Abstract
Aim
An impaired hepatocyte proliferation during severe liver injury causes the proliferation of hepatic progenitor cells (HPCs), also called as the ductular reaction (DR). In the present study, we studied the role of key angiogenic factors in HPC-mediated DR in nonalcoholic steatohepatitis (NASH).
Methods
Liver biopsies from patients with NASH (n = 14) were included in the study. Patients with NASH were divided in two groups, early and late fibrosis (based on fibrosis staging). Biopsies were used to analyze the gene expression by quantitative real-time polymerase chain reaction and immunohistochemical (IHC) staining for two markers of DR, viz, CK19 and epithelial cell adhesion molecule (EpCAM). Cocultures were performed between steatotic human umbilical vein endothelial cells (HUVECs) and LX2 and Huh7 cells. Enzyme-linked immunosorbent assays were performed to measure levels of vascular endothelial growth factor (VEGF) in coculture studies. Next, Huh7 cells were treated with VEGF, and proliferation was investigated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assays. The number of EpCAM-positive cells was analyzed by flow cytometry.
Results
Of all the angiogenic factors, the gene expression of VEGF and angiopoietin 2 (Ang2) was significantly different between patients with NASH in the early and late fibrosis groups (P < 0.05 for both). Both VEGF and Ang2 also correlated significantly with the IHC scores of CK19 and EpCAM in the study group. In the in vitro studies, VEGF levels were significantly increased when Huh7 cells were cocultured with steatotic HUVECs and LX2 cells. The proliferation and percentage of EpCAM-positive cells was increased when Huh7 cells were treated with VEGF.
Conclusion
Our study indicates an important contribution of VEGF toward the activation of HPC-mediated regeneration and DR in NASH.
Keywords: angiogenesis, ductular reaction, hepatic progenitor cells, nonalcoholic steatohepatitis
Abbreviations: Ang2, angiopoietin 2; BSA, bovine serum albumin; CM, conditioned medium; DMEM, Dulbecco's Modified Eagle medium; DR, ductular reaction; ELISA, enzyme-linked immunosorbent assay; EpCAM, epithelial cell adhesion molecule; FBS, fetal bovine serum; H&E, hematoxylin and eosin; HPC, hepatic progenitor cell; HSC, hepatic stellate cell; HUVEC, human umbilical vein endothelial cell; IHC, immunohistochemical; MT, Masson trichrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PCR, polymerase chain reaction; VEGF, vascular endothelial growth factor
The regeneration of the intact liver during physiological conditions takes place through the proliferation of mature hepatocytes. However, hepatocyte proliferation is impeded owing to various insults such as viral infection, toxins, steatosis, and oxidative stress, resulting in chronic liver injury. During chronic injury, normally quiescent resident hepatic progenitor cells (HPCs) are activated and expand from the periportal to the pericentral zone of the liver, giving rise to reactive ductules.1,2 Reactive ductules or ductular reactions (DRs) that refer to an increased number of cholangiocytes or ductules represent a regenerative response of the liver mediated by expansion of HPCs and thus a source of hepatocellular restoration during its damage or senescence. This expansion of HPCs or the emergence of DR is seen during chronic liver injury in both mice and humans.1,3, 4, 5 During DR, various populations of progenitor cells expressing markers of both fetal hepatocytes and biliary cells including epithelial cell adhesion molecule (EpCAM), Thy1, CK7, CK19, CD133, and so on are known to be activated. Studies have illustrated that there is a stringent connection of DR and HPC activation with both liver fibrosis and regeneration.6,7 The microenvironment of HPCs comprises various types of stromal cells and the surrounding extracellular matrix (ECM) that regulate their behavior and characteristics including activation, proliferation, and differentiation.2,8 Different liver pathologies comprise of different microenvironmental signals and elicit a different pattern of HPC niche activation and hence DR. Growth factors/cytokines released from the local microenvironment during liver injury significantly affect the type of HPC population that is activated and are thought to be an important stimulators of DR.2,9,10
In chronic nonalcoholic fatty liver disease (NAFLD), the disease progresses from simple to advanced steatosis and then nonalcoholic steatohepatitis (NASH). NASH is characterized by increased oxidative stress, inflammation, and angiogenesis.11 Angiogenesis or the growth of new vessels has a strong correlation with reactive ductules and HPC activation in primary biliary cirrhosis, but its correlation with NASH remains largely unknown.9,12 Also, the contribution of angiogenic mediators toward DR and HPC activation needs further exploration. In the present study, we therefore investigated the correlation of DR with known proangiogenic factors that are known to be upregulated during liver injury in patients with NASH.
Methods
Human Subjects
Human liver biopsies were obtained from patients with NASH (n = 14) recruited in the studies. These patients were selected from 22 patients with NAFLD, whose liver biopsies were taken and screened. The biopsies were used for immunohistochemical (IHC) studies and mRNA analysis. The study was approved by the ethics committee of the institute, and informed consent was taken from all the subjects. The study was performed in accordance with the guidelines of the Declaration of Helsinki. Liver specimens were embedded in paraffin and stained with hematoxylin and eosin (H&E) and Masson trichrome (MT). NASH was defined as steatosis with lobular inflammation and ballooning degeneration, with or without Mallory-Denk bodies or fibrosis. Steatosis, inflammation, and fibrosis were scored as previously described.13 Progressed or late NASH was regarded as fibrosis stages 2–4, whereas early NASH was defined as fibrosis stages 0–1.14
IHC Staining
DR in patients was measured by staining of the liver biopsies with CK19 and EpCAM. For staining, liver biopsies from patients were fixed in 4% phosphate-buffered formaldehyde solution and embedded in paraffin. From all tissue samples, 4-μm-thick tissue sections were cut using a sliding microtome for histology. Liver sections were deparaffinized. Subsequently, they were incubated overnight at 4 °C with primary antibody and thereafter for 1 h at room temperature with secondary rabbit anti-mouse or anti-goat biotinylated antibody accordingly. HPCs were quantified by counting of positive cells in the portal and periportal areas and expressed as the IHC score. The primary antibodies used were EpCAM (1:200; Santa Cruz Biotechnology) and CK19 (Ready to use antibodies; PathnSitu Biotechnologies). IHC scoring was performed on a scale of 0–4, where grade 0 indicated no evidence of DR and grade 1–4 indicated very weak, weak, moderate, or strong intensity of staining.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA from the liver biopsies was isolated by using the Nucleopore kit as per the manufacturer's instructions. RNA was quantified at 260/280 nm using the Thermo Scientific Nanodrop 2000 Spectrophotometer. First-strand cDNA was synthesized from 1 μg of total RNA using reverse transcriptase (Thermo Scientific Verso cDNA synthesis kit) as per the manufacturer's instructions. Quantitative real-time polymerase chain reaction (PCR) was carried out using the SYBR green PCR master mix (Fermentas Life Sciences) on the ViiA7 instrument PCR system (Applied Biosystems, USA). The following cycling parameters were used: start at 95 °C for 5 min, denaturing at 95 °C for 30 s, annealing at 60 °C for 30 s, elongation at 72 °C for 30 s, and a final 5 min of extra extension at the end of the reaction to ensure that all amplicons were completely extended and repeated for 40 amplification cycles. The genes and primer pairs are given in Table 1.
Table 1.
Gene Primer Sets Used in the Study.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| CCL2 | TCCCAAAGAAGCTGTGATCTTCA | TCTGGGGAAAGCTAGGGGAA |
| VEGFR1 | ATGGTCTTTGCCTGAAATGG | AGCCAGTGTGGTTTGCTTGA |
| VEGFR2 | TGTATGTCCCACCCCAGATT | CTCTTCCTCCAACTGCCAAT |
| VEGF | TGCAGATTATGCGGATCAAACC | TGCATTCACATTTGTTGTGCTGTAG |
| bFGF | CAAACTACAACTTCAAGCAG | GAAACACTCGTCTGTAACAC |
| Ang1 | TATGCCAGAACCCAAAAAGG | AACTCATTCCCCAGCCAATA |
| Ang2 | ATCAGCCAACCAGGAAATGA | TGTGTTCTGCCTCTGTGGATA |
| CXCL8/IL8 | ACTGAGAGTGATTGAGAGTGGAC | ACAACCCTCTGCACCCAGTT |
CCL2, C–C motif chemokine ligand 2; VEGFR1, vascular endothelial growth factor receptor 1; VEGFR2, vascular endothelial growth factor receptor 2; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor, Ang1, angiopoietin 1; Ang2, angiopoietin 2; CXCL8/IL8, C-X-C motif ligand 8.
Cell Cultures
Huh7 cells were cultured in Dulbecco's Modified Eagle medium (DMEM) (Gibco) with 10% fetal bovine serum (FBS; Hyclone), 100 μg/ml streptomycin, and 100 IU/ml penicillin (Gibco) at 37 °C in humidified atmosphere containing 5% CO2. LX2 cells (hepatic stellate cells [HSCs]) were cultured in DMEM with 2% FBS, 100 μg/ml streptomycin, and 100 IU/ml penicillin at 37 °C with 5% CO2. Human umbilical vein endothelial cells (HUVECs) (Gibco) purchased from Invitrogen were grown in endothelial medium (HiMedia Laboratories) with growth factors and 1% antibiotics on gelatin-coated plates.
Treatment of Endothelial Cells and HSCs With Palmitate
HUVECs and LX2 cells were seeded at 10,000 cells per well on 96-well plates and incubated at 37 °C for overnight to allow for cell attachment. About 4 mM palmitate-bovine serum albumin (BSA) conjugate in 10% BSA or 10% BSA was diluted 20× in a complete medium containing 10% FBS to prepare 200 μM palmitate treatment or control medium. The total BSA concentrations in the control and palmitate-containing medium were kept the same to avoid differential protein binding effect on compounds. The following day, the medium was aspirated from plates, and 100 μl of the palmitate treatment or control medium containing compounds was transferred from the compound plates to the cell culture plates, followed by 24-h incubation at 37 °C.15
Staining of HUVECs and HSCs with Nile Red
To confirm lipid uptake in cells, after incubation in the presence or absence of palmitate for 24 h, the confluent cell monolayer was washed with phosphate buffer saline (PBS) twice, fixed in paraformaldehyde (4%) for 20 min, and rinsed with PBS again. Then, the cells were stained with Nile red solution for 30 min on a shaker. The stained cells were finally washed with PBS. The absorbance of the dye-triglyceride complex was measured at 520 nm after suitable dilution.
Coculture of Cells
To study the paracrine effects of steatotic endothelial cells and stellate cells on hepatic cells, coculture studies were performed. Indirect cocultures performed with Huh7 cells were treated with conditioned medium (CM) from HUVECs/LX2 cells. CMs from HUVECs and LX2 cells were prepared after serum starvation of these cells for 24 h and then collecting the supernatants after centrifugation to remove cell debris.17
MTT Assay
The cell proliferation rate in Huh7 cells was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) cell proliferation assay. For the assays, we seeded 10,000 Huh7 cells per well in a 96-well plate and incubated with medium alone and CM from HUVECs or LX2 cells for 24 h. Then, we removed the medium and washed the cells with PBS. MTT was then added to the medium to a final concentration of 0.5 mg/mL and incubated for 3 h at 37 °C, until intracellular purple formazan crystals were visible under the microscope. Finally, MTT was removed, and the solubilizing solution was added. Absorbance was taken at 570 nm after incubation at room temperature or 37 °C for 30 min to 2 h, until the cells had lysed and purple crystals had dissolved.
Enzyme-linked Immunosorbent Assays
HUVECs, LX2 cells, and Huh7 cells were cultured in serum-free medium for 24 h. The supernatant was collected and concentrated on a Speed-Vac, and enzyme-linked immunosorbent assay (ELISA) for vascular endothelial growth factor (VEGF) was performed using ELISA kits (Thermo Fisher Scientific) as per the manufacturer's protocol. The optical density values were measured at 450-nm wavelength using a fluorescence microplate reader (Synergy/H1). Standard curve was plotted to calculate the exact concentrations of VEGF in the culture supernatants.16
Culture of Hepatic Cells With VEGF
Huh7 cells were seeded overnight and were treated with 20 ng/ml VEGF and incubated for 24 h. Furthermore, these hepatic cells were then analyzed for increase in proliferation in the presence of VEGF by MTT assays (as mentioned previously).
Surface Staining and FACS Analysis
VEGF-treated Huh7 cells were investigated for increase in the level of the HPC marker, EpCAM. These cells were incubated with the primary antibody EpCAM (1:100; Santa Cruz) for 1 h at 4 °C, washed with PBS, and again incubated with secondary antibody labeled with phycoerythrin (PE) (1:500; Santa Cruz) for 30 min at 4 °C in dark. The cells were then washed and fixed in 0.4% paraformaldehyde. The cells were further acquired on the BD FACScalibur™ (BD Biosciences, USA) and BD FACSVerse (BD Biosciences, USA) flow cytometer. All the data analysis was performed using FlowJo software, version 8.8.7.
Statistical Analysis
Data analysis was performed using dot plots, bar diagrams, and correlation curves using GraphPad Prism (version 6.01). Student's unpaired t-test was used to analyze difference between the two groups. Pearson's correlation coefficient (r) was used to assess correlations between two variables. Statistical significance between groups was accepted for P < 0.05.
Results
Clinical and Histological Characteristics of the Patients
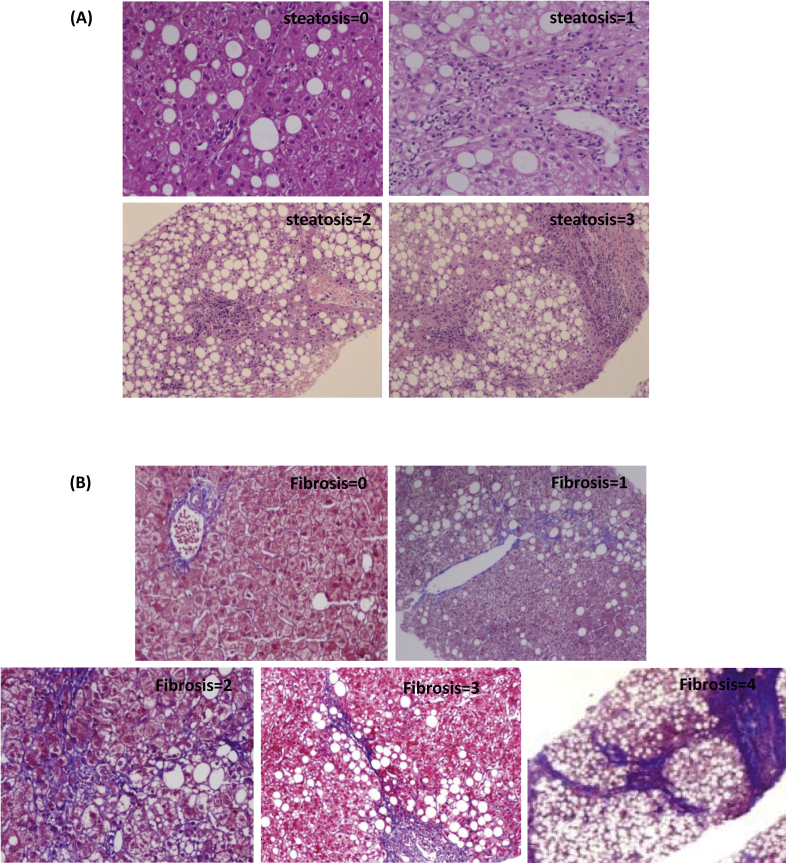
Demographic and clinical characteristics of the patients are summarized in Table 2. H&E staining showed patients having varying degrees of steatosis and inflammation (Figure 1A, Table 2). Fibrosis of varying degrees was well evident by MT staining (Figure 1B, Table 2). About 42% of the patients had early fibrosis (stage 0–1), and 57% of patients had advanced/late fibrosis (stage 2–4) in our study cohort (Table 2).
Table 2.
Clinical Parameters of the Patients.
| Characteristics | Patients with NASH |
|---|---|
| No. of patients (N) | 14 |
| 14 | |
| Male:female | 5:2 |
| 5:2 | |
| Age (years) | 42 (20–63) |
| BMI (kg/m2) | 29 (22–40) |
| Steatosis, n (%) | |
| 1 | 2 |
| 2 | 11 |
| 3 | 1 |
| Ballooning, n (%) | |
| 1 | 4 |
| 2 | 10 |
| Inflammation, n (%) | |
| 1 | 3 |
| 2 | 10 |
| 3 | 1 |
| Fibrosis, n (%) | |
| 0–1 (early) | 6 (42.8%) |
| 2–4 (late) | 8 (57.1%) |
NASH, nonalcoholic steatohepatitis; BMI, body mass index.
Figure 1.
Histological analysis of patients with NASH. (A) H&E staining showed simple steatosis in patients with NASH in grade 1, comparatively more in grade 2, and advanced steatosis in grade 3 and grade 4 (n = 5). (B) MT staining confirms little fibrosis in grade 2, comparatively more in grade 2 and 3, and cirrhosis in grade 4 (n = 5). NASH, nonalcoholic steatohepatitis; H&E, hematoxylin and eosin; MT, Masson trichrome.
Evaluation of Different HPC Populations and DR in Patients
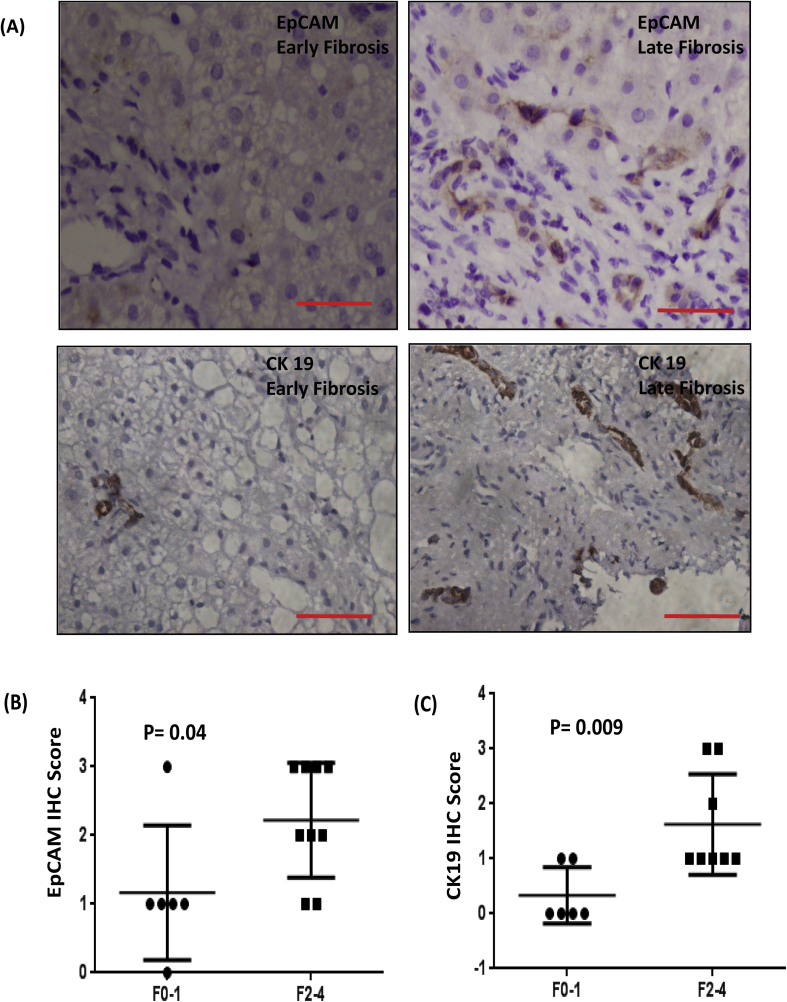
In patients with NASH, markers of DR including CK19 and EpCAM were observed in the portal and adjoining areas by histochemical staining (Figure 2A). IHC staining revealed a significant increase in expression of both CK19 and EpCAM in patients with late fibrosis compared with that present in patients with early fibrosis (P < 0.05 for both markers, Figure 2B, C).
Figure 2.
(A) Immunohistochemical images of EpCAM- and CK19-positive HPCs as markers of DR in NASH biopsies in early (F0–1) and late fibrosis (F2–4). EpCAM- and CK19-positive cells were counted in the portal tracts and scored from 0 to 4; IHC score of (B) EpCAM and (C) CK19 in patients with NASH in the early and late fibrosis groups. The IHC score was calculated as positive cells per field in the portal areas. A score of 0 represented <5% positive cells; a score of 1, 5–25% positive cells; a score of 2, 25–50% positive cells; a score of 3, 50–75% positive cells; a score of 4, >75% positive cells. EpCAM, epithelial cell adhesion molecule; HPC, hepatic progenitor cell; DR, ductular reaction; NASH, nonalcoholic steatohepatitis; IHC, immunohistochemical.
Angiogenic Gene Expression and DR
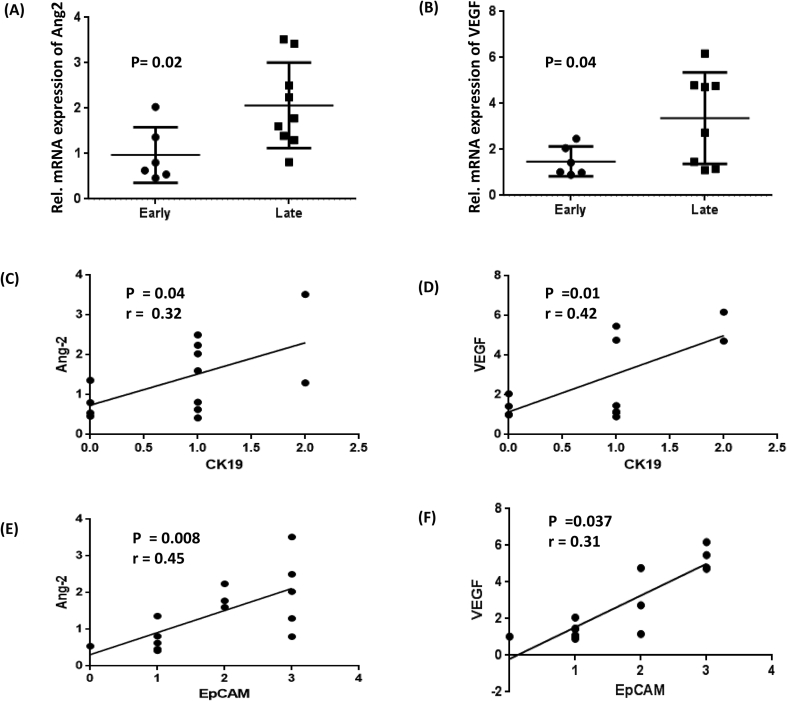
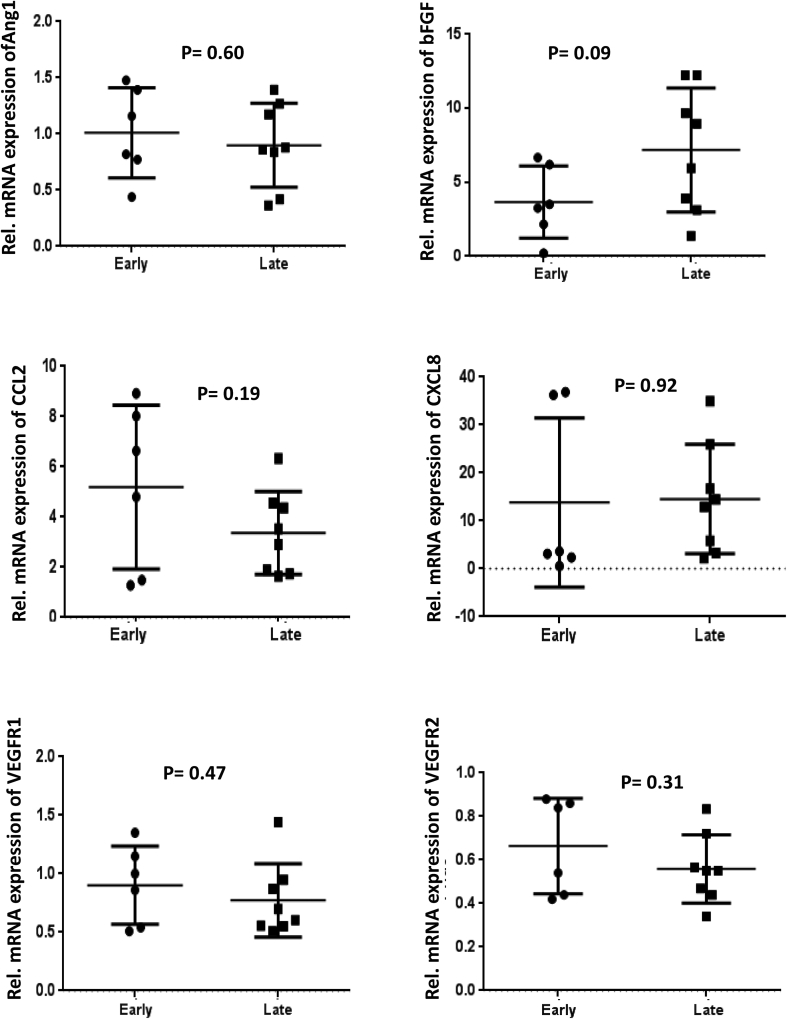
We next investigated the expression of major angiogenic genes in the patients with early and late fibrosis. There was no significant difference in the expression of most of the selected angiogenic genes between early and late injury/fibrosis in the patients. Common angiogenic genes, such as Ang1, basic fibroblast growth factor, C–C motif chemokine ligand 2, C-X-C motif ligand 8, vascular endothelial growth factor receptor 1, and vascular endothelial growth factor receptor 2, did not show any significant difference in their gene expression in the early and late fibrosis groups (Supplementary Figure 1). However, gene expression of angiopoietin 2 (Ang2) and VEGF was significantly upregulated in the advanced fibrosis group compared to the early fibrosis group (P < 0.05 for both, Figure 3A and B). We investigated the relationship between the IHC expression of CK19 and EpCAM with the gene expression of VEGF and Ang2 in our patients. Genes including Ang2 and VEGF showed a significant correlation with both CK19 and EpCAM IHC scores (Figure 3C, D).
Figure 3.
Dot plots showing relative mRNA expression of angiogenic genes (A) Ang2 and (B) VEGF in patients with early and late fibrosis. P < 0.05 denotes significance; Correlation of the angiogenic genes Ang2 and VEGF with (C–D) CK19 and (E–F) EpCAM in the patients with NASH (n = 14). “r” represents Pearson's correlation coefficient, and P < 0.05 denotes significance. Ang2, angiopoietin 2; VEGF, vascular endothelial growth factor; NASH, nonalcoholic steatohepatitis; EpCAM, epithelial cell adhesion molecule.
Enhanced Proliferation of Hepatic Cells When Cocultured With CM From Fat-treated Endothelial and Stellate Cells
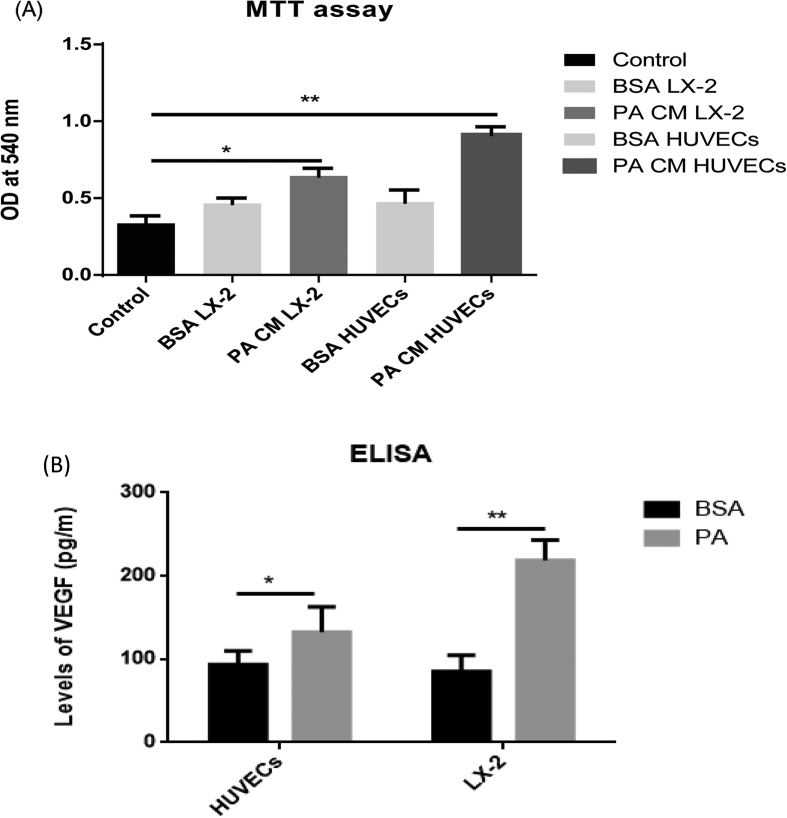
Because the nonparenchymal cells are the major source of angiogenic factors, we next performed coculture studies in the in vitro models of NAFLD to study the effects of steatotic HUVECs and LX2 cells on hepatic cell proliferation (Supplementary Figure 2). There was an increase in proliferation of Huh7 cells when cocultured in the presence of CM from steatotic HUVECs and LX2 cells in comparison with that observed in hepatic cells alone (Figure 4A; P < 0.001 for both). As we observed a significant correlation of VEGF gene expression with HPC markers, we next investigated VEGF levels in supernatants of BSA- and fat-treated HUVECs and LX2 cells. The results illustrated that hepatic cells cocultured with steatotic endothelial and hepatic stellate cells secreted increased amounts of VEGF compared with those treated with BSA (Figure 4B; P < 0.05).
Figure 4.
(A) MTT assay showing proliferation of Huh7 cells alone (untreated), Huh7 + BSA-CM HUVECs, Huh7 + PA-CM HUVECs, Huh7 + BSA-CM LX2, and Huh7 + PA-CM LX2 cocultures. (B) ELISA results revealed increase secretion of VEGF in supernatants of BSA- and PA-treated HUVECs and LX2 cells. ** denotes P < 0.001, and * denotes P < 0.05. BSA, bovine serum albumin; CM, conditioned media; PA, palmitic acid; HUVEC, human umbilical vein endothelial cell; ELISA, enzyme-linked immunosorbent assay; VEGF, vascular endothelial growth factor; OD, optical density.
Enhanced Proliferation and EpCAM in Hepatic Cells Cultured With VEGF
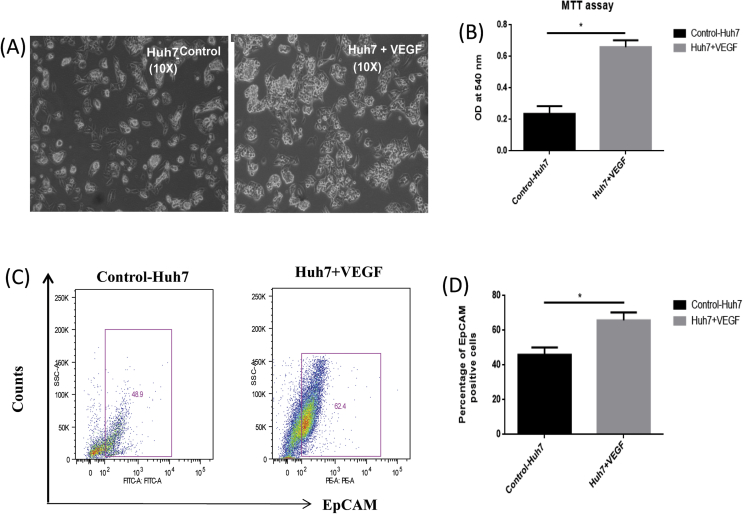
We then investigated the specific effects of VEGF on proliferation and expression of the HPC marker EpCAM on hepatic cells. A substantial increase in the number of Huh7 cells was observed when hepatic cells were treated with VEGF in comparison to untreated hepatic cells (Figure 5A and B; P < 0.05). Flow activated cell sorting (FACS) studies revealed an increase in the number of EpCAM positivity in Huh7 cells (Figure 5C and D; P < 0.05) when treated with VEGF, as compared with controls.
Figure 5.
(A) Representative phase contrast figure of Huh7 cells alone and Huh7-treated with 20 ng/ml VEGF; (B) Bar diagram showing increased proliferation of hepatic cells in the presence of VEGF with respect to the control. (C) FACS plot representing EpCAM-positive cells in the absence and presence of VEGF and (D) bar diagram showing percentage of EpCAM-positive cells in the presence of VEGF with respect to the control. * denotes P < 0.05. VEGF, vascular endothelial growth factor; EpCAM, epithelial cell adhesion molecule.
Discussion
In the present study, we report a significant correlation of Ang2 and VEGF with disease progression and HPC activation/DR in patients with NASH. Similar to earlier studies, we observed a marked increase in the expression of DR markers, CK19 and EpCAM, in patients with NASH, which also correlated significantly with increased fibrosis.14,16,17 It has already been shown that EpCAM-positive hepatic cells become increasingly prominent in the later stages of liver disease in parallel with the emergence of DR.18,19
Next, we investigated the expression of angiogenic genes with disease progression and DR in NASH. A significant correlation of Ang2 and VEGF with EpCAM and CK19 suggests their crucial participation in the perpetuation of DR in NASH. Ang2 is a crucial regulator of neovascularization, vascular remodeling, and maturation, which binds to a kinase receptor, Tie2.20, 21, 22 It is known to be secreted at higher levels in both the regenerating and fibrotic livers by the endothelial cells. Ang2 contributes significantly toward liver regeneration after partial hepatectomy during the late angiogenic phase.23,24 Our study highlights its role in HPC-mediated DR in NASH.
VEGF, a proangiogenic mediator, has been shown to stimulate cholangiocyte proliferation and DR by both autocrine and paracrine mechanisms.25 Alteration in vascular structures with elevation in protein expression of VEGF-A and VEGF-C in HPCs has been correlated with increase in DR in the diseased liver.9 In addition, in patients with liver cirrhosis, increase in both angiogenesis and DR is associated with increase in expression of VEGF in HPCs.12 These studies are in concordance with our work as we also observed an enhancement of DR with increased expression of VEGF. The expression of VEGF was also upregulated in patients with advanced fibrosis in our study group, corroborating its crucial role during progression of disease. Increased expression of VEGF in patients with NASH is a common finding and indicative of its role as a key angiogenic mediator in disease pathogenesis.26 Here, we focused on the contribution of VEGF toward HPC activation and DR. It has been reported that HPC expansion requires a close cooperation with activated Kupffer cells, endothelial cells, and HSCs.27,28 The reactive ductules and nonparenchymal cells of the liver establish paracrine links that are requisite to sustain biliary repair.29 Therefore, we performed in vitro coculture studies of hepatic cells with steatotic endothelial cells and stellate cells. We observed that both steatotic stellate cells and endothelial cells secreted substantially high levels of VEGF in cultures with stellate cells, contributing to more secretion, indicating that these cells may be an important source of VEGF in livers affected with NASH. To study specific effect of VEGF on hepatic cells, hepatic cells were cultured in the presence and absence of VEGF. Hepatic cells showed increased proliferation of EpCAM-positive cells when treated with VEGF in culture, signifying that increased levels of VEGF during liver injury may contribute toward activation of HPCs in vivo in NASH.
In summary, our study reports that VEGF produced from hepatic stellate cells promotes the proliferation of HPC-mediated liver repair during liver pathophysiology. The study highlights the contribution of angiogenic mediators and cellular interactions in the emergence of HPC-mediated DR and liver regeneration in the fibrotic liver affected with NASH.
Conflicts of interest
The authors have none to declare.
Acknowledgments
The study was part of the project funded by ICMR (F. No. 50/9/2013/BMS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.11.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Dot plots showing relative mRNA expression of angiogenic genes in patients with early and late fibrosis (n = 14). P < 0.05 denotes significance. VEGFR, vascular endothelial growth factor receptor; bFGF, basic fibroblast growth factor.
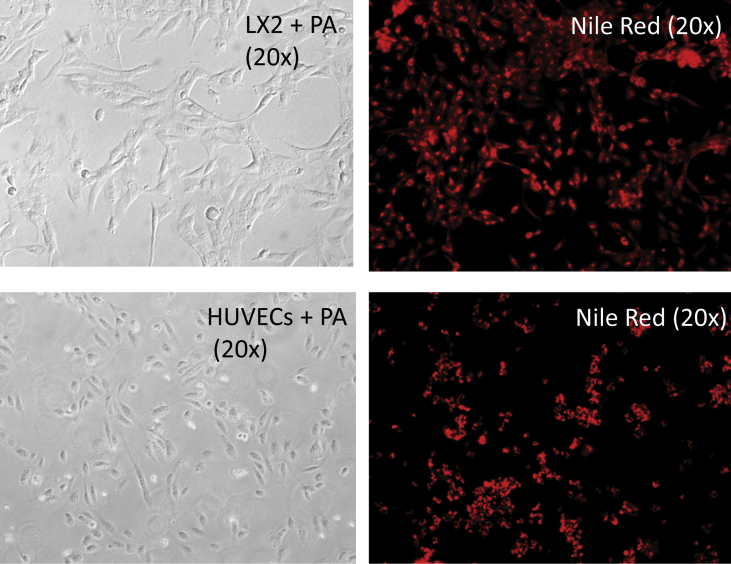
Supplementary Figure 2.
Palmitic acid–treated endothelial cells (HUVECs) and stellate cells (LX2) after 24 h of culture. Accumulation of fat in the cells was tested by Nile red staining. HUVEC, human umbilical vein endothelial cell; PA, palmitic acid.
References
- 1.Raven A., Lu W.Y., Man T.Y. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur S., Siddiqui H., Bhat M.H. Hepatic progenitor cells in action: liver regeneration or fibrosis? Am J Pathol. 2015;1859:2342–2350. doi: 10.1016/j.ajpath.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Gouw A.S., Clouston A.D., Theise N.D. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;545:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 4.Roskams T., Yang S.Q., Koteish A. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang S., Koteish A., Lin H. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 6.Williams M.J., Clouston A.D., Forbes S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;1462:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y., Katagiri H., Wang T. Ductular reactions in the liver regeneration process with local inflammation after physical partial hepatectomy. Lab Investig. 2016;9611:1211–1222. doi: 10.1038/labinvest.2016.97. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Chen L., Zern M.A. The diversity and plasticity of adult hepatic progenitor cells and their niche. Liver Int. 2017;379:1260–1271. doi: 10.1111/liv.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchitto A., Onori P., Renzi A. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg Nutr. 2013;22:68. doi: 10.3978/j.issn.2304-3881.2012.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spirli C., Strazzabosco M. Vascular endothelial growth factors in progenitor cells mediated liver repair. Hepatobiliary Surg Nutr. 2013;22:65–67. doi: 10.3978/j.issn.2304-3881.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammoutene A., Rautou P.E. Role of liver sinusoidal endothelial cells in nonalcoholic fatty liver disease. J Hepatol. 2019 Jun;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Maroni L., Pierantonelli I., Benedetti A., Marzioni M. Angiogenic factors in chronic liver diseases: the effects on hepatic progenitor cells. Hepatobiliary Surg Nut. 2013;22:61. doi: 10.3978/j.issn.2304-3881.2012.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Gadd V.L., Skoien R., Powell E.E. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014 Apr 1;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y., Rana P., Will Y. Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol Sci. 2012 Jun 14;129:346–362. doi: 10.1093/toxsci/kfs208. [DOI] [PubMed] [Google Scholar]
- 16.Carotti S., Vespasiani-Gentilucci U., Perrone G., Picardi A., Morini S. Portal inflammation during NAFLD is frequent and associated with the early phases of putative hepatic progenitor cell activation. J Clin Pathol. 2015;6811:883–890. doi: 10.1136/jclinpath-2014-202717. [DOI] [PubMed] [Google Scholar]
- 17.de Lima V.M., Oliveira C.P., Alves V.A. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol. 2008;496:1055–1061. doi: 10.1016/j.jhep.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Carpino G., Pastori D., Baratta F. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Sci Rep. 2017;71:15756. doi: 10.1038/s41598-017-15943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon S.M., Gerasimidou D., Kuwahara R. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;533:964–973. doi: 10.1002/hep.24122. [DOI] [PubMed] [Google Scholar]
- 20.Scholz A., Plate K.H., Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347:45–51. doi: 10.1111/nyas.12726. [DOI] [PubMed] [Google Scholar]
- 21.Hofer E., Schweighofer B. Signal transduction induced in endothelial cells by growth factor receptors involved in angiogenesis. Thromb Haemost. 2007;9803:355–363. [PMC free article] [PubMed] [Google Scholar]
- 22.Jeltsch M., Leppänen V.M., Saharinen P., Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. 2013;59:a009183. doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R., Huebert R.C., Shah V.H. Sinusoidal endothelial cells coordinate liver regeneration and angiogenesis via angiopoietin-2: an ode to prometheus. Gastroenterology. 2014;1472:533–534. doi: 10.1053/j.gastro.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Pauta M., Ribera J., Melgar-Lesmes P. Overexpression of angiopoietin-2 in rats and patients with liver fibrosis. Therapeutic consequences of its inhibition. Liver Int. 2015;354:1383–1392. doi: 10.1111/liv.12505. [DOI] [PubMed] [Google Scholar]
- 25.Gaudio E., Barbaro B., Alvaro D. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;1304:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Hu J., Srivastava K., Wieland M. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 27.Viebahn C.S., Benseler V., Holz L.E. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;533:500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Kordes C., Sawitza I., Götze S., Herebian D., Häussinger D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Investig. 2014;124:5503–5515. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morell C.M., Fabris L., Strazzabosco M. Vascular biology of the biliary epithelium. J Gastrenterol Hepatol. 2013 Aug;28:26–32. doi: 10.1111/jgh.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]