Abstract
Background
Acute liver failure (ALF) is the leading cause for emergency liver transplantation (LT) all over the world. We looked at the profile of cases who required LT for ALF from a single centre to identify the possible predictors of poor outcomes.
Methodology
During the 10-year period starting from 2007, 320 cases of ALF were treated at our institution, of which 70 (median age 24 years, Male:Female 1:2) underwent LT. Retrospective analyses of these 70 patients were performed.
Results
Etiology was identifiable in 73% (n = 51) of cases (yellow phosphorous [YP] poisoning [n = 16], Hepatitis A virus [HAV] [n = 15], Hepatitis B virus [HBV] [n = 5], Hepatitis E virus [HEV] [n = 1], anti-tubercular therapy [ATT] induced [n = 6], acute Wilson's [n = 3], and autoimmune [n = 5]]. Upon meeting King's College Hospital criteria, 69 had live donor LT (61 right lobe grafts, three left lobe grafts, five left lateral segment grafts) and one had deceased donor LT. Among these, there were five auxiliary partial orthotopic grafts and four ABO-incompatible transplants. Overall, 90-day mortality was 35.7% (n = 25), predominantly due to sepsis. Significant risk factors for mortality on multivariate analysis included indeterminate etiology, pre-op renal dysfunction, and Grade IV hepatic encephalopathy (HE). Cumulative 10-year survival of the remaining survivors was 95.6% (n = 45).
Conclusion
LT for ALF carries high perioperative mortality (35.7%) in those presenting with indeterminate etiology, pre-op renal dysfunction, and Grade IV HE. Nevertheless, if they survive the perioperative period, long-term survival is excellent.
Keywords: acute liver failure, emergency live donor living transplantation, yellow phosphorous poisoning, hepatitis a virus-related acute liver failure, anti-tubercular treatment-induced acute liver failure, paediatric emergency liver transplant, survival following liver transplant for acute liver failure, auxiliary partial orthotopic liver transplant
Abbreviations: ALF, Acute Liver Failure; ALI, Acute Liver Injury; APOLT, Auxiliary Partial Orthotopic Liver Transplant; ATT, Anti-Tubercular Treatment; DDLT, Deceased Donor Liver Transplantation; DILI, Drug-Induced Liver Injury; GRWR, Graft Recipient Weight Ratio; HAV, Hepatitis A Virus; HBV, Hepatitis B Virus; HEV, Hepatitis E Virus; HE, Hepatic Encephalopathy; INR, International Normalised Ratio; LDLT, Living Donor Liver TransplantationPALF; LT, Liver Transplantation; MELD, Model for End-Stage Liver Disease; MODS, Multi-Organ Dysfunction Syndrome; NAC, N-acetylcysteine; PALF, Paediatric Acute Liver Failure; YP, Yellow Phosphorous
Acute liver failure (ALF), previously called fulminant hepatic failure, is defined as coagulopathy (International Normalised Ratio [INR] >1.5) of hepatic onset associated with hepatic encephalopathy (HE) in the absence of pre-existing liver disease. The precise duration for the terminology of ‘acute’ varies between continents. In Europe, while it is 28 days,1 from the onset of the first symptom, in North America, it is 26 weeks.2 Likewise, the definition varies between adults and paediatric age group (≤ 12 years). In adults, in the absence of HE, the condition is termed as acute liver injury (ALI). In paediatric ALF (PALF), however, HE is not mandatory and INR of > 2 of hepatic onset without evidence of chronic liver disease qualifies for the definition of PALF.3
The incidence is fewer than 10 cases per million persons per year,4 usually affecting the younger population. Etiology is multifactorial and shows geographical variations as well. Whilst in the western world, the most common etiology is drug-induced liver failure (DILI),5 in developing countries, vast majority of cases are attributable to viral hepatitis (Hepatitis A virus, Hepatitis B virus, and Hepatitis E virus). Even within India, a regional difference is observable. Whilst in the North, a common cause for ALF is anti-tubercular therapy-induced (ATT-induced) hepatotoxicity;6 in the South and specially in Kerala, yellow phosphorous-related (YP-related) ALF is common.7 Both European Association for Study of the Liver (EASL) and American Association for Study of Liver Diseases (AASLD) recognise acute presentations of Wilson's disease, Hepatitis B, autoimmune hepatitis, and Budd–Chiari syndrome as ALF, fulfilling criteria for super-urgent listing for organ allocation.
If left untreated, mortality in ALF is usually due to cerebral oedema or multi-organ failure. However, the survival of ALF has improved drastically from approximately 17% about four decades ago to almost 90% as reported in 2017.8 This can be attributed to better understanding of the disease and consequent evolution of management options, ranging from good intensive care to timely liver transplantation (LT). The crucial decision in the management of ALF revolves around the possibility of spontaneous resolution with medical management. In approximately 10–20% of patients, the liver damage is extensive and salvage is possible solely by LT. The decision to proceed with LT is usually based on prognostic markers, which vary from country to country, but most popularly used is the King's College Hospital (KCH) criteria.9 The type of transplant carried out for ALFs too varies between the West and the East, predominantly deceased donor LT (DDLT) in Europe and North America, whilst in Asia, including India, living donor LT (LDLT) comprises the majority. Emergency LDLT carries with it a huge social, financial, and emotional burden not only for the afflicted family, but for the treating physician and institution as well.
Literature for emergency LT for ALF in India is scarce, and with this study, we would like to highlight the profile of cases from South India requiring LT for ALF, as well as the factors that could predict mortality following an emergency transplantation.
Methods
This is a retrospective analysis of all patients who underwent emergency LT for ALF between April 2007 and April 2017 at Amrita Institute of Medical Sciences, Kochi.
It included cases from all age groups who presented with ALF based on AASLD definition.2 Thorough search for etiology was carried out based on protocol investigations.2 Etiology was deemed seronegative/indeterminate when none of the diagnostic tests yielded positive results. HE was confirmed and graded as per the West Haven criteria.10 Cerebral oedema was clinically suspected if any of the following was present – bradycardia, hypertension, increased muscle tone, abnormal pupils, neurogenic hyperventilation, myoclonus, and spontaneous decerebrate posturing. Management of cerebral oedema was by elevation of head, correction of hypercapnia/hypoxia, and use of 3% sodium chloride, l-ornithine l-aspartate, and branch chain amino acids. All patients received antibiotics, antifungals, laxatives, correction of hyponatremia, hypoglycaemia, proton pump inhibitors, N-acetylcysteine (N-AC), and prostaglandin E1. Continuous renal replacement therapy and plasmapheresis was carried out towards the latter part of the study period in select cases as recommended in the trial by Larson et al.11
Our primary indication for transplant was any patient meeting the KCH criteria for ALF. However, in the case of YP poisoning, we have developed our own criteria (model for end-stage liver disease [MELD] score > 36 or INR >6 with HE) following an analysis of 41 cases of YP poisoning, comparing patients who survived with those who either died or underwent LT after fulfilling the KCH criteria. We found that HE along with MELD > 36 or INR > 6 had a sensitivity, specificity, positive predictive value, and negative predictive value of 87%, 90%, 93%, and 90%, respectively, for predicting mortality without liver transplant.7 Once they fulfilled the criteria, all cases were listed on the super-urgent Kerala state deceased donor list. Nonetheless, due to extreme deceased donor shortage, counselling was predominantly stressed on the need for first-degree related live donors (spouse/parent/sibling/children/grandparent). Prospective recipients had computerised tomography (CT) / magnetic resonance imaging (MRI) brain, electroencephalography (EEG), and transcranial Doppler to rule out irreversible brain injury prior to transplant.
Live prospective donors within the age group of 18–55 years were evaluated in a protocolised step-wise schema. Quick but thorough assessment was carried out within 12–24 h, beginning with basic blood tests and plain CT. Donors with steatosis > 30% on non-contrast CT were excluded. Those with non-fatty livers were put through triple-phase CT and volumetry for detailed anatomical assessment. Magnetic resonance cholangiopancreatography (MRCP) was done only in cases with Type III portal vein anatomy where biliary anomalies are frequent. Thorough systemic evaluation was carried out with 2D echocardiogram, treadmill test, pulmonary function tests, and risk assessment done by cardiology, pulmonology, endocrinology, psychiatry, and anaesthesiology departments.
We considered auxiliary partial orthotopic LT (APOLT) in few patients with hyper-acute etiology if the liver appeared grossly normal and if the patient was haemodynamically stable at the time of transplant. ABO-incompatible LT too was offered to select, and very motivated families were willing to take the associated risks.
Following transplant, all cases were given standard triple immunosuppression (steroid, tacrolimus, and azathioprine or Mycophenolate Mofetil).
For analysis, we included all patients with ALF who underwent LT. Therefore, the data included patients with Wilson's disease, Hepatitis B virus and autoimmune disease whose first presentation was this acute episode requiring LT. We excluded acute presentations of other causes of chronic liver disease (CLD) like alcoholic hepatitis and patients with previous history of decompensation. We also excluded patients with ALF who met criteria but could not be transplanted due to various reasons (multi-organ failure [MOF], irreversible brain injury, financial or logistical issues).
Statistical Analysis
Continuous variables were described as medians and ranges. Student’s t-test and Mann–Whitney U test were used to evaluate differences in the continuous variables. Categorical variables were compared by means of the chi-square test and Fisher exact test. Cumulative survival curves were analysed by use of the Kaplan–Meier method. To investigate the prognostic factor predicting fatal outcome, univariate and step-wise multivariate regression analyses were performed. Backward conditional binary logistic regression analysis was used to estimate the odds ratio with 95% CI for mortality. P-value was significant if the value was less than 0.05. Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 20.0.
Results
Out of 320 cases of ALF admitted in our hospital during the study period, 120 patients met the KCH criteria for LT. Among them, 35 patients had fulfilled the criteria for LT, but could not be performed due to financial reasons, unwillingness, or lack of timely availability of organs (live or deceased), and unfortunately, all 35 succumbed to their disease. A total of 85 cases were evaluated for LT; however, 15 were excluded at the final stage of workup due to irreversible brain injury (n = 8) and MOF (n = 7; predominantly renal and pulmonary dysfunction). The study flowchart has been provided as Figure 1. Demographical and etiological details of those undergoing LT are given in Table 1.
Figure 1.
Study flowchart.
Table 1.
Demographics, Etiology, and Perioperative Characteristics (n = 70).
| AGE | Mean = 24.7 | SD = 11.52 |
|---|---|---|
| AGE GROUP | ||
| </ = 12 | 9/70 | 12.9% |
| >12 | 61/70 | 87.1% |
| SEX | ||
| Female | 46/70 | 65.7% |
| Male | 24/70 | 34.4% |
| ONSET TOHOSPITAL PRESENTATION | Mean = 14.24 | SD = 8.214 |
| ETIOLOGY | ||
| Seronegative | 19 | 27.1% |
| YP | 16 | 22.9% |
| Hepatitis A virus | 15 | 21.4% |
| Hepatitis B virus | 5 | 7.1% |
| Hepatitis E virus | 1 | 1.4% |
| ATT induced | 6 | 8.6% |
| Autoimmune | 5 | 7.1% |
| Wilson's Disease | 3 | 4.3% |
| TYPE OF TRANSPLANT | ||
| Right lobe | 56 | 80% |
| Left lobe | 3 | 4.2% |
| Left lateral lobe | 5 | 7.1% |
| APOLT | 5 | 7.1% |
| DDLT | 1 | 1.4% |
| ABO incompatible | 4 | 5.7% |
| HOSPITAL STAY | Mean = 23.39 | SD = 13.4 |
| OVERALL MORTALITY | 27/70 | 38.6% |
| Early (< 90 days) mortality | 25 | 92.5% |
| Late (> 90 days) mortality | 2 | 8.0% |
| CAUSES OF MORTALITY | ||
| Sepsis with MOF | 15 | 55.5% |
| Cerebral oedema (IBI) | 2 | 7.4% |
| Graft failure | 2 | 7.4% |
| Primary haemorrhage | 1 | 3.7% |
| Biliary complications | 3 | 11.1% |
| Vascular complications | 3 | 11.1% |
| PTLD | 1 | 3.7% |
ATT – Anti-tubercular therapy; APOLT – Auxiliary partial orthotopic liver transplant; DDLT – Deceased donor liver transplant; MOF – Multi-organ failure; IBI – Irreversible brain injury; PTLD – Post-transplant lymphoproliferative disorder.
Out of the 70 cases, only one underwent DDLT. Among those undergoing LDLTs, 95% were first-degree relatives. This is because LDLT from second-degree relatives mandate a government authorisation process that is rigorous and time-consuming. None of the donors assessed was excluded for anatomic variations.
In five patients, APOLT was performed, three for Hepatitis A (one ABO incompatible) and two for indeterminate causes. Mortality occurred in both the APOLTs performed for indeterminate causes. All three survivors have been weaned off immunosuppression over a period of 1 year and are living a normal life. Further, three more cases had ABO-incompatible transplants. None of them had pre-transplant desensitisation, as their isoagglutinin titres were < 64. Two died in the immediate postoperative period. The lone survivor of pure ABO-incompatible orthotropic liver transplant is on tacrolimus monotherapy and doing well.
Overall mortality was 38%, vast majority of which (92%) occurred within 90 days of transplant. The most common cause for mortality was found to be sepsis with MOF (55.5%). Death due to irreversible brain injury occurred in 7% (n = 2) of cases. CT scan had confirmed gross cerebral oedema as the cause for brain death. Treatment was withdrawn in both cases with consent of family. There were only two late deaths, one due to recurrence of autoimmune disease and another due to biliary sepsis. Details of the type of transplants and perioperative outcomes are provided in Table 1.
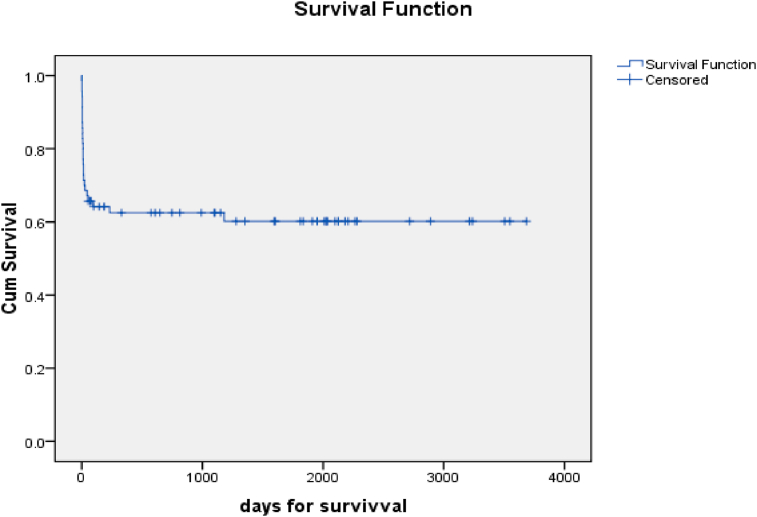
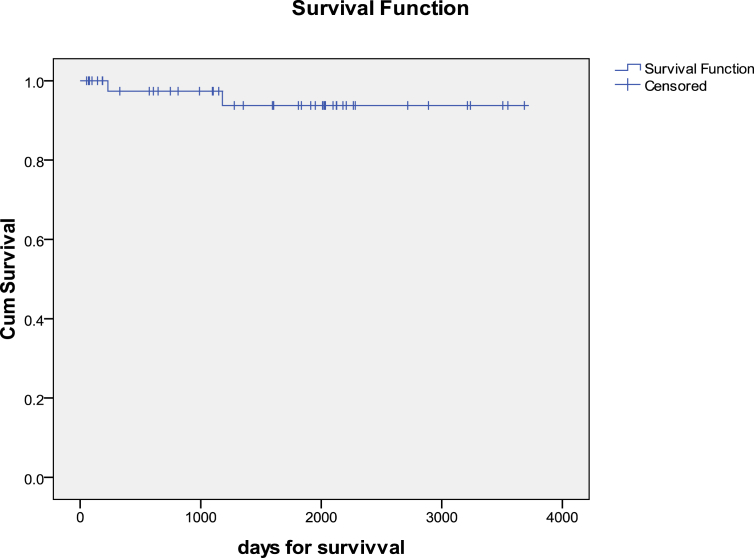
Univariate and multivariate analyses for mortality are shown in Table 2 and Table 3 respectively. Preoperative renal dysfunction, Grade IV HE, and seronegative etiology were the only factors found to be significant for predicting mortality following transplant. The 10-year cumulative survival of recipients over a median period of 6.17 years was 60% (Figure 2). A similar 10-year Kaplan–Maier curve plotted for survivors of the initial 3 months post-transplant demonstrated a survival of 95.6% (Figure 3).
Table 2.
Univariate Analysis of Predictors of Mortality Post-Emergency LT.
| Categorical Factors | Mortality | P-value | OR [CI] |
|---|---|---|---|
| Patient Age | |||
| </ = 12 | 44.4% | 0.726 | 1.32 [0.32–5.56] |
| >12 | 37.7% | ||
| Sex | |||
| Female | 39.1% | 0.894 | 1.07 [0.39–2.96] |
| Male | 37.5% | ||
| Donor age | |||
| </ = 35 years | 23.8% | 0.164 | 2.607 [0.825–8.245] |
| >35 years | 44.9% | ||
| Cerebral oedema | |||
| Absent | 44.4% | 0.333 | 5 [0.492–50.831] |
| Present | 80% | ||
| Hepatic encephalopathy | |||
| Grades I/II/III (n = 49) | 20.4% | < 0.001 | 16.57 [4.554–60.327] |
| Grade IV (n = 21) | 81.0% | ||
| Pre-op Creatinine | |||
| </ = 1.2 mg/dl | 26.0% | 0.002 | 6.641 [2.11–20.896] |
| >1.2 mg/dl | 70.0% | ||
| Etiology of ALF | |||
| Hepatitis A | 60.0% | 0.019 | |
| YP | 12.5% | ||
| Seronegative | 47.4% | ||
| Continuous Factors | Mean (mortality group) | SD | P-value |
|---|---|---|---|
| Presentation to hospital from onset (days) | 13.41 | 6.89 | 0.478 |
| Pre-op INR | 6.87 | 1.90 | 0.358 |
| Pre-op Bilirubin | 17.56 | 8.61 | 0.111 |
| MELD score | 40.93 | 5.39 | 0.387 |
ALF – Acute liver failure; INR – International normalised ratio; MELD – Model for end-stage liver disease.
Table 3.
Multivariate Analysis for Predictors of Mortality Post-emergency LT.
| Factors | OR [95%CI] | p-value |
|---|---|---|
| Seronegative ALF | 8.85 [0.97–80.8] | 0.053 |
| Hepatitis A ALF | 8.04 [0.83–78.03] | 0.072 |
| Grade IV HE | 8.81 [1.45–53.34] | 0.018 |
| Creatinine >1.2 | 5.99 [0.99–36.27] | 0.051 |
Figure 2.
Overall survival over 10-year period (n = 70).
Figure 3.
Long-term survival excluding early (90-day) mortality (n = 45).
Of 41 survivors, 39 are on regular follow-up. They are fully compliant with their medications and are studying or employed. Among them, seven have subsequently married and four have sired children. None of the donors had any Grade IV/V Clavien–Dindo complication.12
Discussion
In this retrospective analysis of emergency LT for ALF, we noted several interesting points. Young females composed the majority of the cases of ALF. Concerning etiology, although majority were indeterminate, YP poisoning reached a close second followed by Hepatitis A virus. This differs from the common etiologies of ALF reported from North India, which include HEV and ATT-induced ALF.13 YP (Zn3P2/ZnP) is an easily available rodenticide in our country, which is commonly used as a means for suicide or at times consumed accidently by children via poison-laden edibles intended for rats. Once ingested, it transforms into phosphine gas, which inhibits cytochrome c oxidase, causing a hepatotoxic effect. The clinical manifestations of liver failure typically present by the 4–5th days after ingestion, and patients can rapidly deteriorate following this. YP is also known to cause renal and cardiovascular failure. There are no antidotes currently known, and mortality rate of YP poisoning is around 37–100%.14 Hepatitis B, E, autoimmune etiology, acute Wilson's, and ATT were the other less common etiological factors found in our study data. It is worth noting that, in contrast to the West, none of the cases transplanted for ALF in our series were for acetaminophen overdose. These cases generally respond well to N-AC treatment and supportive care.
After transplant, cumulative 10-year survival over a median follow-up of 6.17 years was 60.2%. Most of the mortalities (25/27; 92.2%) occurred in the immediate postoperative period (within 90 days) predominantly due to MOF (55.5%). Our high 90-day mortality of 35.7% in this young cohort is worrying. That MOF, secondary to sepsis, was the predominant cause of mortality, probably suggests a prolonged period of intensive care in these sick patients. Similar to a recent study by Kim et al,15 in our study too, pre-op hepatorenal syndrome (HRS) and Grade IV HE were found to be risk factors for mortality, but unlike our study, they found that donor age > 35 years and low graft recipient weight ratio (GRWR) also contributed to mortality following transplant. Whether early resort to renal replacement therapy or plasmapheresis would lessen the risk of mortality merits additional data.11
Sadly, irreversible brain damage was noticed in 7% (n = 2) of cases following transplant, impelling withdrawal of further treatment for them. Considering that in all patients the immediate pretransplant neurological assessment by specialists based on EEG and CT/MRI brain were normal, in these two patients, it is possible that progressive brain damage occurred during the liver transplant procedure. This may be attributed to the massive fluid/electrolyte shifts that occur during hepatectomy, anhepatic phase, as well as during re-perfusion. Whether delay in transplants due to late referrals, fiscal constraints, and time taken for live donor acquiescence contributed to the mortality remains unanswered.
APOLT was performed in 7% (n = 5) cases. APOLT is an acceptable method in select cases of ALF with favourable etiology such as Hepatitis A/B and with stable haemodynamics at the time of transplant.16 APOLT is not suitable for ALF of indeterminate etiology, in subacute liver failure and where liver cell necrosis is massive, resulting in unstable haemodynamics. As organs are scarce and donors are limited, ABO-incompatible transplants also need to be considered at times, provided the family is aware and motivated regarding the augmented risk associated with the procedure. We have performed four emergency ABO-incompatible transplants without desensitisation protocols (three right lobes and one APOLT), out of which two did well postoperatively, whilst other two died in the immediate post-op period due to sepsis and MOF.
It is noteworthy that the long-term prognosis of survivors was excellent. Indeed, only two out of 43 who survived the immediate 90-day period succumbed over the subsequent 10 years. One of them was due to recurrence of autoimmune disease and another due to biliary sepsis. Most of our survivors (95%) are on regular follow-ups, and we find that the quality of life on subjective assessment appears to have been unaffected as borne out by the fact that all are studying or employed and leading a normal family life. It is worth mentioning that none of the donors has reported any medical/surgical issues in the postoperative period.
This study, to our knowledge, is the largest report from India of LT for ALF, majority of which are live donor transplants. The strength of our study is the long-term follow-up of 95% of our survivors. Nonetheless, there are several limitations. Firstly, being a retrospective study, all its inherent defects such as heterogenicity of the study group and incomplete recording of data were unavoidable. Secondly, over the duration of period of our study, several changes have occurred in the medical management of ALF, surgical technique, and postoperative critical care. Such changes in protocols are inevitable given the current rapid progress in the medical field. Therefore, our suggestions from these data merit further scientific rigour.
In conclusion, LDLT inclusive of APOLT and ABO-incompatible transplant is an acceptable life-saving option for patients with ALF failing medical treatment. Nevertheless, the perioperative mortality following transplant is around 35.7%. From this study, we have learnt that Grade IV HE, deranged preoperative renal function, and seronegative ALF are predictive factors for mortality following a transplant; therefore, honest communication and counselling of relatives and donors should not be found wanting in this emotionally charged circumstance. Once the recipients survive the initial postoperative period, they have an excellent long-term survival.
Compliance with ethical standards
Ethics Statement
The authors confirm that the study was performed in a manner that conforms with the Helsinki Declaration of 1975 as revised in 2000 and 2008 concerning Human and Animal Rights.
Author's contribution
Concept and design: S Mallick, K Nair, S. Sudhindran.
Data accrual, analysis and review: S Mallick, Manoj T, Manikandan K, Pulkit S, Madhu SD.
Preparation of first draft: S Mallick, Johns SM, Binoj ST, Critical review, revision and approval of final version: Zubair UM, Ramachandran NM, Dinesh B, Unnikrishnan G, Puneet Dhar, OV Sudheer, S. Sudhindran.
Conflicts of interest
The authors have none to declare.
References
- 1.European Association for the Study of the Liver EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. PMID:28417882. [DOI] [PubMed] [Google Scholar]
- 2.Lee W.M., Stravitz R.T., Larson A.M. Introduction to the revised American Association of Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. PMID:22213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squires R.H., Jr., Schneider B.L., Bucuvalas J. Acute Liver failure in children: the first 348 patients in paediatric acute liver failure study group. J Paediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal William, Wendon Julia. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. https://www.nejm.org/doi/10.1056/NEJMra1208937 PMID:24369077 [DOI] [PubMed] [Google Scholar]
- 5.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. PMID:20638564. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R., Shalimar Bhatia V., Khanal S., Sreenivas V., Gupta S.D. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010 May;51:1665–1674. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 7.Saraf V., Pande S., Gopalakrishnan U. Acute liver failure due to Zinc phosphide containing rodenticide poisoning: clinical features and prognostic indicators of need for liver transplantation. Indian J Gastroenterol. 2015;34:325. doi: 10.1007/s12664-015-0583-2. [DOI] [PubMed] [Google Scholar]
- 8.Shokoohi H., Pourmand A., Teng J., Lucas J. Acute liver failure and emergency consideration for liver transplant. AJEM (Am J Emerg Med) 2017 Nov;35(11):1779–1781. doi: 10.1016/j.ajem.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 9.O'Grady J., Alexander G., Hayllar K. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. PMID:2490426. [DOI] [PubMed] [Google Scholar]
- 10.Vilstrup H., Amodio P., Bajaj J. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the european association for the study of the liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 11.Larson F.S., Schmidt L.E., Bernsmeier C. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J.Hepatol. 2016 Jan;64:69–78. doi: 10.1016/j.jhep.2015.08.018. Epub 2015 Aug 29. PMID:26325537. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. PMID:15273542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya S.K., Panda S.K., Saxena A., Gupta S.D. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000;15:473–479. doi: 10.1046/j.1440-1746.2000.02073.x. PMID:10847431. [DOI] [PubMed] [Google Scholar]
- 14.Oghabian Z., Afshar A., Rahimi H.R. Hepatotoxicity due to zinc phosphide poisoning in two patients: role of N-acetylcysteine. Clin Case Rep. 2016;4:768–772. doi: 10.1002/ccr3.618. Published 2016 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T.S., Kim J.M., Kwon C.H.D. Prognostic factors predicting poor outcome in living donor liver transplantation for fulminant hepatic failure. Transplant Proc. 2017;49:1118–1122. doi: 10.1016/j.transproceed.2017.03.031. PMID:28583539. [DOI] [PubMed] [Google Scholar]
- 16.Rela M., Kaliamoorthy I., Reddy M.S. Current status of auxiliary partial orthotopic liver transplantation for acute liver failure. Liver Transplant. 2016 Sep;22:1265–1274. doi: 10.1002/lt.24509. [DOI] [PubMed] [Google Scholar]