Main Text
Combination antiretroviral therapy (cART) inhibits HIV replication but does not lead to viral eradication in people living with HIV (PLWH) because of the latent reservoir.1 Several studies have shown that interventions that dramatically reduce the number of latently infected CD4+ T cells do not lead to a cure.2, 3, 4, 5 Thus, other therapies may need to be used in conjunction with strategies that target the reservoir if a cure is to be achieved. In this issue of Molecular Therapy, Maldini et al.6 employ CD4+ T cells expressing HIV-specific chimeric antigen receptors (CARs; CAR 4 T cells) and show that some of these engineered cells reduce the magnitude of viremia when cART is discontinued in HIV-infected humanized mice. The CAR 4 T cells proliferate effectively in vivo and also enhance the function of CD8+ T cells expressing CARs (CAR 8 T cells). The study suggests that combinations of CAR 4 T cells and CAR 8 T cells may play an important role in combinatorial strategies designed to cure HIV (Figure 1).
Figure 1.
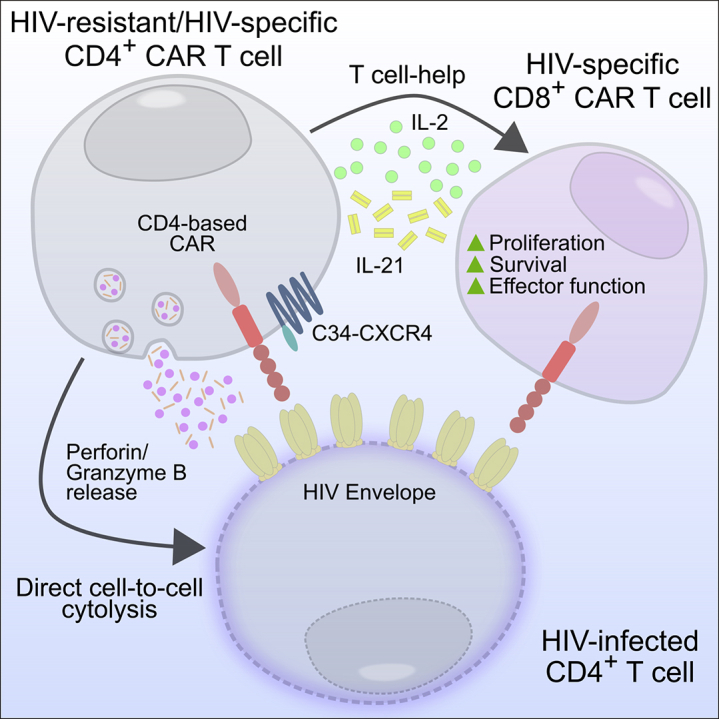
Dual Function of CAR 4 T Cells
41BB-costimulated CAR 4 T cells recognize the HIV envelope expressing infected cells through the CD4 protein, which constitutes part of the CAR. These cells can directly kill infected target cells through the secretion of perforin and granzyme A and B. The cytokines produced by these CAR 4 T cells also lead to the enhanced survival, proliferation, and effector function of CAR 8 T cells.
CAR T cells represent a form of personalized medicine, where patients’ CD4 or CD8 T cells are transduced to express a CAR engineered to recognize a specific cell target. Recently finding successful application in antitumor therapy,7 CARs are “chimeric” because both antigen-binding and T cell-activating functions are combined into a single receptor. In the case of HIV, early CARs consisted of CD4 as the external antigen-binding moiety attached to the zeta chain of the CD3 molecule, which enables T cell activation.8, 9, 10 When these CAR T cells engage their target cells, the CD4 protein binds to the viral gp120 envelope protein expressed on infected cells, leading to activation of the CAR T cells and killing of the infected target cells. These early CARs have been improved over time:11 T cells require additional co-stimulatory signals beyond CD3 signaling to persist after activation and specific effector functions can be directed through the incorporation of customizable costimulatory molecules.12 In this new study, Maldini et al.6 compared the function of CAR 4 T cells incorporating the costimulatory molecules CD27, OX40, 4-1BB, ICOS, or CD28. CD27, OX40, and 4-1BB are members of the same tumor necrosis factor superfamily, and CD28 and ICOS are members of the CD28 superfamily. T cell stimulation through CD28 can elicit the production of various cytokines. In vitro studies using CAR 4 T cells incorporating the different costimulatory molecules co-cultured with HIV-infected CD4 cells showed that CD28-costimulated CAR4 T cells were particularly efficient at generating polyfunctional cytokine responses. These cells were also effective at inhibiting viral replication in a suppression assay and actually eliminated infected CD4+ T cells as effectively as CAR 8 T cells. This is surprising because CD4+ T cells in general are not effective at killing target cells. However, this could be potentially explained by the high levels of the effector proteins granzyme A and granzyme B that were expressed by these CAR 4 T cells. Granzymes are serine proteases normally released by cytoplasmic granules within cytotoxic T cells and natural killer (NK) cells.
Even more compelling are the data from the in vivo studies, in which the effect of various CAR 4 T cells on viral rebound after cART cessation was measured in HIV-infected humanized mice. In this case, Maldini et al.6 used highly immunodeficient NSG (NOD-SCID IL2Rγ−/−) mice reconstituted, or “humanized,” with CD8-depleted peripheral blood mononuclear cells from healthy donors and infused with HIV-infected CD4 T cells in the context of ART, after which ART treatment was then interrupted and HIV-resistant CAR 4 T cells were infused. Establishing durable HIV-resistance in CAR 4 T cells is a crucial step before any CAR 4 T cells can be considered as a viable therapeutic for HIV cure. Maldini et al.6 utilized a 34 amino acid insert into the amino terminus of the HIV co-receptor, CXCR4, that was pioneered by Leslie et al.13 This process allows for retention of normal physiologic chemotaxis while abrogating HIV-1 entry. Surprisingly, the CD28-costimulated CAR 4 T cells that were so effective in controlling HIV replication in vitro were not able to significantly prevent viral rebound in the mice. In contrast, 41BB-costimulated CAR 4 T cells, which demonstrated limited efficacy in controlling HIV replication in vitro, were the most effective at reducing the magnitude of rebound viremia when ART was discontinued. This was probably due to the fact that these cells expanded efficiently in vivo and expressed the lowest levels of the inhibitory molecules PD-1 and TIGIT, which normally provide negative feedback signals to activated T cells. Furthermore co-infusion of CD28- or 4-1BB-costimulated CAR 8 T cells with 4-1BB-expressing CAR 4 T cells dramatically increased the proliferation and persistence of these CAR 8 T cells compared to the proliferation of CAR 8 T cells when they were infused alone. This enhanced persistence of CAR 8 T cells was associated with improved virologic control when cART was discontinued.
The data presented by Maldini et al.6 represent findings that could be important for HIV cure strategies utilizing CAR T cells; however, there is still further work to be done before they are ready for use in clinical trials. And, as demonstrated by the incongruent performance of 4-1BB-costimulated CAR 4 T cells in vitro compared to successful expansion and HIV elimination in vivo, further studies need to include additional animal models. In particular, although humanized mice offer the advantage of in vivo work at relatively low cost compared to primate studies, “humanized mice” is an umbrella term encompassing a wide variety of mouse models, each with their own unique advantages and disadvantages. In this case, Maldini et al.6 used a fairly straightforward, and relatively inexpensive, mouse model utilizing commercially available NSG mice, which are both B and T cell deficient and lacking functional NK cells, that were then reconstituted with healthy mature peripheral blood cells. The disadvantages of this humanized mouse model include extremely high levels of immune activation of engrafted cells and the inevitable development of graft versus host disease, in which engrafted activated human cells attack native mouse tissues. It is also challenging to study HIV latency in the presence of so much immune activation, as the authors acknowledged. Because all humanized mouse models can only provide a facsimile of a normal functional human immune system, studies utilizing more sophisticated humanized mouse models and longer analytical treatment interruptions are warranted.
The first generation of HIV-specific CAR T cells had inconsistent effects on viremia and/or the viral reservoir in PLWH.8, 9, 10 The HIV-resistant 4-1BB-costimulated CAR4 T cells generated in this study should be more effective at controlling viral replication and could potentially be given in clinical trials in conjunction with latency reversal agents. In this context, they could possibly eliminate HIV-infected CD4+ T cells that would be stimulated to express HIV gp120 on their surface. However, to be truly effective, these effector cells will likely need to persist at high frequencies for many years so that they will be capable of eliminating any residual reservoir cells that become activated over time after cART is discontinued. This may be analogous to the potent HIV-specific CD8+ T cell responses that control replication-competent virus present in some patients who maintain undetectable viral loads without cART (elite suppressors).14,15 4-1BB-costimulated CAR4 T cells may be particularly effective at this task given their proliferative capacity and their ability to both directly kill infected cells and to enhance the cytotoxic potential of CAR 8 T cells.
References
- 1.Sengupta S., Siliciano R.F. Targeting the Latent Reservoir for HIV-1. Immunity. 2018;48:872–895. doi: 10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrich T.J., Hatano H., Bacon O., Hogan L.E., Rutishauser R., Hill A., Kearney M.F., Anderson E.M., Buchbinder S.P., Cohen S.E. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017;14:e1002417. doi: 10.1371/journal.pmed.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colby D.J., Trautmann L., Pinyakorn S., Leyre L., Pagliuzza A., Kroon E., Rolland M., Takata H., Buranapraditkun S., Intasan J., RV411 study group Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018;24:923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henrich T.J., Hanhauser E., Marty F.M., Sirignano M.N., Keating S., Lee T.-H., Robles Y.P., Davis B.T., Li J.Z., Heisey A. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzuriaga K., Gay H., Ziemniak C., Sanborn K.B., Somasundaran M., Rainwater-Lovett K., Mellors J.W., Rosenbloom D., Persaud D. Viremic relapse after HIV-1 remission in a perinatally infected child. N. Engl. J. Med. 2015;372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldini C.R., Gayout K., Leibman R.S., Dopkin D.L., Mills J.P., Shan X., Glover J.A., Riley J.L. HIV-Resistant and Q9 HIV-Specific CAR Q10 –Modified CD4+ T Cells Mitigate HIV Disease Progression and Confer CD4+ T Cell Help In Vivo. Mol Ther. 2020;28:1585–1599. doi: 10.1016/j.ymthe.2020.05.012. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettitt D., Arshad Z., Smith J., Stanic T., Holländer G., Brindley D. CAR-T Cells: A Systematic Review and Mixed Methods Analysis of the Clinical Trial Landscape. Mol. Ther. 2018;26:342–353. doi: 10.1016/j.ymthe.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker R.E., Bechtel C.M., Natarajan V., Baseler M., Hege K.M., Metcalf J.A., Stevens R., Hazen A., Blaese R.M., Chen C.C. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96:467–474. [PubMed] [Google Scholar]
- 9.Mitsuyasu R.T., Anton P.A., Deeks S.G., Scadden D.T., Connick E., Downs M.T., Bakker A., Roberts M.R., June C.H., Jalali S. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- 10.Deeks S.G., Wagner B., Anton P.A., Mitsuyasu R.T., Scadden D.T., Huang C., Macken C., Richman D.D., Christopherson C., June C.H. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlmann A.-S., Peterson C.W., Kiem H.-P. Chimeric antigen receptor T-cell approaches to HIV cure. Curr. Opin. HIV AIDS. 2018;13:446–453. doi: 10.1097/COH.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinkove R., George P., Dasyam N., McLellan A.D. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunology. 2019;8:e1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie G.J., Wang J., Richardson M.W., Haggarty B.S., Hua K.L., Duong J., Secreto A.J., Jordon A.P., Romano J., Kumar K.E. Potent and Broad Inhibition of HIV-1 by a Peptide from the gp41 Heptad Repeat-2 Domain Conjugated to the CXCR4 Amino Terminus. PLoS Pathog. 2016;12:e1005983. doi: 10.1371/journal.ppat.1005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veenhuis R.T., Kwaa A.K., Garliss C.C., Latanich R., Salgado M., Pohlmeyer C.W., Nobles C.L., Gregg J., Scully E.P., Bailey J.R. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight. 2018;3:3. doi: 10.1172/jci.insight.122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey J.R., O’Connell K., Yang H.-C., Han Y., Xu J., Jilek B., Williams T.M., Ray S.C., Siliciano R.F., Blankson J.N. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J. Virol. 2008;82:7395–7410. doi: 10.1128/JVI.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


