Abstract
Background
Pelvic multiparametric magnetic resonance imaging (mpMRI)–determined membranous urethral length (MUL) and its surgical maximization have been reported to impact early- and long-term pad-free urinary continence after robot-assisted radical prostatectomy (RARP).
Objective
The objective of this study was to present evidence (data and video) of important effects on post-RARP continence recovery from both innate mpMRI-assessed and surgical preservation of MUL.
Design, setting and participants
Of 605 men undergoing RARP, 580 with complete follow-up were included: Group 1, prior (N = 355), and Group 2, subsequent (N = 225) to technique change of MUL maximization. Effect of innate, mpMRI-assessed MUL on postoperative continence was assessed.
Surgical procedure
Before technique change, the dorsal venous complex was stapled before transection of the membranous urethra. After the change, the final step of extirpation was transection of the dorsal venous complex and periurethral attachments, thus facilitating surgical maximization of MUL.
Measurements
Primary and secondary outcomes for technique change and mpMRI-assessed MUL were both patient-reported 30-day and 1-year pad-free continence after RARP, respectively.
Results
Preoperative prostate-specific antigen, age, and disease aggressiveness were significantly higher in Group 2. After technique change and surgical maximization of MUL, 30-day and 1-year pad-free continence were both significantly improved (p < 0.05). In multivariate analysis, maximization of MUL significantly increased the likelihood of both early- and long-term continence recovery. For men undergoing MUL preservation, mpMRI-assessed MUL>1.4 cm also independently predicted higher 30-day (odds ratio: 4.85, 95% confidence interval: 1.24-18.9) and 1-year continence recovery (odds ratio: 11.26, 95% confidence interval: 1.07-118).
Conclusions
Prostatic rotation and circumferential release of apical attachments and maximization of MUL improves continence after RARP. Separately, innate MUL>1.4 cm independently increased 30-day and 1-year continence recovery.
Patient summary
Surgeon efforts to maximize MUL during radical prostatectomy are highly encouraged, as maximally preserved MUL likely improves post-RARP continence recovery. In addition, individual patients’ mpMRI-assessed MUL (approximately >1.4 cm) independently limits continence recovery.
Keywords: Continence, Magnetic resonance imaging, Prostatectomy, Recovery, Technique, Urethral length
1. Introduction
Early recovery of urinary continence is a central goal of robot-assisted radical prostatectomy (RARP) 1, 2. Incontinence is recognized as the most important long-term outcome limiting the benefit of RARP, such that post-RARP incontinence was cited as a major contributing factor to the 2012 United States Preventive Services Task Force recommendation that complications of prostate cancer treatment outweigh benefits of prostate-specific antigen (PSA) screening 3. Even further, recovery of early continence is closely correlated with the patient quality of life 4.
Membranous urethral length (MUL) has been explored both as a preoperative indicator of early continence recovery after RARP as well as the one which is amenable to surgical manipulation 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. The first suggestion of a preoperative correlation was reported in 2002 by Coakley et al 12: men with longer multiparametric magnetic resonance imaging (mpMRI)–assessed MUL were ostensibly “predetermined” to benefit from faster and more complete recovery of urinary continence after open RARP. Subsequently, in 2004, van Randenborgh et al 13 published the first findings of surgical maximization of MUL. Comparing 610 control RARP cases with 403 procedures performed with systematic preservation of the membranous urethra, they found that pad-free continence significantly improved from 76.02% to 88.4% 13. These findings were revisited seven years later in 2011, by two different groups. First, Schlomm et al 14 demonstrated a significant improvement in 7-day pad-free continence after adoption of MUL maximization techniques during open RARP. The same technique was then applied to RARP by Hakimi et al 33, with similar success. Finally, Mungovan et al 15 published a systematic review that further focused on preexisting mpMRI-assessed MUL, reporting that every extra millimeter of MUL increased the odds of pad-free continence by 9%.
Although the results of the systematic review suggest stepwise benefit of longer innate MUL, we questioned if surgical maximization could trump this effect of predetermined MUL. In this study, we assessed the impact of surgically maximized MUL on early continence with cutting the dorsal venous complex (DVC) without prior stapling and stepwise release of the periurethral attachments, while concurrently evaluating for impact of innate mpMRI-assessed MUL.
2. Methods
2.1. Patients
A total of 605 men underwent RARP by a single surgeon: 370 cases undrwent our standard, previously described procedure (stapling the DVC) 16, 17, and 235 underwent RARP with surgical maximization of the membranous urethra. Of the 370 cases before technique change, 15 men were excluded because of lack of follow-up (Group 1, n = 355). Of the 235 cases subsequent to technique change, 10 men were similarly excluded because of lack of follow-up (Group 2, n = 225). Management protocol for both groups included perioperative Kegel exercises and post-RARP discharge with an indwelling urinary catheter that is removed 6-7 days after RARP.
Data including continence status, patient age, preoperative American Urological Association Symptom Score, PSA level, prostate weight, estimated blood loss, body mass index, pathologic stage, pathologic Gleason score, surgical margin status, and seminal vesicle invasion status were prospectively collected and entered into an electronic database under approval from an institutional review board protocol (HS# 1998-84). Complications were tracked via an institutional Data, Safety, and Monitoring Board and systematically recorded through postoperative Day 30.
2.2. Assessment of Preoperative MRI MUL
The impact of innate or preexisting preoperative MUL was assessed in men undergoing RARP with urethral length preservation. One hundren twenty-three patients underwent standard pelvic T3 MRIs (with and without contrast), and all studies were assessed by a single, specialized pelvic MRI radiologist. MUL was recorded from coronal and sagittal views of the pelvis, as the distance from the prostatic apex to the entry of the urethra into the penile bulb 15. The radiologist was blinded to all clinical and urinary continence outcomes.
2.3. Surgical Technique and Anatomic Considerations
RARP in Group 1 was performed as previously described using an Ethicon Surgical Staplers J&J Medical Devices, Johnson and Johnson 17. The anterior prostatic fat was removed, the endopelvic fascia was incised bilaterally, and the puboprostatic ligaments were transected to maximally release the apex so that the stapler could be easily applied for ligation and transection of the DVC (Video clip 1). The entire prostate was then freed in preparation for the last step: transection of the urethra and bagging the prostate.
In Group 2, to maximize preservation of MUL, the final step of removing the prostate was transection of the DVC and apical attachments (Video clip 2). The DVC is transected without ligation, allowing for full rotation of the prostate and circumferential tension–reduced release of the apical attachments: the external striated muscle and the membranous urethra. As experience dictated, a more definitive attempt was made to extend MUL as much as possible (Video clip 3). Reconstruction was the same throughout 17, using a standard Rocco stitch 18 followed by a van Velthoven anastomosis 19. Per standard technique, no drains were placed in either group.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.prnil.2019.12.005
The following is/are the supplementary data related to this article:
2.4. Pad-free Continence Outcome Assessment
Since 2008, we have maintained a prospective, systematic, and highly redundant protocol to independently assess patient-reported “pad-free” continence status. We have extensively evaluated methods to accurately assess patient-reported time to pad-free continence after RARP. The methods have been externally audited and published and have shown to have reliable response rates of 74.3% to 85.1% 20, 21, 22, 23, 24. Via this system, pad-free continence was assessed in four ways. First, after catheter removal, all patients were asked to keep a daily log of the number of urinary pads used per 24 hours, to be electronically transmitted to our research team once the patient had achieved three consecutive days without use of pads. Second, patients were also given a prestamped “pad-free” postcard that was also returned after three consecutive days of pad-free status. We previously showed excellent correlation between the pad-free cards and daily urinary pad logs in assessment of time-to-continence recovery 24. Third, if we had no response after 30 days to either of the first two methods, patients were called by a nonclinical research associate for pad status. If the patient was not pad-free at 30 days, they were called again at 60 and 90 days, as needed. Fourth, pad status was assessed via patient-reported questionnaires at routine follow-up after RARP.
2.5. Learning Curve Analysis
As experience grew with the new technique, we queried the database for 30-day continence rates for evidence of a learning curve. Because of the need for just 30-day follow-up, we were able to expand this analysis up to 500 men. Thirty-day continence rates were also assessed in a similar manner in Group 1.
2.6. Statistical Analysis
Statistical analysis was conducted in SPSS, version 25, © International Business Machines Corporation (IBM) Corporation. The primary and secondary outcome measures were 30-day and 12-month pad-free continence, respectively. A tertiary outcome measure of time-to-continence recovery was also assessed. Two-sided student t tests were used to compare continuous variables. The Fisher exact and Pearson's chi-square tests were used to compare categorical variables. Univariate associations between patient characteristics and continence recovery at 30 days and 12 months after RARP were examined using Pearson correlations. Multivariate associations between patient characteristics and continence recovery at 30 days and 12 months were analyzed using logistic regression models, adjusting for age, pathologic Gleason score, preoperative PSA, seminal vesicle involvement, pT2-positive margins, preoperative IIEF-5 International index of Erectile Function 5 (IIEF-5) score, and nerve-sparing status.
A p-value of < 0.05 was considered to be statistically significant, and variables that had significant univariate associations were included in the final multivariate models. Finally, time-to-continence recovery, as adjusted for significant covariates, was analyzed in cox regression modeling and plotted as a 1 - [% survival] curve, stratified by technique change. Post hoc, a linear regression model was fitted to 30-day continence recovery curves to assess for learning curve effect after technique change. Goodness of fit was assessed with an R2 coefficient of determination.
To assess the impact of innate MUL on continence, the aforementioned analysis was repeated for Group 2 patients with preoperative, mpMRI-assessed MUL. Receiver operator characteristic curves were generated with 30-day continence as the outcome measure, and a cutoff of 1.4 cm was determined to maximize sensitivity (93.2%) and specificity (88.1%). Using this cutoff, a power analysis was conducted based on level 0.05 (two-sided) test of equality of the probability for overall continence comparing patients with innate MUL>1.4 cm with those with innate MUL≤1.3 cm. A power analysis was performed based on our initial experience, where patients with an MUL>1.4 cm recovered long-term pad-free continence 100% of the time, compared with 85% for patients with MUL≤1.3 cm. Using this effect size, mpMRI-assessed MUL of 120 patients was required to achieve 90% power for detection of a 15% increase in the probability of continence.
3. Results
3.1. Demographics and Safety
Table 1 presents the demographics of both groups. By happenstance, the change in technique occurred approximately at the same time as the Grade D recommendation by the US Task Force against PSA screening in 2012. 25, 26 Hence, preoperative age, PSA levels, clinical Gleason Grade (cGG), the number of cores involved, and % core involvement were higher among Group 2 men presenting for RARP, and pathologically, there was significantly more advanced disease (pGS, p-stage, and positive surgical margins, p < 0.05). Regarding safety of technique change, there were no significant increases in major or minor complications, estimated blood loss, 24-hour change in hemoglobin, or rate of pT2-positive surgical margins.
Table 1.
Baseline characteristics of patients prior (Group 1) and subsequent (Group 2) to surgical technique change
| Demographics | Group 1 (N = 355) | Group 2 (N = 225) | P | ||
| Mean |
SD |
Mean |
SD |
||
| Age (years) | 61.6 | 7.51 | 62.8 | 7.45 | 0.054 |
| Preoperative IIEF-5 | 19.54 | 7.11 | 18.63 | 7.23 | 0.136 |
| PSA (ng/mL) | 6.77 | 6.87 | 10.22 | 17.89 | 0.007 |
| Prostate weight (g) | 53.44 | 20.90 | 54.21 | 22.59 | 0.676 |
| Estimated blood loss | 109 | 39.1 | 96.0 | 36.4 | <0.001 |
| Change in Hgb(a) | 3.0 | 1.4 | 2.5 | 1.4 | <0.001 |
| AUASS | 8.69 | 6.94 | 8.08 | 6.72 | 0.292 |
| Body mass index |
26.79 |
3.51 |
26.73 |
3.61 |
0.968 |
| N |
% |
N |
% |
p |
|
| Pathological stage | 0.049 | ||||
| pT2 | 242 | 68.2 | 135 | 60 | |
| pT3/pT4 | 113 | 31.8 | 90 | 40 | |
| Pathological Gleason score | 0.007 | ||||
| ≤6 | 91 | 25.6 | 41 | 18.6 | |
| 7 | 225 | 63.4 | 135 | 61.1 | |
| ≤8 | 39 | 11 | 45 | 20.3 | |
| SVI | 22 | 6.2 | 32 | 14.2 | 0.002 |
| Overall PSM | 30 | 8.5 | 45 | 20.1 | <0.001 |
| pT2 apical | 8 | 2.3 | 10 | 4.4 | 0.148 |
SD, standard deviations; AUASS, American Urological Association symptom score; PSA, prostate-specific antigen; IIEF-5, International Index of Erectile Function - 5; SVI, Seminal Vesicle Invasion. Bold indicates p<0.05.
Calculated as the difference between preoperative and 24-hour postoperative Hgb values.
3.2. Urinary Continence Recovery
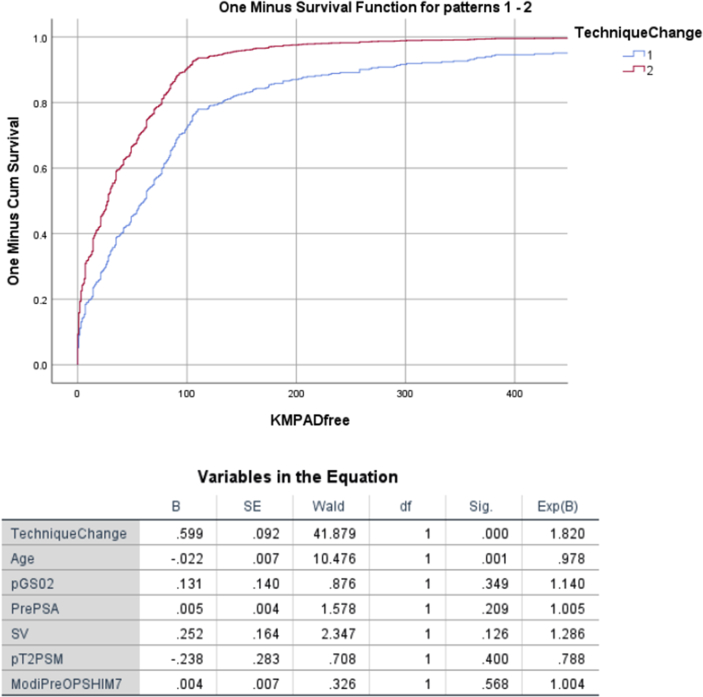
The accumulated patient-reported questionnaire response rates were 96.0% and 95.7% in Groups 1 and 2, respectively. In unadjusted analyses, the 30-day pad-free continence rates in Groups 1 and 2 were 28.2% (100/355) and 55.6% (125/225), respectively (p < 0.0001). The 1-year pad-free continence rates were 88.7% (315/355) and 93.8% (211/225), respectively (p = 0.0441). After adjusting for the aforementioned covariates, technique change improved both 30-day [odds ratio (OR): 3.671, 95% confidence interval (CI): 2.522-5.342] and 1-year continence recovery (OR: 1.886, 95% CI: 1.002-3.592) [Table 2]. Fig. 1 depicts adjusted Kaplan–Meier time-to-continence recovery curves between Group 1 and Group 2 (p < 0.0001). Both age and technique change were the only significant predictors of an earlier time-to-continence and 1-year continence recovery [Table 3] .
Table 2.
Multivariate regression model of variables predicting 30-day continence
| Variables | B | SE | Wald | p | OR | 95% CI |
|
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Technique change | 1.3 | 0.191 | 46.126 | <0.001 | 3.671 | 2.522 | 5.342 |
| Age (cont.) | 0.043 | 0.014 | 9.564 | 0.002 | 1.044 | 1.016 | 1.073 |
| Preoperative PSA (<10 v. >10 [ref]) | −0.005 | 0.008 | 0.373 | 0.541 | 0.995 | 0.979 | 1.011 |
| pT2, positive margins | −0.924 | 0.544 | 2.884 | 0.089 | 0.397 | 0.137 | 1.153 |
| Preoperative IIEF-5 (cont.) | −0.025 | 0.015 | 2.652 | 0.103 | 0.976 | 0.947 | 1.005 |
| Nerve-sparing (any v. none [ref]) | −0.789 | 0.582 | 1.835 | 0.176 | 0.454 | 0.145 | 1.423 |
| Constant | −1.648 | 1.168 | 1.992 | 0.158 | 0.192 | ||
CI, confidence interval; OR, odds ratio; PSA, prostate-specific antigen; SE, standard error.
Fig. 1.
Adjusted analysis Cox regression model of variables predicting time-to-continence recovery is shown.
Table 3.
Multivariate regression model of variables predicting overall continence
| Variables | B | SE | Wald | p | OR | 95% CI |
|
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Technique change | 0.635 | 0.329 | 3.726 | 0.044 | 1.886 | 1.002 | 3.592 |
| Age (cont.) | −0.063 | 0.023 | 7.415 | 0.006 | 0.939 | 0.897 | 0.982 |
| Preoprative PSA (<10 v. >10 [ref]) | −0.232 | 0.411 | 0.32 | 0.572 | 0.793 | 0.354 | 1.774 |
| pT2, positive margins | 0.438 | 0.79 | 0.307 | 0.580 | 1.549 | 0.329 | 7.288 |
| Preoperative IIEF-5 (cont.) | 0.014 | 0.021 | 0.447 | 0.504 | 1.014 | 0.973 | 1.057 |
| Nerve-sparing (any v. none [ref]) | −0.838 | 0.635 | 1.745 | 0.187 | 0.432 | 0.125 | 1.5 |
| Constant | 5.61 | 1.948 | 8.291 | 0.004 | 273.067 | ||
CI, confidence interval; OR, odds ratio; PSA, prostate-specific antigen; SE, standard error.
3.3. Impact of MRI-assessed Preoperative MUL
A total of 123 (34.6%) from Group 2 had a preoperative, MRI-assessed MUL. The average MUL was 1.83 cm, with 16 (13%) having an MUL<1.4 cm and 107 (87%) with MUL≥1.4 cm, and clinical demographics were compared in Supplemental Table 1a. Thirty-day pad-free continence in these groups was 31.3% (5/16) and 59.8% (64/107), respectively (p = 0.033). Similarly, 1-year continence rates were 75% (12/16) and 96.2% (101/105), respectively (p = 0.0174).
In multivariate analysis, both categorical MUL>1.4 cm (OR: 4.851, 95% CI: 1.241-18.961) and age (OR: 0.925, 95% CI: 0.873-0.980) were significant predictors of 30-day continence recovery (Supplemental Table 2a). MUL>1.4 cm (OR: 11.255, 95% CI: 1.070-118.407) was the only significant predictor of 1-year continence recovery (Supplemental Table 2b).
3.4. Learning Curve Assessment
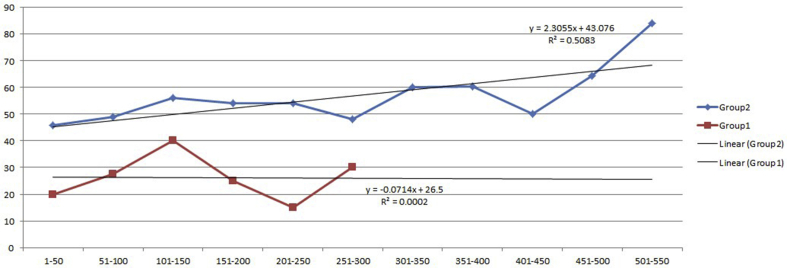
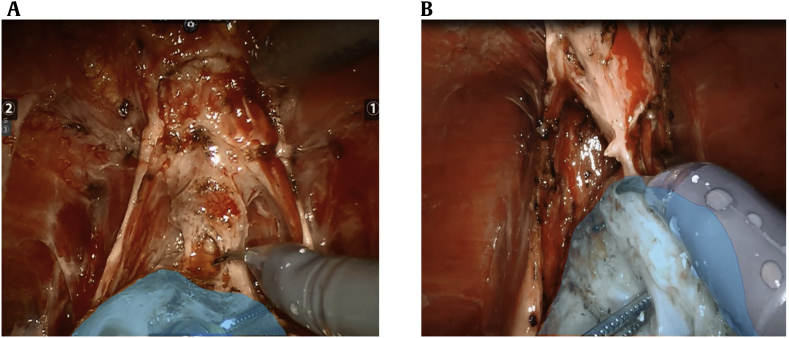
As our surgical experience progressed with the specific intent of increasing MUL (particularly posteriorly), we queried continence outcomes to assess for a learning curve. Fig. 2 illustrates a learning curve effect in Group 2 30-day continence rates (R2 = 0.392) in our initial 500 patients, whereas no learning curve was identified in Group 1 30-day continence rates. In Fig. 3a, the line of transection of the urethra few millimeters from the prostatic apex can be seen. As our experience progressed, we specifically addressed longer urethral length by releasing and transecting the urethra right at the prostatic apex (Fig. 3b). A statistically significant stepwise increase in 30-day continence rates from 50.8% to 67.7% was seen in the most recent 120 patients, p < 0.05.
Fig. 2.
Learning curve of 30-day continence (per 50 patients) is shown.
Fig. 3.
(A) Membranous urethral length (MUL) preparation early after technique change. Compared with (A) (below), there is transection of more striated sphincter, further from the prostatic apex (blue). (B) MUL preparation after learning curve, demonstrating stretching and rotating to maximally preserve MUL. Compared with (A) (above), the urethra is under greater traction, transected right on the prostate (blue), with less striated sphincter included.
4. Discussion
The first major publication regarding MUL by Coakley et al 12 highlighted MRI-assessed MUL as an independent impactor of continence recovery after RARP. In their report, there was no mention that surgical preservation of MUL was a mechanism to increase continence recovery after RARP. It was not until 2004 and 2011 that van Randenborgh et al 13 and Schlomm et al 14 demonstrated convincing evidence that surgeons could physically preserve greater MUL during open RARP, to improve both time-to-continence and overall continence rates. An important question is whether surgical preservation can overcome an unfavorable innate MUL; alternatively, do both factors remain? In this regard, our study is the first to report that both findings, longer innate MUL and the surgical preservation of MUL, dramatically impact continence. Not only do our results further support that preservation of every millimeter of MUL is a critical factor surgeons can adapt but our analysis also supports that an innate component of MRI-assessed MUL 15, independent of surgical preservation, impacts time-to-continence and overall urinary pad-free continence after RARP.
The primary finding of our study is that a technique change of surgical preservation followed by further attempts of maximization of MUL improved early continence—both 30-day and time to pad-free. The major strength of our study was the consistent nature and quality of patient-reported continence data collection before and after the change in technique. Critical to the reliability of the findings is data collection outside the outpatient/hospital setting. To this end, we reduced bias as our patients mailed or faxed their outcomes to us. This highly systematic, prospective, and redundant mechanism corroborated the two means of assessing patient-reported time to pad-free continence 23. Both daily urinary pad logs and/or pad-free continence postcards demonstrated excellent correlation in assessing patient-reported pad-free continence (R2 = 0.98). By happenstance, changes in the United States prostate cancer screening practices in 2012 were coincident 25, 26 with our technique change and created significant demographic differences between Groups 1 and 2. However, even with older men and higher PSA levels in Group 2, the unadjusted 30-day continence rate increased from 28% (Group 1) to 56% (Group 2). After controlling for these factors in multivariate analysis, only age and technique change were significant predictors of continence. The effects of age were modest compared with MUL preservation: a patient in Group 2 was estimated to be 3.77 times more likely to recover pad-free continence at 30 days and 1.81 times more likely to recover by 1 year after RARP.
We observed and suggest that transecting the DVC without prior ligation (i.e., stapled, in our case) enhances tension-free, stepwise, and circumferential release of periurethral attachments. Before the completion of dividing the veins of the DVC, bleeding can impede visualization, complicating this approach. Fortunately, owing to the ambient pressure of the pneumoperitoneum (12 mmHg, which can be safely elevated to 18 mm for 5-10 minutes), once the veins are fully transected, minimal bleeding ensues until the prostate is removed. Although bleeding may initially be unnerving, it was never clinically relevant as no patient required transfusion. As noted in Table 1, the difference in preoperative and postoperative hemoglobin levels reduced from 3.0 to 2.5. Similar to Bianchi et al , we found no impact on our positive surgical margin rate. With experience, we also learned that the more the prostate was rotated, the easier it was to completely dissect the membranous urethra circumferentially, especially posteriorly. Comparing Fig. 2 with Fig. 3, greater MUL (in blue) was maximized as the urethra was transected closer and closer to the prostate. With experience and continued improvement noted in Fig. 2, there was a stepwise increase in 30-day continence rates, further inferring the importance of each millimeter of preservation and supporting the millimeter by millimeter benefit, as predicted by Mungovan et al in 2017 15.
Limitations of the present study include a retrospective, single-surgeon study design. Assessment of technique change in a single surgeon reliably reduced variability in outcomes; it does not allow for the generalized applicability of a multicentered randomized control trial (RCT). However, RCTs are limited, as they are designed to control for a single confounder. By necessity, multicentered surgical RCTs are susceptible to at least two confounders: technique adoption and surgeon variability. Our personal experience with a multicentered high-volume-surgeon RCT revealed a significant impact of surgeon variability on powering 28, 29, 30. Given that we found a 30-fold difference in 30-day continence rates, the present study would require more than 120 patients per surgeon to attenuate the variability in surgical outcomes. The accompanying expenses and administrative oversight are typically prohibitive 31, 32.
Previous studies primarily from Asia and Europe, combined with the present analysis, support the impact of innate MRI-assessed MUL and the importance of surgical maximization. However, given that this practice is more common among Asian centers, our findings suggest the need for increased emphasis of MUL maximization in Europe and the United States. Our findings also demonstrate that regardless of the benefit in maximizing MUL, an innate “predetermined” length of native membranous urethra less than approximately 1.4 cm reduces both 30-day and 1-year continence recovery. This innate MUL threshold appears to exist for about 15% of men.
5. Conclusion
Our findings add further support to both the innate impact of MRI-assessed MUL as well as the impact of each millimeter of MUL spared on early- and long-term pad-free continence. In our experience, the present technique of prostatic rotation and circumferential release of apical attachments effectively maximizes MUL during RARP.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgments
This work was accomplished with special regards to Drs. Edward and Arthur Lui, in memory of their parents Mr. & Mrs. L.H.M. Lui. Special thanks to the research students, Whitney Zhang and Victoria Lee, for their dedication to data management and patient follow-up.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2019.12.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rodriguez E., Skarecky D.W., Ahlering T.E. Post-robotic prostatectomy urinary continence: characterization of perfect continence versus occasional dribbling in pad-free men. Urology. 2006;67:785–788. doi: 10.1016/j.urology.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V., Novara G., Rosen R., Artibani W., Carroll P.R., Costello A. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–417. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Moyer V., on behalf of the U.S Preventive services task force. screening for prostate cancer: u.s. preventive services task force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 4.Liss M.A., Osann K., Canvasser N., Chu W., Chang A., Gan J. Continence definition after radical prostatectomy using urinary quality of life: evaluation of patient reported validated questionnaires. J Urol. 2010;183:1464–1468. doi: 10.1016/j.juro.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Heesakkers J., Farag F., Bauer R., Sandhu J., Ridder D., Stenzl A. Pathophysiology and contributing factors in postprostatectomy incontinence: a review. Eur Urol. 2017;71:936–944. doi: 10.1016/j.eururo.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Cho D.S., Lee E.J., Kim S.K., Kim S. The influence of membranous stretched urethral length and urethral circumference on postoperative recovery of continence after radical prostatectomy: a pilot study. Can Urol Assoc. 2015;9:5–6. doi: 10.5489/cuaj.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satake Y., Kaiho R., Saito H., Yamada T., Kawamorita N., Yamashita S. Estimated minimal residual membranous urethral length on preoperative magnetic resonance imaging can be a new predictor for continence after radical prostatectomy. Urology. 2018:138–144. doi: 10.1016/j.urology.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Song W., Kim C.K., Park B.K., Jeon H.G., Jeong B.C., Seo S.I. Impact of preoperative and postoperative membranous urethral length measured by 3 Tesla magnetic resonance imaging on urinary continence recovery after robotic-assisted radical prostatectomy. Can Urol Assoc J. 2017;11:3–4. doi: 10.5489/cuaj.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadono Y., Nohara T., Kawaguchi S., Naito R., Urata S., Nakashima K. Investigating the mechanism underlying urinary continence recovery after radical prostatectomy: effectiveness of a longer urethral stump to prevent urinary incontinence. BJU Int. 2018;122:456–462. doi: 10.1111/bju.14181. [DOI] [PubMed] [Google Scholar]
- 10.Paparel P., Akin O., Sandhu J., Otero J.R., Serio A.M., Scardino P.T. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009;55:629–639. doi: 10.1016/j.eururo.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 11.Walz J., Epstein J.I., Ganzer R., Graefen M., Guazzoni G., Kaouk J. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70:301–311. doi: 10.1016/j.eururo.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Coakley F.V., Eberhardt S., Kattan M.W., Wei D.C., Scardino P.T., Hricak H. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002;168:1032–1035. doi: 10.1016/S0022-5347(05)64568-5. [DOI] [PubMed] [Google Scholar]
- 13.van Randenborgh H., Paul R., Kubler H., Breul J., Hartung R. Improved urinary continence after radical retropubic prostatectomy with preparation of a long, partially intraprostatic portion of the membranous urethra: an analysis of 1013 consecutive cases. Prostate Cancer Prostatic Dis. 2004;7:253–257. doi: 10.1038/sj.pcan.4500726. [DOI] [PubMed] [Google Scholar]
- 14.Schlomm T., Heinzer H., Steuber T., Salomon G., Engel O., Michl U. Full functional-length urethral sphincter preservation during radical prostatectomy. Eur Urol. 2011;60:320–329. doi: 10.1016/j.eururo.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 15.Mungovan S.F., Sandhu J.S., Akin O., Smart N.A., Graham P.L., Patel M.I. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: a systematic review and meta-analysis. Eur Urol. 2017:368–378. doi: 10.1016/j.eururo.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh L.M., Ahlering T.E. Robot-assisted radical prostatectomy: a step-by-step guide. J Endourol. 2018;32:S28–S32. doi: 10.1089/end.2017.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlering T.E., Eichel L., Edwards R.A., Lee D.I., Skarecky D.W. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology. 2004;64:1224–1228. doi: 10.1016/j.urology.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Patel V.R., Coelho R.F., Paler K.J., Rocco B. Periurethral suspension stitch during robot-assisted radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009;56:472–478. doi: 10.1016/j.eururo.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.van Velthoven R.F., Ahlering T.E., Peltier A., Skarecky D.W., Clayman R.V. Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology. 2003;61:699–702. doi: 10.1016/s0090-4295(02)02543-8. [DOI] [PubMed] [Google Scholar]
- 20.Pick D., Skarecky D.W., Osann K., Narula N., Finley D., Ahlering T.E. The impact of cavernosal nerve preservation on continence following robotic radical prostatectomy. BJU Int. 2011;108:1492–1496. doi: 10.1111/j.1464-410X.2010.10015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarecky D., Morales B., Chang A., Ahlering T. Simple prediction method of return to pad- free continence for men undergoing robotic radical prostatectomy (RARP) J Endourol. 2011;25:1451–1455. doi: 10.1089/end.2011.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlering T., Gordon A., Morales B., Skarecky D. Preserving continence during robotic prostatectomy. Curr Urol Rep. 2013;14:52–58. doi: 10.1007/s11934-012-0295-4. [DOI] [PubMed] [Google Scholar]
- 23.Gordon A., Skarecky D.W., Ahlering T. Long-term outcomes in severe lower urinary tract symptoms in men undergoing robotic-assisted radical prostatectomy. Urology. 2014;84:826–831. doi: 10.1016/j.urology.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Skarecky D., Van T., Morales B., Gordon A., Ahlering T. Continence postcards versus urinary pad logs: simple methods to measure early pad-free urinary continence after radical prostatectomy. Urol Pract. 2014;4:378–382. doi: 10.1016/j.urpr.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Barocas D.A., Mallin K., Graves A.J., Penson D.F., Palis B., Winchester D.P. Effect of the USPSTF grade d recommendation against screening for prostate cancer on incident prostate cancer diagnosis in the United States. J Urol. 2015;194:1587–1593. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 26.Ahlering T., Huynh L.M., Kaler K.S., William S., Osann K., Joseph J. Unintended consequences of decreased PSA-based prostate cancer screening. World J Urol. 2018 doi: 10.1007/s00345-018-2407-3. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Huynh L.M., Skarecky D.S., Porter J., Wagner C., Witt J., Wilson T. A randomized control trial of regional hypothermia on urinary continence during robot-assisted radical prostatectomy. Sci Rep. 2018 doi: 10.1038/s41598-018-34657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianco F.J., Albala D.M., Belkhoff L.H., Miles B.J., Peabody J.O., He W. A randomized, double-blind, solifenacin succinate versus placebo control, phase 4, multicenter study evaluating urinary continence after robotic assisted radical prostatectomy. J Urol. 2015 doi: 10.1016/j.juro.2014.09.106. [DOI] [PubMed] [Google Scholar]
- 30.Freiman J.A., Chalmers T.C., Smith H., Kubler R.R. The importance of beta, the type ii error and sample size in the design and interpretation of the randomized control trial: survey of 71 negative trials. N Engl J Med. 1978;299:690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- 31.Hind D., Reeves B.C., Bathers S., Bray C., Corkhill A., Hayward C. Comparative costs and activity from a sample of UK clinical trials units. Trials. 2017;18:203. doi: 10.1186/s13063-017-1934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frieden T.R. Evidence for health decision making - beyond randomized controlled clinical trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]
- 33.Hakimi A.A., Faleck D.M., Agalliu I. Preoperative and intraoperative measurements of urethral length as predictors of continence after robot-assisted radical prostatectomy. J Endourol. 2011;25:1025–1030. doi: 10.1089/end.2010.0692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.