Abstract
Clear-cell renal cell carcinoma (ccRCC) is the most common histological type of RCC. To investigate the intratumoral heterogeneity of ccRCC, we analyzed single-cell RNA-sequencing data and identified 15 major cell types, along with 39 subgroups of cells derived from tumor or non-malignant tissues, and confirmed their presence by immunofluorescence staining in tissue chips. In this study, we verified that T cell exhaustion was the key factor responsible for the immunosuppressive property of ccRCC tissues, which was significantly related to poor prognosis. We also found that abnormal metabolic patterns occurred not only in cancer cells, but also in tumor-infiltrating stromal cells. Based on the fraction of each cell cluster detected by CIBERSORTx, 533 patients from The Cancer Genome Atlas (TCGA) KIRC dataset were divided into three groups. One group, which showed a lesser proportion of activated CD8+ cells and greater proportion of exhausted CD8+ cells, was associated with a poor prognosis. Hence, the blockade of immunosuppressive checkpoints, not only PD-1, but also LAG3, TIM-3, and other inhibitory checkpoints, could serve as a potential target for ccRCC immunotherapy. Our work will further the understanding of the heterogeneity among ccRCC tissues and provide novel strategies for treating ccRCC.
Keywords: single-cell sequencing, bioinformatics, clear-cell renal cell carcinoma, heterogeneity, tumor microenvironment, T cell exhaustion
Graphical Abstract

Via single-cell transcriptome analysis, we investigated the intra-tumoral heterogeneity of clear-cell renal cell carcinoma (ccRCC) and identified the composition inside tumor tissues. With a computational pipeline, we revealed the clinical significance of tumor-infiltrating cells and provided novel strategies for treating ccRCC.
Introduction
Clear-cell renal cell carcinoma (ccRCC) is the most common histological type of RCC; it causes more than 175,000 deaths every year worldwide.1 About 30%–35% of patients who have undergone surgery have been reported to show distant metastasis. However, almost all adjuvant agents for ccRCC patients have shown no benefits in clinical trials thus far. Only two agents, including renal tumor cell vaccine and sunitinib, which is a type of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI), have shown potential benefits against ccRCC.2 Nevertheless, a considerable number of patients acquire resistance to sunitinib a few years after the adjuvant treatment. Although treatment with anti-PD-1 antibodies has been reported to show a higher overall response rate (ORR) than with TKIs in previous clinical trials, the ORR is still only 37%–58%.3,4 Since then, the discovery of drugs for adjuvant therapy against ccRCC remains an arduous task.
Extensive heterogeneity is an important feature of tumors; it may result in divergent responses to the same treatment among patients. Although a lot of efforts have been dedicated toward elucidating tumor heterogeneity, thus far its understanding is still mainly limited to tumor cells.5 Recently, it has been reported that stromal cells, including tumor-infiltrating immune cells, also demonstrate heterogeneity.6 Moreover, the tumor microenvironment (TME) is increasingly being considered as a target for drug treatment strategies.7 Besides strategies such as anti-PD-1/PD-L1 or anti-CTLA-4 treatments, tumor-associated macrophages (TAMs)8 and cancer-associated fibroblasts (CAFs)9 have also been reported as potential targets for cancer therapy in previous studies. These treatment strategies acquire a deeper understanding of intratumoral heterogeneity.
Since the conventional bulk RNA-sequencing is performed based on the hypothesis that every gene is expressed equally in every cell, it is obviously impossible to investigate intratumoral heterogeneity at the cell-type level. However, the advent of single-cell RNA-sequencing (scRNA-seq) has made it possible to perform transcriptome profiling at a single-cell level. Since then, it seems necessary to perform scRNA-seq, which could disclose the complete TME heterogeneity. Young et al.10 have performed scRNA-seq on 21 samples, including 12 tumor and 9 para-tumor samples. In order to investigate the intratumoral heterogeneity in ccRCC, we performed further bioinformatics analysis using their data, identifying several potential targets for ccRCC therapy. Furthermore, we also investigated the prognostic value of different cell clusters by correlating the scRNA-seq data with the data from TCGA KIRC dataset. Our work will help elucidate the biology of ccRCC and promote the improvement of clinical treatment strategies for ccRCC patients.
Results
Cell Typing of the ccRCC and Paired Para-Tumor Tissues
A total of 21 samples from three ccRCC patients that underwent radical nephrectomy were involved in this study. The quality control (QC) criteria are described in Materials and Methods. Of these, 7,786 single cells originated from normal tissues, while 16,764 were tumor-derived cells (Figure 1B). After removing the batch effects and regressing out the influence of the number of unique molecular identifiers (UMIs) and percentage of mitochondrion-derived UMI counts, 24,550 cells finally passed the quality filtering, in which at least 200 UMIs were detected. These cells were classified into 15 main cell lineages. In addition to cancer cells, we identified nine immune cell lineages (CD45+), including natural killer (NK) cells, CD8+ T cells, CD4+ T cells, regulatory T cells (Tregs), B cells, macrophages, neutrophils, dendritic cells (DCs), and mast cells, along with five non-immune cell lineages (CD45−), including renal tubular cells, collecting duct cells, fibroblasts, endothelial cells, and erythroblasts (Figures 1A and 1C). Known markers (Figure S1) mentioned in the CellMarker database and a previous study by Lake et al.11 were used for this recognition process (refer to Materials and Methods). One cell cluster, which was mainly recognized because of differences in the cell cycle, was not involved in the downstream analysis (Figure S2A). The top five markers of the main cell lineages were visualized as a bubble chart (Figure 1F).
Figure 1.
Overview of Single Cells Derived from ccRCC and Non-malignant Tissues
(A–C) UMAP plot of all the single cells, with each color coded for (A) 15 major cell types, (B) sample origin (normal or tumor), and (C) immune cell (CD45+) or non-immune cell (CD45−). (D) Expression level of four normal tissue-specific genes and immunofluorescence confirmation in tissue chips. Scale bar represents 50 μm. (E) For 39 subgroups identified in this profile (left to right): the fraction of cells that originated from the 9 non-malignant and 15 tumor samples, and the fraction of cells that originated from each of the three patients. (F) Top five marker genes of 15 major cell types identified in this profile.
To identify the cell subclusters of these major cell types, the immune cells and non-immune cells were clustered separately (Figures S2B and S2C). In total, we identified 39 different cell clusters, consisting of 21 non-immune cell clusters and 18 immune cell clusters in the TME in ccRCC. Since macrophage cluster 5 only contained less than 30 cells, this cluster was not included in the subsequent analyses. Interestingly, when the normal tissue- and tumor-derived cells were compared, we noticed that immune cells, especially, CD4+FOXP3+ Tregs, which occur almost only in tumor tissues, were highly enriched in tumor tissues. In addition, we noticed that tumor-derived CD8+ T cells are highly tumor specific. Furthermore, fibroblasts and mast cells also mainly existed in tumor tissues. We also noticed that several cell clusters were enriched in both the ccRCC tumor and para-tumor tissues (Figures 1B, 1C, and 1E).
When screening for differentially expressed genes (DEGs) in the tumor and normal tissues, we observed that four genes (ALDOB, MIOX, GPX3, and MT1G) are especially expressed in normal tissues, and were almost absent in the tumor-derived cells (Figure 1D). These genes were not only expressed in renal tubular cells, but also in normal tissue-derived stromal cells. In order to confirm this phenomenon at the protein level, we performed an immunofluorescence assay using tissue chips. As shown in Figure 1D, the fluorescent signal existed almost only in the tumor-derived tissues. These results revealed that the loss of ALDOB, MT1G, GPX3, and MIOX may be new alarm signals for ccRCC.
ALDOB is a member of the aldolase family of proteins; it catalyzes the reversible conversion of fructose-1,6-bisphosphate to glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. It is a tissue-specific gene and is only expressed in the liver, kidney, and intestine in healthy adults. Since aldolase is an important enzyme for glycolysis, and ALDOB was almost eliminated in ccRCC tissues, we hypothesized that the expression of the other two members of the aldolase family may also be altered in ccRCC. Thus, we performed an immunofluorescence assay for detecting these two proteins using tissue chips. Interestingly, we found that ALDOA and ALDOC were both upregulated in cancer cells, and that ALDOC was expressed almost only in cancer cells (Figure S2D). These results disclosed that compared to normal renal tubular cells, cancer cells may employ a unique glycolysis pattern. This result was consistent with that of a previous study, which stated that the metabolic mode in renal cancer cells is highly abnormal.12
Macrophages Occupy an Important Position and Exhibit M2 Polarization in the TME in ccRCC
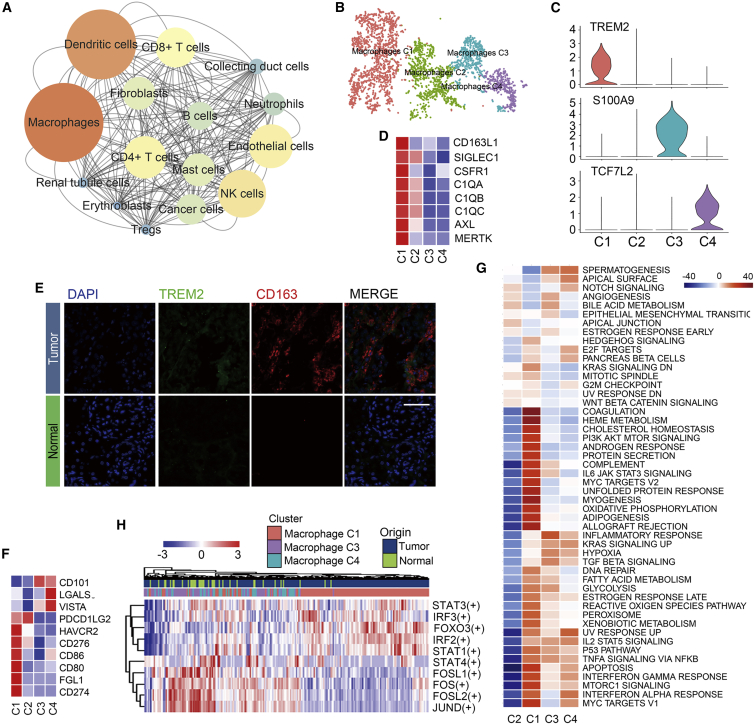
To investigate the interaction network in the TME in ccRCC, we used CellphoneDB, a Python-based tool for analyzing cell-cell communication molecules, to calculate potential ligand-receptor pairs in the TME in ccRCC. Network visualization was performed using Cytoscape. Notably, we found that macrophages possessed the most interaction pairs with cells from other lineages (Figure 2A), revealing the dominant role of macrophages in the TME.
Figure 2.
Macrophage Emerge M2 Polarization in ccRCC
(A) Interaction network constructed by CellPhoneDB; size and color of circles represent interaction counts, brighter color and larger size means more interaction with other cell types. (B) tSNE plot of four subclusters of macrophages. (C) Violin plots of marker genes for three subgroups; cluster 2 emerged as a non-specific marker. (D) Heatmap of already known marker of M2-like TAMs. Mean expression level of each cell cluster was transformed into a row Z score. (E) Heatmap of positive immune checkpoint expression on macrophages. The row Z score was implicated to represent the expression level. (F) Heatmap of the area under the curve (AUC) scores of expression regulation by transcription factors estimated by SCENIC. (G) Differences in 50 hallmark pathway activities scored with GSVA software. Shown are t values calculated by a linear model. (H) Immunofluorescence staining of CD163 and TREM2 in ccRCC tissue chip. CD163+TREM2+ macrophages only emerged in tumor tissues. Scale bar represents 50 μm.
For discussing the heterogeneity among macrophages, 3,698 macrophages were clustered into four subclusters (Figure 2B). As shown in Figure 2C, cells from cluster 1 exhibited the CD68+CD163+TREM2+ phenotype. New markers of TAMs described in recent studies were also highly expressed in cluster 1 (Figure 2D). VEGFA was also expressed in cells from this cluster. In previous studies, it has been reported that macrophages secreting VEGFA promote tumor growth. All of this evidence certified that cluster 1 represented an M2-like TAM cluster. The genes S100A8, S100A9, and S100A12, which encode calcium-binding proteins, were expressed by the cells in cluster 3 (Figure 2C). Release of these proteins by activated mononuclear cells has been shown to promote inflammatory responses in vivo,13 which indicated that cluster 3 may play pro-inflammatory and anti-tumor roles in ccRCC. Cluster 4 represented a CD68+CD163− subgroup. Two transcription factors (TFs), TCF7L2 and HES1, were preferentially expressed in macrophages from cluster 4 (Figure 2C; Figure S3D). In a previous study, TCF7L2 has been shown to participate in the differentiation of macrophages,14 while HES1 acts as a transcriptional repressor that attenuates inflammatory responses.15 We failed to identify markers of cells from cluster 2, and thus these were considered low-quality cells and excluded from the subsequent analyses; however, the biological significance of these cells cannot be ignored.
In order to prove the presence of cells from cluster 1, we performed immunofluorescence staining. As shown in Figure 2E, CD163+TREM2+ macrophages were detected in ccRCC tissues. scRNA-seq revealed that TREM2 was almost only expressed in macrophages from cluster 1 (Figure 2E). Thus, we calculated the Spearman correlation coefficients between TREM2 and other known markers of M2-like TAMs in the case of the patients from TCGA KIRC cohort; all of these Spearman correlation coefficients were found to be greater than 0.5 (Figure S3E). Collectively, we concluded that cluster 1 was a real subgroup of macrophages in the TME in ccRCC.
Next, we examined the distribution of immune checkpoints in the TME in ccRCC. As shown in Figure 2F, compared to other clusters, the expression of CD274 (PD-L1) and PDCD1LG2 (PD-L2) was relatively higher in cluster 1. These molecules have been reported to be able to inhibit the activity of CD8+ T cells by binding to their receptor, PD-1, on the surface of CD8+ T cells. Moreover, FGL1, the major ligand of LAG3, which has been reported by Wang et al.,16 was only expressed in cells from cluster 1. Since all of these immunosuppressive ligands could suppress cytotoxic T lymphocyte (CTL) function, this evidence proved the enhanced immunosuppressive properties of macrophages from cluster 1. Pathway analysis by gene set variation analysis (GSVA) revealed that the complement and interferon (IFN) pathways were enriched in case of cells from cluster 1, while the inflammatory response was significantly suppressed (Figure 2G), which are the hallmarks of M2-like TAMs, as described in a previous study.17 SCENIC analysis revealed that the genes regulated by IRF2, IRF3, STAT1, STAT3, and FOXO3 were upregulated, whereas the STAT4, FOS, FOSL1, FOSL2, and JUND regulons were downregulated in case of macrophages from cluster 1 (Figure 2H). Hitherto, it has been reported that IRF2 plays a immunosuppressive role, while Fos/Jun is able to enhance inflammatory responses in macrophages.18,19 Furthermore, the IRF3/STAT1 pathway has been reported to be associated with M2 polarization in a murine model of sarcoma.17 All of these data supported the M2 polarization phenomenon in ccRCC, and they revealed the potential mechanisms and candidate TFs involved in this process.
CD8+ T Cells Tend to Be Exhausted in the TME in ccRCC
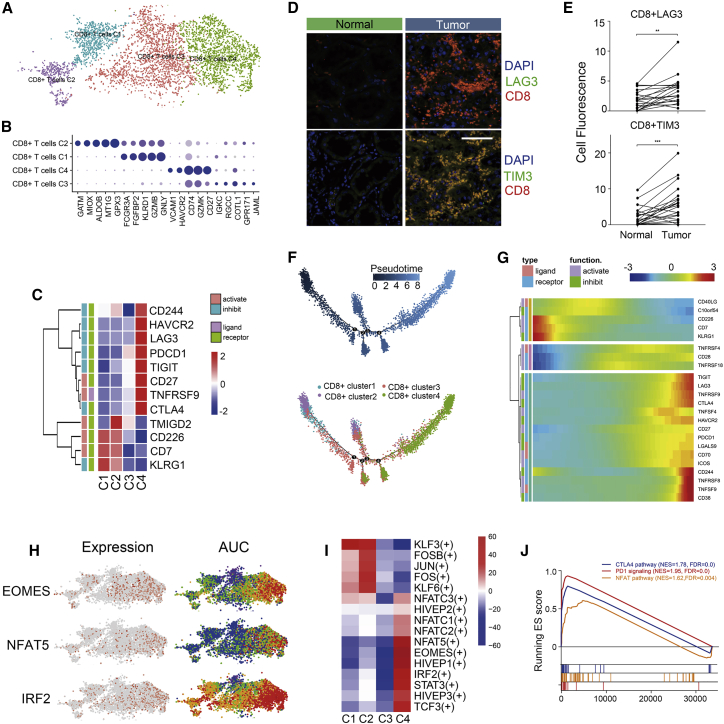
Among the 5,108 cells detected, CD8+ T cells were found to be the most prevalent cell type in the TME in ccRCC. These cells were then clustered into four subgroups. As shown in Figures 1E, 3A, and 3B, cells from cluster 2 (GATM+) were derived from normal kidney tissues, while those from cluster 1 (GNLYhiFCGR3Ahi), cluster 3 (COTL1hi), and cluster 4 (LAG3+HAVCR2+CD27hi) were almost entirely derived from tumor tissues.
Figure 3.
CD8+ T Cells Tend to Be Exhausted in Renal and ccRCC Tissues
(A) tSNE plot of four subgroups of CD8+ T cells. (B) Dotplot of top five markers of each cell cluster; sizes of dots represent abundance, while color represents expression level. (C) Heatmap of immune checkpoints upregulated or downregulated in exhausted T cells. A row Z score was used to represent the expression level. (D) Immunofluorescence of exhausted T cells in tissue chips. CD8+LAG3+ (top) and CD8+TIM3+ (bottom) T cells were enriched in tumor tissues. Scale bar represents 50 μm. (E) Cell fluorescence score of CD8+LAG3+ and CD8+TIM3+ cells was measured by ImageJ. A Student’s t test was employed to recognize the difference between tumor and non-malignant tissues. ∗∗p < 0.01, ∗∗∗p < 0.001. (F) Differentiation trajectory of CD8+ T cells in ccRCC, with each color coded for pseudotime (top) and clusters (bottom). (G) Pseudo-heatmap of immune checkpoints altered in the differentiation process of CD8+ T cells in ccRCC, which was clustered into three clusters. (H) tSNE plot of CD8+ T cells, color coded for the expression level (left) and for the AUC of the estimated regulon activity of these transcription factors (right). (I) Differences of AUC scores of expression regulation by transcription factors estimated by SCENIC. Shown are t values calculated by a linear model. (J) GSEA reveals three pathways enriched in exhausted T cells. FDR <0.01 was considered as significantly enriched.
Subsequently, we examined the immune checkpoints in the cell clusters (Figure 3C). Notably, we found that inhibitory checkpoints, including HAVCR2 (TIM3), LAG3, PDCD1 (PD-1), TIGIT, CD27, CTLA-4, and TNFRSF9, were upregulated in cells from cluster 4. Since these molecules are markers of T cell exhaustion, these data indicated that cells from cluster 4 were exhausted in the TME. To verify this phenomenon, we performed immunofluorescence using tissue chips. As shown in Figures 3D, S4C, and S4D, CD8+ T cells were highly enriched in tumor tissues. Additionally, LAG3 and HAVCR2 were found to be highly expressed in most tumor-infiltrating CD8+ T cells (Figures 3D and 3E). At present, PD-1/PD-L1 and CTLA-4 are the most popular targets of immunotherapy, despite that less than half of the patients suffering from solid tumors benefit from this strategy. Since the expression of LAG3 and HAVCR2 was much higher than that of PD-1 in this exhausted T cell subgroup, our data revealed that LAG3 and HAVCR2 may be better targets for immune therapy in ccRCC.
Although T cell exhaustion has been observed in previous studies, a continuous trajectory has not yet been reported in this regard. Hence, we utilized Monocle 2 for performing pseudotime analysis to infer the co-expression modules. The trajectory was visualized as a t-Distributed Stochastic Neighbor Embedding (tSNE) plot (Figure 3F). Strikingly, we noticed that normal tissue-derived CD8+ T cells from cluster 2 tended to convert into tumor-infiltrating T cell subgroups. In addition, cluster 4, which represented an exhausted T cell cluster, was present at the end of the differentiation trajectory (Figure 3F). In this process, T cell exhaustion-related immune checkpoints (PD-1, CTLA-4, LAG3, TIM3, TNFRSF9, TIGIT) tended to be upregulated, while synergist checkpoints (CD226, CD7) tended to be downregulated (Figure 1G). A total of 468 genes fulfilled the criteria for being considered DEGs (refer to Materials and Methods) were clustered into three modules; these genes were mainly expressed in three different CD8+ T cell subgroups (Figure S4A). Enrichment analysis using Metascape revealed that histone modification, ubiquitin-mediated proteolysis, and cell adhesion pathways were highly enriched in exhausted CD8+ T cells. It was also observed that cytokine production and T cell activation occurred at an earlier stage of ccRCC. These data revealed that CD8+ T cells were activated in the early stage of ccRCC. Following continuous antigen stimulation, activated CD8+ T cells were exhausted; DNA methylation may play an important role in this process. TOX, a critical regulator of tumor-specific T cell differentiation,20 has also been shown to be continuously upregulated in this process (Figure S4B).
SCENIC analysis was performed to determine the alterations of TFs in the process of ccRCC-specific T cell dysfunction (Figures 3H and 3I). Four members of the NFAT family were significantly activated in exhausted CD8+ T cells, while AP-1 (JUN, FOS, FOSB) function was repressed. These data confirmed that NFAT could induce the upregulation of dysfunction-related genes in the absence of AP-1.21 They also revealed that NFAT5 may be the major regulator in ccRCC, while NFAT1 has been shown to play a key role in a murine infection model.21 The gene set enrichment analysis (GSEA) also revealed that the NFAT pathway was enriched in the case of the cells from cluster 4 (Figure 3J). Genes regulated by eomesodermin (EOMES), a TF involved in the regulation of the “terminal” differentiation of T cells, along with the immunosuppression-related TF IRF2, was also upregulated in cells from cluster 4 (Figure 3H). Moreover, genes regulated by STAT3 were also found to be upregulated. Collectively, three out of four modules related to T cell dysfunction that have been reported in a previous review were observed in our scRNA-seq data.22 For the first time, we noticed that the transcriptional activity of genes regulated by members of the HIVEP family was upregulated in exhausted CD8+ T cells. These data provide clues for the identification of new candidate TFs involved in ccRCC-specific T cell dysfunction.
Metabolism Is Extremely Abnormal in ccRCC Cancer Cells
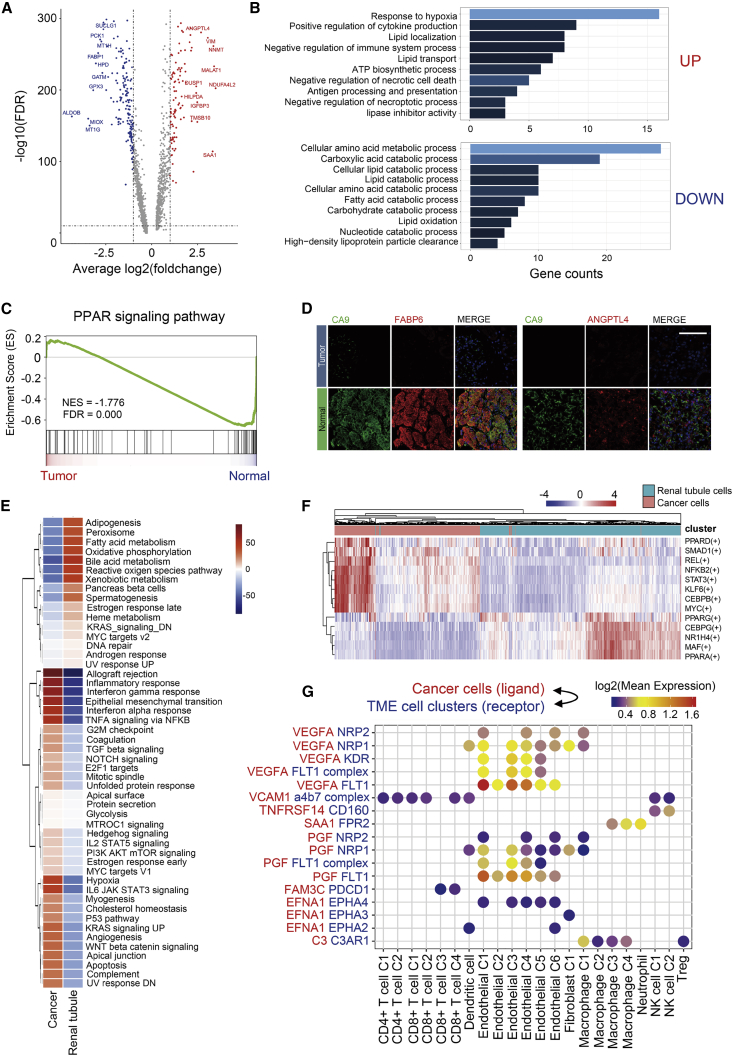
Since ccRCC originated from the renal tubular epithelium, we identified the DEGs among the cancer cells and renal tubular cells. Compared to conventional bulk RNA-seq, scRNA-seq was able to eliminate the differences resulting from the TME; thus, the DEGs exclusively derived from cancer cells could be identified. Strikingly, the top 10 DEGs, either upregulated or downregulated, were mostly related to metabolic processes (Figure 4A). Hence, we performed Gene Ontology (GO) enrichment analysis of the upregulated and downregulated DEGs. As shown in Figure 4B, hypoxia response, lipid biosynthesis, and localization pathways were enriched in cancer cells, while lipid catabolism was suppressed. These data corresponded to the abnormal accumulation of lipid droplets in ccRCC tissues and cell lines.23 In addition, antigen presentation, cytokine production, and immunosuppression pathways were also upregulated in cancers; this may partly contribute to the immunosuppressive property of the TME in ccRCC. Furthermore, amino acid catabolism and carbohydrate catabolism were also repressed in cancer cells.
Figure 4.
Alteration of PPAR Pathway Is an Important Characteristic of ccRCC Metabolic Abnormality
(A) Volcano plot of differentially expressed genes (DEGs) between cancer cells and normal renal tubular epithelium. Upregulated genes (FC >2) were colored in red while downregulated genes (FC less than −2) were colored in blue. Symbols of top 10 upregulated and downregulated genes were annotated, respectively. (B) Gene Ontology analysis of DEGs; upregulated and downregulated DEGs were annotated, respectively. FDR <0.05 was considered as significantly enriched. Brighter color represents a smaller FDR value. (C) GSEA revealed that the PPAR pathway was significantly enriched in normal tubular epithelium. (D) Immunofluorescence staining of ccRCC tissue chips. ANGPTL4 and FABP6 were co-stained with CA9, respectively. Scale bar represents 50 μm. (E) Heatmap of differences in activities of 50 hallmark pathways scored by GSVA. Shown are t values calculated by a linear model. (F) Heatmap of AUC scores of selected regulons altered in cancer cells. AUC scores were measured by SCENIC per cell. (G) Ligand-receptor interaction between cancer cells and TME-infiltrated cell clusters detected by CellPhoneDB 2. Selected ligand-receptor pairs are shown in the bubble plot.
As shown in Figure 4C, the GSEA revealed that the PPAR pathway was enriched in renal tubular cells. In our previous study, the activation of the PPAR pathway was observed to occur via the overexpression of PLCL1, which promoted lipid consumption, thus inhibiting tumor growth, invasion, and migration. Notably, although the PPAR pathway was repressed in ccRCC, three members of the PPAR pathway, including ANGPTL4, FABP6, and FABP7, were upregulated in cancer cells (Figures S5A and S5B). Since almost all members of the FABP family participate in the PPAR pathway, we further investigated their expression in ccRCC-derived cell types. Strikingly, FABP6 and FABP7 were especially expressed in cancer cells, while FABP1 was only expressed in the renal tubular epithelium (Figures S5A and S5B). Moreover, ANGPTL4, FABP6, and FABP7 have been reported to show cancer type-specific expression in samples from TCGA database (Figure S5C). These data indicated the distinctive role of the PPAR pathway in ccRCC.
Hitherto, ccRCC has remained a cancer type for which not many biomarkers have been identified. Because ANGPTL4 and FABP6 were especially expressed in ccRCC cells, these genes may be new biomarkers for ccRCC cells. To confirm their expression at the protein level, we performed immunofluorescence assays using tissue chips. As shown in Figures 4D and S5D, ANGPTL4, FABP6, and FABP7 were co-expressed with a conventional marker of ccRCC, carbonic anhydrase IX (CA9), in cancer cells, but they were almost absent in normal tissues. Thus, we demonstrate that ANGPTL4, FABP6, and FABP7 could be candidate markers for ccRCC.
The SCENIC analysis disclosed the abnormal transcriptional regulation network in ccRCC tumor cells. Notably, genes regulated by PPARα and PPARγ were downregulated, while the PPARδ regulon was upregulated (Figure 4F). These data indicated that PPARδ signaling may be related to the upregulation of ANGPTL4, FABP6, and FABP7 and participate in abnormal lipid accumulation. Genes regulated by other lipid homeostasis-associated TFs, including CEBPB and KLF6,24,25 were upregulated in cancer cells, while CEBPG was downregulated. In addition, genes regulated by NR1H4, which participates in autophagy, were downregulated in cancer cells. These data reveal the presence of new regulatory networks driven by TFs, and they help understand the mechanisms underlying the abnormal accumulation of lipids in cancer cells. In addition, both the SCENIC and GSVA analyses demonstrated that the STAT3 pathway was upregulated in cancer cells. Since STAT3 also participates in T cell exhaustion and M2 polarization of macrophages, in the present study, we hypothesized that STAT3 inhibition may be a new treatment strategy for ccRCC.
Finally, we utilized CellphoneDB to investigate the interactions between cancer cells and cell subgroups in the TME. As shown in Figure 4G, VEGFA, PGF, and EFNA1 secreted by cancer cells interact with receptors expressed on endothelial cells, fibroblasts, and TAMs. These ligand-receptor pairs may be related to angiogenesis and CAF proliferation in the TME. In addition, the adhesion molecule VCAM1 is related to T cell adhesion. C3 secreted by cancer cells interacts with C3AR1, which is expressed in macrophages. In a recent study, it has been proven that C3 secreted by cancer cells is associated with TAM infiltration and tumor growth in a ccRCC murine model.26
Myofibroblasts Are Enriched in ccRCC and Exhibit Heterogeneity
As shown in Figure 5A, most fibroblasts were derived from tumor tissues. Almost all fibroblasts were positive for α-SMA (Figure 5B), which is a conventional marker of myofibroblasts. These myofibroblasts were clustered into subtype 3 (cluster 1, PDGFRB+FABP5+CEBPB+; cluster 3, NNMT+IL32+COL1A1+; cluster 2, no marker) (Figure 5C). Interestingly, as shown in Figure 5C, FABP5 was expressed almost only in fibroblasts. In addition, CEBPB was also found to be related to lipid metabolism. These data revealed that abnormal lipid metabolism may not only occur in cancer cells, but also in tumor-associated fibroblasts. Hence, we performed immunofluorescence staining of ACTA2 and FABP5 to confirm this phenotype. Consistently, FABP5 was almost only detected in tumor-derived fibroblasts, while it was almost absent in normal kidney tissues (Figure 5C).
Figure 5.
Clusters of Fibroblasts and Endothelial Cells in ccRCC
(A and B) tSNE plot of three fibroblast clusters (A) and the expression level of α-SMA (ACTA2) (B). (C) Marker genes of markers of clusters 1 and 3. Cluster 2 showed no specific marker. (D) Immunofluorescence staining of FABP5 and ACTA2 in tissue chip. ACTA2+FABP5+ fibroblasts only occur in ccRCC tissues, while they are almost absent in normal kidney. Scale bar represents 50 μm. (E) Heatmap of AUC scores of selected regulons altered in cancer cells. AUC scores were measured by SCENIC per cell. (F) tSNE plot of endothelial cells color coded for six subgroups of endothelial cells from normal or tumor tissues (left) and sample origins (normal or tumor) (right). (G) Cluster-specific markers of endothelial cells. Cluster 2 has no specific marker. (H) Venn diagram of endothelial-specific markers and DEGs between tumor and normal derived endothelial cells. 14 overlapped genes were recognized (left). Expression levels of VWF and ENPP2 are visualized into violin plots (right). (I) Immunofluorescence staining of ccRCC tissue chips. ENPP2 and VWF were co-stained. Scale bar represents 50 μm. (J) Differences in 50 hallmark pathway activities scored with GSVA software. Shown are t values calculated by a linear model.
The SCENIC analysis revealed that genes regulated by CEBPB, CDBPD, and KLF6, which are associated with lipid accumulation, were upregulated in cells from cluster 1 (Figure 5E). Previous studies on ccRCC have mainly focused on the abnormal metabolic patterns in cancer cells. For the first time, our data indicated that lipid metabolism in tumor-associated fibroblasts also showed anomalies. In addition, the transcriptional activity of AP-1 (JUN, JUNB, JUND, and FOS) was upregulated in cells from cluster 1. Notably, the genes regulated by ZXDC were upregulated in cells from cluster 3. Since ZXDC is a TF related to antigen presentation, cells from cluster 3 may exhibit an antigen-presenting phenotype, as described in a recent study by droplet-based scRNA-seq.27
Tumor-Derived Endothelial Cells Exhibit Two Different Phenotypes in ccRCC
A total of 3,048 endothelial cells derived from tumor or non-malignant tissues were detected in the present study. These cells were categorized into six different clusters. Subsequently, we attempted to identify the markers of each cluster, revealing that almost all endothelial cells were blood endothelial cells (FLT1+, Figure S6C). Three clusters were mostly derived from normal tissues (clusters 3, 4, and 5, SOST+, CCL23+, and SERPINE2+, respectively), while cluster 1 (KCNE3+) and cluster 6 (ACKR1+) were enriched in tumor tissues (Figures 5F and 5G). The ACKR1+ endothelial subgroup has also been discovered in the TME in lung cancer.28 Each cluster was detected in different patient-derived samples (Figure 1E). Since no markers were identified in cells from cluster 2, these cells were considered low-quality cells and excluded from further analyses; however, their biological function cannot be ignored.
To identify tumor-specific markers for endothelial cells, we overlapped the upregulated genes in tumor-derived endothelial cells and the genes preferentially expressed in the endothelial cells; we obtained 14 genes, among which VWF and ENPP2 displayed the best specificity (Figure 5H; Figures S6A and S6B). Next, we performed immunofluorescence analysis to confirm the presence of these cells. As shown in Figure 5I, VWF+ENPP2+ cells emerge almost only in tumor tissues. VWF is a marker of endothelial activation and is upregulated in most cancer types, especially ccRCC (Figure S6D). ENPP2 is also especially expressed in ccRCC, and highly correlated with VWF in the case of patients from TCGA KIRC cohort (Figure S6D). Hence, ENPP2 may serve as a ccRCC-specific marker for tumor-derived endothelial cells.
The GSVA pathway analysis highlighted that two tumor-derived endothelial cell clusters were quite different (Figure 5j). Remarkably, MYC target signatures were highly enriched in the case of cells from cluster 6, which indicates that the cells from this cluster showed a high proliferation phenotype. Interestingly, the inflammation response was significantly higher in the case of cells from cluster 6. Since ACKR1, which is a non-specific receptor for various chemokines and is associated with lymphocyte chemotaxis, was highly expressed in cells from cluster 6, this cluster may participate in maintaining the immunosuppressive infiltration of cells in ccRCC. This could explain why the occurrence of endothelial cells from cluster 6 was related to bad prognosis in the case of patients from TCGA KIRC cohort. Instead, the inflammatory response was repressed in the case of cells from cluster 1, while the WNT-β-catenin, hedgehog, and NOTCH signaling signatures were enriched. However, the biological significance of this occurrence remains unknown. Notably, the KRAS signaling and angiogenesis pathways were enriched in the cells from both cluster 1 and 6. This may be the response to hypoxia in tumors.29
Infiltration of Tumor-Educated Immune Cells Is Associated with a Worse Prognosis in ccRCC
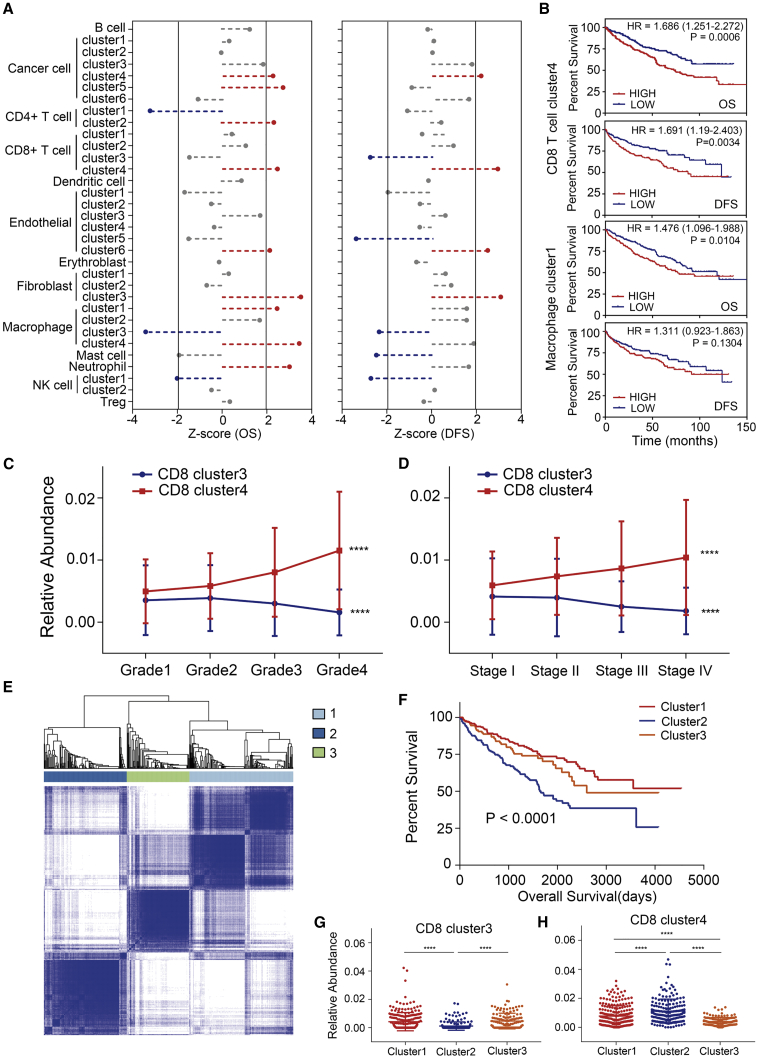
Finally, we evaluated the relative abundance of the cell subgroups in patients from TCGA KIRC cohort by digital flow cytometry (refer to Materials and Methods) to investigate the clinical value of the clusters detected in our present study (Figure S7A). As shown in Figures 6A and 6B, four clusters (cancer cell cluster 4, CD8+ T cell cluster 4, endothelial cell cluster 6, fibroblast cluster 3) were associated with poor overall survival (OS) and disease-free survival (DFS) (p < 0.05); these were mostly tumor-derived cell clusters. In addition, macrophage cluster 3 and NK cell cluster 1 were associated with good outcomes, which demonstrated that M1-like macrophages and NK cells with normal functions could play an anti-tumor role in the TME in ccRCC. Interestingly, mast cells were associated with a better DFS (p = 0.013), which implied that mast cells may participate in the anti-tumor process in ccRCC via unknown mechanisms. Furthermore, M2-like macrophage cluster 1 was associated with a poor OS (p = 0.014).
Figure 6.
Prognostic Role of Cell Clusters Identified by scRNA-Seq in TCGA KIRC Cohort
(A) Association between relative abundance of cell clusters calculated by CIBERSORTx and overall survival (left) and disease-free survival (right). Z score was measured with a Cox regression model in R. Clusters associated with bad outcome (p < 0.05) are colored in red while clusters associated with good outcome (p < 0.05) are colored in blue. (B) Kaplan-Meier survival curve for patients in TCGA KIRC. Hazard ratios (HRs), with their 95% confidence intervals in brackets, are shown. A log rank p value <0.05 was considered as statistically significant. (C and D) Line charts show CD8+ cluster 3 enriched in low-grade (C) and early-stage (D) ccRCC tissues, while cluster 4 shows opposite properties. ∗∗∗∗p < 0.0001, measured with one-way ANOVA test. (E) ccRCC patients in TCGA KIRC were clustered into 3 subgroups by ConsensusClusterPlus based on cell clusters identified in this profile. (F) Kaplan-Meier survival curve of three patient subgroups. Log rank p value was calculated in GraphPad Prism 7. (G and H) Relative abundance of CD8+ T cell clusters 3 (G) and 4 (H) in three patient groups. ∗∗∗∗p < 0.0001, calculated with Tukey’s multiple comparison test.
Notably, the proportion of the CD8+ T cell cluster 3 decreased in higher-grade or later-stage tumor samples, while that of cluster 4 increased (Figures 6C and 6D). This demonstrated that T cell exhaustion was related to the progression of ccRCC.
To further investigate the clinical value of immune infiltration, the 533 ccRCC patients were divided into three different immune subtypes using ConsensusClusterPlus30 (Figure 6E). Compared to the patients from clusters 1 and 3, the patients in cluster 2 showed a significantly worse outcome (Figure 6F). Interestingly, we noticed that CD8+ T cell clusters 3 and 4 showed the most significant difference, as the proportion of the CD8+ cluster 3 decreased, while the CD8+ cluster 4 was enriched in cluster 3 patients (Figures 6G and 6H). These data further demonstrated the key role of T cell exhaustion in ccRCC progression. Thus, the blockade of immune checkpoints participating in this process may serve as a favorable target for ccRCC treatment and drug discovery.
Discussion
Currently, the treatment of ccRCC, especially metastatic ccRCC, after surgery is still a challenge. Although a few patients may benefit from TKI and anti-PD-1 antibody treatment, low efficiency and drug resistance are notable problems that remain to be dealt with. A recent study has revealed that not only cancer cells, but also tumor-infiltrating cells, participate in the process of drug resistance or the lack/absence of the response to cancer treatment strategies.31 However, only little is known about the mechanisms underlying these phenomena. Hence, more attention should be paid toward investigating the intratumoral heterogeneity in cancers. In the present study, we investigated the TME of ccRCC via scRNA-seq, revealed a comprehensive catalog of immune and stromal cells within ccRCC tissues, and verified the presence of important cell subgroups at the protein level by immunofluorescence technology. In the present work, we identified novel cell subgroups and altered pathways, along with novel regulatory networks driven by TFs in ccRCC. These results will aid the understanding of the intratumoral heterogeneity in ccRCC and provide novel targets for cancer therapy.
Because the TME in ccRCC is highly complex, it is impossible to completely describe intratumoral heterogeneity. However, we have noted several key observations. First, M2-like TAMs exhibit the CD68+CD163+TREM2+ phenotype, while another CD68+CD163+TREM2− subgroup showed a pro-inflammatory role similar to M1 macrophages. Notably, the TREM2+ subgroup was associated with bad outcomes in the case of patients from TCGA KIRC cohort, while the TREM2− subgroup showed an opposite association. Thus, the conventional marker of M2-like TAMs, CD68+CD163+, was not efficient in identifying pro-tumoral macrophage subgroups. A similar phenomenon has also been observed in the case of breast cancer.32 By immune checkpoint and pathway analysis, we revealed that M2-like TAMs play an immunosuppressive role in the TME. In addition, several TFs related to the immunosuppressive property were identified via SCENIC analysis. These results provided new targets for immunotherapy.
Second, we revealed that CD8+ T cells were highly exhausted in ccRCC; this was associated with bad clinical outcomes. This could account for why CD8+ T cell infiltration was associated with a worse prognosis in a previous study.33 By pseudotime analysis, we obtained the first differentiation trajectory of T cell dysfunction in ccRCC and disclosed the pathways participating in the different stages of this process. Blockade of these pathways may interrupt T cell dysfunction and cause the anti-tumor activity of CD8+ T cells to resume. This represents a new strategy for immunotherapy against ccRCC. In addition, we discovered novel TF alterations that may underlie T cell exhaustion. These findings will help us further understand this pathological process.
Third, abnormal lipid accumulation has been observed in previous studies, but the mechanism underlying this process remains unclear. Our analysis revealed an important role of the PPAR pathway in the lipid accumulation within cancer cells; this has rarely been discussed previously. In addition, several proteins, including ANGPTL4, FABP6, and FABP7, may be efficient markers for ccRCC and have an important diagnostic value and clinical implications with regard to ccRCC patients. Notably, abnormal metabolic patterns occur not only in cancer cells, but also in fibroblasts. Future research on ccRCC metabolism should not only focus on cancer cells, but also other stromal cell types.
Recognition of the subtypes of ccRCC has always been based on different gene expression patterns, as detected by bulk RNA-seq or microarray technology. For the first time, based on the fractions of cell subgroups identified in the present study, the 533 patients of TCGA KIRC cohort were divided into three different clusters. Significantly, the patients in cluster 2 exhibited worse clinical outcomes. Since cluster 2 was associated with a low proportion of activated CD8+ T cells, but exhausted T cells were enriched in this cluster, the significance of T cell exhaustion in ccRCC was further confirmed. We are planning to verify these results using a larger clinical cohort.
Finally, cell type-specific gene expression patterns revealed in this study will promote the understanding of the intratumoral heterogeneity and biology of ccRCC. Since the marker genes of the different cell subgroups revealed in this study are ccRCC specific, they may serve as more efficient markers for flow cytometry than their conventional counterparts. In summary, this study provides a new perspective for understanding the TME in ccRCC and will aid the development of immunotherapies for treating ccRCC.
Materials and Methods
Data Acquisition
The scRNA-seq count matrix has been described by Young et al.10 in their supplemental data. The expression matrix, along with the clinical information of the patients from TCGA KIRC dataset, which contains 533 tumor samples and 72 normal samples, was obtained from University of California Santa Cruz (UCSC) Xena (http://xena.ucsc.edu/).
QC and Cell Type Recognition
A total of 21 samples, including 12 tumor samples and 9 para-tumor samples from three patients (Figure S7B), were involved in this study. The QC process was performed using Seurat (version 3.0.1).34 Single cells with less than 200 UMIs or with more than 10% mitochondrion-derived UMI counts were considered as low-quality cells and removed. Batch effects among the patients were eliminated using the IntegrateData function in Seurat. The top 30 principal components, along with the top 2,000 variable genes, were used in this process. Influence of the UMI count and the percentage of mitochondrion-derived UMI counts were then regressed out using the ScaleData function. Subsequently, the main cell clusters were identified using the FindClusters function (resolution = 1.1) of Seurat and visualized using 2D uniform manifold approximation and projection (UMAP) or tSNE;35 these are currently the most popular algorithms for the reduction of data dimensions. One cell cluster, which was mainly recognized because of differences in the cell cycle, was not involved in the downstream analysis. Finally, 24,550 single cells, including 7,786 normal tissue-derived cells and 16,764 tumor tissue-derived cells, were subjected to further investigation.
The FindAllMarkers function was used to list the markers of each cell cluster. The major cell types were then recognized based on the markers obtained from the CellMarker database.36 Markers of renal tubules and collecting ducts have been reported in a previous study;11 these were also recognized by droplet-based scRNA-seq. Markers used in this pipeline are addressed in Table S1. Additionally, DEGs of every cell type are listed in Table S2.
Human ccRCC Tissue Array and Immunofluorescence Assay
Tissue chip HKid-CRC060CS-01, which consists of 25 paired tumor and para-tumor samples, along with 5 distant normal tissues and 5 metastatic tissue samples, was purchased from Shanghai Outdo Biotech. To ensure the consistency of the analysis, all immunofluorescence analyses were performed using the same type of tissue chip.
The following antibodies were used to detect specific proteins: anti-ALDOA (rabbit, 1:50, Proteintech, catalog no. 11217-1-AP), anti-ALDOB (rabbit, 1:50, Proteintech, catalog no. 18065-1-AP), anti-ALDOC (rabbit, 1:50, Proteintech, catalog no. 14884-1-AP), anti-MIOX (rabbit, 1:200, Abcam, ab199729), anti-GPX3 (rabbit, 1:100, Proteintech, catalog no. 13947-1-AP), anti-metallothionein (mouse, 1:200, Abcam, ab12228), anti-CD163 (rabbit, 1:50, Proteintech, catalog no. 16646-1-AP), anti-TREM2 (rabbit, 1:50, Proteintech, catalog no. 13483-1-AP), anti-CD8 (mouse, 1:500, Proteintech, catalog no. 66868-1-AP), anti-LAG3 (rabbit, 1:30, Abcam, ab209236), anti-HAVCR2 (rabbit, 1:100, ABclonal, A13444), anti-ALGPTL4 (rabbit, 1:100, Abcam, ab196746), anti-CA9 (rabbit, 1:100, ABclonal, A13682), anti-FABP6 (rabbit, 1:100, ABclonal, A6906), anti-FABP5 (rabbit, 1:200, Proteintech, catalog no. 12348-1-AP), anti-ACTA2 (rabbit, 1:100, Proteintech, catalog no. 14375-1-AP), anti-VWF (rabbit, 1:200, Proteintech, catalog no. 11778-1-AP), and anti-ENPP2 antibodies (mouse, 1:200, Abcam, ab77104).
Immune Checkpoint Analysis
The mean normalized expression levels of immune checkpoints of each cell cluster were calculated and then normalized into row Z scores to represent their relative expression levels among the different cell clusters.
Gene Regulatory Network Analysis
In order to investigate the gene regulatory network of the ccRCC samples, we utilized pySCENIC, a python-based algorithm for the reconstruction of the transcriptional states and regulatory networks from the scRNA-seq data,37 in the Jupyter notebook software (version 1.0.0). Differences of the area under the curve (AUC) of each module (calculated using SCENIC) between the normal tissue-derived and tumor-derived cells, or among cell clusters, were identified with the limma package. Regulons with an adjusted p value (adj. p val) >0.01 were not considered for further investigation.
Pseudotime Trajectory Analysis
In order to reveal the changes of immune cells in the tumor-educating process, we employed Monocle 2, an R package designed for single-cell trajectories by Qiu et al.38 The following parameters were set: mean expression ≥0.125, num_cells_expressed ≥10, qval <0.01 (differentialGeneTest function). The trajectories were visualized as 2D tSNE plots, while dynamical expression heatmaps were constructed using the plot_pseudotime_heatmap function.
Functional Enrichment Analysis
DEGs were identified using the FindMarkers function of Seurat. The following cutoff threshold values were used: adj. p val <0.01 and fold change (FC) >1.5. Then, these DEGs were loaded into clusterProfiler39 for the GO enrichment analysis. Pathways for which the adj. p val was <0.05 were considered significantly enriched.
GSEA analysis was performed to detect which gene set was significantly enriched in each specific cell cluster. Only gene sets with false discovery rate (FDR) p values <0.05 and nominal p values <0.05 were considered significantly enriched.
Gene set variation analysis (GSVA) was performed using 50 hallmark gene sets obtained from the molecular signature database using default sets, as described in the GSVA package.
Cell-Cell Communication Analysis
CellPhoneDB40 is a Python-based computational analysis tool; it enables the analysis of cell-cell communication at the molecular level. A website version was also provided for the analysis of relatively small datasets (https://www.cellphonedb.org/). As described above, first, 24,550 single cells were clustered into 16 cell types. In order to investigate the molecular interaction networks among the cell types or cell clusters, CellphoneDB was used to analyze the 16 major cell types and cell subclusters. Ligand-receptor pairs with p values >0.05, as determined by CellPhoneDB, were filtered, while the others were retained for evaluating the relationship between the different cell clusters.
Correlation to Public Datasets
CIBERSORTx is a new machine-learning method designed for detecting the abundance of cell types in bulk RNA-seq data; this method has been called “digital cytometry” by the authors. To evaluate the roles of the cell clusters identified in the present work, we used CIBERSORTx to analyze TCGA KIRC expression matrix, which was first normalized to obtain log2(TPM+1) values. As the signature matrix was constructed, we calculated the relative abundance of each cell cluster and then divided the patients into two groups: high 50% and low 50%. Subsequently, Cox regression analysis was performed using the R package to evaluate the prognostic value of the cell clusters. Z scores were used to visualize the results of the Cox regression model.
To confirm whether the relative abundance of the cell clusters changed dynamically during the progression of ccRCC, one-way ANOVA was performed using the abundance matrix produced by CIBERSORTx and the results were visualized as a line chart.
To validate the significance of the TME subtype, we employed ConsensusClusterPlus (version 1.46.0), dividing TCGA KIRC cohort into three subgroups. Subsequently, Kaplan-Meier analysis was performed using GraphPad Prism 7.0 software. One-way ANOVA was used to evaluate whether the occurrence of cell clusters in the different ccRCC subgroups showed significant differences. Subsequently, a Tukey’s multiple comparison test was performed to detect the differences between any two subgroups.
Statistical Analysis
Tissue-specific expression analysis was performed using the online tool GEPIA. Spearman correlation analysis was performed using the R package, and the results were then visualized as heatmaps and scatterplots. A Student’s t test was used to detect differences between the fluorescence of the different cell types, which was quantified using the ImageJ software (version 1.52q). p < 0.05 was considered statistically significant.
Author Contributions
K.C. and T.Z. conceived the idea. L.B., L.Z., and Y.H. collected the data. J.H. and Z.C. finished the bioinformatics analysis. L.L., M.X., Y.Z.., and B.W. helped with immunofluorescence staining. J.H. wrote the manuscript. All authors reviewed and approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Servicebio for the support of immunofluorescence scanning. This work was supported by grants from the National Natural Sciences Foundation of China (81672524 and 81602678); the Hubei Provincial Natural Science Foundation of China (2018CFA038); the Natural Science Foundation of Tianjin (17JCQNJC12300); the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20180305164838833); and the Fundamental Research Funds for the Central University (201kfyRCPY0049).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.04.023.
Contributor Information
Zhen Tao, Email: ztao@tmu.edu.cn.
Ke Chen, Email: shenke@hust.edu.cn.
Supplemental Information
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Porta C., Cosmai L., Leibovich B.C., Powles T., Gallieni M., Bex A. The adjuvant treatment of kidney cancer: a multidisciplinary outlook. Nat. Rev. Nephrol. 2019;15:423–433. doi: 10.1038/s41581-019-0131-x. [DOI] [PubMed] [Google Scholar]
- 3.Grimm M.O., Bex A., De Santis M., Ljungberg B., Catto J.W.F., Rouprêt M., Hussain S.A., Bellmunt J., Powles T., Wirth M., Van Poppel H. Safe use of immune checkpoint inhibitors in the multidisciplinary management of urological cancer: the European Association of Urology position in 2019. Eur. Urol. 2019;76:368–380. doi: 10.1016/j.eururo.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha R.R., Motzer R.J., Voss M.H. Towards individualized therapy for metastatic renal cell carcinoma. Nat. Rev. Clin. Oncol. 2019;16:621–633. doi: 10.1038/s41571-019-0209-1. [DOI] [PubMed] [Google Scholar]
- 5.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papalexi E., Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 2018;18:35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 7.Albini A., Sporn M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanley C.J., Mellone M., Ford K., Thirdborough S.M., Mellows T., Frampton S.J., Smith D.M., Harden E., Szyndralewiez C., Bullock M. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J. Natl. Cancer Inst. 2018;110:109–120. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young M.D., Mitchell T.J., Vieira Braga F.A., Tran M.G.B., Stewart B.J., Ferdinand J.R., Collord G., Botting R.A., Popescu D.M., Loudon K.W. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science. 2018;361:594–599. doi: 10.1126/science.aat1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake B.B., Chen S., Hoshi M., Plongthongkum N., Salamon D., Knoten A., Vijayan A., Venkatesh R., Kim E.H., Gao D. A single-nucleus RNA-sequencing pipeline to decipher the molecular anatomy and pathophysiology of human kidneys. Nat. Commun. 2019;10:2832. doi: 10.1038/s41467-019-10861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell J.C., Payne L.B., Rathmell W.K. Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. J. Clin. Invest. 2019;129:442–451. doi: 10.1172/JCI120855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilmann R.M., Nestler J., Schwarz J., Grützner N., Ambrus A., Seeger J., Suchodolski J.S., Steiner J.M., Gurtner C. Mucosal expression of S100A12 (calgranulin C) and S100A8/A9 (calprotectin) and correlation with serum and fecal concentrations in dogs with chronic inflammatory enteropathy. Vet. Immunol. Immunopathol. 2019;211:64–74. doi: 10.1016/j.vetimm.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Baek Y.S., Haas S., Hackstein H., Bein G., Hernandez-Santana M., Lehrach H., Sauer S., Seitz H. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009;10:18. doi: 10.1186/1471-2172-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang Y., Coppo M., He T., Ning F., Yu L., Kang L., Zhang B., Ju C., Qiao Y., Zhao B. The transcriptional repressor Hes1 attenuates inflammation by regulating transcription elongation. Nat. Immunol. 2016;17:930–937. doi: 10.1038/ni.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Sanmamed M.F., Datar I., Su T.T., Ji L., Sun J., Chen L., Chen Y., Zhu G., Yin W. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–347.e12. doi: 10.1016/j.cell.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas S.K., Gangi L., Paul S., Schioppa T., Saccani A., Sironi M., Bottazzi B., Doni A., Vincenzo B., Pasqualini F. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 18.Günthner R., Anders H.J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medzhitov R., Horng T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 20.Scott A.C., Dündar F., Zumbo P., Chandran S.S., Klebanoff C.A., Shakiba M., Trivedi P., Menocal L., Appleby H., Camara S. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez G.J., Pereira R.M., Äijö T., Kim E.Y., Marangoni F., Pipkin M.E., Togher S., Heissmeyer V., Zhang Y.C., Crotty S. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speiser D.E., Ho P.C., Verdeil G. Regulatory circuits of T cell function in cancer. Nat. Rev. Immunol. 2016;16:599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 23.Qiu B., Ackerman D., Sanchez D.J., Li B., Ochocki J.D., Grazioli A., Bobrovnikova-Marjon E., Diehl J.A., Keith B., Simon M.C. HIF2α-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 2015;5:652–667. doi: 10.1158/2159-8290.CD-14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Rong Y., Bao L., Nie B., Ren G., Zheng C., Amin R., Arnold R.D., Jeganathan R.B., Huggins K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017;16:181. doi: 10.1186/s12944-017-0574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syafruddin S.E., Rodrigues P., Vojtasova E., Patel S.A., Zaini M.N., Burge J., Warren A.Y., Stewart G.D., Eisen T., Bihary D. A KLF6-driven transcriptional network links lipid homeostasis and tumour growth in renal carcinoma. Nat. Commun. 2019;10:1152. doi: 10.1038/s41467-019-09116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roumenina L.T., Daugan M.V., Noé R., Petitprez F., Vano Y.A., Sanchez-Salas R., Becht E., Meilleroux J., Clec’h B.L., Giraldo N.A. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res. 2019;7:1091–1105. doi: 10.1158/2326-6066.CIR-18-0891. [DOI] [PubMed] [Google Scholar]
- 27.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A., Teinor J.A., Belleau P., Biffi G., Lucito M.S. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., Bassez A., Decaluwé H., Pircher A., Van den Eynde K. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 29.Zeng M., Kikuchi H., Pino M.S., Chung D.C. Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells. PLoS ONE. 2010;5:e10966. doi: 10.1371/journal.pone.0010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkerson M.D., Hayes D.N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26:1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T., Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 32.Cassetta L., Fragkogianni S., Sims A.H., Swierczak A., Forrester L.M., Zhang H., Soong D.Y.H., Cotechini T., Anur P., Lin E.Y. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35:588–602.e10. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drake C.G., Stein M.N. The immunobiology of kidney cancer. J. Clin. Oncol. 2018;36 doi: 10.1200/JCO.2018.79.2648. JCO2018792648. [DOI] [PubMed] [Google Scholar]
- 34.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becht E., McInnes L., Healy J., Dutertre C.A., Kwok I.W.H., Ng L.G., Ginhoux F., Newell E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Lan Y., Xu J., Quan F., Zhao E., Deng C., Luo T., Xu L., Liao G., Yan M. CellMarker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 2019;47(D1):D721–D728. doi: 10.1093/nar/gky900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aibar S., González-Blas C.B., Moerman T., Huynh-Thu V.A., Imrichova H., Hulselmans G., Rambow F., Marine J.C., Geurts P., Aerts J. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vento-Tormo R., Efremova M., Botting R.A., Turco M.Y., Vento-Tormo M., Meyer K.B., Park J.E., Stephenson E., Polański K., Goncalves A. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.