Abstract
Objectives
The research aims to assess the regenerative potential of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in immature permanent maxillary central incisors with necrotic pulps, clinically and radiographically.
Trial design
Double blinded parallel randomized controlled trial was implemented to identify the results.
Subject & methods
The proposed study was conducted among 30 patients with maxillary necrotic permanent immature central incisors but only 26 patients fulfilled the study requirements. Group I was treated with PRP and Group II with PRF scaffolds. Follow up has been done every 3 months for one year. Primary outcomes were measured clinically: Pain, Mobility, Swelling, and Sinus/fistula. Radiographically: increase root length and width. Secondary outcomes were clinically: Discoloration and Sensibility test. Radiographically: increase in bone density measurements and decrease in apical diameter. Standardized radiographs were collected during the follow up period, and radiographic changes were measured by using Image J software. Statistical analysis was performed on 25 patients who had completed the study.
Results
All 25 patients' teeth were survived during the 12-month follow-up period. PRP showed marginal increase in radiographic root length and width, periapical bone density and a decrease in apical diameter. No statistical significant differences were observed when it was compared with PRF. The teeth which were treated did not respond to sensibility test at the end of the study. PRF displayed statistical significant higher amount of crown discoloration when compared to PRP group.
Conclusions
For necrotic immature teeth, revascularization using PRP is an appropriate alternative to PRF and showed excellent 12-months prognosis.
Keywords: Platelet rich plasma, Platelet‑rich fibrin, Immature necrotic permanent maxillary central incisors, Revascularization/revitalization
1. Introduction
Trauma caries or developmental anomalies may threaten the developing dentition by causing pulp necrosis. It leads to root growth cessation with subsequent open apex and thin dentin walls which can display potential difficulties and complications during and after root canal treatment (Do, 2012).
Apexification is a traditional management used to preserve non vital immature permanent teeth for many decades. It is the process by which a calcific barrier is shaped across the open apex. Because of this process neither further root lengthening nor width are achieved (Rafter, 2005).
Recently, there is a strong interest in endodontic regeneration as a replacement to apexification. It may allow for developing tooth maturogenesis which might lower fracture rate and premature tooth loss related with apexification treatment (Nosrat et al., 2011). Regenerative endodontic procedures (REPs) can be defined as “biologically based procedures designed to replace damaged structures including dentin, root and cells of the pulp-dentin complex”. The basis for regenerative endodontic procedures is the utilization of tissue engineering triad (Lenzi and Trope, 2012).
Stem cells for dental pulp tissue engineering could be divided into two broad strategies: cell-based approaches that are based on the transplantation of stem cells into the root canal, and cell-free approaches or cell homing that rely on the chemo-attraction of host cells into the root canal (Nakashima and Iohara, 2011). The primary choice of cells is typically the resident tissue-specific stem cells. In the case of the dental pulp, the dental pulp stem cells from permanent teeth (DPSC) as well as stem cells from apical papilla (SCAP) are tissue specific stem cells. They actively participate in regenerative endodontic procedures. Stem cells can survive in punitive environment as in bacterial necrotic infected tissue (Casagrande et al., 2010).
Growth factors are required for odontoblastic differentiation and regulation from stem cells. Scaffolds provide an organization structures for stem cells to distribute and arrange for proliferate and differentiate into odontoblasts (Kim et al., 2012). Encouraging bleeding into the root canal by sharp sterile instrument and succeeding clot formation is the most widely used scaffold in regenerative endodontics. Although it is not always possible to evoke bleeding in the root canal, researchers have begun examining other three-dimensional scaffolds. However, platelet concentrates have been proposed as a possible model scaffold for regenerative endodontic techniques (Torabinejad et al., 2011).
Platelet concentrate forms a scaffold and resorbs overtime as they contain elevated platelet count and thus greater amounts of growth factors to assist in stem cell proliferation for healing induction and tissue regeneration. As the first generation of platelet concentrates, Platelet Rich Plasma (PRP) has been revealed as a possibly model scaffold for regenerative endodontic treatment (Alsousou et al., 2013). Being the second generation of platelet concentrates, platelet-rich fibrin (PRF) has many merits over PRP. First, it does not require the addition of anticoagulant. Second, PRF platelets and leukocytes entrapped inside fibrin gel, liberating growth factors in sustained long time. Third, immune cells and cytokines in a PRF clot could counteract infection (Dohan et al., 2006). So it is essential to compare between them to reveal which is superior to be used in regenerative endodontic procedures. In case of no bleeding initiates or better treatment outcomes and best practice desired based on evidence. So we aimed to evaluate & compare the regenerative potential of PRP and PRF scaffolds in maxillary immature permanent central incisors with necrotic pulp, clinically & radiographically.
2. Subjects and methods
2.1. Trial design
The study is a randomized clinical trial (RCT) between two arms parallel groups with allocation ratio 1:1. In addition, it is a double blinded study in which the child and parents/legal guardian of each participant was blinded. Furthermore, the outcome analyzer and the statistician who performed the study analysis were blinded.
2.2. Sample size determination
A total of thirty immature anterior maxillary permanent incisors in thirty subjects of both gender were included. Determined according to sampling method described by Pozos-Guillén et al. (2017).
2.3. Study setting
Subjects were randomly selected from patients seeking treatment from outpatient clinic of Pediatric Dentistry of the institution. Screening of patients continued until the target number to satisfy the inclusion criteria was achieved which took over a period of 19 months from November 1, 2015 to May 25, 2017 and met predetermined selection criteria.
2.4. Eligibility criteria: (AAE, 2018)
Inclusion criteria: subjects were free from any chronic systemic disease, both gender with age range from 8 to 14 years, tooth in question is restorable, permanent necrotic maxillary central incisors with incomplete root development defined by apical foramen ≥1.0 mm, with or without periapical lesions, pulp involvement is either due to caries or trauma and selected teeth have never been subjected to any line of endodontic treatment. Exclusion criteria: any patient showed previously or during the study allergic response to ciprofloxacin, metronidazole or minocycline or against any materials used in the study, radiographic or clinical identification of ankylosis, root resorption, root fracture, uncooperative patients and legal guardians did not consent to participate in the study.
2.5. Randomization & blinding
2.5.1. Sequence generation
The study started with 30 patients. Sequence generation was done for the patient’s number from 1 to 30 using computer sequence generation (www.random.org).
2.5.2. Allocation concealment
The upper central incisors in each group was randomly assigned by a coin toss to be either the PRP on the head side or the PRF on the tail side.
2.5.3. Blinding
It was not possible for the operator to be blinded due to the nature of the treatment received.
2.6. Clinical diagnostic procedure & informed consent
A detailed dental diagnostic chart was utilized to record personal, medical & dental history, clinical and radiographic examination, and treatment plan & follow up. An informed consent was obtained from each patient’s parents before beginning any procedure explaining intended treatment, the possible outcomes, complications, follow up period needed and sequela of no treatment.
2.7. Radiographic diagnostic procedure & standardization
Periapical radiographic images were dimensionally standardized through using a film holding device and radiographic stent to obtain a constant tooth- film-cone relationship through the study. Obtained x-ray images were processed using automatic X-ray film processor) DÜRR NDT GmbH & Co. KG, Germany), scanned and saved in a JPEG format (Hamanaka et al., 2012).
2.8. Treatment procedure
Regenerative Endodontic Treatment has been performed according to the American Association of Endodontics protocol (AAE, 2018).
2.8.1. First appointment
Mechanical instrumentation of the root canal walls was avoided as only loose or necrotic pulp tissue were removed using suitable endodontic files. Irrigation was achieved with sodium hypochlorite 2% NaOCl (CHLORA X D 2%, CERKAMED, ul, POLAND) (20 mL/canal, 5 min). ENDO-TOP irrigation needles (25 gauge) side vented (CERKAMED, ul, POLAND) were used. With needle tip positioned about 1 mm from root end, to reduce the cytotoxic effects of the solution on stem cell and vital tissues. Followed by Irrigation with EDTA 17% (20 mL/canal, 5 min) (MASTER-DENT 60 mL REF: 12-750 Dentonics Inc. USA). Triple antibiotic paste (TAP): freshly prepared TAP consisted of Ciprofloxacin, Metronidazole and Minocycline with a ratio of 1:1:1 by weight. The mixed powder was placed inside a mortar for mixing using pestle with equal amount of distilled sterile water to form a homogenous paste-like consistency with final concentration of 0.1 mg/ml. Access cavity was sealed by dry sterile cotton then temporary filling material (Coltosol F, Colten Whaldent, Switzerland) for coronal seal was placed for 21 days.
2.8.2. Second appointment
Response to initial treatment was assessed along with complete resolution of signs and symptoms under Local Anesthesia without vasoconstrictor & rubber dam isolation. The temporary restoration were removed using ultrasonic scaler. Antibiotic dressing was removed by irrigation with 20 mL sterile physiological saline. Irrigation then was performed with EDTA 17% (20 mL/canal, 5 min). Canals were dried with suitable size sterile paper points; Scaffolds was then created according to the assigned group; Platelet Rich Plasma, PRP (group I) and Platelet rich Fibrin, PRF (group II).
2.8.2.1. Group I: PRP scaffold
PRP was prepared according to Dohan and Choukroun (2007) method. Then the obtained PRP was soaked on 2 × 2 ml of sterile collagen sponge and was introduced into the root canal with sterile tweezer and pushed beyond apical region, flushed to level of cemento-enamel junction.
2.8.2.2. Group II: PRF scaffold
PRF was prepared in accordance with the method given by Dohan and Choukroun (2007). The obtained PRF was cut into small fragments using scalpel blade and placed incrementally inside the canal using suitable endodontic pluggers till the level of the cemento-enamel junction. 2 × 2 ml of sterile collagen sponge was introduced above scaffold.
An MTA orifice plug extending 2–3 mm in the canal was used to seal the canal orifice then glass ionomer (GC America, Alsip, IL) and composite (Z 250, 3 M ESPE) to give an effective and durable seal.
2.9. Post treatment evaluation
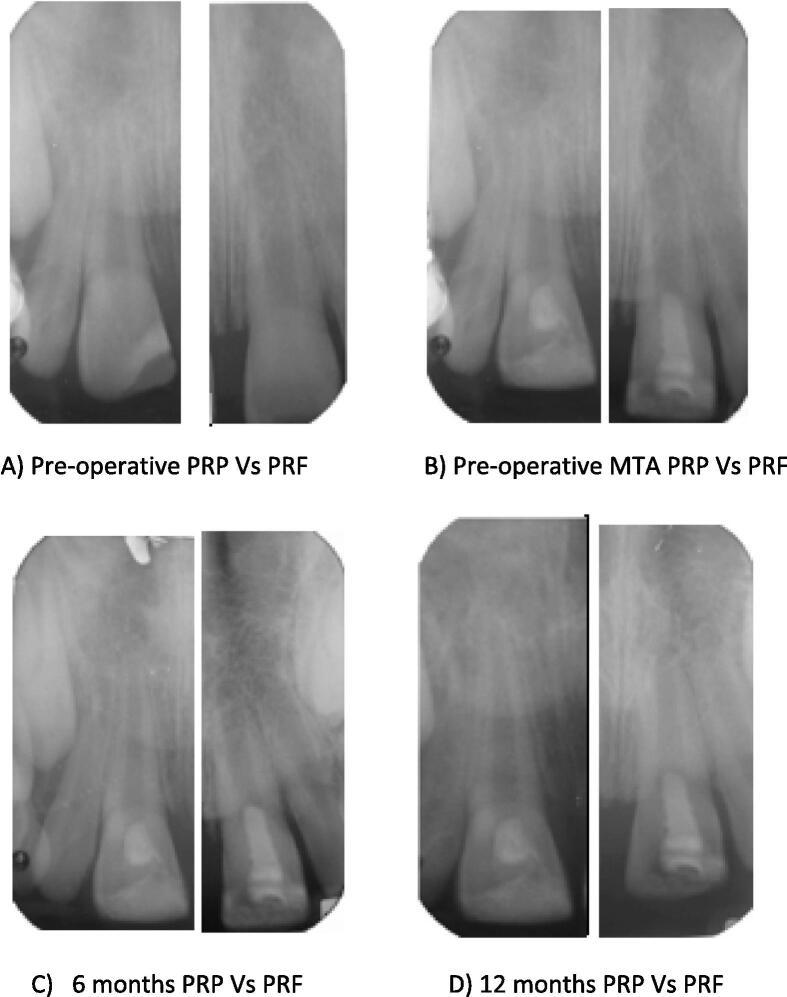
(Fig. 1) All Patients were recalled at 3, 6, 9 and 12 months to evaluate treated teeth. In this study, the primary and secondary outcomes were assessed. The primary outcomes were, clinically: Pain, Mobility, Swelling, and Sinus/fistula. In addition, radiographically: increase root length and width. On the other hand, secondary outcomes were clinically: Discoloration and Sensibility test. Radiographically: increase in bone density measurements and decrease in apical diameter.
Fig. 1.
An example from both groups; PRP tooth #11 vs. PRF tooth #21: Necrotic immature fractured teeth with an open apex in an 9-years-old boy and 8-years old girl respectively. (A) Pre-operative periapical radiograph; (B) After the placement of mineral trioxide aggregate; (C) At 6-month follow-up, with development of the root; (D) At 12-month follow-up, with continued development of the root apex.
2.9.1. Image analysis
Quantitative radiographic judgement of the radiographic outcome was done using Image J software (ImageJ v1.44; US National Institutes of Health, Bethesda, MD). Radiometric measurements and calculations performed by the same method described by Nagy et al. (2014).
2.9.2. Statistical analysis
Statistical analysis was accomplished using software program (SPSS statistical version 19).
3. Results
3.1. Patient flow
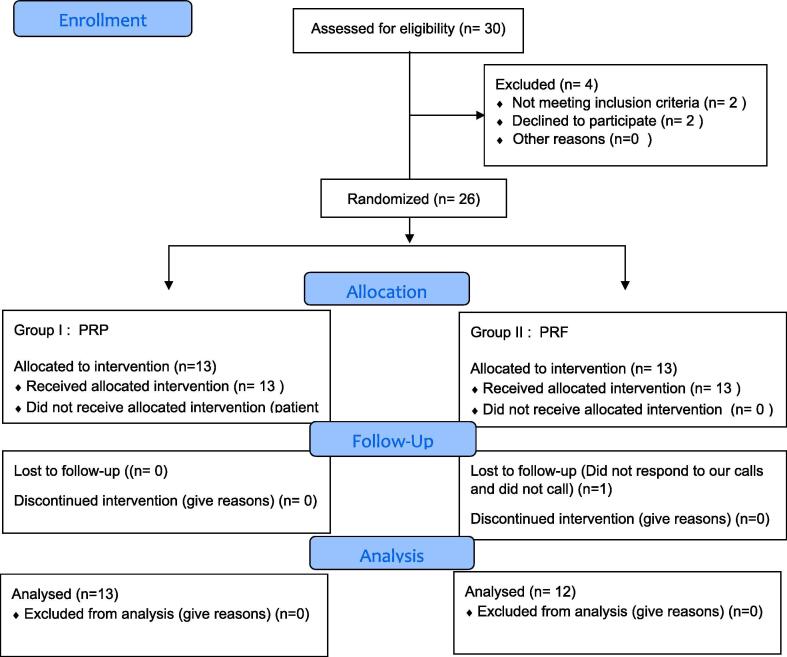
Fig. 2 shows the flow of the patients through the study.
Fig. 2.
CONSORT 2010 flow diagram.
3.2. Demographic variables
Table 1 describes the demographic data for the participants in each group.
Table 1.
Sample description according to the basic characteristics of the patients.
| Variable | PRP | PRF | P value |
|---|---|---|---|
| Mean ± SD | 9.08 ± 1.038 | 9.08 ± 1.165 | 0.989 |
| Male | 7 (53.8%) | 6(50%) | 0.582 |
| Female | 6 (46.2%) | 6 (50%) | |
| Type of Trauma | |||
| Enamel-Dentin-Pulp fracture | 7 (53.8%) | 11(91.7%) | 0.013** |
| Enamel-Dentin | 6(46.2%) | 1 (8.3%) | 0.013** |
| No loss of tooth structure | 0 (0%) | 0 (0%) | |
*Significant at P-value < 0.1.
SD = Standard Deviation
Highly significant at P-value < 0.05.
3.3. Primary & secondary clinical outcomes
No statistically significant differences were found between the two groups in regard to primary clinical outcomes (resolution of pain, swelling, mobility and sinus/fistula), and all cases showed 100% success.
All revascularized/revitalized teeth in the present study did not respond to pulp sensibility tests (thermal (cold/ heat), and electric pulp tester) during follow-up and at the end of the 12-month study.
PRF group showcased higher crown discoloration than PRP group with no statistical significance difference between the groups.
3.4. Primary and secondary radiographic outcomes
Table 2a, Table 2b represent the primary radiographic outcomes (root length and width).
Table 2a.
The increase in root length in millimeters and percentage of the PRP and PRF groups during the four evaluation periods.
| PRP group (mean ± SD) | PRF group (mean ± SD) | p-value | |
|---|---|---|---|
| 3 months (mm, %) | 0.225 ± 0.19 (1.52%±1.43%) | 0.155 ± 0.099 (1.02%±0.673%) | 0.406 |
| 6 months (mm, %) | 0.557 ± 0.23 (3.7%±1.43%) | 0.391 ± 0.187 (2.57%±1.23%) | 0.127 |
| 9 months (mm, %) | 0.996 ± 0.35 (6.6%±2.4%) | 0.793 ± 0.378 (5.2%±2.48%) | 0.174 |
| 12 months (mm, %) | 1.48 ± 0.37 (9.88%±2.85%) | 1.24 ± 0.54 (8.19%±3.64%) | 0.355 |
*Significant at P-value < 0.1.
**Highly significant at P-value < 0.05.
SD = Standard Deviation
Table 2b.
The increase in root width in millimeters and the percentage of the PRP and PRF groups during the four evaluation periods.
| PRP group (mean ± SD) | PRF group (mean ± SD) | p-value | |
|---|---|---|---|
| 3 months (mm, %) | 0.153 ± 0.128 (6.03% ± 5.03%) | 0.19494 ± 0.172 (7.9% ± 6.2%) | 0.47 |
| 6 months (mm, %) | 0.445 ± 0.41 (18.05%±17.45%) | 0.474 ± 0.299 (19.97% ± 12.08%) | 0.503 |
| 9 months (mm, %) | 0.739 ± 0.56 (29.65%±23.9%) | 0.73517 ± 0.34 (30.77% ± 13.26%) | 0.503 |
| 12 months (mm, %) | 0.97 ± 0.75 (39.27% ± 32.04%) | 1.003 ± 0.392 (42.37% ± 16.49%) | 0.574 |
*Significant at P-value < 0.1.
**Highly significant at P-value < 0.05.
SD = Standard Deviation
Table 3a, Table 3b illustrate the secondary radiographic outcomes.
Table 3a.
The increase in bone density in grey value and percentage of the PRP and PRF groups during the four evaluation periods.
| PRP group (mean ± SD) | PRF group (mean ± SD) | p-value | |
|---|---|---|---|
| 3 months (grey value, %) | 24.87 ± 16.63 (27.605% ± 19.89%) | 15.64 ± 11.1 (45.14% ± 74.78%) | 0.345 |
| 6 months (grey value, %) | 34.42 ± 21.03 (42.3% ± 25.46%) | 28.2 ± 16.7 (80.6% ± 145.1%) | 0.345 |
| 9 months (grey value, %) | 52.47 ± 25.39 (57.74% ± 31.36%) | 40.79 ± 19.12 (109.29% ± 171.22%) | 0.414 |
| 12 months (grey value, %) | 65.08 ± 30.043 (71.84% ± 30.043%) | 53.44 ± 22.165 (137.4% ± 203.02%) | 0.345 |
*Significant at P-value < 0.1.
**Highly significant at P-value < 0.05.
SD = Standard Deviation
Table 3b.
The decrease in apical diameter in millimeters and percentage of the PRP and PRF groups during the four evaluation periods.
| PRP group (mean ± SD) | PRF group (mean ± SD) | p-value | |
|---|---|---|---|
| 3 months (mm, %) | 0.25 ± 0.167 (9.91%±6.03%) | 0.34 ± 0.2 (15.7%±8.84%) | 0.246 |
| 6 months (mm, %) | 0.656 ± 0.43 (27.29%±14.1%) | 0.87 ± 0.48 (38.23%±15.03%) | 0.123 |
| 9 months (mm, %) | 2.17 ± 3.86 (51.98%±19.64%) | 1.33 ± 0.57 (58.89%±10.59%) | 0.611 |
| 12 months (mm, %) | 2.49 ± 3.93 (64.83%±18.5%) | 1.73 ± 0.665 (76.75%±8.5%) | 0.437 |
*Significant at P-value < 0.1.
**Highly significant at P-value < 0.05.
SD = Standard Deviation
4. Discussion
The present study was carried out as a double blinded randomized controlled trial on 26 necrotic immature permanent central incisors, and it was divided randomly into two groups to evaluate & compare the regenerative potential of PRP and PRF scaffolds clinically & radiographically.
The mean age of the subjects when the treatment started was (9 ± 1) years. It was in agreement with recommended age suitable for pulp regeneration that range from 5 to 15 years as they may have a greater healing capacity more stem cells regenerative potential (Chueh et al., 2009, Dudeja et al., 2015).
Regarding the fracture type, the present study showed that Enamel-dentin-pulp fracture type presented the highest percentage (72%) (28% in group I and 44% in group II), followed by enamel-dentin fracture type which presented 28% (24% in group I and 4% in group II) with zero percentage to enamel fracture. These findings contradicts Cavalcanti et al., 2009, El-Kenany et al., 2016 and in agreement with Celenk et al. (2002). This could be explained by the fact that all presented cases which sought treatment were presented with necrotic pulp, and the two presented fracture types are common cause of non-vital teeth than enamel fracture type In addition, to reoccurrence of traumatic injury that might be the result of parents’ negligence.
Both groups revealed 100% clinical and radiographic success. The results of clinical outcomes showed that there was no statistically significant difference between the two groups in the clinical outcomes which include resolution of pain, swelling, mobility and sinus/fistula. This could be explained by the standardized and effective disinfection protocol used. During the follow up intervals, all of the cases continued their 100% clinical success rate in all groups with no statistical significant difference between the groups. This data was consistent with the finding presented by Shivashankar et al. (2017).
The study revealed that the mean increase in root length and root dentin width in either mm or percentage of PRP is greater than that of PRF for all time points. Yet, this difference is statistically insignificant for all time points with confident 95%. The findings were in agreement with the results obtained by Shivashankar et al., 2017, Murray, 2018 and disagreement with Narang et al. (2015).They found that PRF has enormous potential to hasten the growth characteristics in immature necrotic permanent teeth when matched to PRP. The difference in results might be attributed to different inclusion criteria as he recruited patients below 20 years of age, and he did not mention their mean age. In addition, different protocol has been used in PRP and PRF preparation. No precise standardization of radiograph was achieved, and he used scoring method instead of measurement.
PRP was carried on collagen carrier (CollaCote), and it is available in the form of a sponge which contains resorbable collagen fibers. Hence, it is easy to carry PRP into the root canal. Furthermore, Collagen can trigger platelets in PRP, so aids in discharging the growth factors. Because it is a bioinductive material itself, so it may improve the rate of revascularization/revitalization (Jadhav et al., 2012, Jadhav et al., 2013).
Badade et al. (2016) evaluated antimicrobial efficacy of PRP and PRF. He found that PRP is more effective against bacteria than PRF. This finding was further confirmed by Kour et al. (2018). Possible explanation that PRF have subordinate concentration of platelets and leukocytes when matched to the PRP. Furthermore, in PRP platelets and cytokines would be completely released once the fibrin meshwork disintegrates. On the other hand, PRF provides a delayed and sustained release of growth factors, as opposed to the single sharp burst of growth factors provided by PRP. Therefore PRF may need longer follow up time to express its effect over PRP.
All revascularized/revitalized teeth did not respond to pulp sensibility tests during follow up intervals and at the end of the study. These findings agreed with Petrino et al., 2010, Torabinejad and Faras, 2012, Saoud et al., 2014. Other studies reported variable positive responses to pulp sensibility tests (Cehreli et al., 2011, Keswani and Pandey, 2013). Negative results could possibly be attributed to coronally present MTA layer which act as an insulator, or the requirement of more than 12 months for complete formation of blood vessels and nerve fibers within the root canal. In addition, thickening of the canal walls is mainly caused by establishment of cementum-like tissue devoid of tubular structure observed in dentin (Yang et al., 2016).
The study revealed that the teeth discoloration between the two groups, the PRF is slightly greater and statistically not significant than PRP. The results were in line with Nagata et al., 2014, McTigue et al., 2013. It is obvious that all studies attributed teeth discoloration in regenerative/revascularization procedures to either TAP or MTA but to our knowledge, the role of the scaffold has not been investigated yet. It needs further investigation as the constituent of the scaffold may play a role in this. Neither PRP nor PRF treated teeth showed any signs of canal calcification/obliteration.
During bone density measurements, it is noticed that the mean of bone density in the PRP group is higher than PRF group at all time points. This difference is statistically non-significant. Our findings is in line with Shivashankar et al., 2017, Narang et al., 2015. PRP exhibited marginal improved results than PRF regarding healing of periapical area. This could be described by liquid consistency of PRP that permitted it to reach the periapical area without any impedance. Unlike PRF, which has gel like consistency that helps transporting the highest amount of growth factors to accelerate the wound healing procedure (Shivashankar et al., 2017).
For apical diameter measurement in group I & II, the decrease in diameter in the PRP is greater than that of PRF at all time points. This difference is statistically non-significant. The findings is in line with Shivashankar et al., 2017, Murray, 2018, Narang et al., 2015. PRP and PRF functional theory is stimulating stem cell proliferation and higher expression of Osteoprotegrin proteins and alkaline phosphatase. These proteins are usually identified as odontoblast differentiation markers (Narang et al., 2015).
Conclusion: For necrotic immature permanent teeth, revascularization/revitalization utilizing PRP/PRF is a highly successful method and showed excellent 12-months prognosis. PRP was valuable as a scaffold in revascularization/revitalization over PRF with no significant difference in primary and secondary outcomes.
Recommendations: PRP should be the first choice used as scaffolding material in regenerative endodontic treatment. Longer follow up period might have been required for pulp sensibility test to display positive reaction.
Limitations: Pulp vitality testing that evaluate blood supply should have been used which done by Laser Doppler Flowmetry & Pulse Oximeter. The histology of tissues formed inside root canal could not be assessed due to ethical reasons.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alsousou J., Ali A., Willett K., Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24:173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- American Association of Endodontics Clinical considerations for a regenerative procedure, 2018. Available at: www.aae.org.
- Badade P.S., Mahale S.A., Panjwani A.A., Vaidya P.D., Warang A.D. Antimicrobial effect of platelet–rich plasma and platelet–rich fibrin. Indian J. Dent. Res. 2016;27:300–304. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- Casagrande L., Demarco F., Zhang Z., Araujo F.B., Shi S., Nör L.E. Dentin-derived BMP2 and odontoblastic differentiation of SHED. J. Dent. Res. 2010;89(6):603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- Cavalcanti A.L., Bezerra P.K.M., de Alencar C.R.B., Moura C. Traumatic anterior dental injuries in 7- to 12-year-old Brazilian children. Dental Traumatol. 2009;25(2):198–202. doi: 10.1111/j.1600-9657.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Cehreli Z.C., Isbitiren B., Sara S., Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J. Endod. 2011;37:1327–1330. doi: 10.1016/j.joen.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Celenk S., Sezgin B., Ayna B., Atakul F. Causes of dental fractures in the early permanent dentition: a retrospective study. J. Endodont. 2002;28(3):208–210. doi: 10.1097/00004770-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Chueh L., Ho Y., Kuo T., Lai W., Chen Y., Chiang C. Regenerative endodontic treatment for necrotic immature permanent teeth. J. Endo. 2009;35(2):160–164. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Do L.G. Distribution of caries in children: variations between and within populations. J. Dent. Res. 2012;91:536–543. doi: 10.1177/0022034511434355. [DOI] [PubMed] [Google Scholar]
- Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endo. 2006;101:37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dohan D.M., Choukroun J. PRP, cPRP, PRF, PRG. How to find your way in the jungle of platelet concentrates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endo. 2007;103:305–316. [Google Scholar]
- Dudeja P., Grover S., Srivastava D., Dudeja K.K., Sharma V. Pulp revascularization- it’s your future whether you know it or not? J. Clin. Diagn. Res. 2015;9(4):1–4. doi: 10.7860/JCDR/2015/10149.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kenany M.H., Awad S.M., Hegazy S.A. Prevalence and risk factors of traumatic dental injuries to permanent anterior teeth among 8–12 years old school children in Egypt. Pediat. Dent. J. 2016;26(2):67–73. [Google Scholar]
- Hamanaka E.F., Poi W.R., Salzedas L.M.P., Alves L.C., Panzarini S.R., Sonoda C.K., Martins C.M. A method for the geometric standardization of intraoral radiographs for long-term follow up of replanted teeth: a case report. Dent. Traumatol. 2012;29(2):121–126. doi: 10.1111/j.1600-9657.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- Jadhav G., Shah N., Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: a pilot clinical study. J. Endod. 2012;38(12):1581–1587. doi: 10.1016/j.joen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Jadhav G.R., Shah N., Logani A. Comparative outcome of revascularization in bilateral, non-vital, immature maxillary anterior teeth supplemented with or without platelet rich plasma: a case series. J. Conserv. Dent. 2013;16:568–572. doi: 10.4103/0972-0707.120932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani D., Pandey R.K. Revascularization of an immature tooth with a necrotic pulp using platelet-rich fibrin: a case report. Int. Endod. J. 2013;46(1):1096–1104. doi: 10.1111/iej.12107. [DOI] [PubMed] [Google Scholar]
- Kim S.G., Zhou J., Solomon C., Zheng Y., Suzuki T., Chen M., Mao J.J. Effects of growth factors on dental stem/progenitor cells. Dent. Clin. North America. 2012;56(3):563–575. doi: 10.1016/j.cden.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour P., Pudakalkatti P.S., Vas A.M., Das S., Padmanabhan S. Comparative evaluation of antimicrobial efficacy of platelet-rich plasma, platelet-rich fibrin, and injectable platelet-rich fibrin on the standard strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp. Clin. Dent. 2018;9(S2):325–330. doi: 10.4103/ccd.ccd_367_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi R., Trope M. Revitalization procedures in two traumatized incisors with different biological outcomes. J. Endod. 2012;38:411–417. doi: 10.1016/j.joen.2011.12.003. [DOI] [PubMed] [Google Scholar]
- McTigue D.J., Subramanian K., Kumar A. Management of immature permanent teeth with pulpal necrosis-a case series. Pediatr. Dent. 2013;35:55–60. [PubMed] [Google Scholar]
- Murray P.E. Mini review of the clinical efficacy of platelet-rich plasma, platelet-rich fibrin and blood-clot revascularization for the regeneration of immature permanent teeth. World J. Stomatol. 2018;6(1):1–5. doi: 10.3389/fbioe.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy M.M., Tawfik H.E., Hashem A.A.R., Abu-Seida A.M. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J. Endod. 2014;40(2):192–198. doi: 10.1016/j.joen.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Nagata J.Y., Gomes B.P., Rocha Lima T.F. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J. Endod. 2014;40:606–612. doi: 10.1016/j.joen.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Nakashima M., Iohara K. Regeneration of dental pulp by stem cells. Adv. Dent. Res. 2011;23(3):313–319. doi: 10.1177/0022034511405323. [DOI] [PubMed] [Google Scholar]
- Narang I., Mittal N., Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemp. Clin. Dent. 2015;6:63–68. doi: 10.4103/0976-237X.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrat A., Seifi A., Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J. Endod. 2011;37:562–567. doi: 10.1016/j.joen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Petrino J., Boda K., Shambarger S., Bowles W., McClanahan S. Challenges in regenerative endodontics: a case series. J. Endo. 2010;36(3):536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Pozos-Guillén A., Chavarría-Bolaños D., Garrocho-Rangel A. Split-mouth design in Paediatric Dentistry clinical trials. Eur. J. Paediat. Dent. 2017;18:1–5. doi: 10.23804/ejpd.2017.18.01.13. [DOI] [PubMed] [Google Scholar]
- Rafter M. Apexification: a review. Dental Traumatol. 2005;21:1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Saoud T.M.A., Zaazou A., Nabil A., Moussa S., Lin L.M., Gibbs J.L. Clinical and radiographic outcomes of traumatized immature permanent necrotic teeth after revascularization/revitalization therapy. J. Endod. 2014;40:1946–1952. doi: 10.1016/j.joen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivashankar V.Y., Johns D.A., Maroli R.K., Sekar M., Chandrasekaran R., Karthikeyan S., Renganathan S.K. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: a triple blind randomized clinical trial. J. Clin. Diagnost. Res. 2017;11(6):34–39. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabinejad M., Corr R., Buhrley M., Wright K., Shabahang S. An animal model to study regenerative endodontics. J. Endo. 2011;37(2):197–202. doi: 10.1016/j.joen.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J. Endo. 2012;38(6):864–868. doi: 10.1016/j.joen.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Yang J., Yuan J., Chen Z. Pulp regeneration: current approaches and future challenges. Front. Physiol. 2016;7(58):1–8. doi: 10.3389/fphys.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]