, and might also calculate the mean particle size using both Scherrer’s equations which depends on the broad ends of the most eminent peaks, where broad ends are equivalent to smaller particle size. Additionally, the intensity performed by comparing experimental to reference peaks allow the quantification of MNPs proportion in a mixture (Faraji et al., 2010).
1.4.3. Magnetic properties
Superconducting Quantum Interference Device (SQUID) is used to assess the magnetic properties of the particles (Faraji et al., 2010), through sensitive detection of the magnetic flux. It is a combination of two superconductivity quantum properties, the Josephson effect, and quantization of flux in a superconducting loop (Xianyu et al., 2018), whereas Vibrating Sample Magnetometry (VSM) evaluates the magnetization of MNPs under the influence of an external magnet. The magnetic behavior then is interpreted based on the VSM curve at room and low temperatures. Both VSM and SQUID are powerful tools used to measure the net magnetization of a given sample (Faraji et al., 2010).
1.4.4. Surface characterization
X-Ray Photoelectron Spectroscopy (XPS) is an interesting technique in studying the reaction mechanisms that happen on the surface of MNPs, it is also quite useful in identifying the bonding characteristics of the several elements involved, as well as determining the chemical composition. Zetasizer is related to characterizing the surface properties of MNPs in physiological conditions, as well as to improve the chemistry of conjugation. Zeta potential analysis is used to characterize the surface charge. Fourier Transform Infrared Spectroscopy (FT-IR) is a beneficial tool to identify organic molecules functional groups, it is commonly acquired in double checking the linkage of these functional groups after each functionalization step (Faraji et al., 2010). Thermal Gravimetric Analysis (TGA) is concerned mostly with organic coatings on the surface of inorganic nanoparticles (Biehl et al., 2018), like polymers or surfactants, to predict and measure their efficacy of binding onto the MNPs surface (Faraji et al., 2010).
1.5. MNPs pharmacokinetics, biodistribution, and biological fate
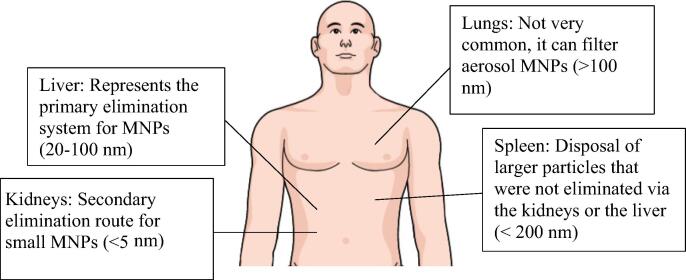
The route taken by MNPs in the body usually consists of three main steps: (1) MNPs are guided to their target by a magnet; (2) an external magnetic field immobilizes the particles for drug release; (3) clearance of MNPs by organs of elimination (Reddy et al., 2012), illustrated in more depth in Fig. 4.
Fig. 4.
The different elimination routes undertaken by MNPs.
Following an IV administration, MNPs travel through the blood and opsonization happen. Opsonization is the process where plasma proteins get adsorbed onto the surface of MNPs, particularly the hydrophobic parts, allowing their recognition by the immune system, and thus their clearance. Opsonization by the Reticuloendothelial System (RES), which represent macrophages of the liver, spleen, or bone marrow, can sometimes be beneficial if the targeted tissue is one of these organs, but otherwise, it is an undesired process (Reddy et al., 2012).
Here, surface modification of MNPs become of an increased importance. This can be achieved through the addition of hydrophilic heads, or coating them with PEG (Reddy et al., 2012), which makes them more water soluble, more biocompatible, and more selective (Singamaneni et al., 2011), with a stealth property. Repeated administration of PEGlylated MNPs had controversial effects on pharmacokinetics (PK). Some studies show no effect while others reported a phenomenon of “Accelerated blood clearance” due to the induction of anti-PEG-IgM antibody by the first dose, resulting in faster clearance of subsequent doses (Reddy et al., 2012).
The distribution and accumulation of MNPs in the site of interest depend on the size, shape and surface characterization (Shapiro et al., 2015), as well as the dose and the time after IV administration, which make time-course profiles at multiple dosing is of utmost importance (Spirou et al., 2018).
MNPs elimination involves both the liver and kidneys. In a study, it was reported that after 6 h of injection with IONPs, >50% of the NPs were located in the liver, indicating the involvement of the reticular endothelial system. The good vascularization and permeability of IONPs are what eases their elimination by the RES (Ali et al., 2016).
1.6. MNPs targeting
Generally, there are two modes of targeting, passive and active targeting. Passive targeting happens when NPs accumulate due to their physicochemical characteristics such as size and surface properties (Reddy et al., 2012), it also depends on enhanced permeation and retention effects (EPR) of the leaky blood vessels at the tumor sites. Although passive targeting served nicely in several applications, its outcome has shown to have poor reliability (Singamaneni et al., 2011). Active targeting is more controlled and provides reproducible delivery patterns to the target (Dolovich and Dhand, 2011). It happens due to either a stimulus or surface functionalization. The stimulus can be an external magnetic field with a force powerful enough to guide and stabilize the particles against the hydrodynamic blood flow and Brownian motion. According to Food and Drug Administration (FDA), magnets that induce up to 8 T do not have any significant risks on adults. In the case of cancer, functionalization of MNPs with specific ligands additionally allow their accumulation in tumor sites due to their high affinity to unique molecules or endothelial cells lining the tumor neovasculature (Reddy et al., 2012).
1.7. Toxicity of MNPs
MNPs as a carrier must ensure the delivery of attached actives to the intended site, and then be cleared out of the body as soon as possible to ensure no toxicity related issues could occur (Reddy et al., 2012).
As previously stated, MNPs clearance involves several organs including the liver and the spleen. When injecting MNPs, the degradation of the MNPs by these organs will release iron. The released iron as shown in Fig. 5, can take place in a Fenton reaction to produce reactive oxygen species (ROS). Iron molecules can be stored in the body by specific proteins, but when the iron levels become too high, the iron stores will be saturated, and this pushes the reaction forward to produce more toxic ROS, causing further damage to the cells (Patil et al., 2015).
Fig. 5.
Illustration of Fenton reaction.
1.7.1. In-vitro toxicity
In vitro toxicity studies use multiple assays like (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT or (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)–2H-tetrazolium) MTS assays that aim to test the function of the mitochondria and the viability of the cells, lactate dehydrogenase (LDH) test that looks for signs of damage to the body’s tissues via the release of LDH enzyme from the injured cells, and Trypan Blue test that is directed to test the integrity of the cell membrane (Patil et al., 2015). When the concern arises from MNPs toxicity, several parameters related to the properties of MNPs are taken into accounts such as size, shape, and surface coating (Patil et al., 2015, Reddy et al., 2012).
Summary of findings about the size suggested that the smaller the particle size is, the higher the toxicity MNPs might produce. In another experiment designed to measure the impact of shape, conducted on mice macrophage cells, the effect of rode shape and spherical shape MNPs was tested, with a conclusion that the rode shape MNPs had a greater toxicity influence than the spherical shape due to their higher surface area/volume ratio, and their ability to damage the cell membrane through ROS production (Lee et al., 2014). Finally, the coating, where most studies displayed results supporting the coating of MNPs, in order to decrease the chances of toxicity arising due to leaching of bare MNPs and the release of iron (Patil et al., 2015).
In-vitro toxicological testing must take into account other variables when being conducted aside from the parameters mentioned above such as the type of cells being tested, as well as the dose given. Table 3 offers a sample of the in-vitro toxicity trials preformed with the different parameters and assays Castaneda et al., 2011, Omidkhoda et al., 2007.
Table 3.
Brief overview on in-vitro toxicological studies of MNPs.
| Types of MNPs | Size | Type of cell used | Dose | Assay used | Results | References |
|---|---|---|---|---|---|---|
| Uncoated MNPs | 150 nm | hMSCs | 50–250 μg/L | Comet assay | Cell viability was not affected at any concentration. | (Omidkhoda et al., 2007) |
| Dextran-coated IONPs | 150 nm | ESCs | 50 μg/L | Trypan Blue | Cell viability was not affected at any concentration | (Castaneda et al., 2011) |
1.7.2. In-vivo toxicity
As though in-vitro experiments are great measures to scan the viability of cells, being quick, inexpensive and reproducible, they can be limited when it comes to mimicking the complex physiology of living entities. This is where in-vivo tests step in, not only to overcome these obstacles, but also to gain FDA approval for drug registration (Patil et al., 2015).
In a study attempted by Prodan et al., male brown Norway rats were used to run in-vivo cytotoxicity assays. The rats were housed in a controlled environment, under restricted pathogen-free conditions, and were divided into control and test groups, treated with peritoneal injections of normal saline and different concentrations of iron oxide NPs (IONPs) respectively. All animals in the test group survived and showed no behavioral changes during the next two days of follow up. Then, they were sacrificed, and different organs were obtained for microscopic observation and histopathological examination. Results at first showed no significant changes between the tested group compared to the control, yet at higher concentrations of 1.7 mL/kg and more, the cellular architecture started to look disfigured, with macrophages build up, pigment deposits, and some tissue degeneration. It was concluded that the concentration of injected IONPs is directly proportional to the level of toxicity, expressing high increase in concentration with a parallel increase in toxicity (Prodan et al., 2013).
It is worth noting that the safety profile of MNPs used for different purposes do not always align in aspect with one another, for instance, MNPs used for hyperthermia are challenged by blood vessels disseminating the heat they generate, thus making them less effective. To cope with such a problem, the force of the magnetic field and the residence time of MNPs in the tissue should increase, that in turn increases the damage on healthy cells. Therefore, another set of toxicity measurements may be needed for MNPs intended for hyperthermia applications (Spirou et al., 2018).
2. Therapeutic applications in cancer
The current trend in the design of new DDS call for the design of multi-functional systems. MNPs as an advanced DDS, are employed in the field of biomedicine not only to counteract the limitations of traditional therapeutics, but also for their biocompatibility, ease of controllability, and their unique physiochemical properties (Hedayatnasab et al., 2017). The use of MNPs in the field of biomedicine can be divided into three subfields: the diagnostic, the therapeutic, and the theranostic field (Dadfar et al., 2019). This review will focus mainly on the therapeutic (including hyperthermia and gene delivery) and the theranostic applications of MNPs. .
2.1. Hyperthermia
The general concept of hyperthermia demonstrates an elevation in the body temperature several degrees above the norm of 37 °C for a certain period of time. Modern hyperthermia limits the thermal therapy to the problematic site, to maximize treatment outcomes and minimize undesired side effects on healthy tissues (GIUSTINI et al., 2010). The known mechanism is that hyperthermia would be lethal to tumor cells by damaging proteins and cellular structures leading to necrosis (Hildebrandt et al., 2002), interestingly, hyperthermia also leads to increased expression in heat shock proteins (HSPs) within and around the tumor tissue. These proteins can be tricky and respond differently depending on the type of treatment provided. For instance, in traditional hyperthermia, HSPs work in favor of the tumor, protecting it against heat-induced cell apoptosis. However, with MNPs induced hyperthermia, it functions in favor of the host, triggering intriguing tumor-specific immune responses. This description is particularly relevant when speaking of metastatic tumors, extending the scope to grab the unheated metastatic tumors at distant sites by activating host immunity against HSPs (Kobayashi et al., 2016).
In many researches, hyperthermia was shown to be promising, singularly and when conjugated with traditional cancer therapies, chemotherapy or radiation. It was believed that high temperatures of 43 °C and above would produce cytotoxic response in cells, as well as augment the cytotoxic effects of chemotherapeutics and radiation (Petryk et al., 2013). The use of MNPs as hyperthermia agents under the influence of an alternating magnetic field can produce damage at tumor sites while preserving the healthy tissues from traditional heating side effects of discomfort, blisters, pain and burns (Falk and Issels, 2001). Following treatment, IONPs were shown to be non-toxic and could be easily cleared by the body within a few weeks (Soo Choi et al., 2007).
In a study performed by Zhao et al., Tu212 cell line was effectuated in mice, acting for human head and neck cancer. Then, a magnetic ferrofluid was delivered to the tumor and then heated by the application of an external alternating magnetic field. Tumor temperatures were controlled between 40 and 50 °C throughout the 20 min procedure, and results noted severe damage to cellular structures post heating (Zhao et al., 2012). Many other in-vivo trials suggested profound inhibitory rates and apoptosis (Yan et al., 2005), with partial or complete successfulness to treat various tumors (Soo Choi et al., 2007). This encouraged researchers to move on to the next step which is clinical trials.
The first clinical trial was carried on a patient suffering from prostate cancer, a direct tumor injection with water-based ferrofluid was made, using variable magnetic field strengths, and a frequency of 100 kHz to retain high temperatures for 60 min. It was noted that with repeated injections, the maximum temperature would drop significantly, corresponding to decreased local concentrations of MNPs due to uncontrolled distribution patterns (Attaluri et al., 2011). Two years later, the same group performed a phase I study, recruiting 10 patients with prostate cancer. Traces of the injected MNPs resided in the prostate for a full year, yet no systemic toxicity nor significant patient-discomfort was noted (Johannsen et al., 2007). The most recent clinical trial so far was made on 66 patients having glioblastoma multiforme (GBM). In some locations within the tumor, temperatures reached as high as 82 °C, yet the distribution was still not optimal, for MNPs tend to travel to peripheries rather than cluster in the center of the tumor (LeBrun and Zhu, 2018). All previously mentioned examples had a similar pattern, and challenges facing the employment of MNPs induced hyperthermia in clinical settings such as difficulty in controlling the deposition of the MNPs to the desired location, non-uniform dispersion patterns (Attaluri et al., 2011), and the difficulty in accurately predicting the temperature–time needed to estimate an appropriate thermal dose (LeBrun and Zhu, 2018), suggesting the need for further testing on animals to try to overcome these hurdles.
2.2. Gene delivery (magnetofection)
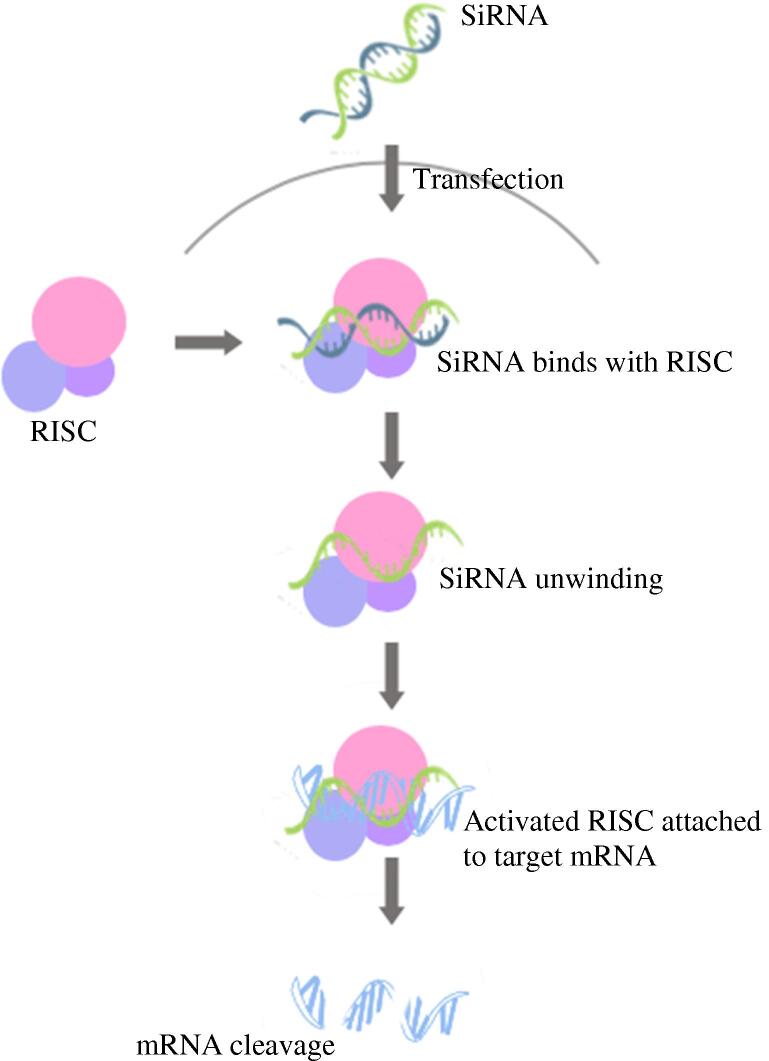
Gene therapy involves the provision of therapeutic agents into diseased cells, whether by providing a functional gene copy to the patient, delivering therapeutic nucleic acids or RNA species, like small interfering Ribonucleic Acid (siRNA), to be first loaded into RNA induced silencing complex (RISC), then onto mRNA for degradation, or expression modulation, as illustrated in Fig. 6, or even delivering suicide gene therapy for cancerous cells, to force the expression of enzymes for the conversion of cytotoxic pro-drugs to active molecules selectively in the cancer region (Cheung et al., 2016, Riley and Vermerris, 2017).
Fig. 6.
RNA-induced silencing complex (RISC) and gene silencing via mRNA cleavage.
When presenting a model for gene delivery, the choice of vector should be very selective to withhold the desired properties, and that can be challenging. Primary systems for gene delivery can be one of three, involving viral vectors like adenovirus and retrovirus, nucleic acid electroporation, and nucleic acid transfection (Haider et al., 2005, Kami et al., 2014).
Viral vectors have been used in transduction successfully for quite some time in research, but that does not make them the ideal system due to many factors, upon which is the arousal of a special phenomenon known as viral tropism (Scherer et al., 2002). Tropism is the viral ability to replicate and produce an infection, and it can be divided to three types: cellular tropism, tissue tropism, and host tropism. For instance, when a particular virus infects a rabbit rather than a human, this is said to be host tropism, likewise, when a virus can only infect lung tissues, not brain tissues, this is said to be tissue tropism, and finally, when a virus is able to infect macrophages, not neurons, this becomes cellular tropism limited to macrophages. Viral immunogenicity is another handful, continuing to be a cause for concern, as it may prohibit their repeated use (McFadden et al., 2009).
Electroporation is another efficient method, but its electrical stimulation result in unmeasured/hard to estimate cytotoxicity (Kami et al., 2014). Alternatively, non-viral gene vectors have been suggested, yet sadly there is only very little that they can do on their own, and they are usually of low efficiency. These boundaries limit the utilization of classic vectors and urge to find finer alternatives. Using super-paramagnetic nanoparticles for that purpose with the application of an external magnetic field served as a turning point in nanoscale delivery of genes, they were shown to have pronounced transfection, and the power to potentiate the efficacy up to several hundred folds, lessening long durations to be a matter of minutes (Riley and Vermerris, 2017, Scherer et al., 2002).
The first study enrolled the use of MNPs for gene delivery was performed by Mah et al., it demonstrated the incorporation of adeno-associated virus (AAV) in MNPs, encoding green fluorescent protein (GFP) on the surface. The transduction efficiency was shown to be higher in-vitro when administered in C12s cells (a derivative of Hela cells) (Mah et al., 2002), and in-vivo when given intramuscularly to 129/svJ mice, not only that, but histological examination of tissues showed no significant immunological response either. Studies by Scherer further explained the vital role of MNPs conjugation in resolving viral carriers tropism phenomenon, in that it can extend the host tropism to non-permissive cells, leading to increased efficiency (McBain et al., 2008).
The conventional methodology in attaching genes to MNPs is by binding to the coat on the surface of MNPs, but this result in gene exposure to the outside environment during delivery, which may lead to gene damage thus reducing the transfection efficiency. In a study conducted by Yang et al., an alternative proposition was made, and that is to envelop the gene together with the MNP inside a liposome vector. This was found to preserve and protect the gene from the external environment until it reaches the location of purpose (Yang et al., 2008).
While designing a delivery system for genes, attaching specific target linkers can be an interesting approach, yet unfortunately, it is not always applicable. A popular alternative was the employment of the cationic polymer polyethyleneimine (PEI), which contains secondary amines to form electrostatic interactions with the negative phosphate backbone of DNA. The principle advantage is its ability to reduce the vector’s dose and transfection time, due to the rapid sedimentation of gene-particle complex onto the target tissues. The mechanism by which PEI-MNPs escape lysosomal degradation following internalization had been heavily debated in a number of publications. Many have attributed it to the “Proton sponge” hypothesis (Haider et al., 2005), which supports PEI buffering capacity in increasing the intra-lysosomal pH, causing their rupture and content release (McBain et al., 2008). However, in a study conducted by Benjaminsen et al., pH sensors were used to measure the lysosomal pH when incorporated with PEI, yet results showed no alkalization of any sort, partially invalidating the hypothesis. It was assumed that perhaps a small portion of PEI managed to escape the endosomal pathway before reaching the lysosomes, and that portion was responsible for gene transfection (Benjaminsen et al., 2013).
In another study by Veronica et al., a multifunctional nanocomplex composed of micro Ribonucleic Acid-Polyethyleneimine-Superparamagnetic Iron Oxide Nanoparticles (miRNA-PEI-SPIONS) was created for angiogenesis manipulation. Normally, the conjugation of SPIONS with PEI result in increased toxicity, but in this study, results showed reduced cytotoxicity, while maintaining high transfection efficiency. The offset of PEI cytotoxicity was explained due to the increase in iron load presented in the included nanoparticles, which ultimately reduced the amount of PEI necessary for miRNA delivery (Riley and Vermerris, 2017, Voronina et al., 2016).
The application of a proper magnetic field has a considerable effect on the final placement of the system’s successfulness. Static magnetism can increase the transfection efficiency, yet applying oscillating magnetic field was shown to increase the level of transfection > 10 fold compared to that of static fields. It was presumed that oscillating magnetic fields provided more energy to the system, which improved the uptake of particles, as well as aided in tissue penetration (McBain et al., 2008), and while there is a wide range of different MNPs, γ-Fe2O4, showing a low toxicity profile, makes it a more promising option (Riley and Vermerris, 2017).
2.3. Theranostics
When a molecule combines a diagnostic feature alongside a therapeutic trait, it gets promoted to hold the title “theranostic.”. Theranostics have gained wide attention over the years as attractive systems to improve cancer therapy, mostly in preclinical trials by integrating imaging techniques to foster monitoring of real time positioning and distribution of the nanoparticles, as well as to support the individuality in patients therapies (Zhou et al., 2016, Zhu et al., 2017). The imaging is performed non-invasively, allowing timely assessment and treatment modification for cancer subjects. Theranostic NPs also have the ability to carry single, or multiples of therapeutics, including chemotherapeutics, small-sized molecules, photosensitizers, as well as siRNA. In a study conducted by Ling et al., the use of pH-sensitive magnetic nano-grenades (PMNs) in targeting tumor cells was explored. The acidic microenvironment of the tumor evokes their disassembling to release MRI contrast and fluorescent signals that ease the detection of small tumors having a 3 mm diameter. The pH change also triggers the generation of singlet oxygen (a reactive radical) as a selective photodynamic therapy against tumor cells. It displays superior activity against multi-drug resistant tumors, providing high hopes and promises for clinical applications (Ling et al., 2014). Multimodal imaging agents represent another intriguing concept under the umbrella of theranostics. It is a growing trend in research that involves the creation of particles with multi-functionalities between the different imaging techniques, to provide precise diagnosis, and overcome their limitations when used independently.
This concept was illustrated nicely in a study done by Zhou et al., where a novel system of Infrared 820-Chitosan Quaternary ammonium Salt-Fe (IR820-CSQ-Fe) was implemented for combined Magnetic Resonance Imaging/Multi-Spectral Optoacoustic Tomography/Fluorescence Imaging (MRI/MSOT/FI) and photodynamic therapy. The IR820 is a Near Infrared fluorescent (NIR) organic dye capable of various types of imaging with low toxicity profile. It contains unique absorption bands within the 600–1000 wavelength range that privileges it to be a photodynamic therapy (PDT) agent. The flexibility in structure due to its meso-halogen center has two effects of increasing the relative stability under physiological environment, like water and lightening conditions, yet decreasing its PDT effect. Surprisingly, with further conjugation with chitosan quaternary ammonium (CSQ) salt, the PDT effect was found to be doubled, alongside increased stability in water for at least three months, accrediting the system for long-term observation (Zhou et al., 2016).
3. Future prospects
Engineering a fully functional DDS that overcomes all challenges would be phenomenal, but is still far from where we stand. Every DDS suffers from a number of limitations, and IONPs are no different. The major limitation of MNPs is the poor conductivity of magnetic oxides, resulting in short residence time, and particle dissipation into non-targeted tissues, which leads to lowered treatment efficiency, and increased chances for adverse effects. This is further affected by the strength of the external magnetic field, and the responsiveness of the particles (Arruebo et al., 2007b). Although iron oxides are known to be of relatively low toxicity, high concentration still pose a problem to the liver, inducing oxidative stress and increasing lipid peroxidation, which cumulatively lead to liver necro-inflammation (Selvam et al., 2018, Yao et al., 2019).
An exciting proposal, which has not been explored sufficiently in the literature, is the creation of metal-hybrid nano-ferrite systems, generally referred to by the formula MFe2O4, where M is a transition metal, with possible catalytic activity (Chaibakhsh and Moradi-Shoeili, 2019).
Metal ferrites NPs have very distinct properties, like high saturation magnetization, and enhanced encapsulation capacity (Chaibakhsh and Moradi-Shoeili, 2019, Selvam et al., 2018), which improves cancer targeting and therapy respectively. They can also overcome the problem of iron toxicity, provided that the used metal substituting iron is less toxic, and cytotoxicity assays confirm the absence of toxicity (Selvam et al., 2018).
Metal ferrites NPs with catalytic activity are nanoenzymes that exhibit enzymatic activity and can function as enzymes mimetics. They are very promising because they usually overcome the intrinsic limitations of natural enzymes, such as limited natural sources, ease of digestion, denaturation by environmental temperatures and pH, cost, and often time-consuming preparation, purification, and storage. These nanozymes can serve in both medical diagnosis and cancer treatment (Chaibakhsh and Moradi-Shoeili, 2019).
Metal ferrite NPs in terms of hyperthermia, have a large heating capacity, and display higher specific loss power (SLP) and a high saturation magnetization compared to IONPs, resulting in similar heating at lower concentrations (Chaibakhsh and Moradi-Shoeili, 2019).
A divalent metal ferrite NP that received a lot of attention recently is magnesium ferrite NPs referred to by the formula MgFe2O4. In a study done by Husein et al, it was shown that MgFe2O4 NPs exhibited greater magnetic heating capacity compared to other ferrites. MgFe2O4 NPs also showed safe metabolism and high biocompatibility because Mg2+ as an ion takes part in the ATP hydrolysis to produce energy, genomic stability, as well as the involvement of catalytic activities of multiple enzymatic systems in the processing of DNA (Hussein et al., 2015). In another study performed by Maehara et al, different metals were tested for thermal coagulation therapy with an alternating magnetic field, where MgFe2O4 samples were most efficient and displayed a considerably higher increase in temperature than did other metal ferrites (Maehara et al., 2005).
The anticancer activity of MgFe2O4 have been also tested on HCT-15 prostate cancer cells, HEK 293 (Selvam et al., 2018) and MCF-7 breast cancer cells (Kanagesan et al., 2013). Further research on magnesium ferrite NPs is required on different cell lines to test the anticancer activity on a wider variety of cancers.
The synthesis of these metal ferrites NPs can be done with similar processes used to synthesize IONPs, most commonly co-precipitation (Zhang et al., 2009), sol–gel (Hussein et al., 2015), and combustion (Selvam et al., 2018) methods, to create nanoparticles with a size range of 10–100 nm in order to effectively cumulate within cancer tissues through passive diffusion and EPR effects (Hussein et al., 2015). Tang et al study explored a unique synthesis strategy using precipitation reaction at room temperature, followed by a solvent thermal process at low temperature with a metal content of 10% to produce ultra-small NPs of 3.7 nm. The self-assembled NaCl cage formed during the precipitation reaction and retained during the thermal decomposition is what confined the metal ferrites growth rate (Tang et al., 2013). This method was convenient because it avoided the common drawbacks encountered with other synthesis processes happening in aqueous solutions, leading to lack of uniformity in size and morphology due to aggregation.
Metal ferrite NPs surface is also flexible to modification, by adding polymeric coating and ligands (Selvam et al., 2018), as well as aptamers, which are short oligonucleotides strands of ribonucleic acid (RNA) and single-strand ribonucleic acid (ssDNA). Aptamers can bind to antigens present on tumor targets, therefore selectively eradicate or image the tumor of interest. On top of that, combinations of metals in the substituted ferrites are expected to have synergistic activity, thereby increased system effectiveness (Chaibakhsh and Moradi-Shoeili, 2019), however there is a very little coverage of that in the literature and more research is required to unravel all possibilities.
In conclusion, the feasibility of the concept has been proven in pre-clinical animal studies and few early phases clinical trials however, a product for magnetic drug targeting may take some time before granting approval for use in humans and reach the market. The scale-up of magnetic field-generating equipment with optimized field parameters tailored for magnetic drug targeting will be not easy, in addition, the complexity of the design of this equipment will depend on the location of the target tissue. Not to mention the increasing awareness of the potential of nanotoxicity expected to impose a challenge for approval of such systems by health authorities.
Footnotes
Peer review under responsibility of King Saud University.
References
Papers of special note have been highlighted as: * of interest ** of considerable interest.
- Ahmed, I.S., El-Hosary, R., Shalaby, S., Abd-Rabo, M.M., Elkhateeb, D.G., Nour, S., 2016. PD-PK evaluation of freeze-dried atorvastatin calcium-loaded poly-ε-caprolactone nanoparticles. Int. J. Pharm. 10.1016/j.ijpharm.2016.03.045. [DOI] [PubMed]
- Ahmed I.S., El Hosary R., Hassan M.A., Haider M., Abd-Rabo M.M. Efficacy and Safety Profiles of Oral Atorvastatin-Loaded Nanoparticles: Effect of Size Modulation on Biodistribution. Mol. Pharm. 2018;15:247–255. doi: 10.1021/acs.molpharmaceut.7b00856. [DOI] [PubMed] [Google Scholar]
- Alexiou C., Tietze R., Schreiber E., Jurgons R., Richter H., Trahms L., Rahn H., Odenbach S., Lyer S. Cancer therapy with drug loaded magnetic nanoparticlesmagnetic drug targeting. J. Magn. Magn. Mater. 2011;323:1404–1407. doi: 10.1016/j.jmmm.2010.11.059. [DOI] [Google Scholar]
- Ali, A., Zafar, H., Zia, M., ul Haq, I., Phull, A.R., Ali, J.S., Hussain, A., 2016. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 9, 49–67. 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed]
- Arruebo M., Fernández-Pacheco R., Ibarra M.R., Santamaría J. Magnetic nanoparticles for drug delivery The potential of magnetic NPs stems from the intrinsic properties of their magnetic cores combined with their drug loading capability and the biochemical properties that can be bestowed on them by means of a suitab. 2007;2:22–32. [Google Scholar]
- Arruebo M., Fernández-Pacheco R., Ibarra M.R., Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today. 2007 doi: 10.1016/S1748-0132(07)70084-1. [DOI] [Google Scholar]
- Attaluri, A., Ma, R., Qiu, Y., Li, W., Zhu, L., 2011. Nanoparticle distribution and temperature elevations in prostatic tumours in mice during magnetic nanoparticle hyperthermia. Int. J. Hyperth. 10.3109/02656736.2011.584856. [DOI] [PubMed]
- Benjaminsen, R. V., Mattebjerg, M.A., Henriksen, J.R., Moghimi, S.M., Andresen, T.L., 2013. The possible “proton sponge ” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed]
- Bhandari P.R. Crocus sativus L. (saffron) for cancer chemoprevention: A mini review. J. Tradit. Complement. Med. 2015;5:81–87. doi: 10.1016/j.jtcme.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia, S., 2016. Natural polymer drug delivery systems: Nanoparticles, plants, and algae, Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae. 10.1007/978-3-319-41129-3. [DOI]
- Biehl P., von der Lühe M., Dutz S., Schacher F.H. Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers (Basel). 2018;10 doi: 10.3390/polym10010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda, R.T., Boddington, S., Henning, T.D., Wendland, M., Mandrussow, L., Liu, S., Daldrup-Link, H., 2011. Labeling human embryonic stem-cell-derived cardiomyocytes for tracking with MRI imaging. Pediatr. Radiol. 10.1007/s00247-011-2130-3. [DOI] [PubMed]
- Chaibakhsh N., Moradi-Shoeili Z. Enzyme mimetic activities of spinel substituted nanoferrites (MFe 2 O 4): A review of synthesis, mechanism and potential applications. Mater. Sci. Eng., C. 2019;99:1424–1447. doi: 10.1016/j.msec.2019.02.086. [DOI] [PubMed] [Google Scholar]
**Substituted ferrites having excellent catalytic performance promote them for multiple applications and biological activities
- Chen Z., Wu C., Zhang Z., Wu W., Wang X., Yu Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chinese Chem. Lett. 2018;29:1601–1608. doi: 10.1016/j.cclet.2018.08.007. [DOI] [Google Scholar]
- Cheung, J., Chino, T., Co, C., Konopka, K., Düzgünes, N., 2016. A nonviral vector with transfection activity comparable with adenoviral transduction. Ther. Deliv. 10.4155/tde-2016-0054. [DOI] [PubMed]
- Dadfar, S.M., Roemhild, K., Drude, N.I., von Stillfried, S., Knüchel, R., Kiessling, F., Lammers, T., 2019. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed]
- Daou T.J., Pourroy G., Bégin-Colin S., Grenèche J.M., Ulhaq-Bouillet C., Legaré P., Bernhardt P., Leuvrey C., Rogez G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006;18:4399–4404. doi: 10.1021/cm060805r. [DOI] [Google Scholar]
- Demirer G.S., Okur A.C., Kizilel S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B. 2015;3:7831–7849. doi: 10.1039/c5tb00931f. [DOI] [PubMed] [Google Scholar]
- Dolovich M.B., Dhand R. Aerosol drug delivery: Developments in device design and clinical use. Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- Falk, M.H., Issels, R.D., 2001. Hyperthermia in oncology. Int. J. Hyperth. 10.1080/02656730150201552. [DOI] [PubMed]
- Fang C., Zhang M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009;19:6258–6266. doi: 10.1039/b902182e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji M., Yamini Y., Rezaee M. Iranian chemical society magnetic nanoparticles : synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010;7:1–37. [Google Scholar]
- Farokhzad O.C., Langer R. Impact of Nanotechnology on Drug Discovery & Development Pharmanext. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- Gao, H., Pang, Z., Jiang, X., 2013. Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm. Res. 10.1007/s11095-013-1122-4. [DOI] [PubMed]
- Giordano S.H., Hortobagyi G.N., Kau S.W.C., Theriault R.L., Bondy M.L. Breast cancer treatment guidelines in older women. J. Clin. Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- Giustini A.J., Petryk A.A., Cassim S.M., Tate J.A., Baker I., Hoopes P.J. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano Life. 2010;01:17–32. doi: 10.1142/s1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider M., Hatefi A., Ghandehari H. Recombinant polymers for cancer gene therapy: A minireview. J. Control. Release. 2005 doi: 10.1016/j.jconrel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Hedayatnasab Z., Abnisa F., Daud W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017;123:174–196. doi: 10.1016/j.matdes.2017.03.036. [DOI] [Google Scholar]
- Hildebrandt, B., Wust, P., Ahlers, O., Dieing, A., Sreenivasa, G., Kerner, T., Felix, R., Riess, H., 2002. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 10.1016/S1040-8428(01)00179-2. [DOI] [PubMed]
- Hussein S.I., Elkady A.S., Rashad M.M., Mostafa A.G., Megahid R.M. Structural and magnetic properties of magnesium ferrite nanoparticles prepared via EDTA-based sol-gel reaction. J. Magn. Magn. Mater. 2015;379:9–15. doi: 10.1016/j.jmmm.2014.11.079. [DOI] [Google Scholar]
- Johannsen, M., Gneveckow, U., Thiesen, B., Taymoorian, K., Cho, C.H., Waldöfner, N., Scholz, R., Jordan, A., Loening, S.A., Wust, P., 2007. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. Eur. Urol. 10.1016/j.eururo.2006.11.023. [DOI] [PubMed]
- Kami, D., Kitani, T., Kishida, T., Mazda, O., Toyoda, M., Tomitaka, A., Ota, S., Ishii, R., Takemura, Y., Watanabe, M., Umezawa, A., Gojo, S., 2014. Pleiotropic functions of magnetic nanoparticles for ex vivo gene transfer. Nanomedicine Nanotechnology, Biol. Med. 10.1016/j.nano.2014.03.018. [DOI] [PubMed]
- Kanagesan S., Hashim M., Tamilselvan S., Alitheen N.B., Ismail I., Bahmanrokh G. Cytotoxic effect of nanocrystalline MgFe2O4 Particles for Cancer Cure. J. Nanomater. 2013;2013 doi: 10.1155/2013/865024. [DOI] [Google Scholar]
- Kim S.Y., Lee Y.M. Taxol-loaded block copolymer nanospheres composed of methoxy poly(ethylene glycol) and poly(ε-caprolactone) as novel anticancer drug carriers. Biomaterials. 2001 doi: 10.1016/S0142-9612(00)00292-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Ito, A., Honda, H., 2016. Magnetic nanoparticle-mediated hyperthermia and induction of anti-tumor immune responses, in: Hyperthermic Oncology from Bench to Bedside. 10.1007/978-981-10-0719-4_13. [DOI]
- LeBrun A., Zhu L. Magnetic Nanoparticle Hyperthermia in Cancer Treatment: History. Mechanism, Imaging-Assisted Protocol Design, and Challenges, in: Theory and Applications of Heat Transfer in Humans. 2018 doi: 10.1002/9781119127420.ch29. [DOI] [Google Scholar]
- Lee, J.H., Ju, J.E., Kim, B. Il, Pak, P.J., Choi, E.K., Lee, H.S., Chung, N., 2014. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 10.1002/etc.2735. [DOI] [PubMed]
- Ling D., Park W., Park S.J., Lu Y., Kim K.S., Hackett M.J., Kim B.H., Yim H., Jeon Y.S., Na K., Hyeon T. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J. Am. Chem. Soc. 2014 doi: 10.1021/ja4108287. [DOI] [PubMed] [Google Scholar]
* Very interesting theranostic application about the use of pH-sensitive magnetic nano-grenades (PMNs) in targeting tumor cells
- Maehara T., Konishi K., Kamimori T., Aono H., Hirazawa H., Naohara T., Nomura S., Kikkawa H., Watanabe Y., Kawachi K. Selection of ferrite powder for thermal coagulation therapy with alternating magnetic field. J. Mater. Sci. 2005;40:135–138. doi: 10.1007/s10853-005-5698-x. [DOI] [Google Scholar]
- Mah, C., Fraites, T.J., Zolotukhin, I., Song, S., Flotte, T.R., Dobson, J., Batich, C., Byrne, B.J., 2002. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 10.1006/mthe.2001.0636. [DOI] [PubMed]
- Maity D., Ding J., Xue J.M. Synthesis of magnetite nanoparticles by thermal decomposition: Time, temperature, surfactant and solvent effects. Funct. Mater. Lett. 2008;1:189–193. doi: 10.1142/S1793604708000381. [DOI] [Google Scholar]
- Majidi S., Sehrig F.Z., Farkhani S.M., Goloujeh M.S., Akbarzadeh A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells, Nanomedicine Biotechnol. 2016;44:722–734. doi: 10.3109/21691401.2014.982802. [DOI] [PubMed] [Google Scholar]
- Mamon H.J., Yeap B.Y., Jänne P.A., Reblando J., Shrager S., Jaklitsch M.T., Mentzer S., Lukanich J.M., Sugarbaker D.J., Baldini E.H., Berman S., Skarin A., Bueno R. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J. Clin. Oncol. 2005;23:1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- McBain, S.C., Yiu, H.H.P., Dobson, J., 2008. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomedicine. 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed]
- McFadden, G., Mohamed, M.R., Rahman, M.M., Bartee, E., 2009. Cytokine determinants of viral tropism. Nat. Rev. Immunol. 10.1038/nri2623. [DOI] [PMC free article] [PubMed]
- Nguyen D.T., Kim K.S. Functionalization of magnetic nanoparticles for biomedical applications. Korean J. Chem. Eng. 2014;31:1289–1305. doi: 10.1007/s11814-014-0156-6. [DOI] [Google Scholar]
- Okoli C., Sanchez-Dominguez M., Boutonnet M., Järås S., Civera C., Solans C., Kuttuva G.R. Comparison and functionalization study of microemulsion-prepared magnetic iron oxide nanoparticles. Langmuir. 2012;28:8479–8485. doi: 10.1021/la300599q. [DOI] [PubMed] [Google Scholar]
- Omidkhoda, A., Mozdarani, H., Movasaghpoor, A., Fatholah, A.A.P., 2007. Study of apoptosis in labeled mesenchymal stem cells with superparamagnetic iron oxide using neutral comet assay. Toxicol. Vitr. 10.1016/j.tiv.2007.03.010. [DOI] [PubMed]
- Patil U.S., Adireddy S., Jaiswal A., Mandava S., Lee B.R., Chrisey D.B. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015 doi: 10.3390/ijms161024417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcharoen K., Sirivat A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012;177:421–427. doi: 10.1016/j.mseb.2012.01.003. [DOI] [Google Scholar]
- Petryk, A.A., Giustini, A.J., Gottesman, R.E., Kaufman, P.A., Hoopes, P.J., 2013. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int. J. Hyperth. 10.3109/02656736.2013.825014. [DOI] [PMC free article] [PubMed]
- Prodan A.M., Iconaru S.L., Ciobanu C.S., Chifiriuc M.C., Stoicea M., Predoi D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J. Nanomater. 2013;2013 doi: 10.1155/2013/587021. [DOI] [Google Scholar]
- Reddy L.H., Arias J.L., Nicolas J., Couvreur P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- Riley M.K., Vermerris W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials. 2017 doi: 10.3390/nano7050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
*Interesting review handling the aspect of nanomaterials in gene delivery
- Scherer F., Anton M., Schillinger U., Henke J., Bergemann C., Krüger A., Gänsbacher B., Plank C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002 doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Selvam R., Ramasamy S., Mohiyuddin S., Enoch I.V.M.V., Gopinath P., Filimonov D. Molecular encapsulator–appended poly(vinyl alcohol) shroud on ferrite nanoparticles. Augmented cancer–drug loading and anticancer property. Mater. Sci. Eng., C. 2018;93:125–133. doi: 10.1016/j.msec.2018.07.058. [DOI] [PubMed] [Google Scholar]
- Shapiro B., Kulkarni S., Nacev A., Muro S., Stepanov P.Y., Weinberg I.N. Open challenges in magnetic drug targeting. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 2015;7:446–457. doi: 10.1002/wnan.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singamaneni S., Bliznyuk V.N., Binek C., Tsymbal E.Y. Magnetic nanoparticles: Recent advances in synthesis, self-assembly and applications. J. Mater. Chem. 2011;21:16819–16845. doi: 10.1039/c1jm11845e. [DOI] [Google Scholar]
- Soo Choi, H., Liu, W., Misra, P., Tanaka, E., Zimmer, J.P., Itty Ipe, B., Bawendi, M.G., Frangioni, J. V., 2007. Renal clearance of quantum dots. Nat. Biotechnol. 10.1038/nbt1340. [DOI] [PMC free article] [PubMed]
- Spirou S.V., Costa Lima S.A., Bouziotis P., Vranješ-Djurić S., Efthimiadou E.K., Laurenzana A., Barbosa A.I., Garcia-Alonso I., Jones C., Jankovic D., Gobbo O.L. Recommendations for in vitro and in vivo testing of magnetic nanoparticle hyperthermia combined with radiation therapy. Nanomaterials. 2018 doi: 10.3390/nano8050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzl A., Cowan N.C., De Santis M., Kuczyk M.A., Merseburger A.S., Ribal M.J., Sherif A., Witjes J.A. Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur. Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Sun C., Lee J.S.H., Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Su Y., Li Q., Gao S., Shang J.K. Well-dispersed, ultrasmall, superparamagnetic magnesium ferrite nanocrystallites with controlled hydrophilicity/hydrophobicity and high saturation magnetization. RSC Adv. 2013;3:13961–13967. doi: 10.1039/c3ra41543k. [DOI] [Google Scholar]
**The aim of this article was to create well-dispersed ultrasmall superparamagnetic magnesium ferrite nanocrystals, with a particle size of ∼3.7 nm, high saturation magnetization, and a low Mg content of 10%
- Tavakoli A., Sohrabi M., Kargari A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007;61:151–170. doi: 10.2478/s11696-007-0014-7. [DOI] [Google Scholar]
- Teja A.S., Koh P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009;55:22–45. doi: 10.1016/j.pcrysgrow.2008.08.003. [DOI] [Google Scholar]
- Tran P.H.L., Tran T.T.D., Vo T. Van. Polymer conjugate-based nanomaterials for drug delivery. J. Nanosci. Nanotechnol. 2014;14:815–827. doi: 10.1166/jnn.2014.8901. [DOI] [PubMed] [Google Scholar]
- Umut, E., 2013. Surface Modification of Nanoparticles Used in Biomedical Applications. Mod. Surf. Eng. Treat. 10.5772/55746. [DOI]
- Voronina, N., Lemcke, H., Wiekhorst, F., Kühn, J.P., Rimmbach, C., Steinhoff, G., David, R., 2016. Non-viral magnetic engineering of endothelial cells with microRNA and plasmid-DNA—An optimized targeting approach. Nanomedicine Nanotechnology, Biol. Med. 10.1016/j.nano.2016.06.015. [DOI] [PubMed]
- Xianyu Y., Wang Q., Chen Y. Magnetic particles-enabled biosensors for point-of-care testing. TrAC - Trends Anal. Chem. 2018;106:213–224. doi: 10.1016/j.trac.2018.07.010. [DOI] [Google Scholar]
- Yan S., Zhang D., Gu N., Zheng J., Ding A., Wang Z., Xing B., Ma M., Zhang Y. Therapeutic effect of Fe 2O 3 nanoparticles combined with magnetic fluid hyperthermia on cultured liver cancer cells and xenograft liver cancers. J. Nanosci. Nanotechnol. 2005 doi: 10.1166/jnn.2005.219. [DOI] [PubMed] [Google Scholar]
- Yang, S.Y., Sun, J.S., Liu, C.H., Tsuang, Y.H., Chen, L.T., Hong, C.Y., Yang, H.C., Horng, H.E., 2008. Ex vivo magnetofection with magnetic nanoparticles: A novel platform for nonviral tissue engineering. Artif. Organs. 10.1111/j.1525-1594.2007.00526.x. [DOI] [PubMed]
- Yao, Y., Zang, Y., Qu, J., Tang, M., Zhang, T., 2019. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int. J. Nanomedicine. 10.2147/IJN.S212907. [DOI] [PMC free article] [PubMed]
- Zhang H., Pan D., Zou K., He J., Duan X. A novel core-shell structured magnetic organic-inorganic nanohybrid involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core for magnetically controlled drug release. J. Mater. Chem. 2009;19:3069–3077. doi: 10.1039/b820176e. [DOI] [Google Scholar]
- Zhao Q., Wang L., Cheng R., Mao L., Arnold R.D., Howerth E.W., Chen Z.G., Platt S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics. 2012 doi: 10.7150/thno.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Hou, X., Liu, Y., Zhao, T., Shang, Q., Tang, J., Liu, J., Wang, Y., Wu, Q., Luo, Z., Wang, H., Chen, C., 2016. Superstable Magnetic Nanoparticles in Conjugation with Near-Infrared Dye as a Multimodal Theranostic Platform. ACS Appl. Mater. Interfaces. 10.1021/acsami.5b11308. [DOI] [PubMed]
* A novel system of (IR820-CSQ-Fe) implemented for combined (MRI/MSOT/FI) and photodynamic therapy
- Zhu L., Zhou Z., Mao H., Yang L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. 2017 doi: 10.2217/nnm-2016-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]