Abstract
Introduction
Adults with Down syndrome (DS) are at high risk for developing Alzheimer's disease (AD) and its associated dementia, warranting the development of strategies to improve early detection when prevention is possible.
Methods
Using a broad battery of neuropsychological assessments, informant interviews, and clinical record review, we evaluated the psychometrics of measures in a large sample of 561 adults with DS. We tracked longitudinal stability or decline in functioning in a subsample of 269 participants over a period of 3 years, all initially without indications of clinically significant aging‐related decline.
Results
Results identified an array of objective measures that demonstrated sensitivity in distinguishing individuals with incident “mild cognitive impairment” (MCI‐DS) as well as subsequent declines occurring with incident dementia.
Discussion
Several instruments showed clear promise for use as outcome measures for future clinical trials and for informing diagnosis of individuals suspected of experiencing early signs and symptoms of a progressive dementia process.
Keywords: aging, Alzheimer's disease, Down syndrome, mild cognitive impairment
1. BACKGROUND
Adults with Down syndrome (DS) have benefited from improvements in medical care, nutritional practices, and public health policies that have occurred over the last century. 1 , 2 These positive developments, along with societal changes that have benefited all people with developmental disorders (eg, deinstitutionalization), have resulted in a dramatic extension of their life expectancy. 3 Life expectancy is now approaching that of the neurotypical population for many of individuals with intellectual disability (ID), although DS continues to contribute to earlier mortality risk. 4 , 5
Dementia is among the most serious public health concerns faced by elderly adults, with disease progression having devastating impacts on independent functioning and quality of life. Alzheimer's disease (AD) is the most common cause 6 currently affecting more than 44 million worldwide. 7 Demographic projections, based on continuing population longevity, indicate that the number of affected individuals will triple by 2050. 8 Thus, the discovery of effective treatment and prevention methods is one of the highest priorities for current biomedical research.
Lifespan development for adults with DS is atypical in many respects and an increased risk for AD is a well‐established phenotype. 9 , 10 , 11 , 12 , 13 The high risk for AD has been attributed largely to the triplication and overexpression of the gene coding for amyloid precursor protein, located on chromosome 21, contributing to the increased production of amyloid beta (Aβ) protein leading to amyloid deposition in the brain. 14
Recognition of the importance of early diagnosis of AD has led to an emphasis on understanding the prodromal stages of disease. Much effort in the field of aging and dementia has been devoted to the early signs and symptoms that could be used as reliable markers of disease progression. 15 Then, as efficacious interventions become available, clinicians can identify at‐risk individuals to prevent or slow progression to severe dementia.
Mild cognitive impairment (MCI) is now recognized as a broad construct referring to the prodromal condition that precedes dementia, having a variety of causes with multiple clinical profiles. 16 This state, intermediate between an individual's “normal” functioning and dementia, is characterized by relatively subtle cognitive decline, with day‐to‐day functioning minimally affected. 16 Though the construct has evolved over the last 25 years, the core criteria have remained unchanged. 17 Petersen et al 18 originally emphasized impairments in memory processes (now known as amnestic‐MCI), but there has been significant broadening of the construct to include non‐amnestic and mixed subtypes. 19 While a decline in cognitive abilities, greater than would be expected with aging per se, is necessary to diagnose MCI in the neurotypical population, diagnostic criteria have relied on performance profiles on norm‐referenced tests that provide standardized scores reflecting the individual's percentile relative to the general population. However, there is no “gold standard” consensus specifying which specific tests to use for diagnosis. There also remains debate regarding the defining quantitative severity of impairment (although there is consensus that it should be insufficient to justify a diagnosis of dementia). Performance 1.5 standard deviations below the neurotypical population mean (in one or more domains) in tests of cognitive abilities has been serving as an operational criterion in broad use. 20 , 21 This condition will often precede a diagnosis of aging‐related dementia and it can persist for an extended period. 22
As research on MCI has progressed, several areas of controversy have arisen concerning the specific boundaries of the condition, its precise definition, and the criteria used in various clinical settings. 23 This complexity is magnified for adults with DS because they have substantial lifelong cognitive deficits, which vary substantially in severity.
While the notion of MCI as a transitional stage between “normal” cognitive aging and AD is easy to grasp, development of an operational definition sufficiently precise to delineate a unique and useful diagnostic entity in individuals with DS has proven challenging. Clearly an objective criterion of performance on standardized testing of 1.5 SDs below the population mean is not applicable for adults with lifelong cognitive impairment(s), who typically perform below that level from early development. In fact, few studies have focused on MCI among adults with DS (MCI‐DS) and none have proposed explicit diagnostic criteria applicable to this population, although reports have emphasized the significance of this condition. 10 , 24 , 25 , 26
Research in Context
Systematic review: The authors reviewed the literature on mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the neurotypical population and in adults with Down syndrome (DS). Few existing studies focused on MCI among adults with DS (MCI‐DS) and none have proposed explicit diagnostic criteria applicable to this population, although they emphasized the significance of this condition. These relevant citations are appropriately cited.
Interpretation: The authors identified objective measures sensitive to the emergence of MCI‐DS in adults with DS and were able to quantify further decline associated with progression of AD to incident dementia.
Future directions: Several instruments: (1) showed considerable promise for use as outcome measures in future clinical trials targeting AD in adults with DS, (2) may inform diagnosis of early clinical impacts of AD for adults with DS, and (3) may serve as critically important tools in discovery studies for biomarkers of preclinical and prodromal AD.
A series of three studies evaluated testability for individuals ranging in the severity of ID, the sensitivity of objective measures of performance to the onset of MCI‐DS, and the ability of these measures to quantify subsequent decline indicative of dementia.
2. OVERALL METHODS
2.1. Participants
A large cohort of adults with DS (N = 687) was recruited for longitudinal studies with follow‐up at ≈14‐ to 22‐month intervals. Enrollment took place in several waves, with some participants examined up to nine times, others for only a single assessment. Inclusion criteria were: (a) a phenotypic or genetic diagnosis of DS, (b) 30 years of age or older, (c) vision and hearing sufficient for compliance with testing procedures, (d) communication ability sufficient to assent, and (e) provision of consent (by the participant or legally authorized representative). Over time, criteria shifted to increased participation of older adults having less severe ID. Mean age of participants at baseline was 51.6 years (SD = 9.1) and mean Full Scale Intelligence Quotient (FSIQ) was 33.3 (SD = 7.3). The overwhelming majority of study participants were white (92%). Additionally, 5% were black/African American, 2% were Hispanic/Latino, and 1% were Asian. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of the New York State Institute for Basic Research in Developmental Disabilities, Columbia University Irving Medical Center, and the New York Psychiatric Institute. Subsamples of these participants were included in Studies 2 and 3, as indicated in Figure 1.
FIGURE 1.
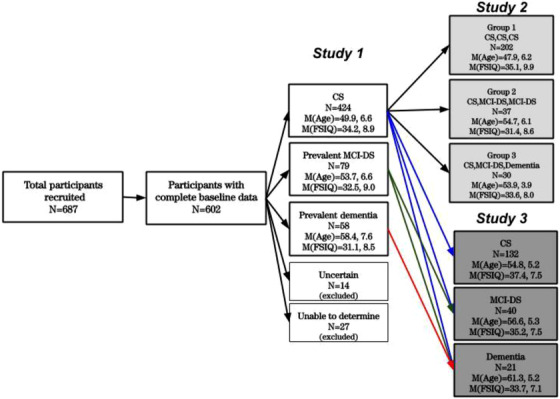
Flowchart of participant characteristics in studies 1–3
2.2. Assessments
All participants were evaluated with a manualized comprehensive evaluation which included: (a) a detailed review of clinical and medical records that included descriptions of health concerns, a list of all diagnoses received, recent histories of psychological assessments, level of intellectual disability (and/or IQ), and medication usage, (b) direct assessment of selected cognitive functions, and (c) structured informant interviews regarding day‐to‐day functioning and neuropsychiatric concerns. (Blood samples were collected via routine phlebotomy from willing participants for the examination of selected biomarkers of risk, but these findings were not considered for the present analyses.) Methods were selected to be generally consistent with guidelines recommended by a previous Working Group for the Establishment for the Criteria for the Diagnosis of Dementia in Individuals with Developmental Disability. 27
Cognitive evaluations took approximately 2 hours to complete and included: (a) a modified version of the Selective Reminding Test (MSRT); 25 , 28 (b) assessments of mental status: (i) a modified version of the original Mini‐Mental Status Examination, 29 the Modified Mini Mental Status Evaluation—Down Syndrome (MMMSE‐DS 30 ), (ii) an enhanced version of the Down Syndrome Mental Status Examination (DSMSE 31 ), (iii) The Test for Severe Impairment (TSI 32 ); (c) an adaptation of the McCarthy Category Fluency Test (CF‐T 33 ); (d) The Block Design subtest from the Wechsler Intelligence Scale for Children (WISC‐Revised 34 ) supplemented with less complex items from the original DSMSE 31 (BLOCK‐T); and (e) the Beery Buktenica Developmental Test of Visual‐Motor Integration (long form [VMI]). 35
Informant‐based interviews focused on participants’ cognitive, adaptive, and neuropsychiatric functioning and were conducted with a caregiver: (a) The Dementia Questionnaire for People with Learning Disabilities (DLD 36 , 37 ); (b) The American Association on Mental Deficiency Adaptive Behavior Scale, Part I (ABSI 38 ). Neuropsychiatric concerns were assessed with the (i) Reiss Screen for Maladaptive Behavior, 39 , 40 (ii) Columbia University Scale to Assess Psychopathology in Alzheimer's Disease, 41 and (iii) Neuropsychiatric Inventory. 42 Findings in the area of neuropsychiatric concerns informed consensus decisions but were not considered for the present analyses. Note that criteria defining clinical dementia status have not been developed for the tests included in this battery, the only exception being the DLD. (However, we did not adhere to the specific DLD scoring criteria in our evaluations.)
2.3. Case consensus review procedures
Determination of overall clinical dementia status was based on profiles of performance which were captured on summary sheets generated by the project coordinator and included all scores for the current test cycle being evaluated and any longitudinal data available from previous test cycles. Demographic information such as sex, age at test cycles, and level of intellectual functioning or IQ was also included. To determine clinical dementia status, profiles of performance across all tests were combined with clinical judgment during case consensus review for each participant that included senior staff members, the study coordinator, and research assistants who had direct contact with the participant(s) under consideration. 25 , 26 , 43 Criteria were conceptually consistent with those of the National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association. 44 Categories were: (a) cognitively stable (CS), indicating with reasonable certainty that significant impairment was absent; (b) (MCI‐DS), indicating that there was indication of subtle cognitive and/or functional decline over and above what would be expected with aging, though of insufficient severity to suggest the presence of dementia; (c) possible dementia, indicating that some signs and symptoms of dementia were present, but declines over time were judged to need further evidence of progression; (d) definite dementia, indicating with a high degree of confidence that dementia was present based upon substantial decline over time; (e) status uncertain due to complications, indicating that declines were observed that might be caused by some other concern unrelated to neuropathology (eg, psychiatric diagnosis, disruptive life event); and (f) indeterminable, indicating that the individual's preexisting developmental disability was of such severity that detection of decline indicative of dementia was not possible. (Note that individuals receiving classifications of “uncertain” or ''indeterminable” were not included in the analyses.) It is important to emphasize that consensus determinations of clinical dementia status were informed by performance on all individual tests. This introduced potential of partial circularity that might cause overestimation of the true sensitivity of these measures to differences in clinical status. This concern was recognized and addressed directly in Study 3, described below; findings indicated that this partial circularity only had an insignificant impact.
3. STUDY 1: TESTABILITY AND CROSS‐SECTIONAL ANALYSES OF AD CLINICAL PROGRESSION
Study 1 examined the appropriateness of specific methods used for the assessment of early AD. Aims included descriptions of the range of performance for adults with varying severity of ID, estimation of test‐retest reliability, and determination of minimum levels of preclinical performance that would allow quantification of subsequent decline. Groups varying in diagnostic status were compared.
3.1. Methods
3.1.1. Participants
Study 1 examined the baseline assessment and the data from the first follow‐up to provide a conservative estimate of test‐retest reliability. Data were available for 561 participants who were identified with either CS, prevalent MCI‐DS, or prevalent dementia (categories of possible and definite dementia were collapsed for purposes of analyses).
3.2. Results
We first examined the relation between measures of cognitive performance and functional status with severity of ID (IQ), followed by these relations with Diagnostic Status, controlling for IQ. Table 1 provides summary statistics stratified by Diagnostic Status and Severity of ID (IQ) and indicates performance on all measures was strongly associated with IQ, ranging from .76 ≥ rs ≥ .57, Ps < .001.
TABLE 1.
Study 1: mean, standard deviation (in italics), and minimum/maximum score [in brackets] for the direct and informant‐based measures of cognition and functioning × diagnostic status × severity of ID for the baseline assessment
| CS (N = 424) | MCI‐DS (N = 79) | Dementia (N = 58) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure (maximum score) | Mild (N = 146) | Moderate (N = 121) | Severe (N = 110) | Profound (N = 44) | Mild (N = 14) | Moderate (N = 24) | Severe (N = 27) | Profound (N = 12) | Mild (N = 7) | Moderate (N = 16) | Severe (N = 23) | Profound (N = 10) |
|
MSRT‐TR (48) |
31.7,9.8 [2‐48] |
23.9,12.0 [0‐44] |
16.1,11.6 [0‐42] |
2.4,5.9 [0‐25] |
16.7,10.1 [3‐33] |
13.4,7.6 [0‐30] |
8.4,9.4 [0‐35] |
2.2,5.3 [0‐13] |
7.3,7.5 [0‐17] |
9.6,9.3 [0‐27] |
1.9,4.2 [0‐13] |
0.3,0.5 [0‐1] |
|
TSI‐T (24) |
21.3,1.9 [16‐24] |
19.9,2.6 [7‐24] |
15.1,5.0 [0‐23] |
6.5,4.6 [0‐17] |
19.4,2.2 [16‐22] |
16.6,5.1 [2‐22] |
13.5,5.4 [2‐23] |
5.3,5.8 [0‐14] |
16.0,2.6 [13‐19] |
12.4,7.8 [0‐22] |
6.2,5.6 [0‐16] |
3.8,4.5 [0‐10] |
|
MMMSE‐DS‐T (74) |
65.0,7.5 [29‐74] |
53.7,13.2 [4‐73] |
32.2,19.9 [0‐66] |
4.1,8.8 [0‐37] |
54.2,10.2 [14‐32] |
37.7,17.9 [0‐30] |
24.4,18.5 [0‐60] |
3.7,8.3 [0‐22] |
19.2,23.3 [0‐56] |
26.1,25.4 [0‐61] |
6.6,11.8 [0‐40] |
0.5,1.6 [0‐5] |
|
DSMSE‐M (24) DSMSE‐NM (79) |
15.0,4.9 [0‐24] 58.6,10.3 [31‐78] |
12.4,4.5 [0‐22] 48.3,11.6 [7‐68] |
8.2,5.5 [0‐22] 34.3,13.4 [0‐69] |
1.3,2.6 [0‐11] 14.1,11.8 [0‐37.5] |
7.6,4.6 [1‐16] 48.1,9.4 [28‐65.5] |
5.3,4.0 [0‐17] 34.5,14.2 [8‐56] |
4.2,4.3 [0‐17] 25.8,13.8 [5‐58] |
0.8,2.5 [0‐8] 11.3,14.0 [0‐33] |
1.8,2.5 [0‐5] 28.1,8.5 [19‐39] |
2.12,3.0 [0‐11] 29.5,21.1 [0‐62] |
0.6,1.4 [0‐6] 13.3,13.1 [0‐41] |
0.2,0.6 [0‐2] 7.8,10.4 [0‐22] |
| CF‐Ta |
8.5,3.2 [1‐17] |
3.8,6.3 [0‐14] |
3.4,3.0 [0‐14] |
.56,1.3 [0‐4] |
5.7,2.8 [1‐10] |
3.7,3.2 [0‐11] |
2.0,3.0 [0‐12] |
.71,1.3 [0‐3] |
2.3,2.6 [0‐5] |
2.3,2.6 [0‐7] |
0.6,1.2 [0‐4] |
0.0 |
|
VMI‐T (27) |
12.8,3.5 [0‐23] |
10.4,2.9 [0‐18] |
7.0,3.8 [0‐16] |
1.2,1.9 [0‐7] |
10.8,1.2 [8‐13] |
7.2,3.6 [0‐12] |
4.5,3.8 [0‐12] |
.50,1.2 [0‐3] |
4.8,4.3 [2‐11] |
5.6,4.7 [0‐13] |
2.3,3.4 [0‐9] |
0.3,0.5 [0‐1] |
|
BLOCK‐T (78) |
19.8,9.4 [0‐53] |
12.7,7.6 [0‐34] |
6.0,6.1 [0‐26] |
0.1,0.4 [0‐2] |
11.9,8.8 [0‐26] |
4.9,4.7 [0‐14] |
3.5,4.6 [0‐13] |
0.0 |
1.5,1.9 [0‐4] |
4.5,5.5 [0‐13] |
0.8,1.8 [0‐6] |
0.0 |
|
DLD‐SCSb (50) DLD‐SOSb (52) |
3.6,4.4 [0‐21] 6.4,5.2 [0‐24] |
8.3,7.2 [0‐36] 7.5,5.5 [0‐29] |
14.3,8.9 [0‐35] 9.3,6.0 [0‐25] |
29.0,9.1 [0‐44] 19.6,8.5 [5‐38] |
10.9,7.3 [1‐24] 10.0,5.4 [3‐25] |
18.6,8.9 [3‐36] 12.2,6.0 [4‐26] |
23.1,8.0 [6‐40] 14.6,6.2 [3‐32] |
33.4,8.3 [15‐44] 25.6,11.3 [10‐42] |
24.3,16.6 [0‐44] 22.5,15.6 [8‐42] |
28.9,10.8 [8‐44] 22.8,11.9 [9‐40] |
35.7,8.1 [17‐44] 27.9,12.2 [9‐46] |
37.8,5.5 [26‐44] 36.2,5.6 [27‐45] |
|
ABSI‐T (280) |
224.8,24.7 [141‐271] |
204.8,26.1 [129‐260] |
183.8,32.6 [66‐245] |
107.3,38.1 [25‐196] |
204.9,24.6 [154‐246] |
177.8,33.1 [97‐228] |
151.5,29.6 [92‐209] |
83.6,50.0 [18‐178] |
99.9,70.5 [18‐204] |
127.8,64.9 [20‐209] |
90.5,51.5 [19‐191] |
50.2,34.6 [5‐109] |
Abbreviations: ABSI‐T, American Association on Mental Deficiency Adaptive Behavior Scale, Part I‐Total Score; BLOCK‐T, Block Design Subtest‐Total Score; CF‐T, Category Fluency Test‐Total Score; CS, cognitively stable; DLD‐SCS, The Dementia Questionnaire for People with Learning Disabilities, Sum of Cognitive Scores; DLD‐SOS, The Dementia Questionnaire for People with Learning Disabilities, Sum of Social Scores; DLD‐T, The Dementia Questionnaire for People with Learning Disabilities, Total Score; DSMSE‐M, Down Syndrome Mental Status Examination‐Memory Score; DSMSE‐NM, Down Syndrome Mental Status Examination‐ Nonmemory Score; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE‐DS‐T, Modified Mini Mental Status Examination‐Down Syndrome‐Total Score; MSRT‐TR, Modifed Selective Reminding Test‐Total Recall Score; TSI‐T, Test for Severe Impairment‐Total Score; VMI‐T, the Beery Buktenica Developmental Test of Visual‐Motor Integration‐Total Score
Analyses of covariance controlling for effects of IQ also verified expectations that performance differed systematically across Diagnostic Status Groups. In all cases, Groups differed significantly with results ranging from F(2,502) = 162.09, ηp 2 = .39 to F(2, 438) = 35.09, ηp 2 = .14 (Table S1 in supporting information). Conservative post‐hoc analyses with Bonferroni correction verified that all pairwise Group differences were significant.
All measures showed an appropriate range of performance with very few participants achieving scores at ceiling (Table 1). However, some individuals, especially those with more severe ID, performed at or near floor or were unable to perform at all, even when we saw no evidence of dementia.
Because measures of clinical progression of AD within any specific individual can only be quantified when their baseline performance is high enough to allow observations of clinically significant decline, we determined the relation between preclinical severity of ID and the ability to perform significantly above the floor level within groups without MCI‐DS or dementia. This performance level is an arbitrary value above the lowest possible score, and we set our criterion at two standard errors of measurement for all direct tests of cognition. This statistic provided an estimate of within‐person variability and values for specific measures calculated using conservative estimates of test‐retest reliability. These values are listed in Table 2, along with the proportion of individuals performing above this level, stratified by Severity of ID.
TABLE 2.
Study 1: percentages of cognitively stable participants scoring at least 2 standard errors of measurement above floor on each test stratified by severity of id, along with estimates of test‐retest reliability
| % of Participants performing above 2 SEMs × Level of functioning | ||||||
|---|---|---|---|---|---|---|
| Measure | Reliability (Cronbach's α) | 2 SEMs | MildID | Moderate | Severe | Profound |
| MSRT‐TR | .896 | 9 | 92.0 | 83.2 | 52.2 | 7.5 |
| TSI‐T | .932 | 3 | 100.0 | 97.4 | 91.6 | 54.2 |
| MMMSE‐DS‐T | .979 | 7 | 98.2 | 90.7 | 67.3 | 14.7 |
| DSMSE‐T | .966 | 8 | 99.4 | 95.7 | 89.4 | 48.9 |
| CF‐T | .865 | 3 | 91.4 | 67.9 | 35.5 | 4.7 |
| VMI‐T | .942 | 2 | 97.5 | 93.0 | 70.5 | 18.6 |
| BLOCK‐T | .938 | 5 | 90.2 | 70.1 | 38.8 | 0.0 |
| ABSI‐T | .934 | 23 | 97.6 | 95.8 | 88.3 | 39.5 |
Abbreviations: ABSI‐T, American Association on Mental Deficiency Adaptive Behavior Scale‐Total Score; BLOCK‐T, Block Design subtest‐Total Score; CF‐T, Category Fluency Test‐Total Score; DSMSE‐T, Down Syndrome Mental Status Examination‐Total Score; ID, intellectual disability; MMMSE‐DS‐T, Modified Mini Mental Status Examination‐Down Syndrome‐Total Score; MSRT‐TR, Modified Selective Reminding Test‐Total Recall Score; SEM, standard error of the mean; TSI‐T, Tet for Severe Impairment‐Total Score; VMI‐T, Beery Buktenica Developmental Test of Visual‐Motor Integration‐Total Score
Informant‐based measures were handled differently. For the ABSI, we arbitrarily set the criterion equal to or greater than 100 points based on our long‐term experience with this instrument. For the two summary scores generated from the DLD (DLD‐SCS and DLD‐SOS), no participant showed a preclinical score poor enough to preclude quantification of further decline and it was unnecessary to estimate a floor value.
3.3. Discussion
The cross‐sectional findings from Study 1 confirmed that our measures are reliable for this target population and are sensitive to clinical progression of AD at the level of group effects. Most participants in the mild/moderate range of ID performed sufficiently well enough on all measures to enable us to document decline. Many of the measures used in direct testing are likely to be uninformative for tracking AD progression for individuals in the severe to profound range of ID. This was especially pronounced for participants with profound ID, where very few scored sufficiently high enough at baseline on the MSRT‐TR (7.5%), the CF‐T (4.7%) and the BLOCK‐T (0.0%) to allow us to track decline on these measures (see Table 2). For these groups, assessment may need to rely solely on informant reports absent explicit documentation of preclinical performance above floor. However, there is always the possibility that future research will be able to demonstrate improved testability within this population using other methods.
Study 1 left unanswered the question of whether these measures would be capable of detecting onset of MCI‐DS or incident dementia. Study 2 addressed this directly by focusing analyses on longitudinal examinations of participants who were initially CS and followed on at least two successive assessment cycles.
4. STUDY 2: LONGITUDINAL ANALYSES TO DETECT MCI‐DS AND DEMENTIA ONSET
4.1. Method
4.1.1. Participants
A subset of Study 1 participants (N = 269) was selected for Study 2 (Figure 1). Participants included in Study 2 were: (a) CS at the time of their baseline assessment; (b) able to achieve a score significantly above floor on at least one measure of performance; (c) unaffected by conditions or concerns that were unrelated to dementia but that might have complicated interpretation of any observed declines; and (d) had to have a minimum of three assessment cycles and in cases developing MCI‐DS, the first assessment cycle had to be immediately prior to MCI‐DS onset and the third immediately after. Diagnostic Status was determined via case consensus review that considered all available data, now including any longitudinal findings.
The present analyses examined data for three subgroups of participants defined by their longitudinal diagnostic status profile. Group 1 adults remained CS for all three cycles. Group 2 adults developed MCI‐DS during follow‐up and maintained that status. Group 3 adults developed MCI‐DS and then progressed to dementia.
4.2. Results
Repeated measure analyses of covariance (ANCOVAs) examined differences between groups in change over time. 45 The between‐subjects variables were Sex and Diagnostic Status Group, Test Cycle was the repeated measure and covariates were Age (at Time 1) and IQ. No effects involving Sex were found; subsequently the data for men and women were combined. Table 3 presents the means for each measure stratified by Cycle and Diagnostic Status Group.
TABLE 3.
Study 2: adjusted least square means and standard deviation (in parentheses) and the minimum and maximum scores [in brackets] generated from core assessments
| Measure | Group 1 | Group 2 | Group 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CycleDiagnostic status | |||||||||
| 1 CS | 2 CS | 3 CS | 1 CS | 2 MCI‐DS | 3 MCI‐DS | 1 CS | 2 MCI‐DS | 3 Dementia | |
| MSRT‐TR |
[9‐48] 30.65 (9.97) |
[1‐47] 31.14 (9.99) |
[0‐48] 30.19 (11.76) |
[13‐43] 24.50 (7.83) |
[0‐41] 17.16 (11.27) |
[0‐41] 16.16 (10.14) |
[9‐41] 20.81 (7.81) |
[0‐25] 12.43 (6.71) |
[0‐17] 5.47 (6.29) |
| TSI‐T |
[4‐24] 19.10 (4.59) |
[2‐24] 18.98 (4.84) |
[3‐24] 19.09 (4.60) |
[8‐24] 17.91 (4.05) |
[3‐23] 16.26 (5.14) |
[0‐24] 15.34 (6.84) |
[11‐23] 17.75 (3.76) |
[0‐23] 14.85 (5.60) |
[0‐24] 12.58 (6.61) |
| MMMSE‐DS‐T |
[12‐74] 57.61 (14.30) |
[0‐74] 58.58 (15.66) |
[0‐74] 58.40 (15.64) |
[24‐71] 48.71 (12.87) |
[5‐73] 45.23 (16.08) |
[0‐69] 40.19 (17.46) |
[10‐71] 44.39 (17.58) |
[0‐70] 38.50 (22.47) |
[0‐67] 24.44 (21.73) |
| DSMSE‐M |
[0‐23] 13.60 (5.44) |
[0‐24] 13.56 (5.77) |
[0‐24] 13.46 (6.08) |
[2‐20] 10.66 (5.08) |
[0‐18] 8.56 (4.75) |
[0‐17] 7.03 (4.93) |
[0‐19] 8.25 (5.13) |
[0‐15] 5.68 (4.16) |
[0‐10] 2.54 (3.04) |
| DSMSE‐NM |
[11‐78] 50.25 (15.47) |
[11‐78] 50.02 (16.64) |
[11‐76.5] 50.28 (16.63) |
[11.5‐71] 41.61 (17.02) |
[0‐73.5] 38.09 (16.02) |
[0‐66.5] 35.83 (19.06) |
[18‐70] 42.70 (14.66) |
[0‐60] 35.20 (15.95) |
[0‐58] 23.74 (15.39) |
| CF‐T |
[3‐17] 8.20 (3.26) |
[0‐18] 8.23 (3.90) |
[0‐18] 8.06 (3.84) |
[3‐12] 6.33 (2.93) |
[0‐13] 5.70 (3.31) |
[0‐12] 4.79 (2.81) |
[3‐11] 5.88 (2.63) |
[0‐8] 4.19 (2.37) |
[0‐10] 2.56 (2.63) |
| VMI‐T |
[3‐23] 11.47 (3.90) |
[2‐24] 11.60 (4.00) |
[1‐21] 11.17 (3.74) |
[3‐14] 9.09 (2.90) |
[2‐15] 9.10 (3.63) |
[0‐13] 8.00 (3.40) |
[3‐13] 9.00 (3.29) |
[0‐16] 7.15 (4.28) |
[0‐12] 4.73 (4.43) |
| BLOCK‐T |
[6‐53] 18.82 (8.78) |
[0‐59] 18.50 (9.55) |
[0‐64] 18.56 (10.06) |
[5‐30] 11.08 (6.31) |
[0‐24] 8.35 (6.71) |
[0‐22] 7.83 (6.02) |
[5‐35] 13.13 (7.83) |
[2‐16] 7.79 (4.58) |
[0‐16] 4.00 (5.45) |
| DLD‐SCS |
[0‐36] 8.72 (9.47) |
[0‐35] 8.14 (8.59) |
[0‐34] 8.57 (9.57) |
[0‐28] 10.85 (8.50) |
[1‐36] 16.03 (8.35) |
[2‐32] 16.67 (8.01) |
[0‐32] 10.28 (7.82) |
[2‐40] 17.50 (9.91) |
[6‐40] 26.90 (8.86) |
| DLD‐SOS |
[0‐34] 7.79 (6.34) |
[0‐31] 7.53 (6.13) |
[0‐32] 7.31 (5.91) |
[0‐25] 10.27 (6.58) |
[0‐24] 12.00 (5.78) |
[1‐27] 12.31 (6.97) |
[0‐16] 6.52 (4.47) |
[2‐32] 12.17 (7.04) |
[2‐44] 18.07 (10.62) |
| ABSI‐T |
[104‐271] 205.91 (37.22) |
[94‐273] 206.66 (38.88) |
[90‐275] 205.11 (39.00) |
[129‐248] 190.90 (31.75) |
[107‐242] 173.95 (35.11) |
[97‐227] 163.56 (32.44) |
[143‐262] 199.27 (29.89) |
[107‐252] 179.27 (32.14) |
[32‐209] 140.55 (42.47) |
Abbreviations: ABSI‐T, American Association on Mental Deficiency Adaptive Behavior Scale, Part I‐Total Score; BLOCK‐T, Block Design subtest‐Total Score; CF‐T, Category Fluency Test‐Total Score; CS, cognitively stable; DLD‐SCS, Dementia Questionnaire for People with Learning Disabilities‐Sum of Cognitive Scores; DLD‐SOS, Dementia Questionnaire for People with Learning Disabilities‐Sum of Social Scores; DLD‐T, Dementia Questionnaire for People with Learning Disabilities‐Sum of Total Scores; DSMSE‐M, Down Syndrome Mental Status Examination‐Memory Score; DSMSE‐NM, Down Syndrome Mental Status Examination‐Nonmemory Score; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE‐T, Modified Mini Mental Status Examination‐Total Score; MSRT‐TR, Modified Selective Reminding Test‐Total Recall Score; TSI‐T, Test of Severe Impairment‐Total Score; VMI‐T, Beery Buktenica Developmental Test of Visual Motor Integration‐Total Score
As expected, IQ was strongly related to performance on all tests (Fs > 100) while age effects were more variable (effects not shown). The Cycle × Diagnostic Group interactions were of primary a priori interest and as predicted, significant multivariate interactions were found for all measures with results ranging from F(4,348) = 4.42, ηp 2 = .05 for CF‐T; to F(4,448) = 50.69, ηp 2 = .31 for the DSMSE‐NM (Table 4). Post‐hoc contrasts (with Bonferroni correction) examined differences in performance over time within Diagnostic Status Groups and showed: (a) no declines for the Group remaining CS, (b) declines with onset for both Groups developing MCI‐DS (for almost all measures), and (c) further declines with onset of dementia. Note the consistency of these findings across all direct testing measures with two exceptions: CF‐T and visuospatial integration (VMI‐T), for which significant declines emerged only with dementia onset. While we expected that not all the measures would be sensitive to MCI‐DS, why these two particular measures were not, cannot explained. However, our findings are consistent with some of those found in the neurotypical population for CF‐T and visual motor integration ( 46 , 47 , respectively).
TABLE 4.
Study 2: The cycle х diagnostic status multivariate interaction and post‐hoc comparison within diagnostic status group (P‐values)
| Measure | Cycle × Diagnostic statusmultivariate interaction | Group 1CS,CS,CS | Group 2CS,MCI‐DS,MCI‐DS | Group 3CS,MCI‐DS, Dementia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison by cycle (P‐values) | ||||||||||
| 1‐2 | 1‐3 | 2‐3 | 1‐2 | 1‐3 | 2‐3 | 1‐2 | 1‐3 | 2‐3 | ||
| MSRT‐TR | F(4,366) = 14.441, ηp 2 = .14 | ns | ns | ns | .001 | .000 | ns | .001 | .000 | .002 |
| TSI‐T | F(4,452) = 20.771, ηp 2 = .16 | ns | ns | ns | .000 | .000 | ns | .000 | .000 | .000 |
|
DSMSE‐M DSMSE‐NM |
F(4,456) = 13.181, ηp 2 = .10 F(4,448) = 50.691 ηp 2 = .31 |
ns ns |
ns ns |
ns ns |
.010 .003 |
.000 .000 |
ns ns |
.003 .000 |
.000 .000 |
.001 .000 |
| MMMSE‐T | F(4,418) = 38.101, ηp 2 = .27 | ns | ns | ns | .030 | .000 | .001 | .000 | .000 | .000 |
| CF‐T | F(4,348) = 4.422, ηp 2 = .05 | ns | ns | ns | ns | .030 | ns | .068 | .000 | ns |
| VMI‐T | F(4,420) = 13.981 ,ηp 2 = .12 | ns | ns | ns | ns | .016 | .006 | .000 | .000 | .000 |
| BLOCK‐T | F(4,352) = 8.911, ηp 2 = .10 | ns | ns | ns | .047 | .051 | ns | .022 | .000 | .026 |
|
DLD‐SCS DLD‐SOS |
F(4,478) = 57.441, ηp 2 = .33 F(4,478) = 26.111, ηp 2 = .18 |
ns ns |
ns ns |
ns ns |
.000 ns |
.000 ns |
ns ns |
.000 .000 |
.000 .000 |
.000 .000 |
| ABSI‐T | F(4,584) = 53.101, ηp 2 = .31 | ns | ns | ns | .000 | .000 | .005 | .000 | .000 | .000 |
F‐ratio was significant at: 1 p < .001 level. 2 p < .01 level. 3 p < .05 level
Abbreviations: ABSI‐T, American Association on Mental Deficiency Adaptive Behavior Scale, Part I‐Total Score; BLOCK‐T, Block Design subtest‐Total Score; CF‐T, Category Fluency Test‐Total Score; CS, cognitively stable; DEM, dementia; DLD‐SCS, Dementia Questionnaire for People with Learning Disabilities‐Sum of Cognitive Scores; DLD‐SOS, Dementia Questionnaire for People with Learning Disabilities‐Sum of Social Scores; DLD‐T, Dementia Questionnaire for People with Learning Disabilities‐Total Score; DSMSE‐M, Down Syndrome Mental Status Examination‐Memory Score; DSMSE‐NM, Down Syndrome Mental Status Examination‐Nonmemory Score; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE‐T, Modified Mini Mental Status Examination‐Down Syndrome‐Total Score; MSRT‐TR, Modified Selective Reminding Test‐Total Recall Scor; ns, not significant; TSI‐T, Test for Severe Impairment‐Total Score; VMI‐T, Beery Buktenica Test of Visual Motor Integration
This pattern was similar for measures based on informant interviews. The two subscales of the DLD showed differential sensitivity to incident MCI‐DS, with the summary score reflecting cognitive skills (DLD‐SCS) showing clear change with MCI‐DS onset and the summary score reflecting social skills (DLD‐SOS) showing only small and statistically insignificant losses until the onset of dementia. While MCI in the neurotypical population is expected to have minimal impacts on functional skills related to everyday activity, we found significant declines in the ABSI at MCI‐DS onset. Decline further progressed for those who developed incident dementia. This finding suggests that, at least for adults with DS, the ADL skills assessed by the ABSI may be as cognitively demanding as instrumental activities of daily living are for neurotypical adults.
Studies 1 and 2 provided convincing evidence of the utility of these measures for distinguishing MCI‐DS, even at onset, from preclinical AD and approximate a true gold standard that is based on consensus review of broad profiles of performance on our longitudinal evaluation. While these findings bolster our confidence in the validity of these classifications, specifics of our procedure raised the possibility that, given consensus decisions were informed by these same measures, partial circularity may have contributed substantially to the strong associations between overall classification and more detailed specifics of performance. This concern should be somewhat mitigated by the fact that specific criterion scores were never considered during consensus determinations and performance on no specific task predicted consensus status with perfect precision. Nevertheless, this possibility of partial circularity needs to be addressed procedurally and this was the aim of Study 3.
5. STUDY 3: ADDRESSING CIRCULARITY IN EVALUATING SPECIFIC MEASURES’ RELATION TO DIAGNOSTIC STATUS
5.1. Method
5.1.1. Participants
The Study 3 sample included all participants assessed at Cycles 7 (N = 187), 8 (N = 173), or 9 (N = 118) of our longitudinal study who received a classification of CS, MCI‐DS, or Dementia (Figure 1). (Note that with rolling enrollment, the number of previous assessments for each individual varied from zero to six and the lower numbers for Cycles 8 and 9 tend to reflect differences in time of enrollment rather than attrition.)
5.1.2. Procedures
Study 3 modified our standard case consensus review procedures. Individual overviews of all data considered in reviews were generated as for Studies 1 and 2. In addition, the database coordinator generated another six sets of summaries for each participant, each excluding data for one assessment instrument, for example, one summary sheet excluding the MSRT‐TR, one excluding the MMMSE‐T and so on for the other measures. This allowed us to relate each measure to a consensus classification made without knowledge of performance on that specific measure. (Note that some procedures generated more than one measure, so only six additional ratings were needed to allow us to examine the impact of partial circularity for the larger variable set.) To minimize raters’ ability to link these summaries for any individual participant, all personal identifiers were removed, and each overview received a unique identifier code for each one of these six ratings. (Only the database coordinator had access to codes linking these identifiers to the original participant numbers, and this person did not participate in any of the modified consensus decisions.) Each coded overview was initially reviewed by three people, two investigators plus the study coordinator or a highly experienced research associate. Each person rated clinical dementia status independently. In each case, 100% agreement defined the reference standard at this point. When there were disagreements, two additional investigators evaluated that case and agreement among four of the five raters was taken as consensus. There were no cases that failed to reach this standard.
5.2. Results
We found a high degree of agreement between our standard consensus decisions, considering all information available and the modified consensus procedures. Table 5 summarizes these results, averaged across the three cycles of testing, stratified by Diagnostic Status Group for each of the six modified classification decisions. Overall, this degree of agreement suggests that differences between our standard and modified consensus procedures had minimal impact on Study 1 or 2 findings. We verified this by repeated analyses of performance associated with Diagnostic Status for the smaller sample of participants in Cycles 7, 8, and 9, with results ranging from F(2,62) = 10.0, ηp 2 = .24; to F(2,184) = 123.9, ηp 2 = .58. We recognize that our consensus determinations are inherently imperfect, especially in the accurate classification of those individuals with possible MCI‐DS. Studies in the neurotypical population have also found inaccuracies in the determination of cognitive status, especially between the boundaries of CS and MCI. 48
TABLE 5.
Study 3: mean percent agreement between the standard consensus conference procedures and alternative consensus conference procedures
| Measure subtracted | % Agreement with original consensus review procedures | |||
|---|---|---|---|---|
| Overall agreement | By diagnostic status | |||
| CS | MCI‐DS | DEM | ||
| MSRT‐TR | 76.6 | 74.6 | 73.2 | 88.6 |
| DSMSE‐T | 77.0 | 74.2 | 75.6 | 88.6 |
| MMMSE‐T | 74.0 | 72.3 | 69.8 | 86.1 |
| CF‐T | 76.2 | 74.6 | 73.2 | 86.1 |
| BLOCK‐T | 75.0 | 72.7 | 72.3 | 87.2 |
| DLD‐T | 77.7 | 76.5 | 74.0 | 87.3 |
Abbreviations: BLOCK‐T, Block Design subtest; CF‐T, Category Fluency Test; CS, cognitively stable; DEM, dementia; DLD‐T, Questionnaire for People with Learning Disabilities‐Total Score; DSMSE‐T, Down Syndrome Mental Status Examination‐Total Score; MCI‐DS, mild cognitive impairment‐Down syndrome; MMMSE‐T, Modified Mini Mental Status Examination‐Down Syndrome‐Total Score; MSRT‐TR, Modified Selective Reminding Test‐Total Recall Score
6. OVERALL DISCUSSION
In individuals with DS, MCI is a conceptually clear but empirically ill‐defined prodromal stage of AD, a gray area between “cognitively‐normal” aging and dementia. From a biomedical science perspective, knowledge regarding the earliest stages of AD is vital in furthering an understanding of how the disease evolves, for adults with DS and more generally. From a clinical perspective, identifying individuals experiencing cognitive decline caused by AD, as early as possible, is imperative for maximizing treatment efficacy. More than two‐thirds of adults with DS have the clinical symptoms of dementia by the time they reach 65 years of age, which speaks to a pressing need for clear and objective standards defining MCI in the largest genetically defined high‐risk population.
The sequence of studies described focused on the evaluation of objective methods we predicted would be sensitive to the onset of MCI‐DS, verified that these methods have utility for recognizing declines associated with the prodromal stage of AD (MCI‐DS) and for tracking further disease progression for adults with mild to moderate ID. As expected, this was the case for most of the measures examined, but not all. Testing of CF‐T and visuospatial organization (VMI‐T), as well as informant measures tapping social rather than cognitive abilities (DLD‐SOS), showed changes only with dementia.
Esbensen 49 reviewed characteristics of informative outcome measures for clinical trials, specifying that measures must be developmentally appropriate, reliable, valid for content and criterion‐related standards, able to detect change, interpretable, and feasible to administer without substantial floor or ceiling effects. These criteria apply to the tests evaluated herein with respect to their proficiency for recognizing early declines in adults with DS, corresponding to MCI for neurotypical elderly adults.
We believe that there are compelling reasons for viewing these findings as significant. Availability of empirically supported measures sensitive to MCI‐DS can inform clinical diagnosis. Individuals at this stage of AD progression will be the best targets for inclusion in clinical trials for treatment with efficacious disease‐modifying drugs once they become available. More immediately, we have direct evidence supporting the use of these measures as outcomes for clinical trials of promising treatments. Finally, these measures can serve as critically important tools in discovery studies targeting biomarkers of preclinical stage of AD prior to MCI‐DS onset with the goal of preventing clinical progression altogether in the highest risk individuals. 50 In‐vitro biomarker technology is rapidly developing and prospective studies with clear relationships of future clinical progression are needed for validation. Studies of sensitivity and specificity are now needed to determine the extent to which group effects extend to specific measures of individual patients.
While our methods showed considerable promise for informing diagnostic decisions, clinical judgements will continue to be key to placing findings from these tests within a framework that includes other sources of information. The insidious character of clinical onset of AD and its variable impacts on performance across individuals guarantees a degree of imprecision prior to more advanced disease progression, and we must accept this reality until highly valid biomarkers of preclinical and prodromal AD are discovered. This is a target of currently ongoing research by our program and that of others. The methods described here will support these efforts. Thus, they provide essential tools to inform current clinical diagnosis and to support biomarker discovery that can provide the field with the true diagnostic gold standard it seeks.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants P01 HD035897, U54 HD079123, U01 AG051412 and R01 AG014673 as well as funds from the New York State Office for People with Developmental Disabilities. As always, we are grateful to all our participants, their families, and the agencies serving the needs of individuals with intellectual and developmental disabilities.
Krinsky‐McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer's dementia in adults with Down syndrome. Alzheimer's Dement. 2020;12:e12044 10.1002/dad2.12044
REFERENCES
- 1. Passarino G, DeRango F, Montesanto A. Human longevity: genetics or lifestyle? it takes two to tango. Immun Ageing. 2016;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woolfe SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959‐2017. JAMA. 2019;322(20):1996‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Q, Rasmussen SA, Friedman J. Mortality associated with Down's syndrome in the USA from 1983‐1997: a population‐based study. Lancet. 2002;359(9311):1019‐1025. [DOI] [PubMed] [Google Scholar]
- 4. Bayen E, Possin KL, Chen Y, Cleret de Langavant L, Yaffe K. Prevalence of aging, dementia, and multimorbidity in older adults with Down syndrome. JAMA Neurol. 2018;75(11):1399‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hithersay R, Startin CM, Hamburg S, et al. Association of dementia with mortality among adults with Down syndrome older than 35 years. JAMA Neurol. 2019;76(2):152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jicha GA, Carr SA. Conceptual evolution in Alzheimer's disease: implication for understanding the clinical phenotype of progressive neurodegenerative disease. J Alzheimers Dis. 2010;19(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alzheimer's Association. 2019 Alzheimer's Disease Facts and Statistics, 2019. https://www.alz.org/media/Documents/ alzheimers‐facts‐and‐figures‐2019‐r.pdf. Accessed November 6, 2019.
- 8. Wimo A, Winblad B, Aquero‐Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17(2):67‐67. [DOI] [PubMed] [Google Scholar]
- 9. Krinsky‐McHale S, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev. 2013;18(1):31‐42. [DOI] [PubMed] [Google Scholar]
- 10. Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer's disease in Down syndrome. Alzheimers Dement. 2019;15(2):245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiseman FK, Al‐Jabani T, Hardy J, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16(9):564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zigman W, Lott I. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. 2007;13(3):237‐246. [DOI] [PubMed] [Google Scholar]
- 13. Zigman W. Atypical aging in adults with Down syndrome. Dev Disabil Res Rev. 2013;18(1):51‐67. [DOI] [PubMed] [Google Scholar]
- 14. Sinai A, Moktysz C, Bernal J, Bohnen I, et al. Predictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimers Dis. 2018;61(2):717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vos SJB, Verhy F, Frölich L, et al. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain. 2015;138(5):1327‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen R, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knopman DS. Petersen RC Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303‐308. [DOI] [PubMed] [Google Scholar]
- 19. Machulda M, Lundt E, Albertson S, et al. Neuropsychological subtypes of incident mild cognitive impairment in the Mayo Clinic Study of Aging. Alzheimers Dement. 2019;15(7):878‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petersen RC. Mild cognitive impairment. Continuum. 2016;22(2):404‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gauthier S, Reisberg B, Zaudig M, et al. International Psychogeriatric Association Expert Conference on mild cognitive impairment.. Lancet. 2006;367(9518):1262‐1270. [DOI] [PubMed] [Google Scholar]
- 23. Petersen RC. The current status of mild cognitive impairment—what do we tell our patients. Nat Clin Pract Neurol. 2007;3(2):60‐61. [DOI] [PubMed] [Google Scholar]
- 24. Ball S, Holland A, Treppner P, Watson P, Huppert F. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer's disease in adults with DS and mild to moderate learning disabilities. Br J Clin Psychol. 2008;47(1):1‐29. [DOI] [PubMed] [Google Scholar]
- 25. Krinsky‐McHale S, Devenny D, Silverman W. Changes in explicit memory associated with early dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46(3):198‐208. [DOI] [PubMed] [Google Scholar]
- 26. Silverman W, Schupf N, Zigman W, et al. Dementia in adults with mental retardation: assessment at a single point in time. Am J Ment Retard. 2004;109(2):111‐125. [DOI] [PubMed] [Google Scholar]
- 27. Aylward EH, Burt DB, Thorpe LU, Lai F, Dalton A. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 1997;41(2):152‐164. [DOI] [PubMed] [Google Scholar]
- 28. Buschke H. Selective Reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12(5):534‐550. [Google Scholar]
- 29. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state, a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 30. Wisniewski KE, Hill AL. Clinical aspects of dementia in mental retardation and developmental disabilities. In Wisniewski H.& M. Janicki, eds. Aging and Developmental Disabilities: Issues and Approaches. Baltimore: Brookes:195‐210. [Google Scholar]
- 31. Haxby JV. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non‐demented old adults. J Intellect Disabil Res. 1989;33(3):193‐210. [DOI] [PubMed] [Google Scholar]
- 32. Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449‐453. [DOI] [PubMed] [Google Scholar]
- 33. McCarthy D. Scales of Children's Abilities. San Antonio: Psychological Corp; 1972. [Google Scholar]
- 34. Wechsler D. Wechsler Intelligence Scale for Children‐Revised. New York: Psychological Corp; 1974. [Google Scholar]
- 35. Beery KE, Buktenica NA. Developmental Test of Visual‐Motor Integration. Cleveland: Modern Curriculum Press; 1989. [Google Scholar]
- 36. Evenhuis H. The natural history of dementia in Down's syndrome. Arch Neurol. 1990;47(3):263‐267. [DOI] [PubMed] [Google Scholar]
- 37. Evenhuis HM. Medical aspects of ageing in a population with intellectual disability: II. Hearing impairment. J Intellect Disabil Res. 1995;39(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 38. Nihira K, Foster R, Shellhaas M, Leland H. AAMD Adaptive Behavior Scale. Washington, DC: American Association on Mental Deficiency; 1974. [Google Scholar]
- 39. Reiss S. Handbook of challenging behavior: Mental health aspects of mental retardation. Worthington: IDS Publishing Corp; 1994. [Google Scholar]
- 40. Urv TK, Zigman W, Silverman W. Psychiatric symptoms in adults with Down syndrome and Alzheimer's disease. Am J Intellect Devel Disabil. 2010;115(4):265‐276. [DOI] [PubMed] [Google Scholar]
- 41. Devanand DP. Use of the Columbia University Scale to assess psychopathology in Alzheimer's disease. Int Psychogeriatr; 1997;9(S1):137‐142. discussion 143‐150. [DOI] [PubMed] [Google Scholar]
- 42. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
- 43. Silverman W, Miezejeski C, Ryan R, Zigman W, Krinsky‐McHale S, Urv T. Stanford‐Binet & WAIS IQ Differences and Their Implications for Adults with Intellectual Disability (aka Mental Retardation). Intelligence. 2010;38(2):242‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnosis guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed Boston: Pearson Education; 2012. [Google Scholar]
- 46. Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol. 2007;20(4):219‐224. [DOI] [PubMed] [Google Scholar]
- 47. Malloy P, Belanger H, Hall S, Aloia M, Salloway S. Assessing visuoconstructional performance in AD, MCI and normal elderly using the Beery Visual‐Motor Integration Test. Clin Neuropsychol. 2003;17(4):544‐550. [DOI] [PubMed] [Google Scholar]
- 48. Overton M, Pihlsgård M, Elmståhl S. Diagnostic stability of mild cognitive impairment, and predictors of reversion to normal cognitive functioning.. Dement Geriatr Cogn Disord. 2020;30:1‐13. [DOI] [PubMed] [Google Scholar]
- 49. Esbensen AJ. Conditions association with aging and end of life of adults with Down syndrome. Int Rev Res Ment Retard. 2010;39(C):107‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartley SL, Handen BL, Devenny D. Cognitive decline and brain amyloid‐β accumulation across 3 years in adults with Down syndrome. Neurobiol Aging. 2017;8:68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


