Abstract
Objective:
To establish a radiomics nomogram by integrating clinical risk factors and radiomics features extracted from digital mammography (MG) images for pre-operative prediction of axillary lymph node (ALN) metastasis in breast cancer.
Methods:
216 patients with breast cancer lesions confirmed by surgical excision pathology were divided into the primary cohort (n = 144) and validation cohort (n = 72). Radiomics features were extracted from craniocaudal (CC) view of mammograms, and radiomics features selection were performed using the methods of ANOVA F-value and least absolute shrinkage and selection operator; then a radiomics signature was constructed with the method of support vector machine. Multivariate logistic regression analysis was used to establish a radiomics nomogram based on the combination of radiomics signature and clinical factors. The C-index and calibration curves were derived based on the regression analysis both in the primary and validation cohorts.
Results:
95 of 216 patients were confirmed with ALN metastasis by pathology, and 52 cases were diagnosed as ALN metastasis based on MG-reported criteria. The sensitivity, specificity, accuracy and AUC (area under the receiver operating characteristic curve of MG-reported criteria were 42.7%, 90.8%, 24.1% and 0.666 (95% confidence interval: 0.591–0.741]. The radiomics nomogram, comprising progesterone receptor status, molecular subtype and radiomics signature, showed good calibration and better favorite performance for the metastatic ALN detection (AUC 0.883 and 0.863 in the primary and validation cohorts) than each independent clinical features (AUC 0.707 and 0.657 in the primary and validation cohorts) and radiomics signature (AUC 0.876 and 0.862 in the primary and validation cohorts).
Conclusion:
The MG-based radiomics nomogram could be used as a non-invasive and reliable tool in predicting ALN metastasis and may facilitate to assist clinicians for pre-operative decision-making.
Advances in knowledge:
ALN status remains among the most important breast cancer prognostic factors and is essential for making treatment decisions. However, the value of detecting metastatic ALN by MG is very limited. The studies on pre-operative ALN metastasis prediction using the method of MG-based radiomics in breast cancer are very few. Therefore, we studied whether MG-based radiomics nomogram could be used as a predictive biomarker for the detection of metastatic ALN.
Introduction
Breast cancer is now the most common malignancy and the leading cancer-related cause of death in females worldwide. For breast cancer patients, the major cause of death is not the primary tumor but the distant organs metastasis.1 And the 5 year survival rate of breast cancer with distant metastasis is reported to decrease as the number of metastatic axillary lymph node (ALN) increases.2 In addition, ALN metastasis is considered as the major indication for neoadjuvant chemotherapy. Therefore, the status of ALN is the most important decisive factor of the prognosis and therapeutic decision-making for breast cancer patients.3,4 Clinically, axillary lymph node dissection (ALND) is the standard method for axillary status evaluation in breast cancer patients with palpable or metastatic ALNs confirmed by biopsy. Sentinel lymph node biopsy (SLNB) is the standard procedure in staging the axilla of breast patients with clinically negative axilla.5 However, both ALND and SLNB are invasive procedures, which are still associated with some unacceptable complications, such as seroma, arm pain, infection and lymphedema.6,7
Clinically, some noninvasive imaging techniques, such as MG, ultrasound, CT, MRI and PET/CT, are usually used to evaluate ALN status according to its morphological and functional abnormalities. However, these methods have high false-negatives.8–10 Ultrasound-guided fine-needle aspiration can effectively improve the dignostic accuracy of ALN metastasis pre-operatively. However, if there has no evidence of cancer cell infiltration, further surgery is still needed to reconfirm the status of ALN. In addition, for some methods, such as MRI and PET/CT, the cost is too high to be suitable for most breast cancer patients. Hence, a non-invasive and cheaper method to predict the ALN status for breast cancer patients is in urgent need.
Radiomics is a new research field based on quantitative imaging technology, and it is also a non-invasive method aimed to utilize the full potential of medical imaging to reflect tissue heterogeneity.11 Mammography (MG) is widely used for breast cancer screening, diagnosis and pre-operative staging, but the value of detecting metastatic ALN is very limited.8 To the best of our knowledge, the studies on preoperative predicting ALN metastasis of breast cancer using the method of MG-based radiomics are very few.12 Therefore, we studied whether MG-based radiomics could be used as a predictive biomarker for the detection of metastatic ALN, and the aim of this study was to establish a MG-based radiomics nomogram by intergrating the clinical risk factors and radiomics signature to predict the probability of ALN metastasis in breast cancer.
Methods and materials
Patient population
Our institutional review board approved this retrospective study, and patient informed consent requirement was waived. We retrospectively collected 307 consecutive patients initial diagnosed as invasive carcinoma of no special type in Henan Provincial People’s Hospital between Jun 2015 and May 2017. And the inclusion criteria were as follows: (1) breast invasive carcinoma of no special type confirmed initially by surgical excision, (2) ALND or SLNB operation must undergo to evaluate the status of ALN, (3) lesions presenting as mass on mammograms, regardless of calcification. Patients with missing data, with the tumor only in the MLO view of mammogram, or who underwent biopsy, chemoradiotherapy before MG examination were excluded. A total of 216 patients were enrolled our study; and the patients were divided into primary cohort (n = 144; mean age, 52.19 ± 10.60) and validation cohort(n = 72; mean age, 53.97 ± 10.21) according to the study date of MG and the ratio of 2:1. Clinically, all patients presented with a breast lump, with a duration ranging from several days to 5 years. Pathologically, all patients were invasive carcinoma of no special type, and 34.7% (75/216) patients had the lesions combined ductal carcinoma in situ (DCIS) components with no more than 20%; immunohistochemistry exam was used to further evaluate the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67 using post-operative histological specimens. Clinical data, such as age, tumor size, pathological grade, ER status, PR status, HER2 status, and Ki-67 proliferation index were recorded both in the primary and validation cohorts, and the molecular subtype of breast cancer were calculated by the status of ER, PR and HER2.13
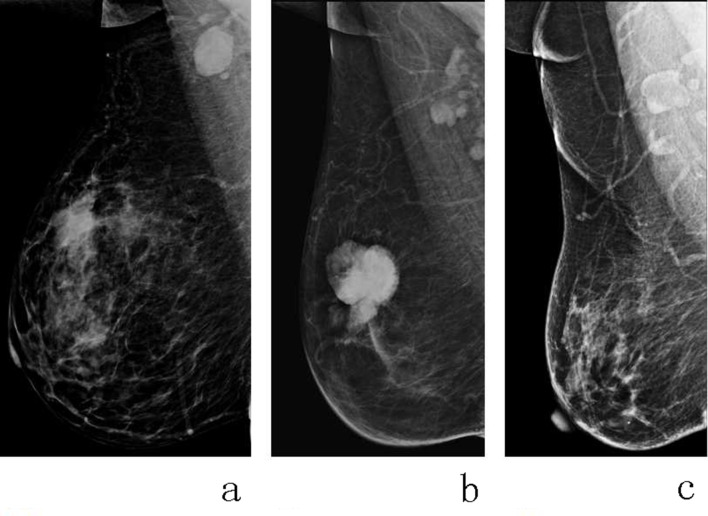
Mammograms were obtained in the routine craniocaudal (CC) and mediolateral oblique (MLO) views for the bilateral breasts. On mammograms, ALNs can be observed at MLO view. Hence, in our study we only use CC view mammogram to analyze radiomics features, and MLO view mammogram was used to evaluate the status of ALN and the mammary gland density by two radiologists with 5 or more years of radiological experience. On the image of MLO view, metastatic ALNs were defined as round enlarged lymph node with the short diameter ≥10 mm (Criterion a), or multiple round high density small lymph nodes with the short diameter<10 mm (Criterion b); and all the lymph nodes with fat density should be excluded (Criterion c) (Figure 1). And the flowchart of this study is shown in Figure 2.
Figure 1.
The criteria of MG-reported metastatic ALNs on the MLO view mammogram. Both round enlarged lymph nodes with the short diameter ≥10 mm (a) and multiple round high density small lymph nodes with the short diameter<10 mm (b) were regarded as metastatic ALNs; and all the lymph nodes with fat density should be considered as benign lymph nodes (c). ALN, axillary lymph node;MG, mammography; MLO, mediolateral oblique.
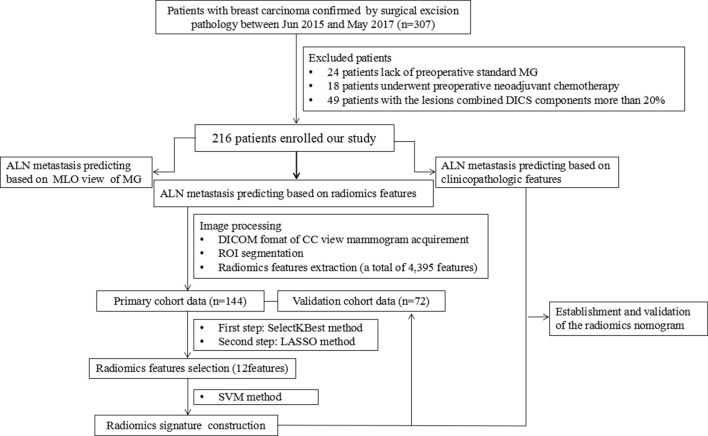
Figure 2.
The flow chart of our study. In our study, a total of 216 patients were enrolled. And there were four methods to pre-operative evaluate ALN metastasis for breast cancer, including MG, radiomics signature, clinical features and the radiomics nomogram. ALN, axillary lymph node;CC, craniocaudal; DICOM, DigitalImaging and Communications in Medicine; LASSO, least absoluteshrinkage and selection operator; MG, mammography; MLO, mediolateral oblique; ROI,region of interest; SVM, support vector machine.
Mammograms acquisition and segmentation
Bilateral digital MG was performed using Hologic Selenia (Hologic Medical Systems, Boston, MA). Images were obtained in the routine CC and MLO views for the bilateral breasts. CC and MLO view mammograms were saved as the format of Digital Imaging and Communications in Medicine archived in the Picture Archiving and Communication System (PACS, Carestream, Canada). CC view mammogram was used to extract radiomics features, and MLO view mammogram was used to evaluate the status of ALN based on the MG-reported criteria in our study.
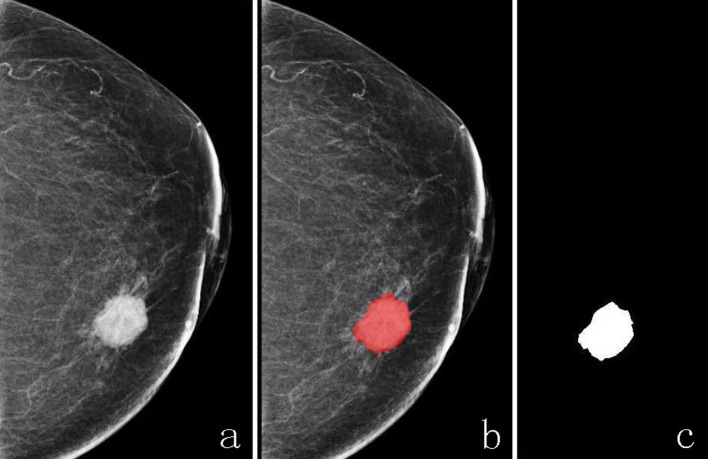
The ITK-SNAP software (open source software; http://www.itk-snap.org) was used for manual image segmentation, then a two-dimensional (2D) region of interest (ROI) that covered the whole lesion was delineated on the CC view of mammogram by two radiologists with 5 or more years of experience in breast imaging diagnosis (Figure 3).
Figure 3.
The ROI of breast cancer lesion was delineated on CC view of mammogram; an irregular breast cancer mass was showed on CC view mammogram (a), and the ROI of breast cancer was delineated manually on the same image before lesion segment (b) and after lesion segment (c). ROI, region of interest.
Radiomics feature extraction
Radiomics feature extraction was performed using in-house software implemented in Matlab 2018b (Mathworks, Natick, MA). Four groups of imaging features were extracted from the CC view of mammogram with manually segmented ROI, including 8 shape features, 17 first-order statistical features, 90 texture features and 4280 gabor features (5 scales and 8 directions are used for gabor filtering, calculating first-order statistical features and texture features for each filter data). A total of 4395 radiomics features were extracted from each patient. More information about the radiomics feature extraction methodology can be found in the Supplementary Data.
Radiomics feature selection
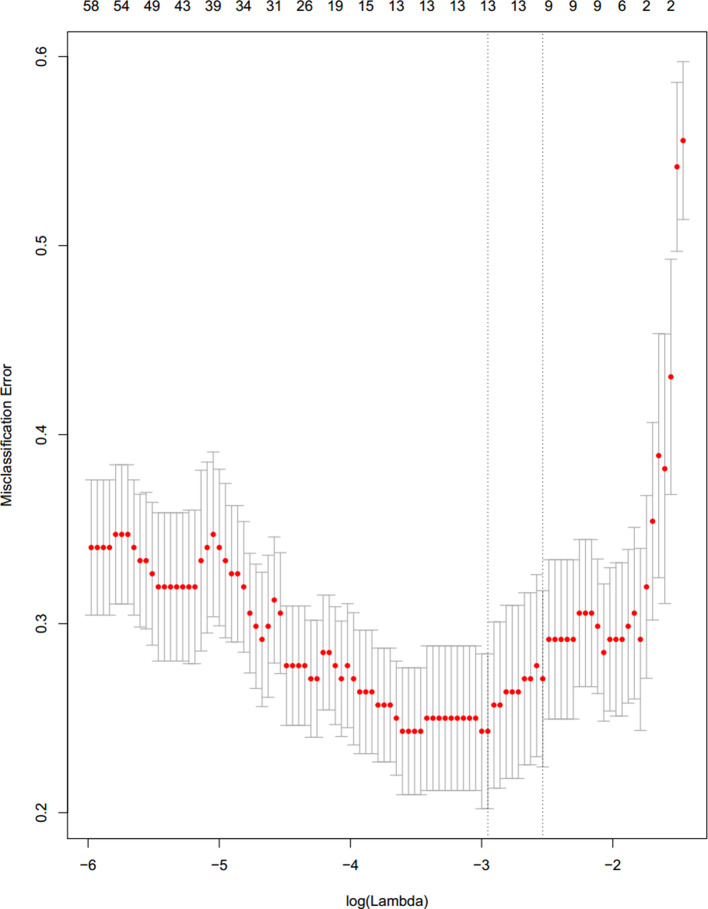
The methods of ANOVA F-value and LASSO were applied to select the ALN metastasis-related radiomics features. The selection method of radiomics features is divided into two steps. The first step is to use the single feature selection method to select features according to the k highest F-value, and 500 features were selected from these 4395 radiomics features. The second step, 12 features were selected based on the first step with the method of LASSO (Figure 4). And these 12 ALN metastasis-related radiomics features were shown in the Supplementary Data.
Figure 4.
Radiomics feature selection using LASSO logistic regression based on CC view of mammogram in the primary cohort. Selection of the tuning parameter (λ) in the LASSO model via 10-fold cross-validation based on minimum criteria. 12 ALN status-related radiomics features were selected using the method of LASSO logistic regression. ALN, axillary lymph node;CC, craniocaudal;LASSO, least absolute shrinkage and selection operator.
Radiomics signature construction
12 metastasis-related features of all 4395 radiomics features were selected in the primary cohort to build a radiomics signature. Linear support vector machine (with a C value 1/16) was used to construct radiomics signature. Radiomics score (Rad-Score) is a manifestation of radiomics signature and contains all the information of the selected features. The Rad-Score was calculated for each patient as a linear fitting of selected features that were weighted by their respective coefficients.
Establishment and validation of the nomogram
MG-based radiomics nomogram was constructed with multivariate logistic regression analysis among the radiomics signature and clinicopathological factors using the likelihood ratio test with Akaike's information criterion (AIC) as a stopping rule.14
Calibration curves were plotted to assess the calibration of the nomogram, accompanied with the Hosmer-Lemeshow test. The C-index and calibration curve were derived based on the regression analysis both in the primary and validation cohorts.
Statistical analysis
To analyze the differences between the primary cohort data and validation cohort data, we conducted a descriptive analysis, using cross-tabulations of pathological grade, ER status, PR status, HER2 status; and a two-tailed Student’s t-test was used to compare the mean age, mean size, Ki-67 proliferation index and Rad-Score. The data were analyzed using Statistical Package for SPSS 22.0 software (SPSS Inc., Chicago, IL). Multivariate logistic regression analysis was applied to construct the prediction models of clinical features and radiomics nomogram, and the statistical analyses were performed with commercially available software (R software, v. 3.4.3). A two-tailed p-value < 0.05 was considered statistically significant. The performance is expressed as the sensitivity, specificity, accuracy and area under the receiver operating characteristic (ROC) curve (AUC) for the predictive models.
Results
Clinical findings
The clinicopathological characteristics of patients whose data are classified into the primary and validation cohorts are shown in Table 1. The differences were not statistically significant between the primary cohort and the validation cohort in terms of age, lesion size, location, pathological grade, ER status, PR status, HER-2 status and Ki-67 proliferation index (all p-values>0.05).
Table 1.
Clinical features of patients with breast cancer in the primary cohort and validation cohort
| Characteristic | Primary cohort (n = 144) |
Validation cohort (n = 72) |
p-value |
|---|---|---|---|
| Age (year, ) | 52.19 ± 10.60 | 53.97 ± 10.21 | 0.241 |
| Size (cm,) | 2.27 ± 0.75 | 2.33 ± 1.03 | 0.147 |
| Location of disease | |||
| Right lobe | 79 (54.9%) | 34 (47.2%) | 0.289 |
| Left lobe | 65 (45.1%) | 38 (52.8%) | |
| Pathological grade | |||
| Grade I | 4 (2.8%) | 1 (1.4%) | 0.775 |
| Grade Ⅱ | 84 (58.3%) | 41 (56.9%) | |
| Grade Ⅲ | 56 (38.9%) | 30 (41.7%) | |
| ER status | |||
| Positive | 98 (68.1%) | 53 (73.6%) | 0.401 |
| Negative | 46 (31.9%) | 19 (26.4%) | |
| PR status | |||
| Positive | 83 (57.6%) | 48 (66.7%) | 0.200 |
| Negative | 61 (42.4%) | 24 (33.3%) | |
| HER-2 status | |||
| Positive | 40 (27.8%) | 18 (25.0%) | 0.664 |
| Negative | 104 (72.2%) | 54 (75.0%) | |
| Ki-67 (%,) | 43.33 ± 22.52 | 44.79 ± 24.84 | 0.664 |
HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
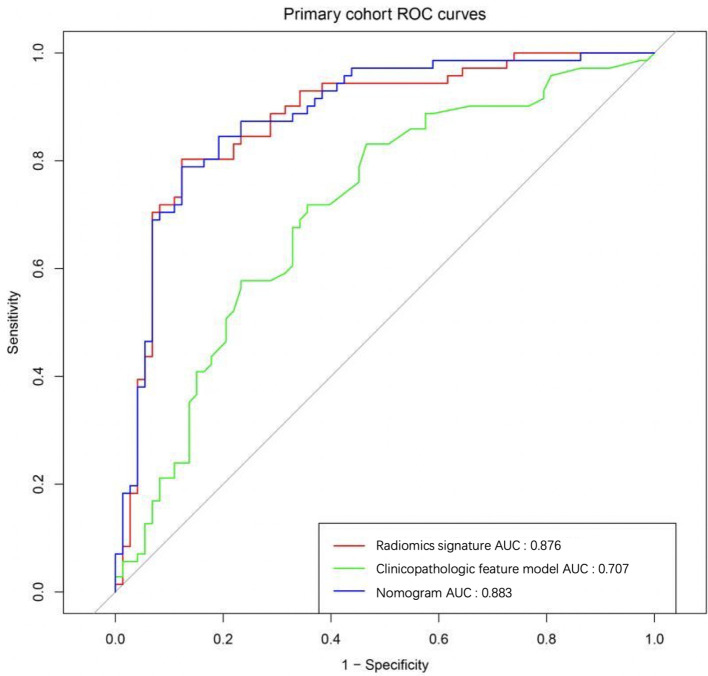
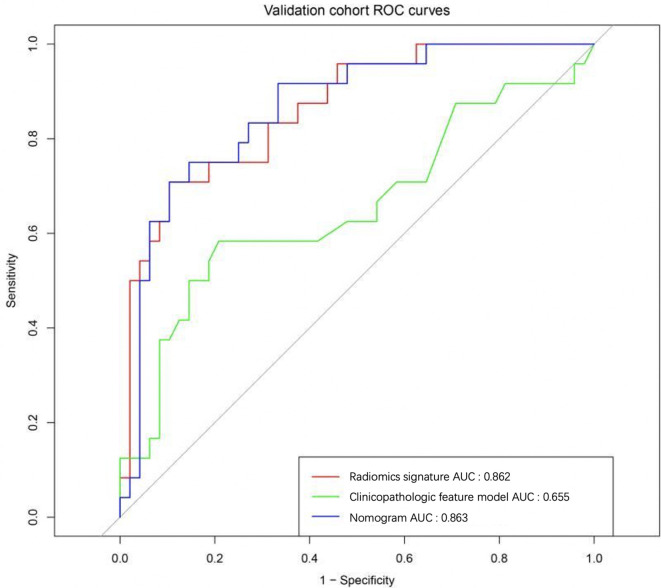
Multivariate logistic regression analysis was used to assess the relationship between the clinical risk factors and ALN metastasis. Three factors (including tumor size, molecular subtype and ER) were selected to construct the clinical features model to predict ALN metastasis. The sensitivity, specificity, accuracy and AUC value for the prediction model of clinical features were 59.16%, 68.49%, 63.89% and 0.707 [95% confidence interval (CI): 0.658–0.752] in the primary cohort and 45.83%, 85.42%,72.22% and 0.657 (95% CI: 0.590–0.708) in the validation cohort, respectively.
ALN status prediction based on MG
According to the criteria of MG-reported metastatic ALNs on the MLO view mammogram, the result of ALN status prediction was shown in the Table 2. Of 216 breast cancer patients, 95 (44.0%, 95/216) cases in all had ALN metastasis confirmed by ALND, and 52 (24.1%, 52/216) cases were diagnosed with metastatic ALNs on mammograms. In the whole cohort, 55 patients were reported to be LN-negative but confirmed to have LN metastases, while 11 patients were reported to be LN-positive but confirmed to have no LN metastases. Then the sensitivity, specificity, accuracy and AUC value of MG were calculated, and they were 42.7%, 90.8%, 24.1% and 0.666 (95% CI: 0.591–0.741), respectively.
Table 2.
The result of ALN status prediction based on MG
| ALN status based on MG* | Pathological ALN status | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 41 | 11 | 52 |
| Negative | 55 | 109 | 164 |
| Total | 96 | 120 | 216 |
ALN, axillary lymph node; MG, mammography.
Note: * Both round enlarged lymph node with the short diameter ≥10 mm (Criterion a) and multiple round high density small lymph nodes with the short diameter<10 mm (Criterion b) were diagnosed as metastatic ALNs according to the criteria of MG-reported.
ALN status prediction based on radiomics signature
12 ALN metastasis-related radiomics features were used to construct radiomics signature using the SVM method. The sensitivity, specificity, accuracy and AUC value for the prediction model of radiomics signature based on CC view of mammogram were 80.28%, 79.45%, 79.86% and 0.876 (95% CI: 0.842–0.920) in the primary cohort and 75.00%, 77.08%, 76.39% and 0.862 (95% CI:0.822–0.896) in the validation cohort, respectively.
Additionally, there was significant difference between the median of Rad-Score between positive ALN and negative ALN cases in the primary and validation cohorts (both p < 0.05). The correlation between Rad-Score and ALN status was shown in Table 3.
Table 3.
Rad-scores for the primary cohort and validation cohort
| Rad-score | Positive ALN Median (IQR) |
Negative ALN Median (IQR) |
p-value |
|---|---|---|---|
| Primary cohort | 0.701 (0.590, 0.781) |
0.303 (0.146, 0.440) |
0.000 |
| Validation cohort | 0.695 (0.438, 0.758) |
0.260 (0.123, 0.448) |
0.000 |
IQR, interquartile range.
Note: p value < 0.05 indicates a significant difference in the median Rad-score between positive and negative ALN patients.
ALN status prediction based on radiomics nomogram
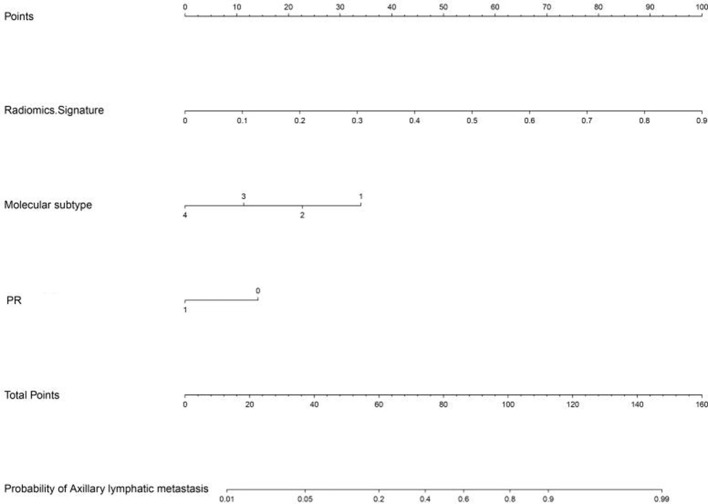
Radiomics signature, PR and molecular subtype were selected as ALN metastasis-related factors and used to construct the nomogram model using multivariate logistic regression analysis (Figure 5). The sensitivity, specificity, accuracy and AUC value for the prediction model of nomogram were 80.28%, 80.82%, 80.56% and 0.883 (95% CI: 0.849–0.920) in the primary cohort and 75.00%, 81.25%, 79.17% and 0.863 (95% CI: 0.821–0.897) in the validation cohort, respectively.
Figure 5.
Nomogram was developed by incorporating radiomics signature with age, size, pathological grade, mammary gland type, molecular subtype, ER, PR, HER2 and Ki-67 using multivariate logistic regression analysis, and radiomics signature, molecular subtype and PR were selected to construct the nomogram in our study. ER, estrogen receptor; HER2,human epidermal growth factor receptor 2; PR, progesterone receptor.
ALN status predictive performance comparison
The performance of four predictive models, including MG-reported, radiomics signature, clinical features and the nomogram, were compared. In our study, MG-based radiomics nomogram was superior to the other three methods, and the results were shown in Table 4. At the same time, the nomogram model was more effective in metastatic ALN prediction than independent radiomics signature and clinical factors both in the primary cohort (Figure 6) and validation cohort(Figure 7).
Table 4.
Predictive performance of MG, radiomics signature, clinical features and the nomogram
| Predictive Model | Metastatic ALN Predictive Performance | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | AUC (95% CI) | |
| MG-reported | 42.7% | 90.8% | 24.1% | 0.666 (0.591 to 0.741) |
| Radiomics signature | 75.0% | 77.1% | 76.4% | 0.862 (0.822 to 0.896) |
| Clinical features | 45.8% | 85.4% | 72.2% | 0.657 (0.590 to 0.708) |
| Radiomics nomogram* | 75.0% | 81.3% | 79.2% | 0.863 (0.821 to 0.897) |
AUC, area under the curve; CI, confidence interval; MG, mammography.
Note: * represent for the model of radiomics signature +clinical features.
Figure 6.
The ROC curves were compared among the methods of the radiomics signature, clinical features, and the nomogram in the primary cohort. AUC, area under the curve; ROC, receiver operating characteristic.
Figure 7.
The ROC curves were compared among the methods of the radiomics signature, clinical features, and the nomogram in the validation cohort. AUC, area under the curve; ROC, receiver operating characteristic.
The validation of the radiomics nomogram
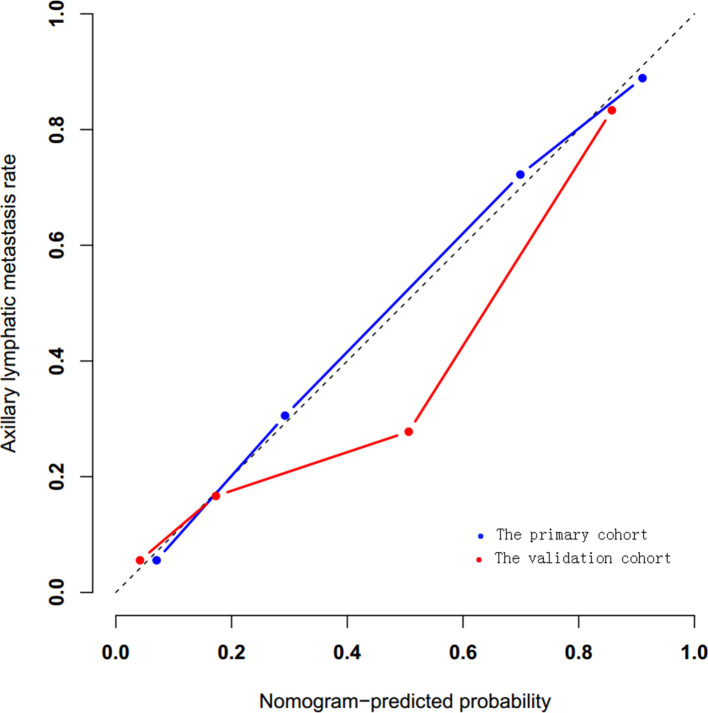
Calibration curves were plotted to assess the consistent between the radiomics nomogram-predicted probability of ALN metastasis and the actual results. On the calibration curves in our study, the p-values in the primary and validation cohorts were 0.923 and 0.269, respectively(Figure 8).
Figure 8.
Calibration curves were plotted to assess the calibration of the nomogram, both p-values>0.05 in the primary and validation cohorts.
Discussion
ALN status remains among the most important breast cancer prognostic factors and is essential for making treatment decisions. ALN is the most common metastatic site of breast cancer, which receives about 70% lymphatic drainage of the breast. Of 216 breast cancer patients in our study, 95 (44.0%, 95/216) cases had ALN metastasis confirmed by ALND. Pre-operative accurate assessment of ALN status is particularly important for making treatment decisions. ALND or SLNB can be used to assess ALN status, but these methods are invasive. Pre-operative imaging examination is common used as non-invasive method to confirm the status of ALN metastasis, but imaging examination has a low diagnostic sensitivity, which may lead to a considerable proportion of ALN metastasis patients to be missed.8,9 Hence, we aimed to develop a non-invasive and high diagnostic sensitivity model to preoperative predict the probability of ALN metastasis to support clinical decision-making.
MG is mainly used for screening and diagnosis of breast cancer, which is also the most basic imaging examination for breast diseases. However, the value of MG on pre-operative detecting metastatic ALNs is very limited. Valente et al reported the sensitivity of metastatic ALNs detected by MG were 21%.8 In our series, 52 (24.1%,52/216) cases were diagnosed as ALN metastasis based on the MG-reported criteria. In the whole cohort, 55 patients were reported to be LN-negative but confirmed to have LN metastasis, while 11 patients were reported to be LN-positive but confirmed to have no metastasis. And the sensitivity, specificity, accuracy and AUC value of MG were 42.7%, 90.8%, 24.1% and 0.666 (95% CI: 0.591–0.741), respectively. The sensitivity of MG in our study is slightly higher than that of the above literature8 ; and this may have some relationship with the different diagnostic criteria for ALN metastasis. In our study, on the MLO view of mammogram, besides round enlarged lymph nodes with the short diameter ≥10 mm, multiple round high density small lymph nodes with the short diameter<10 mm were also defined as metastatic ALNs. While, only round enlarged lymph nodes with the short diameter ≥10 mm were regarded as metastatic LN in mostly studies. Overall, MG has low efficiency in the detection of metastatic ALNs, and this may be related to the absence of the whole view of fossa axillaris because of the influence of projection position on MG. Because only the anterior axillary wall tissue can be displayed on the MLO view, which affects the overall imaging of ALNs.
Radiomics nomogram has been proved to be an useful non-invasive tool for cancer patients in the prediction of lymph node metastasis pre-operatively.15–22 For breast cancer, comparing with MG, more studies about radiomics features/nomogram based on MRI used to pre-operative predict ALN metastasis were reported.12,21–24 Cui and their colleagues have used radiomics features of dynamic contrast enhanced MRI to predict ALN metastasis in breast cancer, and they reported the accuracy, sensitivity, specificity and AUC value were 89.54%, 94.50%, 80.06% and 0.862, respectively.23 Lately, Chai et al reported that the accuracy/AUC of the sequences of T1WI, CE2, T2WI, and DWI for ALNs prediction in breast cancer was 79%/0.87, 77%/0.85, 74%/0.79, and 79%/0.85, respectively; When CE2 was augmented by adding kinetic features, the model achieved the highest performance (accuracy = 0.86 and AUC = 0.91),24 which showed a good prediction efficacy based on MRI radiomics. In our study, the radiomics signature based on 12 ALN metastasis-related features extracted from CC view of mammogram demonstrated more favorite predictive performance than that of independent MG-reported criteria and clinical features (AUC of 0.862 vs 0.666 vs 0.657). The nomogram developed by radiomics signature and clinical risk factors showed a little higher predictive efficacy than that of independent radiomics signature (AUC of 0.883 vs 0.876 in the primary cohort; 0.863 vs 0.862 in the validation cohort), and the specificity and accuracy were also improved compared with those of radiomics signature (81.25 and 79.17 vs 77.1% and 76.4%, respectively). However, no matter in the primary cohort or in the validation cohort, there was little difference in the terms of predictive efficacy between the nomogram and radiomics signature in our study, which was not consistent with the literature reports.21,22 This may be related to the Ultrasound-reported and MRI-reported ALN status added into the construction of nomogram in the above studies21,22 ; however, in our study, the radiomics nomogram was constructed with radiomics signature and clinical risk factors, and MG-reported ALN status was studied separately. On the other hand, this result also demonstrated that the clinical risk factors have little contribution to the prediction of momogram in ALN metastasis. Calibration curves were plotted to assess the consistent between the radiomics nomogram-predicted probability of ALN metastasis and the actual results. On the calibration curves in our study, both p-value were>0.05 in the primary and validation cohorts, which demonstrated that the stability of our model is well. Comparing with the study of Yang and their colleagues,12 the AUC value of radiomics signature in our study is a little lower both in the primary cohort (0.895 vs 0.876) and validation cohort (0.875 vs 0.862). This may have some relationship with only CC view of mammogram used to extract radiomics features in our study, both CC and MLO views of mammograms can provide more radiomics features reflecting the heterogeneity of tumors. While, the AUC value of nomogram in our study is a little higher than that of the study of Yang and their colleagues in the primary cohort (0.883 vs 0.779) and validation cohort (0.863 vs 0.809).12 This may lead by the limited number enrolled the study, and the number enrolled the study in our study is bigger than theirs; otherwise, the AUC value of the validation cohort was a little higher than that of primary cohort (0.809 vs 0.779), larger studies are still needed to further evaluate these findings. Our predictive performance is a little lower than that of the study of Cui and their colleagues,23 especially for the sensitivity and accuracy. This may be related to the radiomics features of breast tumor extracted from dynamic contrast-enhanced MR images reflecting more comprehensive tumor heterogeneity than that of MG.
Our study has several limitations as well. First, the outline of lesion’s ROI is manually delineated by radiologists, and the judgment of lesion’s exact outline is greatly influenced by personal experience; besides, in view of the fact that most invasive breast cancers are prone to combine with calcification, it is impossible to eliminate the influence of calcification when delineating the ROI. Second, we only use CC view of mammogram for the extraction of radiomics features, and both CC and MLO views of mammograms were used to extract radiomics feature in other studies; and only mass lesions, regardless of mass dimensions and calcifications combined, were enrolled in this study, which could not represent the general situation of the whole breast tumor. Third, this is a relatively small retrospective study, then increasing case number and multicentre studies carrying out further study are needed.
In conclusion, our study suggests that quantitative radiomics features extracted from CC view of mammogram are helpful for pre-operative accurate predicting ALN metastasis of breast cancer. The MG-based radiomics nomogram could be used as a non-invasive and reliable tool in predicting ALN metastasis and may facilitate to assist clinicians for pre-operative decision-making.
Footnotes
The authors Hongna Tan and Yaping Wu contributed equally to the work.
Funding: This work was supported by the China Postdoctoral Science Foundation (No.2018M632779), National Natural Scientific Foundation of China (No.81401378, No.81772009) and Henan Provincial Department of Science and Technology Research Project (No.201602221,No.182102310162).
Conflicts of interest: The authors have no conflicts of interest to declare.
Contributor Information
Hongna Tan, Email: natan2000@126.com.
Yaping Wu, Email: ypwu@ha.edu.cn.
Fengchang Bao, Email: fengchangbao8018@126.com.
Jing Zhou, Email: F-zj360567346@sina.com.
Jianzhong Wan, Email: E-jzhwan@ha.edu.cn.
Jie Tian, Email: jytian99@gmail.com.
Yusong Lin, Email: yslin@ha.edu.cn.
Meiyun Wang, Email: mywang@ha.edu.cn.
REFERENCES
- 1.Chen W, Hoffmann AD, Liu H, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol 2018; 2: 4. doi: 10.1038/s41698-018-0047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. . Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983; 52: 1551–7. doi: [DOI] [PubMed] [Google Scholar]
- 3.Rack B, Janni W, Gerber B, Strobl B, Schindlbeck C, Klanner E, et al. . Patients with recurrent breast cancer: does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat 2003; 82: 83–92. doi: 10.1023/B:BREA.0000003955.73738.9e [DOI] [PubMed] [Google Scholar]
- 4.Lale Atahan I, Yildiz F, Ozyigit G, Sari S, Gurkaynak M, Selek U, et al. . Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol 2008; 47: 232–8. doi: 10.1080/02841860701678761 [DOI] [PubMed] [Google Scholar]
- 5.Wernicke AG, Goodman RL, Turner BC, Komarnicky LT, Curran WJ, Christos PJ, et al. . A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat 2011; 125: 893–902. doi: 10.1007/s10549-010-1167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kootstra J, Hoekstra-Weebers JEHM, Rietman H, de Vries J, Baas P, Geertzen JHB, et al. . Quality of life after sentinel lymph node biopsy or axillary lymph node dissection in stage I/II breast cancer patients: a prospective longitudinal study. Ann Surg Oncol 2008; 15: 2533–41. doi: 10.1245/s10434-008-9996-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. . Post-Operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 2006; 95: 279–93. doi: 10.1007/s10549-005-9025-7 [DOI] [PubMed] [Google Scholar]
- 8.Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. . Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 2012; 19: 1825–30. doi: 10.1245/s10434-011-2200-7 [DOI] [PubMed] [Google Scholar]
- 9.van Nijnatten TJA, Ploumen EH, Schipper RJ, Goorts B, Andriessen EH, Vanwetswinkel S, et al. . Routine use of standard breast MRI compared to axillary ultrasound for differentiating between NO, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur J Radiol 2016; 85: 2288–94. doi: 10.1016/j.ejrad.2016.10.030 [DOI] [PubMed] [Google Scholar]
- 10.An Y-S, Lee DH, Yoon J-K, Lee SJ, Kim TH, Kang DK, et al. . Diagnostic performance of 18F-FDG PET/CT, ultrasonography and MRI. detection of axillary lymph node metastasis in breast cancer patients. Nuklearmedizin 2014; 53: 89–94. doi: 10.3413/Nukmed-0605-13-06 [DOI] [PubMed] [Google Scholar]
- 11.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. . Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Wang T, Yang L, Wang Y, Li H, Zhou X, et al. . Preoperative prediction of axillary lymph node metastasis in breast cancer using Mammography-Based Radiomics method. Sci Rep 2019; 9: 4429. doi: 10.1038/s41598-019-40831-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. . Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206–23. doi: 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–23. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 15.Huang Y-Q, Liang C-H, He L, Tian J, Liang C-S, Chen X, et al. . Development and validation of a Radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol 2016; 34: 2157–64. doi: 10.1200/JCO.2015.65.9128 [DOI] [PubMed] [Google Scholar]
- 16.Wu S, Zheng J, Li Y, Yu H, Shi S, Xie W, et al. . A Radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res 2017; 23: 6904–11. doi: 10.1158/1078-0432.CCR-17-1510 [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Pan X, Liu H, Gao D, He J, Liang W, et al. . A new approach to predict lymph node metastasis in solid lung adenocarcinoma: a radiomics nomogram. J Thorac Dis 2018; 10(Suppl 7): S807–19. doi: 10.21037/jtd.2018.03.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao M, Ma F, Li Y, Li Y, Li M, Zhang G, et al. . Multiparametric MRI-based Radiomics nomogram for predicting lymph node metastasis in early-stage cervical cancer. J Magn Reson Imaging 2020;25 Feb 202010.1002/jmri.27101. doi: 10.1002/jmri.27101 [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Gao T, Yang J, Yan X, Wang Y, Zhou X, et al. . Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur J Radiol 2019; 114: 128–35. doi: 10.1016/j.ejrad.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Liu W, Yu Y, Liu J-J, Xue H-D, Qi Y-F, et al. . Ct radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol 2020; 30: 976–86. doi: 10.1007/s00330-019-06398-z [DOI] [PubMed] [Google Scholar]
- 21.Yu F-H, Wang J-X, Ye X-H, Deng J, Hang J, Yang B. Ultrasound-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in early-stage invasive breast cancer. Eur J Radiol 2019; 119: 108658. doi: 10.1016/j.ejrad.2019.108658 [DOI] [PubMed] [Google Scholar]
- 22.Han L, Zhu Y, Liu Z, Yu T, He C, Jiang W, et al. . Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol 2019; 29: 3820–9. doi: 10.1007/s00330-018-5981-2 [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Wang N, Zhao Y, Chen S, Li S, Xu M, et al. . Preoperative prediction of axillary lymph node metastasis in breast cancer using Radiomics features of DCE-MRI. Sci Rep 2019; 9: 2240. doi: 10.1038/s41598-019-38502-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai R, Ma H, Xu M, Arefan D, Cui X, Liu Y, et al. . Differentiating axillary lymph node metastasis in invasive breast cancer patients: a comparison of radiomic signatures from multiparametric breast Mr sequences. J Magn Reson Imaging 2019; 50: 1125–32. doi: 10.1002/jmri.26701 [DOI] [PMC free article] [PubMed] [Google Scholar]