Abstract
While it is well-established that hypoxia is a major factor that affects clinical outcomes in cervical cancer, widespread usage of clinically available methods to detect and evaluate hypoxia during the course of treatment have not been established. This review compares these methods, summarizes their strengths and weaknesses, and assesses the pathways for their useful employment to alter clinical practice. We conducted a search on PubMed for literature pertaining to imaging hypoxic cervical cancer, and implemented keywords related to oxygen measurement tools to improve the relevance of the search results.
Oxygenation level-dependent applications of MRI have demonstrated hypoxia-induced radioresistance, and changes in cervix tumor oxygenation from hyperoxic therapy.
The hypoxic areas within tumors can be indirectly identified in dynamic contrast-enhanced images, where they generally display low signal enhancement, and diffusion-weighted images, which demonstrates areas of restricted diffusion (which correlates with hypoxia). Positron emmision tomography, used independently and with other imaging modalities, has demonstrated utility in imaging hypoxia through tracers specific for low oxygen levels, like Cu-ATSM tracers and nitroimidazoles. Detecting hypoxia in the tumors of patients diagnosed with cervical cancer via medical imaging and non-imaging tools like electron paramagnetic resonance oximetry can be utilized clinically, such as for guiding radiation and post-treatment surveillance, for a more personalized approach to treatment. The merits of these methods warrant further investigation via comparative effectiveness research and large clinical trials into their clinical applications.
Introduction to oxygen measurements
Tissue oxygenation is heterogeneous over time and space. It is upregulated over time, e.g. through the microcirculatory bed during exercise to meet the metabolic demand of skeletal muscle, and over space, as cell-to-cell oxygen levels vary based on the cell’s distance from the microvessels supplying it oxygen.1 There are also macroscopic variations corresponding to physiological function (the heart consumes more oxygen than the kidneys, both in total and per weight of the organ).1
While large differences in oxygen concentration or average pO2 exist among normal tissues, there is often a more profound heterogeneity associated with pathologies, like within cervix tumors, at any given timepoint.1–6 Although tissue oxygenation is an important measurement because it can impact therapeutic response and overall disease outcomes, this utility is limited as real-time microscopic variations in oxygen levels cannot be measured. Current measurement techniques merely present data averaged over time and space.1
Techniques to measure oxygen in vivo include electron paramagnetic resonance (EPR) oximetry, phosphorescent probes, electrodes (i.e. Eppendorf histography), monitoring of hemoglobin or mitochondrial cytochromes, nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH) fluorescence, and imaging methods such as nuclear magnetic resonance (NMR).7 These methods may be clinically applied in different ways, such as through repeating oxygen measurements before and during treatment to determine how therapies affect oxygenation. Which method is most appropriate depends on the clinical needs of the particular circumstance. While these methods have an advantage of accuracy over imaging due to their ability to measure tissue oxygen directly, instead of indirectly assessing parameters correlated with oxygen in tissues, certain imaging tools that are already used to derive benefits such as identifying target regions for radiation therapy can also provide clinical utility as oxygen measurement tools.
Hypoxic cervical cancer: evidence of the need for oximetry
Cervical cancer under hypoxic conditions is associated with metastatic progression, extracellular matrix remodeling, and poor outcomes.8,9 The significance of these consequences is exacerbated by the fact that cervical cancer itself is an independent risk factor for hypoxic tumors.10 Oxygen measurements of cervix tumors are of paramount importance, not only to provide more accurate prognoses, but also to personalize treatments for patients, taking into account the presence of hypoxic areas that are less responsive to radiation therapy. For example, when the Eppendorf histograph was used to directly measure tumor oxygenation, it found poor prognoses post-radiotherapy in poorly oxygenated tumor tissues, particularly in sarcoma, head and neck cancers, and cervical cancer.11–14
Not surprisingly, these poor prognoses post-radiotherapy have been associated with hypoxia-related proteins (such as hypoxia-inducible factor-1α, or HIF-1α).15 While reverse transcription quantitative PCR (RT-qPCR) can be used to confirm hypoxia-related gene expression as a biomarker for aggressive hypoxia tumors, data on suitable reference genes for cervix tumors are sparse, motivating one study to identify 182 genes unaffected by hypoxia in cervical cancer. Three genes have been identified (CHCHD1, SRSF9, and TMBIM6) not associated with tumor volume, stage, lymph node involvement or progression of disease and were thus determined to be a suitable set of reference genes for RT-qPCR evaluation of hypoxia-related gene expression in squamous cervix tumors.16
Studies investigating tissue oxygen tension (pO2) have suggested hypoxia to be common in cervix tumors, though there are large intratumor heterogeneities in their oxygen levels.17 The principal limitations are associated with the region that is measured and potential perturbations in pO2 as a result of the method.7 These hypoxic conditions are dynamic, expanding and diminishing in accordance to tumor growth and treatment.18 One study found that while the oxygenation levels of normal tissues (determined by the pO2 of arterial blood) did not change significantly following 40–45 Gy of radiation, in 15/19 patients with cervical cancer, the tumor region experienced an increase in oxygenation following 40–45 Gy of radiotherapy (delivered in 20 fractions over 4 weeks).19 Changes in oxygenation can also occur over short time periods, as cervical cancer xenografts have been shown to undergo temporal fluctuations in pO2 over durations under 1 hr, and a study on mice found the magnitude of these pO2 fluctuations to be greater in tumors than physiologic tissues like muscle.20,21
In addition to identifying tumor subpopulations that are hypoxic and are thus prone to radioresistance, oxygen measurements in tissue could also allow for the screening of patients more likely to respond well to immunotherapy, as evidence is accumulating that oxygen levels affects immunotherapy efficacy.22,23 Repeated measurements are particularly useful for cancer, where neoangiogenesis results in leaky arteriole vessels that are less efficient in delivering oxygenated blood, growth of the tumor results in the degradation of local vessel integrity, and the metabolic switch to glycolysis characteristic of tumors may all result in regional hypoxia.24,25
EPR oximetry: imaging in preclinical and spectroscopy in clinical studies
EPR oximetry (also called electron spin resonance) is a minimally invasive technique that allows for repetitive in vivo and in vitro oxygen level measurements over a period of time. The technique involves a paramagnetic oxygen sensor or probe that is implanted in tissues or cell cultures, and interacts with the unpaired electrons of oxygen atoms. This interaction broadens the sensors linewidth, a parameter of the EPR’s sensor. The ability of the linewidth to be broadened by oxygen’s electrons determines the sensor’s sensitivity to oxygen. This broadening is directly proportional to the oxygen concentration of the surrounding tissue.
While EPR oximetry is not currently used in clinical practice for reasons such as patient compliance and a lack of a consensus on clinical values, it has been used to quantitatively and directly measure oxygen in vivo, with repeated measurements over time, in both preclinical studies26 27–29 and clinical studies.19,27,30 It has great potential for clinical application particularly because of its ease of long-term repeatability (over days, months or longer) as well as its capability of continuous short-term monitoring (over minutes).
As methods being tested to increase tumor oxygenation to improve tumor response to ionizing radiation are often unsuccessful, repeated EPR measurements before and after hyperoxic interventions could be used to screen for tumors that have responded well to the interventions and are thus more likely to have improved responses to radiation therapy or identify hypoxic tumors that will not respond to these hyperoxic interventions and thus require a different approach.29,31–36 Identifying these subgroups can provide a more accurate prognosis, as patients with hypoxic tumors have lower survival rates and increased recurrence relative to patients with more oxygenated tumors.36–42 EPR oximetry studies have also been used to test the oxygenation techniques themselves, and found sodium hydrosulfide, or NaHS, administration to be potentially effective in oxygenating hypoxic tumors.22,23 Other studies involving human and murine models have also suggested EPR oximetry may be beneficial for cervical cancer patients in order to determine if oxygenation methods will be effective.43–45
Though EPR oximetry serves as a suitable example of how oxygen measurements are clinically useful, similar applications of medical imaging tools are of particular interest in this review, as they allow for the visualization of hypoxia prior to and during treatment, in addition to their traditional benefits of detecting anatomical irregularities and providing information to determine target regions and radiation doses.30,46 While direct methods of measuring tumor oxygenation like EPR (Eppendorf histography) have some advantages over indirect methods like imaging, such as accurately quantifying the response to hyperoxygenation interventions, different imaging methods offer other unique advantages discussed later.
BOLD and TOLD MRI for oximetry
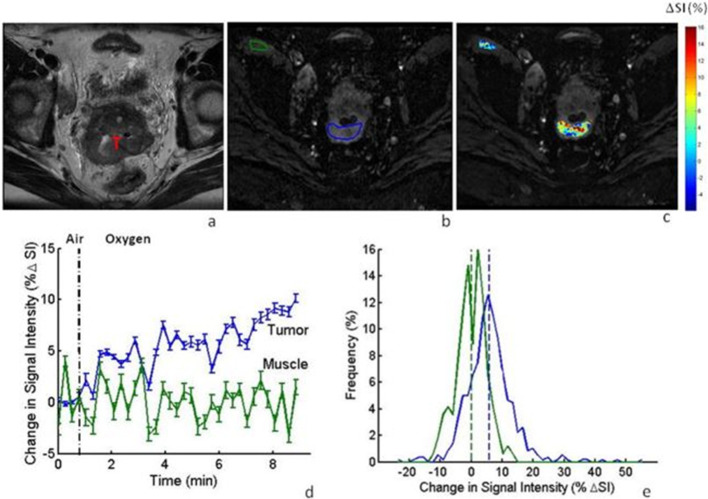
One way in which low tissue oxygenation levels may be detected via MRI is by utilizing the unusually high deoxyhemoglobin (dHb) levels found in hypoxic conditions and the generally low blood oxygen levels in these areas. Because dHb is paramagnetic, an MRI scan of these hypoxic tissues will show an increase in R2, and thus a decrease in the T2 weighted image intensity.47This use of MRI scanning has been described as blood oxygenation level-dependent MRI (BOLD). In one study, BOLD imaging was conducted on patients with cervical cancer both before and during hyperoxygenation treatment (inhaling supplemental, hyperoxic gas), showing a positive shift in the tumor’s signal intensity during the initiation of oxygen breathing.48 Figure 1 shows how a greater change in T2 weighted signal intensity was recorded in cervix tumors compared to the nearby iliacus muscle in response to breathing hyperoxic gas, demonstrating the dynamic nature and heterogeneity of oxygenation associated with cervical cancer.48
Figure1.
Bold MRI in Axial Plane: Cervical Tumor Oxygenation by Hyperoxic Breathing (a) T2-weighted image of a cervical tumor (T) in a patient. (b) T2-weighted images obtained using multi-shot EPI. The tumor and iliacus muscle are outlined in blue and green, respectively. (c) Map of the % in SI for the tumor and muscle, showing a heterogeneous response. (d) The mean tumor SI from hyperoxic breathing (5.4+3.1%) was greater than that of the iliacus muscle (-0.2+2.5%). The vertical bars at each Point indicate one standard error (SE) at each point in time. (e) Maps the distribution of this hyperoxic breathing-induced % in the SI in the tumor (blue) and muscle (green). Reproduced from: Hallac RR. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T.NMR Biomed. 2012;25;1321-1330
The Department of Radiology of the Sungkyunkwan University School of Medicine in Seoul used BOLD for 30 cervical cancer patients to evaluate how radiotherapy changes tumor oxygenation, and found the tumor’s R2, detected quantitatively, was notably higher post-radiotherapy (reflecting an increase in dHb levels and potentially an increase in hypoxia), perhaps as a result of vascular damage induced by the radiation treatment, and that the higher R2 was negatively correlated with the degree of tumor shrinkage.49,50 Imaging-based detection and localization of regions of tumor hypoxia could thus alter the treatment approach, deliver additional radiation dose to such regions in order to overcome their hypoxia-related radioresistance.
Another emerging NMR technique that could detect hypoxic conditions is tissue oxygen-level dependent MRI (TOLD). Though similar to BOLD, this method allows dissolved oxygen to decrease oxygen dependent R1 (increasing T1). When utilized on cervical cancer patients during hyperoxygenation therapy, the signal intensity for the cervix tumors increased greatly.51 This demonstrates the high sensitivity of TOLD imaging to variations in tissue oxygen levels.
TOLD and BOLD imaging have provided illuminating results in studies of cervical cancer, from radiotherapy increasing the tumor’s R2 and dHb levels to how the high R2 associated with hypoxic conditions correlate with radioresistance. These relevant findings support their continued usage in research studies, as well as further investigations into applications of MR-technology to evaluate hypoxic conditions.
Dynamic contrast-enhanced (DCE) and diffusion-weighted (DW) MRI for oximetry
DCE imaging involves a baseline image without contrast enhancement preceding a set of images obtained over time after an intravenous contrast agent (CA) is introduced and transported to the cancerous tissue through the bloodstream. This CA alters the calculated signal intensity for MRI and the measured X-ray attenuation for CT in a non-linear and linear fashion, respectively. The time–concentration curves, which can be constructed from the changes in contrast enhancement over time, allows for the quantification of parameters relevant to tumor tissue oxygenation levels and the local venous and arterial system in the tumor.52
While DCE imaging has not been shown to directly measure oxygenation levels in tumors, its usage with low-molecular weight CAs has allowed for visualization of tumor biology relevant to hypoxic cervix tumors.53 Some semi-quantitative measures, such as the area under the uptake curve (AUC) and the initial slope of the curve (or relative signal increase), have been found to correlate with pO2 or hypoxic fraction in both CT and MRI studies of cervix tumors.53–55 This means that regions of hypoxic cervical cancer generally display low signal enhancement in DCE images. This concept is demonstrated in Figure 2, which depicts a cervix tumor before and 90 s after 0.1 mmol per kg body weight Gd‐DTPA was administered, which enhanced the signal of the images in areas of greater perfusion and oxygenation (as well as greater cell density).54 Gd-DTPA administration dependent signal enhancement improved the identification of more hypoxic areas with less signal.
Figure 2.
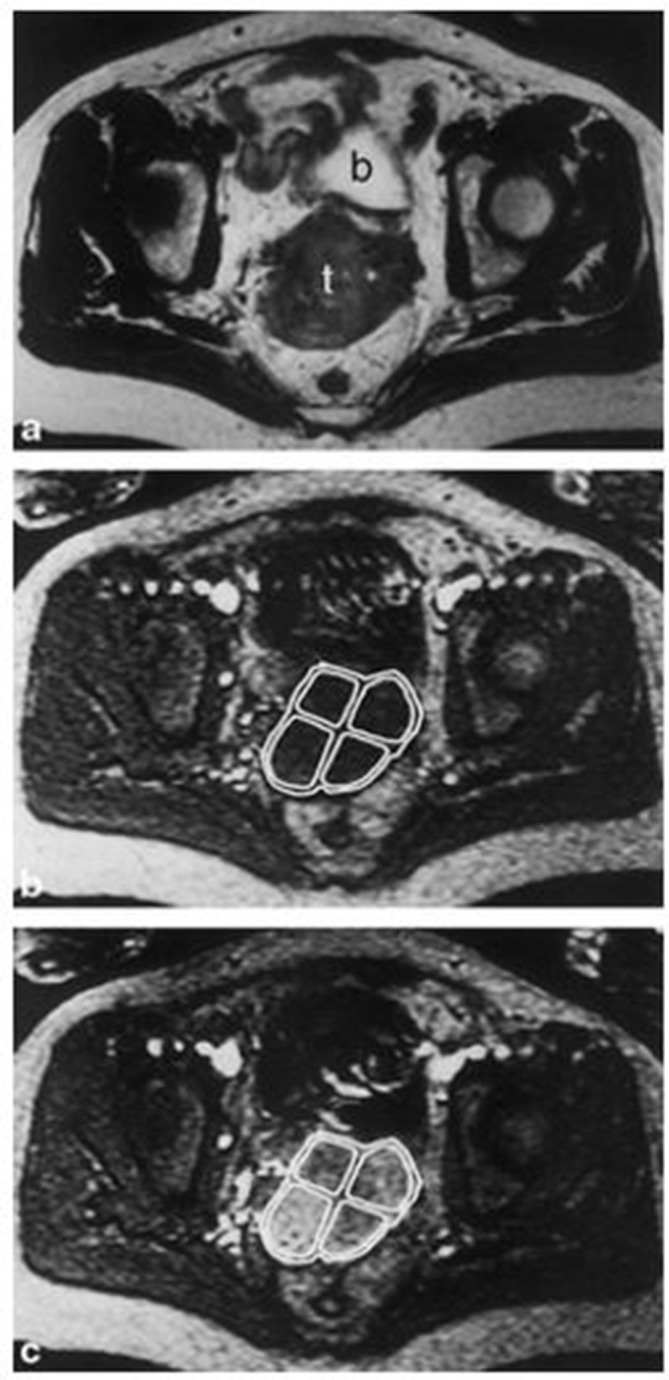
DCE-MRI of Cervical Carcinoma from the Axial Plane a: T2-weighted image of a cervical tumor marked 't' for tumor (posterior to the structure labeled 'b'- the bladder).b: T1-weighted image with the ROIs marked for the Whole tumor and four tumor subregions, before Gd-DTPA administration.Reproduced from: Lyng H. Assessment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J Magn Reason Imaging. 2001;14:750-756.
More quantitative image parameters, specifically Ktrans from the Tofts model (representing blood perfusion) and ABrix from the Brix model (representing a combination of perfusion and the size of the extravascular and extracellular spaces) have been found to be positively correlated with each other and with positive outcomes (as measured by parameters like progression-free survival) in a study including 78 cervical cancer patients.56 Studies have demonstrated an inverse relationship between Ktrans or ABrix and pO2 levels in cervical cancer, and a strong correlation between ABrix and expression of hypoxia-related genes (tumors with low ABrix values had upregulated levels of HIF-1 targets and UPR genes).54,57 Other studies looking at ABrix and Ktrans values have shown a general trend in which low pre-treatment contrast enhancement correlates with poor locoregional control.58,59 The only correlation between the location of local recurrence that has been identified is a cluster analysis showing simple uptake kinetics to be the strongest correlate with location of recurrence.60
Another advanced MRI application involves diffusion-weighted (DW) MRI. The apparent diffusion coefficient (ADC), which measures the magnitude of water diffusion within a tissue, can be calculated clinically using DWI. Like DCE imaging, this DWI-ADC MRI technique has been established for its potential use in evaluating biology relevant to cervix tumor hypoxia. While there are no universal guidelines for how to best utilize DWI-ADC, tumors with low perfusion (low ADC values) are associated with tumor hypoxia, giving ADC clinical value as a potential biomarker for tumor hypoxia. In addition, DWI-ADC has value as a potential means to evaluate the effectiveness of treatment (degree to which ADC values increase), as ADC values increase during treatment in which the tumor responds well and the cervical tissue signal returns towards normal.
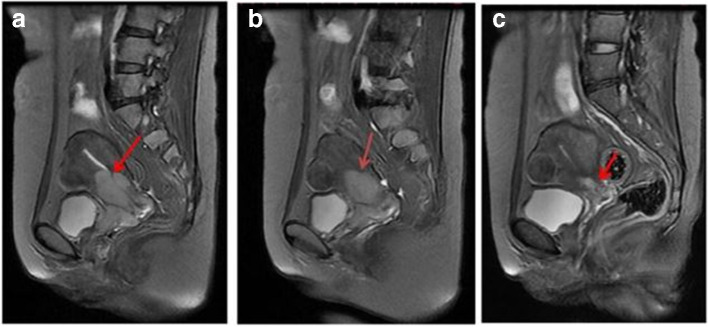
DWI-ADC improves the sensitivity and specificity of conventional MRI and provides qualitative and quantitative information regarding the tumor microenvironment and oxygenation status.61 Figure 3 shows DWI-ADC applied to cervical cancer.62 In this imaged patient and in the other patients in the study who experienced either complete or partial remission following treatment, the ADC values increased after treatment. For example, among patients who experienced complete remission, the ADC value (×10–3 mm2/s) increased from an average pretreatment value of 0.764 ± 0.073 to a value 15 days after treatment of 1.703 ± 0.0.
Figure 3.
Difussion-weighted MRI of cervical squamous cell carcinoma These are sagittal T2 weighted images of a 45-year-old patient before treatment (A),15 days after treatment (B), and 2 months after treatment (c). The tumor (indicated by the arrow) size reduced (and the ADC values increased) after treatment. Reproduced from: Chen J. The Value of DW MRI in predicting the Efficacy of Radiation and Chemotherapy in Cervical Cancer. Open Life Sciences. 2018; 13(1) 305-311
Neither DCE imaging nor DWI-ADC imaging have universal guidelines for how to best utilize the DCE imaging method to derive the most meaningful parameters relevant to hypoxia in cervical cancer. However, DWI-ADC imaging does boast a highly sensitive and specific ability to evaluate properties of a cervix tumor’s microenvironment, such as its degree of hypoxia. In doing so, DWI-ADC may help steer oncology away from a “one-size-fits-all” model, towards a more personalized approach, in the detection, characterization, and post-treatment surveillance of a patient’s specific hypoxic cervix tumors.
Positron emission tomography (PET) and multimodal imaging
Molecular imaging is an emerging field with broad applications, such as the ability to detect molecular events associated with the risk of developing a particular disease before disease-induced anatomic properties are detectable by radiologic imaging, enabling earlier intervention. Molecular methods used to measure tumor oxygenation include the use of hypoxia markers (such as hypoxia-induced expression of GLUT1 and CAIX) and tracers in conjunction with imaging modalities such as PET.63,64 Hypoxic tissues exhibit increased uptake of hypoxia-specific tracers relative to surrounding normal tissues (which is how hypoxic tissues are identified as hypoxic using PET). However, the extent of this uptake can be hindered by radiation-induced vascular damage, which, although can contribute to hypoxia by reducing blood flow, interferes with PET tracer delivery and uptake (radiation therapy may also induce direct cell killing, which reduces uptake to the necrotic areas). Unlike BOLD and TOLD MRI applications, PET does not rely on hyperoxygenation therapy making it more feasible for clinical use. In addition to its feasibility, PET has been shown to be the best direct imaging tool for non-invasive three-dimensional visualization of hypoxic conditions through the use of hypoxia PET tracers: copper labeled diacetyl-bis(N4-methylthiosemicarbazone) analogs, or Cu-ATSM, and fluorine-labeled nitroimidazoles.65,66 Although not yet clinically successful, iodine-124-labeled iodo-azomycin-galactoside has also shown promise as a hypoxia-imaging PET tracer.67
60Cu-ATSM can efficiently diffuse given its high membrane permeability. Under hypoxic conditions, the Cu2+ component may irreversibly reduce to Cu+ and dissociate from ATSM, a dissociation that is prevented by re-oxidation in normoxic tissues, where the complex is cleared.68 Washington University conducted a multicenter trial regarding hypoxia and cervical cancer, in which PET with60Cu-ATSM was used on 38 patients, and data from 0 to 60 min or 30 to 60 min after injection were analyzed.69,70 The results showed that nearly every tumor had significant uptake of Cu-ATSM, and an additional comparison of immunohistochemistry markers revealed patients with tumors that were more hypoxic had significantly higher levels of carbonic anhydrase 9 (a hypoxia-induced cell-surface enzyme involved in regulating the pH of hypoxic tumors).54 However, enthusiasm for Cu-ATSM was diminished in a study from the NIH showing tumor type variability in radiotracer uptake.71,72
Nitroimidazoles, another class of PET tracers that can be used to detect hypoxia, are lipophilic and thus passively diffuses through cell membranes. In the presence of hypoxia, the nitro group experiences a multistep reduction in which the intermediate products are highly reactive and are able to bind macromolecules, allowing for the accumulation of the reduced tracer in hypoxic tissues.73 18F-fluoromisonidazole (18F-FMISO) is the most common nitroimidazole used for imaging hypoxia.74 Intervals of 2 h between 18F-FMISO injection and imaging are generally preferred to distinguish the hypoxic regions from normoxic regions, as the tracer clears from normoxic tissues very slowly.75 A study led by Dr Pinker of the Medical University of Vienna found pretreatment of 18F-FMISO PET demonstrated an accurate diagnosis of hypoxia in every cervical cancer tumor present in the 11 patients who participated in the study, and found the hypoxic subvolume to be independent of overall tumor volume.76
The radiopharmaceuticals used in conjunction with PET, such as 18F-FMISO and 60Cu-ATSM, are not without limitations. 18F-FMISO exhibits a slow cellular washout (it takes 2 h post-injection for the tracer to clear from normal background tissues, thus delaying imaging) and relatively low hypoxia-specificity, manifesting in a limited contrast between hypoxic tumors and normal tissues. 60Cu-ATSM is limited clinically by its short radioactive half-life of 0.40 h (64Cu, which has a longer half-life or 12.7 h and potential to produce superior image quality compared to 60Cu, will likely be the subject of large clinical trials to come).77 Both 18F-FMISO and 60Cu-ATSM can be produced on a medical cyclotron at relatively low costs.
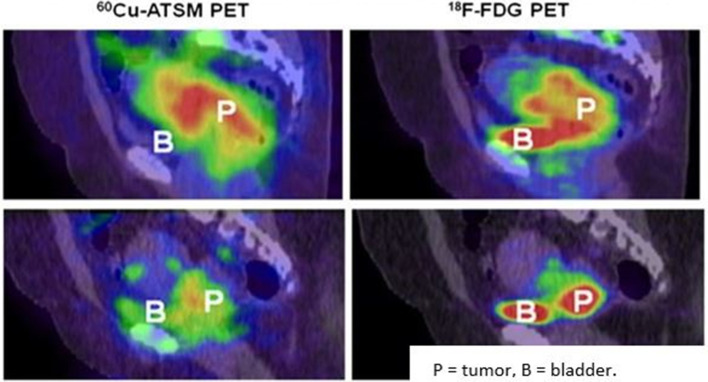
Other nitroimidazoles have also been used, like 18F-fluoroerythronitroimidazole (18F-FETNIM) and 18F-fluoroazomycin-arabinoside (18F-FAZA). The administration of 18F-FETNIM PET to 16 cervical cancer patients prior to definitive chemoradiotherapy showed patients with high tracer uptake (more hypoxic cervix tumors) had reduced survival, though the tumor specificity of the tracer appeared to be low, as the tracer uptake into the tumors were hard to distinguish from nearby normoxic soft tissues.78 The researchers did not observe a correlation between 18F-FETNIM uptake and 18FDG uptake (which was markedly high in every tumor). This nonspecific uptake is demonstrated in Figure 4, which also contrasts it to the high specificity of 60Cu-ATSM PET for hypoxic cervix tumors.45 With regards to 18F-FAZA PET, imaging was conducted before, during, and after external chemoradiotherapy in one study of 15 cervical cancer patients, and the resulting images showed only five patients to have tumors that took up much of the tracer.79 Four of these five patients were observed to still have marked 18F-FAZA uptake during radiotherapy treatment (though post-treatment these patients were PET-negative).
Figure 4.
PET/CT imaging of Cu-ATSM and F-FDG uptake in hypoxic and normoxic cervix tumors Top: These sagittal images are of a patient with a hypoxic cervix tumor. They show high 60Cu-ATSM(left) and high 18F-FDG(right)uptake in the tumor.Bottom: These sagittal images are of a patients with an overall normoxic cervix tumor. There is a sharp decrease in 60Cu-ATSM(left) uptake compared to the hypoxic tumor, but still a fairly high 18F-FDG(right)uptake compared to the hypoxictumors than 18F-FDG.Reproduced from: Lyng H. Hypoxia in cervical cancer: from biology to imaging. Clin Transl Imaging. 2017; 5(4): 373-388.
Combined PET/CT is able to determine the extent of tumor progression and can visually assess regional lymph nodes and distant metastasis sites, allowing this multimodality tool to be helpful in pretreatment evaluation and radiation therapy planning. Quantitative parameters relevant to PET/CT include metabolic tumor volume, maximum standardized uptake value (SUVmax), and total lesion glycolysis. These parameters have been used to predict prognosis and clinical outcome, as well as to detect aggressive cervix tumors and quantify their degree of hypoxia.80 Another study agreed that imaging cervical cancer with PET/CT (this time with 60Cu-ATSM) may be helpful for prognostication, as well as radiation therapy planning, by enabling characterization of the tumor microenvironment, such as hypoxic status.81
Another multimodality tool, PET/MRI, has the potential to improve the detection of primary tumors and metastatic sites as a result of the improved soft tissue contrast resolution of MRI as compared to CT imaging. One study, which involved examining 10 cervical cancer patients scheduled for radiation therapy with combined 3 T multiparametric (MP) PET/MRI, found the multimodality system as able to identify regions of tumor hypoxia (and did so in eight patients).82 A different study incorporated 13 patients and involved repetitive imaging of cervix tumors prior to, during, and after radiochemotherapy using biomarkers with MRI and/or PET to assess spatiotemporal stability of hypoxia and other tumor environment characteristics. This longitudinal MP PET/MRI study concluded that while tumor cell density and perfusion decreased over time, a non-uniform hypoxia change during radiotherapy was observed.83
Another study also looked at spatiotemporal patterns of tumor parameters in the cervical tumors. This study used combined MP PET/MRI with 18F-FMISO to measure tumor hypoxia, perfusion, and microstructure before, twice during, and after chemotherapy in 10 cervical cancer patients. The study concluded PET/MRI was feasible and revealed spatiotemporal patterns (averaged apparent diffusion coefficient, or ADC, values increased, while TBR and Ktrans decreased over time), and that these patterns can help define subtargets for dose painting and response assessment.84 Higher ADC (and SUV) values correlate with Ki-67 expression (a protein upregulated in aggressive tumors), but not directly with tumor hypoxia.85
In conclusion, the specific uptake of compounds like 18F-FMISO and 60Cu-ATSM allows PET and multimodal methods to image hypoxia more directly than the imaging methods previously discussed. 18F-FDG PET/CT has been used to obtain values like metabolic tumor volume, SUVmax, and total lesion glycolysis to assess the tumor microenvironment like hypoxia, and quantify this hypoxia to identify aggressive tumors. PET/MRI also shows great promise, as it can provide reliable target definitions for primary cervix tumors and can identify regions of tumor hypoxia to a high degree of spatial accuracy.
Conclusion
Additional methods for handling hypoxia are needed, particularly for locally advanced stages, where radiotherapy is the main treatment option. While medical imaging and EPR oximetry offer several methods of providing clinical utility via direct and indirect measurements of cervical cancer tissue oxygenation, the benefits of each method vary, pointing to the need for clinical guidelines as to which method is most appropriate for a given circumstance. DWI can be used to identify tumor areas with decreased perfusion and oxygenation, while BOLD and TOLD MRI imaging have been useful for research in showing how radiotherapy can increase hypoxic cervix tumor’s relaxation rate R2 (and that the higher R2 correlates with radioresistance).
These imaging methods have not been preferred for the clinical setting due to their reliance on supplemental oxygen, and the costs of imaging in general. However, recent investigations into the clinical utility of these and other methods for imaging hypoxic cervix tumors show some important advantages over spectroscopy techniques like EPR. For example, imaging can better guide radiotherapy, and can address deeper tumors than EPR.
Unlike BOLD and TOLD MRI, PET is feasible for clinical use, and it allows for the usage of radiopharmaceuticals like Cu-ATSM and nitroimidazoles. While PET may provide the best method of imaging hypoxia, other techniques like DCE imaging can indirectly and quantitatively measure parameters relevant to tumor hypoxia and oxygenation. Multimodality imaging shows great promise, as 18F-FDG PET/CT may help in defining nodal targets and detecting pathological lymph nodes, and PET/MRI shows high potential to provide reliable target definitions in the primary cervix tumor, while both have been used with tracers to specifically contrast hypoxic cervix tumor regions from normoxic regions (Table 1). Particularly, intriguing is the possibility of the use of PET/DCE-MRI. Future research regarding dose-escalation and de-escalation are needed to tailor treatment responses with these imaging modalities in order to balance efficacy with toxicity.
Table 1.
Applications, future directions, and limitations of current imaging modalities
| Modality | Applications/Future directions | Limitations |
|---|---|---|
| Electron paramagnetic resonance oximetry |
|
|
| MRI |
|
- Indirect and requires coordination with supplemental oxygen administration (BOLD and TOLD). |
| Dynamic contrast-enhanced imaging |
|
- Indirect; Ktrans and Abrix measure tissue perfusion and correlate with hypoxic genes, thus indirectly measuring tumor hypoxia. |
| Positron emission tomography | - Compounds such as60Cu-ATSM and 18F-FMISO directly demonstrate increased uptake in hypoxic cervical cancer. | - 18F-FETNIM and 18F-FAZA may lack correlation with traditional 18F-FDG PET. |
| Multimodality imaging |
|
- Higher SUV and ADC values correlate with higher Ki-67 values, but not directly with tumor hypoxia. |
ADC, apparent diffusion coefficient; AUC, area under the curve; BOLD, blood oxygenation level-dependent; DWI, diffusion-weighted imaging; FAZA, fluoroazomycin-arabinoside; FETNIM, fluoroerythronitroimidazole; FMISO, fluoromisonidazole; MTV, metabolic tumor volume; PET, positron emmision tomography; RSI, relative signal increase; SUV, standardized uptake value; TLG, total lesion glycolysis; TOLD, tissue oxygen-level dependent.
The clinical benefits of taking oxygen measurements and visualizing hypoxia through imaging, as well as the utility of screening for patients more likely to respond to radiation and immunotherapy treatments directly through EPR oximetry, demonstrate the need for large clinical trials to investigate the clinical applications of these methods further. As no such method is currently in widespread clinical use, there is also a need for further comparative effectiveness research, which involves analyzing the effectiveness of medical practices realistic to the clinical setting, based on the treatment’s risks and benefits, head-to-head trials, or observational studies.86–90 Comparative effectiveness research would also allow for the meticulous comparison of direct and indirect oxygen measurement methods and could provide additional insight into their clinical utility.
Footnotes
Competing interests: Two of the authors (Ann Barry Flood and Harold M Swartz) are co-owners of Clin-EPR, LLC that manufacturers and sells EPR instruments for investigational use only. However, while our paper mentions EPR, our topic and focus is on imaging, which these instruments do not do.
Contributor Information
Joseph Waller, Email: jwaller14va@gmail.com.
Benjamin Onderdonk, Email: bonderdonk@radonc.uchicago.edu.
Ann Flood, Email: ann.barry.flood@dartmouth.edu.
Harold Swartz, Email: Harold.M.Swartz@dartmouth.edu.
Jaffer Shah, Email: thejaffershah@gmail.com.
Asghar Shah, Email: asgharshah1415@gmail.com.
Bulent Aydogan, Email: baydogen@uchicago.edu.
Howard Halpern, Email: h-halpern@uchicago.edu.
Yasmin Hasan, Email: yhasan@radonc.uchicago.edu.
REFERENCES
- 1.Swartz HM;in press ‘Oxygen level in a tissue’ - What do available measurements really report? Adv Exp Med Bio 2019;. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P. Oxygenation of tumors Encyclopedia of Cancer. 4th ed: Springer; 2017. 978–3662-47424-2. [Google Scholar]
- 3.Carreau A. Why is the partial pressure of tissues a crucial parameter? J Cell Mol Med 2011; 15: 1239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using PO2 histography. Antioxid Redox Signal 2007; 9: 1221–36. doi: 10.1089/ars.2007.1628 [DOI] [PubMed] [Google Scholar]
- 5.Epel B, Bowman MK, Mailer C, Halpern HJ. Absolute oxygen R1e imaging in vivo with pulse electron paramagnetic resonance. Magn Reson Med 2014; 72: 362–8. doi: 10.1002/mrm.24926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A 2002; 99: 2216–21. doi: 10.1073/pnas.042671399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springett R, Swartz HM. Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid Redox Signal 2007; 9: 1295–302. doi: 10.1089/ars.2007.1620 [DOI] [PubMed] [Google Scholar]
- 8.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol 2002; 20: 680–7. doi: 10.1200/JCO.20.3.680 [DOI] [PubMed] [Google Scholar]
- 9.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 2014; 14: 430–9. doi: 10.1038/nrc3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hockel M. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–15. [PubMed] [Google Scholar]
- 11.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 1996; 56: 941–3. [PubMed] [Google Scholar]
- 12.Brizel DM, Rosner GL, Prosnitz LR, Dewhirst MW. Patterns and variability of tumor oxygenation in human soft tissue sarcomas, cervical carcinomas, and lymph node metastases. Int J Radiat Oncol Biol Phys 1995; 32: 1121–5. doi: 10.1016/0360-3016(95)00106-9 [DOI] [PubMed] [Google Scholar]
- 13.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997; 38: 285–9. doi: 10.1016/S0360-3016(97)00101-6 [DOI] [PubMed] [Google Scholar]
- 14.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996; 41: 31–9. doi: 10.1016/S0167-8140(96)91811-3 [DOI] [PubMed] [Google Scholar]
- 15.Vordermark D. Hypoxia as a biomarker and for personalized radiation oncology. Recent Results Cancer Res 2016; 198: 123–42. [DOI] [PubMed] [Google Scholar]
- 16.Fjeldbo CS, Aarnes E-K, Malinen E, Kristensen GB, Lyng H. Identification and validation of reference genes for RT-qPCR studies of hypoxia in squamous cervical cancer patients. PLoS One 2016; 11: e0156259. doi: 10.1371/journal.pone.0156259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong RK, Fyles A, Milosevic M, Pintilie M, Hill RP. Heterogeneity of polarographic oxygen tension measurements in cervix cancer: an evaluation of within and between tumor variability, probe position, and track depth. Int J Radiat Oncol Biol Phys 1997; 39: 405–12. doi: 10.1016/S0360-3016(97)00328-3 [DOI] [PubMed] [Google Scholar]
- 18.Michiels C, Tellier C, Feron O. Cycling hypoxia: a key feature of the tumor microenvironment. Biochim Biophys Acta 2016; 1866: 76–86. doi: 10.1016/j.bbcan.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 19.Cooper RA, West CM, Logue JP, Davidson SE, Miller A, Roberts S, et al. Changes in oxygenation during radiotherapy in carcinoma of the cervix. Int J Radiat Oncol Biol Phys 1999; 45: 119–26. doi: 10.1016/S0360-3016(99)00093-0 [DOI] [PubMed] [Google Scholar]
- 20.Ellingsen C, Ovrebø KM, Galappathi K, Mathiesen B, Rofstad EK. pO₂ fluctuation pattern and cycling hypoxia in human cervical carcinoma and melanoma xenografts. Int J Radiat Oncol Biol Phys 2012; 83: 1317–23. doi: 10.1016/j.ijrobp.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 21.Braun RD, Lanzen JL, Dewhirst MW. Fourier analysis of fluctuations of oxygen tension and blood flow in R3230AC tumors and muscle in rats. Am J Physiol 1999; 277(2 Pt 2): H551–68. doi: 10.1152/ajpheart.1999.277.2.H551 [DOI] [PubMed] [Google Scholar]
- 22.Multhoff G. International Society on oxygen transport to tissue. 2018;isott.org.
- 23.O'Hara JA, Blumenthal RD, Grinberg OY, Demidenko E, Grinberg S, Wilmot CM, et al. Response to radioimmunotherapy correlates with tumor PO2 measured by EPR oximetry in human tumor xenografts. Radiat Res 2001; 155: 466–73. doi: 10.1667/0033-7587(2001)155[0466:RTRCWT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 24.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891–9. doi: 10.1038/nrc1478 [DOI] [PubMed] [Google Scholar]
- 25.Ostergaard L, Tietze A, Nielsen T, Drasbek KR, Mouridsen K, Jespersen SN, et al. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res 2013; 73: 5618–24. doi: 10.1158/0008-5472.CAN-13-0964 [DOI] [PubMed] [Google Scholar]
- 26.Hou H, Khan N, Gohain S, Kuppusamy ML, Kuppusamy P. Pre-Clinical evaluation of OxyChip for long-term EPR oximetry. Biomed Microdevices 2018; 20: 29. doi: 10.1007/s10544-018-0272-x [DOI] [PubMed] [Google Scholar]
- 27.Jarvis LA, Williams BB, Schaner PE, Chen EY, Angeles CV, Hou H, et al. Phase 1 clinical trial of OxyChip, an implantable absolute PO2 sensor for tumor oximetry. Int J Radiat Oncol Biol Phys 2016; 96: S109–10. doi: 10.1016/j.ijrobp.2016.06.268 [DOI] [Google Scholar]
- 28.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, et al. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res 2005; 11(2 Pt 1): 743–50. [PubMed] [Google Scholar]
- 29.Gallez B, Neveu M-A, Danhier P, Jordan BF. Manipulation of tumor oxygenation and radiosensitivity through modification of cell respiration. A critical review of approaches and imaging biomarkers for therapeutic guidance. Biochim Biophys Acta Bioenerg 2017; 1858: 700–11. doi: 10.1016/j.bbabio.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Vikram DS. A comparative evaluation of EPR and OxyLite oximetry using a random sampling of PO2 in a murine tumor. Radiation Research 2015;: 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012; 30: 1777–83. doi: 10.1200/JCO.2011.35.9315 [DOI] [PubMed] [Google Scholar]
- 32.Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 2014; 21: 1516–54. doi: 10.1089/ars.2013.5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26: 225–39. doi: 10.1007/s10555-007-9055-1 [DOI] [PubMed] [Google Scholar]
- 34.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393–410. doi: 10.1038/nrc3064 [DOI] [PubMed] [Google Scholar]
- 35.Menon C, Fraker DL. Tumor oxygenation status as a prognostic marker. Cancer Lett 2005; 221: 225–35. doi: 10.1016/j.canlet.2004.06.029 [DOI] [PubMed] [Google Scholar]
- 36.Dhani N, Fyles A, Hedley D, Milosevic M. The clinical significance of hypoxia in human cancers. Semin Nucl Med 2015; 45: 110–21. doi: 10.1053/j.semnuclmed.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Höckel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–15. [PubMed] [Google Scholar]
- 38.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 2007; 25: 4066–74. doi: 10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]
- 39.Vordermark D, Horsman MR. Hypoxia as a biomarker and for personalized radiation oncology. Recent Results Cancer Res 2016; 198: 123–42. doi: 10.1007/978-3-662-49651-0_6 [DOI] [PubMed] [Google Scholar]
- 40.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. doi: 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 41.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006; 82: 699–757. doi: 10.1080/09553000601002324 [DOI] [PubMed] [Google Scholar]
- 42.Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys 2012; 82: e433–9. doi: 10.1016/j.ijrobp.2011.05.037 [DOI] [PubMed] [Google Scholar]
- 43.De Preter G. a fast hydrogen sulfide-releasing donor increases the tumor response to radiotherapy. Mol Cancer Ther 2016; 1: 154–61. [DOI] [PubMed] [Google Scholar]
- 44.Gallez B, Baudelet C, Jordan BF, Bernard G. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed 2004; 17: 240–62. doi: 10.1002/nbm.900 [DOI] [PubMed] [Google Scholar]
- 45.Image (Figure 4) reproduced from: Lyng H. hypoxia in cervical cancer: from biology to imaging. Clin Transl Imaging 2017; 5: 373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, et al. Electron paramagnetic resonance imaging of tumor pO₂. Radiat Res 2012; 177: 376–86. doi: 10.1667/RR2622.1 [DOI] [PubMed] [Google Scholar]
- 47.Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, et al. Clinical applications of EPR: overview and perspectives. NMR Biomed 2004; 17: 335–51. doi: 10.1002/nbm.911 [DOI] [PubMed] [Google Scholar]
- 48.Hallac RR, Ding Y, Yuan Q, McColl RW, Lea J, Sims RD, et al. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T. NMR Biomed 2012; 25: 1321–30. doi: 10.1002/nbm.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price JM, Robinson SP, Koh DM. Imaging hypoxia in tumours with advanced MRI. Q J Nucl Med Mol Imaging 2013; 57: 257–70. [PubMed] [Google Scholar]
- 50.Khiewvan B. Update of the role of PET/CT and PET/MRI in the management of patients with cervical cancer. Hell J Nucl Med 2016; 19: 254–68. [DOI] [PubMed] [Google Scholar]
- 51.Kim CK, Park SY, Park BK, Park W, Huh SJ. Blood oxygenation level-dependent MR imaging as a predictor of therapeutic response to concurrent chemoradiotherapy in cervical cancer: a preliminary experience. Eur Radiol 2014; 24: 1514–20. doi: 10.1007/s00330-014-3167-0 [DOI] [PubMed] [Google Scholar]
- 52.Choi SH, Kim CK, Park JJ, Park BK. Assessment of early therapeutic changes to concurrent chemoradiotherapy in uterine cervical cancer using blood oxygenation level-dependent magnetic resonance imaging. J Comput Assist Tomogr 2016; 40: 730–4. doi: 10.1097/RCT.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 53.O'Connor JPB, Naish JH, Parker GJM, Waterton JC, Watson Y, Jayson GC, et al. Preliminary study of oxygen-enhanced longitudinal relaxation in MRI: a potential novel biomarker of oxygenation changes in solid tumors. Int J Radiat Oncol Biol Phys 2009; 75: 1209–15. doi: 10.1016/j.ijrobp.2008.12.040 [DOI] [PubMed] [Google Scholar]
- 54.Image (Figure 2) reproduced from: Lyng H. Assissment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J Magn Reson Imaging 2001; 14: 750–6. [DOI] [PubMed] [Google Scholar]
- 55.Loncaster JA, Carrington BM, Sykes JR, Jones AP, Todd SM, Cooper R, et al. Prediction of radiotherapy outcome using dynamic contrast enhanced MRI of carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2002; 54: 759–67. doi: 10.1016/S0360-3016(02)02972-3 [DOI] [PubMed] [Google Scholar]
- 56.O'Connor JPB, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br J Radiol 2011; 84 Spec No 2: S112–20. doi: 10.1259/bjr/55166688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haider MA, Milosevic M, Fyles A, Sitartchouk I, Yeung I, Henderson E, et al. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int J Radiat Oncol Biol Phys 2005; 62: 1100–7. doi: 10.1016/j.ijrobp.2004.12.064 [DOI] [PubMed] [Google Scholar]
- 58.Andersen EKF, Hole KH, Lund KV, Sundfør K, Kristensen GB, Lyng H, et al. Pharmacokinetic parameters derived from dynamic contrast enhanced MRI of cervical cancers predict chemoradiotherapy outcome. Radiother Oncol 2013; 107: 117–22. doi: 10.1016/j.radonc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Halle C, Andersen E, Lando M, Aarnes E-K, Hasvold G, Holden M, et al. Hypoxia-Induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res 2012; 72: 5285–95. doi: 10.1158/0008-5472.CAN-12-1085 [DOI] [PubMed] [Google Scholar]
- 60.Andersen EKF, Hole KH, Lund KV, Sundfør K, Kristensen GB, Lyng H, et al. Dynamic contrast-enhanced MRI of cervical cancers: temporal percentile screening of contrast enhancement identifies parameters for prediction of chemoradioresistance. Int J Radiat Oncol Biol Phys 2012; 82: e485–92. doi: 10.1016/j.ijrobp.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 61.Lund KV, Simonsen TG, Hompland T, Kristensen GB, Rofstad EK. Short-Term pretreatment DCE-MRI in prediction of outcome in locally advanced cervical cancer. Radiother Oncol 2015; 115: 379–85. doi: 10.1016/j.radonc.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 62.Image (Figure 3) reproduced from: Chen J. The value of DW MRI in predicting the efficacy of radiation and chemotherapy in cervical cancer. Open Life Sciences 2018; 13: 305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahajan A, Deshpande SS, Thakur MH. Diffusion magnetic resonance imaging: A molecular imaging tool caught between hope, hype and the real world of “personalized oncology”. World J Radiol 2017; 9: 253–68. doi: 10.4329/wjr.v9.i6.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torheim T, Groendahl AR, Andersen EKF, Lyng H, Malinen E, Kvaal K, et al. Cluster analysis of dynamic contrast enhanced MRI reveals tumor subregions related to locoregional relapse for cervical cancer patients. Acta Oncol 2016; 55: 1294–8. doi: 10.1080/0284186X.2016.1189091 [DOI] [PubMed] [Google Scholar]
- 65.Hoskin PJ, Sibtain A, Daley FM, Wilson GD. Glut1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer 2003; 89: 1290–7. doi: 10.1038/sj.bjc.6601260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer 2015; 112: 238–50. doi: 10.1038/bjc.2014.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang S, Tian M, Zhang H. Positron emission tomography imaging of tumor hypoxia. Curr Med Imaging Rev 2010; 6: 8–16. doi: 10.2174/157340510790693910 [DOI] [Google Scholar]
- 68.Imam SK. Review of positron emission tomography tracers for imaging of tumor hypoxia. Cancer Biother Radiopharm 2010; 25: 365–74. doi: 10.1089/cbr.2009.0740 [DOI] [PubMed] [Google Scholar]
- 69.Zanzonico P, O'Donoghue J, Chapman JD, Schneider R, Cai S, Larson S, et al. Iodine-124-labeled iodo-azomycin-galactoside imaging of tumor hypoxia in mice with serial microPET scanning. Eur J Nucl Med Mol Imaging 2004; 31: 117–28. doi: 10.1007/s00259-003-1322-y [DOI] [PubMed] [Google Scholar]
- 70.Holland JP, Lewis JS, Dehdashti F. Assessing tumor hypoxia by positron emission tomography with Cu-ATSM. Q J Nucl Med Mol Imaging 2009; 53: 193–200. [PMC free article] [PubMed] [Google Scholar]
- 71.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone. J Nucl Med 2008; 49: 201–5. doi: 10.2967/jnumed.107.048520 [DOI] [PubMed] [Google Scholar]
- 72.Grigsby PW, Malyapa RS, Higashikubo R, Schwarz JK, Welch MJ, Huettner PC, et al. Comparison of molecular markers of hypoxia and imaging with 60Cu-ATSM in cancer of the uterine cervix. Mol Imaging Biol 2007; 9: 278–83. doi: 10.1007/s11307-007-0095-2 [DOI] [PubMed] [Google Scholar]
- 73.Matsumoto K-I, Szajek L, Krishna MC, Cook JA, Seidel J, Grimes K, et al. The influence of tumor oxygenation on hypoxia imaging in murine squamous cell carcinoma using [64Cu]Cu-ATSM or [18F]Fluoromisonidazole positron emission tomography. Int J Oncol 2007; 30: 873–81. doi: 10.3892/ijo.30.4.873 [DOI] [PubMed] [Google Scholar]
- 74.O'Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys 2005; 61: 1493–502. doi: 10.1016/j.ijrobp.2004.12.057 [DOI] [PubMed] [Google Scholar]
- 75.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci 2009; 100: 1366–73. doi: 10.1111/j.1349-7006.2009.01195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med 2007; 37: 451–61. doi: 10.1053/j.semnuclmed.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 77.Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992; 22: 199–212. doi: 10.1016/0360-3016(92)91001-4 [DOI] [PubMed] [Google Scholar]
- 78.Pinker K, Andrzejewski P, Baltzer P, Polanec SH, Sturdza A, Georg D, et al. Multiparametric [18F]Fluorodeoxyglucose/ [18F]Fluoromisonidazole Positron Emission Tomography/ Magnetic Resonance Imaging of Locally Advanced Cervical Cancer for the Non-Invasive Detection of Tumor Heterogeneity: A Pilot Study. PLoS One 2016; 11: e0155333. doi: 10.1371/journal.pone.0155333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone. J Nucl Med 2008; 49: 201–5. doi: 10.2967/jnumed.107.048520 [DOI] [PubMed] [Google Scholar]
- 80.Vercellino L, Groheux D, Thoury A, Delord M, Schlageter M-H, Delpech Y, et al. Hypoxia imaging of uterine cervix carcinoma with (18)F-FETNIM PET/CT. Clin Nucl Med 2012; 37: 1065–8. doi: 10.1097/RLU.0b013e3182638e7e [DOI] [PubMed] [Google Scholar]
- 81.Schuetz M, Schmid MP, Pötter R, Kommata S, Georg D, Lukic D, et al. Evaluating repetitive 18F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol 2010; 49: 941–7. doi: 10.3109/0284186X.2010.510145 [DOI] [PubMed] [Google Scholar]
- 82.Khiewvan B, Torigian DA, Emamzadehfard S, Paydary K, Salavati A, Houshmand S, et al. Update of the role of PET/CT and PET/MRI in the management of patients with cervical cancer. Hell J Nucl Med 2016; 19: 254–68. doi: 10.1967/s002449910409 [DOI] [PubMed] [Google Scholar]
- 83.Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol 2013; 3: 34. doi: 10.3389/fonc.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinker-Domenig K. Pet/Mri in cervical cancer: insights into tumor biology. Journal of Clinical Oncology 2017;. [Google Scholar]
- 85.Georg P, Andrzejewski P, Baltzer P, Daniel M, Wadsak W, Mitterhauser M, et al. Changes in tumor biology during chemoradiation of cervix cancer assessed by multiparametric MRI and hypoxia PET. Mol Imaging Biol 2018; 20: 160–9. doi: 10.1007/s11307-017-1087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daniel M, Andrzejewski P, Sturdza A, Majercakova K, Baltzer P, Pinker K, et al. Impact of hybrid PET/MR technology on multiparametric imaging and treatment response assessment of cervix cancer. Radiother Oncol 2017; 125: 420–5. doi: 10.1016/j.radonc.2017.10.036 [DOI] [PubMed] [Google Scholar]
- 87.Surov A, Meyer HJ, Schob S, Höhn A-K, Bremicker K, Exner M, et al. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget 2017; 8: 28285–96. doi: 10.18632/oncotarget.16043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flood AB, Satinsky VA, Swartz HM. Comparing the effectiveness of methods to measure oxygen in tissues for prognosis and treatment of cancer. Adv Exp Med Biol 2016; 923: 113–20. doi: 10.1007/978-3-319-38810-6_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holve E. A first look at the volume and cost of comparative effectiveness research in the United States.. Academy health 2009;. [DOI] [PubMed] [Google Scholar]
- 90.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290: 1624–32. doi: 10.1001/jama.290.12.1624 [DOI] [PubMed] [Google Scholar]