Abstract
Alpha-amylase reputes for starch modification by breaking of 1-4 glycosidic bands and is widely applied in different industrial sectors. Microorganisms express unique alpha-amylases with thermostable and halotolerant characteristics dependent on the microorganism’s intrinsic features. Likewise, genetic engineering methods are applied to produce enzymes with higher stability in contrast to wild types. As there are widespread application of α-amylase in industry, optimization methods like RSM are used to improve the production of the enzyme ex vivo. This study aimed to review the latest researches on the production improvement and stability of α-amylase.
Keywords: Microbial, Alpha-amylase, RMS, Stability, Optimization
Introduction
Compared to the chemical methods that need harsh conditions such as high pressure and temperature, using of microorganism is considered in many purposes including heavy metal absorption,1 gene engeering,2 digestion,3 production of novel anti-microbs4 and particullary for producing of industrial enzymes.5,6 Demand on the high-quality productions leads to development of novel methods to improve industrial products such as protease and amylase that are frequently applied in industry and medical science.
There have been identified three types of amylase including α-amylase, β-amylase, and γ-amylase. Alpha-amylase is an industrial enzyme (EC 3.2.1.1.), which cleaves internal alpha 1-4 glycosidic bands of starch and other polysaccharides to produce several products such as glucose and maltose.7,8 It belongs to the family of GH13 (most of them), GH57, GH119, and GH1269 and is one of the most widely used commercial enzymes.10
Most of alpha-amylases are secreted extracellularly, however some intracellular alpha-amylase have been reported. Regarding the outstanding application of α-amylase, there is an urgent need to develop the cost-effective techniques to produce stable and efficient alpha-amylase.11 Here we aim to present different methods of the amylase production and how it is possible to improve the efficiency of alpha-amylase.
Alpha-amylase production in microbial source
A wide range of organisms, including microorganisms such as aquatic bacteria,12 fungi, actinomycetes, plants, and animals, can produce alpha-amylase.13-15
Regarding the high rate of proliferation and growth, microorganisms are the primary source of alpha-amylase producing a high volume of the enzyme. Also, the genetically manipulated microorganisms are forced to produce alpha-amylase with novel characteristics like thermos-stability.14,16 Additionally, the microorganisms produce large quantity of enzyme, which can be simply optimized by various methods such as response surface methodology.17 The most widely used microorganisms for the production of alpha-amylase include bacteria, actinomycetes, and fungi.15
Several bacteria have been shown are capable of producing a tremendous amount of alpha-amylase for industrial applications, these bacteria include Bacillus amyloliquefaciens, Bacillus licheniformis, and Bacillus stearothermophilus. Some bacteria can produce alpha-amylase in harsh conditions; for instance, some thermophilic bacteria produce alpha-amylase at high temperatures. Most of the starch processing steps, including saccharification, gelatinization, and liquefaction, need high temperature, so the thermostable alpha-amylase is useful to progress the possessing steps in such harsh conditions.18 The most common sources of thermostable α-amylase are Geobacillus bacterium isolated from Manikaran hot springs. The thermophilic alpha-amylases (BLA) have been shown to have more structural flexibility than mesophilic alpha-amylases (BAA). Although the optimum temperature for this enzyme is 80°C, there is an essential need for amylases resistant to other harsh conditions, especially in the industrial process.19-21 The halophilic α-amylase tolerates saline and high-temperature conditions.14 Additionally, this enzyme is resistant to organic solvents and keep its activity at low-water conditions. One of the halophilic bacterial sources of alpha-amylase is Nesterenkonia sp. strain F, which produces enzymes with catalytic activity even in the organic solvents such as chloroform, benzene, cyclohexane, and toluene.22 Due to acidic residues on the surface of halophilic alpha-amylase, this enzyme is stable at low water conditions.23-25
Other types of bacteria can remain alive in low temperatures, such as marine bacteria, and produce cold-active enzymes. Cold-active alpha-amylase is widely used in industry for saving energy26; for example, in detergent contents, it is not needed to heat water for washing and also cold water help to protect clothes’ color. In the backing process, cold-stable amylase efficiently reduces the time of dough fermentation and improves bread quality. Pseudoalteromonas sp. M175 has been isolated from Antarctic sea ice, which is a common source of cold-stable alpha-amylase.27 The microorganisms produce cold-active amylases that have a flexible polypeptide chain to make an easier accommodation of substrates at the low-temperature condition. Also, the enzymes contain lipid composition to maintain greater membrane fluidity.28
Also, some types of actinomycetes such as Nocardiopsis aegyptia can produce cold-adapted enzymes.15 Actinomycetes, such as Streptomyces fragilis DA7-7 produce thermostable alpha-amylase.29
Other microbial sources of alpha-amylase for commercial purposes include Aspergillus niger, Aspergillus awamori, and Aspergillus oryzae.14 Due to the extracellular secretion of alpha-amylase that is easily isolatable from the microbial culture medium, fungi can be a good source for α-amylase production in the industry.30 Also, other fungi species have been reported to possess some unique features making them suitable for industrial goals; for example, an alpha-amylase produced by Aspergillus flavus NSH9 is thermally stable at 50°C.31 Table 1 shows different microbial sources for alpha-amylase production.
Table 1. Microbial sources for alpha amylase production .
| Source | Microbial type | Feature of alpha-amylase |
| Bacillus stearothermophilu s | Bacteria | Thermophile alpha-amylase |
| Geobacillus bacterium | Bacteria | Thermophile alpha-amylase |
| Nesterenkonia sp. strain F | Bacteria | Halophilic enzyme |
| Bacterium Pseudoalteromonas sp. M175 | Bacteria | Cold-active alpha-amylase |
| Nocardiopsis aegypt ia | Actinomycetes | Cold-active alpha-amylase |
| Streptomyces fragil is | Actinomycetes | Cold-active alpha-amylase |
| Aspergillus nige r | Fungi | Commercial production |
| Aspergillus awamo ri | Fungi | Commercial production |
| Aspergillus oryzae | Fungi | Commercial production |
| Aspergillus flavu s | Fungi | Thermophile alpha-amylase |
Application of alpha-amylase
Alpha-amylase is currently used in a broad array of industrial applications such as the production of ethanol and high fructose corn syrup, food, textile, paper, and detergent industries.32-35 Table 2 shows the most current applications of α-amylase in different industries.
Table 2. Industrial applications of α-amylase .
| Industry | Application | Further explanation | Microorganism |
| Detergent (laundry and dish wash) | Starch stain removal | Detergents containing chemicals don't break down easily in waste-water and cause pollution and eutrophication in the rivers/water bodies so the same may be replaced by enzyme which enhances the detergents ability to remove tough stains and making the detergent environmentally safe. The targeted benefit of enzyme inclusion in detergents is due to much milder conditions; 90% of all liquid detergents contain α-amylase. These enzymes degrade the residues of starchy foods | Bacillus orAspergillus (Amylases with activity at lower temperatures, alkaline pH, and oxidative conditions) |
| Starch liquefaction | Dispersion of insoluble starch granules in aqueous solution and decreasing viscosity followed by partial hydrolysis and | This is the most widespread applications of α-amylases (thermostable) which are used for starch hydrolysis into starch hydrolysates such as glucose and fructose. Because of their high sweetening property, these are used in huge quantities in the beverage industry as sweeteners for soft drinks. Liquefaction process causes reduction in viscosity and partial hydrolysis of starch thus avoided during subsequent cooling. |
Bacillus species (thermostable) amyloliquefaciens ; Bacillus stearothermophilus or Bacillus licheniformis |
| Fuel alcohol production | Starch liquefaction and saccharification | Ethanol has been used as fuel a since the early days of the automobile. Ethanol is a significant product of 21st century with its versatile usages and widely consumption across the globe. For the ethanol production, starch is the most used substrate due to its low price and easily available raw material. In this production, starch has to be solubilized and then submitted to two enzymatic steps in order to obtain fermentable sugars. The bioconversion of starch into ethanol involves liquefaction and saccharification, where starch is converted into sugar using an amylolytic microorganism or enzymes such as α-amylase, followed by fermentation, where sugar is converted into ethanol | As yeast Saccharomyces cerevisiae |
| Food industry | Bread softness and volume, flour adjustment; Juice treatment, low calorie beer | Wide applications of α-amylases in food industry include baking, brewing, starch liquefaction as well as a digestive aid (5). They are widely used in baking industry as flavour enhancement and antistaling agent to improve bread quality. α-amylases converts starch to smaller dextrins, which are subsequently fermented by the yeast. improves the taste, crust colour and toasting qualities of the bread; Amylases are also used for the clarification of beer or fruit juices, or for the pretreatment of animal feed to improve the digestibility of fiber | Thermostable maltogenic amylase of Bacillus stearothermophilus |
| Textile industry | De-sizing | Modern production processes for textiles introduce a considerable strain on the warp during weaving. The yarn must, therefore, be prevented from breaking. For this purpose a removable protective layer is applied to the threads. Sizing agents like starch are applied to yarn before fabric production to ensure a fast and secure weaving process. Starch is later removed from the woven fabric in a wet-process in the textile finishing industry. The α-amylases remove selectively the size and do not attack the fibers | Bacillus stain |
| Pulp and paper | De-sizing; Starch-coating, de-inking, drainage improvement | As for textiles, sizing of paper is performed to protect the paper against mechanical damage during processing. It also improves the quality of the finished paper. The size enhances the stiffness and strength in paper. It also improves the erasibilty and is a good coating for the paper. |
- |
Alpha-amylase production
Starch
Starch is known as a carbon source and the main substrate of alpha-amylase, which is comprised of two parts, amylose (25–30%) and amylopectin (70–75%).36,37 Amylose contains glucose monomers that are linked to each other via α (1-4) glycosidic bands and its molecular weight spans 1 × 105– 1×106Da. The other polymer is amylopectin, which is polymerized by α(1-4) glycosidic bands and is branched by α(1-6) glycosidic bands with the molecular weight about 1×107- 1×109 Da.38
According to the digestibility features, starch is divided into three main groups, including rapidly digestible starch (RDS), slowly digestible starch, and resistant starch (RS).39 The most current RDS are used in the foods and gelatinized waxy. RDS in the digestive system is rapidly degraded (20 minutes) into glucose units and so elevates the blood glucose rapidly.10
RS is very resistant against digestive mechanisms because of their low glycemic index and these starches are used mainly by bacteria in the colon to generate short-chain fatty acids, which is essential for human health. RS consists of five different types, including encapsulated starch, resistant granules, retrograded amylose, chemically modified starch, and amylose-lipid complex.37
Production method
Isolated microbial strains used in starch processing must be able to produce the enzyme on the industrial scale. To produce alpha-amylase on the industrial scale, submerged fermentation (SMF), and solid state fermentation (SSF) are frequently utilized. SMF is used to produce bio-products from broth medium such as molasses. This method requires remarkable moisture that is crucial for the growth of microorganisms (mostly bacteria) in the medium to produce alpha-amylase.14 This high moisture also provides easily applicable processes for sterilization, production, purification, controllable temperature, nutrient, pH, and etc; for instance, amylase production as a microbial source using Bacillus sp. 40
SSF is used for the production of alpha-amylase from easily recycled waste materials such as paper. This method needs low moisture, which can be regarded as an advantage. Further advantages of SSF include more straightforward equipment, more production, and less effluent production. However, SSF is slower than SMF in utilizing of substrates by microorganisms. Therefore, SSF is the most common method for the production of alpha-amylase.14 Bacillus thuringiensis and Bacillus cereus have been frequently used in co-culture condition to produce alpha-amylase through SSF.41
Enzyme activity assay
The digestive activity of alpha-amylases is measured via several colorimetric methods, including dinitrosalicylic acid method (DNS), Nelson–Somogyi, and Iodine method.42 DNS is an alkaline reagent that attaches to the reducing sugars and then color changes can be detected by UV absorbance at 540 nm43 (Figure 1). Amylases, xyloglucanases, pectinases, and β-mannanases are assessed by DNS method. A Drawback of this method is the lack of information on its stoichiometric properties with oligosaccharides44 (Figure 1).
Figure 1.
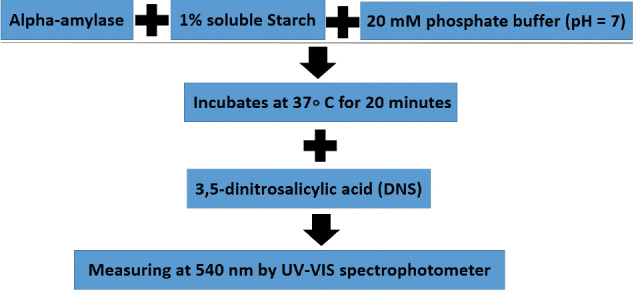
Assaying of alpha-amylase activity by DNS method.
In iodine method, Lugol interacts with starch and forms complexes and alpha-amylase degrades starches and reduces UV absorbance at 580 nm.31 Figure 2 shows measurement of the alpha-amylase activity by iodine method. Also, While Nelson – Somogyi method is used for measuring of α-amylase activity. This method is 10 times more sensitive than DNS method.44 DNS method process is described in Figure 3.
Figure 2.
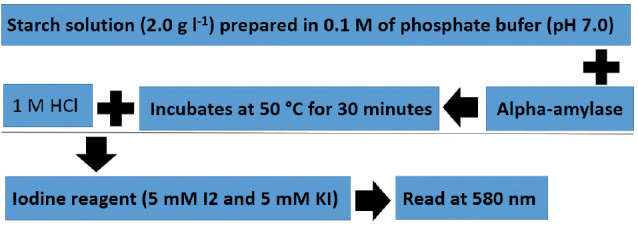
Assaying of alpha-amylase activity by iodine method.
Figure 3.
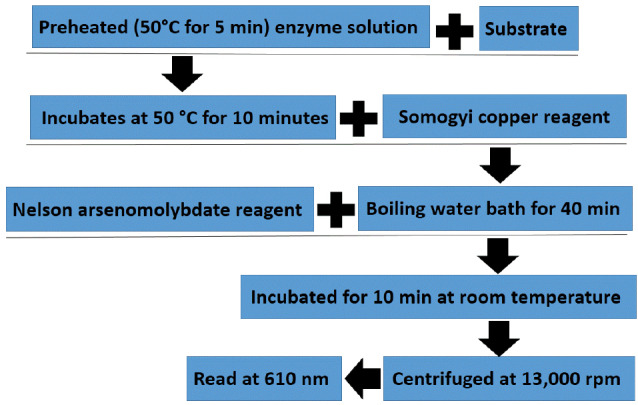
Assaying of alpha-amylase activity by Nelson-Somogyi method.
Medium composition factors
The condition of the culture medium must be regarded for enzyme production. Medium composition and physical conditions can directly affect the production of alpha-amylase. Different factors have been shown to affect enzyme production, here these factors are presented.
Carbon source
Some of the most known substrates as a carbon source for microorganisms to produce alpha-amylase include maltose, glucose, and sucrose. A study on Aspergillus oryzae S2 showed that the proper concentration of starch (10%) are the best carbon source to produce alpha-amylase.45 Penicillium notatum IBGE 03 is another fungus that is used for alpha-amylase production; this fungus uses molasses as its favorable carbon source.42 Bacillus subtilis as another microorganism is used for the production of alpha-amylase in SSF and it needs glucose for optimized production of alpha-amylase is 0.02 g/g.46 Another study on Bacillus family showed that the glucose concentration for optimum production of alpha-amylase by Bacillus amyloliquefaciens was 10.50 g/L.47
pH optimization
pH plays a significant role in the production and secretion of alpha-amylase. Microorganisms need an appropriate growth condition for production of amylase, for example, most fungi grow in the light acidic condition; however, bacteria need a neutral pH (around pH 7).48
Kluyveromyces marxianus IF0 0288 at pH 6.13 produces the highest quantity of alpha-amylase.49 The optimized pH for the production of alpha-amylase by Penicillium notatum IBGE 03 has been reported to be 5.5.42 Also, Bacillus sp. MB6 produces a remarkable quantity of alpha-amylase at pH 6.50
Nitrogen source
As nitrogen contents in culture medium have a significant role in the growth of microorganisms. Different nitrogen sources have been widely studied for the optimization of alpha-amylase production, including the organics such as yeast extract, soybean, and peptone, which are the most applicable nitrogen sources in culture medium; other sources are the inorganics, such as ammonium hydrogen phosphate, ammonium sulfate, and ammonium chloride.14
Bacillus amyloliquefaciens KCP2 produces a significant amount of alpha-amylase using ammonium sulfate (0.2 g) as an inorganic nitrogen source under SSF.51 Also, Bacillus amyloliquefaciens has been shown to use yeast extract (2 g/L), as an organic nitrogen source to produce alpha-amylase.52 A study on Penicillium notatum IBGE 03 used corn steep as the organic nitrogen source to optimize alpha-amylase production.42 Soybean has been used as a source of nitrogen by Aspergillus oryzae CBS 819.72, which produced alpha-amylase on optimized condition.36
Metal ions
Ca2+ ions due to presence in alpha-amylase structure play an important role in alpha-amylase production; In most culture media, calcium chloride (CaCl2) is added to produce alpha-amylase.48
Bacillus amyloliquefaciens has been shown to use CaCl2 (0.0275 M) as a crucial factor to produce alpha-amylase.53 Also, further study by Zhao et al showed that CaCl2 (2 g/L) plays an important role in the production of alpha-amylase by Bacillus amyloliquefaciens.52 Penicillium sp., another microbial source is remarkably dependent on CaCl2 to produce high levels of alpha-amylase.54
Enzyme optimization methods
Hydrolytic enzymes contribute to global business so there is an essential need for optimization of these enzymes. Some methods have been developed for optimization of these enzymes, including response surface methodology55 and Taguchi methods.
Response surface methodology
Due to the vital role for the optimization of factors and developing a novel experiment, RSM is considered as an important part of the experimental design.52 RSM is comprised of statistical and mathematical techniques consisting of two methods for optimization, including Box–Behnken designs and Central Composite Design (CCD).56 Minitab® 16.1.0 is the most commonly used software for the optimization of amylase production by Enterococcus faecium DMF78.57 On the other hand, design expert (version 8.0) is frequently utilized to improve the alpha-amylase production from thermostable and alklophilic alpha-amylase from Bacillus amyloliquefaciens KCP2.51
Box–Behnken designs:The Box–Behnken designs have been used for RSM experiment to enhance alpha-amylase production from Aspergillus oryzae S2, Aspergillus oryzae CBS 819.72, Bacillus laterosporus, and Bacillus amyloliquefaciens36,45,52,58 (Figure 4).
Figure 4.
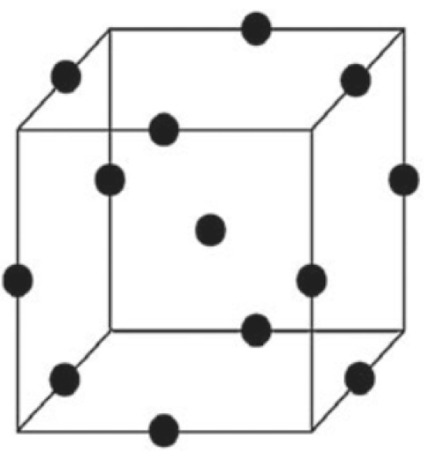
Schemes of Box–Behnken design.
CCD: Figure 5 shows CCD.Bacillus amyloliquefaciens , Streptomyces erumpens MTCC 731, Bacillus amyloliquefaciens KCP2, and Aspergillus oryzae S2 synthesize alpha-amylase by CCD method.51,59-61
Figure 5.
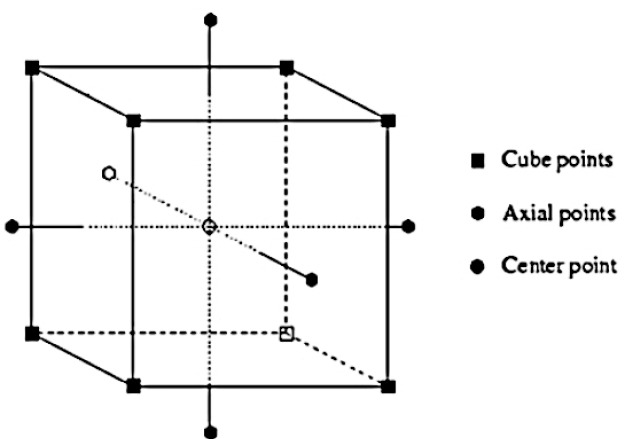
Schemes of Central Composite Design (CCD).
Purification
Purification of the produced enzyme may include the main part of the enzyme production cost, in particular when there is a need for stringent purification. There are different methods of enzyme purification; however, selecting the final method is dependent on the market, cost, final quality, and available technology.62
Mostly, after primary isolation via precipitation or membrane separations, enzymes are purified by chromatographic methods.11,63,64 Regarding the needs for large-scale production of enzyme, the purification methods have been improved to provide higher efficiency, cost-effectiveness, and faster purification in less processing steps.65 Table 3 shows several purification methods for amylase in bacteria.
Table 3. Purification methods for alpha amylase .
| Microorganism | Method of purification | Yield % |
| Thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. Isolate ANT-6 | Partial purification by ethanol precipitation | - |
| Marine Vibrio sp. | Substitute affinity method by insoluble corn stratch | 78 |
| Bacillus subtilis | High-speed counter-current chromatography | 73 |
| Bacillus amyloliquefaciens | Primary precipitation by ammonium sulphate then final purification by ion exchange chromatography | - |
| Thermostable α -amylase from Bacillus sp. PS-7 | Partial purification with ammonium sulphate and further purification by Sephadex G-75 gelfiltration followed by phenyl agarose 4XL hydrophobic interaction chromatography. | - |
| Bacillus amyloliquefaciens | Affinity precipitation by alginate as the affinity matrix. | 96 |
| - | Affinity adsorption chromatography | 95 |
| - | Expanded bed chromatography | 69 |
Stability of alpha-amylase
Industrial enzymes should be stable in harsh conditions. One of the industrial enzymes is alpha-amylase, which needs to be stable against high temperatures, pH, oxidation, and other conditions that are inevitable during starch hydrolysis. So far, several methods have been utilized to achieve a stable amylase; the first, isolation and screening enzyme for extremophile microorganisms such as thermophile bacteria and genetically manipulating of other microorganisms; the second, improving enzyme stability via protein engineering, enzyme immobilization and adding of additives.66
Enzyme recycling maybe regarded as a strategy to save the enzyme. The limitation of recycling of the enzyme was solved by immobilization method.67 Also, immobilization provides higher stability, and continuous operation, and it is suitable for several various engineering designs and improves control of reaction.68 Several methods have been used for Immobilization, including entrapment, crosslinking, covalent attachment, encapsulation, and adsorption.66
Due to fungal alpha-amylases recognized as safe status, the alpha-amylases are more preferred in the starch industry but it needs to be more stable and cost-effective. A study by He et al showed increased stability and longer service life of fungal alpha-amylases (AmyA1) via immobilization on magnetic nanoparticles.69
Another method for enhancing enzyme stability is protein engineering, which is classified into two categories; the site-directed mutagenesis and the random mutagenesis. Improving stability by random mutagenesis within the whole length of a gene uses several methods such as UV irradiation, DNA shuffling, chemical mutation, and error-prone PCR.66 Site-directed mutagenesis is an approach to produce new biocatalyst with a higher stability, activity, expiration, and solubility compared with wild protein. Appropriate amino acid residues are selected and manipulated, is the leading characteristic of the site-directed mutagenesis.70
A useful effect of mutagenesis on the stability of amylase was shown by Wang et al. Most of the Alpha-amylases are active in natural pH. Due to acidic pH of native starch slurry (3.2-45) deactivates the enzyme during starch processing, starch slurry must be adjusted to natural pH. A recent report on alpha-amylase production by Bacillus subtilis showed that the mutated alpha-amylase (in the active site) is more active in acidic condition.71
Another method for improving the alpha-amylase stability is chemical modification.72 In the starch industry, high temperatures are required for synthesis of the product, which inactivates alpha-amylase. Siddiqui et al reported that the modification of carboxyl groups of TAA by L-arginine methyl ester dihydrochloride may improve the hydrolytic activity of alpha-amylase at 60°C.73
Water is crucial for alpha-amylase activity; on the other hand, water can lead to denaturation of the enzyme. some stabilizing agents such as sugars or polyols can be added to alpha-amylase in order to change water structure and improve hydrophobic interaction strength in the enzyme structure.66,74 Samborska et al reported that sucrose, trehalose, and polyols can have a remarkable stabilizing effect on alpha-amylase at thermophilic condition.75
Conclusion
The production of alpha-amylase at a large scale are crucial for industrial goals. Therefore, finding microorganisms with such potential to produce a high quantity of amylase is an important goal of the scientists. Some microorganisms from special environments such as saline soil can be used to achieve such goal. Also according to the microorganism sources, some types of amylases can be used to save energy via the development of the cold-active alpha-amylase. Also, protein engineering methods can be applied to produce enzymes with remarkable stability.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare that there is no conflict of interest.
Acknowledgments
This project (Ph.D.thesis) is financially supported by Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 58056).
References
- 1.Tarhriz V, Akbari Z, Dilmaghani A, Hamidi A, Hejazi MA, Hejazi MS. Bioreduction of iron and biosorption of heavy metals (Ni 2+, Co 2+, Pb 2+) by a novel environmental bacterium, Tabrizicola aquatica RCRI19 t. Asian J Water Environ Pollut. 2019;16(3):73–81. doi: 10.3233/AJW190035. [DOI] [Google Scholar]
- 2.Chapeland-Leclerc F, Dilmaghani A, Ez-Zaki L, Boisnard S, Da Silva B, Gaslonde T. et al. Systematic gene deletion and functional characterization of histidine kinase phosphorelay receptors (HKRs) in the human pathogenic fungus Aspergillus fumigatus. Fungal Genet Biol. 2015;84:1–11. doi: 10.1016/j.fgb.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Dafe A, Etemadi H, Dilmaghani A, Mahdavinia GR. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int J Biol Macromol. 2017;97:536–43. doi: 10.1016/j.ijbiomac.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 4.Tarhriz V, Eyvazi S, Shakeri E, Hejazi MS, Dilmaghani A. Antibacterial and antifungal activity of novel freshwater bacterium Tabrizicola aquatica as a prominent natural antibiotic available in Qurugöl Lake. Pharm Sci. 2020;26(1):88–92. doi: 10.34172/ps.2019.56. [DOI] [Google Scholar]
- 5. Prasad NK. Enzyme Technology: Pacemaker of Biotechnology. New Delhi: PHI Learning Pvt Ltd; 2011.
- 6.Tarhriz V, Hamidi A, Rahimi E, Eramabadi M, Eramabadi P, Yahaghi E. et al. Isolation and characterization of naphthalene-degradation bacteria from Qurugöl Lake located at Azerbaijan. Biosci Biotech Res Asia. 2014;11(2):715–22. doi: 10.13005/bbra/1326. [DOI] [Google Scholar]
- 7.Avwioroko OJ, Anigboro AA, Unachukwu NN, Tonukari NJ. Isolation, identification and in silico analysis of alpha-amylase gene of Aspergillus niger strain CSA35 obtained from cassava undergoing spoilage. Biochem Biophys Rep. 2018;14:35–42. doi: 10.1016/j.bbrep.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delkash-Roudsari S, Zibaee A, Abbasi Mozhdehi MR. Digestive alpha-amylase of Bacterocera oleae Gmelin (Diptera: Tephritidae): biochemical characterization and effect of proteinaceous inhibitor. J King Saud Univ Sci. 2014;26(1):53–8. doi: 10.1016/j.jksus.2013.05.003. [DOI] [Google Scholar]
- 9.Ficko-Blean E, Stuart CP, Boraston AB. Structural analysis of CPF_2247, a novel alpha-amylase from Clostridium perfringens. Proteins. 2011;79(10):2771–7. doi: 10.1002/prot.23116. [DOI] [PubMed] [Google Scholar]
- 10.Tanyildizi MS, Özer D, Elibol M. Optimization of alpha-amylase production by Bacillus sp using response surface methodology. Process Biochem. 2005;40(7):2291–6. doi: 10.1016/j.procbio.2004.06.018. [DOI] [Google Scholar]
- 11.Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp isolate ANT-6. Process Biochem. 2003;38(10):1397–403. doi: 10.1016/S0032-9592(03)00037-2. [DOI] [Google Scholar]
- 12.Tarhriz V, Mohammadzadeh F, Hejazi MS, Nematzadeh G, Rahimi E. Isolation and characterization of some aquatic bacteria from Qurugöl Lake in Azerbaijan under aerobic conditions. Adv Environ Biol. 2011;5(10):3173–8. [Google Scholar]
- 13.Oboh G. Isolation and characterization of amylase from fermented cassava (Manihot esculenta Crantz) wastewater. Afr J Biotechnol. 2005;4(10):1117–23. [Google Scholar]
- 14.Sundarram A, Murthy TP. Alpha-amylase production and applications: a review. J Appl Environ Microbiol. 2014;2(4):166–75. doi: 10.12691/jaem-2-4-10. [DOI] [Google Scholar]
- 15.Abou-Elela G, El-Sersy NA, Wefky SH. Statistical optimization of cold adapted alpha-amylase production by free and immobilized cells of Nocardiopsis aegyptia. J Appl Sci Res. 2009;5(3):286–92. [Google Scholar]
- 16.Konsoula Z, Liakopoulou-Kyriakides M. Co-production of alpha-amylase and beta-galactosidase by Bacillus subtilis in complex organic substrates. Bioresour Technol. 2007;98(1):150–7. doi: 10.1016/j.biortech.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Kizhakedathil MPJ, Chandrasekaran SD. Media optimization for extracellular amylase production by Pseudomonas balearica vitps19 using response surface methodology. Front Biol. 2018;13(2):123–9. doi: 10.1007/s11515-018-1485-3. [DOI] [Google Scholar]
- 18.Gazali FM, Suwastika IN. Thermostable α-amylase activity from thermophilic bacteria isolated from Bora Hot Spring, Central Sulawesi. Journal of Physics: Conference Series. 2018;979:012001. doi: 10.1088/1742-6596/979/1/012001. [DOI] [Google Scholar]
- 19.Yuuki T, Nomura T, Tezuka H, Tsuboi A, Yamagata H, Tsukagoshi N. et al. Complete nucleotide sequence of a gene coding for heat- and pH-stable alpha-amylase of Bacillus licheniformis: comparison of the amino acid sequences of three bacterial liquefying alpha-amylases deduced from the DNA sequences. J Biochem. 1985;98(5):1147–56. doi: 10.1093/oxfordjournals.jbchem.a135381. [DOI] [PubMed] [Google Scholar]
- 20.Sudan SK, Kumar N, Kaur I, Sahni G. Production, purification and characterization of raw starch hydrolyzing thermostable acidic alpha-amylase from hot springs, India. Int J Biol Macromol. 2018;117:831–9. doi: 10.1016/j.ijbiomac.2018.05.231. [DOI] [PubMed] [Google Scholar]
- 21.Prakash O, Jaiswal N. Alpha-amylase: an ideal representative of thermostable enzymes. Appl Biochem Biotechnol. 2010;160(8):2401–14. doi: 10.1007/s12010-009-8735-4. [DOI] [PubMed] [Google Scholar]
- 22.Shafiei M, Ziaee AA, Amoozegar MA. Purification and characterization of an organic-solvent-tolerant halophilic alpha-amylase from the moderately halophilic Nesterenkonia sp strain F. J Ind Microbiol Biotechnol. 2011;38(2):275–81. doi: 10.1007/s10295-010-0770-1. [DOI] [PubMed] [Google Scholar]
- 23.Madern D, Ebel C, Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4(2):91–8. doi: 10.1007/s007920050142. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi S, Yoshimune K, Wakayama M, Moriguchi M, Nishikawa K. Unique amino acid composition of proteins in halophilic bacteria. J Mol Biol. 2003;327(2):347–57. doi: 10.1016/s0022-2836(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 25.Bieger B, Essen LO, Oesterhelt D. Crystal structure of halophilic dodecin: a novel, dodecameric flavin binding protein from Halobacterium salinarum. Structure. 2003;11(4):375–85. doi: 10.1016/s0969-2126(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 26.Dou S, Chi N, Zhou X, Zhang Q, Pang F, Xiu Z. Molecular cloning, expression, and biochemical characterization of a novel cold-active alpha-amylase from Bacillus sp dsh19-1. Extremophiles. 2018;22(5):739–49. doi: 10.1007/s00792-018-1034-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Kan G, Ren X, Yu G, Shi C, Xie Q. et al. Molecular cloning and characterization of a novel alpha-amylase from antarctic sea ice bacterium Pseudoalteromonas sp M175 and its primary application in detergent. Biomed Res Int. 2018;2018:3258383. doi: 10.1155/2018/3258383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuddus M, Roohi Roohi, Arif JM, Ramteke PW. An overview of cold-active microbial alpha-amylase: adaptation strategies and biotechnological potentials. Biotechnology. 2011;10(3):246–58. doi: 10.3923/biotech.2011.246.258. [DOI] [Google Scholar]
- 29.Nithya K, Muthukumar C, Kadaikunnan S, Alharbi NS, Khaled JM, Dhanasekaran D. Purification, characterization, and statistical optimization of a thermostable alpha-amylase from desert actinobacterium Streptomyces fragilis DA7-7. 3 Biotech. 2017;7(5):350. doi: 10.1007/s13205-017-0981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Gautam N, Modi DR. Optimization of alpha-amylase production from free and immobilized cells of Aspergillus niger. J Biotechnol Pharm Res. 2010;1(1):1–8. [Google Scholar]
- 31.Karim KMR, Husaini A, Sing NN, Sinang FM, Roslan HA, Hussain H. Purification of an alpha amylase from Aspergillus flavus NSH9 and molecular characterization of its nucleotide gene sequence. 3 Biotech. 2018;8(4):204. doi: 10.1007/s13205-018-1225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza PM, de Oliveira Magalhaes P. Application of microbial alpha-amylase in industry - a review. Braz J Microbiol. 2010;41(4):850–61. doi: 10.1590/s1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El-Fallal A, Abou-Dobara MA, El-Sayed A, Omar N. Starch and microbial alpha-amylase: from concepts to biotechnological applications. In: Chang CF, ed. Carbohydrates: Comprehensive Studies on Glycobiology and Glycotechnology. Croatia: InTech; 2012. p. 459-89. 10.5772/51571 [DOI]
- 34.Rana N, Walia A, Gaur A. Alpha-amylases from microbial sources and its potential applications in various industries. Natl Acad Sci Lett. 2013;36(1):9–17. doi: 10.1007/s40009-012-0104-0. [DOI] [Google Scholar]
- 35. Demirci A, Izmirlioglu G, Ercan D. Fermentation and enzyme technologies in food processing. In: Clark S, Jung S, Lamsal B, eds. Food Processing: Principles and Applications. 2nd ed. Chichester, West Sussex, UK: Wiley-Blackwell; 2014. p. 107-36. 10.1002/9781118846315.ch6 [DOI]
- 36.Kammoun R, Naili B, Bejar S. Application of a statistical design to the optimization of parameters and culture medium for alpha-amylase production by Aspergillus oryzae CBS 81972 grown on gruel (wheat grinding by-product) Bioresour Technol. 2008;99(13):5602–9. doi: 10.1016/j.biortech.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Andersson M, Andersson R. Resistant starch and other dietary fiber components in tubers from a high-amylose potato. Food Chem. 2018;251:58–63. doi: 10.1016/j.foodchem.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Lee BH, Hamaker BR. Number of branch points in alpha-limit dextrins impact glucose generation rates by mammalian mucosal alpha-glucosidases. Carbohydr Polym. 2017;157:207–13. doi: 10.1016/j.carbpol.2016.09.088. [DOI] [PubMed] [Google Scholar]
- 39.Richardson TH, Tan X, Frey G, Callen W, Cabell M, Lam D. et al. A novel, high performance enzyme for starch liquefaction Discovery and optimization of a low pH, thermostable alpha-amylase. J Biol Chem. 2002;277(29):26501–7. doi: 10.1074/jbc.M203183200. [DOI] [PubMed] [Google Scholar]
- 40.Vidyalakshmi R, Paranthaman R, Indhumathi J. Amylase production on submerged fermentation by Bacillus spp. World J Chem. 2009;4(1):89–91. [Google Scholar]
- 41.Abdullah R, Naeem N, Aftab M, Kaleem A, Iqtedar M, Iftikhar T. et al. Enhanced production of alpha amylase by exploiting novel bacterial co-culture technique employing solid state fermentation. Iran J Sci Technol Trans A Sci. 2018;42(2):305–12. doi: 10.1007/s40995-016-0015-x. [DOI] [Google Scholar]
- 42.Ahmed K, Munawar S, Khan MA. Cultural conditions for maximum alpha-amylase production by Penicillium notatum IBGE 03 using shaken flask technique of submerged fermentation. Pure Appl Biol. 2015;4(3):306–12. doi: 10.19045/bspab.2015.43005. [DOI] [Google Scholar]
- 43.Raul D, Biswas T, Mukhopadhyay S, Kumar Das S, Gupta S. Production and partial purification of alpha amylase from Bacillus subtilis (MTCC 121) using solid state fermentation. Biochem Res Int. 2014;2014:568141. doi: 10.1155/2014/568141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gusakov AV, Kondratyeva EG, Sinitsyn AP. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem. 2011;2011:283658. doi: 10.1155/2011/283658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naili B, Sahnoun M, Bejar S, Kammoun R. Optimization of submerged Aspergillus oryzae S2 alpha-amylase production. Food Sci Biotechnol. 2016;25(1):185–92. doi: 10.1007/s10068-016-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan H, Karim KA. Optimization of alpha amylase production from rice straw using solid-state fermentation of Bacillus subtilis. Int J Sci Environ Technol. 2015;4(1):1–16. [Google Scholar]
- 47.Uygut MA, Tanyildizi MŞ. Optimization of alpha-amylase production by Bacillus amyloliquefaciens grown on orange peels. Iran J Sci Technol Trans A Sci. 2018;42(2):443–9. doi: 10.1007/s40995-016-0077-9. [DOI] [Google Scholar]
- 48.Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A. Alpha-amylases from microbial sources–an overview on recent developments. Food Technol Biotechnol. 2006;44(2):173–84. [Google Scholar]
- 49.Stergiou P-Y, Foukis A, Theodorou L, Papagianni M, Papamichael E. Optimization of the production of extracellular alpha-amylase by Kluyveromyces marxianus IF0 0288 by response surface methodology. Braz Arch Biol Technol. 2014;57(3):421–6. doi: 10.1590/S1516-8913201401485. [DOI] [Google Scholar]
- 50.Paul JS, Lall BM, Jadhav SK, Tiwari KL. Parameter’s optimization and kinetics study of alpha-amylase enzyme of Bacillus sp MB6 isolated from vegetable waste. Process Biochem. 2017;52:123–9. doi: 10.1016/j.procbio.2016.10.005. [DOI] [Google Scholar]
- 51.Prajapati VS, Trivedi UB, Patel KC. A statistical approach for the production of thermostable and alklophilic alpha-amylase from Bacillus amyloliquefaciens KCP2 under solid-state fermentation. 3 Biotech. 2015;5(2):211–20. doi: 10.1007/s13205-014-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao W, Zheng J, Wang YG, Zhou HB. A marked enhancement in production of amylase by Bacillus amyloliquefaciens in flask fermentation using statistical methods. J Cent South Univ Technol. 2011;18(4):1054–62. doi: 10.1007/s11771-011-0803-6. [DOI] [Google Scholar]
- 53.Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, Sukumaran RK, Pandey A. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour Technol. 2008;99(11):4597–602. doi: 10.1016/j.biortech.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 54.Abdullah R, Nadeem S, Iqtedar M, Kaleem A, Iftikhar T, Naz S. Influence of growth conditions on enhanced production of alpha amylase from Penicillium species in solid state fermentation. Indian J Biotechnol. 2017;16(3):426–32. [Google Scholar]
- 55.Magro M, Nauta S, Simsek C, Onuma Y, Garg S, van der Heide E. et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: the MI SYNTAXscore study. Am Heart J. 2011;161(4):771–81. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Khuri AI, Mukhopadhyay S. Response surface methodology. Wiley Interdiscip Rev Comput Stat. 2010;2(2):128–49. doi: 10.1002/wics.73. [DOI] [Google Scholar]
- 57.Duque SM, Dizon EI, Merca FE, Flores DM. Optimization of raw-starch-digesting amylase (RSDA) production medium for Enterococcus faecium DMF78. Int Food Res J. 2016;23(3):1280–8. [Google Scholar]
- 58.Kumar NM, Karthikeyan S, Jayaraman G. Thermostable alpha-amylase enzyme production from Bacillus laterosporus: statistical optimization, purification and characterization. Biocatal Agric Biotechnol. 2013;2(1):38–44. doi: 10.1016/j.bcab.2012.10.005. [DOI] [Google Scholar]
- 59.Sahnoun M, Kriaa M, Elgharbi F, Ayadi DZ, Bejar S, Kammoun R. Aspergillus oryzae S2 alpha-amylase production under solid state fermentation: optimization of culture conditions. Int J Biol Macromol. 2015;75:73–80. doi: 10.1016/j.ijbiomac.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Tanyildizi MS, Elibol M, Özer D. Optimization of growth medium for the production of alpha-amylase from Bacillus amyloliquefaciens using response surface methodology. J Chem Technol Biotechnol. 2006;81(4):618–22. doi: 10.1002/jctb.1445. [DOI] [Google Scholar]
- 61.Kar S, Datta TK, Ray RC. Optimization of thermostable alpha-amylase production by Streptomyces erumpens MTCC 7317 in solid-state fermentation using cassava fibrous residue. Braz Arch Biol Technol. 2010;53(2):301–9. doi: 10.1590/S1516-89132010000200008. [DOI] [Google Scholar]
- 62.Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101(22):8493–501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- 63. Triveni R. Production, Characterization and Utilization of Gelling Polysaccharide of Bacteria [thesis]. University of Mysore; 2000.
- 64.Coronado M, Vargas C, Hofemeister J, Ventosa A, Nieto JJ. Production and biochemical characterization of an alpha-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett. 2000;183(1):67–71. doi: 10.1111/j.1574-6968.2000.tb08935.x. [DOI] [PubMed] [Google Scholar]
- 65.Amritkar N, Kamat M, Lali A. Expanded bed affinity purification of bacterial alpha-amylase and cellulase on composite substrate analogue–cellulose matrices. Process Biochem. 2004;39(5):565–70. doi: 10.1016/S0032-9592(03)00123-7. [DOI] [Google Scholar]
- 66.Dey TB, Kumar A, Banerjee R, Chandna P, Kuhad RC. Improvement of microbial α-amylase stability: strategic approaches. Process Biochem. 2016;51(10):1380–90. doi: 10.1016/j.procbio.2016.06.021. [DOI] [Google Scholar]
- 67.Talekar S, Pandharbale A, Ladole M, Nadar S, Mulla M, Japhalekar K. et al. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): a tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour Technol. 2013;147:269–75. doi: 10.1016/j.biortech.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 68.Cordeiro AL, Lenk T, Werner C. Immobilization of Bacillus licheniformis alpha-amylase onto reactive polymer films. J Biotechnol. 2011;154(4):216–21. doi: 10.1016/j.jbiotec.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 69.He L, Mao Y, Zhang L, Wang H, Alias SA, Gao B. et al. Functional expression of a novel alpha-amylase from Antarctic psychrotolerant fungus for baking industry and its magnetic immobilization. BMC Biotechnol. 2017;17(1):22. doi: 10.1186/s12896-017-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sindhu R, Binod P, Madhavan A, Beevi US, Mathew AK, Abraham A. et al. Molecular improvements in microbial alpha-amylases for enhanced stability and catalytic efficiency. Bioresour Technol. 2017;245(Pt B):1740–8. doi: 10.1016/j.biortech.2017.04.098. [DOI] [PubMed] [Google Scholar]
- 71.Wang CH, Liu XL, Huang RB, He BF, Zhao MM. Enhanced acidic adaptation of Bacillus subtilis Ca-independent alpha-amylase by rational engineering of pKa values. Biochem Eng J. 2018;139:146–53. doi: 10.1016/j.bej.2018.08.015. [DOI] [Google Scholar]
- 72.Habibi AE, Khajeh K, Nemat-Gorgani M. Chemical modification of lysine residues in Bacillus licheniformis alpha-amylase: conversion of an endo- to an exo-type enzyme. J Biochem Mol Biol. 2004;37(6):642–7. doi: 10.5483/bmbrep.2004.37.6.642. [DOI] [PubMed] [Google Scholar]
- 73.Siddiqui KS, Poljak A, De Francisci D, Guerriero G, Pilak O, Burg D. et al. A chemically modified alpha-amylase with a molten-globule state has entropically driven enhanced thermal stability. Protein Eng Des Sel. 2010;23(10):769–80. doi: 10.1093/protein/gzq051. [DOI] [PubMed] [Google Scholar]
- 74.Khajeh K, Nemat-Gorgani M. Comparative studies on a mesophilic and a thermophilic alpha-amylase. Appl Biochem Biotechnol. 2001;90(1):47–55. doi: 10.1385/ABAB:90:1:47. [DOI] [PubMed] [Google Scholar]
- 75.Samborska K, Guiavarc’h Y, Van Loey A, Hendrickx M. The thermal stability of Aspergillus oryzae alpha‐amylase in presence of sugars and polyols. J Food Process Eng. 2006;29(3):287–303. doi: 10.1111/j.1745-4530.2006.00062.x. [DOI] [Google Scholar]


