Abstract
Background
Gestational diabetes mellitus (GDM) is a disorder of glucose metabolism that occurs or is found for the first time during pregnancy. GDM is very harmful and urgently needs drug treatment to improve pregnancy outcome. PPARδ is involved in a variety of biological processes related to glycolipid metabolism in the body, suggesting that it may be closely related to insulin resistance and impaired glucose tolerance. The role of PPARδ agonist GW501516 in gestational diabetes has not been studied.
Methods
Firstly, the rat model of GDM was established. Then, fasting blood-glucose (FGB), fasting insulin (FINS), HOMA-islet resistance index (HOMA-IR) and insulin sensitivity index (ISI) of GDM rats treated with GW501516 were measured on day 3, day 10 and day 17. Glucose tolerance test was performed on the 20th day of gestation to measure glucose tolerance in rats. The expression of PPARδ and Angptl8 in islet tissues of rats was detected by Western blot and immunohistochemistry (IHC). Histopathological changes of islet were detected by HE stain; apoptosis rate of islet cells was detected by Tunel; and expression of apoptosis-related proteins in the cells was detected by Western blot. The biochemical kits were used to detect the expression of lipid metabolism-related factors in blood of GDM rats after the PPARδ agonist GW501516 treatment. Finally, the expression of SREBP-1c and GLUT2 in islet tissues was detected by RT-qPCR and IHC.
Results
The PPARδ agonist GW501516 decreased the expression of FGB, FINS and HOMA-IR in GDM rats, and we found that GW501516 decreased ISI in GDM rats. GW501516 increased glucose tolerance in GDM rats too. In GDM rats, the expression of PPARδ in islet decreased and the expression of Angptl8 increased, which was reversed by GW501516. In addition, we also found that GW501516 can improve the damaged islet tissue of GDM rats, reduce the apoptosis rate of islet cells and inhibit the expression of lipid metabolism-related factors in the blood. Finally, we found that GW501516 inhibited the expression of SREBP-1c and promoted the expression of GLUT2 in the islet tissue.
Conclusion
The PPARδ agonist GW501516 could improve the blood glucose level, damaged islet tissue and increase the insulin content in the rats with GDM, possibly by regulating the SREBP-1c/GLUT2 pathway. Our study provided a new basis for clinical treatment of GDM in pregnant women with PPARδ agonist GW501516.
Keywords: PPARδ, GW501516, gestational diabetes mellitus, GDM, SREBP-1c/GLUT2 pathway
Introduction
Gestational diabetes mellitus (GDM) refers to the normal glucose metabolism before pregnancy and the phenomenon of the decline of potential glucose tolerance and diabetes that occurs during pregnancy. More than 80% of pregnant women with diabetes are GDM.1 GDM is very harmful, not only affect the pregnant woman, which leads to dystocia, retinopathy and an increased risk of type 2 diabetes, but also leads to fetal malformation, stillbirth and neonatal respiratory disease caused by immature fetal lungs.2 Therefore, diabetes treatment and intervention should be taken in time to control the blood glucose level and improve their pregnancy outcomes.
Peroxisome proliferators-activated receptor (PPAR), as a class of nuclear transcription factors, can be activated by corresponding ligands and is considered to be a member of the nuclear receptor superfamily. PPAR is divided into three subtypes: PPARα, PPAR δ and PPARγ3 PPAR δ are widely distributed in adipose tissue, muscle tissue and nerve tissue, especially in tissues related to glucose and lipid metabolism. Activation of PPAR δ stimulates the oxidation of fatty acids in adipose tissue and skeletal muscle, which improve dyslipidemia in mice and humans.4 In BXD mice, the expression of PPAR δ in muscle was positively correlated with endurance performance and activation of PPAR δ can effectively inhibit glucose catabolic metabolism without affecting muscle fiber type or mitochondrial content.5 This suggests that PPAR δ may be closely related to insulin resistance and impaired glucose tolerance. However, the expression and the role of PPAR δ in GDM are unknown.
GW501516 is a selective ligand of PPAR δ, which can reduce apoptosis of pancreatic beta cells of mice caused by palmitate (PAM) and LPS,6,7 and dysfunction of pancreatic beta cells induced by elevated free fatty acid (FFA) cycle now was considered as important pathological reasons for type 2 diabetes.8 However, the role of PPAR δ agonist GW501516 in GDM has not been studied.
Angiopoietin-like protein 8 (Angptl8) is secreted protein factor discovered in recent years, which are closely related to sugar metabolism, lipid metabolism and insulin sensitivity.9,10 Studies have shown that Angptl8 affects plasma lipoprotein levels by regulating glucose homeostasis and insulin resistance.11 So, Angptl8 can also be used as a marker for the development of GDM.
Sterol regulatory element-binding protein 1c (SREBP-1c) is an important transcription factor regulating lipid synthesis and glucose metabolism. Overexpression of SREBP-1c can lead to increased lipid synthesis and disorder of glucose and lipid metabolism, which plays an important role in the occurrence and development of GDM.12 Studies have shown that SREBP-1c in liver cells of diabetic rats can mediate the expression of GLUT2 gene stimulated by glucose.13,14 GLUT2 is mainly expressed in the basolateral membrane of the proximal tubules of the liver and pancreas and plays an important role in glucose homeostasis in the organism.15 In addition, Kramer et al16 reported that GW501516 and GW0742, specific activators of PPAR δ, can directly affect the insulin action of skeletal muscles by targeting the regulation of SREBP 1c/GLUT2 pathway. Therefore, it was speculated that whether GW501516 acted through the SREBP 1c/GLUT2 pathway in GDM rats.
Therefore, the purpose of this study was to detect the expression of PPAR δ in the pancreas of GDM rats and to investigate the role of its agonist GW501516 in lipid metabolism and insulin resistance in GDM rats. Our study provided a new basis for clinical treatment of GDM in pregnant women with PPARδ agonist GW501516.
Materials and Methods
Establishment of Animal Model
Fifty healthy female Wistar rats (180 ~ 220 g) were adaptively fed a basic diet for 1 week before modeling. Then, they were given with a high-fat feed (corn starch 24.15%, casein 20%, dextrin 13.2%, sugar 10%, 0.1% cholesterol, 22% lard, 5% cellulose and 3.5% minerals, vitamins, 1% 0.3% l-cystine, choline chloride 0.25%, bile salts, 0.5%) for 8 weeks. The ratio of males to females in the same cage was 1:2, and they were allowed to spend the night together and the female mice whose sperm was observed by microscopic examination were labeled as pregnant mice. Fasting for 12h on the first morning of pregnancy before treatment, a small dose of streptozotocin (STZ)(35mg/kg) was intraperitoneal injection, which was dissolved with citrate–sodium citrate buffer at pH4.4, while the blank control group was only given buffer injection. After the injection for 72h (the 4th day of gestation), fasting blood glucose was measured to be stable above 13.8mmol/L and gestational diabetes mellitus was considered to have been successfully modeled.
Experimental Grouping and Administration
The animals were divided into normal pregnant female rats (control, n=10), gestational diabetes mellitus (GDM, n=10), GW501516 (1mg/kg, n=10), GW501516 (3mg/kg, n=10), GW501516 (10mg/kg, n=10), and metformin hydrochloride (DMBG, 200mg/kg · d, n=10). Both GW501516 groups and GDM group were feed with high-fat feed. After the successful establishment of the GDM model (the 4th day of gestation), gavage of GW501516 in different doses was given for 20 days, once a day. The control group and the model group were given normal saline.
Hematoxylin–Eosin (HE) Stain
Islet tissue was brought into 4% paraformaldehyde solution for several days and subsequently 5 mm longitudinal sections were cut using a rotary microtome (Fa. Leica Biosystems Nussloch GmbH, Germany). These sections were stained with hematoxylin–eosin (HE) according to the manufacturer ‘s instructions.
Immunohistochemistry (IHC)
The paraffin sections were blocked with 2% milk for 1 h and probed with the primary antibody (1:800) overnight at 4°C. After washing, the sections were incubated for 2 h at room temperature in the appropriate biotinylated secondary antibodies (diluted 1:500; all purchased from Vector Labs). According to the manufacturer’s instructions, the slides were incubated with the DAB kit for 10min. All tissue sections were examined by light microscopy (BX51, Olympus, Japan).
TUNEL Assay
Paraffin-embedded sections were stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (In Situ Cell Death Detection Kit, Roche). In Situ Cell Death Detection Kit with fluorescein-dUTP (Roche) was used to detect TUNEL+ cells. Islet cells were analyzed using Nikon Eclipse E400 Microscope equipped with a Nikon DS-Ri1 camera using NIS-Elements Microscope Imaging Software or Spot Advance Software. The percentage of apoptotic cells was calculated from randomly selected fields.
Western Blot Assay
Islet tissue lysates were prepared using tissue protein extraction reagent (T-PER), which were then subjected to centrifugation at 4 °C with a rotation speed of 10,000 rpm for 10 min. The protein concentration was measured using BCA kit (Beyotime). Samples underwent denaturation at 95 °C for 10 min and store overnight at −80°C. The proteins separated on the gel were transferred onto PVDF membranes and after transfer, membrane containing proteins were blocked with 5% BSA for 1 h at room temperature. Then, the blots were washed effectively with TBST for 3 times and incubated with diluted primary antibodies at 4 °C overnight. The next day, blots were washed and treated with respective secondary antibodies for 1 h at room temperature and expression of the proteins of various experimental groups was obtained using enhanced chemiluminescence reagent (GE Healthcare) and Image J software (version 146; National Institutes of Health, Bethesda) was used to analyze fold-changes of protein levels. Anti-cleaved-caspase3 (1:1000; 9953S), anti-Bcl2 (1:1000; ab32124), anti-Bax (1:1000; ab32503), anti-SREBP-1c (1:1000; ab133125), anti-GLUT2 (1:1000; ab54460), anti-GAPDH (1:1000; ab181602) antibodies were obtained from Abcam.
ELISA Assay
The levels of high-density lipoprotein (HDL) and low-density lipoprotein cholesterol (LDL) and insulin were measured by ELISA according to the manufacturer’s instructions (ThermoFisher, USA). The absorbance was measured using a VersaMax microplate reader (Molecular Devices Corporation, CA, USA).
Determination of Triglyceride (TG) and Total Cholesterol (TC)
TG and TC were measured in the blood of GDM rats using cholesterol and triglyceride assay kits (Cayman Chemical, Ann Arbor, MI, USA) following instructions from the manufacturer.
Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from cultured cells using RNAzol RT (Sigma-Aldrich, USA), following the manufacturer’s protocol. The RNA concentration and quantification were assessed using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.). After DNase I digestion, QuantiTect Reverse Transcription Kit (QIAGEN China, Shanghai, China) was used to carry out reverse transcriptions to synthesize cDNA, according to the manufacturer’s protocols. Subsequently, cDNA was analyzed using QuantiTect SYBR Green PCR Kit (QIAGEN China, Shanghai, China), according to the manufacturer’s protocols. Amplification conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. Primer sequences used in PCR were obtained from GenScript (Piscataway, NJ, USA). The primer sequences are as follows: SREBP-1c forward: 5ʹ- AGTAGCAGCGGTGGAAGT −3ʹ; SREBP-1c reverse: 5ʹ-CTTCCTGTATGTGCACTGGCGGTCCT-3ʹ. GLUT2 forward: 5′- GTCAGAAGACAAGATCACCGGA-3′; GLUT2 reverse: 5′- AGGTGCATTGATCACACCGA-3′. GAPDH forward: 5ʹ-AGCCACATCGCTCAGACAC-3ʹ; GAPDH reverse: 5ʹ-GCCCAATACGACCAAATCC-3ʹ. GAPDH was included as an internal control and 2−ΔΔCq method was used for statistical analysis.17
Statistical Analysis
Data are expressed as mean ± SD. Statistically significant values were compared using T-test, and P-values of less than 0.05 were considered statistically significant.
Results
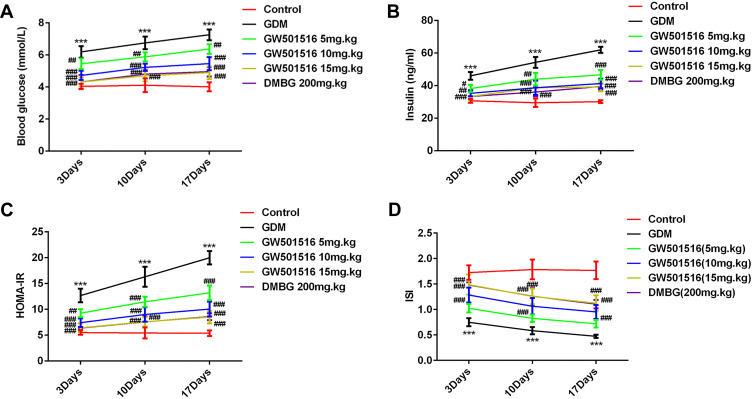
GW501516 Decreased Fasting Blood Glucose (FBG), Fasting Insulin (FINS), HOMA-Islet Resistance Index (HOMA-IR) Expression and Increased Insulin Sensitivity Index (ISI) Expression in GDM Rats
FBG and FINS in each group were examined when GDM rats were treated with GW501516 on 3rd, 10th, an 17th day, as shown in Figure 1, we found that compared with the control group, the level of FBG, FINS and HOMA-IR in GDM rats increased and the level of ISI decreased significantly; Compared with the GDM group, the levels of FBG, FINS and HOMA-IR in rats treated with GW501516 decreased in dose dependence, while the levels of ISI increased significantly. No difference in FBG, FINS, HOMA-IR and ISI levels between the GW501516 (15mg.kg) and DMBG was observed.
Figure 1.
GW501516 decreased fasting blood glucose (FBG), fasting insulin (FINS), HOMA-islet resistance index (HOMA-IR) expression and increased insulin sensitivity index (ISI) expression in GDM rats. (A) Expression level of FBG in rats. (B) Expression level of FINS in rats. (C) Expression level of HOMA-IR. Calculation method: HOMA-IR = FBG ×FINS/22.5. (D) Expression level of ISI. Calculation method: ISI=1/(FBG×FINS), where insulin unit was converted as (1 ng/mL=21.2 mIU/L). n=10, ***p<0.001 vs control; #p<0.05, ##p<0.01, ###p<0.001 vs GDM.
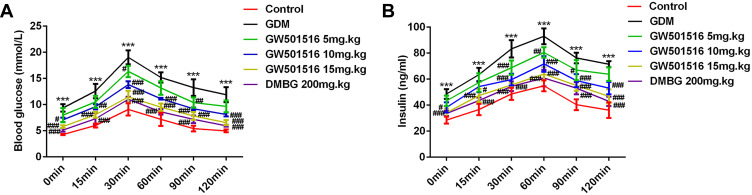
GW501516 Increased Glucose Tolerance in GDM Rats
After 21 days of treatment with GW501516, the FBG levels at 0, 15, 30, 60, 90 and 120 min in the GDM group were higher than those in the control group. The FBG levels at all of the above time points were lower in the GW501516 group than in the GDM group (Figure 2A). For the insulin test, rats in the GDM group had higher FINS levels compared with the control group; It was obvious that this was reversed with GW501516 treatment (Figure 2B).
Figure 2.
GW501516 increased glucose tolerance in GDM rats. OGTT assay measured the expression levels of FBG (A) and FINS (B) in rats. n=10, ***p<0.001 vs control; #p<0.05, ##p<0.01, ###p<0.001 vs GDM.
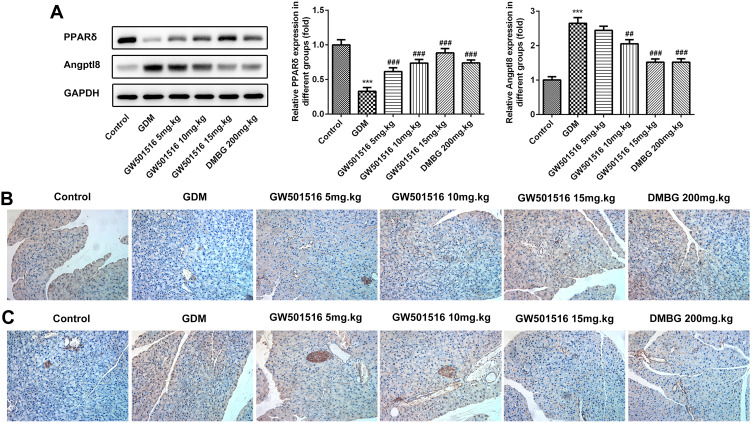
GW501516 Increased the Expression of PPARδ in GDM Rats
We examined the expression of PPARδ in the islet tissues of rats and found that compared with the control group, the expression of PPARδ in the GDM group was significantly decreased, while GW501516 showed a dose-dependent improvement in the decline of PPAR δ, and DMBG also improved the decline (Figure 3A and B). In addition, the expression of angiopoietin-like protein 8 (Angptl 8), which is closely related to carbohydrate metabolism, lipid metabolism and insulin sensitivity, was also detected in pancreatic tissues. We found that compared with the control group, Angptl8 expression increased significantly in the GDM group, while the expression of Angptl8 decreased in a dose-dependent manner after GW501516 treatment, which was consistent with that of the DMBG group (Figure 3A and C).
Figure 3.
GW501516 increased the expression of PPARδ in GDM rats. The expression levels of PPAR δ and Angptl8 in the pancreas were detected by Western blot (A) and IHC of PPAR δ (B) and Angptl8 (C). n=3, ***p<0.001 vs control; ##p<0.01, ###p<0.001 vs GDM.
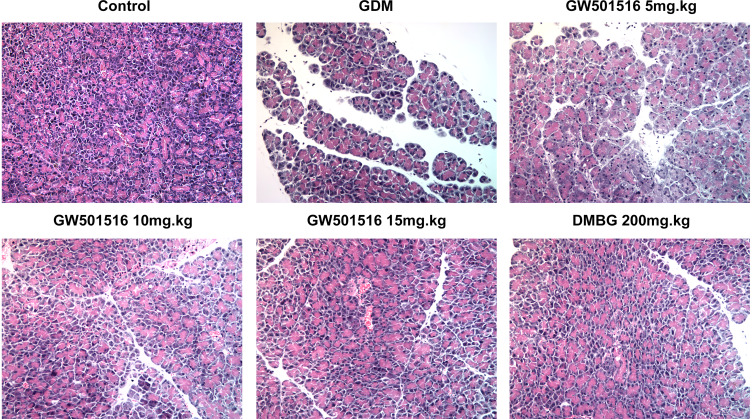
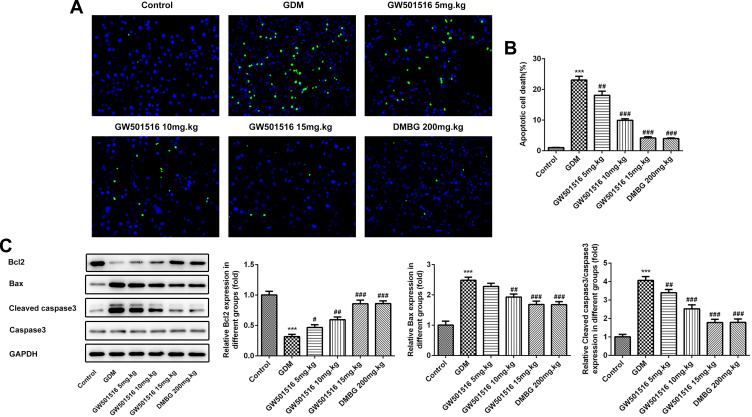
GW501516 Improved the Damaged Islet Tissue and Reduced the Apoptosis Rate of Islet Cells in GDM Rats
We detected the histopathological changes of islet in rats by HE stain, and we found that the islet tissues of the GDM group were severely damaged, while the islet tissues of GDM rats were improved after treatment with GW501516 and DMBG (Figure 4). Subsequently, we detected the apoptosis of islet cells. We found that compared with the control group, the apoptosis of islet cells in the GDM group was severe (Figure 5A and B), accompanied by increased expression of Bax and Cleaved caspase3, and decreased expression of Bcl-2 (Figure 5C). Compared with the GDM group, GW501516 and DMBG showed improved apoptosis, decreased expression of Bax and Cleaved caspase3, and increased expression of Bcl-2.
Figure 4.
GW501516 improved the damaged islet tissue in GDM rats. Histopathological changes of rat islet tissue were detected by HE staining. n=3.
Figure 5.
GW501516 reduced the apoptosis rate of islet cells in GDM rats. (A) Tunel staining detected changes in apoptosis level of islet cells. (B) Statistical analysis of apoptosis rate. (C) Western blot was used to detect the expression of apoptosis-related proteins. n=3, ***p<0.001 vs control; #p<0.05, ##p<0.01, ###p<0.001 vs GDM.
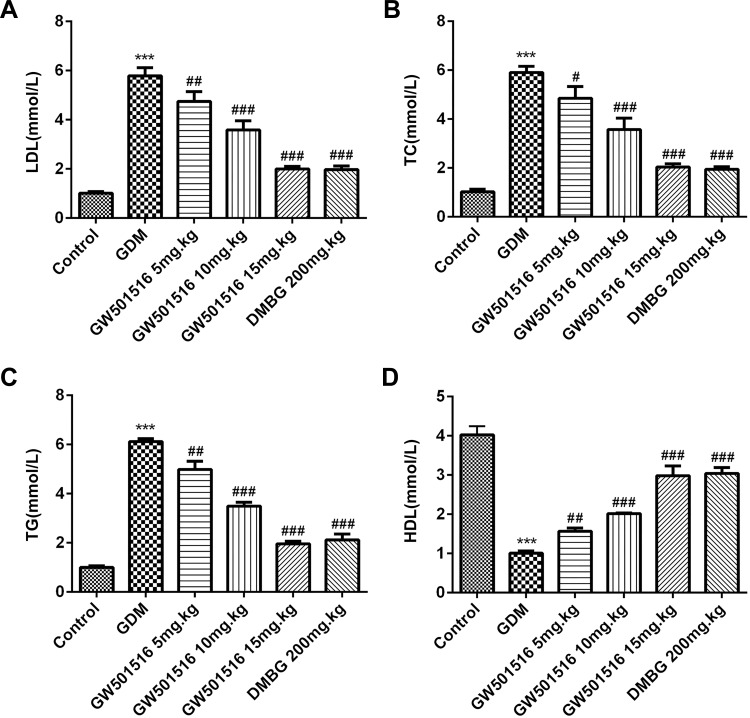
GW501516 Inhibited the Expression of Lipid Metabolism-Related Factors in Blood
We tested the expression of lipid metabolism-related factors: low-density lipoprotein cholesterol (LDL), triglyceride (TG), total cholesterol (TC) and high-density lipoprotein (HDL). The results showed that the expressions of LDL, TG and TC in GDM rats were significantly increased, while the expressions of HDL were decreased. Compared with GDM group, after GW501516 and DMBG treatment, the expressions of LDL, TG and TC decreased, while the expressions of HDL increased obviously (Figure 6).
Figure 6.
GW501516 inhibited the expression of lipid metabolism-related factors in blood. The expressions of LDL (A), TC (B), TG (C) and HDL (D) were determined by the corresponding kit. n=10, ***p<0.001 vs control; #p<0.05, ##p<0.01, ###p<0.001 vs GDM.
GW501516 May Have a Therapeutic Effect on GDM Through Regulating the SREBP-1c/GLUT2 Pathway
To further explore the possible targets of GW501516 in the treatment of GDM, we detected the expression of sterol regulatory element-binding protein 1c (SREBP-1c) and GLUT2. We found that compared with the control group, the expression of SREBP-1c in the GDM group increased, while the expression of SREBP-1c decreased in a dose-dependent manner after GW501516 treatment. The trend of GLUT2 was contrary to that of SREBP-1c (Figure 7A–C). From this, we preliminarily concluded that GW501516 may have a therapeutic effect on GDM by regulating the SREBP-1c/GLUT2 pathway.
Figure 7.
GW501516 may have a therapeutic effect on GDM through regulating the SREBP-1c/GLUT2 pathway. Expression levels of SREBP-1c (A) and GLUT2 (B) in the pancreas were detected by RT-qPCR and immunofluorescence staining of SREBP-1c and GLUT2 (C). The sample size of RT-qPCR was n=5, and the sample size of IHC staining was n=3, ***p<0.001 vs control; ##p<0.01, ###p<0.001 vs GDM.
Discussion
PPAR receptor is a key regulator of adipocyte differentiation and lipid metabolism, which can maintain the stability of blood glucose and lipid, and its activation is necessary for adipogenesis and insulin sensitivity.18 PPAR is divided into three subtypes: PPARα, PPAR δ and PPAR γ. Studies have shown that the expression of PPAR γ in placenta tissues and adipose tissues is decreased.19,20 And PPAR γ is involved in the regulation of genes related to lipid synthesis and storage and the production of adipokines and insulin signaling.21,22 Thiazolidinediones are PPAR γ agonists that can improve the sensitivity of diabetic patients and insulin resistance models.23 At present, there are few studies on PPAR δ, glucose metabolism and lipid metabolism. Doktorova found that activation of PPAR δ stimulates the oxidation of fatty acids in adipose tissue and skeletal muscle, improving dyslipidemia in mice and humans.4 In BXD mice, the expression of PPAR δ in muscle was positively correlated with endurance performance and activation of PPAR δ can effectively inhibit glucose catabolic metabolism without affecting muscle fiber type or mitochondrial content.5 However, studies on the expression and role of PPAR δ in GDM have not been reported.
After successfully establishing the GDM rat model, we found that the expression of PPAR δ was significantly decreased in the GDM rat model, which was consistent with the literature we reviewed, indicating that PPAR δ receptor plays an important role in regulating the balance between lipid metabolism and glucose metabolism in GDM. In addition, angiotensin 8 (Angptl8) expression was significantly increased in GDM rats and decreased in a dose-dependent manner after GW501516 treatment. This is because Angptl8 plays an important role in glucose metabolism during insulin resistance.24 In 2013, Melton also found that Angptl8 could specifically promote the proliferation of pancreatic β cells and promote the production of insulin, thereby lowering blood glucose.25
In this paper, we treated GDM rats with different concentrations of PPAR δ receptor agonist GW501516 and found that the expression levels of FBG, FINS and HOMA-IR in GDM rats increased, while ISI levels in GDM rats decreased. Meanwhile, GW501516 can increase the glucose tolerance, improve the damaged islet tissue, and reduce the apoptosis rate of islet cells of GDM rats. Our results are also consistent with those of Li et al.26 In addition, in our experiment, we only studied the effect of GW501516 on islet tissues and islet cells of GDM rats. Is the effect of GW501516 on other tissues and cells of GDM rats the same as that of islet tissues? Magliano et al27 found that short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system. Early controlled release of peroxisome proliferator-activated receptor β/δ agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment and GW501516 can facilitate keratinocyte migration.28 We will continue to study the effects of GW501516 on other organs of GDM rats in later experiments. We also found that GW501516 inhibited the expression of lipid metabolism-related factors in the blood. The results showed that GW501516 had certain therapeutic effect on gestational diabetes.
We investigated the mechanism of GW501516 in the treatment of GDM. Through literature review, we found that GW501516 could restore the expression level of SREBP 1c and GLUT2 in non-alcoholic fatty liver disease to normal. SREBP 1c and GLUT2 play important roles in fat synthesis and glucose metabolism homeostasis. Meanwhile, Kramer et al16 reported that GW501516 and GW0742, specific activators of PPAR δ, can directly affect the insulin action of skeletal muscles by targeting the regulation of SREBP 1c/GLUT2 pathway. Therefore, we speculated whether the therapeutic effect of GW501516 on GDM rats was related to the SREBP 1c/GLUT2 pathway? In our paper, we found that after GDM rats were treated with GW501516, the expression of SREBP 1c decreased in a dose-dependent manner, while the expression of GLUT2 increased in a dose-dependent manner. SREBP 1c is an important transcription factor regulating lipid synthesis and glucose metabolism. Overexpression of SREBP 1c can lead to increased fat synthesis and disorder of glucose and lipid metabolism. GLUT2 plays an important role in glucose homeostasis in organisms. Therefore, we preliminarily concluded that GW501516 may have a therapeutic effect on GDM rats by regulating the SREBP 1c/GLUT2 pathway. The mechanism will be further discussed in our laboratory later.
Conclusion
The PPAR δ agonist GW501516 could improve the blood glucose level, damaged islet tissue and increase the insulin content in the rats with GDM, possibly by regulating the SREBP-1c/GLUT2 pathway.
Funding Statement
This study was supported by the 2018 Shenzhen Science and Technology Commission International Cooperation Project [grant number GJHZ20180410144638700].
Data Sharing Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by ethics committee of the Shenzhen People’s Hospital. All of the procedures were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Committee on Practice B-O. ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. doi: 10.1097/AOG.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 2.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur J Med Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067 [DOI] [PubMed] [Google Scholar]
- 4.Doktorova M, Zwarts I, Zutphen TV, et al. Intestinal PPARdelta protects against diet-induced obesity, insulin resistance and dyslipidemia. Sci Rep. 2017;7(1):846. doi: 10.1038/s41598-017-00889-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan W, Waizenegger W, Lin CS, et al. PPARdelta promotes running endurance by preserving glucose. Cell Metab. 2017;25(5):1186–1193 e1184. doi: 10.1016/j.cmet.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24(9):934–945. doi: 10.1111/j.1464-5491.2007.02186.x [DOI] [PubMed] [Google Scholar]
- 7.Wan J, Jiang L, Lu Q, Ke L, Li X, Tong N. Activation of PPARdelta up-regulates fatty acid oxidation and energy uncoupling genes of mitochondria and reduces palmitate-induced apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun. 2010;391(3):1567–1572. doi: 10.1016/j.bbrc.2009.12.127 [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Ren J, Tong Y, Hu X, Lv Q, Tong N. Protective role of PPARdelta in lipoapoptosis of pancreatic beta cells. Lipids. 2016;51(11):1259–1268. doi: 10.1007/s11745-016-4190-5 [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Chen X, Chen X, et al. Angiopoietin-like protein 8 in early pregnancy improves the prediction of gestational diabetes. Diabetologia. 2018;61(3):574–580. doi: 10.1007/s00125-017-4505-y [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Perez B, Ejarque M, Gutierrez C, et al. Angiopoietin-like protein 8 (ANGPTL8) in pregnancy: a brown adipose tissue-derived endocrine factor with a potential role in fetal growth. Transl Res. 2016;178:1–12. doi: 10.1016/j.trsl.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 11.Yi P, Park JS, Melton DA. Retraction notice to: betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2017;168(1–2):326. doi: 10.1016/j.cell.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaka T, Shimano H, Yahagi N, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53(3):560–569. doi: 10.2337/diabetes.53.3.560 [DOI] [PubMed] [Google Scholar]
- 13.Im SS, Kang SY, Kim SY, et al. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54(6):1684–1691. doi: 10.2337/diabetes.54.6.1684 [DOI] [PubMed] [Google Scholar]
- 14.Marks J, Carvou NJC, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol. 2003;553(1):137–145. doi: 10.1113/jphysiol.2003.046268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rencurel F, Waeber G, Antoine B, et al. Requirement of glucose metabolism for regulation of glucose transporter type 2 (GLUT2) gene expression in liver. Biochem J. 1996;314(Pt 3):903–909. doi: 10.1042/bj3140903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer DK, Al-Khalili L, Perrini S, et al. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator-activated receptor delta. Diabetes. 2005;54(4):1157–1163. doi: 10.2337/diabetes.54.4.1157 [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 18.Costa V, Foti D, Paonessa F, et al. The insulin receptor: a new anticancer target for peroxisome proliferator-activated receptor- (PPAR) and thiazolidinedione-PPAR agonists. Endocr Relat Cancer. 2008;15(1):325–335. doi: 10.1677/ERC-07-0226 [DOI] [PubMed] [Google Scholar]
- 19.Capobianco E, Martinez N, Fornes D, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1–2):7–15. doi: 10.1016/j.mce.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282(3):E522–E533. doi: 10.1152/ajpendo.00124.2001 [DOI] [PubMed] [Google Scholar]
- 21.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camacho A, Huang JK, Delint-Ramirez I, et al. Peroxisome proliferator-activated receptor gamma-coactivator-1 alpha coordinates sphingolipid metabolism, lipid raft composition and myelin protein synthesis. Eur J Neurosci. 2013;38(5):2672–2683. doi: 10.1111/ejn.12281 [DOI] [PubMed] [Google Scholar]
- 23.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest. 2000;106(4):467–472. doi: 10.1172/JCI10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rong Guo X, Li Wang X, Chen Y, et al. ANGPTL8/betatrophin alleviates insulin resistance via the Akt-GSK3beta or Akt-FoxO1 pathway in HepG2 cells. Exp Cell Res. 2016;345(2):158–167. doi: 10.1016/j.yexcr.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun. 2012;424(4):786–792. doi: 10.1016/j.bbrc.2012.07.038 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Xu S, Liu Y, et al. Activated PPARbeta/delta protects pancreatic beta cells in type 2 diabetic goto-kakizaki rats from lipoapoptosis via GPR40. Lipids. 2019;54(10):603–616. doi: 10.1002/lipd.12182 [DOI] [PubMed] [Google Scholar]
- 27.Magliano DC, Penna-de-Carvalho A, Vazquez-Carrera M, Mandarim-de-Lacerda CA, Aguila MB. Short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system. Endocrine. 2015;50(2):355–367. doi: 10.1007/s12020-015-0590-1 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Sng MK, Foo S, et al. Early controlled release of peroxisome proliferator-activated receptor beta/delta agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment. J Control Release. 2015;197:138–147. doi: 10.1016/j.jconrel.2014.11.001 [DOI] [PubMed] [Google Scholar]