Abstract
Objective:
Standard treatment for progressive gastric cancer with bleeding includes hemostatic radiotherapy (RT); however, the only prospective study using a fixed dose with fractions during hemostatic RT did not introduce re-irradiation. Therefore, we determined the utility of RT including re-irradiation for gastric cancer.
Methods:
In this study, 31 patients with gastric cancer and bleeding were treated with an initial dose of 20 Gy/5 fractions for the whole stomach and a salvage dose of 15 Gy/5 fractions for the partial stomach. Patients achieving hemostasis, defined as a stable hemoglobin level within 30 days following irradiation, were considered responders, whereas those with no cessation of bleeding and those with re-bleeding within 30 days of irradiation were considered non-responders. We evaluated response rate, disease-free survival, overall survival (OS), re-irradiation, and adverse events (AEs).
Results:
The response rate of initial RT was 80% (25/31). 6 of the 25 patients underwent re-irradiation, and all 6 were responders (100%). The median OS was significantly different among the entire cohort and one-time irradiation and re-irradiation groups (91, 76, and 112 days, respectively). No AEs of grade ≥3 were observed. Initial low-dose RT followed by reirradiation was effective in reducing AEs and did not cause any further AEs.
Conclusion:
Hemostatic RT was an effective approach with low toxicity, and re-irradiation was effective and tolerable, with no patients developing severe AEs. Further, randomized controlled studies are warranted to determine the ideal dose and number of fractions for initial RT in patients with gastric cancer and bleeding.
Advances in knowledge:
In this prospective study on hemostatic radiotherapy for gastric cancer, the response rate was 80% using a fixed dose of 20 Gy/5 fractions and the salvage dose of 15 Gy for re-bleeding was effective. Future comparative studies should include other doses with 20 Gy as a control.
Introduction
Surgery is currently the first choice of treatment for gastric cancer, and adjuvant chemotherapy may be performed for treatment of cancer at advanced stages.1 External beam radiotherapy (RT) alone is an effective and well-tolerated modality for local palliative treatment of gastric cancer, with palliation expected to continue for the majority of the patient’s life.2 Moreover, endoscopic hemostatic techniques are available for gastric cancer treatment. The rate of hemostatic response in patients with re-bleeding is high.3–5 Hemostatic RT is a useful treatment strategy for inoperable progressive gastric cancer.6–21 The outcomes of several retrospective reports on the efficacy of hemostatic RT are varied.6–8,12 One prospective study in patients with gastric cancer conducted by Tey et al found that the median survival duration following palliative RT with 36 Gy in 12 daily fractions was 85 days.21 The doses used in hemostatic RT ranged from 6 to 39 Gy in previous retrospective studies, which reported varying response rates. Additionally, in patients for whom re-irradiation is considered, the initial dose should be as low as possible. Importantly, no study to date investigated re-irradiation. Therefore, this prospective pilot study aimed to evaluate the efficacy of a two-step method for the treatment of gastric cancer, with an initial RT dose of 20 Gy/5 fractions and a second salvage RT dose of 15 Gy/5 fractions, and to examine the effects and safety of an initial dose of 20 Gy and a salvage dose of 15 Gy.
Methods and materials
Patients
In total, 33 patients with inoperable advanced gastric cancer and bleeding between 2016 and 2019 were enrolled in this prospective pilot study. Three of the patients underwent bypass surgery to connect the stomach to the intestines due to obstruction, whereas the remaining patients did not undergo any surgical treatment.
Inclusion criteria for initial irradiation
Patients with bleeding identifiable by pathological and endoscopic examinations and those with hemoglobin levels of ≤8 g ml−1 at initial consultation were included in the study. Continued bleeding causing anemia was identified by endoscopic examination. Whole-body contrast-enhanced CT and gastric endoscopy were performed; further, anemia caused by a chronic disease was excluded via blood tests. In case bleeding during surgery could not be stopped, argon plasma laser or clip was evaluated. Patients who cannot maintain hemoglobin level of 8 g ml−1 or more even after surgery, endoscopic treatment or blood transfusion. There were no limitations on age, sex, tumor size, tumor location, initial hemoglobin level, and Eastern Cooperative Oncology Group Performance status score.
Exclusion criteria for initial irradiation
Patients with the following conditions were excluded: high risk of febrile neutropenia (neutrophil count ≤1000/μL, platelet count <30000/mm3), severe distant metastases (brain, lungs, or liver) with a life expectancy of less than 1 month, and medical history of anticoagulant use or RT to the abdomen.
Inclusion and exclusion criteria for re-irradiation
Patients who responded to the initial hemostatic RT were evaluated for their eligibility to receive chemotherapy or best supportive care. Patients with re-bleeding were also were evaluated for their eligibility to undergo re-irradiation or best supportive care. Most of the inclusion criteria were the same as those for the initial irradiation. Informed consent was obtained again for re-irradiation. Additionally, chemotherapy was not scheduled after re-irradiation. Patients with more than three of the following adverse events (AEs) at the time of initial irradiation were excluded from re-irradiation: disorders of the blood, lymphatic system, congenital, gastrointestinal, hepatobiliary, and renal and urinary systems; fatigue; fever; pain; and infections. Patients with abdominal pain and suspicious infections were also excluded.
Definition of responders
After irradiation, blood tests were performed on 7, 15 and 30 days after RT. Responders were defined as those with continued hemoglobin levels > 8 g dl−1 without red blood cell transfusion during the first 30 days after the completion of RT. Although the absence of bleeding can be confirmed via endoscopy, the hemoglobin level also shows a tendency to increase to >8 g dl−1 in patients without bleeding.
Definition of non-responders
Non-responders were defined as patients with a poor general condition such as those with continued melena following RT. In addition, non-responders included those with confirmed bleeding via endoscopy and those whose hemoglobin levels fell to 8 g dl−1 or below despite an expected increase in hemoglobin to 8 g dl−1 or more following blood transfusions administered within 30 days after the completion of RT.
Primary endpoint
In the present study, response rate was the primary endpoint. Definition of no bleeding time, i.e. disease-free survival (DFS), was defined as a steady hemoglobin level of <8 g dl−1.
During follow-ups, we regularly conducted blood tests twice a month. Alternatively, if the patient had symptoms of anemia, a blood test was performed at that time. When the hemoglobin level was <8 g dl−1, we regarded it as rebleeding; the period up to that point was set as the no bleeding time.
Secondary endpoint
Due to the occurrence of re-bleeding, bleeding momentum varies. Quality of life is important because in-hospital treatments such as blood transfusions may be necessary following re-bleeding. Therefore, DFS was defined as the primary endpoint and overall survival (OS) was defined as the secondary endpoint. Briefly, OS was defined as the time from the end of RT until death due to any cause. AEs and the evaluation of salvage re-irradiation were other important secondary endpoints. DFS was defined as the time period from the end of RT to the emergence of symptoms such as dyspnea, anemia, and malaise with a hemoglobin level below 8 g ml−1.
RT planning
One radiation oncologist and one gastroenterologist examined tumor spread in the stomach using endoscopy and contrast-enhanced CT. The initial RT plan comprised irradiation for the whole stomach. Although the endoscopic examination indicated tumor spread, determining the border between normal and malignant tissues was difficult. Additionally, the tumors were often large or located remotely. Furthermore, extramural invasion is common in advanced gastric cancer, whereas the endoscope can only observe the inside of the stomach. Contrast-enhanced CT cannot determine the invasion to the surrounding tissue from the outer wall of the stomach. Finally, first irradiation dose was set as 20 Gy, which does not have a strong impact on organs at risk (OARs). Given that reproducibility is important in interinstitutional variation for pilot studies, the whole stomach was defined as the target for irradiation in the present study. No clip was required during the initial irradiation because the outer wall of the stomach could be identified on CT.
The outer wall of the stomach was contoured using butylscopolamine injection on an empty stomach. RT was performed in the early morning, and all patients consumed a meal after RT. Both hands were lifted up because the irradiation method used is basically from the front, rear, left and right. Cushions are placed under the patient's knees and the feet is placed in a comfortable position. Cone beam CT (CBCT) was taken every time. Three-dimensional (3D) conformal RT was performed using the field–in-field technique. IMRT affects the surrounding organs when the stomach is displaced. These methods provided good reproducibility. The planning target volume (PTV) was a 2 cm margin from the outer stomach wall in all directions. The intestines, liver, kidneys, and spinal cord were contoured as the OAR. The intestinal maximum point dose was <20 Gy, the whole liver mean dose was <20 Gy, and the bilateral whole kidney mean dose was <15 Gy. The OAR regulation was not a concern because the initial RT was 20 Gy/5 fractions, which was equal to a biologically effective dose with α/β = 10 (BED10) of 28 Gy. Irradiation was performed five days a week from Monday to Friday in all patients. AEs were scored every day during RT and 1 week after the conclusion of RT using the Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0.22 When a cancer patient passes away, symptoms such as anemia, nausea, and pain usually occur; therefore, it is not appropriate to apply hemostatic irradiation for AEs during the overall survival period of radiotherapy AEs.
The salvage re-irradiation dose was 15 Gy/5 fractions. Before RT, three or four clips were placed near the gastric tumor via endoscopy. By narrowing the irradiated area, it was possible to reduce the radiation dose to the normal stomach. Therefore, if the clip was placed in the vicinity of the cancer, the extent of the lesion could be clearly determined even by CT. The radiation oncologist contoured the tumor as well as the gastric wall and PTV under endoscopic guidance with the clips. The clinical target volume (CTV) was not determined for the whole stomach, and irradiation was performed only on the partial stomach.13,14 OAR regulation was performed in the same manner as described for the initial RT. An example of 3D conformal RT planning is shown in Figure 1. In our pilot study, hemostatic RT was likely to cause nausea. Therefore, patients with nausea were administered 10 mg domperidone orally every day during irradiation.
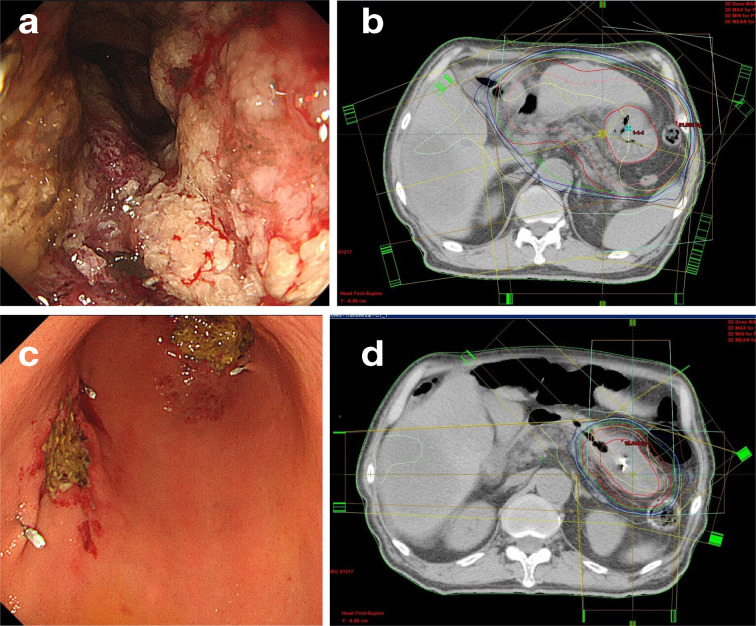
Figure 1.
(a) Initial endoscopic examination of gastric cancer with bleeding (Bormann Type 3). (b) Radiotherapy planning. Inner red line: whole gastric outer wall; pink line: CTV margin, 1 cm from the inner red line; outer red line: PTV margin, 1 cm from the CTV. Most (95%) of the prescribed dose (20 Gy/5 fractions) was administered to the PTV. (c) Re-bleeding from the initial tumor. The amount of bleeding was less than that observed during the initial irradiation. Clipping was performed in four places because the argon plasma coagulation performed did not stop the bleeding. Clips were used for radiotherapy planning as markers for guidance of the tumor spread. (d) Salvage radiotherapy (re-irradiation) and radiotherapy planning. Inner red line: partial gastric outer wall (including clips placed near the tumor using endoscopic guidance); pink line: clinical target volume margin, 5 mm from the inner red line; outer red line: PTV margin, 5 mm from the CTV. Most (95%) of the prescribed dose (15 Gy/5 fractions) was administered to the PTV. CTV, clinical target volume; PTV, planning target volume.
Evaluation
Hemostatic effect was defined as stabilization of the hemoglobin level within 30 days of initial irradiation. The primary endpoint was response rate, and the secondary endpoint was OS. DFS was defined as a hemoglobin level of <8 g dl−1 during the first 30 days after RT. No requirement of red blood cell transfusions after RT and recovery of the hemoglobin level to ≥8 g dl−1 lasting 30 days were defined as the presence of a stable hemoglobin level.
Re-bleeding was determined based on various symptoms such as melena, palpitations, dyspnea, dizziness, headache, general fatigue, and tiredness. In the presence of these symptoms, endoscopic examination and blood tests were performed. However, in patients who refused endoscopic examination, re-bleeding was defined as a reduction in hemoglobin level to <8 g dl−1. Subsequently, the patients decided whether to receive chemotherapy or best supportive care. Re-bleeding was defined as bleeding symptoms such as hematemesis or melena with a decrease in hemoglobin levels to <8 g dl−1. Toxicity was evaluated using CTCAE v. 5.0. AEs were evaluated from the initiation of RT until 30 days after RT.
Statistical analysis
Survival rates were estimated using the Kaplan–Meier analysis and compared using the log-rank test. Univariable Cox proportional hazards regression model was used to determine the association of all-cause death rate, determined from the end of RT, with clinical factors, year, sex, Karnofsky Performance Status, Borrmann type of cancer, pathology, metastasis, pre-RT hemoglobin level, red cell transfusion, and pre-RT treatment. p-values of ≤ 0.05 were considered statistically significant. All statistical analyses were performed using Excel statistical software package (Excel Statistics 2015; Social Survey Research Information, Tokyo, Japan).
Results
In the present study, four gastroenterologists, two surgeons, and one radiation oncologist evaluated each patient. In patients for whom radical surgery was not feasible, endoscopic hemostasis was performed. In patients with a passage disorder, including three patients with stenosis of the pyloric ring bypass, surgery was performed to connect the normal stomach area to the small intestine. Furthermore, we performed treatment with argon plasma laser, clipping, and topically administered tranexamic acid in all patients according to the endoscopic hemostasis method. In the majority of patients, venous bleeding and not arterial bleeding was observed; therefore, the response rate was 80% with hemostatic RT. After hemostatic RT, the requirement of blood transfusion was optional. RT was provided for patients in whom bleeding did not cease after endoscopic hemostasis (Figure 1).
Table 1 presents the results of the analyses by Cox proportional hazards model, which revealed no significant differences. There were no patient characteristics that were significantly associated with the results of hemostasis by initial RT. Of the 25 patients who responded, 19 required transfusion of irradiated red blood cells; the leukocyte counts were reduced to achieve a stabilized hemoglobin level of >8 g dl−1 in these patients. An average of 8 (range, 2–14) red blood cell units were transfused.
Table 1.
Characteristics of the study participants
| Patient characteristics | HR (95% CI) | ||
|---|---|---|---|
| Age, years, median (range) | 74 (62–86) | 1.01 (0.95–1.07) | |
| Sex | Male/female | 21/7 | 2.81 (0.76–10.28) |
| PS | 0–2/3–4 | 21/10 | 2.49 (0.61–10.10) |
| Borrmann type | 1–3/4 and others | 24/7 | 0.73 (0.18–3.01) |
| Pathology | Adenocarcinoma | 30 | 1.02 (0.97–1.12) |
| Unknown malignancy | 1 | ||
| Metastasis | Yes/No 4/27 | 1.36 (0.39–4.68) | |
| Pre-treatment Hb level (g/dL), median (range) | 6.0 (4.7–9.7) | 0.89 (0.69–1.15) | |
| Transfusions, units | 8 (0–14) | 0.96 (0.63–1.45) | |
| Pre-RT treatment | APC | 25 | 0.77 (0.61–0.97) |
| Clipping | 11 | 0.81 (0.61–1.05) | |
| Bypass Op | 3 | 0.98 (0.93–1.01) | |
Hb, hemoglobin; Transfusion, packed red blood cell units transfused before radiotherapy; APC, argon plasma coagulation; clipping, endoscopic clipping; bypass op, gastrointestinal bypass operation; Pre-RT treatment, previous treatment before radiotherapy;
PS, Karnofsky performance status; HR, hazard ratio; CI, confidence interval
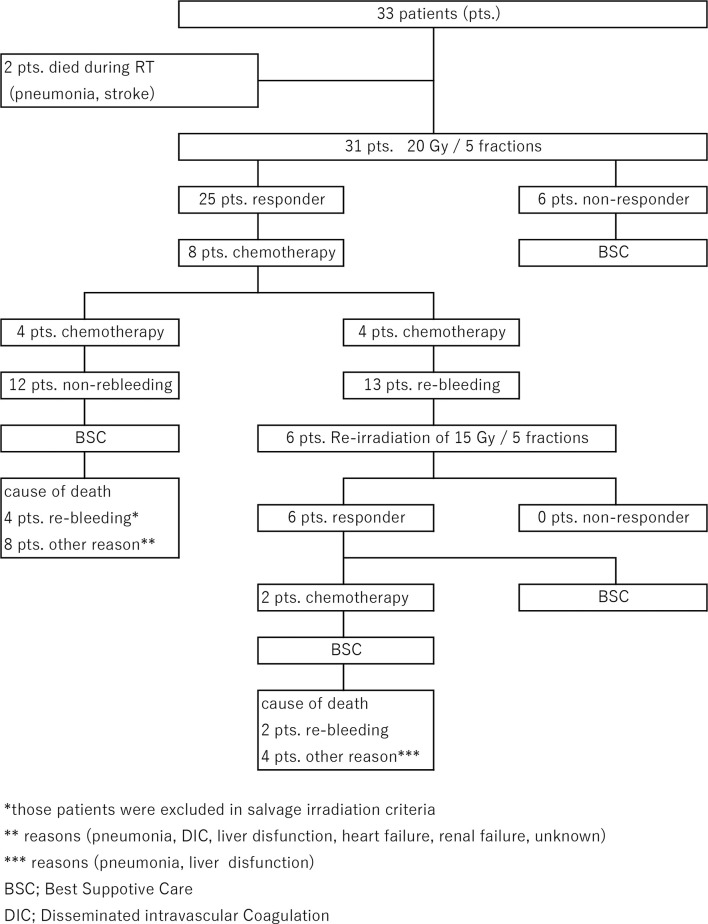
The flow of the treatment regimen is shown in Figure 2. 2 of the 33 enrolled patients were excluded because of pneumonia and stroke that occurred during RT; therefore, 31 patients were included in the final analyses. The overall response rate was 25/31 (80.6%; Figure 3 and Table 2). In these 25 patients with hemostatic effects, i.e. responders, the median time without bleeding was 63 days (range, 33–196 days) and the median OS was 91 days (range, 46–299 days). Conversely, in the non-responder group (n = 6/25), the median OS was 21 days (range, 14–28 days). Furthermore, the median OS rates were 76 days (range, 46–240 days) and 112 days (range, 87–299 days) in the one-time irradiation and re-irradiation (salvage RT) groups, respectively (Figure 4 and Table 2). 8 of the 25 patients who successfully responded to hemostatic RT received chemotherapy after initial RT. Re-bleeding was observed in 13 patients, and re-irradiation was performed in six patients. Four of the six patients who underwent re-irradiation received chemotherapy prior to re-irradiation, and two of these four patients received chemotherapy after re-irradiation as well. 4 of the 12 patients who did not exhibit re-bleeding received chemotherapy after irradiation. Conversely, 6 of the 13 patients with re-bleeding underwent re-irradiation. Although re-irradiation was effective in all six patients with re-bleeding who underwent re-irradiation, two patients died from re-bleeding, whereas the remaining four patients died from other causes. In conclusion, 13 of the 25 patients died due to re-bleeding, whereas the remaining 12 patients died without bleeding.
Figure 2.
Illustrative diagram showing the flow of treatment.
Figure 3.
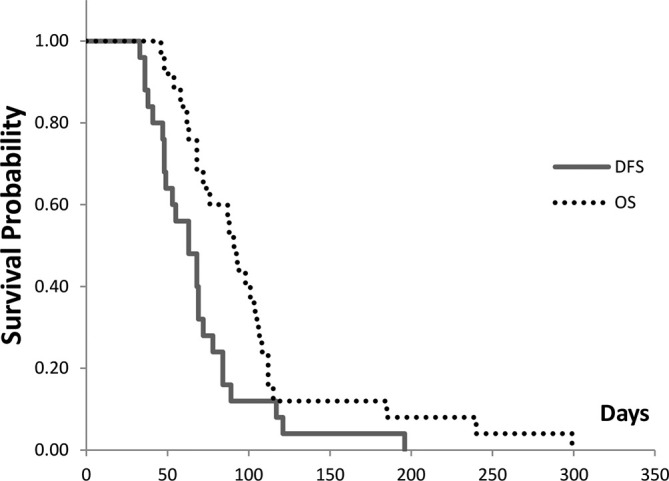
DFS and OS of 25 responders. The median DFS and OS rates are 63 and 76 days, respectively. DFS, disease-free survival; OS, overall survival.
Table 2.
Survival time of 25 responders
| Median, days | |
|---|---|
| One-time irradiation response group (n = 19/25; responder)* | 76 (46–240) |
| Re-irradiation group (n = 6/25; responder)** | 112 (87–299) |
| Overall survival (n = 25) | 91 (46–299) |
| Disease-free survival (n = 25) | 63 (33–196) |
| *Time to death from the end of initial irradiation | |
| **Time to death from the end of re-irradiation |
There is a significant difference in survival time between the one-time irradiation group and the re-irradiation group; log-rank test (p = 0.02).
Figure 4.
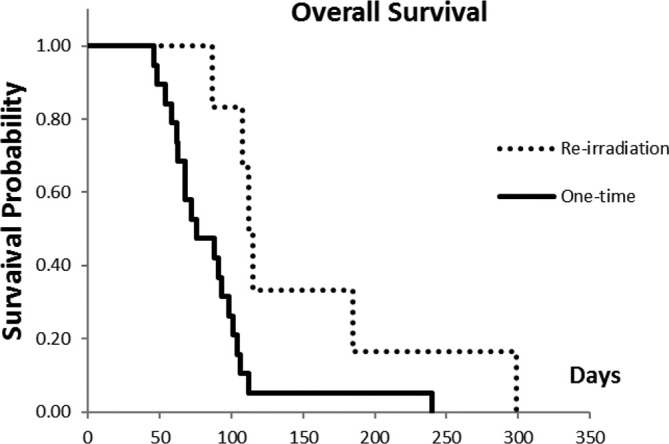
OS is significantly greater in the re-irradiation group (n = 6) than in the one-time irradiation group (n = 19; p = 0.02). Significant differences were observed in the OS time between one-time (initial irradiation only) patients (n = 19) and re-irradiation (n = 6); log-rank test (p = 0.02). OS, overall survival.
In the present study, 16 of the 25 responders were discharged and received supportive care at home before death; the duration of home stay ranged from 4 to 10 weeks. There were no grade ≥3 AEs. Nausea was the universally (100%) reported side-effect (Table 3), which disappeared after 3 days of irradiation in all patients. Prophylactic domperidone was used in some patients (20/31; 65%). Nausea and vomiting were reduced typically after 1 week of RT in patients who underwent re-irradiation. Neutropenia was not observed in any of the patients.
Table 3.
Acute toxicity during radiotherapy according to the CTCAE v. 5.0
| Symptom (n = 25) | Grade 1 | Grade 2 |
| Nausea | 11 | 7 |
| Vomiting | 4 | 0 |
| Gastrointestinal pain | 2 | 0 |
| Fever | 1 | 0 |
| Malaise | 1 | 1 |
| Neutropenia | 0 | 0 |
CTCAE, Common Terminology Criteria for Adverse Events.
We did not find any significant predictive factors associated with response rate, OS, or AEs in the present study (Table 1).
Discussion
The standard dose of RT for bone metastases is 8 Gy rather than 6 or 10 Gy, whereas the optimal dose for hemostatic RT in patients with gastric cancer remains unknown. We therefore conducted a prospective pilot study to determine the efficacy and safety of 20 Gy/5 fractions as the optimal dose for hemostatic RT.
Irradiation field
In gastric cancer, the time to achieve hemostasis might vary if initial RT is applied locally. However, determining the tumor outline without clips is difficult, particularly in tumors with submucosal spread. Previous studies have reported target areas varying from partial to the whole stomach. Our results indicated a relatively high response rate (80.6%) with a low AE rate. Therefore, we recommend that the initial target should encompass the whole stomach if the tumor spread cannot be identified by CT. The hemostatic RT utilized in the present study was a two-step protocol; all six patients with re-bleeding were successfully treated by re-irradiation. The re-irradiation field was contoured with the guidance of 3–4 clips placed using endoscopy (Figure 1). However, it may be acceptable to contour a narrow field based on endoscopic findings and using more precise contrast-enhanced CT imaging if reproducibility can be achieved.
Dose and re-irradiation
In the present study, 13 of the 25 patients developed re-bleeding. Among these, six patients with re-bleeding chose to be treated with re-irradiation whereas the remaining seven patients declined to undergo re-irradiation and received supportive care (Figure 2). Similar to the RT for painful bone metastases wherein re-irradiation is as effective as the initial RT, re-irradiation for gastric cancer may achieve results similar to those of initial RT by reducing tumor mass. Tey et al reviewed seven studies on palliative RT for gastric cancer.12 Lee et al.6 showed that the median RT dose was 40 Gy in responders vs 21 Gy in non-responders (p < 0.001), with the BED10 for responders being significantly higher than that for non-responders (median 48 vs 26.4 Gy, p < 0.001), with the optimal cutoff being 36 Gy. None of the patients in their study received additional RT. The authors evaluated toxicity by the National Cancer Institute Common Toxicity Criteria v. 5.0, wherein grade ≥3 nausea, vomiting, asthenia, dysphagia, or epigastric pain was considered to indicate significant toxicity. Tey et al7 did not observe a significant difference in the response rate for bleeding between the regimens with a high BED of ≥39 Gy and those with a low BED of <39 Gy (p = 0.39). Grade 3–4 toxicities occurred in up to 15% of the patients treated with RT alone and up to 25% of patients treated with chemoradiotherapy. They concluded that a short (<39 Gy BED) RT schedule is adequate for effective symptom palliation. In another retrospective study, Kawabata et al8 analyzed the clinical data of 18 patients who underwent palliative RT and experienced bleeding due to gastric cancer. The radiation dose was 6 Gy/3 fractions, and the treatment success rate was 55%. Therefore, the authors concluded that low-dose hemostatic RT might improve the quality of life in these patients. These results from retrospective studies therefore suggest that a high dose may not be necessary for initial RT.
According to reports from previous studies, the frequency of AEs, such as nausea, increases when a high dose is administered at once. We also performed treatments with 20 Gy/5 fractions or 30 Gy/10 fractions in the actual clinic before this prospective study; however, the lower the dose, the lower the AEs. Considering the tolerable dose to the stomach, if we first irradiate with 20 Gy, we thought that 15 Gy of salvage irradiation could be treated with less AEs. Palliative treatment was started at the lowest possible dose, as established with re-irradiation for bone metastases, and a schedule was set up so that radiation therapy could be performed when relapse occurred. We previously reported that hemostatic RT with 20 Gy/5 fractions was successful in patients with metastatic gastric cancer.9 We also reported two patients with gastric cancer who received an initial dose of 20 Gy followed by 15 Gy as salvage RT10,11 Although short-term irradiation is preferable, high doses are associated with the risk of perforation in the gastrointestinal tract. The re-irradiation group that received 15 Gy responded to hemostasis with a corresponding increase in lifespan. However, although 15 Gy may be sufficient for initial treatment, there is the possibility of early re-bleeding. Considering the previous reports,3–5 bleeding was stopped in 80% of the patients who received 20 Gy as initial RT in the present study. Re-irradiation was performed at 15 Gy as a total BED10 of <50 Gy is considered better for reduced rates of gastric AEs.
In the present study, the OS of the re-irradiation group was better than that of the one-time irradiation group, which might due to several reasons. First, the general condition of the patients was relatively good in the re-irradiation group. Additionally, four patients with a life expectancy of <1 month were excluded from the re-irradiation group. Second, several patients among those who received one-time irradiation did not desire to receive re-irradiation. Third, the actual cause of death included other reasons in the one-time irradiation group. The high hemostasis rate in the re-irradiation group was attributable to bleeding occurring only on the tumor surface; arterial bleeding was unlikely as hemostatic RT might not have been successful in that case. Therefore, it was necessary to observe the state of bleeding by endoscopy.
Considering the toxicity of irradiation, a two-step protocol is preferable. In the present study, eight patients underwent concurrent chemotherapy. Although chemotherapy is a burden for patients with severe anemia, all eight patients recovered from anemia and started chemotherapy 4–8 weeks after RT. Of the 25 patients who responded to the initial RT, 18 patients were discharged and received supportive care at home, although a relatively low dose of 20 Gy/5 fractions for initial RT was used in the present study.
In the present study, the evaluation was started at the time of RT completion. Two patients who died due to causes unrelated to gastric cancer during RT were excluded. While intention-to-treat analysis can certainly be used for detailed assessment, not all patients with inoperable bleeding were treated with RT. Future studies including larger number of eligible patients is necessary to perform intention-to-treat analyses. Following the confirmation of inoperable gastric cancer, 2 weeks are required to complete RT in the radiation oncology department. Therefore, we analyzed only those patients who completed the treatment. Patients who cannot be treated by surgery or endoscopy are often in poor clinical condition. The two patients with pneumonia and stroke were categorized into the non-responder group, the response rate would be decreased; however, in clinical settings, simple results may be easier to explain to patients.
Adverse events
Most patients experienced venous bleeding and not arterial bleeding. After hemostatic RT, the requirement of red blood cell transfusion was optional, and future studies for dose reduction are necessary to reduce AEs.
Conclusions
Initial RT followed by re-irradiation was effective (80%). Re-irradiation was a suitable option for patients in good clinical condition. No grade ≥3 AEs occurred, and most AEs disappeared one week after the RT. Moreover, RT was completed in only 5 days and the re-irradiation option appeared to be favorable for patients. Approximately, two-thirds of the patients could be discharged. The range of effective hemostatic time was wide, and there were no significant predictive factors associated with response rate, OS, or AEs in the present study. Therefore, randomized controlled studies should determine the ideal dose and number of fractions for initial irradiation.
Footnotes
Ethical statements: The research on human subjects were performed in accordance with the standards set out in the Code of Ethics of the World Medical Association (Declaration of Helsinki). The research procedures were approved by internal review board and registered UMIN-CTR number 000026362. All the patients provided written informed consent before enrolling in the study.
Contributor Information
Osamu Tanaka, Email: c.bluered@gmail.com.
Akihiko Sugiyama, Email: akihiko1025@vanilla.ocn.ne.jp.
Tatsushi Omatsu, Email: otamatsushi@gmail.com.
Masahiro Tawada, Email: tawada0316@yahoo.co.jp.
Chiyoko Makita, Email: chioko.makita@gmail.com.
Masayuki Matsuo, Email: matsuo_m@gifu-u.ac.jp.
REFERENCES
- 1.Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol 2015; 12: 676–82. doi: 10.1038/nrclinonc.2015.132 [DOI] [PubMed] [Google Scholar]
- 2.Tey J, Back MF, Shakespeare TP, Mukherjee RK, Lu JJ, Lee KM, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys 2007; 67: 385–8. doi: 10.1016/j.ijrobp.2006.08.070 [DOI] [PubMed] [Google Scholar]
- 3.Kim Y-I, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc 2015; 48: 121–7. doi: 10.5946/ce.2015.48.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y-I, Choi IJ, Cho S-J, Lee JY, Kim CG, Kim M-J, et al. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J Gastroenterol Hepatol 2013; 28: 1489–95. doi: 10.1111/jgh.12262 [DOI] [PubMed] [Google Scholar]
- 5.Koh KH, Kim K, Kwon DH, Chung BS, Sohn JY, Ahn DS, et al. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer 2013; 16: 397–403. doi: 10.1007/s10120-012-0200-3 [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Lee JW, Jang HS. Palliative external beam radiotherapy for the treatment of tumor bleeding in inoperable advanced gastric cancer. BMC Cancer 2017; 17: 541. doi: 10.1186/s12885-017-3508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine 2014; 93: e118. doi: 10.1097/MD.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawabata H, Uno K, Yasuda K, Yamashita M, low-dose Eof. Experience of low-dose, short-course palliative radiotherapy for bleeding from unresectable gastric cancer. J Palliat Med 2017; 20: 177–80. doi: 10.1089/jpm.2016.0141 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka O, Yokoi R, Mukai T, Yamada M, Kato T, Taniguchi T, et al. Radiotherapy for gastric bleeding from tumor invasion of recurrent colon cancer with liver metastasis after resection. J Gastrointest Cancer 2019; 50: 349–52. doi: 10.1007/s12029-017-0026-7 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka O, Matsuura K, Sugiyama A, Kato T, Tomita E, Matsuo M. Hemostatic radiotherapy used twice for inoperable progressive gastric cancer with bleeding. J Gastrointest Cancer 2019; 50: 151–5. doi: 10.1007/s12029-017-9994-x [DOI] [PubMed] [Google Scholar]
- 11.Tanaka O, Yamada M, Kato T, Taniguchi T, Ono K, Matsuo M. Two sessions of radiotherapy were successful in treating gastric cancer with bleeding. J Gastrointest Cancer 2018;: 1–5. [DOI] [PubMed] [Google Scholar]
- 12.Tey J, Soon YY, Koh WY, Leong CN, Choo BA, Ho F, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017; 8: 25797–805. doi: 10.18632/oncotarget.15554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JA, Lim DH, Park W, Ahn YC, Huh SJ. Radiation therapy for gastric cancer bleeding. Tumori 2009; 95: 726–30. doi: 10.1177/030089160909500615 [DOI] [PubMed] [Google Scholar]
- 14.Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist 2004; 9: 561–70. doi: 10.1634/theoncologist.9-5-561 [DOI] [PubMed] [Google Scholar]
- 15.Chaw CL, Niblock PG, Chaw CS, Adamson DJ. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: a single-institution experience. Ecancermedicalscience 2014; 8: 384. doi: 10.3332/ecancer.2014.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto K, Mayahara H, Takashima A, Nakajima TE, Kato K, Hamaguchi T, et al. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single Institute experience. J Cancer Res Clin Oncol 2009; 135: 1117–23. doi: 10.1007/s00432-009-0553-0 [DOI] [PubMed] [Google Scholar]
- 17.Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, et al. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol 2011; 137: 125–30. doi: 10.1007/s00432-010-0866-z [DOI] [PubMed] [Google Scholar]
- 18.Kondoh C, Shitara K, Nomura M, Takahari D, Ura T, Tachibana H, et al. Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: a retrospective cohort study. BMC Palliat Care 2015; 14: 37. doi: 10.1186/s12904-015-0034-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosugi T, Shikama N, Saito T, Nakamura N, Nakura A, Harada H, et al. A nationwide survey in Japan of palliative radiotherapy for bleeding in gastrointestinal and genitourinary tumor patients. World J Oncol 2016; 7(2-3): 29–33. doi: 10.14740/wjon977w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corry J, Peters LJ, Costa Ieta D', Milner AD, Fawns H, Rischin D, et al. The 'QUAD SHOT'--a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol 2005; 77: 137–42. doi: 10.1016/j.radonc.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 21.Tey J, Zheng H, Soon YY, Leong CN, Koh WY, Lim K, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: a phase II trial. Cancer Med 2019; 8: 1447–58. doi: 10.1002/cam4.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute Common terminology criteria for adverse events (CTCAE..