Abstract
Objectives
We conducted this study to test the susceptibility of P. aeruginosa to the routinely used drugs and to the two recently available antimicrobial agents, ceftazidime-avibactam and ceftolozane-tazobactam.
Methods
We isolated the non-replicate strains of P. aeruginosa from inpatients between December 2018 and April 2019. The VITEK® MS system was used for phenotypic identification and VITEK 2 for initial antimicrobial susceptibility testing. We supplemented these tests with determination of the minimum inhibitory concentration (MIC) of four antimicrobials; imipenem, meropenem, ceftazidime-avibactam and ceftolozane-tazobactam. The standards of the Clinical and Laboratory Standards Institute were followed.
Results
A total of 67 strains of P. aeruginosa, including 38 multidrug-resistant strains, were obtained from various specimens. Susceptibility to various tested aminoglycosides and fluoroquinolones was maintained in 49.3–56.7% and 40.0–43.3% of the total isolates. Amongst β-lactams, the strains were susceptible to the following agents in an ascending order: ceftazidime (32.8%), cefepime (37.3%), imipenem (36.0%), piperacillin-tazobactam (39.0%), meropenem (44.8%), ceftazidime-avibactam (61.2%) and ceftolozane-tazobactam (62.7%). The susceptibility rates of the multidrug-resistant strains to both ceftazidime-avibactam and ceftolozane-tazobactam were less than 35%. High levels of resistance to the new agents (MIC > 256 ug/ml) were detected in 21 and 22 isolates.
Conclusion
Our study shows limitation in the empirical use of ceftazidime-avibactam and ceftolozane-tazobactam as therapeutics in serious infections. Moreover, our data highlights the need for prompt antimicrobial susceptibility testing to guide their clinical usage.
Keywords: Antimicrobial susceptibility, Ceftazidime-avibactam, Ceftolozane-tazobactam, KSA, Pseudomonas aeruginosa
Abbreviations: blaVIM, Verona imipenemase; CA, Ceftazidime-avibactam; CT, Ceftolozane-tazobactam; HIV, Human immunodeficiency virus; MBL, Metallo-β-Lactamase; MDR, Multidrug-resistant; MIC, Minimal inhibitory concentration; VIM, Verona integron-mediated metallo-β-lactamase
الملخص
أهداف البحث
اختبار حساسية الزائفة الزنجارية للأدوية المستخدمة بشكل روتيني، واثنين من مضادات الميكروبات المتوفرة مؤخرا، سفتازديم افيباكتام وسفتالوزين تازوباكتام اللذين لم يتم تقييمها بعد في المملكة العربية السعودية.
طرق البحث
تم تضمين سلالات غير متكررة من الزائفة الزنجارية المعزولة من المرضى الداخليين بين ديسمبر ٢٠١٨ وأبريل ٢٠١٩. تم التعرف عليها باستخدام نظام مخبري آلي كما تم تحديد النمط الظاهري وإجراء اختبار الحساسية الأولية للميكروب. استكملنا هذه الاختبارات بتحديد الحد الأدنى من التركيز المثبط لأربعة مضادات حيوية للميكروب هي الاميبنم والميروبنم وسفتازديم افيباكتام وسفتالوزين تازوباكتام وفقا لأنظمة معهد المعايير السريرية والمخبرية.
النتائج
تم الحصول على ما مجموعه ٦٧ سلالة من الزائفة الزنجارية، بما في ذلك ٣٨ سلالة مقاومة للأدوية المتعددة، من عينات مختلفة. تم الحفاظ على القابلية للعديد من الأمينوغليكوزيدات والفلوروكينولونات المختبرة. كانت السلالات عرضة للعوامل التالية بترتيب تصاعدي: سفتازديم (٣٢.٨٪)، سيفيبيم (٣٧.٣٪)، اميبنيم (٣٦٪) وبيبراسلين تازوباكتام (٣٩٪) ثم ميروبينيم (٤٤.٨٪) وسفتازديم افيباكتام (٦١،٢٪) وسفتالوزين تازوباكتام (٦٢.٧٪). كانت معدلات حساسية السلالات المقاومة للأدوية لكل من سفتازديم افيباكتام وسفتالوزين تازوباكتام أقل من ٣٥٪ كما تم اكتشاف مستويات عالية من المقاومة للأدوية الجديدة في ٢١ و٢٢ عزلة بكتيرية.
الاستنتاجات
تظهر نتائج الدراسة محدودية الاستخدام التجريبي لسفتازديم افيباكتام وسفتالوزين تازوباكتام كعلاجات للعدوى الخطيرة. علاوة على ذلك، تسلط بياناتنا الضوء على الحاجة إلى اختبار حساسية سريع للميكروبات لتوجيه استخدامها السريري.
الكلمات المفتاحية: الزائفة الزنجارية, سفتازديم افيباكتام, سفتالوزين تازوباكتام, المملكة العربية السعودية
Introduction
Pseudomonas aeruginosa is a recognized nosocomial pathogen worldwide, responsible for infections associated with prolonged hospitalization, increased costs, and mortality.1 In KSA, it has been implicated in causing challenging infections including those in critical care settings.2 The success of an organism as a healthcare-associated pathogen largely relies on its ability to resist multiple drugs via intrinsic and acquired genetic elements, limiting the efficacy of empirical therapy.3 High variability among the resistance rates of P. aeruginosa was observed in various regions in the global Study for Monitoring Antimicrobial Resistance Trends.4 Various chromosome- or plasmid-encoded resistance mechanisms in P. aeruginosa have been described. These elements can exist simultaneously and be carried by organisms on the same transmissible genetic element, thereby conferring multidrug resistance to different classes of antibiotics and limiting therapeutic options. Carbapenem resistance, which originates from the production of β-lactamases, porin mutations, overexpression of the MexA-MexB-OprM efflux pump, and/or alterations in the penicillin-binding protein targets,5 is of particular concern. Combinations of mechanisms that confer resistance to carbapenems may coexist in an organism; however, the organism may still be susceptible to β-lactam agents.6,7 Risk factors for infections with resistant P. aeruginosa strains include prior use of broad-spectrum antimicrobials, diabetes mellitus, recent surgery, presence of invasive devices, bedridden status, and intensive care unit (ICU) admission.8
P. aeruginosa is intrinsically resistant to several broad-spectrum beta-lactam antibiotics including cefotaxime, ceftriaxone, and ertapenem. Anti-pseudomonal agents effective against the wild-type strain include piperacillin, ceftazidime, cefepime, imipenem, and meropenem. However, P. aeruginosa can acquire resistance to these agents, including carbapenems. In KSA, P. aeruginosa resistance to carbapenems has increased from 6 to 9%, as reported in earlier studies, to more than 30%, according to more recent reports.9,10 A large national study (sample size = 6364) conducted in 24 Saudi hospitals in 2009 found that approximately 16% of P. aeruginosa isolates were resistant to a carbapenem.11 Another ICU-based study performed within the same time frame in central KSA showed an incremental rate of P. aeruginosa resistance to carbapenems, from 34% to 74%, over a 5-year period.2 This represents a major clinical challenge, especially in cases of invasive, serious pseudomonal infections.
Recently, new β-lactam/β-lactamase inhibitor combinations, including ceftolozane-tazobactam (CT) and ceftazidime-avibactam (CA), have demonstrated efficacy in vitro and clinical activity against P. aeruginosa.12 Both drug combinations are clinically accessible and licensed for use in complicated cases of urinary tract and abdominal infections. Moreover, these combinations are promising as effective therapies for infection with carbapenem-resistant P. aeruginosa, provided the strains are susceptible to the drug combinations in vitro, as illustrated in a small multicenter, retrospective study.13 These novel drug combinations are also potentially promising as carbapenem-sparing agents and could help slow the emergence and spread of carbapenem resistance.14 A recent randomized clinical trial has shown that ceftolozane-tazobactam was not inferior to meropenem in treating 519 cases of hospital-acquired pneumonia, including 128 cases in which P. aeruginosa was the implicated pathogen.15 The clinical trial, however, was based on the minimum inhibitory concentration (MIC) and treatment was administered only when the in vitro susceptibility status was proven.
There are no reports available based on which the activity of these newly licensed drugs against local P. aeruginosa isolates could be evaluated. Therefore, this study was undertaken to investigate the antimicrobial susceptibility patterns of clinical P. aeruginosa strains from various infection sites in hospitalized patients, including multidrug-resistant (MDR) isolates, to traditional drugs with potential anti-pseudomonal activity and the newer agents CA and CT. In addition, the drug resistance index (DRI) was determined for the isolated strains.
Materials and Methods
Research settings and identification of isolates
We serially collected all non-replicate strains of P. aeruginosa isolated from clinical specimens belonging to patients of all ages who were admitted to King Fahad University Hospital, a 550-bed academic centre, between December 2018 and April 2019. We included samples from infected patients who received antimicrobial therapy based on the clinical picture, while the patients’ surveillance cultures and colonizing organisms were excluded. The specimens were inoculated on blood and MacConkey agar plates (SPML, Riyadh, KSA) and incubated overnight at 35 °C. Suspected colonies, based on colony morphology and catalase- and oxidase–positive reactions, were further tested to confirm their identity using the VITEK MS system (bioMérieux, US) based on matrix-assisted laser desorption/ionization time-of-flight technology. MDR strains were labelled following international consensus guidelines for describing drug-resistant organisms.16 Community and hospital-acquired infections were defined based on the Centers for Disease Control and Prevention (CDC) criteria.17
Antimicrobial susceptibility testing
This was a diagnostic research study. Susceptibility testing was initially performed in the routine microbiology laboratory by using the VITEK 2 system (bioMérieux, US), which is accepted for routine, high-throughput susceptibly testing in diagnostic settings. Data were extracted for agents recommended by the Clinical and Laboratory Standards Institute (CLSI) for anti-pseudomonal therapy, including both group A and B drugs (CLSI 2019).7 Group A drugs (ceftazidime, gentamicin, and piperacillin-tazobactam) represent antimicrobial agents that are considered appropriate for inclusion in a routine, primary testing panel, as well as for routine reporting of results for a specific organism group. Group B drugs (cefepime, amikacin, ciprofloxacin, levofloxacin, CA, CT, imipenem, and meropenem) are agents that can be included in primary testing but are reported only in selective situations, such as when an organism is resistant to agents of the same antimicrobial class in group A. E tests (AB Biodisk, Solna, Sweden) were additionally used to determine the MICs of two carbapenems, imipenem and meropenem, and two β-lactam/β-lactamase inhibitor combinations, CA and CT. The tests were conducted per the CLSI 2019 guidelines for all antimicrobials tested, because the MICs of the agents could influence the selection of therapy in cases of infection with drug-resistant strains. For analytical quality control, the MICs for the P. aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 strains were determined in each test run after a validation study. The results were accepted if values within the MIC reference range for both CT and CA were obtained.
Antimicrobial resistance index
The aggregated antimicrobial DRI for each strain was determined as previously described by calculating the number of antibiotics to which an isolate was resistant and dividing this value by the total number of antibiotics tested for the isolate based on the CLSI 2019 anti-pseudomonal panel.7,18
Statistical analysis
Categorical variables were expressed as absolute frequencies and percentages and continuous variables as median values and ranges. Categorical and continuous variable comparisons were assessed using the Fisher's exact test and Mann–Whitney U test, respectively. Statistical analyses were performed using GraphPad Prism Version 6.0 for Mac. A two-tailed P-value < 0.05 was considered statistically significant.
Results
Characterization of the isolates
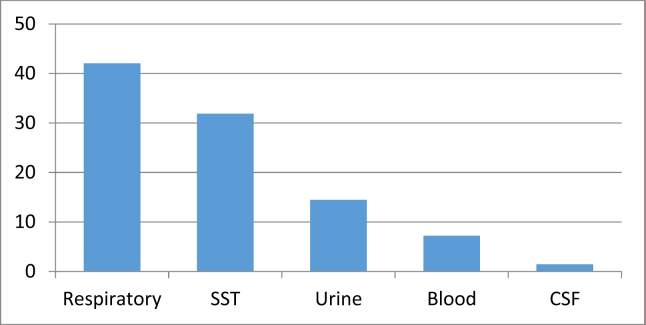
Sixty-seven strains of P. aeruginosa, of which 38 (56.7%) were MDR, were isolated from hospitalized patients. The demographic data of the patients from whom the strains were isolated are summarized in Table 1. Of the 15 (22.4%) community-acquired strains, three were MDR, which were implicated in soft-tissue, respiratory, and urinary tract infections in three patients aged 61, 77, and 91 years. The sample distribution of the total isolates is demonstrated in Figure 1. The most prevalent infections were lower respiratory tract (43%) and soft-tissue (33%) infections.
Table 1.
Characteristics of patients from whom 67 Pseudomonas aeruginosa strains were isolated.
| Epidemiological parameter | Total strains, no. (%) | MDR strains, no (%) | P-value |
|---|---|---|---|
| Sex | 0.2 | ||
| Male | 43 (64.2) | 27 (62.8) | |
| Female | 24 (35.8) | 13 (54.2) | |
| ∗Distribution by age group | 0.0001 | ||
| <15 years | 4 (5.9) | 0 (0) | |
| 15–44 years | 13 (19.4) | 3 (23.1) | |
| 45–64 years | 15 (22.4) | 5 (33.3) | |
| ≥65 years | 35 (40) | 30 (85.7) | |
| Community acquired | 15 (22.4) | 3 (20) | 0.0003 |
| Hospital acquired | 52 (77.6) | 35 (67.3) |
∗The median patient age was 56.5 ± 6.1 years.
The frequency of P. aeruginosa isolation and the occurrence of multidrug resistance show an increasing trend with age (P < 0.05).
MDR: multidrug-resistant.
Figure 1.
Distribution of the sites of 67 Pseudomonas aeruginosa infections. skin and soft tissue infections (SST):; CSF: cerebrospinal fluid.
Antimicrobial susceptibility
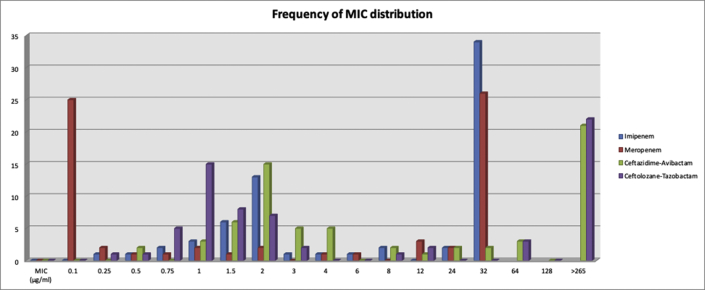
Susceptibility testing results for the commonly tested agents per the CLSI guidelines for all P. aeruginosa and MDR strains are shown in Table 2. All of the 38 strains (100%) that fit the definition of MDR strains were resistant to meropenem. The MICs of four antimicrobial drugs as obtained by the E-test are shown in Table 3 (see Figure 2).
Table 2.
Susceptibility rates of Pseudomonas aeruginosa strains.
| All strains (n = 67) Susceptibility (%) |
MDR strains (n = 38) Susceptibility (%) |
P-value | |
|---|---|---|---|
| Ceftazidime | 32.8 | 0 | 0.0001 |
| Cefepime | 37.3 | 0 | 0.0001 |
| Piperacillin/tazobactam | 39 | 0 | 0.0001 |
| Ciprofloxacin | 43.3 | 5.3 | 0.0004 |
| Levofloxacin | 40 | 2.6 | 0.0001 |
| Gentamicin | 56.7 | 2.6 | 0.0001 |
| Amikacin | 49.3 | 2.6 | 0.0001 |
| Imipenem | 36 | 2.6 | 0.0001 |
| Meropenem | 44.8 | 0 | 0.0001 |
| Ceftazidime-avibactam | 61.2 | 36.8 | 0.52 |
| Ceftolozane-tazobactam | 62.7 | 36.8 | 0.42 |
MDR: multidrug-resistant.
Table 3.
Minimal inhibitory concentrations of four agents against Pseudomonas aeruginosa.
| Antimicrobial drug | All isolates (n = 67) |
MDR (n = 38) |
||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | Resistance rate (%) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | Resistance rate (%) | |
| Imipenem | >32 | >32 | 0.25–32 | 64.0 | >32 | >32 | >32 | 97.4 |
| Meropenem | 1.5 | >32 | 0.1–32 | 55.2 | >32 | >32 | >32 | 100 |
| Ceftazidime-avibactam | 4 | >256 | 0.5 to ≥256 | 38.8 | >256 | >256 | 12 to ≥256 | 63.2 |
| Ceftolozane-tazobactam | 2 | >256 | 0.25 to ≥256 | 37.3 | >256 | >256 | 8 to ≥256 | 63.2 |
The breakpoints for imipenem, meropenem, ceftazidime-avibactam, and ceftolozane-tazobactam are 2, 2, 8, and 4 μg/ml.
MDR: multidrug-resistant; MIC: minimal inhibitory concentration.
Figure 2.
Distribution of the minimal inhibitory concentrations (MICs) of four agents against Pseudomonas aeruginosa (n = 67). The breakpoints for imipenem, meropenem, ceftazidime-avibactam, and ceftolozane-tazobactam are 2, 2, 8, and 4 μg/ml.
Discussion
This study describes the susceptibility patterns of P. aeruginosa strains isolated from inpatients against various antimicrobial agents, including the novel cephalosporin/β-lactamase inhibitor combinations CA and CT. Most of the isolates were obtained from patients with respiratory and soft-tissue infections. P. aeruginosa and MDR strains were more frequently encountered with increasing patient age. Consistent with findings from other studies,19,20 85.7% of the MDR P. aeruginosa strains were isolated from elderly patients (aged >65 years). This finding has implications for the selection of the therapeutic regimen for this age group. In addition, institutions might consider screening this patient population upon admission for MDR organisms, including MDR P. aeruginosa; this is supported by a moderate level of evidence.21 Currently, there are no clear guidelines on the selection of sites for the screening of MDR P. aeruginosa strains, although the best yield is obtained with multiple site screening.22 Most of the MDR strains in this study were hospital acquired. Three cases (20%), however, had a community origin, raising a concern. No previous study in KSA has addressed community-acquired P. aeruginosa infection. The cases detected in this study could have been sources of infection or even an outbreak if they had not been identified. A recent review examined the risk factors for community-acquired respiratory infections with MDR organisms, including P. aeruginosa, and proposed a scoring system to identify them upon admission.23 Such a scoring system, or an equivalent, can be used to help with triaging such cases and implementing effective infection control and treatment strategies.
Ceftazidime is a powerful anti-pseudomonal agent, with strong bactericidal action against most wild-type strains and retained activity against some carbapenem- or fluoroquinolone-resistant isolates.24 The majority of the P. aeruginosa strains tested in our study exhibited resistance to ceftazidime, as well as to cefepime, piperacillin-tazobactam, fluoroquinolones, aminoglycosides, and carbapenems, making empirical therapy difficult and highlighting the importance of rapid antibiotic susceptibility testing.
CA and CT have good coverage for wild-type P. aeruginosa strains. Although these agents were initially licensed for use in cases of complicated intra-abdominal or complicated urinary tract infections, clinical studies are ongoing to support their extended use in other conditions.25,26 We found that less than 35% of the total P. aeruginosa strains were susceptible to these new agents. High levels of resistance to CA and CT (MIC: >256 μg/ml) were detected in a significant proportion of the isolates, which were never exposed to the drugs earlier. Furthermore, the MIC50/90 values for the 67 isolates were >32/>32 μg/ml, 1.5/>32 μg/ml, 4/>256 μg/ml, and 4/>256 μg/ml for imipenem, meropenem, CA, and CT, respectively. The resistance rates to CT and CA in this study were higher than those reported by other groups; however, they were in accordance with those reported in certain other published work, including a recent meta-analysis from Turkey.27, 28, 29 In contrast, studies performed in Asian and East European countries demonstrated that >67% of MDR P. aeruginosa strains maintained susceptibility to CA and CT.30,31 These variable resistance rates for CT and CA may reflect variations in the underlying resistance mechanisms of MDR P. aeruginosa strains that could vary across regions. As expected, the MDR strains demonstrated higher MIC50/90, and none of the four agents can be used for adequate empirical monotherapy if MDR P. aeruginosa is a likely pathogen.
The limited molecular surveillance work conducted thus far has revealed the diverse mechanisms that confer carbapenem resistance in P. aeruginosa isolates from KSA. In those few available studies, Verona imipenemase (blaVIM) was repeatedly described as a leading mechanism underlying the widespread carbapenemases in P. aeruginosa strains in the Gulf Cooperation Council countries, including KSA.32,33 In the United States, more than 90% of MDR P. aeruginosa strains were estimated to be susceptible to both CA and CT.27 However, it is known that novel cephalosporin/β-lactamase inhibitor combinations can overcome resistance to carbapenem and other β-lactams that is mediated by certain mechanisms, for instance by outer membrane porin D downregulation, but not to those encoded by class β metallo-beta-lactamases, as the later are not inhibited by by tazobactam or avibactam.27,34 Ceftolozane is the most active anti-pseudomonal β-lactam to date, and an MIC of >4 ng/ml is limited to those harbouring metallo-β-lactamase (MBL) or other unusual genes such as VEB and GES.35 The high prevalence of MBL-producing P. aeruginosa strains in KSA raises a question about the efficacy of empirical use of CA and CT. In KSA, blaVIM was first detected in 2002 by sequencing the gene from a urine isolate obtained from an HIV-infected patient who presented with a complicated urinary tract infection following admission.36 Since then, few reports have identified blaVIM-like genes during the screening of clinical P. aeruginosa isolates from various regions of KSA.32,33 These data suggest that MBLs are commonly associated with carbapenem resistance among P. aeruginosa strains in KSA with a high prevalence of the VIM type, owing to which the therapeutic options for MDR P. aeruginosa are very limited. The gene is usually carried on mobile gene cassettes inserted into class 1 integrons that are embedded in resistant plasmids and conjugative transposons.36 Since molecular characterization is not routinely performed in diagnostic settings because of the absence of commercial kits for carbapenemase detection in P. aeruginosa, caution needs to be exercised during the routine use of CT and CA in a formulary without prior surveillance in an institution. Another consideration is the well-known emergence of resistance during the treatment of P. aeruginosa infections despite the initial susceptibility. This is encountered in approximately 10% of the infections caused by the organism, with subsequent increased morbidity and mortality along with higher hospitalization costs. This phenomenon, however, is associated more with imipenem use.37 Thus, de-escalation is necessary once in vitro susceptibility testing shows a sensitive strain. The emergence of resistance during therapy has also been observed for CT and CA, which presents another challenge in using these new combinations. It also raises the need for an expert infectious diseases (ID) team and effective communication with the laboratory to remeasure the MIC if the isolate was subsequently grown as a failure to respond or breakthrough infection.39,40 In a small, retrospective cohort,38 resistance to CT was reported to emerge as early as 8 days, and in vitro resistance to the drug was predictive of clinical failure.13,41
Recently, the DRI has been described as an antimicrobial stewardship toolkit.42 It is a composite measure that combines the ability of antibiotics to treat infections within the extent of their use in clinical practice. The tool is sensitive to changes and useful for experts to monitor the trend of resistance over time and to correlate it with interventional measures. It also allows comparisons between regional resistance rates for non-specialist decision makers, whereby aggregate information about antimicrobial resistance and consumption is quantified to predict antibiotic effectiveness over time. To our knowledge, this is the first systematic measurement of the DRI for P. aeruginosa strains in this region. We found an overall high DRI (>0.5) for P. aeruginosa strains isolated from patients with community- and hospital-acquired infections in our institution (Table 4). We also observed susceptibility to CA and CT in isolates with a lower DRI. This finding further demonstrates that the empirical use of these anti-pseudomonal drugs needs to be restricted to cases of low risk of MDR P. aeruginosa infection.
Table 4.
Drug resistance index for 67 Pseudomonas aeruginosa clinical isolates.
| Number (%) | Median DRI | |
|---|---|---|
| Isolates susceptible to both ceftazidime-avibactam and ceftolozane-tazobactam | 41 (61.2) | 0.18 |
| Isolates resistant to both ceftazidime-avibactam and ceftolozane-tazobactam | 25 (37.3) | 1 |
| Community-acquired strains | 15 (22.4) | 0.63 |
| Hospital-acquired strains | 52 (77.6) | 0.9 |
DRI: drug resistance index.
The main limitations of the study are the small number of strains and the non-availability of colistin susceptibility data, which require broth microdilution testing that was not performed for the isolates. Future prospective, larger studies will be useful for providing microbiological evidence about the efficacy of CA and CT against infections with P. aeruginosa and other challenging gram-negative pathogens, mainly Enterobacteriaceae species.
Conclusion
This study provides evidence for the limited anti-pseudomonal activity of CT and CA in the absence of accurate in vitro susceptibility testing. Diagnostic laboratories need to supplement the therapeutic use of the drugs with a timely and quality-assured susceptibility testing tool in institutions where CA and CT are to be administered. Therefore, there is a need for an enhanced national surveillance system with the ability to provide feedback to medical centres, so as to facilitate the effective introduction of new drugs to hospital formularies. For this purpose, a country-specific policy that supports the fight against antimicrobial resistance is necessary. Furthermore, such a policy should be complemented by stringent infection control measures to limit the spread of emerging resistant strains.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The material presented is original, unpublished, and has not been submitted elsewhere. The study has been kept confidential. This study was approved by the Imam Abdulrahman Bin Faisal University institutional review board (IRB-PGS-2019-03-355).
Authors contributions
All authors contributed toward data analysis, drafting, or critical revision of the paper, and agree to be accountable for all aspects of the work. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
We also thank Mr. Untoy Rashan and other technical staff in the Diagnostic Microbiology section for the technical help in some aspects of laboratory work and the data extraction for the purpose of this study.
Data availability
The raw data used to support the findings of this study are available from the corresponding author upon request.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Thaden J.T., Park L.P., Maskarinec S.A., Ruffin F., Fowler V.G., Jr., van Duin D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother. 2017;61(6) doi: 10.1128/AAC.02671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Johani S.M., Akhter J., Balkhy H., El-Saed A., Younan M., Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30(5):364–369. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanj S.S., Kanafani Z.A. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86(3):250–259. doi: 10.4065/mcp.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrissey I., Hackel M., Badal R., Bouchillon S., Hawser S., Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 2013;6(11):1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo R.A., Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 6.Tsai M.H., Wu T.L., Su L.H., Lo W.L., Chen C.L., Liang Y.H. Carbapenem-resistant-only Pseudomonas aeruginosa infection in patients formerly infected by carbapenem-susceptible strains. Int J Antimicrob Agents. 2014;44(6):541–545. doi: 10.1016/j.ijantimicag.2014.07.022. Epub 2014/09/30. [DOI] [PubMed] [Google Scholar]
- 7.CLSI. Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards.
- 8.Raman G., Avendano E.E., Chan J., Merchant S., Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Contr. 2018;7:79. doi: 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alagamy M.H., Shibl A., Zaki S., Tawik A. Antimicrobial resistance pattern and prevalence of metallo-β-lactamases in Pseudomonas aeruginosa from Saudi Arabia. Afr J Microbiol Res. 2011;5(30):5528–5533. [Google Scholar]
- 10.Khan M.A., Faiz A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann Saudi Med. 2016;36(1):23–28. doi: 10.5144/0256-4947.2016.23. Epub 2016/02/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memish Z.A., Shibl A.M., Kambal A.M., Ohaly Y.A., Ishaq A., Livermore D.M. Antimicrobial resistance among non-fermenting Gram-negative bacteria in Saudi Arabia. J Antimicrob Chemother. 2012;67(7):1701–1705. doi: 10.1093/jac/dks091. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti M., Peghin M., Vena A., Giacobbe D.R. Treatment of infections due to MDR gram-negative bacteria. Front Med (Lausanne) 2019;6:74. doi: 10.3389/fmed.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munita J.M., Aitken S.L., Miller W.R., Perez F., Rosa R., Shimose L.A. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2017;65(1):158–161. doi: 10.1093/cid/cix014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson A.P.R. Sparing carbapenem usage. J Antimicrob Chemother. 2017;72(9):2410–2417. doi: 10.1093/jac/dkx181. [DOI] [PubMed] [Google Scholar]
- 15.Kollef M.H., Novacek M., Kivistik U., Rea-Neto A., Shime N., Martin-Loeches I. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2019;19(12):1299–1311. doi: 10.1016/S1473-3099(19)30403-7. [DOI] [PubMed] [Google Scholar]
- 16.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Contr. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Laxminarayan R., Klugman K.P. Communicating trends in resistance using a drug resistance index. BMJ Open. 2011;1(2) doi: 10.1136/bmjopen-2011-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y.Y., Cao J.M., Yang Q., Chen S., Lv H.Y., Zhou H.W. Risk factors for carbapenem-resistant Pseudomonas aeruginosa, Zhejiang Province, China. Emerg Infect Dis. 2019;25(10):1861–1867. doi: 10.3201/eid2510.181699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palavutitotai N., Jitmuang A., Tongsai S., Kiratisin P., Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PloS One. 2018;13(2) doi: 10.1371/journal.pone.0193431. Epub 2018/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacconelli E., Cataldo M.A., Dancer S.J., De Angelis G., Falcone M., Frank U. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 22.Araoka H., Kimura M., Abe M., Takahashi N., Yoneyama A. Appropriate sampling sites for the surveillance of multidrug-resistant Pseudomonas aeruginosa colonization. Jpn J Infect Dis. 2014;67(2):118–119. doi: 10.7883/yoken.67.118. [DOI] [PubMed] [Google Scholar]
- 23.Cilloniz C., Dominedo C., Torres A. Multidrug resistant gram-negative bacteria in community-acquired pneumonia. Crit Care. 2019;23(1):79. doi: 10.1186/s13054-019-2371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiskirchen D.E., Nordmann P., Crandon J.L., Nicolau D.P. Efficacy of humanized carbapenem and ceftazidime regimens against Enterobacteriaceae producing OXA-48 carbapenemase in a murine infection model. Antimicrob Agents Chemother. 2014;58(3):1678–1683. doi: 10.1128/AAC.01947-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirley M. Ceftazidime-avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675–692. doi: 10.1007/s40265-018-0902-x. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher J.C., Satlin M.J., Elabor A., Saraiya N., McCreary E.K., Molnar E. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis. 2018;5(11):ofy280. doi: 10.1093/ofid/ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Duin D., Bonomo R.A. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-Lactam/beta-Lactamase inhibitor combinations. Clin Infect Dis. 2016;63(2):234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acar A., Karaahmetoglu G., Akalin H., Altay A.F. Pooled prevalence and trends of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates over the past 10 years in Turkey: a meta-analysis. J Glob Antimicrob Resist. 2019;18:64–70. doi: 10.1016/j.jgar.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Humphries R.M., Hindler J.A., Wong-Beringer A., Miller S.A. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother. 2017;61(12) doi: 10.1128/AAC.01858-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neall D., Juhász E., Tóth Á., Urbán E., Szabó J., Melegh S. Ceftazidime-avibactam and ceftolozane-tazobactam susceptibility of multidrug resistant Pseudomonas aeruginosa strains in Hungary. Acta Microbiol Immunol Hung. 2020 Mar 26:1–5. doi: 10.1556/030.2020.01152. [DOI] [PubMed] [Google Scholar]
- 31.Rahimzadeh M., Habibi M., Bouzari S., Asadi Karam M.R. First study of antimicrobial activity of ceftazidime-avibactam and ceftolozane-tazobactam against Pseudomonas aeruginosa isolated from patients with urinary tract infection in Tehran, Iran. Infect Drug Resist. 2020 Feb 17;13:533–541. doi: 10.2147/IDR.S243301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Agamy M.H., Jeannot K., El-Mahdy T.S., Samaha H.A., Shibl A.M., Plesiat P. Diversity of molecular mechanisms conferring carbapenem resistance to Pseudomonas aeruginosa isolates from Saudi Arabia. Can J Infect Dis Med Microbiol. 2016;2016:4379686. doi: 10.1155/2016/4379686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zowawi H.M., Syrmis M.W., Kidd T.J., Balkhy H.H., Walsh T.R., Al Johani S.M. Identification of carbapenem-resistant Pseudomonas aeruginosa in selected hospitals of the Gulf Cooperation Council States: dominance of high-risk clones in the region. J Med Microbiol. 2018;67(6):846–853. doi: 10.1099/jmm.0.000730. [DOI] [PubMed] [Google Scholar]
- 34.Juan C., Zamorano L., Perez J.L., Ge Y., Oliver A., Spanish Group for the Study of P Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother. 2010;54(2):846–851. doi: 10.1128/AAC.00834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkey P.M., Warren R.E., Livermore D.M., McNulty C.A.M., Enoch D.A., Otter J.A. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 36.Guerin F., Henegar C., Spiridon G., Launay O., Salmon-Ceron D., Poyart C. Bacterial prostatitis due to Pseudomonas aeruginosa harbouring the blaVIM-2 metallo-{beta}-lactamase gene from Saudi Arabia. J Antimicrob Chemother. 2005;56(3):601–602. doi: 10.1093/jac/dki280. [DOI] [PubMed] [Google Scholar]
- 37.Walsh T.R., Toleman M.A., Poirel L., Nordmann P. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeli Y., Troillet N., Eliopoulos G.M., Samore M.H. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43(6):1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grupper M., Sutherland C., Nicolau D.P. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother. 2017;61(10) doi: 10.1128/AAC.00875-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haidar G., Philips N.J., Shields R.K., Snyder D., Cheng S., Potoski B.A. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis. 2017;65(1):110–120. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis P.O., Cluck D.B., Tharp J.L., Krolikowski M.A., Patel P.D. Failure of ceftolozane-tazobactam salvage therapy in complicated pneumonia with lung abscess. Clin Case Rep. 2018;6(7):1308–1312. doi: 10.1002/ccr3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein E.Y., Tseng K.K., Pant S., Laxminarayan R. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob Health. 2019;4(2) doi: 10.1136/bmjgh-2018-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used to support the findings of this study are available from the corresponding author upon request.