Abstract
Background
Glioma is a common malignant tumor worldwide. Sevoflurane (Sev) has been reported to inhibit the metastasis of glioma cells, but the underlying molecular mechanism needs further exploration.
Methods
Cell Counting Kit-8 (CCK8) assay was used to check cell viability. Flow cytometry assay was hired to check cell apoptosis. The protein levels of B-cell lymphoma-2 (Bcl-2), BCL2-Associated X (Bax), hexokinase 2 (HK2) and magnesium transporter 1 (MAGT1) in samples were measured by Western blot. The abilities of cell migration and invasion were estimated by transwell assay. Glucose colorimetric assay kit and lactate colorimetric assay kit were used to check glucose consumption and lactate production, respectively. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect the levels of circular RNA (circRNA) circ_0002755 (also known as the circRNA1656) and microRNA (miR)-628-5p in samples. The interaction between miR-628-5p and circ_0002755 or MAGT1 was predicated by starBase, which was verified by the dual-luciferase reporter assay. Xenograft tumor model was established to explore the biological role of circ_0002755 in vivo.
Results
Sev inhibited cell viability, migration, invasion and promoted cell apoptosis, and also reduced glucose consumption and lactate production. Circ_0002755 was significantly upregulated in glioma tissues and cells, while its level was notably declined under Sev treatment. Besides, overexpression of circ_0002755 overturned Sev-mediated inhibitory effect on glioma progression. Further research indicated that circ_0002755 targeted miR-628-5p, and miR-628-5p targeted MAGT1, and Sev modulated glioma progression via circ_0002755/miR-628-5p/MAGT1 axis. Moreover, Sev hindered tumor growth in vivo.
Conclusion
Sev mediated glioma progression via circ_0002755/miR-628-5p/MAGT1 axis.
Keywords: Sev, glioma, circ_0002755, miR-628-5p, MAGT1
Introduction
Glioma, starting in the glial cells of the brain or the spine,1,2 comprises nearly 80% of all malignant brain tumors.3 Sevoflurane (Sev), a class of common anesthetics, was reported to inhibit migration and invasion of glioma cells.4 However, the regulatory mechanism of Sev in glioma remains poorly understood.
Circular RNAs (CircRNAs), a class of single-stranded RNA that forms a covalently closed continuous loop, are produced by backsplicing and have the resistance to exonuclease-mediated degradation.5 CircRNAs were verified to be associated with various human cancer,6,7 including glioma.8–10 A previous study showed that circ_0002755 could act as a biomarker in high-grade serous ovarian cancer.11 Nevertheless, the role and function of circ_0002755 in glioma is still poorly understood.
MicroRNAs (MiRNAs) are highly conserved small noncoding RNA molecules (about 22 nucleotides in length), and modulate gene expression mainly through binding to the 3ʹ-untranslated region (3ʹUTR) of messenger RNA (mRNA) at the post-transcriptional level.12 Emerging evidence showed that Sev inhibited cancer progression by regulating miRNAs. Sun et al reported that Sev repressed migration and invasion of colorectal cancer cells via regulating microRNA-34a/ADAM10 axis.13 Gao et al confirmed that Sev suppressed glioma cells proliferation and metastasis by miRNA-124-3p/ROCK1 axis.14 Lately, Xie et al found that miR-628-5p repressed cell proliferation in glioma.15 But the role of miR-628-5p in Sev-mediated glioma progression is little known and worthy of investigation.
Magnesium transporter 1 (MAGT1) was reported to be correlated with diverse human cancers. Zheng et al reported that overexpression of MAGT1 led to the poor prognosis of colorectal cancer.16 Wang et al found that microRNA-199a-5p inhibited glioma progression by inhibiting MAGT1.17 Therefore, MAGT1 might be an attracting drug target for glioma and its function in Sev-mediated glioma progression should be explored.
In this research, we first investigated the effect of Sev on glioma progression. Afterwards, the potential mechanism of Sev in regulating glioma progression was investigated by bioinformatics analysis and subsequent experiments.
Materials and Methods
Specimens and Cell Culture
Glioma tissues and normal brain tissues were collected from The Second Affiliated Hospital of Dalian Medical University. The informed consent was acquired from every participant and our research was authorized by the Ethics Committee of The Second Affiliated Hospital of Dalian Medical University (IRB No.DLMU20190318), the research has been carried out in accordance with the World Medical Association Declaration of Helsinki and all patients had signed the written informed consents.
Normal human astrocytes (NHA) were purchased from Bena Culture Collection (Beijing, China), human glioma cell lines (A-172 and SHG-44) were obtained from MLbio (Shanghai, China). McCoy’s 5A medium (XP Biomed, Shanghai, China), containing 5% CO2 and 10% fetal bovine serum (FBS; Solarbio, Beijing, China)was used to culture cells. For Sev treatment, cells were first treated with various concentrations of Sev (1.7%, 3.4% and 5.1%) for 6 h and then the cells were normally cultured for 24 h for further investigation according to a previous report.4
Cell Transfection
Circ_0002755 overexpression plasmid (named as circ_0002755) and its matched control (named as vector) were acquired from RiboBio (Guangzhou, China). MiR-628-5p mimic (named as miR-628-5p mimic), miR-628-5p inhibitor (named as anti-miR-628-5p) and small interfering RNA against MAGT1 (named as si-MAGT1, sequence: 5ʹ-GAAGAAUGGUACAAAUCCAAG-3ʹ), and the corresponding controls (miR-NC, anti-miR-NC and si-NC, sequence: 5ʹ-UAUCGCCGUAGACCCACU-3ʹ) was obtained from GenePharma (Shanghai, China). Cell transfection experiment was executed using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) following the provided methods.
Counting Kit-8 (CCK8) Assay
A-172 and SHG-44 cells were seeded into 96-well plates and then 10 μL CCK8 solution (Sigma, St Louis, MO, USA)) was added to the well to incubate for 2 h. Afterwards, Optical density (OD) values were measured using a microplate reader (Bio-Rad, Richmond, Virginia, USA) at the 450 nm wavelength.
Flow Cytometry
Cell apoptosis was checked by using the Annexin Apoptosis Detection Kit (Sigma) according to the given instructions. Briefly, after the re-suspension in the binding buffer, cells were incubated with 5 μL Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and 5 μL propidium iodide (PI) for 5 min in the dark. Thereafter, the stained cells were analyzed by the flow cytometry (Countstar, Shanghai, China)
Western Blot
Western blot was performed according to a previous description.18 In brief, proteins were extracted from samples by using the total protein extraction kit (Solarbio) and then the proteins were separated and incubated with the primary antibodies and the secondary antibody. The antibodies used in this research: anti-B-cell lymphoma-2 (Bcl-2) (1:2000, ab196495, Abcam), anti-BCL2-Associated X (Bax) (1:1500, ab199677, Abcam), anti-hexokinase 2 (HK2) (1:5000, ab227198, Abcam), anti-MAGT1 (1:3000, ab90478, Abcam), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2500, ab9485, Abcam) and goat anti-rabbit IgG H&L (HRP) (1:3000, ab205718, Abcam). After the treatment with ECL kit (Solarbio), the protein band was analyzed using the ChemiDoc™ MP Imaging System (Bio-Rad).
Transwell Assay
Transwell chamber pre-treated with Matrigel (Solarbio) or not was hired to estimate the ability of cell invasion or migration. The upper chamber was filled with cells and the lower chamber was filled with the medium containing FBS. Subsequently, the cells were treated with crystal violet (Sigma) and then were analyzed under an inverted microscope (MTX Lab Systems, Bradenton, FL, USA).
Detection of Glucose Consumption and Lactate Production
The levels of glucose and lactate in samples were measured using the glucose colorimetric assay kit (Elabscience, Wuhan, China) and lactate colorimetric assay kit (BioVision, Milpitas, California, USA) according to the instructions, respectively. Briefly, the medium from each treatment group were collected and the levels of glucose and lactate in the medium were marked as controls. Next, the cells were incubated for 14 to 18 hours and then the levels of glucose and lactate in the culture medium was measured.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted by the Total RNA Extraction Kit (Solarbio) and was reversely transcribed to complementary DNA (cDNA) by the PrimeScript™ RT Master Mix kit (Solarbio). For the qRT-PCR, the SYBR Green Realtime PCR Master Mix (Solarbio) was used for the quantitative analysis of circ_0002755 and MAGT1, and the miScript SYBR Green PCR kit (Qiagen, Shanghai, China) was used for the analysis of miR-628-5p. All qRT-PCR data was analyzed by 2−ΔΔCt method. Beta-actin (β-actin) was utilized to normalize the level of circ_0002755 and MAGT1, and U6 was used to normalize the level of miR-628-5p. Primers used in this study: circ_0002755 (forward 5ʹ-CTGCGAGGTGGAGAAGGAGA-3ʹ, reverse 5ʹ-GACACACCCATGGCCATACG-3ʹ); miR-628-5p (forward, 5ʹ-GTCGTATCCAGTGCAGTTATATCC-3ʹ, reverse 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ); MAGT1 (forward, 5ʹ-GTGAACTATATCCATGGAAGC-3ʹ, reverse 5ʹ-TCCTAAAGTAACACCACCATTG-3ʹ); β-actin (forward 5ʹ-GCACCACACCTTCTACAATG-3ʹ, reverse, 5ʹ-TGCTTGCTGATCCACATCTG-3ʹ); U6 (forward, 5ʹ-TCCGGGTGATGCTTTTCCTAG-3ʹ, reverse, 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ).
The Dual-Luciferase Reporter Assay
We used the starBase (http://starbase.sysu.edu.cn/) to predicate the interaction between miR-628-5p and circ_0002755 or MAGT1.19 Afterwards, the dual-luciferase reporter vectors of circ_0002755 WT, circ_0002755 MUT, MAGT1 3ʹUTR WT and MAGT1 3ʹUTR MUT were built and then were co-transfected with miR-628-5p or miR-NC into the A-172 and SHG-44 cells. The Dual-Luciferase Assay kit (Promega, Madison, WI, USA) was utilized to measure the luciferase activity.
Xenograft Mice Model
Six-week-old BALB/c nude mice were purchased from LingChang Biotech (Shanghai, China), and then randomly divided into three groups (n=4 per group). A-172 cells (5×106) treated with Sev or not were injected subcutaneously into the flank of the nude mice. After 7 days of injection, mice were administrated with circ_0002755 or vector. The tumor volume was measured every 7 d according to the formula: 0.5 × length × width.2 The tumor weight was calculated after the mice were euthanized. Subsequently, the mRNA levels of circ_0002755 and miR-628-5p in tumors were detected by qRT-PCR, and the protein level of MAGT1 in tumors was determined by Western blot. Animal studies were performed in compliance with the ARRIVE guidelines and the Basel Declaration. The animal experiment was approved by the Animal Care and Use Committee of The Second Affiliated Hospital of Dalian Medical University. All animals received humane care according tothe National Institutes of Health (USA) guidelines.
Statistical Analysis
The GraphPad Prism (GraphPad, La Jolla, CA, USA) was employed to dissect the experimental data, which were presented by mean ± standard deviation (SD). The Student’s t-test or the one-way analysis of variance (ANOVA) was utilized to assess the difference of different groups. Every experiment was performed at least three replicates. P< 0.05 indicated the statistical significance.
Results
Sev Regulated Viability, Apoptosis, Migration, Invasion and Glucose Metabolism in Glioma Cells
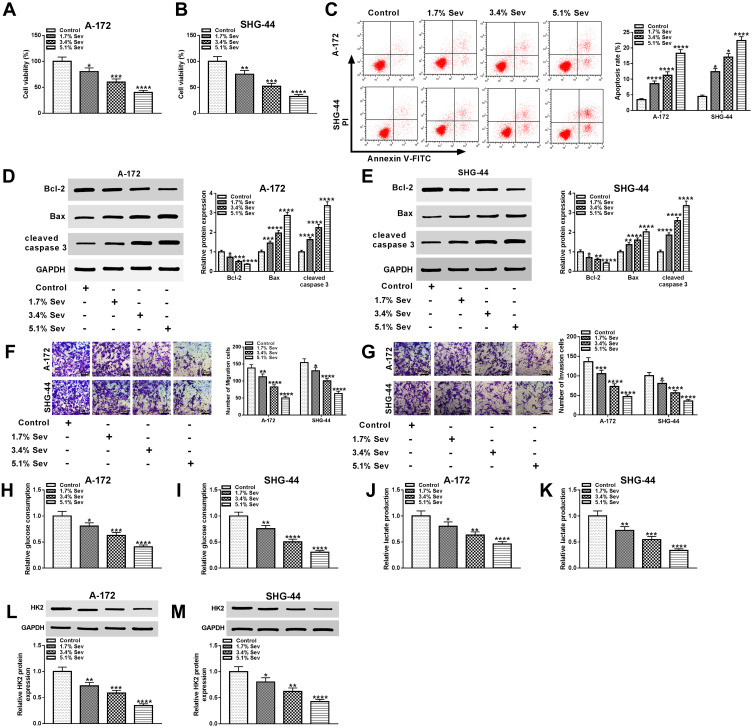
To explore the function of Sev in glioma, we first measured the viability of glioma cells and CCK8 assay showed that the higher concentration of Sev led to the lower cell viability (Figure 1A and B). Flow cytometry assay showed that high concentration of Sev significantly induced cell apoptosis (Figure 1C). Moreover, previous literature suggested that Bcl-2 acted as an anti-apoptosis protein, and Bax worked as a pro-apoptosis protein in glioma.20 Hence, these protein levels were further detected to verify the effect of Sev on apoptosis. Our data suggested that the protein level of Bcl-2 was obviously declined in glioma cells treated with Sev, while the protein levels of Bax and cleaved caspase 3 was notably elevated (Figure 1D and E). Besides, transwell assay indicated that the abilities of migration and invasion were markedly inhibited in glioma cells treated with Sev (Figure 1F and G). As glucose metabolism was associated with cancer progression,21–23 we checked the levels of glucose and lactate in glioma cells. The data showed that the rates of glucose consumption and lactate production were gradually decreased in glioma cells with the growing concentrations of Sev (Figure 1H–K). Meanwhile, Sev strikingly decreased the protein level of HK2, which played a pivotal role in glycolysis.24 Our data showed that Sev remarkedly reduced the expression level of HK2 in glioma cells (Figure 1L and M). Altogether, these results demonstrated Sev could inhibit the progression of glioma.
Figure 1.
Sev inhibited the progression of glioma. (A and B) The viability of glioma cells treated with Sev (1.7%, 3.4% and 5.1%) or not was checked by CCK8 assay. (C) Cell apoptosis was measured by flow cytometry assay. (D and E) The protein levels of Bcl-2, Bax and cleaved caspase 3 in samples were detected by Western blot. (F and G) Transwell assay was hired to assess the abilities of cell migration and invasion. (H–K) The rates of glucose consumption and lactate production in cells were measured by glucose colorimetric assay kit and lactate colorimetric assay kit, respectively. (L and M) The protein level of HK2 in samples was measured by Western blot. *P< 0.05, **P< 0.01. ***P< 0.001. ****P< 0.0001.
Sev Reduced the Expression Level Ofcirc_0002755 in Glioma Cells
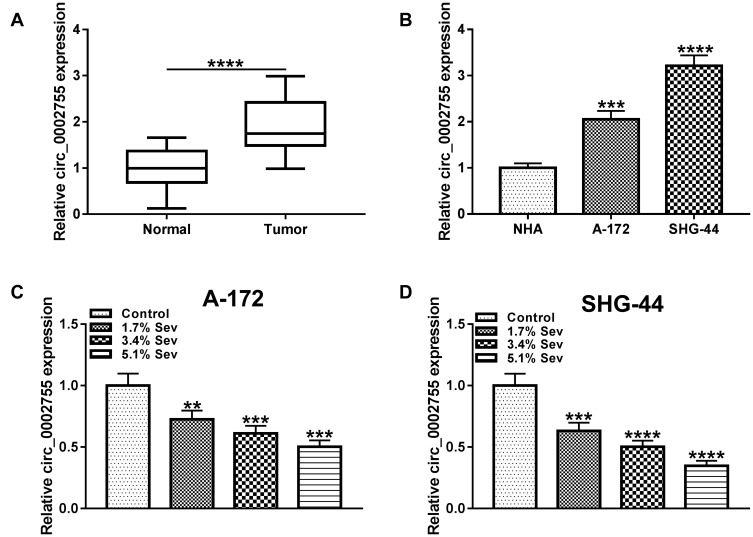
To investigate the role of circ_0002755 in glioma, we checked its level and the results showed that circ_0002755 was significantly upregulated in glioma tissues and cells compared with matched normal brain tissues and NHA cells (Figure 2A and B). However, a significant decline level of circ_0002755 was observed in glioma cells treated with Sev (Figure 2C and D). All in all, these results disclosed that circ_0002755 might act an oncogene in glioma and could be regulated by Sev.
Figure 2.
Sev decreased the level of circ_0002755 in glioma cells. (A and B) The level of circ_0002755 in glioma tissues and cells, as well as matched controls was detected by qRT-PCR. (C and D) The level of circ_0002755 in glioma cells treated with Sev (1.7%, 3.4% and 5.1%) or not was measured by qRT-PCR. **P< 0.01. ***P< 0.001. ****P< 0.0001.
Overexpression of Circ_0002755 Inverted Sev-Mediated Repressive Impact on Glioma Progression
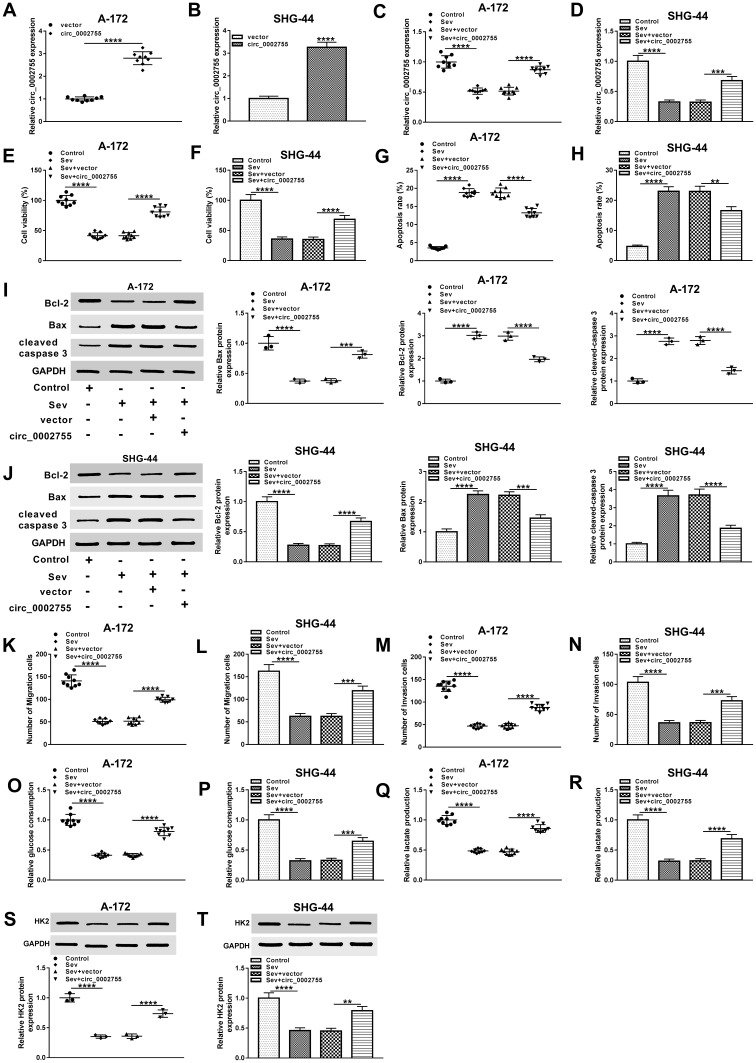
To explore the function of circ_0002755, we first checked its expression level in glioma cells transfected with vector or circ_0002755. Results presented that circ_0002755 level was obviously increased in circ_0002755-transfeced glioma cells relative to vector-transfected cells (Figure 3A and B). Then, glioma cells were treated with Sev or Sev + circ_0002755, as well as matched controls. The data showed that the declined mRNA level of circ_0002755 in Sev group was reversed following the transfection with circ_0002755 (Figure 3C and D). Also, CCK8 assay indicated that overexpression of circ_0002755 reversed Sev-mediated inhibitory effect on cell viability (Figure 3E and F). Flow cytometry assay showed that upregulation of circ_0002755 overturned Sev-mediated promoted effect on cell apoptosis (Figure 3G and H). Meanwhile, the protein levels of apoptosis-related proteins (Bcl-2, Bax and cleaved caspase 3) in Sev group were reversely changed after the extra transfection with circ_0002755 (Figure 3I and J). In addition, transwell assay showed that overexpression of circ_0002755 transposed Sev-mediated suppressive impact on cell migration and invasion (Figure 3K–N). Moreover, Sev-induced declined rates of glucose consumption and lactate production were reversed by circ_0002755 overexpression (Figure 3O–R). Simultaneously, the decreased protein level of HK2 in Sev group was inverted after the additional transfection with circ_0002755 (Figure 3S and T). Broadly, these results illustrated that Sev-mediated glioma progression by regulating circ_0002755.
Figure 3.
Sev inhibited glioma progression by regulating circ_0002755. (A and B) Circ_0002755 level was detected in A-172 and SHG-44 cells transfected with vector or circ_0002755 by qRT-PCR assay. (C and D) The level of circ_0002755 in glioma cells treated with Sev or Sev + circ_0002755, as well as corresponding controls was detected by qRT-PCR. (E and F) The viability of glioma cells was checked by CCK8 assay. (G and H) Flow cytometry assay was used to check the apoptosis of glioma cells. (I and J) The protein levels of Bcl-2, Bax and cleaved caspase 3 in samples were determined by Western blot. (K–N) The abilities of cell migration and invasion were estimated by transwell assay. (O–R) The rates of glucose consumption and lactate production were evaluated by glucose colorimetric assay kit and lactate colorimetric assay kit, respectively. (S and T) The protein level of HK2 in glioma cells under different conditions was checked by Western blot. **P< 0.01. ***P< 0.001. ****P< 0.0001.
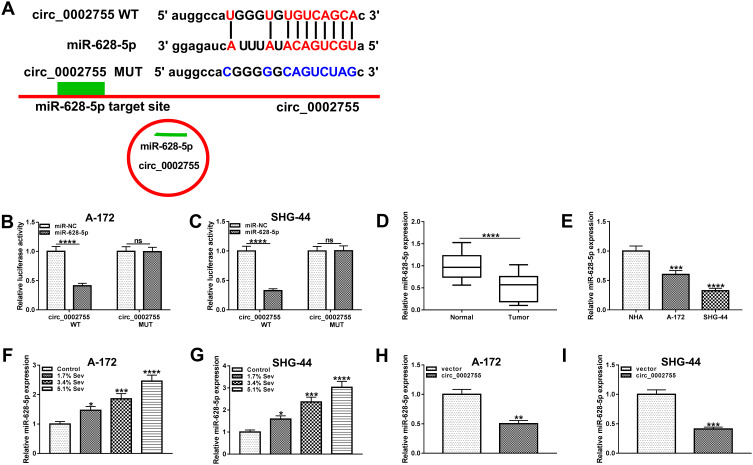
Circ_0002755 Targeted miR-628-5p and Negatively Regulated the Expression of miR-628-5p in Glioma Cells
Previous studies indicated that circRNAs could serve as sponges of miRNAs in various human cancers.25,26 In this study, we found that miR-628-5p contained the target sites of circ_0002755 by using starBase (Figure 4A). To verify this interaction, the dual-luciferase reporter assay was conducted and the result showed that miR-628-5p apparently diminished the luciferase activity of circ_0002755 WT in glioma cells, rather than circ_0002755 MUT (Figure 4B and C). Afterwards, the level of miR-628-5p was measured and the result showed that miR-628-5p was clearly downregulated in glioma tissues and cells compared with corresponding controls (Figure 4D and E). Besides, the elevated level of miR-628-5p was observed in glioma cells treated with Sev (Figure 4F and G). Further analysis indicated that overexpression of circ_0002755 significantly reduced the level of miR-628-5p in glioma cells (Figure 4H and I). To sum up, these results elucidated that miR-628-5p was a target of circ_0002755 and negatively regulated by circ_0002755 in glioma cells.
Figure 4.
Circ_0002755 directly targeted miR-628-5p in glioma cells. (A) The target sites between circ_0002755 and miR-628-5p were predicated by starBase. (B and C) The dual-luciferase reporter assay was employed to verify the interaction between circ_0002755 and miR-628-5p in glioma cells. (D and E) The qRT-PCR was performed to detect the level of miR-628-5p in glioma tissues and cells, as well as normal brain tissues and NHA cells. (F and G) The level of miR-628-5p in control and Sev-treated glioma cells was determined by qRT-PCR. (H and I) The level of miR-628-5p in glioma cells transfected with circ_0002755 or vector was measured by qRT-PCR. *P< 0.05, **P< 0.01. ***P< 0.001. ****P< 0.0001.
Sev Regulated Glioma Progression by Circ_0002755/miR-628-5p Axis
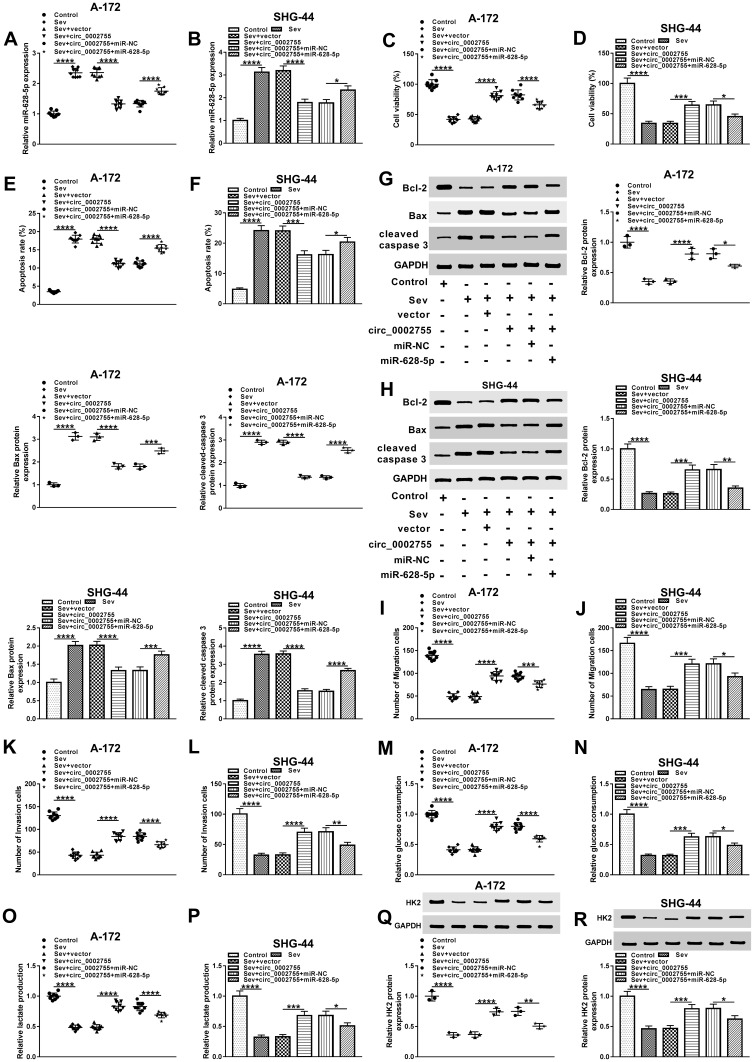
To investigate whether circ_0002755 and miR-628-5p were involved in the regulation of Sev-mediated glioma progression, we first checked the level of miR-628-5p in glioma cells treated with Sev, Sev + circ_0002755 or Sev + circ_0002755 + miR-628-5p, as well as matched controls. The data showed that the declined level of miR-628-5p in glioma cells treated with Sev + circ_0002755 was reversed after the extra transfection with miR-628-5p mimic (Figure 5A and B). Afterwards, CCK8 assay was carried out and the result indicated that overexpression of miR-628-5p transposed circ_0002755 overexpression-mediated boosted impact on the viability of Sev-treated glioma cells (Figure 5C and D). Meanwhile, flow cytometry assay indicated that miR-628-5p mimic inverted circ_0002755 overexpression-mediated repressive effect on the apoptosis of Sev-treated glioma cells (Figure 5E and F). Also, the protein levels of apoptosis-related proteins (Bcl-2, Bax and cleaved caspase 3) in glioma cells treated with Sev + circ_0002755 were inverted following the transfection with miR-628-5p(Figure 5G and H). Moreover, transwell assay indicated that upregulation of miR-628-5p transposed circ_0002755 overexpression-mediated promoted effect on the migration and invasion of Sev-treated glioma cells (Figure 5I–L). In addition, the elevated rates of glucose consumption and lactate production in Sev + circ_0002755 were inverted by miR-628-5p overexpression (Figure 5M–P). Concurrently, the increased protein level of HK2 in Sev + circ_0002755-treated glioma cells was overturned after the extra transfection with miR-628-5p mimic (Figure 5Q and R). Collectively, these results demonstrated that Sev mediated glioma progression via regulating circ_0002755/miR-628-5p axis.
Figure 5.
Circ_0002755/miR-628-5p axis functioned in Sev-mediated glioma progression. (A and B) The level of miR-628-5p in glioma cells treated with Sev, Sev + circ_0002755 or Sev + circ_0002755 + miR-628-5p, as well as matched controls was detected by qRT-PCR. (C and D) CCK8 assay was executed to check cell viability. (E and F) Cell apoptosis was checked by flow cytometry. (G and H) Western blot was conducted to measure the protein levels of Bcl-2, Bax and cleaved caspase 3 in samples. (I–L) Transwell assay was utilized to assess the abilities of cell migration and invasion. (M–P) The rates of glucose uptake and lactate production were detected by corresponding colorimetric assay kit. (Q and R) Western blot was utilized to determine the protein level of HK2 in samples. *P< 0.05, **P< 0.01. ***P< 0.001. ****P< 0.0001.
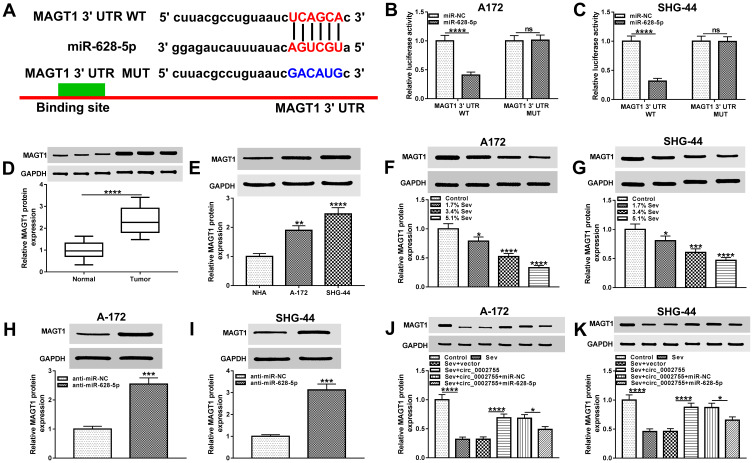
MiR-628-5p Bound to the 3ʹUTR of MAGT1 and Negatively Regulated MAGT1 Expression in Glioma Cells
To further study the regulatory mechanism of miR-628-5p, we predicated its target gene by starBase and the result showed that miR-628-5p could bind to the 3ʹUTR of MAGT1(Figure 6A). Afterwards, the interaction between miR-628-5p and MAGT1 was corroborated by the dual-luciferase reporter assay (Figure 6B and C). We then checked the protein the level of MAGT1 and found that MAGT1 was significantly upregulated in glioma tissues and cells compared with matched controls (Figure 6D and E). Moreover, the level of MAGT1 was remarkably decreased in glioma cells after the treatment with Sev (Figure 6F and G). In addition, downregulation of miR-628-5p apparently elevated the protein level of MAGT1 in glioma cells (Figure 6H and I). Further investigation showed that overexpression of miR-628-5p reversed the protein level of MAGT1 in glioma cells treated with Sev + circ_0002755 (Figure 6J and K). To sum up, these results elucidated that miR-628-5p was a target of circ_0002755 and negatively regulated by circ_0002755 in glioma cells. In summary, these results testified that circ_0002755 regulated the expression of MAGT1 via interacting with miR-628-5p.
Figure 6.
MiR-628-5p targeted MAGT1 in glioma cells. (A) The binding sites between miR-628-5p and MAGT1 were forecasted by starBase. (B and C) The interaction between miR-628-5p and MAGT1 was checked by the dual-luciferase reporter assay in glioma cells. (D and E) The Western blot was carried out to check the protein level of MAGT1 in glioma tissues and cells, as well as normal brain tissues and NHA cells. (F and G) The protein level of MAGT1 in control and Sev-treated glioma cells was measured by Western blot. (H and I) The protein level of MAGT1 in glioma cells transfected with anti-miR-628-5p or anti-miR-NC was detected by Western blot. (J and K) The protein level of MAGT1 in glioma cells transfected with Sev, Sev + circ_0002755 or Sev + circ_0002755 + miR-628-5p, as well as the corresponding controls was measured by Western blot. *P< 0.05, **P< 0.01. ***P< 0.001. ****P< 0.0001.
Sev Modulated Glioma Progression by miR-628-5p/MAGT1 Axis
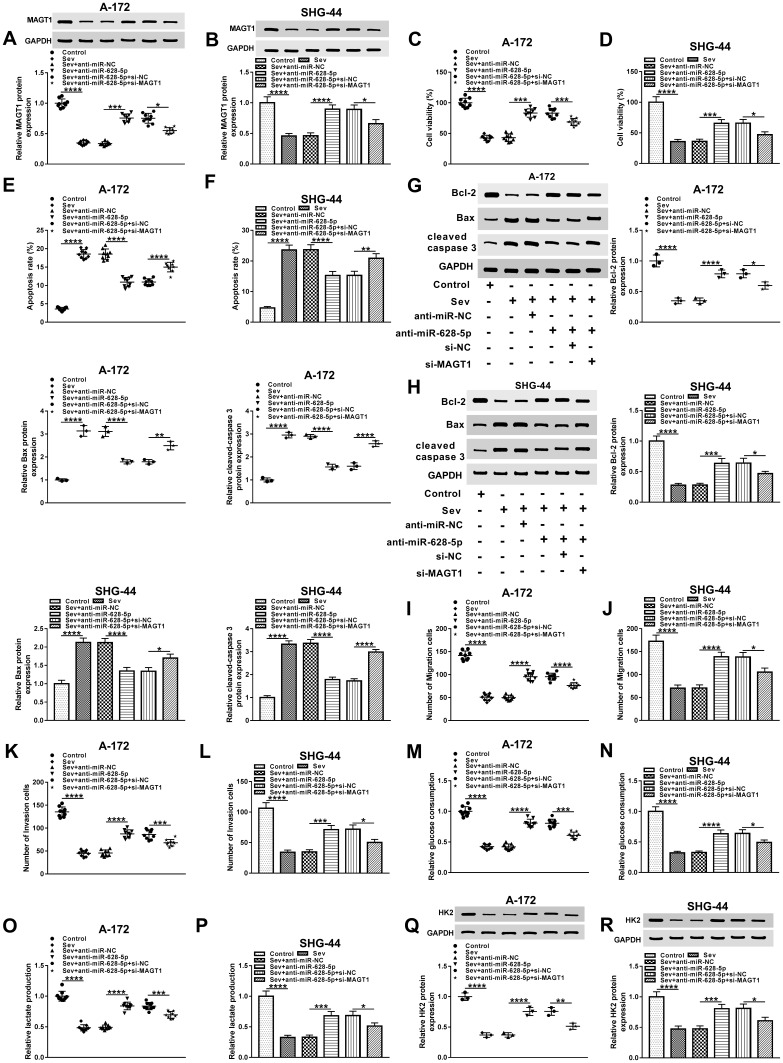
To explore the role of MAGT1 in Sev-mediated glioma progression, we checked its protein level in glioma cells treated with Sev, Sev + anti-miR-628-5p or Sev + anti-miR-628-5p + si-MAGT1, as well as corresponding controls. The results showed that the elevated protein level of MAGT1 in Sev + anti-miR-628-5p group was reversed after the additional transfection with si-MAGT1 (Figure 7A and B). We then checked cell viability and the data showed that downregulation of MAGT1 inverted miR-628-5p depletion-mediated promoted effect on the viability of Sev-treated glioma cells (Figure 7C and D). Besides, flow cytometry assay indicated that the silence of MAGT1 transposed miR-628-5p depletion-mediated repressive effect on the apoptosis of Sev-treated glioma cells (Figure 7E and F). Simultaneously, the protein levels of Bcl-2, Bax and cleaved caspase 3 in glioma cells treated with Sev + anti-miR-628-5p were overturned following the transfection with si-MAGT1 (Figure 7G and H). Afterwards, transwell assay was performed and the results indicated that MAGT1 silencing rescued miR-628-5p depletion-mediated boosted impact on the migration and invasion of Sev-treated glioma cells (Figure 7I–L). Furthermore, the enhanced rates of glucose consumption and lactate production in Sev + anti-miR-628-5p group were reversed by the knockdown of MAGT1 (Figure 7M–P). Meanwhile, the elevated protein level of HK2 in glioma cells treated with Sev + anti-miR-628-5p was inverted following the transfection with si-MAGT1 (Figure 7Q and R). Taken together, our results suggested that Sev mediated glioma progression via regulating miR-628-5p/MAGT1 axis.
Figure 7.
MiR-628-5p/MAGT1 axis functioned in Sev-mediated glioma progression. (A and B) The protein level of MAGT1 in glioma cells treated with Sev, Sev + anti-miR-628-5p or Sev + anti-miR-628-5p + si-MAGT1, as well as matched controls was detected by Western blot. (C and D) The cell viability was assessed by CCK8 assay. (E and F) Cell apoptosis was detected by flow cytometry. (G and H) Western blot was hired to check the protein levels of Bcl-2, Bax and cleaved caspase 3 in samples. (I–L) Transwell assay was done to estimate the capabilities of cell migration and invasion. (M–P) The rates of glucose uptake and lactate production were checked by corresponding colorimetric assay kit. (Q and R) Western blot was performed to measure the protein level of HK2 in samples. *P< 0.05. **P< 0.01. ***P< 0.001. ****P< 0.0001.
Sev Retarded Tumor Growth in vivo
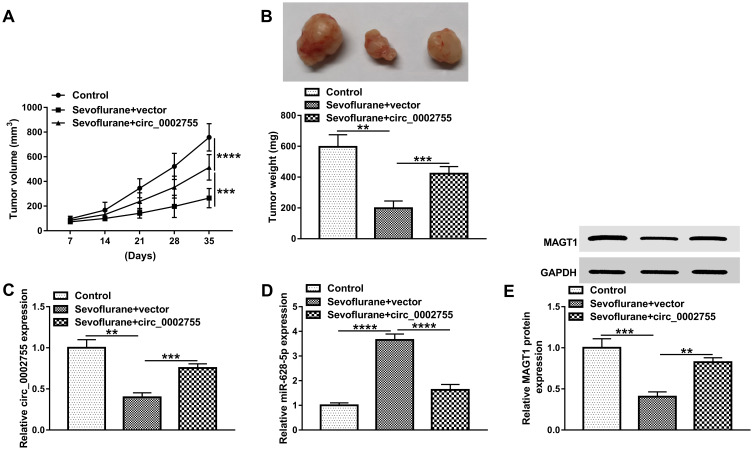
To confirm the anti-tumor function of Sev in vivo, the xenograft mouse model using the A-172 cells treated with Sev (named as Sevoflurane) or not (named as Control), followed by administration with vector or circ_0002755. The results showed that the tumor volume was obviously shrank in the Sevoflurane group compared with the Control group, which was reverted due to the introduction of circ_0002755 (Figure 8A). Also, the tumor weight was obviously declined in Sevoflurane group compared with the Control group, while the overexpression of circ_0002755 restored the inhibitory effect (Figure 8B). These data suggested that circ_0002755 upregulation relieved the anti-tumor effect of Sevoflurane. Subsequently, the levels of circ_0002755 and miR-628-5p were checked in tumors, and the results showed that circ_0002755 was significantly downregulated in the Sevoflurane group compared with the Control group, opposite to the level of miR-628-5p (Figure 8C and D). In addition, the protein level of MAGT1 was notably decreased in the Sevoflurane group compared with the Control group (Figure 8E). By and large, these results suggested that Sev inhibited tumor growth by altering the levels of circ_0002755, miR-628-5p and MAGT1 in vivo.
Figure 8.
Sev obstructed tumor growth in vivo. (A) Tumor volume was measured every 7 d. (B) The weight of the resected tumor was examined after the mice were killed. (C and D) The levels of circ_0002755 and miR-628-5p in tumors were measured by qRT-PCR. (E) The protein level of MAGT1 in tumors was detected by Western blot. **P< 0.01. ***P< 0.001. ****P< 0.0001.
Discussion
Glioma is an aggressive tumor and the average survival time in patients with high-grade glioma is not more than one year.27–30 Sev was reported to be involved in the regulation of glioma progression4,31 and our data showed that Sev inhibited glioma progression in vitro and tumor growth in vivo. However, the current studies were mostly focused on the regulatory mechanisms of miRNAs and long noncoding RNAs in Sev-mediated human diseases.3,32 Little was known about the role of circRNAs in Sev-mediated human diseases. Growing evidence showed that circRNAs played a vital role in regulating cancer progression.33 Huang et al reported that circular RNA circERBB2 promoted gallbladder cancer progression by regulating PA2G4-dependent rDNA transcription.6 Jin et al confirmed that circRNA circHIPK3 could act as a prognostic marker to boost glioma progression.8 In our research, we found that circ_0002755 was strikingly upregulated in glioma tissues and cells, but its level was then clearly declined in glioma cells after the treatment with Sev. Further investigation indicated that Sev-mediated effect on cell viability, apoptosis, migration, invasion and glucose metabolism was reversed by the overexpression of circ_0002755. All in all, these results demonstrated that Sev mediated glioma progression possibly via regulating circ_0002755.
Emerging evidence confirmed that circRNAs exerted their functions mainly by interacting with miRNAs in multiple human cancers.25,26 In this research, by using bioinformatics analysis and cell experiments, we found that miR-628-5p was a target of circ_0002755. In addition, miR-628-5p was apparently downregulated in glioma tissues and cells, which was in line with a previous report,15 but its expression level was notably increased in glioma cells treated with Sev. Meanwhile, miR-628-5p was negatively regulated by circ_0002755 in glioma cells. The in-depth studies showed that Sev modulated glioma progression via circ_0002755/miR-628-5p axis. Subsequently, we found that miR-628-5p could bind to the 3ʹUTR of MAGT1, which was closely associated with human cancer progression.16,17 We next measured the level of MAGT1 and found was highly expressed in glioma tissues and cells, whereas its level was conspicuously downregulated in glioma cells treated with Sev. Besides, circ_0002755 modulated MAGT1 expression via interacting with miR-628-5p. Further exploration illustrated that Sev regulated glioma progression by miR-628-5p/MAGT1 axis. Taken together, our results suggested that Sev mediated glioma progression via modulating circ_0002755/miR-628-5p/MAGT1 axis.
In conclusion, our research showed that circ_0002755 and MAGT1 were downregulated in glioma cells after the treatment with Sev, contrary to the expression level of miR-628-5p. And Sev hampered the progression of glioma via mediating circ_0002755/miR-628-5p/MAGT1 axis. The novel regulatory mechanism might provide an experimental basis for choosing more reasonable anesthetics for cancer patients.
Funding Statement
None.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv. 2007;4(2):175–186. doi: 10.1517/17425247.4.2.175 [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Yan Y, Zhou J, et al. Clinical prognostic value of isocitrate dehydrogenase mutation, O-6-methylguanine-DNA methyltransferase promoter methylation, and 1p19q co-deletion in glioma patients. Ann Transl Med. 2019;7(20):541. doi: 10.21037/atm.2019.09.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. doi: 10.1016/j.cancergen.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Wang J, Fu Z, et al. Sevoflurane suppresses migration and invasion of glioma cells by regulating miR-146b-5p and MMP16. Artif Cells Nanomed Biotechnol. 2019;47(1):3306–3314. doi: 10.1080/21691401.2019.1648282 [DOI] [PubMed] [Google Scholar]
- 5.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7 [DOI] [PubMed] [Google Scholar]
- 6.Huang X, He M, Huang S, et al. Circular RNA circERBB2 promotes gallbladder cancer progression by regulating PA2G4-dependent rDNA transcription. Mol Cancer. 2019;18(1):166. doi: 10.1186/s12943-019-1098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Fang K, Chen TQ, et al. circRNA circAF4 functions as an oncogene to regulate MLL-AF4 fusion protein expression and inhibit MLL leukemia progression. J Hematol Oncol. 2019;12(1):103. doi: 10.1186/s13045-019-0800-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin P, Huang Y, Zhu P, Zou Y, Shao T, Wang O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun. 2018;503(3):1570–1574. doi: 10.1016/j.bbrc.2018.07.081 [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Gao X, Zhang M, et al. Novel Role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–315. doi: 10.1093/jnci/djx166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Zhang S, Chen X, et al. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 2018;78(17):4812–4825. doi: 10.1158/0008-5472.Can-18-0532 [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Zhang C, Liu Y, Wang M. Circular RNA profiling reveals circRNA1656 as a novel biomarker in high grade serous ovarian cancer. Biosci Trends. 2019;13(2):204–211. doi: 10.5582/bst.2019.01021 [DOI] [PubMed] [Google Scholar]
- 12.Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3ʹUTRs. PLoS One. 2011;6(3):e18067. doi: 10.1371/journal.pone.0018067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SQ, Ren LJ, Liu J, Wang P, Shan SM. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma. 2019;66(6):887–895. doi: 10.4149/neo_2018_181213N962 [DOI] [PubMed] [Google Scholar]
- 14.Gao C, Shen J, Meng ZX, He XF. Sevoflurane inhibits glioma cells proliferation and metastasis through miRNA-124-3p/ROCK1 Axis. Pathol Oncol Res. 2019. doi: 10.1007/s12253-019-00597-1 [DOI] [PubMed] [Google Scholar]
- 15.Xie P, Wang Y, Liao Y, et al. MicroRNA-628-5p inhibits cell proliferation in glioma by targeting DDX59. J Cell Biochem. 2019;120(10):17293–17302. doi: 10.1002/jcb.28991 [DOI] [PubMed] [Google Scholar]
- 16.Zheng K, Yang Q, Xie L, et al. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncol Lett. 2019;18(4):3857–3862. doi: 10.3892/ol.2019.10710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Li Y, Li J, et al. microRNA-199a-5p suppresses glioma progression by inhibiting MAGT1. J Cell Biochem. 2019;120(9):15248–15254. doi: 10.1002/jcb.28791 [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Qiu J, Yang C, et al. Identification of a novel estrogen receptor beta1 binding partner, inhibitor of differentiation-1, and role of ERbeta1 in human breast cancer cells. Cancer Lett. 2009;278(2):210–219. doi: 10.1016/j.canlet.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 19.Li JH, S L, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:92–97. doi: 10.1093/nar/gkt1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Wang X, Chen X, et al. Identification of Aloperine as an anti-apoptotic Bcl2 protein inhibitor in glioma cells. PeerJ. 2019;7:e7652. doi: 10.7717/peerj.7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose S, Le A. Glucose Metabolism in Cancer. Adv Exp Med Biol. 2018;3–12. doi: 10.1007/978-3-319-77736-8_1 [DOI] [PubMed] [Google Scholar]
- 22.Chai XX, Le YF, Wang JC, et al. Carpesium abrotanoides (L.) root as a potential source of natural anticancer compounds: targeting glucose metabolism and PKM2/HIF-1α axis of breast cancer cells. J Food Sci. 2019;84(12):3825–3832. doi: 10.1111/1750-3841.14953 [DOI] [PubMed] [Google Scholar]
- 23.Allen AE, Locasale JW. Glucose metabolism in cancer: the saga of pyruvate kinase continues. Cancer Cell. 2018;33(3):337–339. doi: 10.1016/j.ccell.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeWaal D, Nogueira V, Terry AR, et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9(1):446. doi: 10.1038/s41467-017-02733-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong D, Lu C, Zhang B, et al. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18(1):25. doi: 10.1186/s12943-019-0958-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yang C, Yuan W, Yang X, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17(1):19. doi: 10.1186/s12943-018-0771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamza MA, Mandel JJ, Conrad CA, et al. Survival outcome of early versus delayed bevacizumab treatment in patients with recurrent glioblastoma. J Neurooncol. 2014;119(1):135–140. doi: 10.1007/s11060-014-1460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue L, Wang Y, Yue S, Zhang J. The expression of miRNA-221 and miRNA-222 in gliomas patients and their prognosis. Neurol Sci. 2017;38(1):67–73. doi: 10.1007/s10072-016-2710-y [DOI] [PubMed] [Google Scholar]
- 29.Yan Y, Xu Z, Chen X, et al. Novel Function of lncRNA ADAMTS9-AS2 in promoting temozolomide resistance in glioblastoma via upregulating the FUS/MDM2 ubiquitination axis. Front Cell Dev Biol. 2019;7:217. doi: 10.3389/fcell.2019.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Xu Z, Zeng S, et al. SIRT5 downregulation is associated with poor prognosis in glioblastoma. Cancer Biomark. 2019;24(4):449–459. doi: 10.3233/CBM-182197 [DOI] [PubMed] [Google Scholar]
- 31.Yi W, Li D, Guo Y, Zhang Y, Huang B, Li X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int J Mol Med. 2016;38(6):1857–1863. doi: 10.3892/ijmm.2016.2797 [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Ai Y. Overexpression of lncRNA Gm15621 alleviates apoptosis and inflammation response resulting from sevoflurane treatment through inhibiting miR-133a/Sox4. J Cell Physiol. 2020;235(2):957–965. doi: 10.1002/jcp.29011 [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Yan Y, Zeng S, Dai S. Circular RNAs: clinical relevance in cancer. J Cell Physiol. 2018;9(1):1444–1460. doi: 10.18632/oncotarget.22846 [DOI] [PMC free article] [PubMed] [Google Scholar]