Abstract
Social determinants of health that have been examined in relation to prostate cancer incidence, stage at diagnosis, and survival include socioeconomic status (income, education), neighborhood disadvantage, immigration status, social support, and social network. Other social determinants of health include geographic factors such as neighborhood access to health services. Socioeconomic factors influence risk of prostate cancer. Prostate cancer incidence rates tend to be positively associated with socioeconomic status. On the other hand, low socioeconomic status is associated with increased risk of poorer survival. There are well-documented disparities in prostate cancer survival by socioeconomic status, race, education, and census tract–level poverty. The results of this review indicate that social determinants such as poverty, lack of education, immigration status, lack of social support, and social isolation play an important role in prostate cancer stage at diagnosis and survival. To address these social determinants and eliminate cancer disparities, effective interventions that account for the social and environmental contexts in which patients with cancer live and are treated are needed.
Keywords: African Americans, Education, Prostate cancer, Poverty, Unemployment
Social determinants of health that have been examined in relation to prostate cancer incidence, stage at diagnosis, and survival include socioeconomic status (income, education), neighborhood disadvantage, immigration status, social support, and social network.1, 2, 3, 4 Other social determinants of health include geographic factors such as neighborhood access to health services.5 Socioeconomic factors such as lack of education, poverty, and income inequality are among the most important social determinants of health. Low-income people are at increased risk of an array of adverse health outcomes and more likely to die prematurely.
It is well established that socioeconomic factors influence risk of prostate cancer. Prostate cancer incidence rates tend to be positively associated with socioeconomic status. On the other hand, low socioeconomic status is associated with increased risk of poorer survival. There are well-documented disparities in prostate cancer survival by socioeconomic status, race, education, and census tract–level poverty.
Using data from the SEER-Medicare linked database, Du et al.6 examined the relationship between socioeconomic status and prostate cancer survival. Low socioeconomic status was significantly associated with decreased survival in men with prostate cancer. Those living in a community with the lowest quartile of socioeconomic status were 31% more likely to die than those living in the highest quartile [hazard ration (HR) = 1.31, 95% confidence interval (CI) = 1.25, 1.36]. Compared with whites, the risk of mortality in African American men with prostate cancer was not significantly different after adjusting for poverty, income, or composite socioeconomic variable.
Using prostate cancer incidence and mortality data from Alameda County, California, Ernster et al.7 examined whether the higher occurrence of prostate cancer among African Americans than among whites could be explained by racial differences in socioeconomic status. Each death or case of prostate cancer was assigned to a social class based on census tract of residence. Comparison of age-specific morality and incidence rates by socioeconomic status revealed no gradient in either whites or African Americans.
Using data from the California Cancer Registry from 1995 to 2004, Robbins et al.8 examined whether black–white disparities in prostate cancer mortality are reduced or eliminated after accounting for differences in socioeconomic status and other prognostic factors. The age-adjusted HR for prostate cancer death (blacks vs. whites) was 1.61 (95% CI = 1.50, 1.72). The racial difference in survival was completely eliminated after additional adjustment for stage, treatment, grade, socioeconomic status, and year of diagnosis.
Using data from the California Cancer Registry, Cheng et al.9 examined prostate cancer incidence and mortality according to socioeconomic status. Each prostate cancer case and death was assigned a multidimensional neighborhood socioeconomic index using US Census data. For prostate cancer incidence, higher levels of socioeconomic status were significantly associated with increased risk of the disease (quartile 1 vs. quartile 5: relative risk = 1.28, 95% CI = 1.25, 1.30). Higher levels of socioeconomic status were associated with lower rates of prostate cancer death (quartile 1 vs. quartile 5: relative risk = 0.88, 95% CI = 0.92, 0.94). African Americans had a twofold to fivefold increased risk of prostate cancer deaths in comparison with non-Hispanic whites across all levels of socioeconomic status.9
Using data from the New Jersey State Cancer Registry between 1986 and 1999, Niu et al.10 examined prostate cancer survival disparities by race/ethnicity and socioeconomic status. Compared with those residing in the wealthiest areas, patients with prostate cancer residing in areas of high poverty had increased risks of cancer death. African American and Hispanic patients with prostate cancer had higher death rates than non-Hispanic whites did (p < 0.01). After adjustment for poverty level, the higher risk of death among African American patients was attenuated.
Freeman et al.11 conducted a retrospective cohort study of African American and non-Hispanic white men diagnosed with prostate cancer at four Chicago area medical centers between 1986 and 1990. Census tract–level socioeconomic status was associated with increased risk of prostate cancer–specific mortality (highest vs. lowest quartile, HR = 2.37, p < 0.0001). Using data from the Vitamins and Lifestyle study, Hastert et al.12 examined the relationship between a block group socioeconomic status index and prostate cancer incidence. Lower area–level socioeconomic status was weakly associated with lower prostate cancer risk.
Ellis et al.13 analyzed data from the California Cancer Registry between 2000 and 2013 to estimate prostate cancer–specific survival for each racial/ethnic group. A composite index of neighborhood socioeconomic status was derived using US Census or American Community Survey data on education, occupation, employment, household income, poverty, and rent and house values. Prostate cancer–specific mortality among African American men with prostate cancer was 60% higher than that among non-Hispanic white men (HR = 1.60, 95% CI = 1.52, 1.69). About 7% of this survival disparity was explained by neighborhood socioeconomic status. An additional 14% was explained by differences in marital status.
In a population-based case–control study in Montreal, Canada, Nicolau et al.14 examined socioeconomic position over the life course among men. Four hundred cases of prostate cancer were included. Socioeconomic position in childhood increased the risk of prostate cancer, suggesting that early childhood may be a critical period for exposures associated with socioeconomic position.
Other studies have examined the relation between socioeconomic status and prostate cancer stage at diagnosis. Schwartz et al.15 examined whether racial differences in prostate cancer stage at diagnosis are explained by differences in socioeconomic status. A socioeconomic status variable was calculated for each case using aggregate US Census data for education, poverty status, and occupation specific to each case's census block group. Socioeconomic status was an independent predictor of prostate cancer stage at diagnosis, with cases from the highest socioeconomic status block group more likely to present with local stage disease than those from the lowest socioeconomic status group. Race independently predicted stage at prostate cancer diagnosis. Greenlee and Howe16 examined the relationship between county-level poverty and late-stage prostate cancer using data from the North American Association of Central Cancer Registries from 1997 to 2001. Higher county poverty was associated with increased late-stage disease (OR = 1.7, 95% CI = 1.5, 1.9). Weiner et al.17 analyzed prostate cancer data from the National Cancer Database from 2004 to 2013. Men presenting with and without metastatic disease were compared using a 4-level composite score of socioeconomic status created using Census-based income and education data. Lower socioeconomic status (first vs. fourth quartile; adjusted OR = 1.39, 9% CI = 1.35, 1.44) was associated with higher odds of presenting with metastatic prostate cancer.
1. Methods
The present review is based on bibliographic searches in PubMed and CINAHL and relevant search terms. Articles published in English from 1970 through April 1, 2019, were identified using the following Medical Subject Headings search terms and Boolean algebra commands: prostate cancer AND (incidence OR stage OR mortality) AND (social determinants OR neighborhood disadvantage OR racial discrimination OR immigration OR social support). The searches were limited to neither words appearing in the title of an article nor studies in a particular country or geographic region of the world. The references of review articles were also reviewed. Information obtained from bibliographic searches (title and topic of article, information in abstract, study design, and key words) was used to determine whether to retain each article identified in this way. Only studies written in English that examined social determinants of breast cancer risk, stage, and survival were eligible for inclusion.
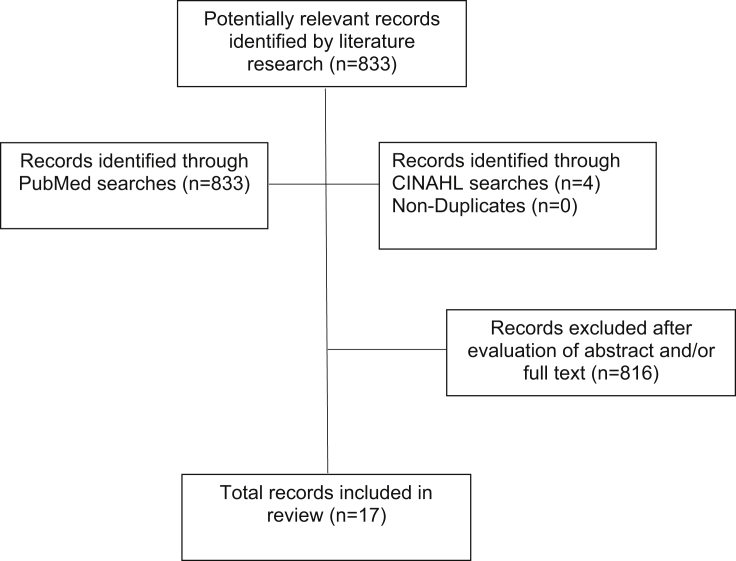
A total of 833 articles were identified in the bibliographic searches. Of these, 17 met the study criteria (Fig. 1). A variety of study designs were identified including case–control studies, cohort studies, and population-based studies of cancer registry data.
Fig. 1.
Flowchart of record selection process is shown.
2. Results
2.1. Neighborhood disadvantage and prostate cancer
In an analysis of cancer registry data from Connecticut and Massachusetts, between 1994 and 1988, DeChello et al.18 found no association between racial segregation and prostate cancer incidence (Table 1).
Table 1.
Studies of neighborhood disadvantage and prostate cancer risk, stage, and survival
| Author | Design | Outcomes | Sample size | Results |
|---|---|---|---|---|
| DeChello et al., 2006 | Analysis of cancer registry data from Connecticut and Massachusetts, between 1994 and 1988 | Prostate cancer incidence | 30,687 men with prostate cancer | No association between racial segregation and prostate cancer incidence was observed |
| Li et al., 2012 | Analysis of data linked to the Swedish Cancer Registry, between 1958 and 2008 | Prostate cancer mortality | 73,159 men with prostate cancer | The prostate cancer mortality rate was 1.5 times higher in men living in high-deprivation neighborhoods than in those living in the most affluent neighborhoods. There was a strong association between prostate cancer mortality and being unmarried, having a low income, and educational attainment. |
| Haas et al., 2008 | Analysis of SEER-Medicare data, between 1992 and 2002 | Prostate cancer stage at diagnosis | 151,142 older men with prostate cancer | Area of residence was categorized into 4 groups: low segregation/high income (potentially the most advantaged), high segregation/high income, low segregation/low income, and high segregation/low income (possibly the most disadvantaged). No association between racial segregation and prostate cancer stage at diagnosis was observed. |
| Major et al., 2012 | Analysis of data from the National Institutes of Health-American Association of Retired Persons Study | Prostate cancer incidence | More than 500,000 men at risk of prostate cancer | A statistically nonsignificant association was observed between neighborhood deprivation and advanced prostate cancer among African American men (HR = 1.13, 95% CI = 0.79, 1.63). |
CI, confidence interval; HR, hazard ratio.
In an analysis of data linked to the Swedish Cancer Registry conducted by Li et al.,19 the prostate cancer mortality rate was 1.5 times higher in men living in high-deprivation neighborhoods than in those living in the most affluent neighborhoods. There was a strong association between prostate cancer mortality and being unmarried, having a low income, and educational attainment.
Haas et al.20 examined the relation between neighborhood disadvantage and prostate cancer stage at diagnosis using SEER-Medicare linked data. Area of residence was categorized into 4 groups: low segregation/high income (potentially the most advantaged), high segregation/high income, low segregation/low income, and high segregation/low income (possibly the most disadvantaged). No association between racial segregation and prostate cancer stage at diagnosis was observed.
In an analysis of data from the National Institutes of Health-American Association of Retired Persons Study, Major et al.1 found a statistically nonsignificant association between neighborhood deprivation and advanced prostate cancer among African American men (HR = 1.13, 95% CI = 0.79, 1.63).
2.2. Immigration status and prostate cancer
McCredie et al.21 conducted a study of immigration status and prostate cancer mortality in New South Wales, Australia, as summarized in Table 2. Compared with those born in Australia, migrants had a significantly lower risk of dying from prostate cancer (Table 3).
Table 2.
Studies of immigration status and prostate cancer risk, stage, and survival
| Author | Design | Outcomes | Sample size | Results |
|---|---|---|---|---|
| McCredie et al., 1999 | Population-based study in New South Wales, Australia | Prostate cancer mortality | 11,545 deaths from prostate cancer | Compared with those born in Australia, migrants had a significantly lower risk of dying from prostate cancer. |
| Lee et al., 2007 | Analysis of SEER data (U.S.) and IARC data (South Korea) | Prostate cancer incidence | Prostate cancer risk was higher among Korean American men than among their Korean counterparts. | |
| Schupp et al., 2014 | Analysis of data from the California Cancer Registry, from 1995 through 2008 | Prostate cancer survival | 35,427 Hispanic men diagnosed with prostate cancer | Foreign-born Hispanics had a significantly lower risk of prostate cancer survival (HR = 0.81, 95% CI = 0.75, 0.87) than U.S.-born Hispanics. |
| Lichtensztajn et al., 2014 | Analysis of data from the California Cancer Registry, from 2004 through 2010 | Low-, intermediate-, or high-risk group based on clinical stage, Gleason score, and PSA value at diagnosis | 90,845 men diagnosed with prostate cancer | In addition to non-Hispanic blacks, six Asian American groups (U.S.-born Chinese, foreign-born Chinese, U.S.-born Japanese, foreign-born Japanese, foreign-born Filipino, and foreign-born Vietnamese) were more likely to have an unfavorable risk profile than non-Hispanic whites. |
| Feletto & Sitas, 2015 | Analysis of cancer incidence and mortality data for New South Wales, Australia residents, for 2004–2008 | Prostate cancer incidence | Prostate cancer incidence was lower in non–Australian-born men than in Australian-born men. | |
| Lynch et al., 2017 | Neighborhood-wide association study, utilizing Pennsylvania Cancer Registry data linked to US Census data, from 1995 to 2005 | Prostate cancer aggressiveness variable defined by high tumor stage (Stage 3 or 4) and high tumor grade (Grade 7+) | 77,086 white men with prostate cancer | The most significant variables in principal component analysis included immigration status (OR = 0.93, 95% CI = 0.87–0.99). |
| McDonald et al., 2017 | Analysis of data from Statistics Canada that links Census information with administrative data on cancer and mortality, between 1991 and 2003 | Prostate cancer diagnosis | Men diagnosed with prostate cancer | Recent immigrants to Canada were significantly less likely than nonimmigrant Canadians to be diagnosed with prostate cancer (OR = 0.472, p-value = 0.000). This gap declined with additional years in Canada for immigrant men. |
| Kaucher et al. 2018 | Analysis of data from two cohorts in Germany, including ethnic Germans who had immigrated from the Russian federation and other countries of the former Soviet Union | Prostate cancer incidence, mortality, and stage at diagnosis | 16,033 and 28,744 men at risk of prostate cancer | Compared with the general German population, ethnic resettlers had lower incidence and mortality from prostate cancer. |
CI, confidence interval; HR, hazard ratio; IARC, International Agency for Research on Cancer; OR, odds ratio; PSA, prostate-specific antigen.
Table 3.
Studies of social support and prostate cancer risk, stage, and survival
| Author | Design | Outcomes | Sample size | Results |
|---|---|---|---|---|
| Bergelt et al., 2009 | Cohort study | Prostate cancer risk | 3,838 men at risk of prostate cancer | Men with the highest social network scores had slightly but not significantly decreased risk for prostate cancer. |
| Aizer et al., 2013 | Analysis of SEER cancer registry data from 2004 to 2008 | Total survival | Men with prostate cancer | Married patients were less likely to present with metastatic disease (OR = 0.52, 95% CI = 0.50, 0.55) and less likely to die as a result of prostate cancer (OR = 0.74, 95% CI = 0.67, 0.81) than unmarried patients. |
| Rottenberg et al., 2014 | Historical prospective study in an Israeli community | Total survival | 69 men with prostate cancer | No statistically significant association was observed between social networks and total survivor (HR = 0.63, 95% CI = 0.28–1.42, p = 0.2). |
| Li et al., 2014 | Case–control study in China | Odds of prostate cancer | 250 patients with prostate cancer and 500 controls | Marital separation was associated with increased risk of prostate cancer (OR = 1.94, 95% CI = 1.29, 2.91). |
| Lynch et al., 2017 | Neighborhood-wide association study, utilizing Pennsylvania Cancer Registry data, from 1995 to 2005, linked to US Census data | Prostate cancer aggressiveness variable defined by high tumor stage (Stage 3 or 4) and high tumor grade (Grade 7+) | 77,086 white men with prostate cancer | The most significant variables in principal component analysis included two variables related to social support [% male householder living alone (OR = 1.06, 95% CI = 1.01, 1.11) and % male householder older than 65 years living alone in nonfamily household (OR = 1.07, 95% CI = 1.02–1.13)]. |
CI, confidence interval; HR, hazard ratio; OR, odds ratio.
In analysis of surveillance, epidemiology, and end-results (SEER) cancer registry data (U.S.) and International Agency for Research on Cancer data (South Korea), Lee et al.3 found that prostate cancer risk was higher among Korean American men than their Korean counterparts.
Schupp et al.22 conducted a study of immigration status and prostate cancer survival using data from the California Cancer Registry. Foreign-born Hispanics had a significantly lower risk of prostate cancer survival (HR = 0.81, 95% CI = 0.75, 0.87) than U.S.-born Hispanics.
Lichtensztajn et al.2 examined the relation between immigration status and prostate cancer risk profile using data from the California Cancer Registry. The cases were categorized as low-, intermediate-, or high-risk group based on clinical stage, Gleason score, and prostate-specific antigen value at diagnosis. In addition to non-Hispanic blacks, six Asian American groups (U.S.-born Chinese, foreign-born Chinese, U.S.-born Japanese, foreign-born Japanese, foreign-born Filipino, and foreign-born Vietnamese) were more likely to have an unfavorable risk profile than non-Hispanic whites.
Feletto and Sitas23 examined the relation between immigration status and prostate cancer incidence using data for New South Wales Australia residents. Prostate cancer incidence was lower in non–Australian-born men than in Australian-born men.
Lynch et al.24 conducted a neighborhood-wide association study to examine predictors of prostate cancer aggressiveness. The prostate cancer aggressiveness variable was defined by high tumor stage (Stage 3 or 4) and high tumor grade (Grade 7+). The most significant variables in principal component analysis included immigration status (OR = 0.93, 95% CI = 0.87–0.99).
McDonald et al.25 examined the relation between immigration status ad prostate cancer diagnosis using data from Statistics Canada that links Census information with administrative data on cancer and mortality. Recent immigrants to Canada were significantly less likely than nonimmigrant Canadians to be diagnosed with prostate cancer (OR = 0.472, p-value = 0.000). This gap declined with additional years in Canada for immigrant men.
Kaucher et al.26 analyzed data from two cohort studies in Germany, including ethnic Germans who had immigrated from the Russian federation and other countries of the former Soviet Union. Compared with the general German population, ethnic resettlers had lower incidence and mortality from prostate cancer.
2.3. Social support and prostate cancer
In a cohort study, Bergelt et al.4 examined the relation between social network and prostate cancer risk. Men with the highest social network scores had slightly but not significantly decreased risk for prostate cancer.
In an analysis of SEER cancer registry data, Aizer et al.27 examined the relation between marital status and prostate cancer survival. Married patients were less likely to present with metastatic disease (OR = 0.52, 95% CI = 0.50, 0.55) and less likely to die as a result of prostate cancer (OR = 0.74, 95% CI = 0.67, 0.81) than unmarried patients.
In an historical prospective study in an Israeli community, Rotteberg et al.28 examined the relation between social network and prostate cancer survival. No statistically significant association was observed between social networks and total survivor (HR = 0.63, 95% CI = 0.28–1.42, p = 0.2).
Li et al.29 conducted a case–control study of prostate cancer in China. Marital separation was associated with increased risk of prostate cancer (OR = 1.94, 95% CI = 1.29, 2.91).
Lynch et al.24 conducted a neighborhood-wide association study to examine predictors of prostate cancer aggressiveness. The prostate cancer aggressiveness variable was defined by high tumor stage (Stage 3 or 4) and high tumor grade (Grade 7+). The most significant variables in principal component analysis included two variables related to social support [% male householder living alone (OR = 1.06, 95% CI = 1.01, 1.110 and % male householder older than 65 years living alone in nonfamily household (OR = 1.07, 95% CI = 1.02–1.13)].
3. Discussion
The results of this review indicate that social determinants such as poverty, lack of education, immigration status, lack of social support, and social isolation play an important role in prostate cancer survival. Outcomes such as incidence, stage at diagnosis, and survival correspond to different stages in cancer carcinogenesis (i.e., initiation, promotion, and progression).30 Social determinants may contribute to cancer disparities at each of these stages.
Although prostate cancer incidence rates have been found to be lower among immigrants, the healthy immigrant effect diminishes over several generations. Studies have shown that country of origin is associated with prostate cancer survival.21, 22, 26 The later stage at diagnosis among immigrants may be due to lower prostate cancer screening rates or population differences in environmental exposures such as diet, physical activity, or occupational exposures.
Several factors may account for the inverse association between social support and advanced stage at prostate cancer diagnosis.4, 27, 24, 29 Men may be influenced by their spouse, other relatives, or friends within their social network to undergo prostate cancer screening. In addition, married status is positively associated with health insurance and household income. Men who are married may have greater access to health care associated with prostate cancer screening.
Some,1, 19 but not all studies have found an association between neighborhood disadvantage and higher prostate cancer stage at diagnosis and mortality. The inconsistency between studies may be due to differences in study design or population differences in prostate cancer screening rates. Social stressors associated with neighborhood disadvantage and low socioeconomic status may increase the risk of prostate cancer incidence and mortality through biological and behavioral pathways.31
To address these social determinants and eliminate cancer disparities, effective interventions that account for the social and environmental contexts in which patients with cancer live and are treated are needed.5 Of particular concern are access to health care among immigrant populations and health communication about the early detection and treatment of prostate cancer for men who are unmarried or socially isolated.
Conflict of interest
The author has no conflicts of interest to report.
References
- 1.Major J.M., Oliver M.N., Doubeni C.A., Hellenbeck A.R., Graubard B.I., Sinha R. Socioeconomic status, health care density, and risk of prostate cancer among African-American and Caucasian men in a large prospective study. Cancer Causes Control. 2012;23:1185–1191. doi: 10.1007/s10552-012-9988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtensztajn D.Y., Gomez S.L., Sieh W., Chung B.I., Cheng I., Brooks J.D. Prostate cancer risk profiles in Asian Americans: disentangling the effects of immigration status and race/ethnicity. J Urol. 2014;191:952–956. doi: 10.1016/j.juro.2013.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Demissie K, Lu S-E, Rhodes GG. Cancer incidence among Korean-American immigrants in the United States and Native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 4.Bergelt C., Prescott E., Gronbaek M., Koch U., Johansen C. Social ties and risk for cancer—a prospective cohort study. Acta Oncol. 2009;48:1010–1018. doi: 10.1080/02841860903036230. [DOI] [PubMed] [Google Scholar]
- 5.Dean L.T., Gehlert S., Neuhouser M.L., Oh A., Zanetti K., Goodman M. Social factors matter in cancer risk and survivorship. Cancer Causes Control. 2018;29:611–618. doi: 10.1007/s10552-018-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du X.L., Fang S., Coker A.L., Sanderson M., Aragaki C., Cormier J.N. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma. Findings from a large community-based cohort. Cancer. 2006;106:1276–1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 7.Ernster V.L., Winkelstein W., Jr., Selvin S., Brown S.M., Sacks S.T., Austin D.F. Race, socioeconomic status, and prostate cancer. Cancer Treat Rep. 1977;61:187–191. [PubMed] [Google Scholar]
- 8.Robbins A.S., Yin D., Parikh-Patel A. Differences in prognostic factors and survival among white men and Black men with prostate cancer, California, 1995-2004. Am J Epidemiol. 2007;166:71–78. doi: 10.1093/aje/kwm052. [DOI] [PubMed] [Google Scholar]
- 9.Cheng I., Witte J.S., McClure L.A., Shema S.J., Cockburn M.G., John E.M. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. 2009;20:1431–1440. doi: 10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu X., Pawlish, Roche L.M. Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. J Health Care Poor Underserved. 2010;21:144–160. doi: 10.1353/hpu.0.0263. [DOI] [PubMed] [Google Scholar]
- 11.Freeman V.L., Ricardo A.C., Campbell R.T., Barrett R.E., Warnecke R.B. Association of census tract-level socioeconomic status with disparities in prostate cancer-specific survival. Cancer Epidemiol Biomark Prev. 2011;20:2150–2159. doi: 10.1158/1055-9965.EPI-11-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastert TA, Beresford SAA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. 2015;69:168–176. doi: 10.1136/jech-2014-204417. [DOI] [PubMed] [Google Scholar]
- 13.Ellis L., Canchola A.J., Spiegel D., Ladabaum U., Haile R., Gomez S.L. Racial and ethnic disparities in cancer survival: the contribution of tumor, socioeconomic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolau B., Madathil S.A., Castonguay G., Rousseau M.C., Parent M.E., Siemiatycki J. Shared social mechanisms underlying the risk of nine cancers: a life course study. Int J Cancer. 2019;144:59–67. doi: 10.1002/ijc.31719. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz K.L., Crossley-May H., Vigneau F.D., Brown K., Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14:761–766. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 16.Greenlee R.T., Howe H.L. County-level poverty and distant stage caner in the United States. Cancer Causes Control. 2009;20:989–1000. doi: 10.1007/s10552-009-9299-x. [DOI] [PubMed] [Google Scholar]
- 17.Weiner A.B., Matulewicz R.S., Tosoian J.J., Feinglass J.M., Shaeffer E.M. The effect of socioeconomic status, race, and insurance type on newly diagnosed metastatic prostate cancer in the United States (2004-2013) Urol Oncol. 2018;36:91.e1–91.e6. doi: 10.1016/j.urolonc.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeChello L.M., Gregorio D.I., Samociuk H. Race-specific geography of prostate cancer incidence. Int J Health Geogr. 2006;5:59–67. doi: 10.1186/1476-072X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Sundquist K., Sundquist J. Neighborhood deprivation and prostate cancer mortality: a multilevel analysis from Sweden. Prostate Cancer Prostatic Dis. 2012;15:128–134. doi: 10.1038/pcan.2011.46. [DOI] [PubMed] [Google Scholar]
- 20.Haas J.S., Earle C.C., Orav J.E., Brawarsky P., Neville B.A., Williams D.R. Racial segregation and disparities in cancer stage for seniors. J Gen Intern Med. 2008;23:699–705. doi: 10.1007/s11606-008-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCredie M., Williams S., Coates M. Cancer mortality in migrants from the British Isles and continental Europe to New South Wales, Australia, 1975-1995. J Cancer. 1999;83:179–185. doi: 10.1002/(sici)1097-0215(19991008)83:2<179::aid-ijc6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Schupp C.W., Press D.J., Gomez S.L. Immigration factors and prostate cancer survival among Hispanic men in California: Does neighborhood matter? Cancer. 2014;120:1401–1408. doi: 10.1002/cncr.28587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feletto E., Sitas F. Quantifying disparities in cancer incidence and mortality of Australian residents of New South Wales (NSW) by place of birth: an ecological study. BMC Public Health. 2015;15:823. doi: 10.1186/s12889-015-2141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch S.M., Mitra N., Ross M., Newcomb C., Dailey K., Jackson T. A neighborhood-wide association study (NWAS): example of prostate cancer aggressiveness. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald J.T., Farnworth M., Liu Z. Cancer and the healthy immigrant effect: a statistical analysis of cancer diagnosis using a linked Census-cancer registry administrative database. BMC Public Health. 2017;17:296. doi: 10.1186/s12889-017-4190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaucher S., Kajuter H., Becher H., Winkler V. Cancer incidence and mortality among ethnic German migrants from the former Soviet Union. Front Oncol. 2018;8:378. doi: 10.3389/fonc.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizer A.A., Chen M.-H., McCarthy E.P., Mendu M.L., Koo S., Wilhite T.J. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rottenberg Y., Litwin H., Manor O., Paltiel A., Barchana M., Paltiel O. Prediagnostic self-assessed health and extent of social networks predict survival in older individuals with cancer: a population-based cohort study. J Geriatr Oncol. 2014:400–407. doi: 10.1016/j.jgo.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Li M.L., Lin J., Hou J.G., Xu L., Cui X.G., Xu X.X. Environmental and psycho-social factors related to prostate cancer risk in the Chinese population: a case-control study. Biomed Environ Sci. 2014;27:707–717. doi: 10.3967/bes2014.089. [DOI] [PubMed] [Google Scholar]
- 30.Hossain F., Danos D., Prakash O., Gilliland A., Ferguson T.F., Simonsen N. Neighborhood social determinants of triple negative breast cancer. Front Public Health. 2019;7:1–7. doi: 10.3389/fpubh.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuevas A.G., Trudel-Fitzgerald C., Cofie L., Zaitsu M., Allen J., Williams D.R. Placing prostate cancer disparities within a psychosocial context: challenges and opportunities for future research. Cancer Causes Control. 2019;30:443–456. doi: 10.1007/s10552-019-01159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]