Abstract
Purpose
Hypertension is a growing public health problem with a remarkable contribution to morbidity and mortality. It is a common condition which usually coexists with diabetes and aggravates its complications. The objective of this study was to assess the prevalence and determinants of hypertension among diabetic patients attending their follow-up at Jimma University Medical Center (JUMC) from June 1 to August 30, 2019.
Methods
A hospital-based cross-sectional study was conducted in diabetic patients attending their follow-up at JUMC. Systematic random sampling and a pretested interviewer-administered structured questionnaire were used to collect information. Data were entered into EPI data 3.1 and exported to SPSS version 20. A variable having a p-value of <0.25 in the bivariate model was subjected to multivariate analysis to avoid the confounding variable’s effect. Adjusted odds ratios were calculated at the 95% confidence interval and considered significant with a p-value of <0.05.
Results
A total of 366 diabetic patients were included in the study. Their mean age was 50.1 ± 14.28 years, and the mean duration of diabetes was 6.8 ± 5.3 years. The study finding showed that the prevalence of hypertension among diabetic patients was 37.4%. According to the multivariate logistic regression analysis, age of ≥50 years [AOR = 4.79; 95% CI: 1.4, 16.4], having body mass index (BMI) of ≥25 [AOR = 3.11; 95% CI: 1.58, 6.12] and khat chewing [AOR =19.34; 95% CI: 10.26, 36.44] were independent predictors of hypertension among diabetic patients.
Conclusion
Our study found that there is high prevalence of hypertension among diabetic patients. Age of ≥50 years, having BMI of ≥25 kg/m2 and khat chewing were associated with hypertension among participants. Early detection and appropriate interventions should be an important action among patients with age ≥ 50 years, having BMI ≥ 25kg/m2 and khat chewers.
Keywords: prevalence, determinants, hypertension, diabetes, Southwest Ethiopia
Introduction
Hypertension (HTN) is the persistent elevation of systemic arterial blood. There is no sharp demarcation between normal blood pressure (BP) and HTN. However, for clinical purposes, it is defined as systolic blood pressure (SBP) of ≥140 millimeters of mercury (mmHg) and/or diastolic BP (DBP) of ≥90 mmHg or any prior diagnosis of HTN made by a health professional, and taking antihypertensive drugs.1,2
Hypertension, among diabetic patients, is a worldwide public-health challenge.3 The frequency of HTN among the diabetic population is almost twice that of non-diabetic patients.4 Compared with other cardiovascular disorders, HTN is the most common comorbid disease in diabetic patients and its effects are devastating if not controlled.5,6 Hypertension is estimated to cause 7.5 million deaths annually, accounting for 57 million disability-adjusted life years, and accounts for about 6% of deaths worldwide.2,7 Globally, nearly one billion people have HTN; of these, two-thirds are in developing countries.8
The coexistence of HTN and diabetes mellitus (DM) is a major contributor to the development and progression of microvascular and macrovascular complications9. Furthermore, the development of HTN in diabetic individuals complicates the treatment strategy and increases healthcare costs.10 Up to 80% of people with diabetes will die of cardiovascular disease, especially HTN and stroke.9,11 The coexistence of hypertension in DM is attributed to the risk of death and cardiovascular events by 44% and 41%, respectively, as compared to 7% and 9% of these risks in people with diabetes alone.12
The United Kingdom Prospective Diabetes Study showed that BP control helps to prevent cardiovascular complications in patients with diabetes. Each 10 mmHg decrease in mean SBP was associated with a 12% reduction in the risk for any complication related to diabetes and a 15% reduction in deaths related to diabetes.13 Older age, overweight/obesity, unhealthy diet, lack of physical exercise, smoking and family history of hypertension are major risk factors for HTN.14,15 Focusing on detecting and managing hypertension in patients with diabetes is one of the most effective things that can be done to prevent diabetes complications.
Many countries in sub-Saharan Africa, including Ethiopia, lack detailed basic data on the prevalence and determinants of hypertension.16 Only a few studies have reported on the proportion of persons with hypertension who are aware of their hypertension and were not given due attention. To date, there is no established evidence regarding the prevalence and determinants of hypertension among diabetic patients in Jimma. Hence, this study was aimed at assessing the prevalence and determinants of hypertension among diabetic patients for better management and risk minimization in Jimma University Medical Center (JUMC), Southwest Ethiopia. This study will help provide further information regarding the magnitude of hypertension and its determinants to plan further interventions.
Methods and Materials
Study Setting, Design and Period
An institution-based cross-sectional study was conducted on 366 diabetic patients attending their follow-up at the chronic illness clinic of JUMC, Southwest Ethiopia. It is located in Jimma city 352 km southwest of Addis Ababa. It is a teaching and referral hospital in our country serving a very large catchment area in the Southwestern Oromia region. The data collection period was from June 1 to August 30, 2019.
Population
The source population includes all adult diabetic patients at the follow-up clinic at JUMC, while the study population was all adult diabetic patients who were under routine follow-up at the JUMC during the data collection period. A total of 2500 DM patients constituted the source population.
Eligibility Criteria
Participants of age ≥ 18 years who had follow-up for at least 1 month were included and those who were critically ill, had incomplete medical records, with newly diagnosed diabetes who were not registered, with hearing difficulty and pregnant women were excluded.
Sample Size Determination
The sample size was calculated using a single population proportion formula with 95% confidence interval, 50% proportion and a margin of error 5%. This gives an initial sample size of 384. Since the estimated total population of diabetes patients was less than 10,000, we employed a population correction formula for a finite population. The final sample size according to this equation yields 333, and then adding 10% for nonresponse the final sample size was 366.
Sampling Technique
A systematic random sampling technique was employed to select study participants. There were 2500 diabetic patients registered at the follow-up clinic at JUMC. These patients were our sampling frame and the patients included in the sample were selected every six intervals based on their medical record registration numbers. The first patient was selected randomly from the first six by a lottery method, and the next patient was interviewed and examined every sixth interval until the required sample was attained (Figure 1).
Figure 1.
Flow chart to select study participants at JUMC 2019, Jimma, Ethiopia.
Data Collection Tool and Procedure
Data were collected using a structured interviewer-administered questionnaire through face to face interviews, patient record reviews and physical examination. Behavioral variables were assessed based on the WHO STEPwise approach for chronic disease risk factor surveillance.17 Clinical variables were taken from the patient record review and physical measurements were conducted. Body weight was measured to an accuracy of 0.1 kg using a portable weight scale machine. Subjects were barefoot and wearing light indoor clothing. Height was measured in meters, standing upright on a flat surface by a stadiometer. Body mass index (BMI) was calculated as the ratio of weight in kilograms (kg) to the square of height in meters (m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured from the left arm at the level of the heart using a mercury-based sphygmomanometer after the subjects take a rest for more than 10 minutes and 30 minutes for those who take hot drinks like coffee. For those study subjects with SBP ≥ 140 mm of mercury (mmHg) and DBP ≥ 90 mmHg, blood pressure was measured again and finally the average value was taken. Three BSC nurses and two medical interns collected the data with the supervision of two supervisors and principal investigator.
Operational Definition
Hypertension
A study participant was classified as hypertensive if the average SBP/DBP was ≥140/90 mmHg and/or taking antihypertensive drugs.
BMI ≥ 25 kg/m2
Overweight or obese.
Critically Ill
Patients who are unable to communicate and abnormal consciousness.
Controlled Fasting Blood Sugar
Fasting blood sugar ≤130 mg/dl.18
Uncontrolled Blood Sugar
Fasting blood sugar >130 mg/dl.18
Diabetes Patient
Known diabetic patient on follow-up who has a medical record number at JUMC.
Diabetes Duration
The duration of DM was calculated as age at data collection minus age at onset of DM.
Khat Chewer
Participants who chewed khat for 4 or more days in a week.
Non-Chewers
Those who never chewed khat.
Data Quality Management and Statistical Analysis
Before data analysis took place, training was given to the data collectors by the principal investigator on how to use the questionnaire and guideline, objectives of the study on chart review and measurement techniques. A pretest was conducted in 5%18 of the sample size in Shenen Gibe Hospital and amendment of the questionnaire was done. After analyzing pretest results, necessary corrections and modifications were made. Continuous follow-up and supervision were made by the two supervisors and principal investigator and collected data were reviewed and checked daily for completeness, clarity and consistency. After that, data were entered into Epidata version 3.1 and then exported to SPSS, IBM 20 for analysis. Frequency, proportion and mean were computed to describe the relevant variables. The data output was presented using tables. A logistic regression model was computed to see the association of independent variables and dependent variables. Bivariate analysis was done and those independent variables whose p-values less were than <0.25 were shifted into the multivariate logistic model. Finally, significance of statistical association was assured or tested using the 95% confidence interval and a p-value of <0.05 was considered significant in multivariable regression. Model fitness was also checked through the Hosmer and Lameshow test, p > 0.05.
Result
Socio-Demographic Characteristics of Participants
A total of 366 participants were involved in this study. More than half (55.5%, 203) of the respondents were males. The mean age of the respondents was 50.1 ± 14.28 years. Of the respondents, 44.3% were Muslim followed by orthodox 39.6% in religion. Regarding the marital status of the respondents, more than three-quarters (289, 79%) were married followed by single (60, 16.4%) (Table 1).
Table 1.
Socio-Demographic Characteristics of Patients with Diabetes Mellitus at JUMC 2019, Jimma, Ethiopia
| Variables | Category | Number | Percentage |
|---|---|---|---|
| Sex | Male | 203 | 55.5 |
| Female | 163 | 44.5 | |
| Age | <30 years | 43 | 11.7 |
| 30 to 39 years | 30 | 8.2 | |
| 40 to 49 years | 87 | 23.8 | |
| ≥50 years | 206 | 56.3 | |
| Marital status | Married | 289 | 79 |
| Single | 60 | 16.4 | |
| Othersa | 17 | 4.6 | |
| Religion | Muslim | 162 | 44.3 |
| Orthodox | 145 | 39.6 | |
| Protestants | 46 | 12.6 | |
| Othersb | 13 | 3.6 | |
| Educational status | Illiterate | 108 | 29.5 |
| Primary | 167 | 45.6 | |
| Secondary | 44 | 12 | |
| College and above | 47 | 12.8 | |
| Occupational status | Housewife | 108 | 29.5 |
| Farmer | 113 | 30.9 | |
| Employer | 78 | 21.3 | |
| Private worker | 51 | 13.9 | |
| Othersc | 16 | 4.4 | |
| Residence | Urban | 177 | 48.4 |
| Rural | 189 | 51.6 | |
| Family history of hypertension | Yes | 94 | 25.7 |
| No | 272 | 74.3 | |
| Average monthly income (ETB) | <1000 | 96 | 26.2 |
| 1000 to 1999 | 40 | 10.9 | |
| 2000 to 2999 | 95 | 26 | |
| ≥3000 | 135 | 36.9 |
Notes: aWidowed, divorced. bCatholic, Wakefata. cRetired, unemployed.
Clinical and Behavioral Characteristics of Participants
Almost half (54.9%) of study participants were diagnosed with diabetes for less than 5 years. A total of 231 (63.1%) study participants were in the normal category of BMI. Fifty-four (14.8%) participants were active smokers currently, while 244 (66.7%) had never smoked (Table 2).
Table 2.
Clinical and Behavioral Characteristics of Patients with Diabetes Mellitus at JUMC 2019, Jimma, Ethiopia
| Variables | Category | Number | Percentage |
|---|---|---|---|
| Type of DM | 1 | 70 | 19.1 |
| 2 | 296 | 80.9 | |
| Duration of DM | <5 years | 201 | 54.9 |
| ≥5 years | 165 | 45.1 | |
| Hypertension | Yes | 137 | 37.4 |
| No | 229 | 62.6 | |
| Treatment regimen | Noninsulin | 244 | 66.7 |
| Insulin | 99 | 27 | |
| Both | 23 | 6.3 | |
| BMI (kg/m2) | 18.5 to 24.9 | 231 | 63.1 |
| <18.5 | 30 | 8.2 | |
| ≥25 | 84 | 23 | |
| Alcohol intake | Current | 44 | 12 |
| Former | 31 | 8.5 | |
| Never | 291 | 79.5 | |
| Smoking | Current | 54 | 14.8 |
| Former | 68 | 18.6 | |
| Never | 244 | 66.7 | |
| Physical exercise | Active | 179 | 48.9 |
| Inactive | 187 | 51.1 | |
| Other comorbid diseasea | Yes | 42 | 11.5 |
| No | 324 | 88.5 | |
| Fasting blood sugar (mg/dl) | <130 | 263 | 71.9 |
| ≥130 | 103 | 28.1 | |
| Khat chewing | Yes | 157 | 42.8 |
| No | 209 | 57.1 |
Notes: a24 chronic kidney disease, 9 congestive heart failure, 5 asthma, 4 cancer.
Prevalence of Hypertension Among Diabetic Patients
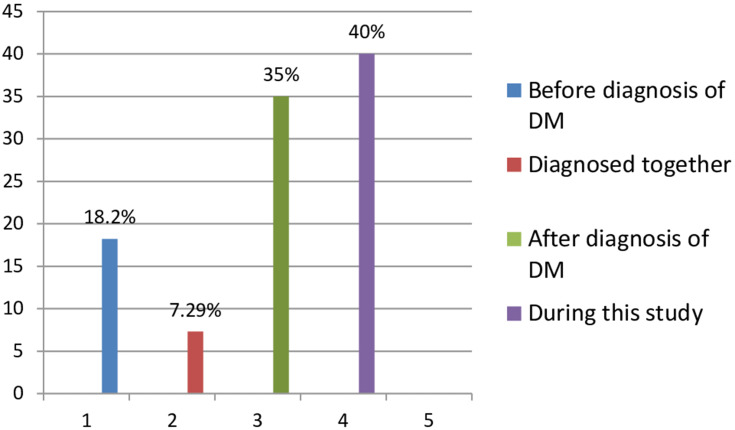
The overall prevalence of hypertension among diabetic patients was found to be 37.4% (137) [95% CI: 32.8, 42.6]. Out of hypertensive diabetics, most (40%) of them were newly discovered during this study period and the least (7.29%) of them were diagnosed together with DM simultaneously (Figure 2).
Figure 2.
Prevalence rate of HTN based on the time it was diagnosed.
Factors Independently Associated with Hypertension
A multivariable logistic regression model was fitted to identify factors independently associated with hypertension among DM patients at p < 0.05. On bivariate evaluation, 10 variables showed evidence of some association with the outcome at a p-value <0.25, hence were included in the multivariate logistic regression analysis. These variables include educational level, age, duration of diabetes, physical inactivity, smoking, type of DM, family history of hypertension, khat chewing, BMI ≥ 25 kg/m2 and treatment regimen. The Hosmer and Lemeshow goodness of fit test gave a p-value of 0.145, indicating evidence of fitness of the model.
From those variables, age of participant was one of the independent factors in predicting hypertension. Participants of age ≥ 50 years were 4.57 times more likely to develop hypertension compared to patients younger than 30 years [AOR = 4.57; 95% CI: 1.40, 16.4, p = 0.012] controlling for all other factors in the model. The other factor identified was BMI; clients whose BMI was ≥25 kg/m2 were 3.11 times more likely to develop hypertension compared to patients with those normal BMI [AOR = 3.11; 95% CI: 1.58, 6.12, p = 0.001] controlling for all other factors in the model. Finally, diabetic patients who were khat chewers were 19.3 times more likely to develop hypertension as compared with non-chewers [AOR = 19.3; 95% CI: 10.26, 36.44, p < 0.001] provided other factors remain the same (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Factors Associated with Hypertension Among Diabetes Patients in JUMC, 2019
| Variables | Category | HTN | Bivariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|---|
| Yes | No | P-value | COR (95% CI) | P-value | AOR (95% CI) | ||
| Age (years) | <30 | 5 | 39 | 1 | 1 | 1 | |
| 30 to 39 | 7 | 23 | 0.178 | 2.37 [0.67, 8.35] | 0.226 | * | |
| 40 to 49 | 26 | 61 | 0.023 | 3.32 [1.17, 9.388] | 0.082 | * | |
| ≥50 | 99 | 106 | 0.00 | 7.28 [2.76, 19.226] | 0.012a | 4.79 [1.4, 16.4] | |
| Educational level | Illiterate | 49 | 59 | 0.072 | 1.95 [0.942, 4.066] | 0.088 | * |
| Primary | 60 | 107 | 0.435 | 1.32 [0.65, 2.66] | 0.118 | * | |
| Secondary | 14 | 30 | 0.834 | 1.1 [0.451, 2.68] | 0.111 | * | |
| College and above | 14 | 33 | 1 | 1 | 1 | 1 | |
| Type of DM | 1 | 12 | 58 | 1 | 1 | 1 | 1 |
| 2 | 125 | 171 | 0.00 | 3.53 [1.82, 6.85] | 0.197 | * | |
| Smoking | Current | 20 | 47 | 0.392 | 0.77 [0.429, 1.39] | 0.587 | * |
| Former | 35 | 33 | 0.019 | 1.927 [1.116, 3.33] | 0.138 | * | |
| Never | 82 | 149 | 1 | 1 | 1 | 1 | |
| Family history of HTN | Yes | 43 | 51 | 0.054 | 1.59 [0.991, 2.57] | 0.124 | * |
| No | 94 | 178 | 1 | 1 | 1 | 1 | |
| Physical exercise | Active | 56 | 123 | 1 | 1 | 1 | 1 |
| Inactive | 81 | 106 | 0.018 | 1.678 [1.094, 2.58] | 0.999 | * | |
| Khat chewing | No | 29 | 180 | 1 | 1 | 1 | |
| Yes | 49 | 108 | ≤.001 | 13.6 [8.1, 22.95] | ≤.001a | 19.34 [10.26, 36.44] | |
| BMI (kg/m2) | 18.5 to 24.9 | 71 | 153 | 1 | 1 | 1 | 1 |
| <18.5 | 9 | 18 | 0.863 | 1.07 [0.461, 2.516 | 0.487 | * | |
| ≥25 | 57 | 55 | 0.001 | 2.233 [1.40, 3.55] | 0.001a | 3.11 [1.58, 6.12] | |
| Duration of DM (years) | <5 | 65 | 136 | 1 | 1 | 1 | 1 |
| ≥5 | 72 | 93 | 0.027 | 1.62 [1.057, 2.48] | 0.275 | * | |
| Treatment regimen | Insulin | 31 | 68 | 1 | 1 | 1 | 1 |
| Noninsulin | 94 | 150 | 0.209 | 1.375 [0.836, 2.26] | 0.894 | * | |
| Both | 12 | 11 | 0.064 | 2.393 [0.95, 6.01] | 0.635 | * | |
Notes: aValue statistically significant. *Value not statistically significant. AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; 1, reference.
Discussion
The overall prevalence of hypertension among diabetic patients was found to be 37.4% [95% CI: 32.8, 42.6]. This result was in line with several studies conducted so far. Accordingly, studies conducted in Pakistan 40.45%,19 38% in Bahrain,20 Taiwan 39%21 and Jos, Nigeria 35%.22 However, the finding of the current study is lower than a study conducted in Botswana 61.2%,23 85.6% in Benghazi, Libya,24 70.4% in Morocco,25 in Jordan 76%26 and 89.6% in Iraq 89.6%.27 The possible reason for such discrepancy might be due to differences in study population used, socio-demographic characteristics, study settings, study design, the habit of visits to health setups and difference in lifestyle of study participants.
In contrast, the finding of the current study is higher than a study conducted in Southern Ethiopia at Sidama zone which reported 18.8%,28 29% in Turkey29 and 25.6% in India.30 The possible reasons for difference with our finding might be due to differences in socio-demographic, study design, type of study population, sample size variation and/ or awareness differences.
Our study showed that age ≥ 50 years was independently associated with HTN among diabetic patients. This report is in line with the previous studies.23,24,27 This might be due to aging being generally associated with a decline in various physiological functions and non-communicable diseases including HTN. Furthermore, increasing age has also been linked with a higher incidence of disease.31,32
In addition, our study showed that patients with higher BMI (≥25 kg/m2) have a higher risk of developing hypertension than ones with normal BMI. This finding agreed with the findings of previous studies.23,25,27 The possible explanation for this is excess weight gain leads to enhanced cardiovascular risk, endothelial dysfunction, inflammation, hemodynamic changes, and atherosclerosis.33 In addition, when an individual becomes overweight he/she will have insulin resistance, excess bad cholesterol in blood vessels and this makes narrow blood vessels, and progressively the person develops HTN. Besides, as BMI levels increase, the risk of chronic diseases increases including hypertension.34,35
Finally, our study indicated that hypertension is associated with khat (Catha edulis) chewing. This result is consistent with prior studies.36,39 The possible reason might be cathinone, which is the main active ingredient in khat leaves, releases noradrenaline, which might have a sustained effect as a peripheral vasoconstrictor among regular khat chewers.38,40
Limitation of the Study
The cross-sectional design of this study was unable to identify a causal relationship. Since the study was hospital based, it may not be representative of the community and some questions were exposed for recall bias. Finally, we did not include concomitant medications.
Conclusion
The study found that a majority of diabetes patients suffer from coexisting hypertension. So active search for early detection of hypertension and related cardiovascular risk factors should be an important part of diabetes follow-up. Furthermore, the study pointed out that BMI ≥ 25 kg/m2, khat chewing and older age were associated with hypertension coexisting with DM, which may be used in tailoring management of hypertension among diabetics.
Acknowledgment
We would like to thank Jimma University for funding this study and JMC staff for their cooperation while conducting this study. Also, we would like to convey heartfelt gratitude for the study participants for their kind and unlimited cooperation, support and participation in the study.
Abbreviations
AOR, adjusted odds ratio; BMI, body mass index; BSC, Bachelor of Science; CI, confidence interval; COR, crude odds ratio; DBP, diastolic blood pressure; DM, diabetes mellitus; ETB, Ethiopian birr; FBS, fasting blood sugar; HTN, hypertension; JUMC, Jimma University Medical Center; mmHg, millimeter of mercury; SBP, systolic blood pressure; WHO, World Health Organization.
Ethical Consideration
The study approved and ethical clearance was obtained from Jimma University institutional review board. Official letter of permission was obtained from Jimma Institute of Health Ethical review board and given to Jimma University Medical Center director office to conduct the study. Then selected respondents were well informed about the purpose, benefit and method of the study. Then information was collected after written consent from each participant was obtained. Information was recorded anonymously and confidentiality and beneficence were assured throughout the study period.
Disclosure
There are no conflicts of interest.
References
- 1.WHO A. Global Brief on Hypertension. Silent killer, global public health crisis; 2013. [Google Scholar]
- 2.Kotchen TA. Hypertension control: trends, approaches, and goals. Hypertension. 2007;49(1):19–20. doi: 10.1161/01.HYP.0000250394.05703.06 [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Jaramillo P, Lopez-Lopez J, Lopez-Lopez C, Rodriguez-Alvarez MI. The goal of blood pressure in the hypertensive patient with diabetes is defined: now the challenge is go from recommendations to practice. Diabetol Metab Syndr. 2014;6(1):31. doi: 10.1186/1758-5996-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul B, Sapra B, Maheshwari S, Goyal R. Role of losartan therapy in the management of diabetic hypertension. J Assoc Physicians India. 2000;48(5):514–518. [PubMed] [Google Scholar]
- 5.Mendis S. Challenges for the management of hypertension in low-resource settings. Ethn Dis. 2003;13(2 Suppl 2):S67– 70. [PubMed] [Google Scholar]
- 6.Kahya NE, Harman E, Dolek D, et al. Rate of blood pressure control and antihypertensive treatment approaches in diabetic patients with hypertension. Turk Kardiyol Dern Ars. 2014;42(8):7. [DOI] [PubMed] [Google Scholar]
- 7.Geneva W. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 8.World Health Organization. Regional Office for Southeast Asia. Hypertension fact sheet. [Google Scholar]
- 9.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28(11):2668–2686. doi: 10.2337/diacare.28.11.2668 [DOI] [PubMed] [Google Scholar]
- 10.Aroda VR, Knowler WC, Crandall JP, et al. Metformin for diabetes prevention: insights gained from the diabetes prevention program/diabetes prevention program outcomes study. Diabetologia. 2017;60(9):1601–1611. doi: 10.1007/s00125-017-4361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. A Global Brief on Hypertension. World Health Organization Press; 2013. [Google Scholar]
- 12.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574 [DOI] [PubMed] [Google Scholar]
- 13.Prospective Diabetes Study Group UK, United Kingdom Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317,(7160):703–713. doi: 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwan A. Global Status Report on Noncommunicable Diseases 2010. World Health Organization; 2011. [Google Scholar]
- 15.Koly KN, Biswas T, Islam A. Increasing prevalence of hypertension in Bangladesh: a review. Cardiovasc J. 2015;8(1):59–64. doi: 10.3329/cardio.v8i1.24771 [DOI] [Google Scholar]
- 16.Hendriks ME, Wit FWNM, Roos MTL, et al. Hypertension in sub- Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS One. 2012;7(3):e32638. doi: 10.1371/journal.pone.0032638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley L, Guthold R, Cowan M, et al. The World Health Organization STEPwise approach to non-communicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106(1):74–78. doi: 10.2105/AJPH.2015.302962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansson SP, Svärdsudd K, Andersson DK. Effects of fasting blood glucose levels and blood pressure and treatment of diabetes and hypertension on the incidence of cardiovascular disease: a study of 740 patients with incident Type 2 diabetes with up to 30 years’ follow‐up. Diabetic Med. 2014;31(9):1055–1063. doi: 10.1111/dme.12514 [DOI] [PubMed] [Google Scholar]
- 19.Arshad AR. Control of blood pressure in hypertensive patients with diabetes mellitus type 2. Pak Hear J. 2014;47(02):78–83. [Google Scholar]
- 20.Al-mahroos F, Al-roomi K, Mckeigue PM. Relation of high blood pressure to glucose intolerance, plasma lipids and educational status in an Arabian Gulf population. Int J Epidemiol. 2000;29(1):71–76. doi: 10.1093/ije/29.1.71 [DOI] [PubMed] [Google Scholar]
- 21.Tseng CH. Higher risk of hypertension in indigenous type 2 diabetic patients in Taiwan. J Hypertens. 2006;24(9):1817–1821. doi: 10.1097/01.hjh.0000242406.76085.c4 [DOI] [PubMed] [Google Scholar]
- 22.Chuhwak EK, Puepet FH, Okeahialam BN, Ohwovoriole AE. Hypertension and Diabetes in Jos, Nigeria. Diabetes Int. 2002;12:25–26. [Google Scholar]
- 23.Mengesha AY. Hypertension and related risk factors in type 2 diabetes mellitus (DM) patients in Gaborone City Council (GCC) clinics, Gaborone. Afr Heal Sci. 2007;7(4):244–245. [PMC free article] [PubMed] [Google Scholar]
- 24.Nouh F, Omar M, Younis M. Prevalence of Hypertension among Diabetic Patients in Benghazi: A Study of Associated Factors. Asian J Med Heal. 2017;6(4):1–11. doi: 10.9734/AJMAH/2017/35830 [DOI] [Google Scholar]
- 25.Berraho M, Youness E. Achhab, Hypertension and type 2 diabetes: a cross-sectional study in Morocco (EPIDIAM Study) Mohamed. Pan Afr Med J. 2012;11. [PMC free article] [PubMed] [Google Scholar]
- 26.Alqudah BM, Mahmoud H, Alhusamia S, Sh A, Al L, Alawneh ZE. Prevalence of hypertension among diabetic type 2 patients attending medical clinic at Prince Hashem bin Abdullah Hospital in Aqaba. Indian J Med Res Pharm Sci. 2017;4:(June):47–54. [Google Scholar]
- 27.Mansour AA, Prevalence and control of hypertension in Iraqi diabetic patients: a prospective cohort study. Open Cardiovasc Med J. 2012;1:68–71. doi: 10.2174/1874192401206010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giday A, Wolde M, Yihdego D. Hypertension, obesity and central obesity in diabetics and non-diabetics in Southern Ethiopia. Ethiop J Health Dev. 2010;24. [Google Scholar]
- 29.Satman I, Yilmaz T. Population-based study of diabetes and risk characteristics in Turkey. Diabetes Care. 2002;25(9):1551–1556. doi: 10.2337/diacare.25.9.1551 [DOI] [PubMed] [Google Scholar]
- 30.Venugopal K, Mohammed MZ. Prevalence of hypertension in type-2 diabetes mellitus. J Heal Res. 2014;1:4. [Google Scholar]
- 31.Satman I, Omer B, Tutuncu Y. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol. 2013;28(2):169–180. doi: 10.1007/s10654-013-9771-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colosia AD, Roberto P, Shahnaz K. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: A systematic literature review. Diabetes Metab Syndr Obes. 2013;6(1):327–338. doi: 10.2147/DMSO.S51325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–619. doi: 10.1038/oby.2000.79 [DOI] [PubMed] [Google Scholar]
- 35.Velásquez-Rodríguez CM, Velásquez-Villa M, Gómez-Ocampo L, Bermúdez-Cardona J. Abdominal obesity and low physical activity are associated with insulin resistance in overweight adolescents: a cross-sectional study. BMC Pediatr. 2014;14(1):258. doi: 10.1186/1471-2431-14-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145–1154. doi: 10.1517/14740338.4.6.1145 [DOI] [PubMed] [Google Scholar]
- 37.Hassan NA, Gunaid AA, Murray-Lyon IM. Khat (Catha edulis): health aspects of Khat chewing. East Mediterr Health J. 2007;13(3):706–718. [PubMed] [Google Scholar]
- 38.Getahun W, Gedif T, Tesfaye F. Regular Khat (Catha edulis) chewing is associated with elevated diastolic blood pressure among adults in Butajira, Ethiopia: A comparative study. BMC Public Heal. 2010;10:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toennes SW, Harder S, Scharmm M, Niess C, Kauert GF. Pharmacokinetics of Cathinone and norephedrine after the chewing of Khat leaves. Br J Clin Pharmacol. 2003;56(1):125–130. doi: 10.1046/j.1365-2125.2003.01834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan NA, Gunaid AA, Abdo-Rabbo AA, Abdel-Kader ZY. The effect of Qat chewing on blood pressure and heart rate in healthy volunteers. Trop Doct. 2000;30(2):107–108. doi: 10.1177/004947550003000219 [DOI] [PubMed] [Google Scholar]