Abstract
Objective:
This study evaluated the performance of the novel liquid fiducial marker (BioXmark®) in IGRT for bladder cancer.
Methods:
20 patients with muscle invasive bladder cancer were entered in this prospective, single center, Phase I-II study. The novel BioXmark® liquid markers were injected around the tumor using a flexible cystoscopy. Visibility and stability of the markers were evaluated on planning-CT and CBCT. Prospectively defined threshold for success was set at a visibility of 75%.
Results:
In total, 76 markers were implanted in 20 patients. Of those, 60 (79% 95% CI ± 9%) were visible on CT scan. Due to the learning curve of the technique, the visibility improved in the last 75% of patients (86% visibility) compared to the first 25% of patients with 58% visibility. Concerning stability of the BioXmark® marker, all visible markers after CT acquisition were still detectable at the last CBCT without displacement. In 15/20 (75%) of the patients, three or more markers were visible on CT. No BioXmark® related adverse events were reported.
Conclusion:
The success rate of this novel fiducial marker was 79%, which is above the prospectively defined threshold rate. A distinct learning curve of the injection of the liquid marker was seen over the study period. The marker showed sustained visibility and positional stability during treatment phases and also appears to be safe and easy to inject.
Advances in knowledge:
This novel liquid BioXmark® marker seems to be a very promising tool in daily-adaptive IGRT for bladder preserving chemoradiotherapy in muscle invasive bladder cancer.
Introduction
Bladder-preserving treatment is gaining interest as a suitable option for patients with muscleinvasive bladder cancer. Where it was originally reserved for inoperable patients, advancements in radiation delivery (Intensity Modulated or Volumetric Modulated Arc RadioTherapy (IMRT or VMAT) in combination with radio-sensitizing drugs gave rise to chemoradiation as equivalent treatment alternative to a radical cystectomy. The study by James et al1 in patients with T2-4 N0 M0 muscle invasive bladder cancer patients showed that disease-free survival after bladder-preserving treatment with chemoradiation was 67% after 2 years and overall survival was 48% after 2 years. Muscle-invasive recurrences were only seen in 18%, toxicity was acceptably low and functional bladder preservation was 89%. The GETUG 97–015 study showed similar results with even a longer follow-up of 8 years.2 Direct comparison between cystectomy and chemoradiotherapy is not (yet) available since randomized controlled trials are lacking. However, indirect comparison based on meta-analyses data demonstrated similar survival and loco-regional tumor control rates.3,4
Accurate tumor delineation and image-guided radiotherapy are essential when using a focal bladder boost in terms of sparing of normal tissue as well as delivering an adequate dose to the primary tumor. Since the bladder is a distensible organ, with only some fixed ligamentous connections at the bladder neck, movement of the bladder wall by more than 1.5 cm has been documented in up to 60% of the patients during the radiation course.5 Therefore, large margins are needed for adequate coverage, which inevitably will lead to more healthy tissue being exposed to radiation and causing more radiation-induced side effects.
Reduced high-dose volume was shown to be non-inferior regarding toxicity and recurrence in the British BC2001 trial.6 Patients in this trial were treated with empty bladder and 3D-conformal radiotherapy.
Image-guided adaptive radiotherapy (IGART) like plan of the day with a library of plans and a focal boost lead to smaller margins, lower dose to healthy tissue—less toxicity and thereby also the possibility of dose-escalation in the tumor which could result in better tumor control. Because the macroscopic tumor boundaries are hard to determine on the CT scan endoscopically implanted fiducial markers with visibility on CT scans are of help in tumor delineation, but also in daily online IGART to determine the most suitable plan of the day. The currently applied fiducial markers (e.g., gold seeds, titanium clips, hydrogel, or lipiodol) all have their own advantages and disadvantages. Gold seeds and titanium seeds are safe and feasible, but up to 40% of the markers are lost in verification imaging.7–9 Also, implantation of these markers needs a rigid cystoscope, which is not comfortable for the patient and it can be difficult to reach all locations in the bladder. Liquid markers such as hydrogel10 and lipiodol11–15 are found to be safe and easy to inject. The disadvantage of hydrogel, however, is the low density that makes the marker less visible on CBCT and the technical difficulty of adequate volume injection. The disadvantage of lipiodol is fading or blurring of the spots, which makes it less useful to accurately delineate the tumor boundaries and online image guidance during the treatment period.
Therefore, there is a need for a safe, easy to inject, well visible and positionally stable fiducial marker during the planning and treatment phases to optimize IGART for bladder conserving treatment with a focal boost. BioXmark® is a novel liquid injectable and adherent fiducial marker that showed promising results for IGRT in lung and esophageal cancer.16,17 BioXmark® has a higher density compared to hydrogel and is expected not to blur or fade during the radiation course. The aim of this study was to evaluate safety, feasibility, visibility, and stability of the BioXmark® liquid fiducial marker for the use in IGART for muscle invasive bladder cancer.
Patients and methods
The trial was approved by the ethical board and registered. Written informed consent was obtained in all study participants. Since the study product was not CE-marked, the trial was also registered at Health and Youth Care Inspectorate of the Ministry of Health, Welfare and Sports.
Patient selection
Between July 2018 and July 2019, 20 patients with non-metastasized unifocal muscle invasive bladder cancer, suitable for chemoradiation, were accrued to this prospective Phase I–II trial. The trial was conducted at a tertiary university medical center. Patients were excluded when there was any contraindication for undergoing an outpatient cystoscopy procedure.
Liquid fiducial marker
BioXmark® is an injectable liquid fiducial marker, which increases in viscosity after injection. The liquid marker consists of three components: sucrose acetate isobutyrate (SAIB), x-SAIB (electron dense SAIB analogue), and ethanol (ETOH). ETOH diffuses out of the gel-like matrix upon injection, thereby forming a hydrophobic marker that has a Hounsfield Units (HU) density of 800–1240 depending on the surrounding tissue. This marker produces a high contrast-to-noise ratio on CT and gives equivalent artifacts compared to other liquid or solid markers.18 BioXmark® is also visible on MRI.
Cystoscopy
Patients underwent in an outpatient setting flexible cystoscopy with a 17 French flexible OlympusVisera Elite CLV-S190 system with a CYF-V2/VA2 cysto-nephro videoscope. It has an inner channel (for instrumentation) diameter 6.6 Fr (diameter 2.2 mm) and angulation range in bending section: up 210/down 120 degrees. It was planned as if two urologists performed the procedures.
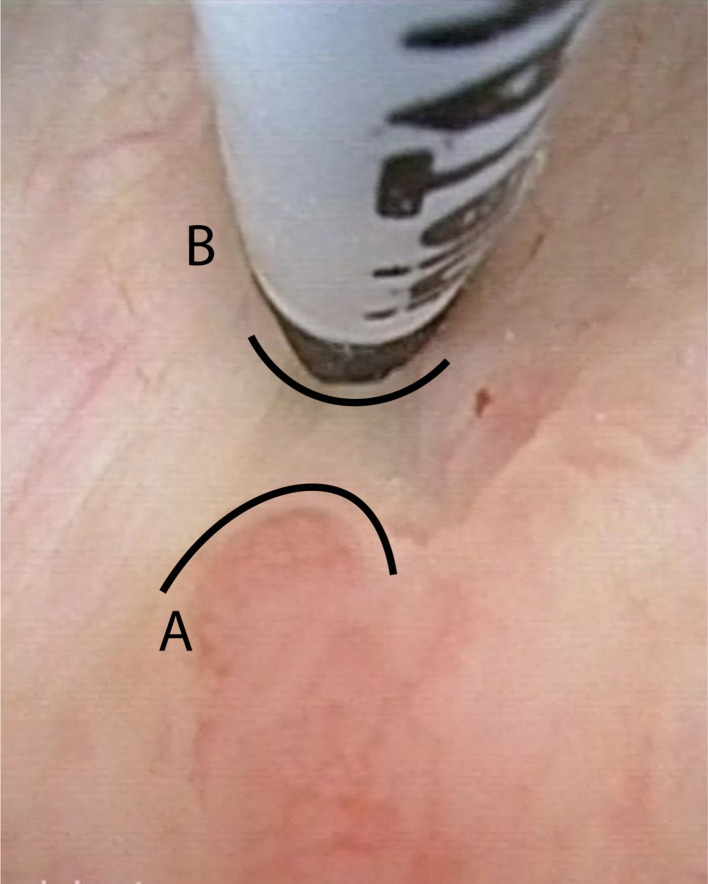
Just before the cystoscopy, every patient got an antiseptic lubricating gel placed intraurethrally (Instillagel® 10 ml, comprised of chlorhexidine gluconate with lidocaine hydrochloride). After that, a cystoscopy was performed in which the residual tumor was located, and the marker-positions were chosen at the border of the tumor with 0–0,5 cm margin. With an endoscopic 4.8 French injection needle (InjeTAK® Adjustable Tip Needle, 23 Gauge, 70 cm), placed through the cystoscope preferably 3–4 dots of 0.1 ml of BioXmark® were submucosally injected in a circumferential pattern around the bladder tumor including the TUR-B resection scar (Figure 1). During this study, fluoroscopy after injection as visual quality control measurement was used only in the beginning of the trial because there was a good outcome of the endoscopic technique in which the fluoroscopy, in our opinion, had no additional value. After the injection procedure, patients were advised to empty their bladder (by spontaneous voiding) and if they felt well, they could leave the hospital within 30 min after the procedure. The procedure was evaluated with a multiple-choice question: How did the implant procedure went? easy/normal/hard
Figure 1.
Image of cystoscopy injection of BioXmarker at the margin of a bladder tumor. A = border of the tumor. B = location of the BioXmark injection at 5 mm of the tumor margin.
Treatment
Within 2 days after fiducial marker implant a treatment planning CT-scan (with full and empty bladder) was made. Based upon the written cystoscopy report, CT findings, and fiducial markers, a gross tumor volume (GTV) was delineated.
Two clinical target volumes (CTV1 and CTV2) are created. CTV1 covers the entire bladder with a 5 mm extravesical margin around the GTV and CTV2 is the GTV with a 5 mm margin. In case there were less than two visible markers, the CTV margin in the direction of the bladder wall was extended to 10 mm. Depending on the difference between full and empty bladder, 3–5 different plans are created based upon interpolated bladder filling to create a library of plans. An isotropic PTV margins of 8 mm around CTV1 and 5 mm around CTV2 is created around each of the plans. A total dose of 40 Gy/20 fractions was prescribed to the PTV1 with a simultaneous integrated boost (SIB) of 15 Gy/20 fractions to PTV2(total dose of 55 Gy/20 fractions). Concomitantly chemotherapy was given (Mitomycine C 12 mg/m2 only on day 1 and bd Capecitabine 825 mg/m2 on day 1 until day 20). A daily online CBCT is used to define the best-suited plan of the day. Fiducial markers are used to check the SIB position in relation to the expanded or contracted bladder wall.
Study endpoints and follow-up
Primary endpoints of the trial were safety of the marker implantation procedure, marker visibility, and positional stability of the fiducial markers over time. Visibility was evaluated on radiotherapy planning CT acquisition and weekly CBCT during treatment. Visibility was scored dichotomously (visible/non-visible). In our study, the definition for non-visibility was that the marker was not reliably visible for delineation (CT scan) or positional verification (CBCT scan). Each image was scored once by one of two radiation oncologists. Positional stability was scored on CT-scan and latest CBCT (with comparable bladder filling). If bladder filling on the last CBCT was not comparable with the CT-scan, the CBCT before the last one was used, if it was a case of comparable bladder filling.
Serious adverse events (SAE) associated with BioXmark®marker were recorded from the moment of injection until the end of chemoradiation treatment or at least 30 days after the marker implantation. Patients were assessed weekly during treatment and 4 weeks following treatment.
Secondary endpoints were the appearance of blurring (i.e., diffuse spread out of the liquid through the bladder wall instead of a single dot), time needed for implantation and possibility for automatic online matching for IGRT (XVI Elekta).
Statistical analysis
Accepted criteria for performance and clinical applicability were that 75% of the markers had to remain visible and positionally stable from the CT acquisition for RT planning to the last CBCT, without causing grade three toxicity (CTCAE v4.0).
Formally this corresponds to test the hypothesis H0: p ≤ 0.75 against the alternative HA: p > 0.75. H0 is rejected if the lower limit of the 95% CI for the observed performance success in the trial is lower than 0.75.
For the secondary endpoints, descriptive statistics are presented. This includes proportions for frequency data, and medians or means and standard deviations for scale variables. Point estimates are presented along with measures of precision such as CIs.
Sample size calculation
Sample size calculation is based on the primary endpoint. Performance is sufficient if in at least 75% the markers remain visible and positionally stable from the CT acquisition for RT planning to the last CBCT. Binomial distribution was used with a 95% CI that does not contain 0.75 as the lower limit. Based on clinical experience with BioXmark® fiducial markers in other tissues, only minor migration is expected—if any—and for sample size calculation, the expected sample proportion of non-visible or migrating markers is set at 8%. Using Clopper-Pearson, a sample size of 40 and 60 markers would produce the CI [0.7634; 0.9721] and [0.8161; 0.9724], respectively. Both are well above the set criteria to show that the markers perform at least as good as other markers.
The sample size should account for possible dropouts of patients, and estimating a 20% dropout rate and a proportion of non-visible or migrating markers at 8%, the resulting CI would be [0.8002; 0.9768], which does not include 0.75. Based on this, the sample size was set to 20 subjects as it was planned to implant at least three markers per subject.
Results
Patient characteristics
In total, 76 markers were implanted in 20 patients with histologically proven muscle invasive urothelial carcinoma of the bladder. All patients had a T2G3 N0 M0 urothelial carcinoma of the bladder. Median age of the patients was 83 years (range 55–90 years). Most of the patients were unfit for cystectomy, although a few were fit but refused cystectomy and preferred bladder preservation. Median number of implanted markers per patient was 4, with a minimum of 3 and maximum of 5 markers. After CT acquisition, but before start of the treatment, one patient died of an intercurrent disease cause (reported as SAE and considered non-study procedural related). This resulted in 20 evaluable patients for marker visibility on CT scan and 19 evaluable patients for marker visibility and stability on CBCT. All 19 patients finished treatment as planned.
Procedure
During the first two procedures, it was noted that the digital pressure applied to the end of the syringe had to be much higher compared to other known liquid markers, due to the high viscosity of this specific marker (BioXmark®). This was probably the cause of misplaced markers in the first two patients. Because of this technical difficulty, we changed the procedure by using a syringe with a dual-lock system, and by keeping the needle in the right submucosal position for approximately 5 s, the pressure inside the needle could be raised slowly so that the BioXmark®liquid marker could be pushed out of the system into the right position in the bladder wall. After these changes in injection-technique, the placement of the markers in this implantation procedure became easier. At the start of the study, the implantation of the markers was performed under fluoroscopy control so that the urologist could learn the use of this specific marker and be able to check the result at the end of the procedure.Two urologists performed the procedures (urologist 1: five cases, urologist 2: 15 cases, of which one was performed by a resident under direct supervision of the urologist). After these small adaptations, successful implantation increased. This was also reflected in the subjective score on how the procedure went; only the first procedure was scored as hard, the other 19 were scored normal (n = 8) or easy (n = 11) by the urologist(s). After the fifth patient, no fluoroscopy was deemed necessary anymore. Mean duration of the implantation procedure was 12 min, calculated from the moment the injection needle was inserted in the scope system until removal of the cystoscope (range 8–16 min).
Visibility, positional stability, and feasibility (Table 1)
Table 1.
Overview of markers per patient and visibility on CT/CBCT
| Patient | No. of markers placed | No. of markers visible on CT | No. of markers visible on CBCT week 1 | No. of markers visible on CBCT week 4 |
|---|---|---|---|---|
| 1 | 4 | 1 | 1 | 1 |
| 2 | 4 | 4 | 4 | 4 |
| 3a | 3 | 2 | a | a |
| 4 | 5 | 1 | 1 | 1 |
| 5 | 3 | 3 | 3 | 3 |
| 6 | 3 | 3 | 3 | 3 |
| 7 | 4 | 4 | 4 | 4 |
| 8 | 4 | 4 | 4 | 4 |
| 9 | 4 | 4 | 4 | 4 |
| 10 | 5 | 5 | 5 | 5 |
| 11 | 3 | 3 | 3 | 3 |
| 12 | 3 | 3 | 3 | 3 |
| 13 | 4 | 4 | 4 | 4 |
| 14 | 5 | 3 | 3 | 3 |
| 15 | 4 | 4 | 4 | 4 |
| 16 | 3 | 0 | 0 | 0 |
| 17 | 4 | 4 | 4 | 4 |
| 18 | 4 | 3 | 3 | 3 |
| 19 | 4 | 2 | 2 | 2 |
| 20 | 3 | 3 | 3 | 3 |
| Total | 76 | 60 | 58 | 58 |
CT = computer tomography; CBCT = cone beam computer tomography
patient died of an unrelated event between CT acquisition and start of therapy
Of the 76 markers implanted, 60 (79% (95% CI 70–88%) were visible on treatment planning CT scan. In 15/20 (75%) of the patients, three or more markers were visible on CT. In one patient, there was one marker visible on the CT scan with a full bladder but disappeared on the empty bladder. Apparently, it was excreted during voiding, possibly because of a very superficial position in the mucosa. Noteworthy, there was a patient with bilateral hip prostheses in which the bladder wall itself was hardly visible on CT, but all three markers were visible and useful for delineation and IGART.
All visible markers after CT acquisition were still detectable at the last CBCT in the same position. All separate spots were continuously classified as clearly visible without artifacts.
Migration did not occur, nor did the density of the markers fade during the treatment course.
All markers appeared as a circumscribed single dot on the treatment planning CT scan and not any blurring occurred. All markers present on CT\CBCT scans could be used for delineation, patient setup and for automatic marker matching at least three detectable markers are necessary. In vivo images of BioXmark® in the bladder are shown in Figure 2.
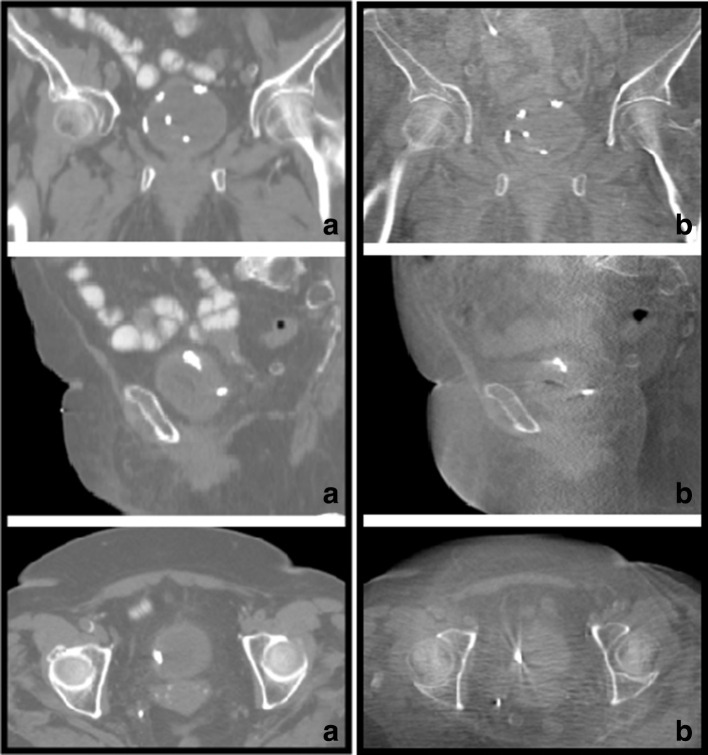
Figure 2.
Illustrative examples of BioXmark® spots in one patient on CT (A) and CBCT at end of treatment (B). Top images are in the coronal plane, middle images in the sagittal plane and the bottom images are in the axial plane. Note: the fifth, most central dot in the top images is not a BioXmark® dot, but a catheter.
Of the 76 implanted markers, 16 were not seen at CT acquisition (21%). In the first five patients, this percentage was 42%, whereas in the last 15 patients 14% of the markers were not detectable. Of the 19 patients that underwent treatment, all markers could be used for delineation and patient setup. In 15/19 (79%) patients, automatic marker matching was possible, based upon at least three detectable markers.
Safety
No grade three or higher toxicity was reported. Two patients (10%) reported acute grade two toxicity (urinary tract infection (n = 1) and haematuria (n = 1). The haematuria was already present before the implantation and stopped during the radiation course. No long-term toxicity was reported. None of the patients reported urethral obstruction or voiding problems following the procedure. The short-term moment of introduction of the needle in the bladder wall was felt by most patients, but no specific pain was indicated at the moment of injection of the (liquid) BioXmark® marker; all patients endured the procedure well.
Discussion
This prospective single-institute clinical trial showed that 79% of all implanted BioXmark® liquid fiducial markers were visible at CT acquisition for radiotherapy treatment planning. Of those visible markers on CT scan, all (100%) of the markers remained detectable until the end of the treatment, defined as visible on the last CBCT at week four. The preset threshold of 75% was overlapped by the CIs, thereby this Phase 1 study failed to show a statistical difference with the prospectively defined test. However, the bar was set up high and this study showed a clear learning curve. The lost markers were mainly due to the learning curve of the implanting technique. In patients where it was injected successfully, the BioXmark ® liquid fiducial marker was an easy and clinically very applicable tool for IGRT in bladder-preserving chemoradiotherapy. Thereby, blurring, migration, and fading did not occur in our study during treatment.
One patient in our study was defined as a SAE because of his death after inclusion into our study. He did have the BioXmark markers implanted but died before starting treatment, because of an ischaemic cerebrovascular event. It was considered unrelated because the patient had multiple vascular risk factors, was not on anticoagulant medication and the brain CT scan did not show any signs of BioXmark in the head or neck. Two patients reported adverse effects; both most likely related to the cystoscopy procedure itself and not to the implantation of the BioXmark® marker.
Previous studies using BioXmark®in lung and esophagus showed similar results as those we found in this present study in bladder cancer. Rydhog et al17 studied the effect of BioXmark® in lung cancer and found that all placed markers were visible and stable during treatment. Three-dimensional interfraction variation for marker position relative to the GTV position or in marker registration relative to carina registration were less than 1 mm. Machiels et al16 studied BioXmark® in esophageal cancer and found that it is technically feasible, safe and it has excellent visibility and positional stability.
At the start of our study, some practical learning problems were found, as was also the case in the esophagus study by Machiels et al.16 It was found that, due to its high viscosity, resistance to release the liquid BioXmark®from the needle is relatively high. So, this caused lack of time between injection of the needle with subsequently pushing the syringe and true release of the gel in the bladder/esophageal wall. We hypothesized this for the reason that in the first patients some marker implantations failed and were, therefore, not visible at CT scanning. We changed our needle system with dual-lock as well as our injection technique and after that, implant success rate, defined by visibility on the planning CT scan, almost reached 100%. There was only one patient (patient #16) later in the study in which surprisingly none of the markers were visible. A new urological resident and a different endoscopy assisting nurse did this specific procedure. Although the injections were supervised by the urologist, all markers failed, and we suspected that his inexperience with the technique and the specific characteristics of BioXmark® were the cause of this failure. A more guided training would probably improve the results and increase the steepness of the learning curve.
The fluoroscopy that we used at the beginning of the study certainly helped to raise the steepness of the learning curve for the marker-implanting urologist. After these fluoroscopy-controlled implantations, the urologist had enough experience to perform implantation without fluoroscopy. A second reason to stop performing fluoroscopy in these patients was that fluoroscopy is not always available and, therefore, could not be arranged as quickly as the treatment team wished. But, because of the good outcomes of our marker placements, we decided that fluoroscopy was not that important anymore in the injecting procedure.
This novel liquid marker, BioXmark®, was safe to use in our study, with only two patients experiencing grade two toxicity. This seemed to be related to the implantation procedure itself and not as such related to the liquid marker itself. This is in line with the previously published papers on the use and safety of this marker.16,17 For the implantation in lung cancer patients, the long-term data (up to 38 months) showed similar results: no marker-related toxicity.19
Compared to other liquid markers available for endoscopic procedures, the use of BioXmark®seems to need somemore training, as discussed before. But, in our view, there are many advantages of this marker compared to other markers, i.e. no blurring, fading, or migration, with all visible BioXmark® marker dots on CT acquisition remaining detectable and stable on CBCTs during the whole treatment period. Implantation in the wall of the bladder can be performed in an outpatient setting by every urologist after following a short learning curve and getting used to the specific characteristics of the liquid marker and the injection technique. Fluoroscopy could be useful in the beginning of the learning curve but seems to be unnecessary after multiple implantations. Continuous visibility of the fiducial markers also allows automatic marker matching during radiotherapy treatment. Specifically, given the more adaptive solutions and daily plan reoptimization automatic detectable and reliable markers are essential. Optimal online-IGRT will lead to a more accurate treatment delivery with increased dose sparing of normal bladder tissue and possibly less geographical misses. Future research will point out whether results of organ-preserving chemoradiotherapy will be improved with this novel, well-performing liquid marker by delivering higher dose to the bladder tumor.
Conclusion
The clinical performance of BioXmark was 79%, which was above the preset threshold rate although within the confidence limits of significance. A distinct learning curve of the injection of the liquid marker was seen. The marker showed sustained visibility and positional stability during treatment phases and also appears to be safe and easy to inject. In conclusion, this novel liquid BioXmark® marker seems to be a very promising tool in daily-adaptive IGRT for bladder preserving chemoradiotherapy in muscle invasive bladder cancer.
Footnotes
Funding: Study product and financial contribution only for access to study data upon trial completion was provided by Nanovi A/S Denmark.
Ethical approval: Was obtained from the Ethical Board (NL65305.018.18 - 2018_065).
Informed consent: Was obtained for all study participants. Netherlands trial register no:Trial NL7396 (NTR7605) [https://www.trialregister.nl/]
Contributor Information
Mischa de Ridder, Email: m.de_ridder.rt@lumc.nl.
Lara C Gerbrandy, Email: l.c.schreuders@amsterdamumc.nl.
Theo M de Reijke, Email: t.m.deReyke@amsterdamumc.nl.
Karel A Hinnen, Email: k.a.hinnen@amsterdamumc.nl.
Maarten C.C.M. Hulshof, Email: m.c.hulshof@amsterdamumc.nl.
REFERENCES
- 1.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012; 366: 1477–88. doi: 10.1056/NEJMoa1106106 [DOI] [PubMed] [Google Scholar]
- 2.Lagrange J-L, Bascoul-Mollevi C, Geoffrois L, Beckendorf V, Ferrero J-M, Joly F, et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: results of a multicenter prospective study (GETUG 97-015. Int J Radiat Oncol Biol Phys 2011; 79: 172–8. doi: 10.1016/j.ijrobp.2009.10.038 [DOI] [PubMed] [Google Scholar]
- 3.Arcangeli G, Strigari L, Arcangeli S. Radical cystectomy versus organ-sparing trimodality treatment in muscle-invasive bladder cancer: a systematic review of clinical trials. Crit Rev Oncol Hematol 2015; 95: 387–96. doi: 10.1016/j.critrevonc.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 4.Vashistha V, Wang H, Mazzone A, Liss MA, Svatek RS, Schleicher M, et al. Radical cystectomy compared to combined modality treatment for muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2017; 97: 1002–20. doi: 10.1016/j.ijrobp.2016.11.056 [DOI] [PubMed] [Google Scholar]
- 5.Turner SL, Swindell R, Bowl N, Marrs J, Brookes B, Read G, et al. Bladder movement during radiation therapy for bladder cancer: implications for treatment planning. Int J Radiat Oncol Biol Phys 1997; 39: 355–60. doi: 10.1016/S0360-3016(97)00070-9 [DOI] [PubMed] [Google Scholar]
- 6.Huddart RA, Hall E, Hussain SA, Jenkins P, Rawlings C, Tremlett J, et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: results of the BC2001 trial (CRUK/01/004. Int J Radiat Oncol Biol Phys 2013; 87: 261–9. doi: 10.1016/j.ijrobp.2013.06.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia MM, Gottschalk AR, Brajtbord J, Konety BR, Meng MV, Roach M, et al. Endoscopic gold fiducial marker placement into the bladder wall to optimize radiotherapy targeting for bladder-preserving management of muscle-invasive bladder cancer: feasibility and initial outcomes. PLoS One 2014; 9: e89754. doi: 10.1371/journal.pone.0089754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangar S, Thompson A, Miles E, Huddart R, Horwich A, Khoo V. A feasibility study of using gold seeds as fiducial markers for bladder localization during radical radiotherapy. Br J Radiol 2007; 80: 279–83. doi: 10.1259/bjr/54321311 [DOI] [PubMed] [Google Scholar]
- 9.Hulshof MCCM, van Andel G, Bel A, Gangel P, van de Kamer JB. Intravesical markers for delineation of target volume during external focal irradiation of bladder carcinomas. Radiother Oncol 2007; 84: 49–51. doi: 10.1016/j.radonc.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 10.Nolan CP, Forde EJ. A review of the use of fiducial markers for image-guided bladder radiotherapy. Acta Oncol 2016; 55: 533–8. doi: 10.3109/0284186X.2015.1110250 [DOI] [PubMed] [Google Scholar]
- 11.Baumgarten AS, Emtage JB, Wilder RB, Biagioli MC, Gupta S, Spiess PE. Intravesical lipiodol injection technique for image-guided radiation therapy for bladder cancer. Urology 2014; 83: 946–50. doi: 10.1016/j.urology.2013.09.058 [DOI] [PubMed] [Google Scholar]
- 12.Chai X, van Herk M, van de Kamer JB, Remeijer P, Bex A, Betgen A, et al. Behavior of lipiodol markers during image guided radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys 2010; 77: 309–14. doi: 10.1016/j.ijrobp.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 13.Freilich JM, Spiess PE, Biagioli MC, Fernandez DCC, Shi EJ, Hunt DC, et al. Lipiodol as a fiducial marker for image-guided radiation therapy for bladder cancer. Int Braz J Urol 2014; 40: 190–7. doi: 10.1590/S1677-5538.IBJU.2014.02.08 [DOI] [PubMed] [Google Scholar]
- 14.Pos F, Bex A, Dees-Ribbers HM, Betgen A, van Herk M, Remeijer P. Lipiodol injection for target volume delineation and image guidance during radiotherapy for bladder cancer. Radiother Oncol 2009; 93: 364–7. doi: 10.1016/j.radonc.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Søndergaard J, Olsen Kasper Ørding, Muren LP, Elstrøm UV, Grau C, Høyer M. A study of image-guided radiotherapy of bladder cancer based on lipiodol injection in the bladder wall. Acta Oncol 2010; 49: 1109–15. doi: 10.3109/02841861003789491 [DOI] [PubMed] [Google Scholar]
- 16.Machiels M, Voncken FEM, Jin P, van Dieren JM, Bartels-Rutten A, Alderliesten T, et al. A novel liquid Fiducial marker in esophageal cancer image guided radiation therapy: technical feasibility and visibility on imaging. Pract Radiat Oncol 2019; 9: e506–15. doi: 10.1016/j.prro.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 17.Rydhög JS, Mortensen SR, Larsen KR, Clementsen P, Jølck RI, Josipovic M, et al. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother Oncol 2016; 121: 64–9. doi: 10.1016/j.radonc.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 18.Scherman Rydhög J, Irming Jølck R, Andresen TL, Munck Af Rosenschöld P. Quantification and comparison of visibility and image artifacts of a new liquid fiducial marker in a lung phantom for image-guided radiation therapy. Med Phys 2015; 42: 2818–26. doi: 10.1118/1.4919616 [DOI] [PubMed] [Google Scholar]
- 19.de Blanck SR, Rydhög JS, Larsen KR, Clementsen PF, Josipovic M, Aznar MC, et al. Long term safety and visibility of a novel liquid fiducial marker for use in image guided radiotherapy of non-small cell lung cancer. Clin Transl Radiat Oncol 2018; 13: 24–8. doi: 10.1016/j.ctro.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]