In many group-living mammals, mothers may increase the reproductive success of their daughters even after they are nutritionally independent and fully grown [1]. However, whether such maternal effects exist for adult sons is largely unknown. Here we show that males have higher paternity success when their mother is living in the group at the time of the offspring’s conception in bonobos (N = 39 paternities from 4 groups) but not in chimpanzees (N = 263 paternities from 7 groups). These results are consistent with previous research showing a stronger role of mothers (and females more generally) in bonobo than chimpanzee societies.
The effects of maternal health, nutritional and social status, and experience on offspring development and fitness are strongest during the energetically demanding stages of gestation and lactation [1]. However, maternal effects can also be present for older, more independent offspring. For example, in group-living animals, mothers can support their adult offspring during competitive interactions with conspecifics, thereby infl uencing their social rank or access to resources [2]. As most social mammals are female philopatric, maternal support and fitness benefits of co-residence with mothers have often been described for independent daughters [2]. Mothers may also behave in ways to enhance the fitness of their adult sons when they co-reside in the same group. For example, orca mothers lead their sons to attractive foraging grounds, a potential mechanism explaining the increased survivorship of males living with their mothers [3]. To our knowledge, no study (outside of humans [4]) has investigated the effect of mothers’ presence on male fertility (paternities per unit time/opportunities), which is typically a large component of variance in lifetime reproductive success in male mammals [5]. Another limitation of previous research is genetic confounding: offspring with living mothers might have higher fitness not because of their mother’s behavior, but because genes that increase the mother’s survival (for example, through increased body size or health) also increase the fitness of her offspring. Although large, multi-generational pedigrees can disentangle the genetic and environmental components of maternal effects, these are not often available for wild populations, especially in the long-lived, group-living species where we might expect social relationships to most strongly affect fitness. However, if mothers’ presence and offspring fitness are associated in a species where mothers routinely behave in ways that plausibly increase offspring fitness, but not in a closely related species where mothers do not often behave this way, this would increase our confidence that the observed maternal effect is at least partly environmental rather than solely genetic.
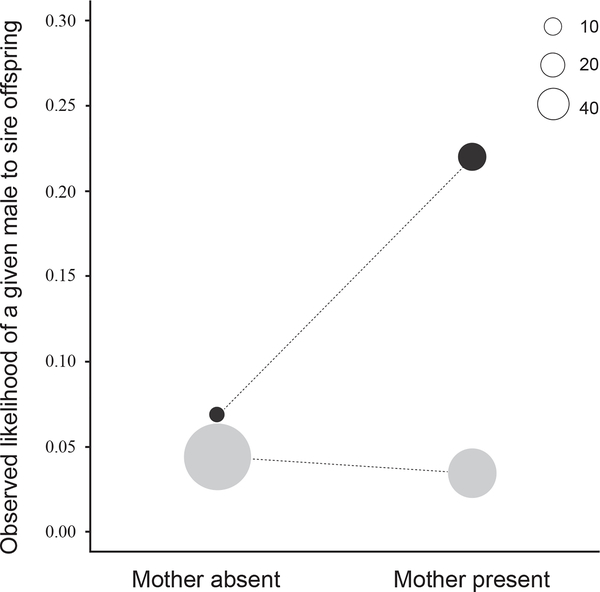
Here we examine the relationship between mother presence and paternity success in bonobos and chimpanzees, two closely related male-philopatric and female dispersal species. In both species, although mothers live alongside their sons for their entire adult lives and help them in male–male competition, a large body of evidence suggests that bonobo mothers also behave in ways that potentially increase the paternity success of their sons. For example, bonobo mothers frequently bring their sons into close spatial proximity with estrous females [6], protect their sons’ mating attempts from interference by other males [6], interfere in the mating attempts of other males [6], and form coalitions with their sons to help them acquire and maintain high dominance rank [7]. Such maternal behavior is more likely to be effective in bonobos, where the sexes are co-dominant and the highest ranks are consistently occupied by females, than in chimpanzees, where all adult males are dominant over all females [8]. We found that bonobo males with a mother living in the group at the time of the conception were about 3 times (odds ratio: 3.14) more likely to sire offspring than males that did not (Figure 1). In contrast, mothers’ presence had no strong relationship with siring probability in chimpanzees (males with mother present were 1.26 times less likely to sire offspring; Figure 1; Figure S1). This species difference in the relationship between mothers’ presence and paternity success was statistically significant (two-way interaction between species and mother presence, GLMM estimate ± SE = −1.54 ± 0.50, p < 0.01; see the Supplemental Information), and was observed while controlling for species differences in the number of males that had a mother present (55% of bonobos and 41% of chimpanzees), the number of competing males (averages of group averages were: = 6.9; = 15.5), and male age (average sire age: bonobos = 21.8y; chimpanzees = 23.3y) at the time of conception. Overall, the sire’s mother was present more than twice as frequently during conception in bonobos (31/39 = 79.5%) than in chimpanzees (92/263 = 34.9%) (Table S1).
Figure 1. Observed average likelihood of a male to sire offspring in the presence and absence of their mothers in the group.
Bonobos are represented in black and chimpanzees in grey. Circle sizes represent the number of offspring. The generally higher likelihood of a male to sire a given offspring in bonobos is due to the smaller number of males in the group compared to chimpanzees.
Findings in humans and orcas linking mothers’ presence and behavior to the fitness of lineal descendants (offspring and grandoffspring) have been interpreted as contributing to the evolution of the unusual pattern of extended longevity and a substantial female post-reproductive lifespan observed in these taxa [3,4]. Although long-term survivorship data are not yet available for wild bonobos, data from captivity suggesting that female longevity may be higher in bonobos than chimpanzees are consistent with this hypothesis [9]. In addition, theory predicts that a female post-reproductive lifespan is more likely to evolve under mating and dispersal systems (including male philopatry and female dispersal) where the expected number of close relatives in the group, and thus the expected benefits of ceasing reproduction to assist them, increase with a female’s age [10]. However, although bonobo females live in male-philopatric and female-dispersal societies, and can increase the number of grandoffspring they have through their sons, they apparently do not have a substantial post-reproductive lifespan. More research on interspecific variation in the costs and benefits of breeding and helping will be necessary to explain why a substantial female post-reproductive lifespan only occurs in some of the species where the dispersal system and resulting age structure of relatedness would appear to favor its evolution [10].
Supplementary Material
ACKNOWLEDGEMENTS
We thank the responsible authorities in the host countries for permitting our research, including: Institut Congolaise pour la Conservation de la Nature, Ministère de l’Education Nationale, Democratic Republic of Congo; Ministry of Scientific Research and Technology and Centre de Recherche en Ecologie et Foresterie, Democratic Republic of Congo; Ivorian Ministry of Environment and Forests and Ministry of Higher Education and Scientific Research and the Office Ivoirien des Parcs et Reserves; the Tanzania National Parks, Wildlife Research Institute, and Commission for Science and Technology; the Uganda Wildlife Authority, Uganda National Council for Science and Technology and Makerere Biological Field Station. We thank Roger Mundry for statistical advice and the field staff of all projects for making long-term data collection possible. M.S. was supported by the Max Planck Society, the National Geographic Society and the Wenner-Gren Foundation, and partially supported by SNF. Work at LuiKotale was supported by the Max Planck Society, the L.S.B. Leakey Foundation, and National Geographic Society. Work in Lomako was supported by the Max Planck Society, the German Academic Exchange Service and the German Science Foundation. The study at Wamba is financially supported by the Japanese Ministry of the Environment Global Environment Research Fund (D-1007 to T.F.), the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (26257408 and 22255007 to T.F., 25304019 to C. Hashimoto., and 16H02753 and 25257407 to T. Yumoto), the JSPS Core-to-Core Program (2009–2011, 2012–2014, and 2015–2017 to T.F.), the JSPS HOPE Project of the PRI of Kyoto University (to T. Matsuzawa), the JSPS Strategic Young Overseas Visits Program for Accelerating Brain Circulation (S2508), the JSPS Grant-in-aid for JSPS fellows (17J09827 to S.I.) and the Leading Graduate Program in Primatology and Wildlife Science of Kyoto University. Long-term sampling at Kanyawara was supported by the U.S. National Science Foundation (grants BCS-0849380, BCS 1355014 and IOS-LTREB 1052693), National Institutes of Health grants AI058715 and R01AG049395, the Leakey Foundation, the National Geographic Society, and the Wenner-Gren Foundation. Long-term research at Ngogo was supported by NIH Grant R01AG049395, the Leakey Foundation, the President’s Strategic Initiative Fund of Arizona State University, the Max Planck Society, and the Institute of Human Origins. The Royal Zoological Society of Scotland provides core support for the Budongo Conservation Field Station. The Max Planck Society supports research at Taï. C.C. is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 679787). Fieldwork and sampling at Gombe is funded primarily by the Jane Goodall Institute with additional support from grants from the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0648481, IOS- 1052693, IOS-1457260) and the National Institutes of Health (R01 AI50529, R01 AI58715, P30 AI27767). E.W. was supported by the Elmer C. Birney and Florence Rothman Fellowships, the Dayton and Wilkie Natural History Fund and the NIH/ NIAID, Ruth L. Kirschstein National Research Service Award (NRSA) (F32 AI085959).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes one figure, one table, experimental procedures, author contributions, and supplemental references, and can be found with this article online at https://doi.org/10.1016/j.cub.2019.03.040.
REFERENCES
- 1.Maestripieri D, and Mateo JM (2009). Maternal Effects in Mammals. (Chicago: University of Chicago Press; ). [Google Scholar]
- 2.Clutton-Brock TH (2016). Mammal Societies. (Chichester, UK: John Whiley & Sons; ). [Google Scholar]
- 3.Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, and Croft DP (2012). Adaptive prolonged postreproductive life span in killer whales. Science 337, 1313. [DOI] [PubMed] [Google Scholar]
- 4.Lahdenperä M, Lummaa V, Helle S, Tremblay M, and Russell AF (2004). Fitness benefits of prolonged post-reproductive lifespan in women. Nature 428, 178. [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock T (1988). Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. (Chicago: University of Chicago Press; ). [Google Scholar]
- 6.Surbeck M, Mundry R, and Hohmann G (2011). Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). Proc. Biol. Sci 278, 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuichi T (2011). Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. Issues News Rev 20, 131–142. [DOI] [PubMed] [Google Scholar]
- 8.Stumpf RM (2007). Chimpanzees and bonobos: Diversity within and between species In Primates in Perspective, Campbell CJ, Fuentes A, MacKinnon KC, Panger M, and Bearder S, Eds., (Oxford: Oxford University Press; ) pp. 321–344. [Google Scholar]
- 9.Schubert G, Vigilant L, Boesch C, Klenke R, Langergraber K, Mundry R, Surbeck M, and Hohmann G (2013). Co-residence between males and their mothers and grandmothers is more frequent in bonobos than chimpanzees. PLoS One 8, e83870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnstone RA, and Cant MA (2010). The evolution of menopause in cetaceans and humans: the role of demography. Proc. Biol. Sci 277, 3765–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.