Acute interstitial nephritis (AIN) is a leading cause of acute kidney injury (AKI) in hospitalized patients, and β-lactam antibiotics are a common cause.1 Early diagnosis of β-lactam−induced AIN is essential to direct changes to therapy, but it is often difficult to distinguish AIN from other causes of AKI, such as acute tubular necrosis. A kidney biopsy is the gold-standard diagnostic test, but carries significant risks and is often delayed. Mislabeling patients with a β-lactam allergy, in the absence of a biopsy, also restricts future antibiotic choice and drives inappropriate prescribing.2 Clinical features alone cannot reliably differentiate AIN from other causes of AKI, and the temporal relationship between drug exposure and onset of AKI remains poorly understood. Some reports suggest that β-lactam−induced AIN typically occurs more than 8 days after exposure; however, data are limited, and existing studies use nonstandardized definitions for AKI.3, 4, 5 We aimed to characterize the time between β-lactam exposure and AKI, defined by doubling of baseline serum creatinine, in a series of hospitalized patients with biopsy-proven AIN across 2 health services in Melbourne, Australia.
Results
A total of 72 cases of biopsy-proven AIN were identified, of which 49 were attributed to drugs and 14 to β-lactams specifically (Figure 1). I.v. flucloxacillin, piperacillin−tazobactam, and benzylpenicillin were used in 8 cases, 5 cases, and 1 case, respectively. Among the 14 patients with β-lactam−induced AIN, the median age was 57 years (interquartile range [IQR], 40−68 years); 6 patients were female; mean baseline creatinine was 78 μmol/l; and median duration of β-lactam use was 8 days (IQR, 3−12 days). The indications for β-lactams included cellulitis (n = 5), methicillin-susceptible Staphylococcus aureus (MSSA) osteomyelitis (n = 2), MSSA bacteremia (n = 2), fever of unknown origin (n = 2), dog bite, pneumonia, and cholangitis. In 7 patients (50%) there were concurrent histopathological features on the kidney biopsy specimen that could contribute to AKI (i.e., acute renal pathology), 6 with acute tubular necrosis and 1 with IgA co-dominant postinfectious glomerulonephritis (PIGN). Three had features of background mild chronic damage. The median time to AKI was 2.5 days (IQR, 2−8 days). There was no appreciable difference in the time to AKI between centers (Figure 2). The mean time to AKI among 7 patients with concurrent acute renal pathology was 3.6 days (range, 1−13 days), whereas among 7 patients without concurrent acute renal pathology it was 6.1 days (range, 1−11 days); however, this difference was not statistically significant (P = 0.27, 2-sided t test). Two patients, 1 patient with and 1 patient without concurrent acute renal pathology, had a known pre-existing allergy to penicillin. One patient reported an unknown reaction, and the other reported prior kidney injury following penicillin exposure. Acute kidney injury occurred within 2 days of re-exposure for both patients. The median time to biopsy after AKI was 9 days (IQR, 5−11), and 6 patients required acute hemodialysis. All 14 patients had recovery of renal function within 1 year, but the mean change in creatinine at 1 year, compared to the pre-AKI baseline, was 28.1 μmol/l (95% confidence interval, 11.2−45.0 μmol/l). Five patients (35.7%; 4 from Austin Health and 1 from Monash Health), had re-exposure to an alternative nonpenicillin β-lactam (4 to cephalosporins and 1 to carbapenem) during subsequent inpatient admissions, without developing an AKI, although 1 patient had serum creatinine measured for only 4 days following exposure to ceftriaxone. One patient also reported exposure to amoxicillin clavulanate on 2 occasions in 2019, and in both cases did not develop an AKI, as determined by measurements of serum creatinine at 7 days after exposure.
Figure 1.
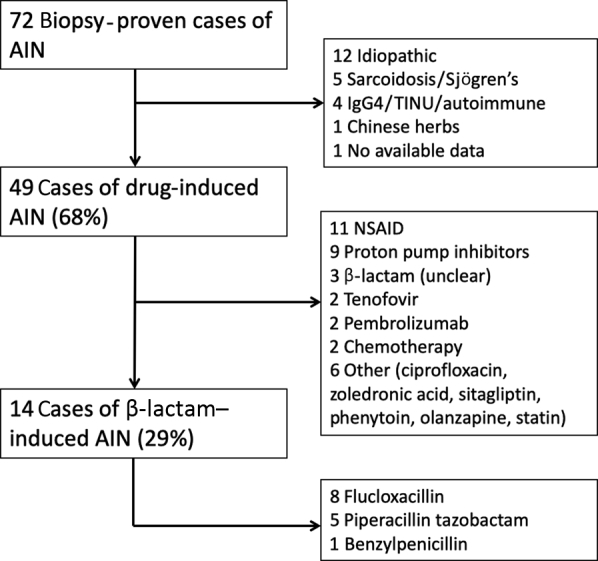
Etiology of biopsy-proven acute interstitial nephritis (AIN) at Monash Health and Austin Health between 2011 and 2017. NSAID, nonsteroidal anti-inflammatory drug.
Figure 2.
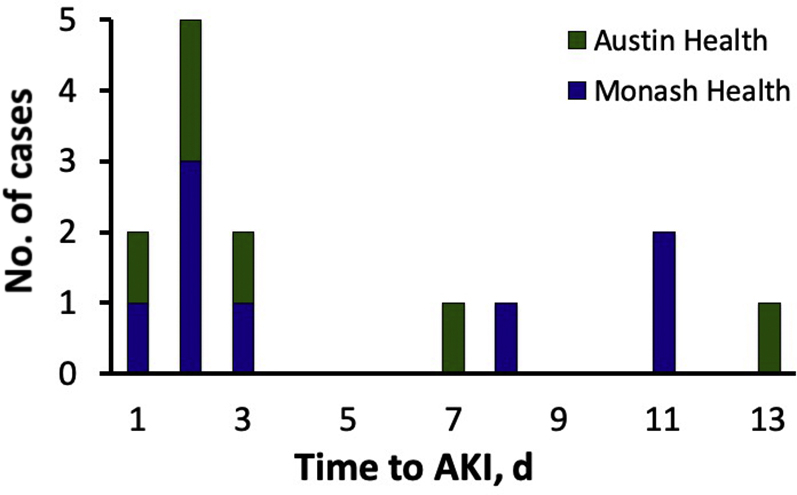
Time to acute kidney injury (AKI), defined by doubling of serum creatinine, in 14 cases of biopsy-proven β-lactam−induced acute interstitial nephritis.
Discussion
In our retrospective multicenter case series of 14 patients with β-lactam−induced biopsy-proven acute interstitial nephritis, the median time to doubling of serum creatinine was 2.5 days, which is earlier than previous estimates and shorter than typically expected in primary T-cell−mediated hypersensitivities. Our finding likely reflects the realities of clinical practice, in which a combination of other factors may contribute to earlier onset of AKI, and selection bias toward more severe cases that warrant biopsy.
Our study is consistent with previous case series of drug-induced AIN, affirming that β-lactams, particularly flucloxacillin and piperacillin−tazobactam, nonsteroidal anti-inflammatory drugs, and proton pump inhibitors are the leading culprit agents in Australia.6 Cephalosporin and carbapenem antibiotics were not identified as culprit agents in either study. Recovery of renal function was observed in all cases, despite 6 of the 14 patients requiring hemodialysis. Serum creatinine at 1 year following AKI was approximately 30 μmol/l higher than baseline, suggesting that AKI related to β-lactam AIN may also contribute to chronic kidney damage and fibrosis.7
Subsequent exposure to another class of β-lactam antibiotics after an episode of penicillin-induced AIN was common among the 6 cases at Austin Health, with 5 of them later receiving an alternative penicillin, cephalosporin, or carbapenem. Only 1 patient at Monash Health was identified to have subsequent exposure, although true re-exposures may be underestimated in the absence of electronic prescribing. No adverse events were observed in any case of re-exposure, which may suggest a possible absence of cross-reactivity seen in other hypersensitivities.8 Another possible explanation is that β-lactams with structurally different R side chains may be less likely to induce a recurrence of AIN. Structural side chain differences are thought to contribute to the variable rates of cross reactivity seen in IgE and T-cell−mediated penicillin hypersensitivity reactions.9
Small sample size is an important limitation of our study. Larger studies would help to validate our findings, to assess the risks of re-exposure to different β-lactams, and to further characterize the contribution that AIN has to the development of chronic kidney disease. Selection bias may also limit the generalizability of our findings, as patients who undergo kidney biopsy are likely to have had a more severe kidney injury. A median time to AKI onset of 2.5 days may therefore not be representative of all β-lactam−induced AIN. The timing of baseline and follow-up measurements of serum creatinine were not prospectively defined, and thus not standardized, but in most cases serum creatinine was measured on a daily basis during the inpatient stay. Flucloxacillin was the most common culprit agent identified in our case series; however, the frequency with which each of the β-lactams was used is unknown, and prospective studies are required to determine the relative incidence of AIN induced by different β-lactams.
In conclusion, a diagnosis of β-lactam−induced AIN should be considered in hospitalized patients who sustain an AKI following β-lactam use, even if doubling of serum creatinine occurs within 2 or 3 days of exposure. This time frame may reflect more severe or atypical cases, because only cases in which patients underwent biopsy were available for our analysis. Larger studies are required to determine risk factors for early-onset AKI in β-lactam−induced AIN. A case-control study would be a logical next step to investigate this issue. Furthermore, we believe that the standard practice of avoiding all β-lactams following an episode of penicillin-induced AIN should be revisited, because re-exposure to alternative β-lactam antibiotics was not associated with recurrence of AKI in our case series.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Methods.
Supplementary Material
References
- 1.Perazella M.A. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol. 2014;81:381–388. doi: 10.5414/CN108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal K.G., Peter J.G., Trubiano J.A., Phillips E.J. Antibiotic allergy. Lancet. 2019;393:183–198. doi: 10.1016/S0140-6736(18)32218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin D.S., Levine B.B., McCluskey R.T., Gallo G.R. Renal failure and interstitial nephritis due to penicillin and methicillin. N Engl J Med. 1968;279:1245–1252. doi: 10.1056/NEJM196812052792302. [DOI] [PubMed] [Google Scholar]
- 4.Raghavan R., Shawar S. Mechanisms of drug-induced interstitial nephritis. Adv Chron Kidney Dis. 2017;24:64–71. doi: 10.1053/j.ackd.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60:804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson G.J., Kark A.L., Francis L.P. The increasing rates of acute interstitial nephritis in Australia: a single centre case series. BMC Nephrol. 2017;18:329. doi: 10.1186/s12882-017-0747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minhas J.S., Wickner P.G., Long A.A. Immune-mediated reactions to vancomycin: a systematic case review and analysis. Ann Allergy Asthma Immunol. 2016;116:544–553. doi: 10.1016/j.anai.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard M., Robitaille G., Karam F. Cross-reactivity to cephalosporins and carbapenems in penicillin-allergic patients: two systematic reviews and meta-analyses. J Allergy Clin Immunol Pract. 2019;7:2722–2738. doi: 10.1016/j.jaip.2019.05.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


