Abstract
In recent past, revolution in medical technology resulted in improved survival rates and outcomes of critically ill children. Unfortunately, its impact relating to morbidity is not well documented. Although survival rates of these critically ill children who are medically fragile and technology-dependent have improved, we as health professionals are still in the learning curve to improve the quality of life of these children at home. Factors such as support from society, infrastructure, and funding play an important role in technology-dependent child care at home. In this review, commonly prescribed home-based medical technologies such as home ventilation, enteral nutrition, renal replacement therapy, and peripherally inserted central catheter, which are useful for quick revision, are described.
Keywords: Technology, Children
1. Introduction
In comparison to previous decades, undoubtedly marked improvements in pediatric medical technology and care resulted in improved survival rates and outcomes for critically ill children in India. Unfortunately, this improvement is not quantified in figures in our setup. In developed nations such as Australia, mortality of children below 5 years decreased from 2604/1,00,000 to 137/1,00,000 in boys and from 2214/1,00,000 to 111/1,00,000 in girls from 1907 to 1998(1). Sophisticated medical treatments and technology saved lives of many premature infants [2]. However, its impact relating to childhood disability is not well documented even in developed countries. Inspite of improvement in survival rates of these medically fragile and technology-dependent children over the years, this has imposed heavy responsibility on hospital resources. The ability to transfer medical technology into homes, patient and parent preferences and to high hospital costs made pathways to send these children home. Consequently, community-based patient care is increasing [3,4]. However, conflicts between hospital and society regarding the management of children who depend on home technology need to be considered. On the one hand, hospitals and health care professionals think that children who are medically stable enough to receive long-term medical support can be discharged back home rather than chronically hospitalized [5,6]. On the other hand, inadequate social support, infrastructure, funding are the major obstacles to home care [[7], [8], [9], [10]]. Obstacles to transition from hospital to home negatively influence the quality of life of these patients [[11], [12], [13], [14]]. The United States Congress' Office of Technology defined technology-dependent child as the one who requires “a medical device to compensate for the loss of a vital bodily function and substantial and ongoing nursing care to avert death or further disability” [15].
2. Home ventilation
Common conditions that require home ventilation are shown in Table 1. Goals of home ventilation are to reverse or ameliorate the cause of respiratory failure, extend life span, reduce morbidity, and promote growth and development. Before discharging a child on ventilator support wholistic assessment of the child, family dynamics, family education and expectations, care provider's confidence to handle emergencies, finances, electricity supply, telephone facilities, suitable entrances for wheel chairs, and local availability of medical facilities for emergencies needs to be considered. Home ventilation includes oxygen therapy, noninvasive ventilation, and invasive ventilation through tracheostomy. Before discharging a child on home ventilation, medical stability of the child should be assessed in acute care setting (Table 2) [16,17].
Table 1.
Conditions that warrant home ventilation.
| Airway problems |
| Airway malacias (Tracheo-bronchomalacia) |
| Craniofacial malformations |
| Obstructive sleep apnea syndrome |
| Pulmonary parenchymal problems |
| Chronic lung diseases such as bronchopulmonary dysplasia |
| Recurrent aspiration syndromes |
| Advanced lung disease in cystic fibrosis |
| Respiratory drive problems |
| Congenital central hypoventilation syndrome |
| Brain/brainstem insult |
| Metabolic disorders |
| Respiratory pump problems |
| Neuromuscular weakness |
| Spinal cord injury |
| Chest wall deformity/kyphoscoliosis |
Table 2.
Assessment of the child on ventilation prior to discharge from ICU.
| To Home | To general hospital ward or transitional care facility |
|---|---|
| Clinical | |
| Gaining weight | No need for 1:1 nursing care |
| Improving stamina | No need for invasive vital monitoring |
| No frequent fever or infection | No need for vasoactive/inotropicdrug support |
| Physiologic | |
| Patent and stable airway | Matured tracheostomy stoma (≥1 week of surgery) |
| PaO2≥ 60 mmHg on FiO2≤ 0.4 | SpO2> 92% on FiO2≤ 40% |
| PaCO2< 50 mmHg (parenchymal disease) or < 45 mmHg (neuromuscular weakness or chest wall problem) | Blood gases within appropriate ranges per diagnosis |
| No need for frequent ventilator setting changes | Stable ventilator settings for ≥ 1 week |
3. Oxygen [18]
Supplemental home oxygen is used for children with chronic lung diseases, such as bronchopulmonary dysplasia. Consider factors such as availability of space at home, mobility, expenses, and FiO2 requirement prior to discharge. Home oxygen can be supplied as liquid oxygen, oxygen cylinders, or oxygen concentrators. Advantages of liquid oxygen are: (1) liquid oxygen tanks are light and portable; (2) the duration of use is longer than oxygen cylinders; (3) they can be filled at home; (4) no need for electricity; and (5) no generation of noise or heat. Disadvantages are: (1) expensive and (2) companies often do not manufacture liquid oxygen.
Oxygen cylinders are commonly used for home oxygen therapy. Advantages are: (1) cylinders are cheaper compared to liquid oxygen, (2) various sizes of cylinders are available for use, (3) it is easy to carry smaller cylinders, (4) larger cylinders can be used for few weeks if the required flow is less than 1 L/min, and (5) no electricity required and no generation of noise or heat. Disadvantages are: (1) heaviness and need for frequent refilling.
Oxygen concentrators can be used in place of liquid oxygen or cylinders. Advantages are: (1) generate required oxygen concentration and (2) less expensive than liquid oxygen or oxygen cylinders.
Disadvantages: (1) not portable, (2) generation of heat and noise, and (3) high electricity costs.
In general, combination of oxygen sources is better to provide appropriate emergency backup and portability.
4. Noninvasive ventilation (NIV)
NIV refers to correction of hypoxemia or alveolar hypoventilation without the use of invasive artificial airways such as tracheostomy or endotracheal tubes. Interface is required for the delivery of NIV. Providing appropriate sized mask is one of the most difficult tasks in providing NIV to infants and children; as a result, a forced decision is made to perform a tracheotomy for providing ventilation. Interface include nasal pillow, nasal cannula, face mask, oronasal mask, helmet, etc. Usually NIV is used during nighttime unless the child suffers with progressive respiratory disorder or develops an acute decompensation where NIV requirement may increase to 24 h in a day. Patients with neuromuscular disorder or restrictive lung disease develop hypercapnic respiratory failure. NIV at nighttime can reduce daytime hypercapnea and symptoms of sleep-disordered breathing by reducing respiratory muscle fatigue, improving lung and chest wall mechanics, reversing microatelectasis, increasing chest wall excursion, and resetting CO2 sensitivity to central chemoreceptors in brain stem. Contraindications for NIV include inability to maintain patent airway, claustrophobia, inability to provide high pressures to maintain oxygenation or ventilation noninvasively, and inability of caregiver to provide NIV. Complications of NIV include poorly fitting interfaces, inadequacy of ventilation, eye irritation, pressure ulceration, and midfacial flattening. High flows may lead to nasal congestion, mouth dryness, aerophagia, and gastric distension. To avoid pressure injuries over the face, it is advised to have different styled interfaces to interchange with the primary nasal or oronasal interfaces. Small children who sweat excessively and who have difficulty in controlling oral secretions should have backup headgear available for noninvasive interfaces. Fixation devices for noninvasive interfaces are often designed for adults; it is advised to alter strap size or provide an extra hook to fit the child's head and stabilize the interface. Adequate padding must be ensured, and frequent inspection for avoiding skin injury is needed.
5. Invasive ventilation
5.1. Tracheostomy
Invasive ventilation via tracheostomy is used for hypoxemia/hypoventilation caused by lung parenchymal disease for children who require continuous mechanical ventilation support, children with severe craniofacial malformations (upper airway obstruction that cannot be improved with NIV), and children with developmental delay who cannot maintain their secretions. According to a report, tracheostomy was performed in children for neurologic impairment with airway hypotonia, obstructive sleep apnea, and recurrent pneumonia [18]. In general, a tracheostomy is performed as a last choice, when noninvasive measures fail to resolve the problem and the treating team and family are convinced. Complications associated with tracheotomy (early and late) are listed in Table 3 [19,20]. Children with tracheotomy need to be under care of caregivers well trained in tracheotomy care, as emergency situations can develop at any time and require immediate action. Tracheostomy tube care includes adequate air humidification and suctioning of tracheostomy tube to avoid blocade and tracheostomy tube changes at predetermined frequency and in emergency as well [21,22]. It was seen that parents have the stress of taking the responsibility for providing tracheostomy care and in dignation by other healthy siblings when they receive less parental attention [23,24]. Recommendations for changing tracheostomy tube vary across the globe from once a week to once a month [25,26]. Frequently changing the tracheostomy tube reduces the risk for tube blockade and, in turn, infection [27]. Patients ventilated via tracheostomy require suction equipment. Portable suction machines are capable of developing pressures of 60–150 mmHg, which is recommended for airway suctioning. All children with tracheostomy should have self-inflating AMBU bag for use during emergencies. At authors' unit, decision for tracheostomy is shared between the clinician and patient's relatives. Following which, tracheostomy procedure is initiated and child remains admitted in hospital for 7 days to allow the tracheostomy stoma to mature. Parents' ability and determination to take care of the tracheostomy is thoroughly assessed by the clinician before discharging the child. Parents are taught the basic skills for tracheostomy tube care by the attending clinician, which include suction, change of the tracheostomy tube, and assessment of tube displacement and obstruction. The child's clinical condition is explained to local otolaryngologist prior to discharge where the child resides.
Table 3.
Complications of tracheostomy.
| Bleeding (intraoperative, early, and late) |
| Intraoperative bleeding |
| Postoperative bleeding |
| Late arterial erosion |
| Loss of airway/ability to ventilate (intraoperative, early, and late) |
| Intraoperative inability to ventilate |
| Decannulation before first tube change |
| Inability to recannulate |
| Tube blockade/disconnection |
| Peritubal leak causing ineffective ventilation |
| Respiratory arrest |
| Air leak (intraoperative, early, and late) |
| Pneumomediastinum |
| Pneumothorax |
| Subcutaneous emphysema |
| Infection (early and late) |
| Tracheitis |
| Aspiration pneumonia |
| Stomal issues (early and late) |
| Skin erosion |
| Infection |
| Bleeding |
| Breakdown |
| Granulation tissue formation |
| Keloid formation |
| Tracheal problems (late) |
| Tracheal/subglottal stenosis |
| Tracheomalacia |
| Tracheocutaneous fistula |
| Others |
| Esophageal injury (intraoperative) |
| False tract creation (intraoperative, early, and late) |
Whenever possible, it is recommended to use relatively small tracheostomy tubes to allow peritubal leak to avoid tracheal damage and facilitate speech therapy. However, it should also be considered that large peritubal leak may lead to compromised delivery of airway pressures during ventilation. Peritubal leak can be tackled using pressure control mode of ventilation or using a cuffed tracheostomy tube with intermittent deflation. There should be a second tracheostomy tube of same size available for emergencies such as dislocation and blockage and another tracheostomy tube of one size smaller in case there is difficulty in reinserting during an unplanned tube change. For children who drool in excessive amounts may require extra tracheostomy tube holders to decrease skin maceration underneath the ties and allow for frequent tube changes. Positive pressure ventilation can be delivered by bi-level positive airway pressure (BiPAP) device, continuous positive airway pressure (CPAP) device, or portable ventilators. Differences between BiPAP/CPAP and portable ventilator are depicted in Table 4.
Table 4.
Differences between BiPAP and portable ventilator.
| BiPAP/CPAP | Portable ventilator |
|---|---|
| Uses blower to generate flow and achieve desired pressure | Uses pistons or turbines to generate desired volume or pressure |
| More easy to carry | Less easy to carry compared to BiPAP/CPAP |
| Better compensation for leaks | Compensation for leak is not as good as BiPAP/CPAP |
| Cannot generate high-peak pressures in case of worsening hypoxemia | Can generate high-peak pressures in case of worsening hypoxemia |
| High rates of energy consumption | Low energy consumption |
| No internal battery | Internal battery is present |
| Rebreathing may be present due to single limb circuit for both inspiration and expiration | No rebreathing due to two limbs circuit for both inspiration and expiration |
No single type of ventilator design is ideal for all patients. Patient's clinical condition and ventilator characteristics are to be taken into consideration whenever a child is planned to discharge on mechanical ventilation.
5.2. Follow up
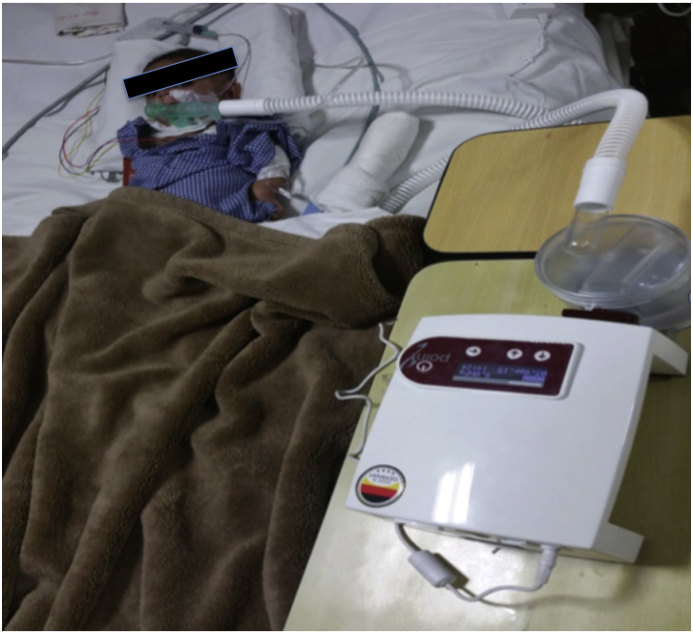
The frequency with which children need to be seen by the health care team depends on disease process, comfort level of treating team, and the ability of family to perform emergency interventions at home. At authors' unit, once the child tolerates reduction in ventilator support in outpatient visit, the family is given appropriate instructions for reduction in respiratory support as well as trained to identify clinical indicators of intolerance after reduction of respiratory support. During every visit, changes in vital parameters, weight gain, tolerance for physical activity, sleep patterns, and overall mood are assessed, and if the child tolerates the reduction in support, orders are given for continued slow reduction of ventilator support and report to emergency room in case the child is uncomfortable with titration of ventilation support at home. Reductions in ventilator parameter setting are being done 1–2 times weekly. A 20% increase in heart rate or respiratory rate from baseline or failure to maintain adequate gas exchange as identified by oximetry and CO2 are indicators to stop further weaning. At authors' unit, weaning from mechanical ventilation in case of reversible diseases begin either to CPAP or completely off support for 1–2 h in a day as per the child's tolerance. The weaning trials are gradually lengthened in the awake state until the child breathes independently in waking state. Once the child tolerates weaning in the wake-up period, further reduction of support is done during naps and finally during sleeping hours overnight. As a point of care in authors' unit, every child on tracheostomy undergoes flexible bronchoscopy to evaluate larynx, the main trachea above the tracheostomy tube, for any evidence of granulation, patency, and movement of vocal cords. A 3-month old infant with hypertrophic cardiomyopathy discharged on home ventilation from authors' unit is represented in Fig. 1.
Fig. 1.
A 3 month old child with tracheostomy in-situ with invasive ventilation to be discharged from ICU.
6. Enteral nutrition
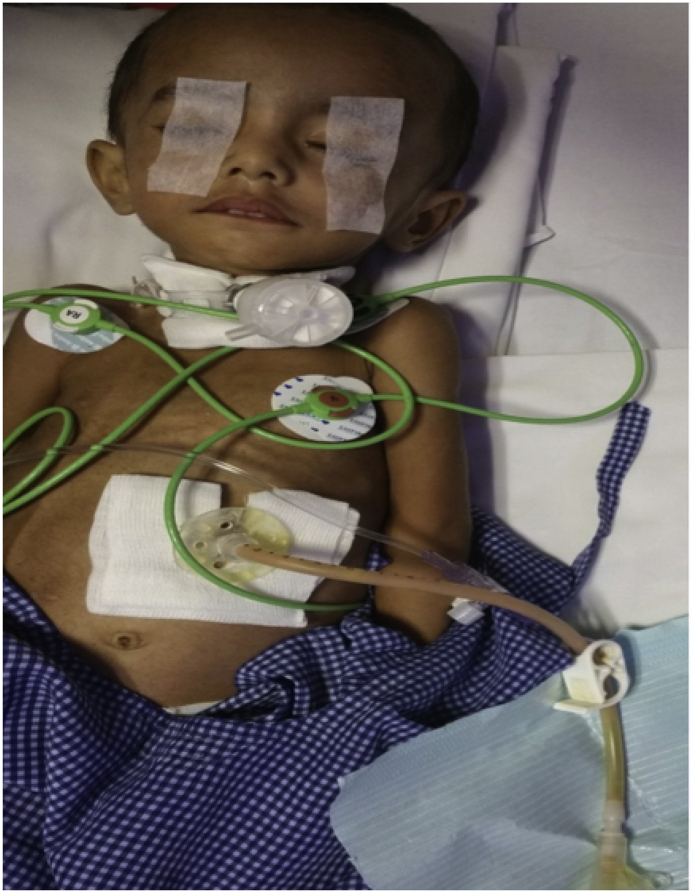
Children with global developmental delay, malignancy, cystic fibrosis, or mechanical ventilation dependency often do not get appropriate nutrition for maintenance and growth by oral intake. As these children have an intact gastrointestinal mucosa for proper absorption of nutrients, it is appropriate to initiate tube feeding when there is an inability to take oral nutrition or poor nutritional status [28]. Involvement of pediatric gastroenterologist and pediatric dietician is often helpful to make a diet plan for the child. At authors' unit, route of enteral nutrition, that is nasogastric (NG)/nasojejunal (NJ)/gastrostomy (percutaneous or surgical) etc., is determined by the patient clinical condition and expected duration of enteral nutrition. Different modes of enteral nutrition delivery are presented in Table 5 [[29], [30], [31], [32], [33], [34]]. Nasogastric, nasoduodenal, or nasojejunal tubes are frequently used for short-term (up to 8 weeks) feeding [35]. Gastrostomy tube can be placed either surgically or percutaneously under endoscopic or radiographic guidance where a permanent tract is created and a tube is introduced through the abdominal wall into the stomach [36]. Gastrojejunostomy can also be performed where a longer tube from the gastrostomy opening is passed through the pylorus and then into the jejunum [37]. Surgical or percutaneous jejunostomy, where tube is passed through the abdominal wall into the jejunum, can also be used for feeding [35]. The choice about which part of the GI tract should be used (stomach, duodenum, or jejunum) for feeding depends on the mechanism of aspiration into lungs (aspiration into lungs from above or below), presence of gastroesophageal reflux, feeding tolerance, and need for tube permanency. The stomach is typically the preferred target unless there is a gastroesophageal reflux or stomach content aspiration risk (e.g., when combined with noninvasive ventilation) [38]. At authors' unit, percutaneous endoscopic gastrostomy (PEG) is performed under conscious sedation under endoscopic guidance to avoid puncture of underlying bowel. In PEG, there is direct apposition of anterior wall of stomach to anterior abdominal wall, with the PEG tube creating a stoma tract. Once the tract matures in about 6–8 weeks, the tube is usually replaced with skin-level gastric button [39]. Most common problem after PEG tube placement is gastroesophageal reflux (GER), which occurs predominantly in neurologically impaired children [40]. Severe GER is a contraindication to perform PEG [41]. Few centers perform PEG in all patients who require long-term enteral feeds and then perform fundoplication in case of severe GER [42]. Other options for children with severe GER are percutaneous gastrojejunostomy or surgical jejunostomy. PEG is contraindicated in children with epidermolysis bullosa due to the risk for esophageal trauma and perforation [43]. PEG-related complications are not seen in children with previous abdominal surgery, ventriculo-peritoneal shunts, or peritoneal dialysis [[44], [45], [46]]. With the appropriately sized equipment, the procedure can be performed safely even in infants weighing less than 4 kg [47]. The PEG tube is usually ready for use within 4–24 h after placement. At authors' unit, neurological disability is the most indication for PEG tube insertion, and liquid diet is initiated within 12 h of PEG. Once the child tolerates liquid feeds well, parents are trained to give feed through PEG tube by clinical nurse. Before discharge, clinician ensures proper way of feeding and comfortable levels of the parents are achieved. A case of 1-year-old child, with tubercular meningitis and tracheostomy, PEG tube, and ventriculoperitoneal shunt in situ, from authors’ unit is shown in Fig. 2.
Table 5.
Various feeding methods used in children.
| Oral feeding | Nasogastric feeding | Gastrostomy feeding | |
|---|---|---|---|
| Clinical benefits | Simple to insert Relatively easy to feed |
Presumed similar to gastrostomy tube, but minimal data specific to nasogastric tube delivery |
Improved nutrition status Lesser mealtimes Easy for the care giver to feed the child Low chances of aspiration from above Better comfort, alertness, and mood of the patient |
| Clinical risks | Risk of aspiration from below Only liquid diet can be given, which results in suboptimal nutrition | Tube dislodgement Nasal damage from long-term use |
Peritonitis, local abscess formation Tube blockage/dislodgement Peristomal leak Increase in gastroespophageal reflux |
| Quality-of-life considerations | Important sensory Experience Mealtime struggles to maintain adequate nutrition |
Improved quality of life of caregiver Decreased stress on caregiver Increased family costs |
Fig. 2.
A 1 year old child with Percutaneous Endoscopic Gastrostomy tube in sit.
7. Dialysis
The usual criteria for the initiation of emergency dialysis are medically refractory hyperkalemia, hyperphosphatemia, acidosis, and fluid overload (>10%). In chronic renal failure, subtle symptoms of uremia such as nausea, vomiting, weakness, and failure to thrive despite adequate calorie intake also make the clinician to consider the initiation of dialysis. Although renal transplant is the ideal treatment for end-stage renal disease, the treatment of most children begins with hemodialysis or peritoneal dialysis.
7.1. Chronic peritoneal dialysis (PD)
For chronic PD, catheter must be placed surgically with subcutaneous tunneling. Chronic PD can be done manually or using automated machines. At authors' unit, child who requires chronic PD is discharged on automated machines and parents are trained by nephrologists to perform at home with confidence. This modality is associated with better outcomes in children, most likely due to preservation of residual renal function, better control of hypertension and anemia, and fewer infection rates at home. In India, chronic PD is more expensive than intermittent hemodialysis, as the patient's family has to pay for the PD catheter insertion and for daily dialysate fluid and its maintenance. For successful initiation and follow-up of chronic PD, a team of surgeons, nephrologists, intensivists, nurses, dieticians, and social worker is important. As a result, specialized pediatric chronic PD programs are few in Indian setup. The most common complication is peritonitis, which is common in children younger than 2 years of age, and approximately 50% of peritonitis episodes are caused by Staphylococcus aureus or coagulase-negative staphylococci [48]. Peritonitis generally responds to intravenous or intraperitoneal antibiotic therapy. However, in case of catheter colonization and peritoneal membrane damage, it is better to revise catheter or convert to hemodialysis.
7.2. Chronic hemodialysis (HD)
Access for HD is obtained via insertion of an appropriately sized hemodialysis double lumen catheter or creation of an arteriovenous (AV) fistula. An AV fistula is the best option for long-term HD, but is difficult to create in young children as the blood flow in young children cannot maintain the patency of AV fistula. Technical expertise is needed for pediatric HD, as children require low flow rates and smaller dialyzer as well as heparin dose as per the body weight compared to adults. Dialysis is performed at least 3 times per week for 3–4 h per session. Children are more prone to malnutrition in overly aggressive dialysis. Although HD is less expensive than PD, difficulty in obtaining and maintaining vascular access and risk of catheter-related infection make clinicians to consider ambulatory PD for children. At authors’ unit, HD is done in pediatric ICU under the supervision of a nephrologist, dialysis nurse, and intensivist.
8. Central venous catheters
At authors’ unit, patients with oncological diseases often are discharged on peripherally inserted central catheter (PICC) for chemotherapy and intravenous antibiotics. Hence, the management of PICC is described in the following section.
Handling a child with PICC line may be uncomfortable at first for parents, as nurses usually take care of the line and give the medicines when the child is admitted to the hospital. As the child improves and is fit to be at home, clinician explains parents regarding the care of PICC line in terms of [1] infection prevention [2], flushing of PICC line [3], injecting medicines [4], trouble shooting, and [5] when and whom to call for help when they face problems. Hand washing is the utmost important step in taking care of PICC line. Hand washing is to be done using one of the following methods [1]: using an alcohol-based hand sanitizer [2]; rubbing antibacterial soap for at least 15 s on all surfaces, including between fingers and under fingernails [3]; using a paper towel or clean hand towel to dry hands; and [3] cleaning the injection cap for at least 30 s with chlorhexidine/alcohol wipe before flushing or putting any medicine into the PICC line and should not blow on it to make the area dry. The PICC line should be flushed with normal saline before and after the medicine is given. Anticipated problems with PICC line at home and required management are depicted in Table 6.
Table 6.
Problems and trouble shooting in PICC at home.
| Problem | Possible cause | Solution |
|---|---|---|
| Fever, erythema, tenderness, or pus at the catheter insertion site Swollen limb |
Infection | Inform health care professionals |
| Difficulty in flushing the PICC | Catheter clamp, kink, and thrombus | Unclamp it (if clamp is present). Remove the kink If the catheter is not kinked or clamped, do not force the solution into the tube and inform |
| Fluid leaking from the catheter | Injection cap is not screwed properly or hole in the catheter | Tighten the injection cap If a leak is seen in the line, fold the catheter over and pinch, apply disposable clamp between the damaged area and the skin, and inform |
| Missing injection cap | Injection cap became loose and fell off | Replace the injection cap using sterile technique and scrub the catheter hub prior to replacing the injection cap |
| Skin redness where the tape or dressing was applied | Sensitivity to tape or dressing | Change the dressing size or the type of tape or dressing used |
| PICC line accidently comes out | Place a sterile gauze pad on the site and press firmly until the bleeding has stopped After the bleeding stops, apply antibiotic ointment and bandage snugly and inform |
Authors’ contribution
KMG: Prepared the manuscript.
TS: Prepared the manuscript.
AS: Reviewed manuscript.
Conflicts of interest
None.
Ethical approval
None.
Funding source
None.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Stanley F.J. Australian Bureau of Statistics; Canberra: 2001. Health: centenary article child health since federation. Year Book Australia. [Google Scholar]
- 2.Donoghue D., Cust A. AIHW National Perinatal Statistics Unit; Sydney: 1998. Australian and New Zealand neonatal network. [Google Scholar]
- 3.Mcfadden E.A. In: Case studies in the nursing of children and families. McFadden Ellen A., editor. Williams &Wilkins; Baltimore, MD: 1989. Hospital, Community, and Home Care. [Google Scholar]
- 4.Smith S.J. Promoting family adaptation to the home care of the technologydependentchild. Issues Compr Pediatr Nurs. 1991;14:249258. doi: 10.3109/01460869109009042. [DOI] [PubMed] [Google Scholar]
- 5.Jardin E., O'Toole M., Paton J.Y., Wallis C. Current status of long termventilation of children in the United Kingdom: questionnaire survey. Br Med J. 1999;318:295299. doi: 10.1136/bmj.318.7179.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noyes J. 'Ventilator-dependent' children who spend prolonged periods oftime in intensive care units when they no longer have a medical need or want to bethere. J Clin Nurs. 2000;9:774783. [Google Scholar]
- 7.Kirk S. Families' experiences of caring at home for a technology-dependentchild: a review of the literature. Child Care Health Dev. 1998;24:101114. doi: 10.1046/j.1365-2214.1998.00043.x. [DOI] [PubMed] [Google Scholar]
- 8.Midgren B., Olofson J., Harlid R., Dellborg C., Jacobsen E., Norregaard O. Home mechanical ventilation in Sweden, with reference to Danish experiences. Respir Med. 2000;94:135138. doi: 10.1053/rmed.1999.0699. [DOI] [PubMed] [Google Scholar]
- 9.Townsley R., Robinson C. More than just a health issue: a review of current issues in the care of enterally-fed children living in the community. Health andSocial Care in the Community. 1999;7:216224. doi: 10.1046/j.1365-2524.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 10.Oslen R., Maslin-Prothero P. Dilemmas in the provision of own-home respite support for parents of young children with complex health care needs:evidence from an evaluation. Issues and Innovations in Nursing Practice. 2001;34:603610. doi: 10.1046/j.1365-2648.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- 11.Capen C.L., Dedlow E.R. Discharging ventilator-dependent children: a continuing challenge. J Pediatr Nurs: Nursing Care of Children andFamilies. 1998;13:175184. doi: 10.1016/S0882-5963(98)80076-6. [DOI] [PubMed] [Google Scholar]
- 12.Gamblian V., Hess D.J., Kenner C. Early discharge from the NICU. J Pediatr Nurs: Nursing Care of Children and Families. 1998;13:296301. doi: 10.1016/S0882-5963(98)80015-8. [DOI] [PubMed] [Google Scholar]
- 13.Fitch M.I., Ross E. Living at home on a ventilator. Canadian Associationof Critical Care Nurses. 1998;19:1824. [PubMed] [Google Scholar]
- 14.Pehrsson K., Olofson J., Larsson S., Sullivan M. Quality of life of patientstreated by home mechanical ventilation due to restrictive ventilatory disorders. Respir Med. 1994;88:2126. doi: 10.1016/0954-6111(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 15.Office of technology assessment technology-dependent children:hospital v. Home care a technical memorandum (report No. OTA-TM-H-38) USGovernment Printing Office; Washington, DC: 1987. [Google Scholar]
- 16.Make B.J., Hill N.S., Goldberg A.I. Mechanical ventilation beyond the intensive care unit. Report of a consensus conference of the American College of Chest Physicians. Chest. 1998;113:289S–344S. doi: 10.1378/chest.113.5_supplement.289s. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosio I.U., Woo M.S., Jansen M.T. Safety of hospitalized ventilator-dependent children outside of the intensive care unit. Pediatrics. 1998;101:257–259. doi: 10.1542/peds.101.2.257. [DOI] [PubMed] [Google Scholar]
- 18.Lawrason A., Kavanagh K. Pediatric tracheotomy: are the indications changing? Int J Pediatr Otorhinolaryngol. 2013;77:922–925. doi: 10.1016/j.ijporl.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Mahadevan M., Barber C., Salkeld L. Pediatric tracheotomy: 17 year review. Int J Pediatr Otorhinolaryngol. 2007;71:1829–1835. doi: 10.1016/j.ijporl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Carr M.M., Poje C.P., Kingston L. Complications in pediatric tracheostomies. The Laryngoscope. 2001;111:1925–1928. doi: 10.1097/00005537-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Fiske E. Effective strategies to prepare infants and families for home tracheostomycare. Adv Neonatal Care. 2004;4:42–53. doi: 10.1016/j.adnc.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher T.Q., Hartnick C.J. Pediatric tracheotomy. Adv Oto Rhino Laryngol. 2012;73:26–30. doi: 10.1159/000334294. [DOI] [PubMed] [Google Scholar]
- 23.Flynn A.P., Carter B., Bray L. Parents' experiences and views of caring for achild with a tracheostomy: a literature review. Int J Pediatr Otorhinolaryngol. 2013;77:1630–1634. doi: 10.1016/j.ijporl.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Carnevale F.A., Alexander E., Davis M. Daily living with distress and enrichment: the moral experience of families with ventilator-assisted children at home. Pediatrics. 2006;117:e48–e60. doi: 10.1542/peds.2005-0789. [DOI] [PubMed] [Google Scholar]
- 25.Gluth M.B., Maska A., Nelson J. Postoperative management of pediatric tracheostomy: results of a nationwide survey. Otolaryngol Head Neck Surg. 2000;122:701. doi: 10.1016/S0194-5998(00)70200-2. [DOI] [PubMed] [Google Scholar]
- 26.Storgion S.A. Care of the technology-dependent child. Pediatr Ann. 1996;25:677. doi: 10.3928/0090-4481-19961201-06. [DOI] [PubMed] [Google Scholar]
- 27.Sherman J.M., Davis S., Albamonte-Petrick S. Care of the child with a chronic tracheostomy. Am J Respir Crit Care Med. 2000;161:297. doi: 10.1164/ajrccm.161.1.ats1-00. [DOI] [PubMed] [Google Scholar]
- 28.Klawitter B.M. Pediatric enteral nutrition support. In: Williams C.P., editor. Pediatric manual of clinical dietetics. American Dietetic Association; Chicago: 1998. p. 479. [Google Scholar]
- 29.Mahant S., Friedman J.N., Connolly B. Tube feeding and quality of life in childrenwith severe neurological impairment. Arch Dis Child. 2009;94:668–673. doi: 10.1136/adc.2008.149542. [DOI] [PubMed] [Google Scholar]
- 30.Canadian Paediatric Society Nutrition in neurologically impaired children. Paediatr Child Health. 2009;14:395–401. [PMC free article] [PubMed] [Google Scholar]
- 31.Marchand V., Motil K.J., NASPGHAN Committee on Nutrition Nutrition support forneurologically impaired children: a clinical report of the North American Societyfor pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2006;43:123–135. doi: 10.1097/01.mpg.0000228124.93841.ea. [DOI] [PubMed] [Google Scholar]
- 32.Heine R.G., Reddihough D.S., Catto Smith A.G. Gastro-oesophageal reflux andfeeding problems after gastrostomy in children with severe neurological impairment. Dev Med Child Neurol. 1995;37:320–329. doi: 10.1111/j.1469-8749.1995.tb12010.x. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan P., Juszczak E., Bachlet A. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev Med Child Neurol. 2004;46:796–800. doi: 10.1017/s0012162204001392. [DOI] [PubMed] [Google Scholar]
- 34.Smith S., Camfield C., Camfield P. Living with cerebral palsy and tube feeding: a population-based follow-up study. J Pediatr. 1999;135:307–310. doi: 10.1016/s0022-3476(99)70125-3. [DOI] [PubMed] [Google Scholar]
- 35.Nijs E., Cahill A. Pediatric enteric feeding techniques: insertion, maintenance,and management of problems. Cardiovasc Interv Radiol. 2010;33:1101–1110. doi: 10.1007/s00270-010-9837-7. [DOI] [PubMed] [Google Scholar]
- 36.Friedman J.N., Ahmed S., Connolly B. Complications associated with imageguided gastrostomy and gastrojejunostomy tubes in children. Pediatrics. 2004;114:458–461. doi: 10.1542/peds.114.2.458. [DOI] [PubMed] [Google Scholar]
- 37.Albanese C.T., Towbin R.B., Ulman I. Percutaneous gastrojejunostomy versus Nissen fundoplication for enteral feeding of the neurologically impaired child with gastroesophageal reflux. J Pediatr. 1993;123:371–375. doi: 10.1016/s0022-3476(05)81734-2. [DOI] [PubMed] [Google Scholar]
- 38.Soscia J., Friedman J.N. A guide to the management of common gastrostomy and gastrojejunostomy tube problems. Paediatr Child Health. 2011;16:281–287. doi: 10.1093/pch/16.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobak G.E., McClenathan D.T., Schurman S.J. Complications of removing percutaneous endoscopic gastrostomy tubes in children. J Pediatr Gastroenterol Nutr. 2000;30:404. doi: 10.1097/00005176-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Khattak I.V., Kimber C., Kiely E.M. Percutaneous endoscopic gastrostomy in paediatric practice: complications and outcome. J Pediatr Surg. 1998;33:67. doi: 10.1016/s0022-3468(98)90364-5. [DOI] [PubMed] [Google Scholar]
- 41.Jolley S.G., Smith E.I., Tunell W.P. Protective antireflux operation with feeding gastrostomy: experience with children. Ann Surg. 1985;201:736. doi: 10.1097/00000658-198506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson P.M., Catto-Smith A.G., Beasley S.W. Technique and complications of percutaneous endoscopic gastrotomy in children. Aust N Z J Surg. 1995;65:194. doi: 10.1111/j.1445-2197.1995.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 43.Haynes L., Atherton D.J., Ade-Ajayi N. Gastrostomy and growth in dystrophic epidermolysis bullosa. Br J Dermatol. 1996;134:872. [PubMed] [Google Scholar]
- 44.Khattak I.V., Kimber C., Kiely E.M. Percutaneous endoscopic gastrostomy in paediatric practice: complications and outcome. J Pediatr Surg. 1998;33:67. doi: 10.1016/s0022-3468(98)90364-5. [DOI] [PubMed] [Google Scholar]
- 45.Graham S.M., Flowers J.L., Scott T.R. Safety of percutaneous endoscopic gastrostomy in patients with a ventriculo-peritoneal shunt. Neurosurgery. 1993;32:932. doi: 10.1227/00006123-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Ramage I.J., Harvey E., Geary D.F. Complications of gastrostomy feeding in children receiving peritoneal dialysis. Pediatr Nephrol. 1999;13:249. doi: 10.1007/s004670050603. [DOI] [PubMed] [Google Scholar]
- 47.Gauderer M.W.L. An updated experience with percutaneous endoscopic gastrostomy in children. Gastrointest Endosc Clin North Am. 1992;2:195. [Google Scholar]
- 48.Lerner G.R., Warady B.A., Sullivan E.K. Chronic dialysis in children and adolescents. The 1996 report of the North American pediatric renal transplant cooperative study. Pediatr Nephrol. 1999;13:404. doi: 10.1007/s004670050631. [DOI] [PubMed] [Google Scholar]