Abstract
Introduction
Acute kidney injury (AKI) is prevalent in low- and middle-income countries (LMIC) and is associated with significant morbidity and mortality, particularly among hospitalized patients. Successful strategies for the prevention and management of AKI in these countries are dependent on the capacity of primary care centers to provide optimal initial management of patients at risk for this disorder.
Methods
From December 2018 to February 2019, using mixed methods, we assessed hospital capacity and the knowledge of clinicians relevant to the prevention, diagnosis, and management of AKI in Rwanda. A checklist based on Kidney Disease: Improving Global Outcomes (KDIGO) guidelines and clinical vignette-based assessment tool were used to assess hospital capacity and provider knowledge base, respectively. Data were analyzed using stata 13 with findings reported as simple frequencies or means with standard deviation. Multivariate analysis was used to assess factors associated with a higher knowledge score among clinicians.
Results
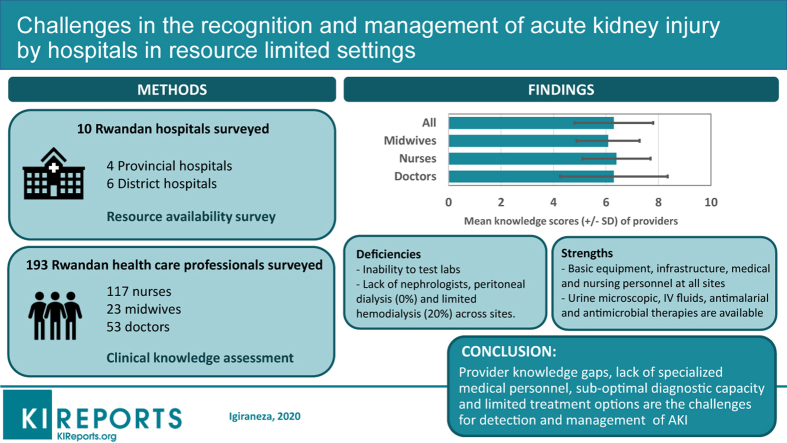
Ten hospitals and 193 health care providers from sites throughout Rwanda participated in the survey. Surveyed hospitals were equipped with basic general medical equipment but were deficient in diagnostic tools and medical supplies that would allow the diagnosis and nondialytic management of AKI. Although 20% of the hospitals could offer hemodialysis services, peritoneal dialysis services were nonexistent. With regard to knowledge base, the health care providers demonstrated significant deficiencies in the diagnosis and management of AKI. The mean knowledge score for all health providers was 6.3 (±1.5) of a maximum of 11, with a mean (±SD) score for doctors, nurses, and midwives of 6.3 ± 2.05, 6.4 ± 1.3, and 6.08 ± 1.2, respectively. On multivariate analysis, the length of clinical experience and age of the respondents were significantly associated with participants’ knowledge score.
Conclusion
This study documents significant barriers to providing optimal management of AKI in primary health care settings in Rwanda, a resource-limited setting. These include lack of specialized medical personnel, significant knowledge gaps among primary health care providers, suboptimal diagnostic capacity, and limited treatment options for detection and management of AKI.
Keywords: acute kidney injury, diagnostic capacity, resource-limited setting, therapeutic options
Graphical abstract
See Commentary on Page 977
Acute kidney injury is a significant cause of mortality in the developed and developing world. The worldwide incidence of AKI in hospitalized patients is estimated at 22%, with a mortality rate of 21%.1 Of an estimated 1.7 million deaths attributed to AKI worldwide annually, 1.4 million occur in low- and middle-income countries (LMIC). The causes of AKI in high-income countries differ from those in limited-resourced settings, and clinical outcomes are worse in the latter settings.2 Adverse outcomes are attributable to late presentation, insufficient diagnostic tools, and other infrastructural deficiencies, as well as insufficient numbers of clinicians able to provide optimal management.3 Recent studies have shown that not only is AKI an independent risk factor for in-hospital mortality, it is also associated with progression to chronic kidney disease (CKD) and end-stage renal disease (ESRD) as well as an increase in long-term mortality and cardiovascular events.4,5
To decrease adverse outcomes associated with AKI worldwide, Kidney Disease: Improving Global Outcomes (KDIGO), a nonprofit foundation established in 2003 to improve the care of patients with kidney disease worldwide, developed guidelines in 2012 on the definition, prevention, diagnosis, and management of AKI. In the era of evidence-based medicine and management by protocol,6 clinical practice guidelines, by standardizing patient care, have improved patient outcomes.7,8
Previous studies from different countries in sub-Saharan Africa, including Rwanda, have reported high mortality rates of up to 34% as well as prolonged hospital stays and increased health care costs among AKI patients.9, 10, 11, 12, 13, 14 Studies on AKI conducted in Rwanda, as in many low resource countries, have focused only on tertiary care facilities.14, 15, 16 There are, however, no data from lower-tier centers (including provincial and district hospitals), which play an important role in the Rwandan and other limited-resource health care systems. Thus, the present study was conducted to assess the challenges that Rwandan subtertiary hospitals face in implementing interventions designed to improve patient care and to decrease AKI-related mortality and morbidity.
The objective of the present study was to document the current infrastructural deficiencies and logistical challenges encountered in subtertiary hospitals, and to assess health care providers’ knowledge about prevention, diagnosis, and management of AKI. This study will help to provide actionable information to policy makers to guide developing programs to improve the care of patients with acute kidney injury in Rwanda and other similar resource-limited settings.
Methods
This is a mixed-method study that assessed the institutional capacity of district and provincial hospitals and knowledge of clinicians relevant to the prevention, diagnosis, and management of AKI. We used the KDIGO clinical practice guideline for AKI as our reference. The study was conducted from December 2018 to February 2019. Specific aims of the study were to assess the following: (i) laboratory capacity and availability of medical equipment and consumables that are necessary for the prevention, diagnosis, and management of AKI; and (ii) knowledge of health care providers (medical practitioners and nurses) regarding AKI risk factors, diagnosis, and management.
Description of Rwandan Health System
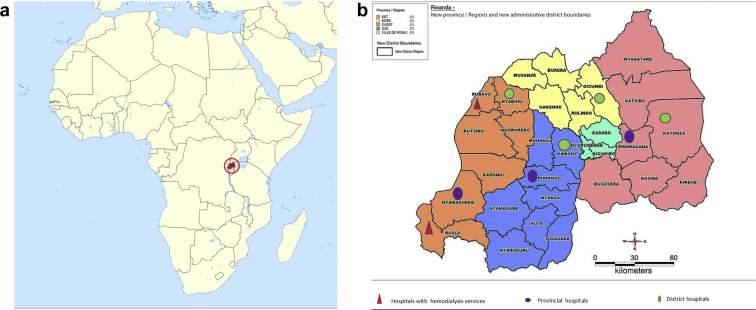
Rwanda is a land-locked country located in East Africa with a surface area of 26,338 km2. It comprises 4 administrative provinces (East, West, South, and North) and Kigali city (Figure 1a). Each province is divided into districts, and there are 30 administrative districts. Each district has at least 1 district hospital, whereas each province has a regional hospital. The health care system is built on a solid primary care system: patients are usually seen at the health centers/posts, and only those who need hospital admission and/or need advanced investigations and/or treatment are sent to district, provincial, and teaching hospitals through a well-defined referral pathway. Provincial and district hospitals oversee health care services in their catchment areas17 but also serve as first-line referral systems for patients in need of more advanced health care services.
Figure 1.
(a) Location of Rwanda and hospitals surveyed. (b) Location of hospitals participating in the study.
Sampling, Data Collection Methods, and Tools
To have a representative sample nationwide, the survey was conducted in 10 hospitals, that is, all 4 provincial and 6 district hospitals (Figure 1b). One district hospital was randomly selected in each province by using the last hospital in alphabetical order. In addition, 2 district hospitals (Gihundwe and Gisenyi District Hospitals) were included in the study because they were able to provide hemodialysis at the time of the study and were therefore providing a higher level of care to patients with AKI.
The preselected hospitals were visited by the primary study investigator (GI) to assess resources relevant to the care of patients with AKI as well as the knowledge of providers at those locations. To determine the capacity of the hospitals for the detection and management of AKI, a checklist based on KDIGO guidelines was used to assess the diagnostic and treatment capacity, including nutritional support and the ability to transfer to higher-level care when needed (see Supplementary Appendix S4). The presence or absence of staff with nephrology training was also assessed. Information on available resources was provided by a combination of hospital administrative authority, a laboratory technician, a pharmacist, and/or a responsible nurse at each hospital. The checklist was completed by the study investigator based on collected information and visual inspection.
As there is no standardized knowledge assessment tool for AKI, a clinical vignette−based questionnaire was developed by the study team and adapted to the level of training of the participants (nurses, midwives, and doctors). The questionnaire was tested by several faculty members of the School of Medicine and nurses working in the Department of Medicine at the University Teaching Hospital of Kigali (CHUK). Their feedback and input were used to create the questionnaire that was used in the study (see Supplementary Appendix S1–S3).
The questionnaire was composed of 3 sections: demographic information, clinical vignette−based questions, and questions assessing the health care practitioners’ perceptions about the use of a clinical practice guideline on the management of AKI. The clinical vignettes were designed to assess knowledge about the prevention, risk stratification, presenting symptoms, diagnosis, and management of AKI. The questionnaire was in English, which is the official language in nursing, midwifery, and medical schools in Rwanda. However, when needed, the study investigator provided more explanation at the request of the study participants.
The study team recruited general practitioners (nonspecialist doctors), registered nurses, and registered midwives, as they are at the frontline of the primary care service delivery at district and provincial hospitals. A convenient sample ranging from 13 to 24 consenting participants was recruited at each hospital. After completing the questionnaire, a teaching session based on the clinical vignettes was conducted by the primary study investigator as a learning opportunity for the participants.
Statistical Analysis
Data collected using questionnaires and checklists were entered in a Microsoft Excel database (Microsoft Corp., Redmond, WA). Stata software version 13 (StataCorp, College Station, TX) was used for data cleaning and statistical analysis. Respondents’ demographics were recorded and reported as simple frequencies. A score of 1 point was given to each correct answer, and a total score was calculated for each respondent. The maximum total score was 11, and respondents were arbitrarily categorized according to their performance. A score of 4 or below was considered as very poor, 5 to 6 poor, 7 to 8 average, and 9 to 11 as having good knowledge. A linear regression (univariate and multivariate) model was used to assess for factors that were associated with a higher score. A P value of ≤0.05 was considered statistically significant. A t test was used to assess for differences between categorical variables. With regard to the institutional survey, the availability of medical equipment, supplies, and infrastructure were categorized using a Likert scale and reported as always available, frequently available, or never available (see Supplementary Appendix S4).
Ethical Considerations
Authorization to conduct the study and approval for use of study tools were obtained from the Institutional Review Board of the College of Medicine and Health Sciences, University of Rwanda, and the Yale University School of Medicine Human Investigations Committee. After adequate explanation about the objectives of the study, informed written consent was obtained from each health care provider who participated in knowledge assessment. Participants and institutional confidentiality as well as data safety were maintained by the study investigators.
Results
The study was conducted from December 2018 to February 2019. A total of 10 hospitals (i.e., Kinihira provincial hospital, Rwamagana provincial hospital, Bushenge provincial hospital, Ruhango provincial hospital, Rwinkwavu district hospital, Rutongo district hospital, Remera Rukoma district hospital, Shyira district hospital, Gisenyi district hospital, and Gihundwe district hospital) participated in the study (Figure 1).
Hospitals’ Capacity, Equipment, and Utilities
Of the 10 hospitals that were assessed in the study, 4 (40%) were provincial hospitals whereas 6 (60%) were district hospitals. Generally, all the hospitals were equipped with basic equipment and medical supplies that would allow the initial diagnosis and nondialytic management of patients with AKI (Table 1). Of the surveyed hospitals, 20% could offer hemodialysis services, but none could offer peritoneal dialysis. Urea and creatinine measurement were usually available; however, electrolyte measurement was often unavailable.
Table 1.
Available equipment and resource to diagnose and manage acute kidney injury (AKI)
| Variables | Hospitals (N = 10) |
Total, % | |
|---|---|---|---|
| Provincial hospitals (n = 4) |
District hospitals (n = 6) |
||
| Frequency | Frequency | ||
| Human resources | |||
| Internal medicine doctor(s) | Present | Present | |
| Nephrologists | Not available | Not available | |
| Nephrology nurses | Not available | Not available | |
| Laboratory tests | |||
| Serum urea | |||
| Always available | 2 (20) | 4 (40) | 60 |
| Frequently available | 2 (20) | 1 (10) | 30 |
| Rarely available | 0 | 1 (10) | 10 |
| Serum creatinine | |||
| Always available | 3 (30) | 6 (60) | 90 |
| Frequently available | 1 (10) | 0 | 10 |
| Serum potassium | |||
| Always available | 2 (20) | 2 (20) | 40 |
| Frequently available | 2 (20) | 2 (20) | 40 |
| Rarely available | 0 | 2 (20) | 20 |
| Serum sodium | |||
| Always available | 2 (20) | 2 (20) | 40 |
| Frequently available | 2 (20) | 2 (20) | 40 |
| Rarely available | 0 | 2 (20) | 20 |
| Urine dipstick for proteinuria | Always available | Always available | |
| Urine microscopic examination | Always available | Always available | |
| Microscopes | Always available | Always available | |
| Centrifuges | Always available | Always available | |
| AKI-related medications and medical supplies | |||
| i.v. Calcium gluconate | Always available | Always available | |
| Calcium /sodium kayexalate | Never available | Never available | |
| i.v. Sodium bicarbonate | |||
| Always available | 1 (10) | 3 (30) | 40 |
| Frequently available | 0 | 2 (20) | 20 |
| Never available | 3 (40) | 1 (10) | 40 |
| Crystalloids (normal saline and Ringer’s lactate) | Always available | Always available | |
| Antimalarial (artemisinin-based therapy) | Always available | Always available | |
| Third-generation cephalosporins | Always available | Always available | |
| Foley catheters and urine bags | Always available | Always available | |
| Other equipment and facilities | |||
| Hemodialysis services | |||
| Available | 0 | 2 (20) | 2 (20) |
| Not available | 4 (40) | 4 (40) | 8 (80) |
| Peritoneal dialysis | Not available | Not available | |
| Ambulance for patient transportation | Always available | Always available | |
| Patient meals provided by hospital | |||
| Yes | 0 | 1 (10) | 10 |
| No | 4 (40) | 5 (50) | 90 |
| Clean water source | Always available | Always available | |
| Electricity | Always available | Always available | |
Always available, available 7 of 7 days; frequently available, available 5 to 6 of 7 days; rarely available, available 1 to 2 of 7 days.
Data are n (%) unless otherwise noted.
With regard to the workforce, all the hospitals were staffed by general practitioners and nurses who made daily ward rounds except on weekends, when only patients deemed critical were seen by the on-call doctor. All the hospitals had at least one internal medicine specialist who oversaw inpatient and outpatient medical services, but none had a nephrologist or nephrology nurse on their staff. Basic utilities such as clean water and electricity were reported to be always present. All the hospitals had piped water and water reservoir tanks that provided water when the public water source was temporally unavailable. All hospitals also had generators that could be used in case of loss of power. All the hospitals had at least 2 ambulances that were used for patient transportation. Only 1 (10%) hospital provided meals; at other facilities, patients were fed by their families.
Knowledge About AKI
A total of 193 health care providers participated in the knowledge assessment. This included 53 general practitioners, 117 nurses, and 23 midwives. The participants had a mean age of 34.5 ± 7 years, and women accounted for 55% of the group. The participants were mostly in the early years of their career, reflecting staffing in these hospitals. The mean years of experience was 5 ± 5 years, and 47.1% had a clinical career of ≤3 years. Table 2 summarizes relevant demographic information about this cohort.
Table 2.
Demographic characteristics of the study participants
| Variable | Frequency/mean (N = 193) | ± SD or percentage |
|---|---|---|
| Age, yr | 34.5 | ±7 |
| Sex | ||
| Male | 86 | 45 |
| Female | 107 | 55 |
| Education | ||
| Medical doctors | 53 | 27.46 |
| Registered nurses | 117 | 60.62 |
| Registered midwives | 23 | 11.92 |
| Department | ||
| Internal medicine | 30 | 15.54 |
| Surgery | 21 | 10.88 |
| Obstetrics/gynecology | 33 | 17.10 |
| Pediatrics | 25 | 12.95 |
| Outpatient | 14 | 7.25 |
| Emergency | 20 | 10.3 |
| All | 39 | 20.21 |
| Othersa | 11 | 5.71 |
| Clinical experience, yr | ||
| ≤3 | 91 | 47.15 |
| 4−8 | 68 | 35.23 |
| ≥9 | 34 | 17.62 |
| Training about AKI | ||
| Undergraduate training | 106 | 54.92 |
| In-service training | 23 | 11.92 |
| Never trained about AKI | 50 | 25.91 |
| Both in-service and undergraduate | 14 | 7.25 |
AKI, acute kidney injury.
Others include registered nurses who previously worked on wards but were appointed in other departments such as pharmacy or nursing administration at the time of the study.
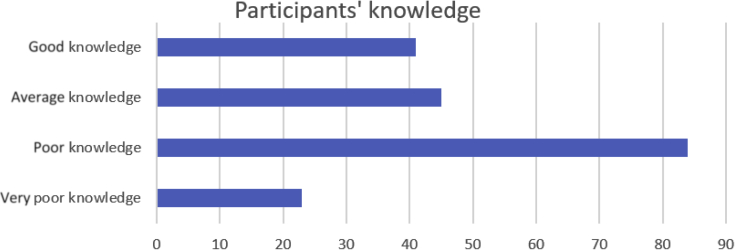
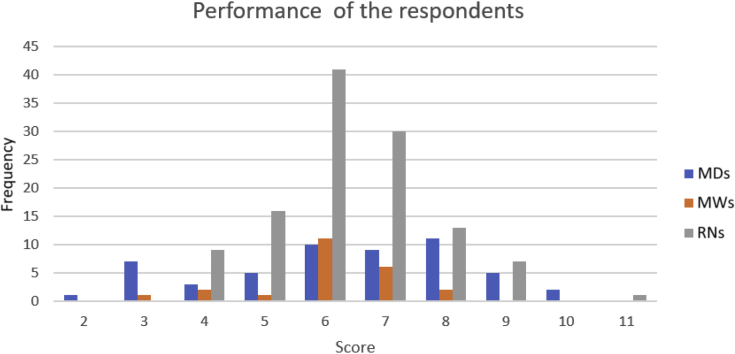
The mean knowledge score for all participants was 6.3 ± 1.5. The mean score for doctors, nurses, and midwives was 6.3 ± 2.05, 6.4 ± 1.3, and 6.08 ± 1.2, respectively (Figures 2 and 3). Interestingly, nurses and midwives performed better on the on AKI management questions, with more than 70% correct answers, versus 30% correct answers on diagnostic questions. There was no difference in the performance of doctors based on the type of questions.
Figure 2.
Acute kidney injury knowledge score among study participants.
Figure 3.
Performance of the respondents. MDs, medical doctors; MWs, registered midwives; RNs, registered nurses.
In addition, doctors were asked how often they see AKI patients on the wards, using 3-point Likert scales with responses of “frequently,” “rarely,” and “never.” Of the doctors, 57% reported that they saw AKI patients frequently. Half of nurses and midwives (58%) reported having cared for at least 1 AKI patient in their clinical experience. With regard to how often they thought that they had missed the diagnosis of AKI in the previous 6 months, most of the doctors thought that they rarely (68%) or never (13%) missed the diagnosis of AKI. However, 43% reported feeling less comfortable caring for AKI patients.
Participants who reported having had in-service training on AKI in addition to training as undergraduates scored higher (6.92 ± 1.8) compared to those who reported training in AKI only an undergraduates (6.3 ± 1.6), those who received AKI training during in-service training only (6.26 ± 1.7), or those who received no AKI training at all (6.28 ± 0.9). However, the difference did not reach statistical significance (P = 0.54).
Subgroup, Univariate, and Multivariate Analysis
Using a logistic linear model, we found that clinical experience and age of the respondents were associated with participants’ scores. Participants were then divided into 2 categories based on years of clinical experience (≤3 years, limited experience; ≥4 years, average experience) and 3 categories based on age (≤30 years, 31−40 years, and ≥41 years). Doctors with “limited experience” had nonsignificantly higher scores than those who were more experienced (6.75 vs. 5.65, P = 0.056), whereas nurses with “average experience” scored higher than those with limited experience (6.62 vs. 6.12, P = 0.039). There was no difference in scores among midwives based on their years of clinical experience. Interestingly, younger participants tended to perform better than older ones, but statistical significance was reached only in the subgroup analysis of doctors alone (P = 0.0025). The difference remained significant on multivariate analysis, in which age was associated with performance score, with younger doctors performing better than their older colleagues (P ≤ 0.006) (Table 3).
Table 3.
Univariate and multivariate analysis
| Variable | Univariate |
Multivariate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean score/n (all) | P | Mean score/n (MDs) | P | Mean score/n (RNs) | P | Coefficient | Confidence interval | P | |
| Clinical experience, yr | |||||||||
| ≤3 | 6.35 (91) | 0.99 | 6.75 (33) | 0.056 | 6.12 (50) | 0.039 | 1.69 | −0.382 to 3.77 | 0.107 |
| >3 | 6.35 (102) | 5. 65 (20) | 6.62 (67) | ||||||
| Age, yr | |||||||||
| ≤30 | 6.5 (69) | 0.12 | 7.1 (29) | 0.0025 | 6.39 (33) | 0.925 | |||
| 31−40 | 6.20 (89) | 5.6 (18) | 6.37 (58) | −3.0 | −5.01 to −1.00 | 0.004 | |||
| ≥41 | 6.14 (35) | 4.5 (6) | 6.5 (26) | −3.9 | −6.29 to −1.62 | 0.006 | |||
| Previous AKI training | |||||||||
| Undergraduate | 6.3 (106) | 0.54 | 6.4 (38) | 0.054 | 6.28 (59) | 0.129 | |||
| In-service | 6.26 (23) | 6.7 (7) | 7.13 (15) | ||||||
| Never | 6.28 (50) | − | 6.27 (37) | ||||||
| Both | 6.92 (14) | 7.1 (8) | 6.41 (6) | ||||||
AKI, acute kidney injury; MD, medical doctor; RN, registered nurse.
Respondents’ Perceptions About Use of a Clinical Practice Guideline for Management of AKI
Most of the respondents (68%) reported that they had never used any clinical practice guideline (CPG) for the management of AKI. Interestingly, almost all of the respondents strongly agreed that a CPG was needed in the hospitals (99%), that it would improve clinicians’ knowledge (97%), improve clinical outcome in patients with AKI (98%), and reduce the number of unnecessary transfers to higher levels of care (91%). In addition, participants expressed excellent commitment (98%) to follow a CPG on the management of AKI if it were available in the hospital.
Discussion
The identification, diagnosis, and management of patients with AKI has recently been a focus of the International Society of Nephrology (ISN) with the establishment of the 0 by 25 initiative, which has set a goal that no one would die of preventable and treatable AKI worldwide by the year 2025. However, before developing programs to achieve this goal, it is important to understand the barriers and challenges present in each region or country. Therefore, it is an opportune time to reflect on how best to accomplish this goal, focusing attention on the unique challenges present in limited-resource countries. The present study is helpful in providing a way of approaching how to implement 0 by 25 by initially identifying the deficiencies at different levels of the health care system and thus permitting appropriate changes to be implemented to improve AKI mortality outcomes. It is important to note that previous studies have reported a high mortality rate in hospitalized AKI patients in Rwanda.14,16
Rwanda is a low- to middle-income country in East Africa. Its annual GDP per capita is less than 800 USD, and total expenditure on health per capita is 53 USD. There is competition by different sectors for the available resources. To improve access to health care, Rwanda has designed and adopted a universal health care access model, and 80% of the Rwandan population are covered by that community-based health insurance scheme. The out-of-pocket spending as a percentage of total health expenditure constitutes only 0.08%.18 Despite the tremendous efforts of the government to improve health care delivery and access, limited financial resources lead to limited diagnostic and treatment capacity of the public health facilities.
The current study found that the Rwandan subtertiary hospitals were reasonably well equipped to manage patients at risk for AKI. The inpatient wards are staffed with trained medical personnel and possess the basic equipment that allow daily monitoring of patients. Medical supplies needed to measure vital signs are readily available except for the weighing scale, which is rarely or not all available on the wards in some hospitals. The diagnostic capacity, however, is limited. All the hospitals were able to perform urine sediment analysis and dipstick urine for proteinuria.
In all surveyed facilities, centrifuges and microscopes were available for urine microscopic examination. Although laboratory technicians are trained to review urine sediments and to report their findings, these are typically focused on findings suggestive of the presence or absence of infection, and other findings (e.g., granular or red blood cell casts) that could provide valuable information to clinicians as to the underlying etiology of renal disease are not reported.
With regard to electrolytes, measurements of serum creatinine and urea are not always available. Serum electrolytes were measured infrequently, and urine electrolytes were not measured at all. To address this problem of the absence, out-of-stock status of laboratory reagents, a strong collaboration between clinicians and hospital administration team is key. Involvement of front-line clinicians in procurement processes will allow them to inform regarding the demand and need for specific infrastructure, equipment, and supplies to support provision of standard-of-care management for AKI patients. Interaction with and involvement of hospital administrators and/or procurement staff by clinicians will be critical to facilitate supporting nephrology units or programs and provision of required resources, particularly in the face of other competing priorities in their health care facilities. Finally, development, adoption, and implementation of national guidelines that can be adapted to individual facilities in consideration of their unique resources will also serve as a benchmark for health care facilities to assess their ability and capacity to offer adequate care to renal patients.
Generally, limited diagnostic capacity has previously been identified as one of the barriers to timely and accurate diagnosis of AKI in resource-constrained settings.3,19,20 A recent survey that assessed laboratories accredited to internationally recognized quality standards in sub-Saharan Africa reported that only 12 of 49 sub-Saharan African countries had accredited clinical laboratories. Of those, only 17% were in public institutions, whereas others were either in private institutions or in research laboratories.19 The above-mentioned limited diagnostic tools of the surveyed hospitals clearly reflect laboratory deficiencies that may hinder timely diagnosis of AKI.
Acute kidney injury in LMIC is frequently related to prerenal injury due to fluid loss. Importantly, the survey found that i.v. crystalloid solutions as well as oral rehydration solutions were regularly available in Rwandan hospitals. Both of these solutions are on the list of essential medications that are always required to be available in the hospitals. However, in many other developing countries, the lack of sufficient i.v. fluids represents a major challenge in caring for AKI patients.3 As diarrheal diseases leading to fluid loss have been identified as one of the main causes of AKI in resource-constrained countries, having rehydration solutions in health facilities would allow timely correction of fluid loss, thereby preventing the progression to more severe forms of kidney injury. In such settings, interventions aimed at improving the availability and timely administration of crystalloids in health care facilities is important to avoid kidney injury due to fluid loss.
With respect to dialysis treatment, as noted, only a limited percentage (20%) of the surveyed hospitals had access to hemodialysis services, and peritoneal dialysis services were nonexistent. This illustrates the limited availability and access to dialysis services in the regional and district hospitals. Currently, in Rwanda, hemodialysis treatment is offered at all national tertiary hospitals, 2 district hospitals, and a few private centers. It is important to highlight that when patients are referred to the public dialysis centers for severe AKI, the hospital bill including cost of dialysis is covered by community-based health insurance at either 90% or 100% of the cost, depending on the social and economic status of the patient. The coverage of hemodialysis for AKI patients by a government-run health insurance scheme has tremendously improved access to dialysis services in Rwanda and has improved renal care.
Thus, in a small country such as Rwanda, where all the hospitals are equipped with sufficient ambulances and where a tertiary hospital is easily accessible within an estimated time of 4- to 5-hour drive, a timely diagnosis at the front-line health care facilities in combination with timely referral to tertiary centers would be an effective, realistic, and achievable solution to reduce AKI-related mortality and to meet the 0 by 25 target.21 On the other hand, the urgent initiation of peritoneal dialysis in critically ill patients at district or regional hospitals may be an important addition to the management of these patients, as has been advocated by the Saving Young Lives Program.22, 23, 24 Peritoneal dialysis, which is less complicated, requires less equipment and infrastructure, and is usually less expensive than hemodialysis, has proved to be effective in the treatment of AKI patients in African countries such as Tanzania,25 Sudan,26 Nigeria,27,28 and Ivory Coast.29 One must recognize that in some countries where PD fluids are not locally produced, it is difficult to import PD fluid and equipment, which can make PD more expensive and potentially unaffordable to most patients in need of renal replacement therapy.
The knowledge gaps about AKI among health care providers, coupled with the lack of nephrologists and nephrology nurses countrywide, constitute additional barriers to providing optimal renal care in Rwanda. A significant number of participants in the current study reported not having had formal training on AKI in undergraduate or in-service training, leading to lack of confidence in managing patients with acute renal dysfunction. The mean knowledge score decreased with years of clinical experience among doctors, whereas the opposite effect was observed among the nurses. Limited knowledge of AKI risk factors impairs the stratification of patients at risk for development of AKI, and results in missed opportunities to diagnosis AKI in the early stage and to initiate timely interventions. The poor knowledge scores of more experienced doctors reported in this study may reflect the lack of continuing education programs. Regular education sessions in the form of conferences or seminars about kidney diseases will inform practitioners about topical issues including the clinical burden of AKI in developing countries, its risk factors, diagnostic criteria, and therapeutic approaches.
This finding is not unique to Rwanda, as the lack of adequate knowledge regarding clinical presentation, diagnosis, and management of patients with AKI has been previously documented in African countries. Studies in Malawi30 and Nigeria31 have reported knowledge gaps in both practicing doctors and nurses. Therefore, this emphasizes the need for ongoing training of clinicians to update their knowledge about diagnosis and management of AKI in developing countries. In the short term, hands-on-training to upgrade competencies of medical personnel would be helpful and is indeed a critical and achievable goal to improve the management of AKI patients. On the other hand, a more rigorous and sustainable effort that would require additional resources with long-term dividends would be to create a regional or national nephology training program. Hands-on training would provide clinicians the opportunity for training on diagnosis and management of AKI, including training through regular continuing medical education conferences. The few trained nephrologists who are currently based in tertiary level hospitals could be engaged to participate in the program and to develop simplified training curricula, and, through organized mentorship programs, could have an impact on providers at the primary care level, equipping them with the necessary skills to improve the care of AKI patients.
Conclusions and Recommendation
This study underscores the need for and importance of understanding the barriers and challenges to developing an effective program to manage AKI in low-resource countries. Importantly, each country and/or region needs to understand the unique barriers in their area so that effective programs for AKI prevention can be initiated and the ISN’s 0 by 25 goals can be met. The present study documents the major issues that need to be addressed in the early identification and treatment of patients with AKI in Rwanda.
Institutional deficiencies in terms of lack of specialized medical personnel, knowledge gaps among primary health care providers, suboptimal diagnostic capacity, and limited treatment options for detection and management of AKI were identified as the main barriers to providing optimal care to these patients. This should inform actions aiming to improve renal care in Rwanda.
Based on these findings, we recommend ongoing training about AKI among health care providers, and strengthening of hospitals’ diagnostic and therapeutic capacity, to enable clinicians to offer standard of care to AKI patients. In addition, it would be reasonable to develop country-specific guidelines that could be implemented in developing countries while efforts are being made to scale up the standard of care for these patients. Finally, the potential role of peritoneal dialysis in subtertiary care hospitals in limited-resource regions should be explored, as this may obviate the need to transfer patients with AKI to tertiary care hospitals.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We acknowledge all the directors and administration teams of the surveyed hospitals for facilitating the meetings with different hospital staff members. We are also grateful for all the doctors, nurses, and midwives who participated in the study, gave their precious time to complete the study questionnaires, and participated in teaching discussions afterward. We are grateful to the International Society of Nephrology (ISN); we recognize that this work has been made possible through an ISN-funded fellowship.
Footnotes
Appendix S1. Knowledge assessment form for doctors.
Appendix S2. Knowledge assessment for nurses and midwives.
Appendix S3. Assessment of availability, importance, and acceptability of AKI clinical practice guideline.
Appendix S4. Assessment form for Hospital capacity to diagnose and manage AKI.
Supplementary Material
References
- 1.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerda J., Bagga A., Kher V. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 3.Lunyera J., Kilonzo K., Lewington A. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67:834–840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta S., Chauhan K., Patel A. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018;19:91. doi: 10.1186/s12882-018-0876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivers E., Nguyen B., Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.Hoste E.A., De Corte W. Implementing the Kidney Disease: Improving Global Outcomes/acute kidney injury guidelines in ICU patients. Curr Opin Crit Care. 2013;19:544–553. doi: 10.1097/MCC.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 8.Eckardt K.U., Kasiske B.L. Kidney Disease: Improving Global Outcomes. Nat Rev Nephrol. 2009;5:650–657. doi: 10.1038/nrneph.2009.153. [DOI] [PubMed] [Google Scholar]
- 9.Bagasha P., Nakwagala F., Kwizera A. Acute kidney injury among adult patients with sepsis in a low-income country: clinical patterns and short-term outcomes. BMC Nephrol. 2015;16:4. doi: 10.1186/1471-2369-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello B.T., Busari A.A., Amira C.O. Acute kidney injury in Lagos: pattern, outcomes, and predictors of in-hospital mortality. Niger J Clin Pract. 2017;20:194–199. doi: 10.4103/1119-3077.183258. [DOI] [PubMed] [Google Scholar]
- 11.Cerda J., Lameire N., Eggers P. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 12.Esezobor C.I., Ladapo T.A., Lesi F.E. Clinical profile and hospital outcome of children with severe acute kidney injury in a developing country. J Trop Pediatr. 2015;61:54–60. doi: 10.1093/tropej/fmu066. [DOI] [PubMed] [Google Scholar]
- 13.Evans R.D., Hemmila U., Craik A. Incidence, aetiology and outcome of community-acquired acute kidney injury in medical admissions in Malawi. BMC Nephrol. 2017;18:21. doi: 10.1186/s12882-017-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igiraneza G., Ndayishimiye B., Nkeshimana M. Clinical profile and outcome of patients with acute kidney injury requiring hemodialysis: two years' experience at a tertiary hospital in Rwanda. Biomed Res Int. 2018;2018:1716420. doi: 10.1155/2018/1716420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igiraneza G., Hategekimana T., Manzi O.M. Obstructive uropathy as initial presentation of genitourinary tuberculosis and masquerading as a postsurgical complication. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nsengiyumva V.I.G., Lameire N. Definition and epidemiology of acute kidney injury. Rwanda Med J. 2018;75:17–23. [Google Scholar]
- 17.Binagwaho A, Harward SH, Dushime T, et al. Extending the right to health to the moment of death: end of life care and the right to palliation in Rwanda. HealthHum Rights J. Available at: https://www.hhrjournal.org/2015/12/extending-the-right-to-health-to-the-moment-of-death-end-of-life-care-and-the-right-to-palliation-in-rwanda/. Published December 4, 2015. Accessed March 17, 2020.
- 18.Ministry of Health Government of Rwanda. Key health indicators, 2016/2017, 2017. Available at: https://www.moh.gov.rw/index.php?id=514. Accessed March 30, 2020.
- 19.Schroeder L.F., Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141:791–795. doi: 10.1309/AJCPQ5KTKAGSSCFN. [DOI] [PubMed] [Google Scholar]
- 20.Anand S., Cruz D.N., Finkelstein F.O. Understanding acute kidney injury in low resource settings: a step forward. BMC Nephrol. 2015;16:5. doi: 10.1186/1471-2369-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 22.Cullis B., Abdelraheem M., Abrahams G. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34:494–517. doi: 10.3747/pdi.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smoyer W.E., Finkelstein F.O., McCulloch M. Saving Young Lives: provision of acute dialysis in low-resource settings. Lancet. 2015;386:2056. doi: 10.1016/S0140-6736(15)00971-X. [DOI] [PubMed] [Google Scholar]
- 24.Smoyer W.E., Finkelstein F.O., McCulloch M.I. “Saving Young Lives” with acute kidney injury: the challenge of acute dialysis in low-resource settings. Kidney Int. 2016;89:254–256. doi: 10.1016/j.kint.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Kilonzo K.G., Ghosh S., Temu S.A. Outcome of acute peritoneal dialysis in northern Tanzania. Perit Dial Int. 2012;32:261–266. doi: 10.3747/pdi.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdelraheem M., el Ali T., Osman R. Outcome of acute kidney injury in Sudanese children—an experience from a sub-Saharan African unit. Perit Dial Int. 2014;34:526–533. doi: 10.3747/pdi.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ademola A.D., Asinobi A.O., Ogunkunle O.O. Peritoneal dialysis in childhood acute kidney injury: experience in southwest Nigeria. Perit Dial Int. 2012;32:267–272. doi: 10.3747/pdi.2011.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esezobor C.I., Ladapo T.A., Lesi F.E. Peritoneal dialysis for children with acute kidney injury in Lagos, Nigeria: experience with adaptations. Perit Dial Int. 2014;34:534–538. doi: 10.3747/pdi.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diarrassouba G., Adonis-Koffy L., Niamien E. Acute peritoneal dialysis in African pediatric area experience of pediatric nephrology unit of Yopougon University Hospital (Abidjan, Cote d'Ivoire) Blood Purif. 2015;39:141–144. doi: 10.1159/000368938. [DOI] [PubMed] [Google Scholar]
- 30.Evans R., Rudd P., Hemmila U. Deficiencies in education and experience in the management of acute kidney injury among Malawian healthcare workers. Malawi Med J. 2015;27:101–103. doi: 10.4314/mmj.v27i3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adejumo O., Akinbodewa A., Alli O. Assessment of knowledge of acute kidney injury among non-nephrology doctors in two government hospitals in Ondo City, Southwest Nigeria. Ethiop J Health Sci. 2017;27:147–154. doi: 10.4314/ejhs.v27i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.