Abstract
Environmental allergens elicit complex immune responses in the lungs that can promote the development of asthma or exacerbate preexisting asthma in susceptible individuals. House dust mites are one of the most common indoor allergens and are a significant driver of allergic disease. Respiratory infections are known factors in acute exacerbations of asthma but the impact of allergen on the pathogen is not well understood. We investigated the pathogenesis of influenza A infection following exposure to house dust mites. Mice exposed to house dust mites lose less weight following infection and had more transcription of interferon-lambda than controls. These data correlated with less transcription of the influenza polymerase acidic gene suggesting diminished viral replication in house dust mite exposed mice. Altogether, these data suggest that exposure to environmental allergens can influence the pathogenesis of influenza infection.
Keywords: House dust mite, influenza, allergy, asthma, inflammation
1.0. Introduction
Asthma and allergic rhinitis are common allergic diseases impacting millions of people worldwide [1–3]. Atopic individuals are sensitized to environmental allergens, proteins in the environment that can elicit an immune reaction upon subsequent exposure [4]. The nature of the pulmonary immune response to environmental substances is dictated by the composition, physical properties of the material, and host-intrinsic properties. In the case of atopic individuals, re-exposure to environmental allergens can lead to an acute exacerbation of disease. Exacerbation of pulmonary allergic inflammation is characterized by physiologic changes including airway hyper responsiveness, goblet cell hyperplasia and mucus hyper secretion, airway edema, and exaggerated allergic inflammation [4].
The allergic immune response is typically dominated by type-2 cytokines such as interleukins (IL) −4, 5, 13, and 33. Additionally, allergen specific IgE and type-2 cellular responses involving innate lymphocytes, eosinophils, mast cells, and T-cells are hallmarks of pulmonary allergic inflammation [5]. However, it is not uncommon to have a mixed inflammatory response following allergen exposure that includes IL-17 and a mixed eosinophilic-neutrophilic response [6]. Both host-intrinsic and allergen-specific factors likely influence the nature of the immune response to environmental allergens.
House dust mites (HDM) are the most common indoor environmental allergens [7–9]. A number of allergenic proteins have been identified in dust mites and common house dust contains these HDM proteins among other molecules known to activate innate immune responses [10–12]. In mouse models of allergic inflammation, HDM are known to induce robust type-2 inflammatory responses [13]. Interestingly, HDM are also known to induce a pattern of gene expression that has the features of an interferon-induced antiviral response in rodents and humans[14–18]. Type I interferon (IFN-α) and type III (IFN-λ) are the major innate antiviral cytokines responsible for inducing a program of gene expression that provides initial protection from viral infection.
Viral and bacterial microbes are known to have a significant interplay with the lung influencing the development of asthma or acute asthma exacerbations [19]. For example, severe early childhood respiratory syncytial virus-induced bronchiolitis is strongly linked with subsequent asthma development [20]. Moreover, rhinovirus infection is the most frequent cause of acute exacerbations of asthma and asthmatics are at higher risk of serious complications from infection with seasonal influenza [19]. Infection with Chlamydia pneumoniae and Mycoplasma pneumoniae, are common atypical bacterial infections known to exacerbate asthma [21]. Microbial products can also significantly impact the development or worsening of allergic inflammation. We previously reported that the Community Acquired Respiratory Distress Syndrome toxin produced by M. pneumoniae induced allergic-type inflammation in naïve rodents and primates [22–24]. Many environmental allergens, including HDM, are complex mixtures that include both the allergenic proteins and other molecules such as lipopolysaccharide capable of stimulating immune responses [11, 12]. The innate immune stimulus provided by the non-allergen components of an allergen mixture potentiate allergic immune responses and likely have yet to be defined influences on the pathogenesis of asthma.
Although it is clear that infection can exacerbate the clinical manifestations of allergic disease, the influence of allergen exposure on the pathogenesis of subsequent pulmonary infections are poorly understood. In particular, the influences on pathogen burden and overall disease are not well understood. To further investigate the impact of HDM exposure on viral pathogenesis, we evaluated H1N1 influenza A infection after a single or multiple exposures to HDM in a mouse model of asthma-associated seasonal influenza infection.
2.0. Materials and Methods
2.1. Mouse strains and procedures
Male and Female 5 week-old BALB/cJ, C57BL/6J, C3H/HeJ, or C3H/HeOuJ mice were purchased from Jackson Laboratory (Bar Harbor ME). Mice were acclimatized to the vivarium for a minimum of 10 days after arrival and prior to experimental use. All animals were housed in an ALAAC-approved facility under specific pathogen-free conditions in isolator cages with soft bedding, a 12-hr light/dark schedule, and free access to food and water. All procedures were approved by the Institutional Animal Care at the University of Texas Health Sciences Center San Antonio. During experimental procedures, animals were monitored by laboratory staff daily and general health was evaluated by monitoring body weight, coat appearance, and mobility. Mice were considered morbibund if they lost 30% of their body weight for two consecutive days. Mice maintaining a 30% body weight loss were subsequently euthanized by carbon dioxide exposure. Animal suffering was minimized by providing free access to food and water, animals had soft bedding for the duration of the experiments and all procedures were done under inhaled general anesthesia, 3% isoflurane and oxygen.
2.2. Allergen exposure and infection with influenza
The D. pteronyssinus house dust mite extract (HDM) was obtained from Greer Laboratories (Lenoir, NC) and was resuspended in sterile PBS at a concentration of 1 mg(protein)/ml. Groups of male or female 6–7 week old mice were anesthetized with 3% isoflurane in oxygen (1L / min) and randomly assigned to be treated intratreacheally with HDM (100 μg per dose in 100μl) or PBS (100μl per dose) (Figure 1). Influenza A (PR8/H1N1) challenges were performed intratreacheally with 400 plaque forming units (PFU) in 100 μl PBS. Mice were challenged either 18hrs following HDM exposure for single exposures or 48hrs after the last HDM exposure for the multiple exposure studies. Depending on the experiment, body weight was monitored for 10 or 14 days following infection.
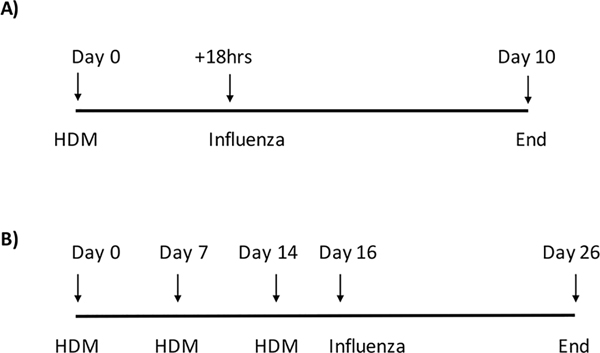
Figure 1. House dust mite exposure scheme.
A) short term exposure model, mice are exposed once to 100 μg of HDM IT and are infected with 400 PFU of influenza 18 hrs later. Control mice are treated with 100 μl saline IT and are then infected with influenza. B) long term exposure model, mice are exposed IT to 100 μg of HDM once a week for three weeks and are infected with 400 PFU of influenza virus 48hrs after the last HDM treatment. Control mice are exposed three times IT with 100 μl of saline and infected with 400 PFU 48hrs after the last exposure to saline.
2.3. Influenza A Virus
The H1N1 influenza A virus (A/PR/8/34) was purchased from the American Type Culture Collection (Manassas, VA). Mice were infected intratreacheally under sedation with 400 or 4000 PFU of virus, 400 PFU is at the LD50 for this PR8 isolate in BALB/cJ mice.
2.4. POLY I:C
Polyinosinic:polycytidylic acid (poly(I:C)) was purchased from Millipore (Darmstadt, Germany; 528906) and resuspended in sterile PBS at a concentration of 1mg/ml.
2.5. Histopathological analysis
BALB/cJ (3 mice/treatment/time point) mice were treated once or 3x with HDM or saline. Mice treated once with HDM were infected with 400 PFU influenza A 18hrs after HDM exposure and mice treated 3x were infected with 400 PFU of influenza 48hrs after the last HDM exposure. On days 2, 5, 8, and 10 after infection, lungs were harvested and fixed 2–3 days in 10% formalin, and embedded in paraffin. Lungs were cut (4μm thin sections) and stained with hematoxylin and eosin by Histology & Immunohistochemistry Laboratory of the University of Texas Health Sciences Center San Antonio. Histopathology was evaluated by two individuals blinded to the treatments. Images of the representative conducting airways were taken on a Zeiss microscope using an Axiocam HR color digital camera and the Axiovision suite of software V.4.5. Pulmonary pathology was quantitatively evaluated using the panel of standards method as we have previously described [23–25].
2.6. qRT-PCR
Female 6–7 week-old BALB/cJ mice were randomly assigned into groups, they were anesthetized with isflorane and challenged with HDM or PBS once or three times with the dose described above. Eighteen hours following HDM exposure, lungs were harvested and preserved in Invitrogen RNAlater Stabilization Solution as per manufacturer’s instructions and stored in liquid nitrogen prior to RNA extraction. Total RNA from each lung was extracted by TRI reagent (Sigma-Aldrich, MO) and chloroform (Sigma-Aldrich, MO) phase separation method and further purified with RNeasy Mini Kit (Qiagen, MA). Nanodrop ND-1000 (NanoDrop Technologies, USA) was used for RNA quality and concentration determinations. The cDNA was reverse transcribed with High Capacity cDNA Reverse Transcription Kit (Applied Biosysyems, NL) and PCT-200 Peltier Thermal Cycler. Real-time qRT-PCR was performed in StepOnePlus Real-Time Pcr System (Applied Biosystems, NL) with TaqMan gene expression assays (Applied Biosystems, NL) for the following genes: GAPDH, IFN-λ and IFN-β. Data was analyzed by using the comparative ΔCt method and control condition were used for comparison.
2.6.1. Determination of viral burden
To determine viral burden, three mice from each group were euthanized four days after infection and total RNA was prepared from their lungs as described above. The RNA was used for determining viral load with Real-Time PCR as described [26, 27]. The H1N1 influenza PA gene on plasmid pHW:193 was used to generate standard curves for calculating the copies of the polymerase (PA) genes per 1 mg lung cDNA. The pHW:193 plasmid was a kind gift from Dr. Richard Webby at St. Jude’s Children’s Hospital. The following primers were used: forward primer, 5’-CGGTCCAAATTCCTGCTGA-3’; reverse primer, 5’-CATTGGGTTCCTTCCATCCA-3’; probe, 5’−6-FAM-CCAAGTCATGAAGGAGAGGGAATACCGCT-3’.
2.7. Statistics
Results are expressed as the mean ± S.D. Statistical differences were determined using a two-way ANOVA and post test analysis by using GraphPad Prism version 5.04 (GraphPad Software, San Diego CA).
3.0. Results
3.1. Exposure to HDM induced expression of interferon β and λ
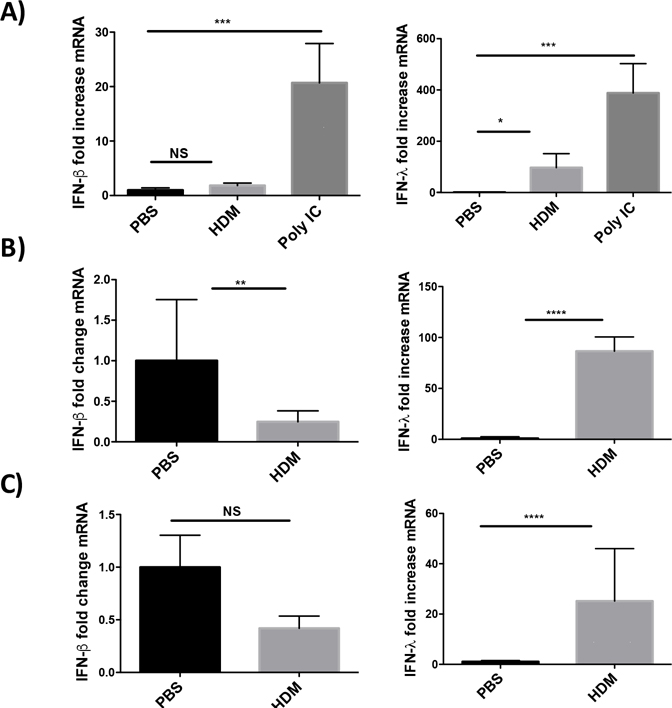
Others have reported that airway cells exposed to HDM respond with a pattern of gene expression consistent with an antiviral response [17]. To test expression of antiviral genes after lung exposure to HDM, C57BL/6J mice were exposed either once or three times intratracheally to 100 μg of HDM extract as outlined in Figure 1. Lungs from singly exposed mice were collected at either 8 or 18 hours following HDM exposure. Lungs from mice exposed three times to HDM were collected 48 hrs following final HDM exposure. At 8, 18 or 48 hours after the last exposure to HDM, lungs were harvested and RNA prepared for qRT-PCR. The expression of the major antiviral interferons (IFN), β and λ were determined using Taqman qRT-PCR. As shown in Figure 2, exposure to HDM results in significant changes in IFN expression. IFN-β expression is not significantly different than controls at 8 and 48hrs after exposure to HDM (Fig. 2A and C) and is significantly down regulated in the lungs of mice 18hrs after one exposure to HDM extract (Fig. 2B). In contrast, IFN-λ is significantly up-regulated at all the time points tested. These data suggest that mice exposed to HDM up-regulate the expression of IFN-λ in vivo while not changing expression or modestly down-regulating IFN-β expression at the time points tested. Importantly, to confirm our ability to detect IFN expression in the lungs of mice at early time points, mice were treated IT with 50μg the TLR3 agonist polyI:C (Fig. 2A). PolyI:C is a potent stimulator of IFN and antiviral gene expression. The polyI:C treated mice expressed readily detectable IFNs β and λ suggesting the lack of IFN-β expression was not due to an inability to detect expression. As shown in Fig. A.1, BALB/cJ showed similar patterns of interferon expression following HDM exposure suggesting this pattern of gene expression occurs in several species of mice.
Figure 2. House dust mite exposure induces interferon expression.
C57BL/6J mice were treated once with 100 μg of HDM or 100 μl of saline and lungs were harvested at 8hrs (A), 18hrs (B), or three times weekly (C) and lungs were harvested 48hrs after the last HDM treatment. Lungs were prepared for qRT-PCR and the expression of IFN-β and IFN-λ was determined using Taqman qRT-PCR. For the 8hr time point a positive control was included and mice were treated IT with 50ug of Poly I:C. Data is representative of two independent experiments with 3 mice per group. Data was analyzed by ANOVA with Dunnett’s multiple comparisons test **p=0.005, ***p=0.0005, NS not significant.
3.2. Mice treated with HDM are partially protected from H1N1 influenza A infection
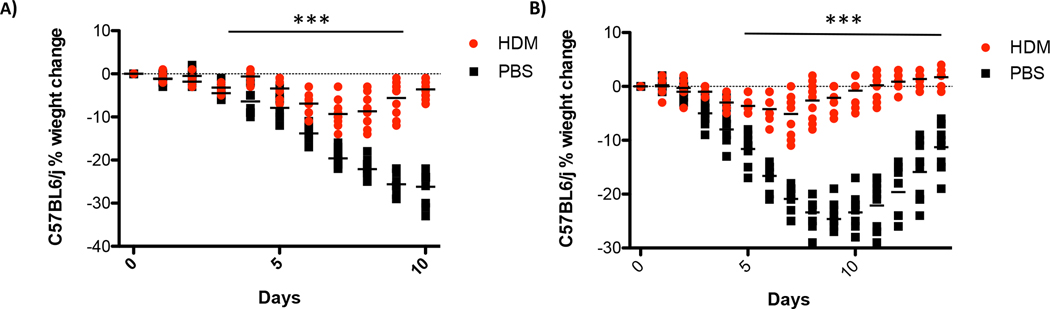
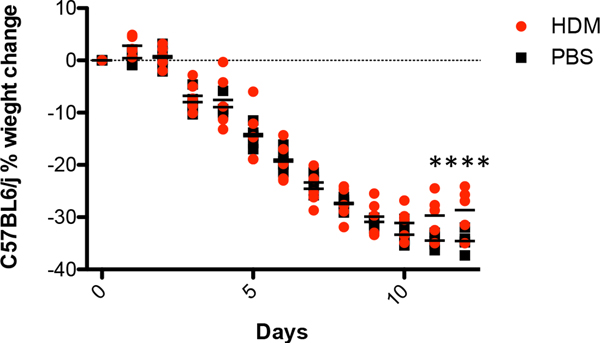
IFN expression following HDM exposure suggests that these animals are more resistant to respiratory viral infections. To test this hypothesis, C57BL/6J mice were exposed to HDM and then 18hrs later they received a sub-lethal dose of H1N1 influenza A. Mice were then observed for changes in body weight as a surrogate measure of general health. As shown in Figure 3A and Fig. A.2, male and female mice treated with saline and then infected with influenza lost significantly more weight than mice exposed to HDM. Similar results were observed when mice were exposed to HDM three times prior to influenza exposure (Fig. 3B). Mice treated three times with HDM extract lost substantially less weight than mice treated once. Altogether these data suggest that mice exposed to HDM are more resistant to influenza infection than control animals.
Figure 3. House dust mite exposure protects against influenza A infection.
C57BL/6J mice were treated once for 18hrs (A) or three times (B) with 100 μg of HDM or 100 μl of saline. Mice were challenged IT with 400 PFU of influenza and body weight was determined daily. Data represents 10 mice and was analyzed by ANOVA***p=0.005, ****p=0.0005.
3.3. Mice treated with HDM have lower viral burdens than controls
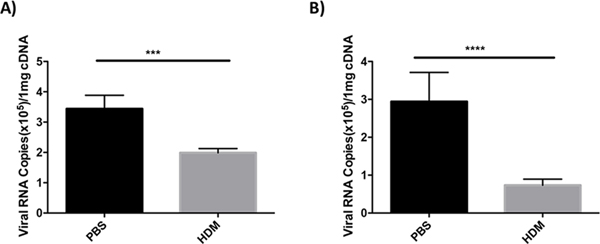
Mice treated with HDM and subsequently infected with influenza lose less body weight than control animals. These observations suggest that HDM-induced antiviral gene expression is impacting viral burdens in the lung. To evaluate viral burdens in the lungs of mice infected with influenza virus, we determined the levels of polymerase acidic (PA) gene mRNA in infected lung tissues by Taqman qRT-PCR. Mice were treated once or three times with HDM or saline as a control, and subsequently infected with 400 PFU of influenza A virus. Four days after infection, lungs were harvested for qRT-PCR analysis of PA mRNA. As shown in Figure 4, animals treated with saline had significantly more PA mRNA present in lung tissues than animals treated with HDM suggesting that HDM treatment reduces viral burden.
Figure 4. Exposure to house dust mite reduces viral load.
C57BL/6J mice were exposed to HDM or saline IT once (A) or three times (B). Mice were infected IT with 400 PFU of influenza A and lungs were harvested 4 days after infection and prepared for Taqman qRT-PCR. As a marker for viral replication we determined the levels of polymerase acidic (PA) gene mRNA in the lungs of infected mice. Data is representative of two experiments N=3/group. Statistical significance was determined by ANOVA ***p=0.005, ****p=0.0005.
3.4. HDM-mediated partial protection from viral infection is dose and timing dependent
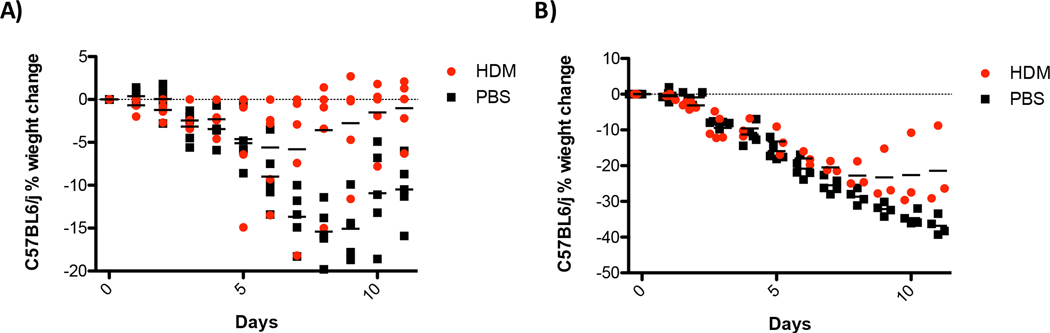
Although HDM exposure partially protects mice from influenza after one or three exposures, it was unclear if animals exposed to high-dose influenza, with chronic HDM exposure, or re-exposed to allergen would maintain protection. To test protection from high-dose influenza infection, mice were exposed to HDM or saline three times and then challenged with a lethal dose of influenza (4,000 PFU). As shown in Figure 5, when mice are challenged with a lethal dose of influenza virus, pre-exposure to HDM does not protect against disease and animals lose weight with kinetics similar to the saline-treated control mice. To evaluate HDM-mediated protection from influenza virus in the context of chronic allergic inflammation, mice were treated once a week for eight weeks with HDM or saline and then challenged with 400 or 4,000 PFU of influenza. As shown in Figure 6A, mice with chronic allergic inflammation are significantly protected from infection with 400 PFU of influenza. When challenged with 4000 PFU mice that had been exposed to HDM eight times initially lost weight with the same kinetics as the saline controls but began to recover faster (Fig. 6B). These data did not reach statistical significance but animals exposed to HDM demonstrated a rate of recovery in animals treated 8X with HDM that was faster than animals treated 3X (Figs. 3 and 5). These data suggest that additional exposures to HDM provide limited benefit to mice challenged with high-dose influenza compared to the significant protection provided against sub-lethal infection.
Figure 5. House dust mite exposure does not protect against a lethal influenza challenge.
C57BL/6J mice were exposed three times to 100 μg of house dust mites or saline as a control. Forty-eight hours after the last exposure to HDM or saline mice were infected with 4,000 PFU of influenza A. Body weight was monitored daily. N=5 mice/group, ANOVA determined statistical significance **** p=0.0005.
Figure 6. Chronic exposure to house dust mite protects against influenza infection.
Mice were exposed to HDM or saline weekly for eight weeks prior to infection with 400 or 4,000 PFU of influenza A. Body weights were monitored daily. N=5 mice/group ANOVA determined statistical significance ****p=0.0005. Note that statistical significance with a multiple measures ANOVA could not be determined in (B) due to unequal group sizes after day 5.
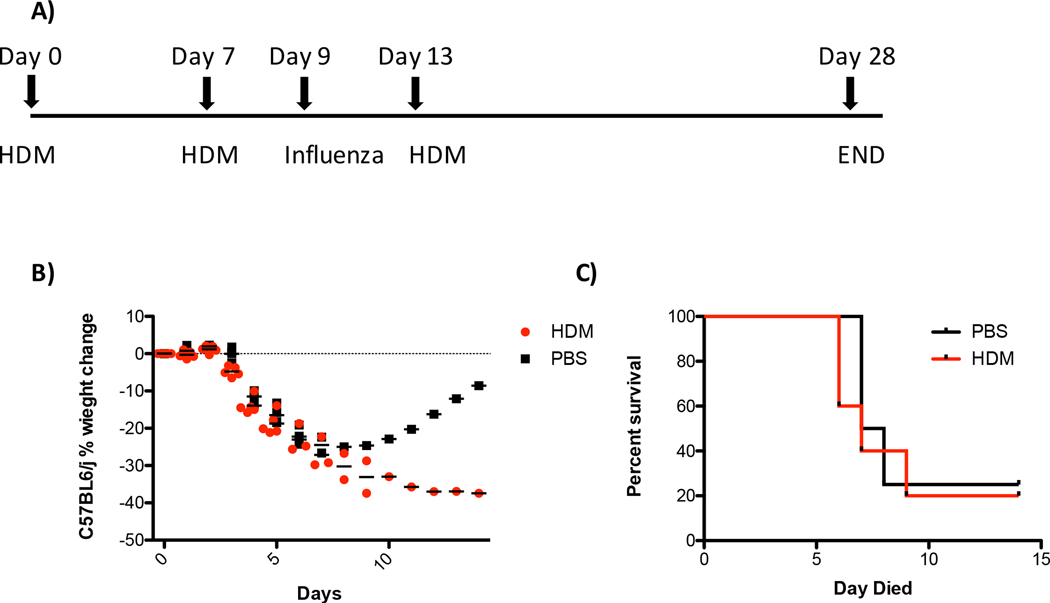
It is clear that viral infections are a critical driver of acute exacerbations of human asthma [19]. To evaluate the effect of HDM exposure after virus infection, mice were sensitized to HDM through two exposures to HDM. Mice were subsequently infected with 400 PFU of influenza virus and challenged with HDM five days later (Fig. 7A). Most of the mice in both the HDM and control groups had to be euthanized between days 6 and 7 due to excessive body weight loss. One animal in each group survived past 7 days with the PBS treated animal ultimately recovering while the HDM treated animal required euthanasia (Figs. 7B and C). Altogether, these data suggest that HDM exposure does not protect against high-titer viral challenge and that challenge with allergen shortly after viral infection exacerbates disease.
Figure 7. House dust mite exposure of sensitized animals after influenza infection worsens outcomes.
Mice were exposed to 100 μg of HDM or 100 μl of saline twice a week apart. Mice were then infected IT with 400 PFU of influenza virus 48hrs after the second exposure to HDM or saline. Four days after influenza virus infection mice were challenged with 100 μg of HDM or 100 μl of saline. Body weight was monitored daily. Mice were euthanized if body weight loss ≥ 30% of the initial body weight. (A) schematic of experiment (B) Body weight loss (C) data from (B) plotted as a survival graph. N=5 mice for the HDM group and N=4 for the saline group.
3.5. HDM-mediated protection from influenza virus infection is independent of TLR4 signaling and occurs in multiple strains of mice
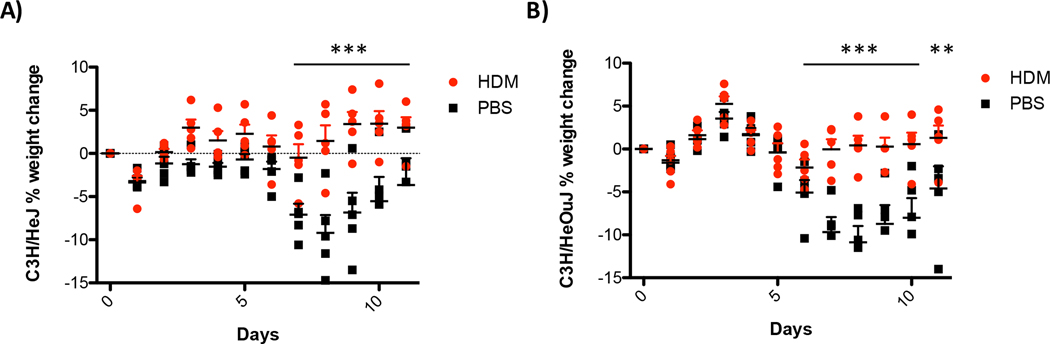
House dust and HDM extracts are complex mixtures with numerous molecules capable of stimulating innate immune responses. One of the most potent innate immune-stimulating components of HDM extracts is lipopolysaccharide (LPS). To test if LPS-stimulated responses were responsible for HDM-mediated protection from influenza infection, we evaluated responses in C3H/HeJ mice. C3H/HeJ mice have a spontaneous mutation in TLR4 (Tlr4Lps-d) that inactivates the signaling functions of TLR4 [28]. C3H/HeJ mice were sensitized three times to HDM or saline and were then challenged with 400 PFU of influenza virus. As shown in Figure 8A, C3H/HeJ mice respond similarly to the TLR4 sufficient C3H/HeOuJ mice (Fig. 8B). C3H/HeOuJ are genetically very similar C3H/HeJ mice but they are capable of signaling through TLR4. These data suggest that TLR4 signaling does not significantly contribute to HDM-mediated resistance to influenza infection. As shown in Figure 3, C57BL/6J mice display HDM-mediated resistance to influenza infection. However, BALB/cJ mice are more susceptible to developing allergic inflammation relative to the C57BL/6J mouse [13]. To test the ability of HDM to protect BALB/cJ mice, BALB/cJ mice were treated once or three times with HDM or saline and were subsequently challenged with 400 PFU of influenza virus. As shown in Figure 9, exposure to HDM provided significant protection from influenza infection in BALB/cJ mice. Similar to what was observed with C57BL/6J mice, BALB/cJ mice exposed to HDM express significantly more IFN-λ than controls while the levels of IFN-β are not substantially different at the time points tested (supplemental Figure 1). Altogether, these data suggest that TLR4 is not required for HDM-mediated protection from influenza virus infection and that HDM can induce protection in multiple strains of mice (BALB/cJ, C57BL/6J, C3H/HeJ, and C3H/HeOuJ).
Figure 8. House dust mite-mediated protection from influenza infection is independent of TLR-4.
(A) TLR-4 deficient C3H/HeJ mice were exposed IT to 100 μg of HDM or 100 μl of saline weekly for three weeks. Forty-eight hours after the last exposure, mice were challenged with 400 PFU of influenza A. (B) TLR-4 sufficient C3H/HeOuJ were treated similarly. Body weight was monitored daily. N=5 mice/group ANOVA determined statistical significance **p=0.05, ****p=0.0005
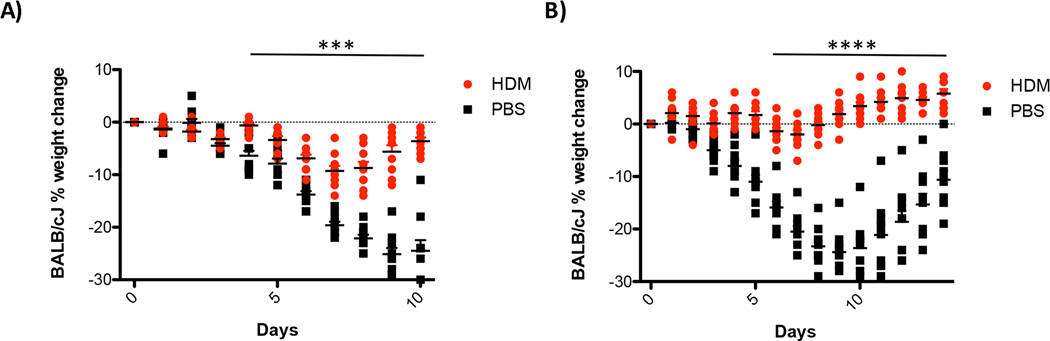
Figure 9. HDM exposure protects BALB/cJ mice from influenza infection.
Mice prone to allergic inflammation, BALB/cJ, were evaluated for protection from influenza infection. Mice were treated as outlined in Figure 1. Both mice exposed once (A) or three times (B) were protected from influenza A virus infection. N= 10 mice/group statistical significance was determined by ANOVA ***p=0.005, ****p=0.0005.
3.6. Mice exposed to HDM and Influenza A virus have histological evidence of mixed inflammatory responses
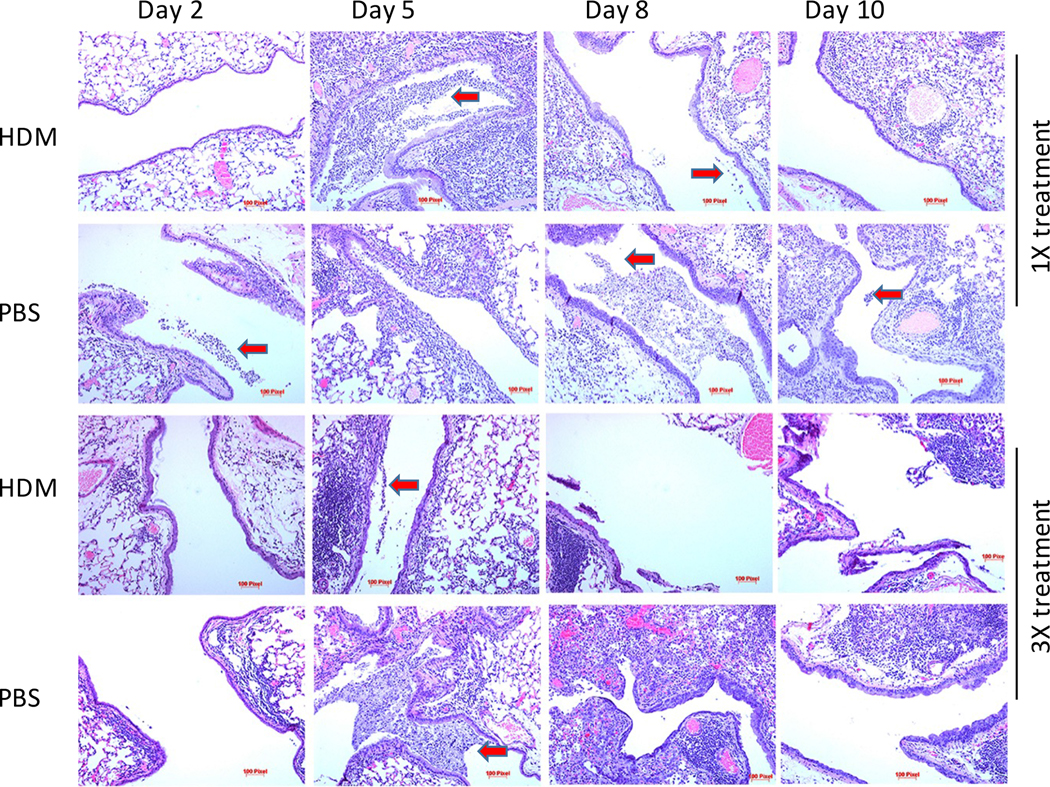
As described above, mice exposed to HDM extracts have better outcomes compared to animals exposed to saline. To further test the pulmonary response to influenza A infection following HDM exposure, BALB/cJ mice were treated once or three times with HDM or saline and then challenged with 400 PFU influenza A virus Mice were randomized and cohorts of HDM treated or controls were euthanized on days 2, 5, 8, and 10. Lungs were removed and prepared for histology. Thin sections (4μm) were stained with hematoxylin and eosin and inflammatory pathology was evaluated microscopically (Fig. 10 and Fig. A.3). Animals treated once with HDM and then infected with influenza virus had no evidence of allergic inflammation. These animals developed evidence of viral pneumonia including inflammatory infiltrates, denuded epithelium, areas of consolidation, and exudate in the conducting airways by day 5 post-viral infection. Animals treated with saline had more severe disease that encompassed greater areas of lung tissue however, these data did not reach statistical significance (Fig. A.3A). Animals treated with HDM three times, as expected, had histology consistent with mild allergic inflammation including, peribronchiolar and perivascular lymphocytic and leukocytic cellular inflammation, eosinophilia, and epithelial hyperplasia. Following infection with influenza virus, both the HDM-treated and control mice had evidence of viral pneumonia. However, the control animals had more severe pneumonia based on the extent of tissue involvement and damage that was statistically significant on day 5 (Fig. A.3B). These findings are consistent with the differences in viral burdens and body weight maintenance.
Figure 10. Inflammatory histopathology of BALB/cJ mice infected with influenza.
A. BALB/cJ mice were exposed to saline or HDM once or three times prior to being infected IT with 400 PFU of influenza A. Mice were randomized and 3 mice/treatment/time point were euthanized at the indicated time and lungs were removed for histological analysis. Inflammatory pathology was evaluated by investigators blinded to the treatment groups. Images of representative large airways were obtained at 10x magnification. Red arrows indicate inflammatory exudate in the conducting airways.
4.0. Discussion
The lung is an organ that is in constant contact with the outside environment. This exposes lung tissues to a variety of insults including potentially allergenic materials, chemical pollutants, and pathogenic organisms amongst others. How the respiratory system responds to these encounters depends on the nature of the material, genetics, immune status, and comorbid conditions. Allergic disease affects a significant proportion of the population with an estimated 40–50% of children worldwide being sensitized to at least 1 common allergen[1–3, 29]. Although these rates encompass all manifestations of allergic disease, allergic diseases impacting the respiratory tract, allergic rhinitis and asthma, account for a large fraction of allergic disease in the US. There are an estimated 26 million Americans with asthma [1]. Many of these individuals will have an acute exacerbation of their disease associated with a microbial infection, most often a rhinovirus infection.
Although it is clear that infection can exacerbate features of allergic disease [19, 22–25], the consequences for the pathogenesis of the infectious agent are less understood. Others have suggested that exposure to HDM inhibits antiviral responses but this was not tested with an actual viral infection in vivo [30]. To investigate responses to a natural environmental allergen, we utilized a mouse model of HDM mucosal sensitization. This was coupled with sub-lethal influenza A infection to model viral infection secondary to allergen exposure. Although the most common virus linked to asthma exacerbation is rhinovirus [31, 32], the mouse poorly models human rhinovirus infection. The mouse model of influenza virus infection recapitulates many aspects of human seasonal influenza but also has some limitations in terms of human disease pathogenesis [33]. The goal of this study was to determine the impact of allergen exposure on viral infection. We did not investigate physiological aspects of asthma exacerbation such as hypoxia, changes in pulmonary function, and changes in airway remodeling.
Surprisingly, we observed that a single exposure to HDM significantly reduced the weight loss associated with influenza infection. This correlated with decreased viral mRNA expression and the increased expression of antiviral interferon λ in the lungs of animals exposed to HDM with no change or diminished expression of interferon β at the time points tested. These data suggest that dust mite induced antiviral responses are mediated by IFN-λ yet this needs to be formally tested. The ability of HDM to induce both IFN-λ and antiviral gene expression has been reported by others and suggests innate immune sensors recognize materials in HDM [14, 16]. IFN-λ is predominantly expressed by the epithelium in a TLR3-dependent manner [34]. Although IFN-λ is an antiviral cytokine, it is also known to have anti-inflammatory properties [14–16, 18, 35–37] and it is currently unknown if the improved outcomes observed with HDM-exposed mice infected with influenza virus is due to the antiviral effects of interferon or reduced inflammation. The pathogen associated molecular pattern molecule (PAMP) present in HDM that induces IFN is currently not known. However, both HDM extracts and house dust contain LPS which is known to induce type I and II interferons and antiviral gene expression from monocyte derived cells.
To test the impact of TLR4 signaling on HDM-mediated resistance to influenza infection, we treated TLR4 deficient C3H/HeJ mice with HDM or saline and then infected the mice with influenza. Similar to the very closely related TLR4 sufficient C3H/HeOuJ mice, as well as BALB/cJ, C57BL/6J, C3H/HeJ mice showed significantly less body weight loss when treated with HDM and infected with influenza relative to controls. These data suggest LPS is not a significant factor in HDM-mediated protection from influenza infection. These data are consistent with the limited expression of IFN-β following HDM exposure since the TLR4/TRIF pathways predominantly mediate LPS-dependent type-I IFN responses [38].
Multiple strains of mice (BALB/cJ, C3H/HeJ, C3H/HeOuJ, and C57BL/6J) are protected from influenza infection following one or three exposures to HDM. We tested whether this protection could be bypassed if animals were exposed to a lethal dose of influenza (4,000 PFU). HDM-exposed mice challenged with 4,000 PFU of influenza had the same rate of body weight loss as control animals losing 25–30% of their initial body weight by day seven post infection. The HDM-exposed animals did begin to regain weight faster than controls but these data suggest that high-dose exposure to influenza can overcome protection provided by three exposures to HDM. Likewise, to test if chronic allergic inflammation impacted resistance to influenza infection, mice were treated eight times with HDM prior to influenza challenge. Mice treated eight times with HDM did significantly better with a reduced peak weight loss compared to animals treated once or three times when challenged with 400 or 4,000 PFU of influenza. These data suggest that protection from influenza infection is maintained in a chronic model of inflammation. However, when sensitized mice were infected with influenza and then challenged with HDM they developed severe disease and succumbed quickly suggesting that an allergic challenge worsens disease during an active viral infection. It is currently unknown why mice are protected from viral infection if they are exposed to virus after an allergen challenge but succumb quickly if the allergen challenge occurs during active viral infection.
Although using the mouse model of HDM-induced allergic inflammation we observed protection from influenza infection, this mouse model does not reflect human severe refractory asthma [13]. Severe asthmatics are at highest risk of significant complications from respiratory infection [39]. Additionally, these mice were not treated with medications such as inhaled corticosteroids or bronchodilators that are commonly taken daily by individuals with asthma. The severity of disease, medication usage and effectiveness, and other comorbid conditions all impact the ability of individuals with asthma to respond to respiratory infections.
During the 2009 pandemic influenza outbreak it was noted that asthmatics were more likely to be hospitalized but were less likely to have severe outcomes [40–43]. The reasons for these observations are not clear but it has been associated with inhaled corticosteroid use [42] and eosinophilia [44]. We did not evaluate steroids in our model. Eosinophils were shown to be sensitive to influenza infection and to function as antigen presenting cells to promote anti-viral CD8+ T-cell responses [44]. We did not specifically test the role of eosinophils in HDM-mediated protection from influenza infection. The protection observed in mice treated three times or eight times with HDM could be consistent with eosinophil-mediated responses as these animals have moderate to robust allergic inflammation respectively. However, animals treated once with HDM for eighteen hours are protected from influenza infection and there is no histologic evidence of allergic inflammation. Moreover, when we examined expression of cytokines and chemokines critical for recruitment of eosinophils to the lungs, IL-5, eotaxin-1 and 2, and saw no differences in expression levels in mice treated once with saline or HDM for 18 hours (not shown). These data suggest that there are multiple factors contributing to the HDM-mediated anti-viral response.
Supplementary Material
Acknowledgements
This work was partially supported by grants awarded to PHD from the NIH (AI070412), the Cancer Prevention and Research Institute of Texas (RP140565), and the William and Ella Owens foundation. RPG is supported by NIH grant 5T32DE014318-16. QH was supported by a fellowship from the Third Xiangya Hospital.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief. 2012;(94):1–8. . [PubMed] [Google Scholar]
- 2.Pawankar R Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12. doi: 10.1186/1939-4551-7-12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring J, Akdis C, Lauener R, Schappi G, Traidl-Hoffmann C, Akdis M, et al. Global Allergy Forum and Second Davos Declaration 2013 Allergy: Barriers to cure--challenges and actions to be taken. Allergy. 2014;69(8):978–82. doi: 10.1111/all.12406. [DOI] [PubMed] [Google Scholar]
- 4.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. Epub 2009/07/07. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017;38(12):942–54. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korsgaard J House-dust mites and asthma. A review on house-dust mites as a domestic risk factor for mite asthma. Allergy. 1998;53(48 Suppl):77–83. [DOI] [PubMed] [Google Scholar]
- 8.Pate AD, Hamilton RG, Ashley PJ, Zeldin DC, Halsey JF. Proficiency testing of allergen measurements in residential dust. J Allergy Clin Immunol. 2005;116(4):844–50. doi: 10.1016/j.jaci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Shedd AD, Peters JI, Wood P, Inscore S, Forkner E, Smith B, et al. Impact of home environment characteristics on asthma quality of life and symptom scores. J Asthma. 2007;44(3):183–7. Epub 2007/04/25. doi: 777187079 [pii] 10.1080/02770900701209699. [DOI] [PubMed] [Google Scholar]
- 10.Calderon MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol. 2015;136(1):38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet A The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011;17(10):604–11. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Jacquet A The role of the house dust mite-induced innate immunity in development of allergic response. Int Arch Allergy Immunol. 2011;155(2):95–105. doi: 10.1159/000320375. [DOI] [PubMed] [Google Scholar]
- 13.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L401–10. Epub 2009/06/30. doi: 00027.2009 [pii] 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MR, Johnston SL. Interferon-lambda as a new approach for treatment of allergic asthma? EMBO Mol Med. 2011;3(6):306–8. doi: 10.1002/emmm.201100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6(4):797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marichal T, Bedoret D, Mesnil C, Pichavant M, Goriely S, Trottein F, et al. Interferon response factor 3 is essential for house dust mite-induced airway allergy. J Allergy Clin Immunol. 2010;126(4):836–44 e13. doi: 10.1016/j.jaci.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, Upham JW. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol. 2012;188(12):5898–905. doi: 10.4049/jimmunol.1103507. [DOI] [PubMed] [Google Scholar]
- 19.Darveaux JI, Lemanske RF Jr. Infection-related asthma. The journal of allergy and clinical immunology In practice. 2014;2(6):658–63. doi: 10.1016/j.jaip.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemanske RF Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest. 2007;132(6):1962–6. doi: 10.1378/chest.06-2415. [DOI] [PubMed] [Google Scholar]
- 22.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4(10):e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maselli DJ, Medina JL, Brooks EG, Coalson JJ, Kannan TR, Winter VT, et al. The Immunopathologic Effects of Mycoplasma pneumoniae and Community-acquired Respiratory Distress Syndrome Toxin. A Primate Model. Am J Respir Cell Mol Biol. 2018;58(2):253–60. doi: 10.1165/rcmb.2017-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina JL, Coalson JJ, Brooks EG, Winter VT, Chaparro A, Principe MF, et al. Mycoplasma pneumoniae CARDS toxin induces pulmonary eosinophilic and lymphocytic inflammation. Am J Respir Cell Mol Biol. 2012;46(6):815–22. doi: 10.1165/rcmb.2011-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina JL, Coalson JJ, Brooks EG, Le Saux CJ, Winter VT, Chaparro A, et al. Mycoplasma pneumoniae CARDS toxin exacerbates ovalbumin-induced asthma-like inflammation in BALB/c mice. PLoS One. 2014;9(7):e102613. doi: 10.1371/journal.pone.0102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202(5):697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre JS, Lorenzo EC, Masters AR, Hopkins JW, Eaton SM, Smiley ST, et al. Vaccine efficacy and T helper cell differentiation change with aging. Oncotarget. 2016;7(23):33581–94. doi: 10.18632/oncotarget.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, et al. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172(10):6202–8. [DOI] [PubMed] [Google Scholar]
- 29.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24 Epub 2009/05/21. doi: 1471–2466-9–24 [pii] 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy. 2018;73(5):1053–63. doi: 10.1111/all.13378. [DOI] [PubMed] [Google Scholar]
- 31.Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev. 1999;12(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gern JE, Lemanske RF Jr., Busse WW. Early life origins of asthma. J Clin Invest. 1999;104(7):837–43. doi: 10.1172/JCI8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouvier NM, Lowen AC. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses. 2010;2(8):1530–63. doi: 10.3390/v20801530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity. 2015;43(1):15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, et al. IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production. J Exp Med. 2015;212(6):845–53. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, et al. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3(6):348–61. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritchard AL, White OJ, Burel JG, Upham JW. Innate interferons inhibit allergen and microbial specific T(H)2 responses. Immunol Cell Biol. 2012;90(10):974–7. doi: 10.1038/icb.2012.39. [DOI] [PubMed] [Google Scholar]
- 38.Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC. Type I interferon signaling contributes to the bias that Toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal. 2014;7(351):ra108. doi: 10.1126/scisignal.2005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelaia G, Vatrella A, Gallelli L, Renda T, Cazzola M, Maselli R, et al. Respiratory infections and asthma. Respir Med. 2006;100(5):775–84. doi: 10.1016/j.rmed.2005.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. The New England journal of medicine. 2009;361(20):1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 41.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 42.Myles P, Nguyen-Van-Tam JS, Semple MG, Brett SJ, Bannister B, Read RC, et al. Differences between asthmatics and nonasthmatics hospitalised with influenza A infection. Eur Respir J. 2013;41(4):824–31. doi: 10.1183/09031936.00015512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaillant L, La Ruche G, Tarantola A, Barboza P, epidemic intelligence team at In VS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33). [DOI] [PubMed] [Google Scholar]
- 44.Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, et al. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J Immunol. 2017;198(8):3214–26. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.