Abstract
Purpose of Review
Molecular imaging with positron emission tomography (PET) is a powerful tool to visualize breast cancer characteristics. Nonetheless, implementation of PET imaging into cancer care is challenging, and essential steps have been outlined in the international “imaging biomarker roadmap.” In this review, we identify hurdles and provide recommendations for implementation of PET biomarkers in breast cancer care, focusing on the PET tracers 2-[18F]-fluoro-2-deoxyglucose ([18F]-FDG), sodium [18F]-fluoride ([18F]-NaF), 16α-[18F]-fluoroestradiol ([18F]-FES), and [89Zr]-trastuzumab.
Recent Findings
Technical validity of [18F]-FDG, [18F]-NaF, and [18F]-FES is established and supported by international guidelines. However, support for clinical validity and utility is still pending for these PET tracers in breast cancer, due to variable endpoints and procedures in clinical studies.
Summary
Assessment of clinical validity and utility is essential towards implementation; however, these steps are still lacking for PET biomarkers in breast cancer. This could be solved by adding PET biomarkers to randomized trials, development of imaging data warehouses, and harmonization of endpoints and procedures.
Keywords: Breast cancer, Molecular imaging, Positron emission tomography, Technical validation, Clinical validation, Clinical utility
Introduction
Over the last decade, there has been an increasing interest in molecular imaging with positron emission tomography (PET), in particular in the field of oncology. PET imaging is a noninvasive tool to obtain qualitative and quantitative whole-body information of biological processes. Molecular imaging in breast cancer (BC) is of particular interest, as it can visualize the estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2), and proliferation. However, molecular imaging with PET has not been widely adopted in clinical practice of BC. Only two radiotracers (2-[18F]-fluoro-2-deoxyglucose ([18F]-FDG) and sodium [18F]-fluoride ([18F]-NaF)) are incorporated in cancer management guidelines, such as National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO). In order to improve successful implementation of PET imaging biomarkers into clinical practice, it is essential to identify potential hurdles. Recently, an international consensus meeting resulted in the “imaging biomarker roadmap,” describing the steps of imaging biomarkers towards clinical practice [1••]. In this review, we describe the current status of PET biomarkers for BC, according to this roadmap. We identify specific challenges for each tracer individually and make recommendations for next steps towards clinical implementation.
Development Stages of Imaging Biomarkers
The imaging biomarker roadmap describes three parallel tracks, towards biomarker implementation in clinical practice [1••]. Technical validity, i.e., whether the test can be trusted, requires harmonization and standardization of techniques as an assessment of repeatability and reproducibility. Clinical validity, i.e., whether the test is clinically meaningful, addresses the discriminatory value to predict diagnosis, prognosis, or therapy response. Finally, clinical utility, i.e., whether the test improves patient outcome and is cost-effective, is determined by health-related measurements. Successful progress through these tracks is essential for a test to pass from analytical to clinical research stage, and subsequently to routine clinical practice [1••].
Search Strategy
For this literature review, the database PubMed was searched until September 2019. PET tracers were included if Food and Drug Administration (FDA) approved or at least two prospective clinical articles, including ≥ 50 BC patients, were published within the past 5 years. As a result, four radiotracers were selected ([18F]-FDG, [18F]-NaF, 16α-[18F]-fluoroestradiol ([18F]-FES), and zirconium-89 [89Zr]-trastuzumab). Search terms were repeatability, reproducibility, inter- and intra-observer, diagnosis, prognosis, response to treatment, survival, metastases, technical and clinical validity/utility, cost-effectiveness, BC, PET, and meta-analysis.
Development Stages of [18F]-FDG-PET/CT
Technical Validity
[18F]-FDG-PET/computed tomography (CT) can detect increased glucose metabolism in cancer cells and is indicated for multiple oncological indications [2, 3]. [18F]-FDG is phosphorylated by the enzyme hexokinase and trapped inside (tumor) cells [4]. The reproducibility and repeatability of [18F]-FDG-PET/CT were assessed for various cancer types (see Table 1 for overview) [58]. One meta-analysis of 5 studies, including 102 cancer patients of which 6 had metastatic BC (MBC), assessed the repeatability of [18F]-FDG-PET(/CT) by measuring the standardized uptake value (SUV)max/mean in the same patient on two separate occasions with an interval of 1–4 days [5]. A high test-retest interclass correlation coefficient (ICC) of 0.90 and 0.91 was found for SUVmax and SUVmean, respectively. Reproducibility across different scanners was assessed in 23 patients, 17 with BC [13]. Patients underwent two [18F]-FDG-PET/CT scans within 15 days on the same scanner or on different scanners at different sites. Cross-calibration of PET/CT scanners and dose calibrator was performed. The average difference in SUVmax between test-retest [18F]-FDG-PET/CT, using the same scanner, was 8% versus 18% on different scanners. International standardization efforts to improve reproducibility resulted in the European Association of Nuclear Medicine (EANM) guideline for 18F imaging procedures, followed in 2010 by the Research Ltd. (EARL) accreditation program to assure independent quality control, comparable scanner performance, and reproducible assessments [3, 59]. Since 2010, the number of accredited centers has increased over time in Europe and beyond [60].
Table 1.
Development stages of [18F]-FDG-PET/CT, [18F]-NaF-PET/CT, [18F]-FES-PET/CT, and [89Zr]-trastuzumab PET/CT
| Checklist | Article | No. of patients | Study type | Scanner | PET measurement | Reference standard | Level of evidence¶ |
|---|---|---|---|---|---|---|---|
| Technical validity* [18F]-FDG-PET (FDA approved) | |||||||
| Repeatability | Van Langen,2012 [5] | 102, 5 studies | Meta-analysis (prospective/retrospective) | PET and PET/CT | Semi-quantitative (SUV) | II | |
| Repeatability | Kramer, 2016 [6] | 9 | Prospective | PET/CT | Semi-quantitative (SUV, TLG, MATV) | III | |
| Repeatability | Weber, 2015 [7] | 74 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability | Rockall, 2016 [8] | 21 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability | Fraum, 2019 [9] | 14 | Prospective | PET/CT | Semi-quantitative (SUV, SUL) | III | |
| Repeatability | Frings, 2014 [10] | 34 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability | Hoang, 2013 [11] | 17 | Prospective | PET/CT | Semi-quantitative ((Δ)SUV) | III | |
| Repeatability | Van Velden, 2014 [12] | 29 | Prospective | PET/CT | Semi-quantitative (SUV, TLG) | III | |
| Reproducibility | Kurland, 2019 [13] | 23 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Reproducibility | Goh, 2012 [14] | 25 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability/reproducibility | Heijmen, 2012 [15] | 20 | Prospective | PET/CT | Semi-quantitative (SUV, TLG, volume) | III | |
| Repeatability/reproducibility | Kolinger, 2019 [16] | 10 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability/reproducibility | Rasmussen, 2015 [17] | 30 | Prospective | PET/CT | Semi-quantitative (SUV, MTV, TLG) | III | |
| Technical validity* [18F]-NaF-PET (FDA approved) | |||||||
| Repeatability | Lin, 2016 [18] | 35 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Repeatability | Wassberg, 2017 [19] | 10 | Prospective | PET/CT | Visual and semi-quantitative (SUV, FTV, TLF) | III | |
| Repeatability | Kurdziel, 2012 [20] | Subgroup of 21 | Prospective | PET/CT | Semi-quantitative (SUV) | III | |
| Reproducibility | Zacho, 2019 [21] | 219 | Prospective | PET/CT | Visual | III | |
| Technical validity* [18F]-FES-PET | |||||||
| Reproducibility | Chae, 2019 [22••] | 90 | Prospective | PET/CT | Visual | III | |
| Technical validity† [89Zr]-trastuzumab-PET: no data are available | |||||||
| Clinical validity† [18F]-FDG-PET (FDA approved) | |||||||
| Diagnosis - primary tumor | Bertagna, 2013 [23] | NR, 13 studies | Meta-analysis (prospective/retrospective) | PET and PET/CT | NR | Partly based on pathology | II |
| Diagnosis - primary tumor | Zhang, 2018 [24•] | 2890, 39 studies | Meta-analysis (NR) | PET and PET/CT | NR | Pathology | II |
| Diagnosis - axillary nodes | Cooper, 2011 [25] | 2591, 26 studies | Meta-analysis (prospective/ retrospective) | PET and PET/CT | Visual | SLNB, ALND | II |
| Diagnosis - axillary nodes | Liang, 2016 [26•] | 1887, 21 studies | Meta-analysis (prospective/retrospective) | PET/CT | Semi-quantitative (SUV) | Fine needle aspiration biopsy, SLNB, ALND | II |
| Diagnosis - axillary nodes | Pritchard, 2012 [27] | 325 | Prospective | PET and PET/CT | Visual | SLNB, ALND | III |
| Diagnosis - recurrence | Xiao, 2016 [28] | 1752, 26 studies | Meta-analysis (prospective/retrospective) | PET and PET/CT | Visual | Pathology, clinical or imaging | II |
| Diagnosis - metastases | Hong, 2013 [29] | 748, 8 studies | Meta-analysis (prospective/retrospective) | PET/CT | Visual, semi-quantitative (not specified) | Pathology, clinical or imaging | II |
| Diagnosis - bone metastases | Rong, 2013 [30] | 668, 7 studies | Meta-analysis (prospective/retrospective) | PET/CT | Visual, semi-quantitative (not specified) | Pathology, clinical, or imaging | II |
| Prognosis - clinicopathological | Groheux, 2011 [31] | 131 | Prospective | PET/CT | Semi-quantitative (SUV) | Pathology | III |
| Prognosis - survival | Diao, 2018 [32•] | 3574, 15 studies | Meta-analysis (prospective/retrospective) | PET and PET/CT | Semi-quantitative (SUV) | Not specified | II |
| Prognosis - survival | Evangelista, 2017 [33] | 275 | Prospective | PET/CT | Visual, semi-quantitative (SUV) | Pathology or imaging | III |
| Prognosis - survival | Zhang, 2013 [34] | 244 | Prospective | PET/CT | Semi-quantitative (SUV) | Pathology, clinical, or imaging | III |
| Therapy response - neoadjuvant | Liu, 2015 [35] | 382, 6 studies | Meta-analysis (prospective/retrospective) | PET/CT | Semi-quantitative (ΔSUV) | Pathology | II |
| Therapy response - neoadjuvant | Tian, 2017 [36•] | 1119, 22 studies | Meta-analysis (prospective/retrospective) | PET/CT | Semi-quantitative (ΔSUV) | Pathology | II |
| Therapy response - neoadjuvant | Coudert, 2014 [37] | 142 | Randomized, prospective | PET/CT | Semi-quantitative (SUV) | Pathology | II |
| Clinical validity† [18F]-NaF-PET (FDA approved) | |||||||
| Diagnosis | Withofs, 2011 [38] | 24 | Prospective | PET/CT | Visual | MRI or CT | III |
| Diagnosis | Damle, 2013 [39] | 72 | Prospective | PET/CT | Visual | Pathology or imaging | III |
| Diagnosis | Liu, 2019 [40•] | Subgroup of 125 (3 studies) | Meta-analyses (prospective/retrospective) | PET/CT | Visual | Pathology, clinical, or imaging | II |
| Prognosis - survival | Peterson, 2018 [41] | 28 | Prospective | PET/CT | Semi-quantitative ((Δ)SUV) | Not specified | III |
| Therapy response | Azad, 2019 [42] | 12 | Prospective | PET/CT | Semi-quantitative ((Δ)metabolic flux, SUV) | Clinical or imaging | III |
| Therapy response | Azad, 2019 [43] | 16 | Prospective | PET/CT | Semi-quantitative ((Δ)SUV, TLM, MTV, SD, entropy, uniformity, kurtosis, skewness) | Clinical or imaging | III |
| Therapy response | Azad, 2019 [44] | 22 | Prospective | PET/CT | Semi-quantitative (ΔSUV) | Clinical or imaging | III |
| Clinical validity† [18F]-FES-PET | |||||||
| Diagnosis | Evangelista, 2016 [45] | 238, 9 studies | Meta-analysis (prospective/retrospective) | PET and PET/CT | Semi-quantitative (SUV) | Partly based on pathology | II |
| Diagnosis | Chae, 2019 [22••] | 90 | Prospective | PET/CT | Visual and semi-quantitative (SUV) | Pathology | III |
| Diagnosis | Venema, 2017 [46] | 13 | Prospective | PET/CT | Semi-quantitative (SUV) | Pathology | III |
| Diagnosis | Gupta, 2017 [47] | 10 | Prospective | PET/CT | Visual and semi-quantitative (SUV) | Pathology | III |
| Prognosis | Kurland, 2017 [48] | 90 | Prospective | PET and PET/CT | Visual and semi-quantitative (SUV, SUL) | Clinical or imaging | III |
| Therapy response | Evangelista, 2016 [45] | 183, 6 studies | Meta-analysis (prospective) | PET and PET/CT | Semi-quantitative (SUV) | Clinical or imaging | II |
| Therapy response | Chae, 2017 [49•] | 26 | Randomized, prospective | PET/CT | Semi-quantitative (SUV) | Pathology | II |
| Therapy response | Van Kruchten, 2015 [50] | 19 | Prospective | PET/CT | Semi-quantitative (SUV) | Clinical or imaging | III |
| Therapy response | Park, 2016 [51] | 24 | Prospective | PET/CT | Semi-quantitative (SUV) | Pathology, clinical, or imaging | III |
| Therapy response | Gong, 2017 [52] | 22 | Prospective | PET/CT | Semi-quantitative ((Δ)SUV) | Imaging | III |
| Clinical validity† [89Zr]-trastuzumab-PET | |||||||
| Diagnosis | Dehdashti, 2018 [53] | 51 | Prospective | PET/CT | Visual and semi-quantitative (SUV) | Pathology, clinical or imaging | III |
| Therapy response | Gebhart, 2016 [54] | 56 | Prospective | PET/CT | Visual and semi-quantitative (SUV) | [18F]-FDG-PET | III |
| Clinical utility† [18F]-FDG-PET (FDA approved) | |||||||
| Cost-effectiveness | Koleva-Kolarova, 2015 [55] | 5073 | Computer simulation | PET/CT | Costs and ICER | § | |
| Clinical utility† [18F]-NaF-PET (FDA approved): no data are available | |||||||
| Clinical utility† [18F]-FES-PET | |||||||
| Cost-effectiveness | Koleva-Kolarova, 2015 [55] | 5073 | Computer simulation | PET/CT | Costs and ICER | § | |
| Cost-effectiveness | Koleva-Kolarova, 2018 [56] | Hypothetical cohort of 1000 | Computer simulation | PET/CT | Costs, LYG and ICER | § | |
| Clinical utility† [89Zr]-trastuzumab-PET | |||||||
| Cost-effectiveness | Koleva-Kolarova, 2018 [56] | Hypothetical cohort of 1000 | Computer simulation | PET/CT | Costs, LYG, and ICER | § | |
Articles are included if they met in- (prospective study design) and exclusion criteria (trials using PET only scanners or including less than 10 (breast cancer) patients (except clinical validity [18F]-FDG-PET ≥ 100 breast cancer patients)
PET positron emission tomography, CT computed tomography, NR not reported, SUV standardized uptake value, TLG total lesion glycolysis, MATV metabolic active tumor volume, SUL SUV normalized by lean body mass, MTV metabolic tumor volume, TLM total lesion metabolism, FTV functional tumor volume, TLF skeletal tumor burden, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, SD standard deviation, QALY quality-adjusted life year, ICER incremental cost-effectiveness ratio, LYG life years gained
¶According to ESMO guidelines (I: large randomized trials of good methodological quality or meta-analyses of randomized trials, II: (small) randomized trials or meta-analyses of (small) trials, III: prospective studies, IV: retrospective studies, V: expert opinion) [57]
§Level of evidence does not fit the ESMO criteria
*The study can be performed in various solid tumors, not necessarily breast cancer. Repeatability: refers to measurements performed multiple times in the same subject using the same equipment, software and observers over a short timeframe. Reproducibility: refers to measurements performed using different equipment, different software or observers, or at different sites and times, either in the same or in different subjects
†Always performed in breast cancer patients
Clinical Validity
For [18F]-FDG-PET/CT, we focused on clinical validity studies with at least 100 BC patients. A meta-analysis of 13 studies (see Table 1) reported incidental and unexpected breast uptake detected by [18F]-FDG-PET(/CT) [23]. Overlap between SUVs in malignant and benign breast incidentalomas was found, and not all lesions were further histologically examined. Therefore, [18F]-FDG-PET/CT is not routinely used for diagnosis of primary BC. With regard to diagnosis of axillary lymph node metastases in BC, a meta-analysis was performed of studies comparing [18F]-FDG-PET(/CT) to the reference standard: axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB) [25]. In 7 out of 26 studies involving 862 BC patients, [18F]-FDG-PET/CT sensitivity was 56% and specificity 96%, compared to 52% and 95% for ALND and/or SLNB [25]. Another meta-analysis (21 studies including 1887 BC patients), using ALND and/or SLNB as reference standard, showed a sensitivity and specificity of 64% and 93%, respectively, for detection of axillary lymph node metastases by [18F]-FDG-PET/CT [26•]. Based on these data, [18F]-FDG-PET/CT is not recommended in the EANM, NCCN, or ESMO guidelines for detection of axillary lymph node metastases. However, as axillary BC management has evolved over the last decades, the use of [18F]-FDG-PET/CT in this setting may change as well. For instance, according to the Dutch BC guideline, [18F]-FDG-PET/CT can be considered for staging of BC patients prior to neoadjuvant chemotherapy, although a biopsy of axillary lymph nodes with high [18F]-FDG uptake is advised to avoid false positive results [61]. With regard to [18F]-FDG-PET/CT for diagnosis of recurrent or distant metastases in BC, two meta-analyses including a total of 2500 patients (2 studies with overlapping subjects) showed both high sensitivity (92–96%) and specificity (82–95%) [28, 29]. For the detection of bone metastases, [18F]-FDG-PET/CT showed a sensitivity and specificity of 93% and 99%, versus 81% and 96% respectively, for conventional bone scintigraphy, as determined in a meta-analysis involving 668 BC patients in 7 studies [30]. According to the EANM, ESMO, and NCCN guidelines, [18F]-FDG-PET/CT should be considered in cases of suspected recurrence or equivocal findings on standard imaging and can be used for staging in high-risk BC patients [2, 3, 62, 63••, 64, 65••].
Despite the non-specific uptake of [18F]-FDG, preoperative [18F]-FDG uptake, expressed as SUVmax, was found to be related to prognostic pathological characteristics assessed on core biopsy in primary BC. SUVmax was higher in ER− than ER+ tumors (7.6 versus 5.5); higher uptake was also observed in triple-negative tumors, tumor grade 3, ductal carcinoma, and p53 mutated tumors [31]. A meta-analysis of 15 studies with 3574 BC patients evaluated the prognostic value of [18F]-FDG uptake in primary breast lesions [32•]. High SUVmax was related to a higher risk of recurrence or progression compared with a low SUVmax. However, the SUVmax cutoff values varied widely between studies, ranging from 3.0 to 11.1 [32•]. Lower baseline SUVmax predicted more favorable survival outcomes than higher SUVmax (analyzed as a continuous variable) [34]. The lack of clear cutoff values has so far precluded the use of [18F]-FDG-PET as a prognostic tool in BC. This is partly due to the fact that SUV calculations can depend on the PET camera systems used. To harmonize the acquisition protocols and the quantification process between different camera systems, the EARL harmonization program was introduced.
Clinical validity of serial [18F]-FDG-PET/CT to monitor therapy response to neoadjuvant treatment was analyzed in two meta-analyses (see Table 1), showing a pooled sensitivity of 82–86% and specificity of 72–79%, using histopathology as reference standard for pathological (non-)response [35, 36•]. Possibly differences between the pace of disease response between BC subtypes may play a role in this setting. In the randomized neoadjuvant study AVATAXHER in 142 patients with HER2+ BC, [18F]-FDG-PET/CT at baseline and after 1 cycle of docetaxel/trastuzumab was used for further treatment decisions [37]. Patients with a ΔSUVmax of ≥ 70% (n = 69) continued docetaxel/trastuzumab. Patients with a ΔSUVmax of < 70% (n = 73) were randomized for continued docetaxel/trastuzumab or addition of bevacizumab. In all patients receiving docetaxel/trastuzumab, this ΔSUVmax cutoff of 70% showed a positive and negative predictive value of 53% and 75%, respectively, to detect pathological complete response. Recently, preliminary data from the neoadjuvant PREDIX HER2 trial showed that pathological response was related to decreased uptake on early [18F]-FDG-PET/CT compared to baseline, in HER2+ primary BC [66]. For MBC, no well-designed large study to assess the clinical value of [18F]-FDG-PET/CT has been performed, only small studies with varying endpoints [67, 68]. The optimal cutoff value and interval between [18F]-FDG-PET/CT scans for response measurement in BC are still unknown and may limit implementation of [18F]-FDG-PET/CT as a tool for early response prediction in clinical practice. Attempts to integrate [18F]-FDG-PET/CT in the Response Evaluation Criteria in Solid Tumors (RECIST) criteria have not been successful so far, and [18F]-FDG-PET/CT is not routinely used for response evaluation in BC, due to the absence of sufficient clinical validation data [69••, 70].
Clinical Utility
Evidence on the cost-effectiveness of [18F]-FDG-PET/CT in BC is limited (Table 1). A Dutch computer simulation study by Koleva-Kolarova et al. evaluated the effect of [18F]-FDG-PET/CT on the number of performed biopsies and additional costs compared to the standard clinical workup for diagnosing ER+ MBC patients, using the incremental cost-effectiveness ratio (ICER) to avoid a biopsy [55]. This study demonstrated a 38 ± 15% increase in biopsies, and higher costs for [18F]-FDG-PET/CT compared to standard workup.
Conclusions and Recommendations of [18F]-FDG-PET/CT
While the technical validity track for [18F]-FDG-PET/CT has been completed successfully with international EARL and EANM standardization and harmonization of the technique itself, this harmonization is still lacking regarding clinical validity and utility. This has hampered routine use of [18F]-FDG-PET/CT in BC management worldwide. First, studies establishing a receiver operating characteristic (ROC) curve, sensitivity, and specificity in well-defined large cohort trials are needed, with biopsy as gold standard. The IMPACT breast trial (NCT01957332), in which baseline [18F]-FDG-PET/CT was performed in 200 MBC patients of all subtypes, including biopsy of a metastasis and conventional imaging, is likely to provide these data in the near future. Second, factors affecting [18F]-FDG-PET/CT results other than treatment effects should be standardized as much as possible (such as time of the scan after therapy). Finally, clinical utility assessment by integrating imaging biomarkers into randomized trials, developing an imaging data warehouse for EARL [18F]-FDG-PET/CT scans, and performing meta-analyses of these data may provide the final support for full implementation of [18F]-FDG-PET/CT into clinical practice (Fig. 1).
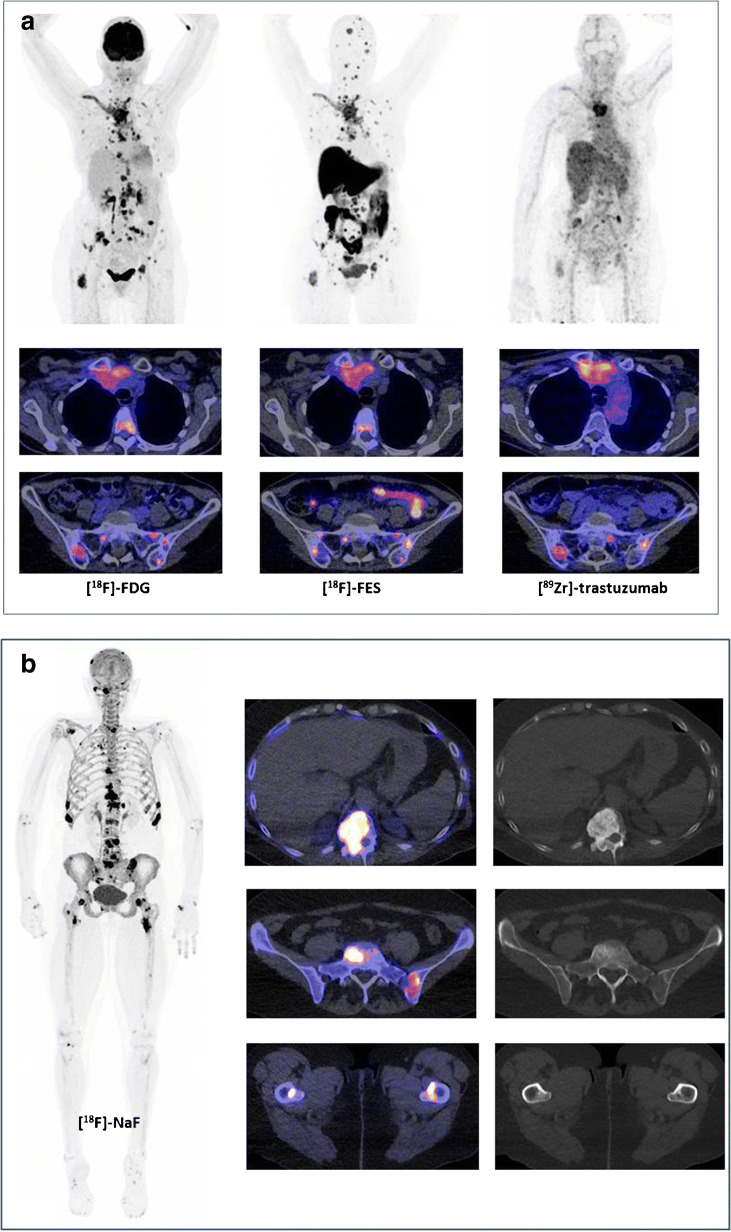
Fig. 1.
Upper image: three PET scans ([18F]-FDG-PET, [18F]-FES-PET, and [89Zr]-trastuzumab-PET) in the same patient showing mediastinal and hilar lymph node metastases, as well as intrapulmonary lesions visible on both [18F]-FDG-PET and [18F]-FES-PET, but not on [89Zr]-trastuzumab-PET. The large mediastinal mass (first row of transversal fused images) was visible on all three imaging modalities. Bone metastases (second row of transversal fused images) were clearly visualized on [18F]-FES-PET, for example, skull lesions, and to a lesser extent on [18F]-FDG-PET and [89Zr]-trastuzumab-PET. Lower image: [18F]-NaF-PET in another patient showing bone metastases in the skull, vertebrae, costae, pelvis, and proximal femora. The increased uptake in the joint was related to degeneration
Development Stages of [18F]-NaF-PET/CT
Technical Validity
Bone is the most common site of metastasis in BC. Two PET tracers ([18F]-FDG and [18F]-NaF) are included in EANM and NCCN guidelines to identify bone metastases in BC patients. [18F]-NaF, approved by the FDA in 1972, reflects enhanced bone metabolism due to bone metastases but also due to degeneration, arthritis, or fractures [71, 72]. The repeatability of [18F]-NaF-PET/CT was evaluated in a prospective multicenter study by Lin et al. in 35 prostate cancer patients with bone metastases who underwent two pretreatment [18F]-NaF-PET/CT scans (test-retest interval 3 ± 2 days), with SUVmean as most repeatable endpoint (overview: Table 1) [18]. Repeatability of SUVmean/max, functional tumor volume (FTV50%), and total lesion [18F]-fluoride uptake (TLF) measured with [18F]-NaF-PET/CT was confirmed by Wassberg et al. [19]. Moreover, a high inter-observer agreement at the patient level was found by using three scales to define [18F]-NaF-PET/CT findings [21]. How to correctly perform and interpret [18F]-NaF-PET/CT scans is published in EANM and Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines, supporting technical standardization and harmonization [73, 74].
Clinical Validity
At present, no comparison has been performed of [18F]-NaF-PET/CT with a bone biopsy as the gold standard for the entire study population, but it has been compared with other imaging modalities. [18F]-NaF-PET/CT has a higher sensitivity to detect bone metastases than either [18F]-FDG-PET/CT or conventional bone scintigraphy with 99mTc-labeled diphosphonates (planar and SPECT) (97–100% versus 74% versus 91%, respectively). However, although the specificity of [18F]-NaF-PET/CT was higher than that of bone scintigraphy, it was slightly lower than [18F]-FDG-PET/CT (71–85% versus 63% and 97%, respectively) [39, 40•]. In general, a negative [18F]-NaF-PET/CT can be used to exclude bone metastases, but in case of positive findings, [18F]-NaF-PET/CT should be carefully interpreted and correlated with CT findings. With regard to the prognostic value of [18F]-NaF-PET/CT, one prospective study was performed in 28 BC patients with bone-dominant disease, showing no correlation between baseline SUVmax and skeletal-related events, time-to-progression or overall survival (OS) [41]. However, ΔSUVmax of 5 lesions between baseline and ~ 4 months of systemic treatment was associated with OS [41]. With regard to the predictive value of [18F]-NaF-PET/CT, two small studies showed that lack of endocrine treatment efficacy was related to an increase in metabolic flux to mineral bone or SUVmax in BC patients with bone only disease (see Table 1) [42, 44]. The national prospective oncologic PET registry of the USA showed that [18F]-NaF-PET/CT altered the treatment plan in 39% of BC patients [75]. However, the impact of [18F]-NaF-PET/CT for therapy response on clinical decision-making remains unclear due to varying endpoints and experimental procedures.
Clinical Utility
The cost-effectiveness of [18F]-NaF-PET(/CT) to detect bone metastases was assessed in a meta-analysis of 11 trials, including 425 patients (7 BC patients) [76]. It was concluded that the average cost-effective ratio was less favorable for [18F]-NaF-PET(/CT) than for conventional bone scintigraphy.
Conclusions and Recommendations of [18F]-NaF-PET/CT
While the technical validation of [18F]-NaF-PET/CT is completed, clinical validation with comparison to a biopsy as reference standard is still warranted. Also, clinical validity of [18F]-NaF-PET/CT should be further assessed with uniform endpoints. Therefore, [18F]-NaF-PET/CT has not yet passed through the necessary steps towards routine clinical practice according to the imaging biomarker roadmap. Although in bone-trope cancers such as BC, an optimal tool for diagnosis and treatment evaluation is still needed and it is unclear whether this tool could be [18F]-NaF-PET/CT.
Development Stages of [18F]-FES-PET/CT
Technical Validity
[18F]-FES-PET/CT enables the visualization of ER expression, with [18F]-FES behaving very similar to estradiol [77]. A large prospective cohort study of 90 BC patients with first recurrence/metastatic disease and preliminary results from a prospective study in 10 ER+ MBC patients showed an excellent inter-observer agreement for [18F]-FES uptake (0.90 and 0.98, respectively) [22••, 78]. Although limited data about repeatability and reproducibility are available, a recent guideline paper does provide recommendations regarding standardization of scanning time, control of pre-analytical factors that influence [18F]-FES uptake (such as discontinuation of estrogen receptor degraders > 5 weeks prior to scanning), visual analysis, and quantification of [18F]-FES uptake [77].
Clinical Validity
A meta-analysis of 9 studies (all prospective, except one) involving 238 patients reported a pooled sensitivity of 82% and specificity of 95% to detect ER+ tumor lesions by quantitative assessment of [18F]-FES uptake (overview: Table 1) [45]. A similar sensitivity and specificity was found in direct comparison of [18F]-FES uptake and ER expression on biopsy (in 5 studies including 158 BC patients) [45]. Recently, a large prospective cohort study was published involving 90 BC patients with first recurrence/metastatic disease, comparing the correlation between qualitative [18F]-FES-PET/CT results and immunohistochemistry (IHC) of ER status of the same metastatic lesion. This resulted in a positive and negative predictive value of 100% and 78%, respectively [22••]. A quantitative analysis was also performed, showing a positive and negative agreement of [18F]-FES-PET/CT (threshold SUVmax 1.5) with ER IHC equaling 85% and 79%, respectively. Despite the importance of this well-defined prospective cohort trial, its impact is likely limited due to exclusion of bone metastases, the most common metastatic site in ER+ MBC. Furthermore, an optimal SUVmax cutoff to distinguish benign from malignant lesions by [18F]-FES-PET/CT has not been established. Although SUVmax 1.5 is most commonly used for this distinction, ranges of 1.0 to 2.0 have also been described. Yang et al. determined an ROC curve in 46 ER+ BC patients, showing an optimal SUVmax cutoff of 1.8, with a sensitivity of 88% and specificity of 88% (optimal SUVmean cutoff: 1.2) [79]. The study of Nienhuis et al. in 91 ER+ MBC patients found that physiological background uptake could exceed SUVmax 1.5, for example, in the lumbar spine [80]. [18F]-FES-PET/CT scans performed in 108 individuals showed that irradiation could induce atypical (non-malignant) enhanced [18F]-FES uptake in the lungs [81]. These issues should be taken into account in interpreting [18F]-FES-PET/CT scans for the diagnosis of BC. However, these data are retrospective and should be interpreted with caution. Nonetheless, two trials have indicated usefulness of [18F]-FES-PET(/CT) for the physician by improving diagnostic understanding compared to conventional assessments in 88% of patients, and causing a treatment change in 48–49% of patients enrolled in the studies [82, 83]. Therefore, [18F]-FES-PET/CT may be a useful diagnostic tool in exceptional diagnostic dilemmas when added to a conventional workup. A prospective study involving 90 ER+ BC patients treated with endocrine therapy found that [18F]-FES-PET(/CT) may be a useful prognostic biomarker for [18F]-FDG avid tumors, demonstrating a higher median progression-free survival (PFS) in the high [18F]-FES uptake group compared to low [18F]-FES uptake group (7.9 versus 3.3 months, respectively) [48]. With regard to response prediction, a meta-analysis including 6 prospective trials and 183 patients found a pooled sensitivity of 64% and specificity of 29% to predict early or late response to hormonal therapy, with an SUVmax cutoff of 1.5, and a sensitivity of 67% and specificity of 62% with SUVmax of 2.0 [45]. In 26 patients with primary ER+ BC, randomized to neoadjuvant chemotherapy or endocrine treatment, no differences in baseline SUVmax were found between post-treatment pathological (non-) responders [49•]. In another small trial (including 18 patients), pathological response to neoadjuvant chemotherapy was related to low rather than high baseline SUVmax (1.8 versus 4.4) [84]. Overall, it is difficult to compare this data due to the heterogeneity of the trials, i.e., different endpoints, and imaging procedures.
Clinical Utility
Two computer simulation studies described the impact of [18F]-FES-PET/CT on health-related measurements, such as life years gained (LYG), ICER, and total costs (Table 1) [55, 56]. One study selected first-line treatment in MBC patients based on biopsy results or [18F]-FES-PET/CT imaging findings and showed higher diagnostic and treatment costs in the PET/CT imaging group [56]. A second study determined the number of avoided biopsies to assess MBC after the introduction of [18F]-FES-PET/CT and showed that the number of biopsies (39 ± 9%) was lower in the [18F]-FES-PET/CT imaging group [55].
Conclusions and Recommendations of [18F]-FES-PET/CT
While [18F]-FES-PET/CT is currently used in a limited number of hospitals worldwide, mostly in a research setting, but also as a diagnostic tool in exceptional diagnostic dilemmas, consistent data to support its clinical validity and utility are still lacking. Only in France is [18F]-FES approved for routine clinical use to determine ER status in MBC. In order to implement [18F]-FES-PET/CT more broadly in routine clinical practice, additional studies are needed. Within two prospective cohort trials, the multicenter IMPACT breast trial and the ECOG-ACRIN trial (NCT02398773; 99 newly diagnosed MBC patients), the analysis of baseline [18F]-FES uptake related to treatment response or PFS is ongoing. In the ongoing ET-FES TRANSCAN trial (EUDRACT 2013-000-287-29), the treatment choice is based on [18F]-FES-PET/CT (high versus low 18F-FES uptake) [85]. [18F]-FES-PET/CT is also added as integrated biomarker to another randomized controlled trial, the SONImage trial (NCT04125277). With these additional studies, sufficient evidence could potentially be generated to support implementation of [18F]-FES-PET/CT in routine clinical practice.
Development Stages of [89Zr]-Trastuzumab-PET/CT
Technical Validity
The [89Zr]-labeled antibody trastuzumab binds to the HER2-receptor and has a relatively long half-life (t ½ = 78 h). This enables imaging at late time points but also limits repeatability testing as radiation dose is high and repeated scans would require a 2-week interval [86]. To optimize the acquisition protocol, imaging at multiple time points (after 1–7 days) was performed after a single tracer injection [87, 88]. The optimal time point was found after 4–5 days, due to lower background uptake and higher contrast. Recently, a [89Zr]-PET/CT EARL accreditation program was established, similar to [18F]-FDG-PET/CT accreditation [60, 89, 90••].
Clinical Validity
No comparison of [89Zr]-trastuzumab-PET/CT with biopsy has been performed so far. In a prospective study including 34 HER2+ and 16 HER2− BC patients, an SUVmax cutoff of 3.2 showed a sensitivity of 76% and specificity of 62% to distinguish HER2+ from HER2− lesions [53]. The HER2 status was based on the primary tumor or metastatic lesion; however, a recent biopsy of a tumor lesion was not performed in all patients. Despite this relatively low discriminative value, [89Zr]-trastuzumab-PET/CT did support diagnostic understanding and resulted in a treatment change in 90% and 40% of patients respectively, in whom HER2 status could not be determined by standard workup [91]. With regard to the prognostic value of [89Zr]-trastuzumab-PET/CT no data are available, but its value to predict therapy response was assessed in the ZEPHIR trial (see Table 1) [54]. In 56 HER2+ MBC patients, qualitative analysis of baseline PET/CT scans indicated that [89Zr]-trastuzumab uptake was related to longer trastuzumab emtansine treatment duration, compared to no uptake (11.2 versus 3.5 months) [54].
Clinical Utility
A computer simulated study of a hypothetical cohort of 1000 MBC patients assessed whether [89Zr]-trastuzumab-PET/CT could replace biopsy [56]. This study concluded that total costs were higher with [89Zr]-trastuzumab-PET/CT. However, biopsy effects on quality of life were not included in the analysis.
Conclusions and Recommendations of [89Zr]-Trastuzumab-PET/CT
Although technical standardization and harmonization is supported by the recently introduced [89Zr]-PET/CT EARL accreditation program, at present, still significant knowledge gaps exist (for instance regarding the relation between biopsy and uptake on [89Zr]-trastuzumab-PET/CT) [89]. Therefore, multiple steps according to the imaging biomarker roadmap have to be taken before [89Zr]-trastuzumab-PET/CT can be implemented in clinical practice. It is expected that the previously mentioned multicenter IMPACT breast study will provide information that can advance the validation of [89Zr]-trastuzumab-PET/CT.
Other PET Tracers for Molecular Imaging in BC
Multiple new tracers of potential interest in BC can be identified (see Table 2). PET imaging of additional receptors may be the next step, for example, the hormone receptor tracer [18F]-dihydrotesterone ([18F]-FDHT)-PET, which is commonly used in prostate cancer trials. This tracer provides information about androgen receptor (AR) expression, which is a potential new target for BC treatment [46]. Moreover, cell proliferation can be detected by [18F]-fluorothymidine ([18F]-FLT)-PET, and post-neoadjuvant chemotherapy [18F]-FLT uptake may be correlated with the proliferation marker Ki-67 measured by IHC in primary BC patients [92]. In light of the current developments in BC immunotherapy, assessment of the programmed death-ligand 1 (PD-L1) with [89Zr]-labeled atezolizumab is clearly of interest. Recently, a first-in-human study with 22 patients (including 4 with triple-negative BC) showed a better correlation of [89Zr]-atezolizumab uptake to treatment response, PFS and OS at patient level than the commonly used SP142 IHC marker [93]. Currently, one recruiting [89Zr]-atezolizumab-PET study is available for lobular BC (NCT04222426). Furthermore, a combination of molecular imaging techniques, such as [18F]-FES-PET, [89Zr]-trastuzumab-PET with [18F]-FDG-PET, may be useful in identifying disease heterogeneity or differentiating between indolent and aggressive disease [48, 54, 94]. This could help to select the best therapeutic strategy.
Table 2.
Ongoing PET imaging based clinical trials including breast cancer patients (n = 48)
| Radiotracer | Target | Description of disease characteristics | Estimated enrollment | Phase | Trial ID | (estimated) Study start year | Status |
|---|---|---|---|---|---|---|---|
| [18F]-FES | ER | ER+, HER2− MBC | 60 | NA | NCT03442504 | 2017 | Recruiting |
| ER+, HER2− MBC | 8 | I/II | NCT04150731 | 2020 | Not yet recruiting | ||
| ER+ (M)BC | 60 | III | NCT03544762 | 2017 | Recruiting | ||
| ER+, HER2− MBC | 75 | II | NCT02409316 | 2015 | Recruiting | ||
| ER+ MBC | 68 | NA | NCT03768479 | 2017 | Recruiting | ||
| ER+, HER2− MBC | 104 | I | NCT03455270 | 2018 | Recruiting | ||
| ER+, HER2− locally advanced and locoregional recurrent BC | 40 | NA | NCT03726931 | 2018 | Recruiting | ||
| ER−, HER2+ MBC | 33 | NA | NCT03619044 | 2019 | Not yet recruiting | ||
| ER+ MBC | 100 | NA | NCT04125277 | 2019 | Recruiting | ||
| ER+ MBC | 99 | II | NCT02398773 | 2016 | Recruiting | ||
| ER+, HER2− MBC | 25 | NA | NCT03873428 | 2020 | Not yet recruiting | ||
| ER+ (M)BC | 100 | I | NCT01916122 | 2013 | Recruiting | ||
| ER+ recurrent BC or MBC | 100 | NA | NCT00816582 | 2010 | Active, not recruiting | ||
| Regardless of ER/HER2 status, MBC | 217 | NA | NCT01957332 | 2013 | Active, not recruiting | ||
| ER+ (M)BC | 29 | NA | NCT02149173 | 2010 | Active, not recruiting | ||
| ER+, HER2− MBC | 16 | I | NCT02650817 | 2016 | Active, not recruiting | ||
| ER+ MBC | 15 | NA | NCT01720602 | 2012 | Active, not recruiting | ||
| [18F]-FDHT | AR | AR+, HER2− MBC | 22 | II | NCT02697032 | 2016 | Active, not recruiting |
| [18F]-FTT | PARP-1 | (M)BC | 30 | NA | NCT03846167 | 2019 | Recruiting |
| BC | 30 | I | NCT03083288 | 2017 | Active, not recruiting | ||
| [18F]-ISO-1 | Sigma-2 receptor | MBC | 30 | NA | NCT03057743 | 2016 | Recruiting |
| BC | 30 | I | NCT02284919 | 2014 | Active, not recruiting | ||
| [18F]-FLT | Thymidine kinase activity | Regardless of ER/HER2 status, Rb + MBC | 20 | I | NCT02608216 | 2015 | Recruiting |
| MBC | 17 | NA | NCT01621906 | 2012 | Active, not recruiting | ||
| [18F]-FMISO | Hypoxic cells | ER−, HER2− MBC | 126 | II | NCT02498613 | 2016 | Recruiting |
| [18F]-GE-226 | HER2 | MBC | 16 | NA | NCT03827317 | 2019 | Recruiting |
| [18F]-F-GLN | Glutamine metabolism | (M)BC | 30 | NA | NCT03863457 | 2019 | Recruiting |
| [18F]-αvβ6-BP | αvβ6 | (M)BC | 27 | I | NCT03164486 | 2016 | Recruiting |
| [18F]-Var3 | Extracellular pH | MBC | 10 | I | NCT04054986 | 2019 | Recruiting |
| [18F]-Flutemetamol | Amyloid beta | BC | 15 | NA | NCT02317783 | 2015 | Recruiting |
| [18F]-FSPG | Amino acid transporter xc− | BC | 120 | NA | NCT03144622 | 2016 | Recruiting |
| [18F]-FAZA | Hypoxic cells | BC | 25 | I | NCT03168737 | 2017 | Recruiting |
| [18F]-ASIS | Tissue factor | (M)BC | 10 | I | NCT03790423 | 2019 | Recruiting |
| [89Zr]-Trastuzumab | HER2 | Regardless of ER/HER2 status, MBC | 217 | NA | NCT01957332 | 2013 | Active, not recruiting |
| [89Zr]-Atezolizumab | PD-L1 | ER−, HER2− MBC | 54 | NA | NCT02453984 | 2016 | Recruiting |
| Lobular ER+ MBC | 10 | NA | NCT04222426 | 2019 | Recruiting | ||
| [89Zr]-CED88004S | CD8 | ER−, HER2− MBC | 40 | I/II | NCT04029181 | 2019 | Recruiting |
| [89Zr]-Bevacizumab | VEGF | Inflammatory HER2− (M)BC | 10 | I | NCT01894451 | 2015 | Active, not recruiting |
| [68Ga]-ABY-025 | HER2 | HER2+ (M)BC | 120 | NA | NCT03655353 | 2018 | Recruiting |
| [68Ga]-RM2 | Gastrin-releasing peptide receptor | ER+ BC | 80 | III | NCT03731026 | 2018 | Not yet recruiting |
| [68Ga]-NOTA-Anti-HER2 VHH1 | HER2 | MBC | 20 | II | NCT03924466 | 2019 | Recruiting |
| MBC | 30 | II | NCT03331601 | 2017 | Recruiting | ||
| [68Ga]-FAPI-46 | Fibroblast activated protein | (M)BC | 30 | I | NCT04147494 | 2019 | Not yet recruiting |
| [68Ga]-PSMA-11 | Prostate specific membrane antigen | (M)BC | 30 | I | NCT04147494 | 2019 | Not yet recruiting |
| [64Cu]-DOTA-Trastuzumab | HER2 | HER2+ BC | 20 | II | NCT02827877 | 2016 | Recruiting |
| HER2+ MBC | 18 | NA | NCT01093612 | 2011 | Active, not recruiting | ||
| HER2+ MBC | 10 | NA | NCT02226276 | 2015 | Active, not recruiting | ||
| [64Cu]-DOTA-alendronate | Mammary microcalcifications | BC | 6 | I | NCT03542695 | 2020 | Not yet recruiting |
| [64Cu]-M5A | Carcinoembryonic antigen | (M)BC | 20 | NA | NCT02293954 | 2015 | Active, not recruiting |
| [13N]-NH3 | Glutamine synthetase | Locally advanced BC | 124 | II | NCT02086578 | 2014 | Active, not recruiting |
Searched for breast cancer and positron emission tomography in ClinicalTrials.gov. Only trials which have not been published and had a recruitment status of active, (not yet) recruiting were included. Combined PET/MRI scans and [18F]-FDG-PET scans were excluded
(M)BC (metastatic) breast cancer, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, AR androgen receptor, NA not applicable, PARP poly ADP ribose polymerase, PD-L1 programmed death-ligand 1, VEGF vascular endothelial growth factor
Conclusions
In this review, we identified hurdles based on the biomarker roadmap for the four most commonly used PET tracers in BC and made recommendations for the next steps towards clinical implementation. This review has summarized several important steps to be considered to successfully implement molecular biomarkers for BC patients in clinical practice. In general, support for clinical utility is still pending for PET tracers in BC, but also assessment of clinical validity is hampered by varying endpoints and procedures. Improving trial designs can contribute to solve this matter; for instance, multicenter trials require standardization and harmonization of procedures. International collaboration is essential, as this would also potentially allow building warehouses of data to overcome a plethora of small solitary single center studies. Based on these warehouses, clinical validation can be established in line with the RECIST guidelines. In this setting, considering all aspects of the biomarker roadmap at an early stage is important. Smart trial designs adding imaging biomarkers to randomized controlled trials (integrated biomarker) are desirable, as imaging biomarker–based randomized controlled trials (integral biomarker) are usually not feasible due to the large numbers of patients required [95]. From a regulatory point of view, the evidence required for implementation is still unclear, although European Medicines Agency and FDA acknowledge that a microdose radiopharmaceutical is not similar to a therapeutic drug in this respect [96, 97]. Nonetheless, establishing whether patient outcome is truly improved is essential to justify implementation of a complex, expensive tool with radiolabeled PET tracers. A considerable international, collaborative effort could potentially make this possible.
Compliance with ethical standards
Conflict of interests
Jorianne Boers, Andor W.J.M. Glaudemans and Carolina P. Schröder declare no conflict of interest. Erik F.J. de Vries has received research funding through grants from ZonMw (for PET imaging of T cells in patients with cancer), the Dutch Cancer Foundation (KWF) (for PET imaging of T cells in patients with cancer), and ZonMw/MS Research Foundation (for validation of novel tracer for PET imaging of myelin); has assisted in conducting contracted research studies funded by Rodin Therapeutics (for PET imaging of the brain in health volunteers and patients with Alzheimer's Disease), Lysosomal Therapeutics Ltd. (for PET imaging of the brain in patients with Parkinson's Disease), Hoffmann-La Roche (for dosimetry of a tracer in rats), and Ionis Pharmaceuticals (for PET imaging in patients with Alzheimer's Disease). Geke A.P. Hospers has received research funding from The Seerave Foundation and Bristol-Myers Squibb, and has served in a consulting/advisory role for Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, and Roche.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Breast Cancer
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Medicines Agency. Guideline on core SmPC and package leaflet for fludeoxyglucose (18F). Londen. 2012.
- 3.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol. 2000;126:549–559. doi: 10.1007/PL00008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, Boers M, Smit EF, Stroobants S, Weber WA, Hoekstra OS. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–708. doi: 10.2967/jnumed.111.095299. [DOI] [PubMed] [Google Scholar]
- 6.Kramer GM, Frings V, Hoetjes N, Hoekstra OS, Smit EF, de Langen AJ, Boellaard R. Repeatability of quantitative whole-body 18F-FDG PET/CT uptake measures as function of uptake interval and lesion selection in non-small cell lung cancer patients. J Nucl Med. 2016;57:1343–1349. doi: 10.2967/jnumed.115.170225. [DOI] [PubMed] [Google Scholar]
- 7.Weber WA, Gatsonis CA, Mozley PD, Hanna LG, Shields AF, Aberle DR, Govindan R, Torigian DA, Karp JS, Yu JQ, Subramaniam RM, Halvorsen RA, Siegel BA, ACRIN 6678 Research team. MK-0646-008 Research team Repeatability of 18F-FDG PET/CT in advanced non-small cell lung cancer: prospective assessment in 2 multicenter trials. J Nucl Med. 2015;56:1137–1143. doi: 10.2967/jnumed.114.147728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockall AG, Avril N, Lam R, Iannone R, Mozley PD, Parkinson C, Bergstrom D, Sala E, Sarker SJ, McNeish IA, Brenton JD. Repeatability of quantitative FDG-PET/CT and contrast-enhanced CT in recurrent ovarian carcinoma: test-retest measurements for tumor FDG uptake, diameter, and volume. Clin Cancer Res. 2014;20:2751–2760. doi: 10.1158/1078-0432.CCR-13-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraum TJ, Fowler KJ, Crandall JP, Laforest RA, Salter A, An H, Jacobs MA, Grigsby PW, Dehdashti F, Wahl RL. Measurement repeatability of (18)F-FDG PET/CT versus (18)F-FDG PET/MRI in solid tumors of the pelvis. J Nucl Med. 2019;60:1080–1086. doi: 10.2967/jnumed.118.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frings V, van Velden FHP, Velasquez LM, Hayes W, van de Ven PM, Hoekstra OS, Boellaard R. Repeatability of metabolically active tumor volume measurements with FDG PET/CT in advanced gastrointestinal malignancies: a multicenter study. Radiology. 2014;273:539–548. doi: 10.1148/radiol.14132807. [DOI] [PubMed] [Google Scholar]
- 11.Hoang JK, Das SK, Choudhury KR, Yoo DS, Brizel DM. Using FDG-PET to measure early treatment response in head and neck squamous cell carcinoma: quantifying intrinsic variability in order to understand treatment-induced change. AJNR Am J Neuroradiol. 2013;34:1428–1433. doi: 10.3174/ajnr.A3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Velden FHP, Nissen IA, Jongsma F, Velasquez LM, Hayes W, Lammertsma AA, Hoekstra OS, Boellaard R. Test-retest variability of various quantitative measures to characterize tracer uptake and/or tracer uptake heterogeneity in metastasized liver for patients with colorectal carcinoma. Mol Imaging Biol. 2014;16:13–18. doi: 10.1007/s11307-013-0660-9. [DOI] [PubMed] [Google Scholar]
- 13.Kurland BF, Peterson LM, Shields AT, Lee JH, Byrd DW, Novakova-Jiresova A, Muzi M, Specht JM, Mankoff DA, Linden HM, Kinahan PE. Test-retest reproducibility of (18)F-FDG PET/CT uptake in cancer patients within a qualified and calibrated local network. J Nucl Med. 2019;60:608–614. doi: 10.2967/jnumed.118.209544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh V, Shastry M, Engledow A, Kozarski R, Peck J, Endozo R, Rodriguez-Justo M, Taylor SA, Halligan S, Groves AM. Integrated (18)F-FDG PET/CT and perfusion CT of primary colorectal cancer: effect of inter- and intraobserver agreement on metabolic-vascular parameters. AJR Am J Roentgenol. 2012;199:1003–1009. doi: 10.2214/AJR.11.7823. [DOI] [PubMed] [Google Scholar]
- 15.Heijmen L, De Geus-Oei LF, De Wilt JHW, Visvikis D, Hatt M, Visser EP, et al. Reproducibility of functional volume and activity concentration in 18F-FDG PET/CT of liver metastases in colorectal cancer. Eur J Nucl Med Mol Imaging. 2012;39:1858–1867. doi: 10.1007/s00259-012-2233-6. [DOI] [PubMed] [Google Scholar]
- 16.Kolinger GD, Vallez Garcia D, Kramer GM, Frings V, Smit EF, de Langen AJ, et al. Repeatability of [(18)F]FDG PET/CT total metabolic active tumour volume and total tumour burden in NSCLC patients. EJNMMI Res. 2019;9:14. doi: 10.1186/s13550-019-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen JH, Fischer BM, Aznar MC, Hansen AE, Vogelius IR, Löfgren J, Andersen FL, Loft A, Kjaer A, Højgaard L, Specht L. Reproducibility of (18)F-FDG PET uptake measurements in head and neck squamous cell carcinoma on both PET/CT and PET/MR. Br J Radiol. 2015;88:20140655. doi: 10.1259/bjr.20140655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C, Bradshaw T, Perk T, Harmon S, Eickhoff J, Jallow N, Choyke PL, Dahut WL, Larson S, Humm JL, Perlman S, Apolo AB, Morris MJ, Liu G, Jeraj R. Repeatability of quantitative 18F-NaF PET: a multicenter study. J Nucl Med. 2016;57:1872–1879. doi: 10.2967/jnumed.116.177295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassberg C, Lubberink M, Sörensen J, Johansson S. Repeatability of quantitative parameters of 18F-fluoride PET/CT and biochemical tumour and specific bone remodelling markers in prostate cancer bone metastases. EJNMMI Res. 2017;7:42. doi: 10.1186/s13550-017-0289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurdziel KA, Shih JH, Apolo AB, Lindenberg L, Mena E, McKinney YY, et al. The kinetics and reproducibility of 18F-sodium fluoride for oncology using current PET camera technology. J Nucl Med. 2012;53:1175–1184. doi: 10.2967/jnumed.111.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zacho HD, Fonager RF, Nielsen JB, Haarmark C, Hendel HW, Johansen MB, et al. Observer agreement and accuracy of (18)F-sodium-fluoride PET/CT in the diagnosis of bone metastases in prostate cancer. J Nucl Med. 2020;61:344–9. [DOI] [PMC free article] [PubMed]
- 22.Chae SY, Ahn SH, Kim S-B, Han S, Lee SH, Oh SJ, et al. Diagnostic accuracy and safety of 16alpha-[(18)F]fluoro-17beta-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol. 2019;20:546–555. doi: 10.1016/S1470-2045(18)30936-7. [DOI] [PubMed] [Google Scholar]
- 23.Bertagna F, Treglia G, Orlando E, Dognini L, Giovanella L, Sadeghi R, Giubbini R. Prevalence and clinical significance of incidental F18-FDG breast uptake: a systematic review and meta-analysis. Jpn J Radiol. 2014;32:59–68. doi: 10.1007/s11604-013-0270-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X-H, Xiao C. Diagnostic value of nineteen different imaging methods for patients with breast cancer: a network meta-analysis. Cell Physiol Biochem. 2018;46:2041–2055. doi: 10.1159/000489443. [DOI] [PubMed] [Google Scholar]
- 25.Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, Wyld L, Ingram C, Wilkinson ID, Lorenz E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37:187–198. doi: 10.1016/j.ejso.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Liang X, Yu J, Wen B, Xie J, Cai Q, Yang Q. MRI and FDG-PET/CT based assessment of axillary lymph node metastasis in early breast cancer: a meta-analysis. Clin Radiol. 2017;72:295–301. doi: 10.1016/j.crad.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard KI, Julian JA, Holloway CMB, McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F, Elavathil L, O'Malley FP, Down N, Bodurtha A, Shelley W, Levine MN. Prospective study of 2-[18F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274–1279. doi: 10.1200/JCO.2011.38.1103. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Y, Wang L, Jiang X, She W, He L, Hu G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun. 2016;37:1180–1188. doi: 10.1097/MNM.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 29.Hong S, Li J, Wang S. 18FDG PET-CT for diagnosis of distant metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22:139–143. doi: 10.1016/j.suronc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22:86–91. doi: 10.1016/j.suronc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 32.Diao W, Tian F, Jia Z. The prognostic value of SUVmax measuring on primary lesion and ALN by (18)F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol. 2018;105:1–7. doi: 10.1016/j.ejrad.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Evangelista L, Cervino AR, Michieletto S, Saibene T, Ghiotto C, Guarneri V, Conte P, Reccia P, Saladini G. Diagnostic and prognostic impact of fluorine-18-fluorodeoxyglucose PET/CT in preoperative and postoperative setting of breast cancer patients. Nucl Med Commun. 2017;38:537–545. doi: 10.1097/MNM.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Jia Z, Zhou M, Ragaz J, Zhang Y-P, Wang B-Y, Wang ZH, Hu XC, Zhang YJ. The SUVmax for (18)F-FDG correlates with molecular subtype and survival of previously untreated metastatic breast cancer. Clin Nucl Med. 2013;38:256–262. doi: 10.1097/RLU.0b013e3182816318. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Wang C, Li P, Liu J, Huang G, Song S. The role of (18)F-FDG PET/CT and MRI in assessing pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2016;2016:3746232. doi: 10.1155/2016/3746232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol. 2017;27:4786–4796. doi: 10.1007/s00330-017-4831-y. [DOI] [PubMed] [Google Scholar]
- 37.Coudert B, Pierga J-Y, Mouret-Reynier M-A, Kerrou K, Ferrero J-M, Petit T, Kerbrat P, Dupré PF, Bachelot T, Gabelle P, Giard S, Coeffic D, Bougnoux P, Prevost JB, Paintaud G, Thibault G, Hernandez J, Coudert M, Arnould L, Berriolo-Riedinger A. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]
- 38.Withofs N, Grayet B, Tancredi T, Rorive A, Mella C, Giacomelli F, Mievis F, Aerts J, Waltregny D, Jerusalem G, Hustinx R. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun. 2011;32:168–176. doi: 10.1097/MNM.0b013e3283412ef5. [DOI] [PubMed] [Google Scholar]
- 39.Damle NA, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, Lata S. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol. 2013;31:262–269. doi: 10.1007/s11604-013-0179-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Sheng J, Dong Z, Xu Y, Huang Q, Pan D, et al. The diagnostic performance of (18)F-fluoride PET/CT in bone metastases detection: a meta-analysis. Clin Radiol. 2019;74:196–206. doi: 10.1016/j.crad.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Peterson LM, O’Sullivan J, Wu Q, Novakova-Jiresova A, Jenkins I, Lee JH, et al. Prospective study of serial 18F-FDG PET and18F-fluoride PET to predict time to skeletal-related events, time to progression, and survival in patients with bone-dominant metastatic breast cancer. J Nucl Med. 2018;59:1823–1830. doi: 10.2967/jnumed.118.211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azad GK, Siddique M, Taylor B, Green A, O’Doherty J, Gariani J, Blake GM, Mansi J, Goh V, Cook GJR. Is response assessment of breast cancer bone metastases better with measurement of 18 F-fluoride metabolic flux than with measurement of 18F-fluoride PET/CT SUV? J Nucl Med. 2019;60:322–327. doi: 10.2967/jnumed.118.208710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azad GK, Cousin F, Siddique M, Taylor B, Goh V, Cook GJR. Does measurement of first-order and heterogeneity parameters improve response assessment of bone metastases in breast cancer compared to SUVmax in [18F]fluoride and [18F]FDG PET? Mol Imaging Biol. 2019;21:781–789. doi: 10.1007/s11307-018-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azad GK, Taylor BP, Green A, Sandri I, Swampillai A, Harries M, Kristeleit H, Mansi J, Goh V, Cook GJR. Prediction of therapy response in bone-predominant metastatic breast cancer: comparison of [(18)F] fluorodeoxyglucose and [(18)F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging. Eur J Nucl Med Mol Imaging. 2019;46:821–830. doi: 10.1007/s00259-018-4223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evangelista L, Guarneri V, Conte PF. 18F-Fluoroestradiol positron emission tomography in breast cancer patients: systematic review of the literature & meta-analysis. Curr Radiopharm. 2016;9:244–257. doi: 10.2174/1874471009666161019144950. [DOI] [PubMed] [Google Scholar]
- 46.Venema CM, Mammatas LH, Schröder CP, van Kruchten M, Apollonio G, Glaudemans AWJM, Bongaerts AHH, Hoekstra OS, Verheul HMW, Boven E, van der Vegt B, de Vries EFJ, de Vries EGE, Boellaard R, Menke van der Houven van Oordt CW, Hospers GAP Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med. 2017;58:1906–12. [DOI] [PubMed]
- 47.Gupta M, Datta A, Choudhury P, Dsouza M, Batra U, Mishra A. Can 18F-fluoroestradiol positron emission tomography become a new imaging standard in the estrogen receptor-positive breast cancer patient: a prospective comparative study with 18F-fluorodeoxyglucose positron emission tomography? World J Nucl Med. 2017;16:133–139. doi: 10.4103/1450-1147.203071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurland BF, Peterson LM, Lee JH, Schubert EK, Currin ER, Link JM, Krohn KA, Mankoff DA, Linden HM. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+breast cancer. Clin Cancer Res. 2017;23:407–415. doi: 10.1158/1078-0432.CCR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chae SY, Kim S-B, Ahn SH, Kim HO, Yoon DH, Ahn J-H, et al. A randomized feasibility study of (18)F-fluoroestradiol PET to predict pathologic response to neoadjuvant therapy in estrogen receptor-rich postmenopausal breast cancer. J Nucl Med. 2017;58:563–568. doi: 10.2967/jnumed.116.178368. [DOI] [PubMed] [Google Scholar]
- 50.van Kruchten M, Glaudemans AWJM, de Vries EFJ, Schröder CP, de Vries EGE, Hospers GAP. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42:1674–1681. doi: 10.1007/s00259-015-3107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JH, Kang MJ, Ahn JH, Kim JE, Jung KH, Gong G, Lee HJ, Son BH, Ahn SH, Kim HH, Shin HJ, Moon DH, Kim SB. Phase II trial of neoadjuvant letrozole and lapatinib in Asian postmenopausal women with estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2)-positive breast cancer [Neo-ALL-IN]: highlighting the TILs, ER expressional change after neoadjuvant treatment and FES-PET as potential significant biomarkers. Cancer Chemother Pharmacol. 2016;78:685–695. doi: 10.1007/s00280-016-3107-6. [DOI] [PubMed] [Google Scholar]
- 52.Gong C, Yang Z, Sun Y, Zhang J, Zheng C, Wang L, Zhang Y, Xue J, Yao Z, Pan H, Wang B, Zhang Y. A preliminary study of 18F-FES PET/CT in predicting metastatic breast cancer in patients receiving docetaxel or fulvestrant with docetaxel. Sci Rep. 2017;7:6584. doi: 10.1038/s41598-017-06903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehdashti F, Wu N, Bose R, Naughton MJ, Ma CX, Marquez-Nostra BV, Diebolder P, Mpoy C, Rogers BE, Lapi SE, Laforest R, Siegel BA. Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat. 2018;169:523–530. doi: 10.1007/s10549-018-4696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, Oyen WJG, Vugts DJ, Hoekstra OS, Schröder CP, Menke-van der Houven van Oordt CW, Guiot T, Brouwers AH, Awada A, de Vries EGE, Flamen P. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 55.Koleva-Kolarova RG, Greuter MJW, van Kruchten M, Vermeulen KM, Feenstra T, Buskens E, Glaudemans AWJM, de Vries EFJ, de Vries EGE, Hospers GAP, de Bock GH. The value of PET/CT with FES or FDG tracers in metastatic breast cancer: a computer simulation study in ER-positive patients. Br J Cancer. 2015;112:1617–1625. doi: 10.1038/bjc.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koleva-Kolarova RG, Greuter MJW, Feenstra TL, Vermeulen KM, de Vries EFJ, Parkin D, Buskens E, de Bock GH. Molecular imaging with positron emission tomography and computed tomography (PET/CT) for selecting first-line targeted treatment in metastatic breast cancer: a cost-effectiveness study. Oncotarget. 2018;9:19836–19846. doi: 10.18632/oncotarget.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ESMO Guidelines Committee. Standard operating procedures (SOPs) for authors and templates for ESMO clinical practice guidelines (CPGs) and ESMO-MCBS scores Available from: https://www.esmo.org/content/download/77789/1426712/file/ESMO-Clinical-Practice-Guidelines-Standard-Operating-Procedures.pdf. Accessed January 2020.
- 58.Huang EP, Wang X-F, Choudhury KR, McShane LM, Gönen M, Ye J, et al. Meta-analysis of the technical performance of an imaging procedure: guidelines and statistical methodology. Stat Methods Med Res. 2015;24:141–174. doi: 10.1177/0962280214537394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.EARL. Available from: http://earl.eanm.org. Accessed September 2019.
- 61.Federatie Medisch Specialisten. Borstkanker - FDG-PET-CT bij PA-bevestigde borstkanker. Available from: https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html. Accessed September 2019.
- 62.Caresia Aroztegui AP, Garcia Vicente AM, Alvarez Ruiz S, Delgado Bolton RC, Orcajo Rincon J, Garcia Garzon JR, et al. 18F-FDG PET/CT in breast cancer: evidence-based recommendations in initial staging. Tumour Biol. 2017;39:1–23. doi: 10.1177/1010428317728285. [DOI] [PubMed] [Google Scholar]
- 63.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3) Ann Oncol. 2017;28:16–33. doi: 10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, ESMO Guidelines Committee Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30. [Google Scholar]
- 65.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Cancer Netw. 2017;15:433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 66.Bergh JCS, Andersson A, Bjohle J, Bosch A, Carlsson L, Dreifaldt AC, et al. Docetaxel, trastuzumab, pertuzumab versus trastuzumab emtansine as neoadjuvant treatment of HER2-positive breast cancer: results from the Swedish PREDIX HER2 trial identifying a new potential de-escalation standard? J Clin Oncol. 2019;37(15):501.
- 67.Lin NU, Guo H, Yap JT, Mayer IA, Falkson CI, Hobday TJ, Dees EC, Richardson AL, Nanda R, Rimawi MF, Ryabin N, Najita JS, Barry WT, Arteaga CL, Wolff AC, Krop IE, Winer EP, van den Abbeele A. Phase II study of lapatinib in combination with trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: clinical outcomes and predictive value of early [18F]fluorodeoxyglucose positron emission tomography imaging (TBCRC 003) J Clin Oncol. 2015;33:2623–2631. doi: 10.1200/JCO.2014.60.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulaner GA, Saura C, Piha-Paul SA, Mayer I, Quinn D, Jhaveri K, Stone B, Shahin S, Mann G, Dujka M, Bryce R, Meric-Bernstam F, Solit DB, Hyman DM. Impact of FDG PET imaging for expanding patient eligibility and measuring treatment response in a genome-driven basket trial of the Pan-HER kinase inhibitor, Neratinib. Clin Cancer Res. 2019;25:7381–7387. doi: 10.1158/1078-0432.CCR-19-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litiere S, Collette S, de Vries EGE, Seymour L, Bogaerts J. RECIST - learning from the past to build the future. Nat Rev Clin Oncol. 2017;14:187–192. doi: 10.1038/nrclinonc.2016.195. [DOI] [PubMed] [Google Scholar]
- 70.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Isakoff SJ, Ljung BM, Mankoff DA, Marcom PK, Mayer IA, McCormick B, Pierce LJ, Reed EC, Smith ML, Soliman H, Somlo G, Theriault RL, Ward JH, Wolff AC, Zellars R, Kumar R, Shead DA, National Comprehensive Cancer Network Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2012;10:821–829. doi: 10.6004/jnccn.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Medicines Agency. Guideline on core SmPC and package leaflet for sodium fluoride (18F). Londen. 2015.
- 72.Bastawrous S, Bhargava P, Behnia F, Djang DSW, Haseley DR. Newer PET application with an old tracer: role of 18F-NaF skeletal PET/CT in oncologic practice. RadioGraphics. 2014;34:1295–1316. doi: 10.1148/rg.345130061. [DOI] [PubMed] [Google Scholar]
- 73.Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, Smith GT, SNM SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–1820. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 74.Beheshti M, Mottaghy FM, Paycha F, Behrendt FFF, Van den Wyngaert T, Fogelman I, et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur J Nucl Med Mol Imaging. 2015;42:1767–1777. doi: 10.1007/s00259-015-3138-y. [DOI] [PubMed] [Google Scholar]
- 75.Hillner BE, Siegel BA, Hanna L, Duan F, Quinn B, Shields AF. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: results from the National Oncologic PET Registry. J Nucl Med. 2015;56:222–228. doi: 10.2967/jnumed.114.150391. [DOI] [PubMed] [Google Scholar]
- 76.Tateishi U, Morita S, Taguri M, Shizukuishi K, Minamimoto R, Kawaguchi M, Murano T, Terauchi T, Inoue T, Kim EE. A meta-analysis of (18)F-fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24:523–531. doi: 10.1007/s12149-010-0393-7. [DOI] [PubMed] [Google Scholar]
- 77.Venema CM, Apollonio G, Hospers GAP, Schröder CP, Dierckx RAJO, De Vries EFJ, et al. Recommendations and technical aspects of 16α-[18F]fluoro-17β-estradiol PET to image the estrogen receptor in vivo: the Groningen experience. Clin Nucl Med. 2016;41:844–851. doi: 10.1097/RLU.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 78.Mammatas LH, Venema CM, Schröder CP, van Kruchten M, Apollonio G, Glaudemans AWJM, et al. Qualitative and quantitative analyses of 18F-FES and 18F-FDHT uptake in patients with metastatic breast cancer: an interobserver variability study. Eur J Nucl Med Mol Imaging. 2017;44:s232–s233. [Google Scholar]
- 79.Yang Z, Sun Y, Xu X, Zhang Y, Zhang J, Xue J, Wang M, Yuan H, Hu S, Shi W, Zhu B, Zhang Y. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F-fluoroestradiol PET/CT. Clin Nucl Med. 2017;42:421–427. doi: 10.1097/RLU.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 80.Nienhuis HH, van Kruchten M, Elias SG, Glaudemans AWJM, de Vries EFJ, Bongaerts AHH, et al. (18) F-fluoroestradiol tumor uptake is heterogeneous and influenced by site of metastasis in breast cancer patients. J Nucl Med. 2018;59:1212–1218. doi: 10.2967/jnumed.117.198846. [DOI] [PubMed] [Google Scholar]
- 81.Venema CM, de Vries EFJ, van der Veen SJ, Dorrius MD, van Kruchten M, Schröder CP, Hospers GAP, Glaudemans AWJM. Enhanced pulmonary uptake on (18)F-FES-PET/CT scans after irradiation of the thoracic area: related to fibrosis? EJNMMI Res. 2019;9:82. doi: 10.1186/s13550-019-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y, Yang Z, Zhang Y, Xue J, Wang M, Shi W, Zhu B, Hu S, Yao Z, Pan H, Zhang Y. The preliminary study of 16alpha-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS One. 2015;10:e0116341. doi: 10.1371/journal.pone.0116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Kruchten M, Glaudemans AWJM, de Vries EFJ, Beets-Tan RGH, Schröder CP, Dierckx RA, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53:182–190. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 84.Yang Z, Sun Y, Xue J, Yao Z, Xu J, Cheng J, Shi W, Zhu B, Zhang Y, Zhang Y. Can positron emission tomography/computed tomography with the dual tracers fluorine-18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer? A pilot study. PLoS One. 2013;8:e78192. doi: 10.1371/journal.pone.0078192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gennari A, Brain E, Nanni O, Muñoz Couselo E, Harbeck N, Geiss R, et al. Molecular imaging with 18F-fluoroestradiol (18F-FES) to assess intra-patient heterogeneity in metastatic breast cancer (MBC): a European TRANSCAN program. Ann Oncol. 2017;28(suppl 5). 10.1093/annonc/mdx363.030
- 86.Jauw YWS, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse HN, Vugts DJ, Zijlstra JM, et al. Immuno-positron emission tomography with zirconium-89-labeled monoclonal antibodies in oncology: what can we learn from initial clinical trials? Front Pharmacol. 2016;7:131. doi: 10.3389/fphar.2016.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schröder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 88.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, Burkemper J, Wright BD, Frye J, Frye S, Siegel BA, Dehdashti F. [(89)Zr]Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18:952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makris NE, Boellaard R, Visser EP, de Jong JR, Vanderlinden B, Wierts R, van der Veen BJ, Greuter HJNM, Vugts DJ, van Dongen GAMS, Lammertsma AA, Huisman MC. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–267. doi: 10.2967/jnumed.113.130112. [DOI] [PubMed] [Google Scholar]
- 90.Kaalep A, Huisman M, Sera T, Vugts D, Boellaard R. Feasibility of PET/CT system performance harmonisation for quantitative multicentre (89)Zr studies. EJNMMI Phys. 2018;5:26. doi: 10.1186/s40658-018-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, de Vries EGE, Schröder CP. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–2306. doi: 10.1007/s00259-018-4099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kostakoglu L, Duan F, Idowu MO, Jolles PR, Bear HD, Muzi M, Cormack J, Muzi JP, Pryma DA, Specht JM, Hovanessian-Larsen L, Miliziano J, Mallett S, Shields AF, Mankoff DA, on behalf of the ACRIN 668 Investigative Team A phase II study of 3′-deoxy-3’-18F-fluorothymidine PET in the assessment of early response of breast cancer to neoadjuvant chemotherapy: results from ACRIN 6688. J Nucl Med. 2015;56:1681–1689. doi: 10.2967/jnumed.115.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, Oosting SF, Schröder CP, Hiltermann TJN, van der Wekken AJ, Groen HJM, Kwee TC, Elias SG, Gietema JA, Bohorquez SS, de Crespigny A, Williams SP, Mancao C, Brouwers AH, Fine BM, de Vries EGE. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–1858. doi: 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
- 94.Boers J, Venema CM, de Vries EFJ, Glaudemans AWJM, Kwee TC, Schuuring E, et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer. 2019;126:11–20. doi: 10.1016/j.ejca.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 95.de Vries EGE, Kist de Ruijter L, Lub-de Hooge MN, Dierckx RA, Elias SG, Oosting SF. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat Rev Clin Oncol. 2019;16:241–255. doi: 10.1038/s41571-018-0123-y. [DOI] [PubMed] [Google Scholar]
- 96.European Medicines Agency. Concept paper on the development of guidance on the non-clinical evaluation of radiopharmaceuticals. Londen. 2017.
- 97.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Microdose radiopharmaceutical diagnostic drugs: nonclinical study recommendations guidance for industry. Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed September 2019.