Abstract
Positive‐social emotions mediate one's cognitive performance, mood, well‐being, and social bonds, and represent a critical variable within therapeutic settings. It has been shown that the upregulation of positive emotions in social situations is associated with increased top‐down signals that stem from the prefrontal cortices (PFC) which modulate bottom‐up emotional responses in the amygdala. However, it remains unclear if positive‐social emotion upregulation of the amygdala occurs directly through the dorsomedial PFC (dmPFC) or indirectly linking the bilateral amygdala with the dmPFC via the subgenual anterior cingulate cortex (sgACC), an area which typically serves as a gatekeeper between cognitive and emotion networks. We performed functional MRI (fMRI) experiments with and without effortful positive‐social emotion upregulation to demonstrate the functional architecture of a network involving the amygdala, the dmPFC, and the sgACC. We found that effortful positive‐social emotion upregulation was associated with an increase in top‐down connectivity from the dmPFC on the amygdala via both direct and indirect connections with the sgACC. Conversely, we found that emotion processes without effortful regulation increased network modulation by the sgACC and amygdala. We also found that more anxious individuals with a greater tendency to suppress emotions and intrusive thoughts, were likely to display decreased amygdala, dmPFC, and sgACC activity and stronger connectivity strength from the sgACC onto the left amygdala during effortful emotion upregulation. Analyzed brain network suggests a more general role of the sgACC in cognitive control and sheds light on neurobiological informed treatment interventions.
Keywords: amygdala, anxiety, connectivity, dorsomedial prefrontal cortex, emotion regulation, functional magnetic resonance imaging, intrusive thought suppression, positive‐social emotions, subgenual anterior cingulate cortex
1. INTRODUCTION
Positive emotions facilitate creative thinking, decision‐making, problem‐solving, and social bounds (Ashby, Isen, & Turken, 1999; Carpenter, Peters, Vastfjall, & Isen, 2013; Fredrickson, 2004; Gross, 2002; Nadler, Rabi, & Minda, 2010). A more comprehensive understanding of the mechanisms underlying positive emotion upregulation in social situations (positive‐social emotion upregulation) is critical as impairment of this ability might constitute a key factor contributing to the severity and maintenance of psychiatric disorders (Aldao, Nolen‐Hoeksema, & Schweizer, 2010; Etkin, Buchel, & Gross, 2015). For example, anhedonic symptoms are associated with depression, and emotion dysregulation symptoms are associated with posttraumatic stress disorder (PTSD) (Disner, Beevers, Haigh, & Beck, 2011; Etkin et al., 2015; Nicholson et al., 2017; Treadway & Zald, 2011; Young et al., 2017). Research investigating the psychological and neural processes underlying emotion regulation collectively point to the role of specific brain regions involved in the control of the emotional experience, regulation, and expression (Ochsner & Gross, 2005; Ochsner & Gross, 2008; Ochsner, Silvers, & Buhle, 2012; Schlosser et al., 2008; Smith, Stephan, Rugg, & Dolan, 2006). According to emotion regulation models, successful regulation is achieved through the modulation of emotional bottom‐up responses by higher‐order cognitive top‐down processes (Etkin et al., 2015; Gross, 2002; Ochsner et al., 2009; Ochsner et al., 2012; Taylor & Liberzon, 2007; Zilverstand, Parvaz, & Goldstein, 2017). Interestingly, numerous studies suggest that brain networks involved in the upregulation of positive versus negative emotions have both common and distinct components (Davidson & Irwin, 1999; Fossati et al., 2003; Kim & Hamann, 2007; Koush et al., 2019; Lane, Reiman, Ahern, Schwartz, & Davidson, 1997; Ochsner et al., 2012; Vrticka, Sander, & Vuilleumier, 2012; Yang, Tsai, & Li, 2020).
On balance, the sgACC, amygdala, and PFC areas typically show aberrant emotion regulation engagement associated with mood and anxiety disorders (Drevets, 2003; Gotlib & Hamilton, 2008; Lanius et al., 2018; Ressler & Mayberg, 2007; Yehuda et al., 2015). The amygdala plays a central role in conscious and unconscious emotion processing (Glascher & Adolphs, 2003), and has been shown to be highly dysregulated in psychiatric illness, correlated to symptoms across a wide range of disorders (Etkin & Wager, 2007; Fenster, Lebois, Ressler, & Suh, 2018; Phelps & LeDoux, 2005). Likewise, abnormalities in sgACC gray matter volume, activity, and connectivity, are associated with depressive and hyper‐emotionality symptoms across a range of psychiatric illnesses (Disner et al., 2011; Drevets, 2001; Drevets & Savitz, 2008; Etkin et al., 2015; Fenster et al., 2018; Wu et al., 2016; Yehuda et al., 2015). The sgACC has been used as a target region for treating chronic pharmaco‐resistant depression with deep brain stimulation (Hamilton, Glover, Hsu, Johnson, & Gotlib, 2011; Ressler & Mayberg, 2007). The sgACC has been suggested to transfer emotion information from the limbic system to higher‐order cognitive structures (Disner et al., 2011; Greicius et al., 2007). It has been implicated in functions related to emotion reappraisal (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), positive emotion upregulation (Wager et al., 2008), fear extinction (Phelps, Delgado, Nearing, & LeDoux, 2004), monitoring internal states (Gillath, Bunge, Shaver, Wendelken, & Mikulincer, 2005), anxiety and mood disorders (Drevets & Savitz, 2008; Etkin & Wager, 2007), sadness induction (Ramirez‐Mahaluf, Perramon, Otal, Villoslada, & Compte, 2018), social interactions and social decision‐making (Lockwood & Wittmann, 2018), mediating autonomic arousal (Zhang et al., 2014) and reward mechanisms (Azab & Hayden, 2018; Rudebeck et al., 2014; Stevens, Hurley, & Taber, 2011). In addition, the sgACC and rostral ACC have been shown to be implicated in the processing of negative and positive emotions, respectively (Etkin, Egner, & Kalisch, 2011; Grone et al., 2014; Vogt, 2005), and together are involved in social cognition (Lockwood & Wittmann, 2018; Palomero‐Gallagher et al., 2015). Critically, both the sgACC and amygdala are densely connected (Beckmann, Johansen‐Berg, & Rushworth, 2009; Etkin et al., 2011; Johansen‐Berg et al., 2008), and are thought to be a part of an automatic emotion regulation circuit, whereas the dmPFC has often been implicated in social‐emotion processing (Amodio & Frith, 2006; Bzdok et al., 2013; Goldberg, Harel, & Malach, 2006; Lockwood & Wittmann, 2018; Ochsner & Gross, 2008; Vrticka et al., 2012), and has been associated with the voluntary regulation of motions (Braunstein, Gross, & Ochsner, 2017; LeDoux & Brown, 2017; Phillips, Ladouceur, & Drevets, 2008; Stevens et al., 2011). Patterns of anatomical and functional connectivity collectively support an important role of the sgACC in interacting with limbic regions, including the amygdala, and in communicating with prefrontal areas important for top‐down forms of regulation (dmPFC), where the amygdala also has direct structural connections with the dmPFC (Eickhoff, Laird, Fox, Bzdok, & Hensel, 2016; Etkin et al., 2011; LeDoux, 2007).
The classification of emotion regulation differentiates self‐regulation and social regulation of emotion (Reeck, Ames, & Ochsner, 2016). Self‐regulation of emotion suggests that the regulator and the target are the same person, that is, how people regulate their own emotions. Conversely, the social regulation of emotion suggests that the regulator alters the emotional response of another targeted individual, that is, how people regulate the emotions of others. Positive stimuli with social content have been used to stimulate self‐regulation of emotions in participants (Koush et al., 2019). This was accomplished by passive viewing of displayed situations or effortful imagination of experiencing the depicted positive‐social situations from a first‐person perspective in an active and pleasant manner (termed as positive‐social emotion regulation). Specifically, for positive‐social scenes, participants were instructed to imagine a natural (spontaneous) interaction with a displayed single (multiple) person(s), for example, interactively carrying out a pleasant conversation, or leisure and sport activities. For neutral inanimate scenes (no landscapes or food) participants were instructed to imagine oneself using the depicted neutral objects in an active and pleasant manner, for example, carrying out the favorite activity. This conceptualization is different from (a) instructions to naturally experience the exposed (non)social positive/neutral/negative scenes by watching and evaluating (Vrticka et al., 2012; Vrticka, Sander, & Vuilleumier, 2011), (b) instructions to increase emotional reactions to displayed positive stimuli (Kim & Hamann, 2007), and (c) instructions associated with the social regulation of emotion (Reeck et al., 2016).
It has been shown that regulating positive emotions is associated with increased top‐down signals that stem from the prefrontal cortices (PFCs), which concomitantly modulate bottom‐up emotional responses in the amygdala (Koush et al., 2017; Koush et al., 2019; Young et al., 2018; Zotev, Phillips, Young, Drevets, & Bodurka, 2013). Consistently, positive‐social emotion upregulation with a self‐referential first‐person perspective was associated with the direct influence of the superior frontal gyrus (SFG, lateral to the dmPFC), the ventromedial PFC and the dmPFC exerted onto the bilateral amygdala (Koush et al., 2019). In addition, it has been shown that the top‐down influence of the dmPFC onto the bilateral amygdala could also be voluntarily regulated using positive emotion upregulation neurofeedback (Koush, Meskaldji, et al., 2017). However, it remains unclear if positive‐social emotion regulation of the limbic system occurs through direct connections with PFCs or via indirect connections with the subgenual anterior cingulate cortex (sgACC), an area densely connected with the amygdala, which is often described as an important “gatekeeper” between the cognitive and the emotional system (Disner et al., 2011; Greicius et al., 2007; Palomero‐Gallagher et al., 2015). This highlights the need to investigate further the specific role of brain areas implicated in positive‐social emotion regulation and contribute to the identification of potential targets for future neurofeedback interventions based on positive‐social emotion upregulation (Koush, Meskaldji, et al., 2017; Sitaram et al., 2017; Stoeckel et al., 2014). We hypothesized that a neural model engaging the sgACC in the dmPFC–amygdala neural circuitry would dominate over alternative models without this sgACC engagement, suggesting a gatekeeping role of the sgACC during the regulation of positive‐social emotions.
To test this hypothesis, we investigated positive‐social emotion regulation conditions, where participants effortfully enhanced their positive emotions by imagining to engage actively and pleasantly in social scenes in a first‐person perspective, and different passive viewing conditions. We examined functional networks of two independent data sets, pertaining to (a) previously published data that consisted of positive‐social and neutral nonsocial emotion upregulation conditions, and positive‐social and neutral nonsocial passive viewing conditions of the same condition duration (Koush et al., 2019), and (b) newly acquired data consisting of a similar positive‐social emotion upregulation condition, a positive‐social rapid passive viewing condition, and neutral nonsocial passive viewing and rapid passive viewing conditions. Passive viewing and rapid passive viewing conditions, attenuated and limited effortful emotion upregulation, respectively, that is, self‐referential engagement and active imagination. This design also uniquely allowed us to observe the role of the sgACC engagement in emotion upregulation with regard to dmPFC‐amygdala neural circuitry interactions, and to confirm experimental findings within independent data sets. We also explored the link between psychometric scores associated with the regulation of emotions and network activity and connectivity involved in positive‐social emotion regulation.
2. MATERIALS AND METHODS
2.1. Participants
In this study we examined (a) previously published experimental data (Koush et al., 2019), with n = 23 healthy individuals (11 female, age 27.7 ± 6.2 years), denoted as the first experiment, and (b) newly collected experimental data with n = 14 healthy individuals (7 female, age 24.3 ± 3.6 years), denoted as the second experiment. With regard to the second, unpublished data set, healthy human volunteers were recruited from the local student/research community and gave written informed consent to participate in our study, which was approved by the ethics committee at the University of Geneva. All participants had normal or corrected‐to‐normal vision, and had no prior history of neurological or psychiatric illnesses. Before the experiment, participants received written instructions describing the positive‐social and neutral images they were about to see and the experimental tasks. They were instructed to either passively look at these images, or to imagine positively experiencing the depicted positive‐social situation as one of the main protagonists in a first‐person perspective. After the experiment, participants were asked to fill in several questionnaires and inventories. Participants were paid 20 CHF/hour for their participation.
2.2. Experimental stimuli
Emotional stimuli of both experiments consisted of two sets of photographs taken from the International Affective Picture Set (IAPS; [Lang, Greenwald, Bradley, & Hamm, 1993]), the Nencki Affective Picture System (NAPS; [Marchewka, Zurawski, Jednorog, & Grabowska, 2013]) and the Geneva Affective Picture Database (GAPED; [Dan‐Glauser & Scherer, 2011]). Images in the first set depicted positive‐social situations, and images in the second set depicted nonsocial neutral scenes and objects (no landscapes or food images were selected). For the first experiment, we used 112 photographs per set (normative mean ± SD; positive‐social images of scenes with people: valence 6.92 ± .74, arousal 4.94 ± .75; nonsocial neutral scenes and objects: valence 5.21 ± .60, arousal 3.52 ± 1.05). For the second experiment, we used 150 photographs per set (normative mean ± SD; positive‐social images of scenes with people: valence 6.71 ± .87, arousal 4.38 ± 1.04; nonsocial neutral scenes and objects: valence 5.37 ± .69, arousal 3.61 ± .96). The number of images per set slightly differed between the experiments (112 vs. 150) due to their block‐design differences (Figure S1). Importantly, because present research targets unveiling the role of sgACC using different emotion regulation paradigms, we do not compare associated brain activity and connectivity patterns between both experiments. Thus, to complicate the effortful upregulation of positive emotions so that participants had to apply more effort to induct positive feelings, positive‐social images of the second experiment were selected slightly less positive (two‐sample, two‐tailed t‐test, t = 2.01, p = .045) and less arousing (two‐sample, two‐tailed t‐test, t = 4.73, p < .001) as compared to the first experiment. For both experiments, the order of stimuli presentation was pseudo‐randomized, and each image was shown only once to a given participant to ensure a uniform distribution of stimuli valence and arousal in the picture subsets, and to balance potential color, intensity and scenery differences between pictures from the different databases. The images were presented centrally with a diameter of ~12° visual angle. To facilitate better stimuli recognition in both experiments, images depicting positive‐social situations were framed in green, and images depicting neutral objects were framed in white. The order of passive viewing and upregulation epochs (first experiment) and runs (second experiment) was randomized across participants.
2.3. Experimental procedures
The first experiment consisted of two alternating functional runs with a 2 × 2 factorial design based on the factors stimuli (positive‐social vs. neutral nonsocial scenes) and task (passive viewing vs. effortful emotion upregulation) (Koush et al., 2019). The experimental runs were built of two epochs (Figures 1a and S1a), with each epoch consisting of either effortful emotion upregulation or passive viewing conditions (14 randomized blocks per epoch, 7 blocks per each stimuli category per epoch, 4 images per block, 6 s image display duration, 11.3 min total run duration). The 6 s image presentation time was chosen to facilitate imagination during the emotion regulation runs (Ochsner et al., 2009; Vrticka et al., 2012). During passive viewing conditions, participants passively viewed the presented images. During effortful positive‐social and neutral nonsocial emotion upregulation conditions, participants imagined experiencing the depicted positive‐social and neutral nonsocial scenes in a self‐referential pleasant manner (Koush et al., 2019). During effortful positive‐social emotion upregulation condition, participants were instructed to imagine a spontaneous interaction with a displayed single (or multiple) person(s), for example, interactively carrying out a pleasant conversation, favorite leisure and sport activities. During neutral nonsocial emotion upregulation condition, participants were asked to imagine carrying out the favorite activity with the object and ensure that other people are not being engaged during imagination. For instance, if a bicycle is displayed, outdoor cycling could be imagined without other people.
FIGURE 1.
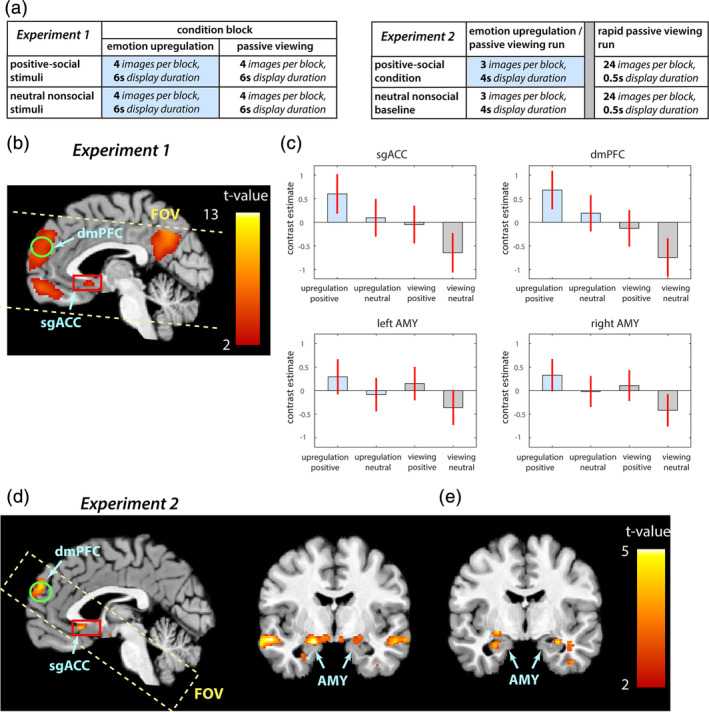
Brain activity related to positive‐social emotion upregulation and passive viewing conditions. (a) In both experiments, Tables resume the number and duration of displayed images per condition block. Blue boxes indicate conditions by which participants were instructed to effortfully upregulate. (b) For the first experiment (Koush et al., 2019), the dmPFC and sgACC activations are illustrated (the main effect of stimuli, positive > neutral), and (c) the bar plots for target ROIs showcase contrast estimates of modeled conditions with their 90% confidence intervals (CI, red error bars). Shaded blue color denotes emotion upregulation conditions. (d) For the second experiment, positive‐social emotion upregulation was associated with increased activity in the left amygdala, the dmPFC, and the sgACC, as compared to baseline. Passive or rapid passive viewing of neutral nonsocial stimuli conditions were selected as control (denoted baseline) conditions in the experimental runs. (b, d) The sgACC and dmPFC ROIs are highlighted as red rectangular box and green circle, respectively. (e) Rapid passive viewing of the depicted positive‐social situations was associated with bilateral amygdala activation, as compared to baseline. For illustration purposes, activation maps were thresholded (p < .005 unc., 10 voxels extent) and overlaid onto the structural template using Mango software (ric.uthscsa.edu/mango). AMY, amygdala; dmPFC, dorsomedial prefrontal cortex; sgACC, subgenual anterior cingulate cortex; FOV, field of view
The second experiment consisted of two alternating functional runs with periodic baseline and regulation conditions (Figures 1a and S1b). The effortful emotion upregulation run consisted of 10 blocks of effortful positive‐social emotion upregulation conditions interleaved with 11 blocks of neutral nonsocial passive viewing conditions (4 s image display duration, 3 images per block, 4.2 min total run duration). Thus, as compared to the first experiment, in the second experiment effortful emotion upregulation run, we slightly reduced the positive‐social stimuli valence and arousal, reduced the stimuli display duration (6 vs. 4 s), the number of stimuli per block (4 vs. 3 images per block), and presented baseline and condition blocks periodically to probe the feasibility of such a challenging experimental design. The rapid passive viewing run consisted of 10 blocks of positive‐social rapid passive viewing conditions interleaved with 11 blocks of neutral nonsocial rapid passive viewing conditions (0.5 s image display duration, 24 images per block, 4.2 min total run duration). The effortful positive‐social emotion upregulation, and positive‐social and neutral nonsocial passive viewing conditions were the same as for the first experiment described above. A critical manipulation was that the images in the rapid passive viewing run were presented more rapidly, and hence, were referred to as the rapid passive viewing conditions. This rapid passive viewing run was designed to limit participant self‐referential engagement with the content of presented scenes, with the goal to simulate an automatic emotion regulation process (Braunstein et al., 2017; Diano, Celeghin, Bagnis, & Tamietto, 2016). The latter renders the rapid passive viewing condition with 0.5 s image display duration different from the passive viewing condition in the first experiment with 6 s image display duration.
2.4. MRI data acquisition
Both experiments were performed on a 3 T MRI scanner (Trio Tim, Siemens Medical Solutions, Erlangen, Germany) at the Brain and Behavior Laboratory (University of Geneva). At the beginning of the scanning session, we acquired for each participant a T1‐weighted structural image (32 channel receive head coil, 3D MPRAGE, voxel size = 1 mm3 isotropic, flip angle α = 9°, TR = 1900 ms, TI = 900 ms, TE = 2.27 ms), and a double‐echo FLASH field map (TE1 = 5.19 ms, TE2 = 7.65 ms, voxel size = 3 × 3 × 2.2 mm3). For the first experiment, functional images were acquired with a whole‐brain single‐shot gradient‐echo T2*‐weighted EPI sequence with 345 repetitions (TR = 2050 ms, 32 slices volume, matrix size = 120 × 120, 2 mm3 isotropic voxel size, flip angle α = 75°, bw = 1.57 kHz/ pixel, TE = 35 ms, GRAPPA, iPAT = 3)(Koush et al., 2019).
The EPI protocol of the second experiment was configured to ensure the optimal sensitivity and precise segregation of the bilateral amygdala, dmPFC and sgACC brain areas (Weiskopf, Hutton, Josephs, & Deichmann, 2006; Weiskopf, Hutton, Josephs, Turner, & Deichmann, 2007). Thus, for the second experiment, functional images were acquired with a partial field of view (FOV) single‐shot gradient‐echo T2*‐weighted EPI sequence with 252 repetitions (TR = 1100 ms, 18 slices volume, matrix size = 120 × 120, 1.8 mm3 isotropic voxel size, flip angle α = 70°, bw = 1.54 kHz/ pixel, TE = 30 ms, GRAPPA, iPAT = 3). The partial FOV was chosen so that it covered our regions of interest (ROIs) given selected spatial and temporal resolution. In addition, data collection of the second experiment was optimized towards rapid data acquisitions typically required for real‐time fMRI and neurofeedback applications (Koush et al., 2013; Koush et al., 2017a; Koush et al., 2017b; Koush, Meskaldji, et al., 2017). This implies a compromise between the partial acquisition field of view (FOV) to cover target ROIs, relatively high spatial resolution (1.8 mm3 isotropic voxels) and short repetition time (TR = 1100 ms) as compared to the whole‐brain data acquired for the first experiment (TR = 2050 ms, isotropic voxel size of 2 mm3). The short TR also facilitates negligible EPI slice timing differences (Kiebel, Kloppel, Weiskopf, & Friston, 2007; Sladky et al., 2011).
Visual stimuli and instructions were displayed using a rectangular projection screen at the rear of the scanner bore with a mirror positioned within the head‐coil. All participants were instructed to breathe steadily and to remain as still as possible.
2.5. Activity analysis
For both experiments, data analyses were performed using SPM12 (Wellcome Trust Centre for Neuroimaging, Queen Square, London, UK). The fMRI analysis of the first experiment is described in detail elsewhere (Koush et al., 2019).
For the second experiment, a similar fMRI analysis was performed. The first 10 EPI volumes were discarded to account for T1 saturation effects. The remaining images were spatially realigned to the mean scan of each session, coregistered to the standard MNI structural template using DARTEL (Ashburner, 2007), corrected for geometric distortions (Jenkinson, 2003; Jezzard & Balaban, 1995), and smoothed with an isotropic Gaussian kernel with moderate 5 mm full‐width‐at‐half‐maximum (FWHM). The regressors were modeled as boxcar functions convolved with the canonical hemodynamic response function (HRF). For the subject‐level analysis, we specified general linear models (GLM) with regressors for positive‐social emotion upregulation and passive viewing conditions, and the neutral nonsocial passive and rapid passive viewing baseline conditions, respectively. The model included covariates derived from head movement parameters.
For the whole‐brain group level analysis of emotion upregulation and rapid passive viewing runs of both experiments, we performed a factorial ANOVA with a fixed factor “condition” and a random factor “subject”. Statistical maps were corrected for multiple comparisons using family‐wise error correction (FWE, p < .05). Small volume correction (SVC) at the peak‐level (FWE, p < .05) was also applied to the dmPFC using a sphere of 10 mm radius (Koush et al., 2019; Pichon, de Gelder, & Grezes, 2012; Poldrack et al., 2008) centered on independent coordinates defined with Neurosynth database ([2, 58, 32], MNI coordinates for association test, entry “emotion regulation”), to the amygdala using Talairach Daemon atlas (Lancaster et al., 2000), and to the sgACC using anatomically referenced rectangular 16 × 27 × 12 mm box centered at [0, 14, −6] (Beckmann et al., 2009; Palomero‐Gallagher et al., 2015). The SVC correction was based on the corresponding contrast maps (p < .005 unc.) with 10 voxels extent threshold. Recent findings suggest that cognitive reappraisal of social emotions might recruit the left amygdala more than the right, where additionally, the interaction between valence and social content is more pronounced in the right amygdala (Vrticka et al., 2011; Vrticka et al., 2012; Young et al., 2014). Hence, we tested for lateralization of amygdala activation during emotion upregulation conditions. To accomplish this, we compared individual hemisphere contrast images with their contralateral counterparts using paired t‐test in SPM12.
2.6. ROIs definitions and time‐series processing for DCM analysis
For both experiments, we considered four ROIs: the bilateral amygdala, the dmPFC, and the sgACC. The dmPFC and bilateral amygdala ROIs were the same as in the first experiment (Koush et al., 2019). Specifically, the dmPFC ROI was defined as a sphere with a 10 mm radius around the corresponding cluster center of gravity (main effect of stimuli, positive > neutral). The bilateral amygdala ROIs were defined anatomically based on the Talairach Daemon atlas (Lancaster et al., 2000) because it is a small region for which a spherical ROI would have likely included non‐amygdala voxels in close proximity. The centers of gravity were defined for the clusters of the thresholded activation maps (p < .005 unc.) as implemented in the Anatomy toolbox (Eickhoff et al., 2005). Based on the generic functional and anatomical labeling of the sgACC (Beckmann et al., 2009; Palomero‐Gallagher et al., 2015), the sgACC ROI was defined as 16 × 27 × 12 mm box centered at [0, 14, −6]. For each ROI, we extracted the first principal component of the individual local multivariate time‐series from both experimental groups using singular value decomposition (Friston, Rotshtein, Geng, Sterzer, & Henson, 2006; Stephan et al., 2010) and corrected for their low‐frequency drift, high‐frequency noise and spikes (Koush et al., 2019; Koush, Zvyagintsev, Dyck, Mathiak, & Mathiak, 2012).
2.7. Effective connectivity analysis
To assess directional connectivity as a function of positive‐social emotion upregulation, we used dynamic causal modeling (DCM)(Friston, Harrison, & Penny, 2003). DCM is a Bayesian framework that can model a functional brain network as a set of differential equations describing not only the architecture of the network (i.e., the ROIs and their directional connections, matrix A), but also the dynamic influences between ROIs within the network due to external inputs to the ROIs (matrix C) and due to contextual modulations of connections between the ROIs (matrix B). For external and modulatory inputs in corresponding DCM analyses, we analyzed either conditions of (rapid) passive viewing or engaging in positive emotion upregulation for positive‐social scenes. Note that external and modulatory inputs can reflect either the image presentation, the task, or both, being inherently linked to one another in our experimental design. By using Bayesian model comparison, DCM allows for quantitatively testing which model architecture and dynamics best explains the observed data (Penny, Stephan, Mechelli, & Friston, 2004). DCM also allows for estimating the individual model parameters and can thus shed light on the strength and impact of connectivity changes during an experiment.
We performed a hierarchical DCM analysis, in that we first estimated all possible model alternatives and then applied family‐level inference procedures to investigate which general model structure underlay the emotion upregulation condition. To generalize the results to the population, we used a random effect (RFX) approach for DCM analysis (Stephan, Penny, Daunizeau, Moran, & Friston, 2009). We estimated the expected posterior model family probability and the model family exceedance probability, which indicate the probability how likely is that a specific model family generated the data of a randomly chosen subject and the probability that a model family explains the data better than any other model family, respectively (Stephan et al., 2009; Stephan et al., 2010).
Thus, to investigate which general model structure underlays effortful and passive viewing emotion regulation processes, we partitioned the model space in subsets of five model families that differed in the connectivity pattern between our four ROIs (Figure 2a). The first model family contained all models where there was a connection between the amygdala and the dmPFC as well as between the dmPFC and the sgACC (112 models); the second family contained all models where there was a connection between the amygdala and the sgACC as well as between the sgACC and the dmPFC (112 models); the third family contained all models with a direct connection between only the amygdala and the dmPFC (12 models); the fourth family contained all models with a direct connection between only the amygdala and the sgACC (12 models), and finally the fifth family contained all models with direct connections between all ROIs (448 models). Due to a large number of possible models within each family, we did not include direct connections between the left and right amygdala and took all possible combinations of modulatory inputs and external inputs for each model family into account. We assumed bilateral amygdala connections, and that external and modulatory inputs affect both hemispheres in the same way (Koush et al., 2019). To investigate model parameters of the winning model family, we applied Bayesian model averaging (BMA). BMA computes a weighted average of each model parameter within the model family, where the weighting depends on the evidence for each of the contributing models, that is, the posterior probability (Penny et al., 2010). We used DCM as implemented in SPM12.
FIGURE 2.
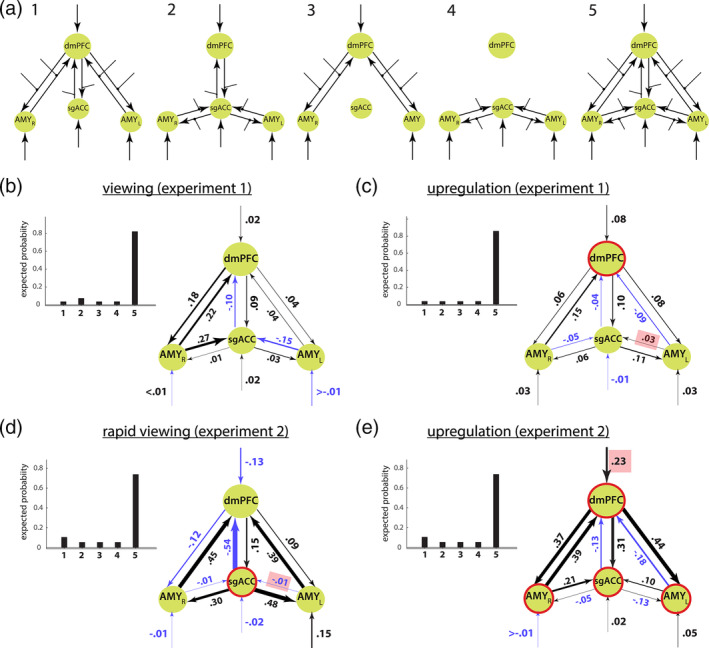
Estimation of general network structures. (a) All models consisted of four ROIs, reciprocal connections between the ROIs (arrows between nodes), modulatory inputs (lines with dotted end points), and external inputs into the ROIs (arrows). For family‐level inference, the model space was partitioned into five subsets with different connectivity patterns between the bilateral amygdala, the dmPFC, and the sgACC. For the first experiment, expected and exceedance family probabilities revealed that the fully connected model dominated during positive‐social (b) passive viewing and (c) emotion upregulation (6 s stimuli display duration) conditions. For the second experiment, expected and exceedance family probabilities showed the same dominance of the fully connected model family during positive‐social (d) rapid passive viewing (0.5 s stimuli display duration) and (e) emotion upregulation (4 s stimuli display duration) conditions. (b–e) Blue arrows highlight negative values. (c–e) Red circles around network nodes highlight corresponding experiment and condition brain areas that showed a significant correlation with psychometric scores, as well as red rectangles highlight the connectivity parameters that showed a significant correlation with psychometric scores (Figure 3). For simplicity, the reciprocal connections (matrix A in DCM) and the modulatory inputs (matrix B in DCM) between the ROIs are added and depicted as arrows between the ROIs. The BMA parameters are indicated next to the corresponding connections and illustrated by the proportional arrow thickness. The external input can reflect either the image presentation, the task, or both, being inherently linked to one another in our experimental designs. AMY, amygdala; dmPFC, dorsomedial prefrontal cortex; sgACC, subgenual anterior cingulate cortex; L, left; R, right
2.8. Correlations between psychometric scores and functional activity and connectivity
In addition to the first experiment data from established standardized psychometric questionnaires and inventories (Koush et al., 2019), we collected similar data in the second experiment to explore differences in individual emotion upregulation habits and in psychometric scores that might characterize changes in brain activity and connectivity during emotion regulation. Specifically, participants were asked to complete the behavioral inhibition system (BIS) and behavioral activation system reward (BAS‐R), drive (BAS‐D), fun‐seeking (BAS‐F) scales (Carver & White, 1994), the Emotion Regulation Questionnaire (ERQ, Gross & John, 2003), the White Bear Suppression Inventory (WBSI, Wegner & Zanakos, 1994) and the State–Trait Anxiety Inventory (STAI‐T, Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) after the experiment. We then correlated psychometric scores with percent signal changes between emotion regulation conditions and corresponding reference (baseline) conditions (Figure 1a) using two‐tailed Spearman correlations. Percent signal changes were extracted separately for each ROI using GLM beta values as provided by SPM12. We also computed two‐tailed Spearman correlations between psychometric scores and effective connectivity parameters, as well as cross‐correlations between psychometric scores. The statistical significance was corrected for multiple comparisons using false‐discovery‐rate (FDR, q < .05) applied to the number of ROIs (four), number of effective connectivity parameters (14 given added matrices A and B), or questionnaires/inventories assessed (eight).
3. RESULTS
We found complementary results in the first and second experiments that suggest the engagement of the sgACC in positive‐social emotion regulation. Below we report activation results between conditions within both experiments, while focusing primarily on the separate DCM analyses revealing model structure between the dmPFC, sgACC and amygdala.
3.1. Brain activation associated with emotion upregulation and passive viewing
For the first full factorial experiment, the interaction between task and stimuli was characterized by activation in the temporoparietal junction (TPJ), and the main effect of task (upregulation > viewing) was characterized by activation in the superior frontal gyrus (SFG) (Table 1, Figure 1b,c) (Koush et al., 2019). The main effect of the stimulus (positive > neutral) was additionally characterized by widespread activations, highlighting the TPJ, dmPFC, ventromedial PFC (vmPFC) and bilateral amygdala, among others. For the first experiment, we additionally contrasted the positive‐social emotion upregulation condition (imagining the experience of those situations) to the positive‐social passive viewing condition, which revealed activation in the SFG (Table 1, upregulate positive > viewing positive), and positive‐social emotion upregulation condition in comparison to the neutral nonsocial passive viewing condition that revealed activation in the dmPFC (Table 1, upregulate positive > viewing neutral). The contrast of the positive‐social passive viewing condition and the neutral nonsocial passive viewing condition mirrored the widespread activation associated with the main effect of stimuli, among others, in the dmPFC, sgACC and bilateral amygdala (Table 1, viewing positive > viewing neutral).
TABLE 1.
Brain areas related to experimental conditions
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Condition/contrast | Area | x | y | z | t‐value | p‐value | |
| Experiment 1 | |||||||
| Interaction task × stimuli | TPJ | −58 | −56 | 26 | 4.00 | .014+ | |
| Main effect of task (upregulation > viewing) | SFG | −14 | 56 | 36 | 3.91 | .018+ | |
| Main effect of stimuli (positive > neutral) | AMY | −18/18 | −8/−6 | −14/−14 | 6.28/6.94 | .002/<.001 | |
| dmPFC | 6 | 50 | 34 | 5.48 | .025 | ||
| sgACC | 2 | 12 | −8 | 4.70 | .002+ | ||
| TPJ | −42/44 | −54/−56 | 24/18 | 3.76/10.28 | .025+/<.001 | ||
| vmPFC | 4 | 58 | −12 | 6.29 | .002 | ||
| PVC | −22/28 | −102/−96 | −2/−6 | 9.37/10.11 | <.001/<.001 | ||
| FFA | −42/42 | −52/−52 | −20/−18 | 9.52/13.43 | <.001/<.001 | ||
| Precuneus | 2 | −58 | 40 | 8.42 | <.001 | ||
| STS | −68/54 | −12/−10 | −12/−12 | 5.64/6.44 | .016/.001 | ||
| V5/MT | −48/48 | −72/−74 | 12/4 | 10.93/16.96 | <.001/<.001 | ||
| IFG | 36 | 16 | 26 | 5.33 | .041 | ||
| Upregulate positive > viewing positive | SFG | −8 | 50 | 40 | 3.82 | .023+ | |
| Upregulate positive > viewing neutral | dmPFC | −2/6 | 56/50 | 24/36 | 4.06/4.64 | .002+/.002+ | |
| Viewing positive > viewing neutral | AMY | −18/20 | −4/−4 | −14/−14 | 4.91/6.43 | .001+/.001 | |
| dmPFC | 6 | 50 | 32 | 4.83 | .001+ | ||
| sgACC | 0 | 12 | −8 | 3.80 | .030 | ||
| TPJ | 42 | −56 | 18 | 9.35 | <.001 | ||
| vmPFC | 4 | 58 | −12 | 4.60 | .002+ | ||
| PVC | −24/28 | −100/−96 | −2/−6 | 6.61/ 7.86 | .001/<.001 | ||
| FFA | −42/42 | −50/−54 | −20/−18 | 9.43/12.40 | <.001/<.001 | ||
| Precuneus | 4 | −56 | 40 | 5.59 | .019 | ||
| STS | 54 | −6 | −12 | 5.48 | .027 | ||
| V5/MT | −50/50 | −74/−74 | 12/4 | 10.21/14.82 | <.001/<.001 | ||
| IFG | 38 | 12 | 26 | 5.50 | .025 | ||
| Experiment 2 | |||||||
|
Upregulation positive > viewing neutral (baseline) |
AMY | −18 | −7 | −16 | 6.10 | .008 | |
| dmPFC | −4/2 | 63/58 | 27/29 | 5.41/4.67 | .001+/.008+ | ||
| sgACC | 2 | 22 | −9 | 4.49 | .016+ | ||
| STS | −54 | −9 | −14 | 5.66 | .027 | ||
|
Rapid viewing positive > rapid viewing neutral (baseline) |
AMY | −23/18 | −9/0 | −9/−16 | 5.46/3.81 | .045/.038+ | |
Note: Reported are the main peak coordinates of areas that survived whole‐brain FWE correction (p < .05, voxel‐level inference). +Plus denotes activity peaks that survived SVC statistics (p < .05, peak‐level FWE). Note that the sgACC activity peak also survived the whole‐brain FDR correction (Experiment 1, main effect of stimuli) (Koush et al., 2019).
Abbreviations: AMY, amygdala; dmPFC, dorsomedial prefrontal cortex; FFA, fusiform face area; IFG, inferior frontal gyrus; MT, middle temporal gyrus; PVC, primary visual cortex; SFG, superior frontal gyrus; STS, superior temporal sulcus; TPJ, temporoparietal junction; vmPFC, ventromedial prefrontal cortex.
For the second experiment, we identified brain areas related to positive‐social emotion upregulation in comparison to the neutral nonsocial passive viewing (baseline) condition (Table 1, upregulation positive > viewing neutral), and brain areas recruited during rapid passive viewing of images depicting positive‐social situations in comparison to the neutral nonsocial rapid passive viewing (baseline) condition (Table 1, rapid viewing positive > rapid viewing neutral). During the positive‐social emotion upregulation condition, activation was found in the left amygdala, superior temporal sulcus (STS), and most critically, in both the sgACC and dmPFC, as compared to the control baseline (Table 1, Figure 1d). During the positive‐social rapid passive viewing condition, the bilateral amygdala was significantly active as compared to the control baseline. Significant dmPFC and sgACC activation was not observed during the rapid passive viewing condition as compared to the control baseline, which suggests a preferential involvement of both areas in effortful positive‐social emotion upregulation (Table 1, Figure 1e). Through laterality analyses during positive social emotion upregulation conditions, we observed higher activation in the left amygdala as compared to the right amygdala (peak at [−16–2 ‐13], SVC FWE, p = .037) during this condition as compared to baseline.
3.2. Effective connectivity underlying positive‐social emotion regulation
The key question investigated in our study concerned how positive‐social emotion regulation modulated the functional interactions between the subgenual ACC, the prefrontal cortex, and limbic brain areas. Using data of both experiments, analyzed separately, we determined which model architecture linking the sgACC with the amygdala and dmPFC could best explain the pattern of fMRI data during the upregulation of positive‐social emotions, and how this may differ from passive viewing conditions with the same or more rapid stimuli display rate. To this aim, we partitioned the model space in subsets of five possible model families that differed in the connectivity pattern between the bilateral amygdala, the dmPFC, and the sgACC (Figure 2a). Each model corresponded to different structural and functional hypotheses about the underlying connectivity between these regions: model families assumed direct interactions of the amygdala with either dmPFC or sgACC or both (Figure 2a). Using a Bayesian approach, we then calculated for each model family the expected (P) and exceedance (Pe) probability, and estimated winning model family parameters using Bayesian model averaging (BMA, Figure 2b–e).
For the first experiment, we found that the fully connected fifth model family explained the data much better than other concurrent model families during emotion upregulation (Figure 2c, winning model family expected probability p = .86) and during passive viewing (Figure 2b, winning model family expected probability p = .82). For the second experiment, our results revealed the same fifth winning model family dominated during emotion upregulation (Figure 2e, winning model family expected probability p = .74) and during passive viewing with rapid stimuli presentation (Figure 2d, winning model family expected probability p = .74). All exceedance probabilities for winning model families where > .99.
The BMA analysis revealed that during positive‐social emotion upregulation condition of both experiments, the modeled network parameters were similar and driven by applied emotion upregulation as indicated by dmPFC response to the emotion upregulation, and positive connection strength from the dmPFC onto the sgACC and bilateral amygdala (Figure 2c,e). Conversely, during emotion upregulation, the sgACC displayed negative connections onto the dmPFC in both experiments and negative connections on the bilateral amygdala in the second experiment (Figure 2e). This data may suggest that the sgACC is engaged in interactions with the bilateral amygdala and the dmPFC, and that the emotion upregulation condition induces distinct recruitment of the dmPFC, the sgACC, and amygdala responses. Interestingly, the emotion upregulation network was more strongly driven by the experimental conditions of the second experiment as indicated by their stronger connection strengths (Figure 2e). In addition, the external input on the sgACC and connections between the sgACC and bilateral amydala showed a slightly different pattern of results when comparing emotion upregulation conditions of both experiments (Figure 2c,e). This could be attributed to the sign modulation, that is, negative input onto the sgACC during emotion regulation of the first experiment was conveyed onto bilateral amygdala without changing the sign (Figure 2c), while positive external input on the sgACC was negatively conveyed onto the bilateral amygdala during emotion regulation of the second experiment (Figure 2e). Similar sign modulation was observed for the external input on the right amygdala and its influence on the sgACC during the emotion upregulation of both experiments (Figure 2c,e, emotion upregulation). During the passive viewing condition in the first experiment (Figure 2b), we did not observe a substantial modulation of model network parameters by external passive viewing inputs, but rather a positive interaction between network nodes and negative modulation of the dmPFC by the sgACC. Interestingly, during rapid passive viewing within the second experiment (Figure 2d), the connection strengths substantially increased, with the sgACC exerting positive modulation on the bilateral amygdala, and negative modulation on the dmPFC, in addition to bilateral amygdala exerting positive modulation on the dmPFC. These results suggest that the emotion upregulation condition induced distinct interactions between the dmPFC, sgACC and amygdala as compared to passive viewing when effortful engagement is attenuated, and that these distinct interactions are more evident when effortful engagement is limited due to shortened stimuli duration, that is, the network upregulation is more automated.
3.3. Correlations between psychometric scores and functional activity and connectivity
In the first experiment, we found that emotion suppression scores (ERQ‐S) correlated negatively to percent signal change between positive emotion upregulation and neutral passive viewing conditions in the dmPFC (Figure 3a, rho = −.52, adjusted p = .045, FDR correction across ROIs, q < .05) (Koush et al., 2019). Interestingly, in the first experiment, we also found that intrusive thought suppression scores (WBSI) correlated positively with the connectivity strengths from the left amygdala onto the sgACC during upregulation condition (Figure 3b, rho = .69, adjusted p = .004; FDR correction across connectivity parameters, q < .05).
FIGURE 3.
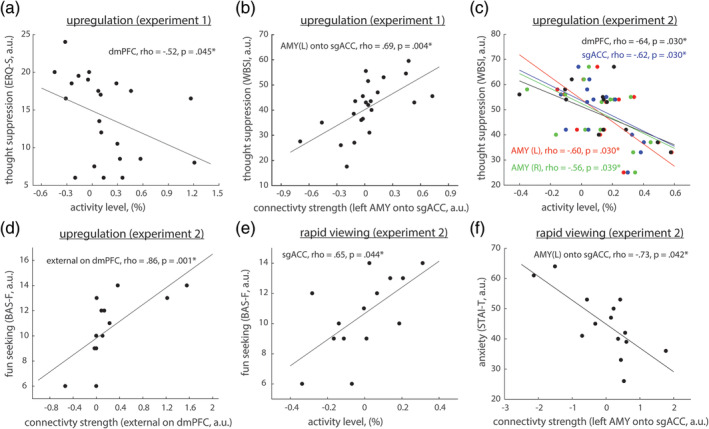
Correlation between the psychometric scores and observed activity and connectivity patterns. In the first experiment, during positive‐social emotion upregulation condition, we observed (a) that the tendency to suppress emotions (ERQ‐S) was negatively correlated with activity levels in the dmPFC (Koush et al., 2019) and (b) that the tendency to suppress intrusive thoughts (WBSI) was positively correlated with the connectivity strengths from the left amygdala onto the sgACC. In the second experiment, during positive‐social emotion upregulation condition, we observed (c) that the tendency to suppress intrusive thoughts correlated negatively with the activity levels in the dmPFC, the sgACC and bilateral amygdala, and (d) that fun seeking scores (BAS‐F) correlated positively with the external connectivity strengths on the dmPFC. In the second experiment, during positive‐social emotion rapid passive viewing condition, we observed (e) that the fun seeking scores correlated positively with the activity levels in the sgACC, and (f) the negative correlation between the anxiety scores and the connectivity strengths from the left amygdala onto the sgACC. In the first experiment, we did not observe significant correlations between psychometric scores and activity levels and connectivity strengths during positive‐social passive viewing conditions. *Survived FDR correction for multiple comparisons
In the second experiment, we found that intrusive thought suppression scores (WBSI) correlated negatively with percent signal changes between positive emotion upregulation and neutral passive viewing conditions in all four ROIs (Figure 3c, Table S1; dmPFC: rho = −.64, adjusted p = .030; sgACC: rho = −.62, adjusted p = .030; left amygdala: rho = −.60, adjusted p = .030; right amygdala: rho = −.56, adjusted p = .039; FDR correction across ROIs, q < .05). Thus, individuals with a higher tendency to suppress emotions (ERQ‐S) and intrusive thoughts (WBSI) exhibited weaker upregulation effects within dmPFC and all these brain regions, respectively, yet exhibited higher modulation of the sgACC by the left amygdala. Higher WBSI scores also suggest worse emotion regulation capabilities (Wegner & Zanakos, 1994). During positive‐social emotion upregulation in the second experiment, we also found a positive correlation between fun‐seeking scores (BAS‐F) and external connectivity strengths on the dmPFC (Figure 3d, rho = .86, adjusted p = .001; FDR correction across connectivity parameters, q < .05), which implies that individuals with a higher desire for new rewards and rewarding events exhibited higher external modulation of the dmPFC.
In the first experiment, we did not find significant correlations between psychometric scores and activity levels and connectivity strengths during positive‐social passive viewing conditions. However, in the second experiment, we found that fun‐seeking (BAS‐F) scores positively correlated with the sgACC percent signal changes between rapid positive‐social and rapid neutral passive viewing conditions (Figure 3e, rho = .65, adjusted p = .044, FDR correction across ROIs, q < .05), as well as anxiety scores (STAI‐T) negatively correlated with the connectivity strength from the left amygdala onto the sgACC (Figure 3f, rho = −.73, adjusted p = .042; FDR correction across connectivity parameters, q < .05).
Cross‐correlations between questionnaires in the first experimental group revealed a significant positive correlation between reward responsiveness (BAS‐R) and fun seeking (BAS‐F) scores (rho = .64, adjusted p = .025, FDR corrected across questionnaires, q < .05). In the second experimental group, the same analysis revealed a significant positive correlation between trait anxiety (STAI‐T) and intrusive thought suppression (WBSI) scores (rho = .88, adjusted p < .001, FDR corrected across questionnaires, q < .05). No effects were found in other cross‐correlations of the questionnaires.
4. DISCUSSION
Using fMRI and DCM with Bayesian model selection procedures, we investigated the engagement of the sgACC within the dmPFC‐amygdala affective circuit during positive‐social self‐referential emotion regulation. With both experiments, we found that positive‐social emotion upregulation was associated with activation in the dmPFC, sgACC and bilateral amygdala, and with an increase of direct and indirect top‐down connections from the dmPFC on bilateral amygdala via the sgACC. During rapid passive viewing runs where the stimuli were presented briefly as to limit the effortful engagement in the self‐referential emotion upregulation task, the sgACC and dmPFC were not significantly activated as compared to baseline conditions, yet were characterized by distinct connectivity patterns. We also explored correlations between neuroimaging measures and individual psychometric scores.
4.1. dmPFC‐sgACC‐amygdala circuit activity
Our results revealed that engaging oneself in upregulation of self‐referential positive‐social emotions activated target brain regions similar to those that are recruited when regulating negative and positive emotions reported elsewhere (Beauregard, Levesque, & Bourgouin, 2001; Herwig et al., 2007; Kim & Hamann, 2007; Koush et al., 2019; Ochsner et al., 2012; Vrticka et al., 2012; Zotev et al., 2013; Zotev et al., 2018; Zotev, Phillips, Yuan, Misaki, & Bodurka, 2014), particularly the dmPFC, sgACC, and the bilateral amygdala (Table 1, Figure 1). This is well in line with the suggested role of the sgACC in transferring emotional feedback from the limbic system to higher‐order cognitive structures (Azab & Hayden, 2018; Banks et al., 2007; Disner et al., 2011; Greicius et al., 2007; Lockwood & Wittmann, 2018; Rudebeck et al., 2014; Stevens et al., 2011; Wager, Barrett, et al., 2008; Wager, Davidson, et al., 2008). For instance, the sgACC has been found to be activated when participants were asked to modulate negative emotional responses (Banks et al., 2007; Wager, Davidson, et al., 2008) and attend and rate core affective feelings (Lindquist, Wager, Kober, Bliss‐Moreau, & Barrett, 2012). Indeed, positive emotion upregulation via real‐time fMRI neurofeedback of the amygdala has previously been found to be associated with increased activation in the rostral ACC and dmPFC (Koush, Meskaldji, et al., 2017; Zotev et al., 2013). Also on balance with previous findings, in both experiments, we found that the dmPFC displayed increased activation when participants cognitively upregulated emotions associated with positive‐social situations. In particular, dmPFC has been shown to be implicated in the upregulation of negative (Ochsner et al., 2004) and positive (Ochsner & Gross, 2005) emotions (for review, see [Ochsner & Gross, 2005; Ochsner et al., 2012]).
Interestingly, activation within the sgACC did not always mirror that of the dmPFC, which was prominently involved in the effortful emotion upregulation processes of both experiments. The dmPFC and sgACC co‐activations were observed (a) in the main effect of stimuli in the first experiment, (b) during passive viewing of the positive‐social stimuli condition as compared to the passive viewing of neutral nonsocial stimuli condition in the first experiment, and (c) during the positive‐social emotion upregulation condition as compared to the passive viewing of neutral nonsocial stimuli condition in the second experiment. However, we did not observe sgACC activation during the positive‐social emotion upregulation condition as compared to the passive viewing of neutral nonsocial stimuli condition in the first experiment, and the sgACC and dmPFC activations during rapid passive viewing of positive‐social emotion stimuli in comparison to the rapid passive viewing of neutral nonsocial scenes. These results support previous findings that link the sgACC with the engagement of effortful positive‐social emotion upregulation, and together with other findings, suggests a potential role of the sgACC as a gatekeeper between cognitive control areas (involving dmPFC) and bilateral amygdala (Disner et al., 2011; Greicius et al., 2007; Palomero‐Gallagher et al., 2015). Overall, brain areas that we found to be associated with positive emotion regulation largely overlap with those involved in the regulation of negative emotions in previous studies (Herwig et al., 2007; Kim & Hamann, 2007; Ochsner et al., 2012; Vrticka et al., 2012).
Identified activity in dmPFC, sgACC, and bilateral amygdala is also consistent with their relevance to social cognition. For instance, it has been suggested (a) that amygdala activation is independent of valence for social images but is dependent on valence of nonsocial images, and (b) that the amygdala is more strongly activated for neutral social than nonsocial stimuli (Vrticka et al., 2012). Similarly, dmPFC has been shown to be more active in social as compared to nonsocial conditions regardless of the emotional content (Vrticka et al., 2012), and has an integral role in emotion regulation and social cognition (Bzdok et al., 2013; Ochsner et al., 2012; Reeck et al., 2016). Finally, the sgACC has also been shown to be implicated in both emotion regulation (Banks et al., 2007; Wager, Barrett, et al., 2008; Wager, Davidson, et al., 2008), and social cognition (Lockwood & Wittmann, 2018; Palomero‐Gallagher et al., 2015).
4.2. dmPFC‐sgACC‐amygdala circuit connectivity
Further evidence supporting the gatekeeping role of the sgACC was provided by our analysis of connectivity and dynamic causal interactions between the ROIs, where a network hierarchy including the sgACC as a relay between the bilateral amygdala and dmPFC was found to be the most likely architecture during the upregulation condition (Figure 2, model family 5). The brain network architecture that emerged during the emotion upregulation condition involved the dmPFC as the predominant entry point for external inputs into the network, which then was conveyed to the bilateral amygdala in a top‐down fashion via direct dmPFC‐amygdala connections and indirectly via the sgACC. Although passive viewing and rapid passive viewing conditions of positive‐social stimuli as compared to the corresponding (rapid) passive viewing of neutral nonsocial stimuli conditions revealed distinct dmPFC and sgACC activations (Table 1), these areas were characterized with strong intra‐network connections (Figure 2b,d). In particular, during rapid passive viewing, the sgACC exerted strong positive modulation on the bilateral amygdala, and strong negative modulation on the dmPFC, in addition to the bilateral amygdala exerting strong modulation on the dmPFC (Figure 2d), which suggests more prominent engagement of the sgACC and amygdala into the automated network upregulation processes.
Indeed, the sgACC appears to constitute a key station relaying bottom‐up emotion information from low‐level areas to the dmPFC, and vice versa conveying top‐down signals from the latter to the amygdala. Previous studies are consistent with a major role of the sgACC during emotion regulation, where this area is associated with the top‐down control of amygdala reactivity (Banks et al., 2007; Ghashghaei, Hilgetag, & Barbas, 2007; Wager, 2008), and is further implicated in negative mood and depressive symptoms (George et al., 1995). Importantly, previous studies have found that the up‐regulation of emotions has been shown to increase effective coupling between the amygdala, ACC, and dmPFC, which was correlated to the individual success of regulation (Morawetz, Bode, Baudewig, & Heekeren, 2017).
The sgACC has been shown to be hyperactive in patients with depression and anxiety disorders (Drevets & Savitz, 2008; Gotlib et al., 2005; Laxton et al., 2013; Ressler & Mayberg, 2007), where successful treatment and recovery of mood disorders has been associated with a normalization of this hyperactivity (Escolano et al., 2014; Mayberg et al., 2005; Nobler et al., 2001; Ressler & Mayberg, 2007; Zotev et al., 2011). Furthermore, normalization of this neural architecture involving hyperactive limbic regions (including the amygdala) and hypoactive emotion regulation areas (including the dmPFC) is also a key target for interventions in trauma‐related disorders (Doll et al., 2016; Koch et al., 2016; Nicholson et al., 2017; Nicholson et al., 2018; Zotev et al., 2018). Interestingly, the sgACC is intimately connected to regions involved in emotion processing and interoception, such as the amygdala, the hypothalamus (Beckmann et al., 2009; Freedman, Insel, & Smith, 2000; Johansen‐Berg et al., 2008), as well as the hippocampus, insula, orbitofrontal cortex, and both the dorsolateral and dorsomedial PFC (Beckmann et al., 2009; Carmichael & Price, 1996; Craig, 2011; Johansen‐Berg et al., 2008; Vogt & Pandya, 1987). This pattern of connectivity is compatible with a role of the sgACC as a gatekeeper between cognitive control areas in the dmPFC and lower‐level areas of the limbic system responsible for the elicitation of emotional responses (Disner et al., 2011; Greicius et al., 2007; Palomero‐Gallagher et al., 2015).
Overall, our results suggest that the dmPFC and sgACC are important hubs for cognitively engaging in positive‐social emotions in a self‐relevant, first‐person perspective, which substantially extends recent findings in positive‐social emotion regulation (Kim & Hamann, 2007; Koush et al., 2019; Ochsner et al., 2012; Vrticka et al., 2012). This is in line with the proposal that the dmPFC may be crucially involved in evaluating social information (Amodio & Frith, 2006; Bzdok et al., 2013; Gallagher & Frith, 2003; Kampe, Frith, & Frith, 2003; Lieberman, 2007; Vrticka et al., 2012) and situating oneself in social contexts (Amodio & Frith, 2006; Gallagher & Frith, 2003; Kampe et al., 2003; Lieberman, 2007; Northoff & Bermpohl, 2004). However, prefrontal regions implicated in emotion upregulation and reappraisal are also commonly involved in other nonemotional tasks and may subserve more general cognitive control processes (Braunstein et al., 2017; Etkin et al., 2011; Ochsner et al., 2012).
4.3. Amygdala activity and connectivity
In our study, the bilateral amygdala was active during positive‐social passive viewing and emotion upregulation conditions (i.e., the main effect of the stimulus), but did not show a functional lateralization within this main effect (Koush et al., 2019). However, during the emotion upregulation condition in the second experiment, activity in the bilateral amygdala shifted predominantly toward the left side, as indicated by our laterality analysis. This laterality might reflect a putative hemispheric difference in encoding the valence of emotional experiences, with a principle role of the left amygdala in positive emotions (Kim & Hamann, 2007; Lanteaume et al., 2007; Sergerie, Lepage, & Armony, 2006; Vrticka et al., 2012). This result also adds to the recent findings that suggest that cognitive reappraisal of social emotions might recruit the left amygdala more than the right (Vrticka et al., 2012; Young et al., 2014). This would, in turn, be consistent with models proposing that the left amygdala is involved in elaborated cognitive information processing and the manipulation of affective representations with linguistic content (Glascher & Adolphs, 2003; Phelps et al., 2001). We therefore hypothesize that the observed shift in activity toward the left amygdala may represent an increased cognitive evaluation of the social scene stimuli due to positive upregulation, however, further research is needed to test more directly these speculations. Our results also showed that during positive‐social emotion upregulation conditions in both experiments, the amygdala received top‐down modulating inputs directly from the dmPFC, as well as indirectly via the sgACC (Figure 2). This is generally in accordance with previous work showing that the amygdala plays a major role in the detection and encoding of emotional information, in which the amygdala is modulated by cognitive reappraisal strategies (Banks et al., 2007; Goldin, Mcrae, Ramel, & Gross, 2008; Ochsner & Gross, 2005; Vrticka et al., 2012; Wager, Davidson, et al., 2008).
4.3.1. Linking psychometric scores with observed activity and connectivity patterns
The modulation of functional activity within the dmPFC‐sgACC‐amygdala circuit was mediated by individual characteristics, as indexed by standard psychometric scores which may influence emotion regulation capacities (Figure 3). In the first experiment, the higher emotion suppression scores were found to predict lower activity in the dmPFC during positive‐social emotion upregulation conditions (ERQ‐S, Koush et al., 2019) and higher positive connectivity strengths from the left amygdala onto the sgACC (WBSI). In the second experiment, the higher tendency to suppress intrusive thoughts (WBSI) was found to predict the lower activity in bilateral amygdala, the sgACC, and dmPFC during positive‐social emotion upregulation conditions. This indicates that individuals with higher tendencies to suppress emotions and intrusive thoughts achieve less effective recruitment of these areas in positive‐social emotion upregulation. During rapid passive viewing conditions in the second experiment, we found that trait anxiety was negatively correlated with connectivity strength from the left amygdala onto the sgACC. Taken together with a positive correlation between intrusive thought suppression and anxiety scores in the second experiment, these findings suggest that more anxious individuals, with a greater tendency to suppress emotions and intrusive thoughts, were likely to display decreased amygdala, dmPFC, sgACC activity, and stronger modulation of the sgACC by the left amygdala during effortful emotion upregulation. In addition, individuals with lower anxiety scores were likely to display higher modulation of the sgACC by the left amygdala during more automated emotion regulation processes. In the second experiment, we also found that individuals with a higher desire for new rewards and rewarding events exhibited higher positive external modulation of the dmPFC during positive‐social emotion upregulation condition, and higher sgACC activity during the rapid passive viewing condition, although the sgACC did not reach the whole‐brain group‐level significance in this condition (Table 1, rapid viewing positive > rapid viewing neutral). These findings are consistent with an important role of the sgACC in emotion processing and mood (Drevets & Savitz, 2008; Gotlib & Hamilton, 2008; Wager, Davidson, et al., 2008) as well as self‐referential representations (Lockwood & Wittmann, 2018). Furthermore, these results add to the view that neural correlates of cognitive control and emotion regulation are reduced in anxiety disorders (Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010).
4.3.2. Experimental design implications
We applied experimental design settings which could be useful for designing future emotion regulation experiments. In the first experiment, we used emotion regulation conditions with conventional stimuli display duration (6 s) and the number of stimuli per block (4 images, 24 s block duration) (Figure S1a). However, in the second experiment, we tested the feasibility of two functional run designs with reduced stimuli display duration. Here, in positive‐social emotion upregulation runs, we reduced image display duration to 4 s and the number of images per block to 3, which resulted in a twofold decrease in condition block duration as compared to the first experiment (24 vs. 12 s) (Figure S1b). This shortening was done to check the feasibility of the periodic functional run design with relatively short experimental blocks. Our results validated the feasibility of such periodic functional runs with reduced valence and arousal of positive‐social stimuli, which could be of specific benefit for real‐time fMRI and neurofeedback paradigms targeting effortful positive‐social emotion upregulation with the dmPFC, bilateral amygdala and sgACC engagement (Koush, Meskaldji, et al., 2017).
As compared to effortful upregulation conditions with the same image display duration, the positive‐social passive viewing condition could be considered an attenuated emotion upregulation condition which does not exclude activation of the dmPFC and sgACC. Therefore, in rapid passive viewing runs in the second experiment, we further reduced stimuli duration to limit the participants' engagement with the content of presented scenes (0.5 s image display duration, 24 images per block, 12 s block duration) (Figure S1b). We showed that sgACC and dmPFC were not significantly activated in rapid passive viewing runs as compared to positive‐social emotion upregulation runs (Table 1). However, rapid passive viewing of positive‐social stimuli was still associated with activations in bilateral amygdala, suggesting that the role of self‐referential engagement with the content of presented scenes in emotion regulation processes could be reduced and that automatic emotion regulation processes could be stimulated (Braunstein et al., 2017; Diano et al., 2016). Thus, rapid passive viewing runs with minimized participant engagement in the context of presented scenes could be recommended for checking the feasibility and sensitivity of MR sequences vulnerable to gradient dropouts in bilateral amygdala (Weiskopf et al., 2006). The rapid passive viewing condition could be further studied in different emotion regulation domains and clinical populations.
4.4. Clinical implications
The brain circuits identified in our study might particularly be important for the mechanisms of projecting oneself in emotional scenarios with a self‐relevant, first‐person perspective. Such first‐person projections are important in constituting an effective strategy within therapeutic settings (Holmes, Coughtrey, & Connor, 2008; Philippot & Segal, 2009; van der Velden et al., 2015). Appraisals of self‐relevance also constitute a key ingredient of emotional experiences that determine their intensity irrespective of valence and emotion type (Sander, Grandjean, & Scherer, 2005; Scherer, 1982). Moreover, self‐referential processing also recruits medial prefrontal, sgACC and amygdala regions that partly overlap with those associated with emotion regulation (Buckner & Carroll, 2007; Koush et al., 2019; Reeck et al., 2016; Zaki & Ochsner, 2012), and is implicated in mood and trauma‐related disorders (Disner et al., 2011; Drevets, 2001; Drevets & Savitz, 2008; Wagner, Schachtzabel, Peikert, & Bar, 2015; Wu et al., 2016; Yehuda et al., 2015; Zaki & Ochsner, 2012).
Critically, the current study informs neurobiological treatment interventions for psychiatric disorders, for example, real‐time fMRI neurofeedback studies in PTSD and depression that aim to regulate the amygdala activity (Nicholson et al., 2018; Nicholson, Friston, et al., 2017; Nicholson, Rabellino, et al., 2017; Young et al., 2014; Young et al., 2017; Zotev et al., 2013; Zotev et al., 2018). Results from the current study suggest that optimal upregulation may be achieved by modulating the amygdala from both the dmPFC and the sgACC, when using effective connectivity as a feedback signal. Indeed, Young and colleagues have previously shown therapeutic effects in depressed patients by upregulating the amygdala during positive emotion regulation in a randomized clinical trial, which also specifically recruited areas of the ACC. Future studies might extend our work to patients suffering from anxiety, mood, and trauma‐related disorders, thereby helping to identify the brain dynamics that prevent positive emotions and affect dysregulation in these patients. Brain‐based measures of these functional interactions might also serve to evaluate the success or failure of specific treatments in patients with abnormal network dynamics, and be used as targets for neurofeedback training (Sitaram et al., 2017) to recover the impaired network dynamics (Bruhl et al., 2014; Keynan et al., 2019; Koush et al., 2019; Koush, Ashburner, et al., 2017a; Linden et al., 2012; Misaki et al., 2018; Nicholson et al., 2018; Sulzer et al., 2013; Young et al., 2014; Young et al., 2018; Zotev et al., 2018).
4.5. Limitations
In the current study, we focused on engaging oneself in positive‐social emotion upregulation. While this is a realistic scenario of therapeutic relevance, our study design does not allow for separating self‐referential, affective, and social aspects in applied emotion regulation. Nevertheless, as discussed above, identified dmPFC, sgACC, and bilateral amygdala activity is highly relevant to both social cognition and emotion regulation. In addition, affective (positive/neutral/negative) and social/nonsocial content relevance to emotion regulation processes in the bilateral amygdala has been previously characterized (Vrticka et al., 2012). In particular, the amygdala is engaged in both social and emotion regulation (Vrticka et al., 2012). Future research could further delineate the social aspect from applied positive‐social emotion regulation paradigms when contrasting positive social and positive nonsocial conditions. An interesting extension of our study would be the direct comparison of the sgACC role in positive‐social and negative‐social emotion regulation conditions. Although our study does not allow for directly contrasting these two conditions, it revealed that positive‐social emotion upregulation recruited a distributed cortico‐limbic network that overlaps with areas involved in regulating negative emotions (Kim & Hamann, 2007; Ochsner et al., 2012; Vrticka et al., 2012).
Future studies might also include more diverse participant populations to assess if the present results generalize beyond the population included in this study (which consisted predominantly of young students and researchers). Here, the purpose of our study was to examine dynamic mechanisms underlying positive‐social emotion upregulation in a healthy population, where future studies are warranted in the aforementioned clinical populations hypothesized to exhibit disrupted emotion regulation networks.
4.5.1. Conclusion
In sum, the present study complements and extends previous research with the sgACC engagement in positive‐social emotion regulation processes and dmPFC‐amygdala interactions. We provide evidence that the sgACC is involved in effortful positive‐social emotion upregulation and more automated positive‐social passive viewing and rapid passive viewing conditions. These findings support a potential role of the sgACC as a gatekeeper between cognitive control areas and emotional limbic areas, which interactions within prefrontal‐limbic network increase in more automated emotion regulation processes as compared to effortful emotion upregulation. We found that more anxious individuals with a greater tendency to suppress emotions and intrusive thoughts were likely to display decreased amygdala, dmPFC, sgACC activity, and stronger modulation of the sgACC by the left amygdala during effortful emotion upregulation. During more automated emotion regulation, less anxious individuals were also likely to display higher modulation of the sgACC by the left amygdala. Our findings may have significant therapeutic relevance for those suffering from psychiatric illnesses, in which disrupted emotion regulation network capacities are a key mechanism contributing to many forms of psychopathology. Finally, our study informs neurobiological treatment interventions, such as neurofeedback, in how optimal emotion regulation of target neural network may be achieved when using effective connectivity as a feedback signal by providing insights on specific prefrontal‐limbic neural network interactions.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This study was supported in part by the Center for Neuroscience of the University of Geneva, the Swiss National Science Foundation (F. S.: PZ00P3‐131932, PP00P2‐146318, BSSG10_155915, 100014_178841, 32003B_166566; Y. K.: P300PB_161083), the Baugarten Stiftung, the Foundation for Research in Science, the Humanities at the University of Zurich (STWF‐17‐012) and the European Union. A. N. is also supported by the Marie Skłodowska‐Curie individual fellowship from the European Research Commission. We thank Camille Piguet for helpful discussions. The authors do not have permission to share data. The authors declare no competing interests.
Scharnowski F, Nicholson AA, Pichon S, et al. The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal–amygdala neural circuitry during positive‐social emotion regulation. Hum Brain Mapp. 2020;41:3100–3118. 10.1002/hbm.25001
Funding information European Research Council; European Union; Foundation for Research in Science, the Humanities at the University of Zurich, Grant/Award Number: STWF‐17‐012; Baugarten Stiftung; Swiss National Science Foundation, Grant/Award Numbers: P300PB_161083, 32003B_166566, 100014_178841, BSSG10_155915, PP00P2‐146318, PZ00P3‐131932
Contributor Information
Frank Scharnowski, Email: frank.scharnowski@univie.ac.at.
Yury Koush, Email: yury.koush@yale.edu.
DATA AVAILABILITY STATEMENT
The authors do not have permission to share data.
REFERENCES
- Aldao, A. , Nolen‐Hoeksema, S. , & Schweizer, S. (2010). Emotion‐regulation strategies across psychopathology: A meta‐analytic review. Clinical Psychology Review, 30, 217–237. [DOI] [PubMed] [Google Scholar]
- Amodio, D. M. , & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews. Neuroscience, 7, 268–277. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Ashby, F. G. , Isen, A. M. , & Turken, U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106, 529–550. [DOI] [PubMed] [Google Scholar]
- Azab, H. , & Hayden, B. Y. (2018). Correlates of economic decisions in the dorsal and subgenual anterior cingulate cortices. The European Journal of Neuroscience, 47, 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, S. J. , Eddy, K. T. , Angstadt, M. , Nathan, P. J. , & Phan, K. L. (2007). Amygdala ‐ frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard, M. , Levesque, J. , & Bourgouin, P. (2001). Neural correlates of conscious self‐regulation of emotion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21, RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, M. , Johansen‐Berg, H. , & Rushworth, M. F. (2009). Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein, L. M. , Gross, J. J. , & Ochsner, K. N. (2017). Explicit and implicit emotion regulation: A multi‐level framework. Social Cognitive and Affective Neuroscience, 12, 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl, A. , Scherpiet, S. , Sulzer, J. , Stampfli, P. , Seifritz, E. , & Herwig, U. (2014). Real‐time neurofeedback using functional MRI could improve Down‐regulation of amygdala activity during emotional stimulation: A proof‐of‐concept study. Brain Topography, 27, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , & Carroll, D. C. (2007). Self‐projection and the brain. Trends in Cognitive Sciences, 11, 49–57. [DOI] [PubMed] [Google Scholar]
- Bzdok, D. , Langner, R. , Schilbach, L. , Engemann, D. A. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2013). Segregation of the human medial prefrontal cortex in social cognition. Frontiers in Human Neuroscience, 7, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, S. T. , & Price, J. L. (1996). Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of Comparative Neurology, 371, 179–207. [DOI] [PubMed] [Google Scholar]
- Carpenter, S. M. , Peters, E. , Vastfjall, D. , & Isen, A. M. (2013). Positive feelings facilitate working memory and complex decision making among older adults. Cognition & Emotion, 27, 184–192. [DOI] [PubMed] [Google Scholar]
- Carver, C. S. , & White, T. L. (1994). Behavioral‐inhibition, behavioral activation, and affective responses to impending reward and punishment ‐ the Bis bas scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- Craig, A. D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- Dan‐Glauser, E. S. , & Scherer, K. R. (2011). The Geneva affective picture database (GAPED): A new 730‐picture database focusing on valence and normative significance. Behavior Research Methods, 43, 468–477. [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. , & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21. [DOI] [PubMed] [Google Scholar]
- Diano, M. , Celeghin, A. , Bagnis, A. , & Tamietto, M. (2016). Amygdala response to emotional stimuli without awareness: Facts and interpretations. Frontiers in Psychology, 7, 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner, S. G. , Beevers, C. G. , Haigh, E. A. , & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews. Neuroscience, 12, 467–477. [DOI] [PubMed] [Google Scholar]
- Doll, A. , Holzel, B. K. , Mulej Bratec, S. , Boucard, C. C. , Xie, X. , Wohlschlager, A. M. , & Sorg, C. (2016). Mindful attention to breath regulates emotions via increased amygdala‐prefrontal cortex connectivity. NeuroImage, 134, 305–313. [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. (2001). Neuroimaging and neuropathological studies of depression: Implications for the cognitive‐emotional features of mood disorders. Current Opinion in Neurobiology, 11, 240–249. [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. (2003). Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences, 985, 420–444. [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. , & Savitz, J. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13, 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. T. , Bzdok, D. , & Hensel, L. (2016). Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex, 26, 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Escolano, C. , Navarro‐Gil, M. , Garcia‐Campayo, J. , Congedo, M. , De Ridder, D. , & Minguez, J. (2014). A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Frontiers in Behavioral Neuroscience, 8, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Buchel, C. , & Gross, J. J. (2015). The neural bases of emotion regulation. Nature reviews. Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Prater, K. E. , Hoeft, F. , Menon, V. , & Schatzberg, A. F. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. The American Journal of Psychiatry, 167, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , & Wager, T. D. (2007). Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster, R. J. , Lebois, L. A. M. , Ressler, K. J. , & Suh, J. (2018). Brain circuit dysfunction in post‐traumatic stress disorder: From mouse to man. Nature Reviews. Neuroscience, 19, 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati, P. , Hevenor, S. J. , Graham, S. J. , Grady, C. , Keightley, M. L. , Craik, F. , & Mayberg, H. (2003). In search of the emotional self: An fMRI study using positive and negative emotional words. The American Journal of Psychiatry, 160, 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fredrickson, B. L. (2004). The broaden‐and‐build theory of positive emotions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 359, 1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, L. J. , Insel, T. R. , & Smith, Y. (2000). Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. The Journal of Comparative Neurology, 421, 172–188. [PubMed] [Google Scholar]
- Friston, K. J. , Harrison, L. , & Penny, W. (2003). Dynamic causal modelling. NeuroImage, 19, 1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Rotshtein, P. , Geng, J. J. , Sterzer, P. , & Henson, R. N. (2006). A critique of functional localisers. NeuroImage, 30, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Gallagher, H. L. , & Frith, C. D. (2003). Functional imaging of 'theory of mind’. Trends in Cognitive Sciences, 7, 77–83. [DOI] [PubMed] [Google Scholar]
- George, M. S. , Ketter, T. A. , Parekh, P. I. , Horwitz, B. , Herscovitch, P. , & Post, R. M. (1995). Brain activity during transient sadness and happiness in healthy women. The American Journal of Psychiatry, 152, 341–351. [DOI] [PubMed] [Google Scholar]
- Ghashghaei, H. T. , Hilgetag, C. C. , & Barbas, H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage, 34, 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath, O. , Bunge, S. A. , Shaver, P. R. , Wendelken, C. , & Mikulincer, M. (2005). Attachment‐style differences in the ability to suppress negative thoughts: Exploring the neural correlates. NeuroImage, 28, 835–847. [DOI] [PubMed] [Google Scholar]
- Glascher, J. , & Adolphs, R. (2003). Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 10274–10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, I. I. , Harel, M. , & Malach, R. (2006). When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron, 50, 329–339. [DOI] [PubMed] [Google Scholar]
- Goldin, P. R. , Mcrae, K. , Ramel, W. , & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib, I. H. , & Hamilton, J. P. (2008). Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science, 17, 159–163. [Google Scholar]
- Gotlib, I. H. , Sivers, H. , Gabrieli, J. D. E. , Whitfield‐Gabrieli, S. , Goldin, P. , Minor, K. L. , & Canli, T. (2005). Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport, 16, 1731–1734. [DOI] [PubMed] [Google Scholar]
- Greicius, M. D. , Flores, B. H. , Menon, V. , Glover, G. H. , Solvason, H. B. , Kenna, H. , … Schatzberg, A. F. (2007). Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone, M. , Dyck, M. , Koush, Y. , Bergert, S. , Mathiak, K. A. , Alawi, E. M. , … Mathiak, K. (2015). Upregulation of the rostral anterior cingulate cortex can Alter the perception of emotions: fMRI‐based Neurofeedback at 3 and 7 T. Brain Topography, 28, 197–207. [DOI] [PubMed] [Google Scholar]
- Gross, J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39, 281–291. [DOI] [PubMed] [Google Scholar]
- Gross, J. J. , & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. Journal of Personality and Social Psychology, 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Glover, G. H. , Hsu, J. J. , Johnson, R. F. , & Gotlib, I. H. (2011). Modulation of subgenual anterior cingulate cortex activity with real‐time neurofeedback. Human Brain Mapping, 32, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig, U. , Baumgartner, T. , Kaffenberger, T. , Bruhl, A. , Kottlow, M. , Schreiter‐Gasser, U. , … Rufer, M. (2007). Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage, 37, 652–662. [DOI] [PubMed] [Google Scholar]
- Holmes, E. A. , Coughtrey, A. E. , & Connor, A. (2008). Looking at or through rose‐tinted glasses? Imagery perspective and positive mood. Emotion, 8, 875–879. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. (2003). Fast, automated, N‐dimensional phase‐unwrapping algorithm. Magnetic Resonance in Medicine, 49, 193–197. [DOI] [PubMed] [Google Scholar]
- Jezzard, P. , & Balaban, R. S. (1995). Correction for geometric distortion in Echo‐planar images from B‐0 field variations. Magnetic Resonance in Medicine, 34, 65–73. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg, H. , Gutman, D. A. , Behrens, T. E. , Matthews, P. M. , Rushworth, M. F. , Katz, E. , … Mayberg, H. S. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment‐resistant depression. Cerebral Cortex, 18, 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe, K. K. , Frith, C. D. , & Frith, U. (2003). "hey John": Signals conveying communicative intention toward the self activate brain regions associated with "mentalizing," regardless of modality. The Journal of Neuroscience, 23, 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan, J. N. , Cohen, A. , Jackont, G. , Green, N. , Goldway, N. , Davidov, A. , … Hendler, T. (2019). Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nature Human Behaviour, 3, 63–73. [DOI] [PubMed] [Google Scholar]
- Kiebel, S. J. , Kloppel, S. , Weiskopf, N. , & Friston, K. J. (2007). Dynamic causal modeling: A generative model of slice timing in fMRI. NeuroImage, 34, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Hamann, S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19, 776–798. [DOI] [PubMed] [Google Scholar]
- Koch, S. B. , van Zuiden, M. , Nawijn, L. , Frijling, J. L. , Veltman, D. J. , & Olff, M. (2016). Aberrant resting‐state brain activity in posttraumatic stress disorder: A meta‐analysis and systematic review. Depression and Anxiety, 33, 592–605. [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Ashburner, J. , Prilepin, E. , Sladky, R. , Zeidman, P. , Bibikov, S. , … De Ville, D. V. (2017a). OpenNFT: An open‐source python/Matlab framework for real‐time fMRI neurofeedback training based on activity, connectivity and multivariate pattern analysis. NeuroImage, 156, 489–503. [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Ashburner, J. , Prilepin, E. , Sladky, R. , Zeidman, P. , Bibikov, S. , … Van De Ville, D. (2017b). Real‐time fMRI data for testing OpenNFT functionality. Data in Brief, 14, 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush, Y. , Meskaldji, D. E. , Pichon, S. , Rey, G. , Rieger, S. W. , Linden, D. E. , … Scharnowski, F. (2017). Learning control over emotion networks through connectivity‐based Neurofeedback. Cerebral Cortex, 27, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Pichon, S. , Eickhoff, S. B. , Van De Ville, D. , Vuilleumier, P. , & Scharnowski, F. (2019). Brain networks for engaging oneself in positive‐social emotion regulation. NeuroImage, 189, 106–115. [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Rosa, M. J. , Robineau, F. , Heinen, K. , Rieger S, W. , Weiskopf, N. , … Scharnowski, F. (2013). Connectivity‐based neurofeedback: Dynamic causal modeling for real‐time fMRI. NeuroImage, 81, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush, Y. , Zvyagintsev, M. , Dyck, M. , Mathiak, K. A. , & Mathiak, K. (2012). Signal quality and Bayesian signal processing in neurofeedback based on real‐time fMRI. NeuroImage, 59, 478–489. [DOI] [PubMed] [Google Scholar]
- Lancaster, J. L. , Woldorff, M. G. , Parsons, L. M. , Liotti, M. , Freitas, E. S. , Rainey, L. , … Fox, P. T. (2000). Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. D. , Reiman, E. M. , Ahern, G. L. , Schwartz, G. E. , & Davidson, R. J. (1997). Neuroanatomical correlates of happiness, sadness, and disgust. The American Journal of Psychiatry, 154, 926–933. [DOI] [PubMed] [Google Scholar]
- Lang, P. J. , Greenwald, M. K. , Bradley, M. M. , & Hamm, A. O. (1993). Looking at pictures ‐ affective, facial, visceral, and behavioral reactions. Psychophysiology, 30, 261–273. [DOI] [PubMed] [Google Scholar]
- Lanius, R. A. , Boyd, J. E. , McKinnon, M. C. , Nicholson, A. A. , Frewen, P. , Vermetten, E. , … Spiegel, D. (2018). A review of the neurobiological basis of trauma‐related dissociation and its relation to cannabinoid‐ and opioid‐mediated stress response: A transdiagnostic, translational approach. Current Psychiatry Reports, 20, 118. [DOI] [PubMed] [Google Scholar]
- Lanteaume, L. , Khalfa, S. , Regis, J. , Marquis, P. , Chauvel, P. , & Bartolomei, F. (2007). Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex, 17, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Laxton, A. W. , Neimat, J. S. , Davis, K. D. , Womelsdorf, T. , Hutchison, W. D. , Dostrovsky, J. O. , … Lozano, A. M. (2013). Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biological Psychiatry, 74, 714–719. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. (2007). The amygdala. Current Biology: CB, 17, R868–R874. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. E. , & Brown, R. (2017). A higher‐order theory of emotional consciousness. Proceedings of the National Academy of Sciences of the United States of America, 114, E2016–E2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M. D. (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. [DOI] [PubMed] [Google Scholar]
- Linden, D. E. , Habes, I. , Johnston, S. J. , Linden, S. , Tatineni, R. , Subramanian, L. , … Goebel, R. (2012). Real‐time self‐regulation of emotion networks in patients with depression. PLoS ONE, 7, e38115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A. , Wager, T. D. , Kober, H. , Bliss‐Moreau, E. , & Barrett, L. F. (2012). The brain basis of emotion: A meta‐analytic review. The Behavioral and Brain Sciences, 35, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood, P. L. , & Wittmann, M. K. (2018). Ventral anterior cingulate cortex and social decision‐making. Neuroscience and Biobehavioral Reviews, 92, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka, A. , Zurawski, L. , Jednorog, K. , & Grabowska, A. (2013). The Nencki affective picture system (NAPS): Introduction to a novel, standardized, wide‐range, high‐quality, realistic picture database. Behavior Research Methods, 46, 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg, H. S. , Lozano, A. M. , Voon, V. , McNeely, H. E. , Seminowicz, D. , Hamani, C. , … Kennedy, S. H. (2005). Deep brain stimulation for treatment‐resistant depression. Neuron, 45, 651–660. [DOI] [PubMed] [Google Scholar]
- Misaki, M. , Phillips, R. , Zotev, V. , Wong, C. K. , Wurfel, B. E. , Krueger, F. , … Bodurka, J. (2018). Real‐time fMRI amygdala neurofeedback positive emotional training normalized resting‐state functional connectivity in combat veterans with and without PTSD: A connectome‐wide investigation. NeuroImage. Clinical, 20, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Bode, S. , Baudewig, J. , & Heekeren, H. R. (2017). Effective amygdala‐prefrontal connectivity predicts individual differences in successful emotion regulation. Social Cognitive and Affective Neuroscience, 12, 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler, R. T. , Rabi, R. , & Minda, J. P. (2010). Better mood and better performance. Learning rule‐described categories is enhanced by positive mood. Psychological Science, 21, 1770–1776. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A. , Friston, K. J. , Zeidman, P. , Harricharan, S. , McKinnon, M. C. , Densmore, M. , … Lanius, R. A. (2017). Dynamic causal modeling in PTSD and its dissociative subtype: Bottom‐up versus top‐down processing within fear and emotion regulation circuitry. Human Brain Mapping, 38, 5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A. , Rabellino, D. , Densmore, M. , Frewen, P. A. , Paret, C. , Kluetsch, R. , … Lanius, R. A. (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real‐time fMRI neurofeedback. Human Brain Mapping, 38, 541–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A. , Rabellino, D. , Densmore, M. , Frewen, P. A. , Paret, C. , Kluetsch, R. , … Lanius, R. A. (2018). Intrinsic connectivity network dynamics in PTSD during amygdala downregulation using real‐time fMRI neurofeedback: A preliminary analysis. Human Brain Mapping, 39, 4258–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobler, M. S. , Oquendo, M. A. , Kegeles, L. S. , Malone, K. M. , Campbell, C. C. , Sackeim, H. A. , & Mann, J. J. (2001). Decreased regional brain metabolism after ECT. The American Journal of Psychiatry, 158, 305–308. [DOI] [PubMed] [Google Scholar]
- Northoff, G. , & Bermpohl, F. (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8, 102–107. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , & Gross, J. J. (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Ray, R. D. , Cooper, J. C. , Robertson, E. R. , Chopra, S. , Gabrieli, J. D. E. , & Gross, J. J. (2004). For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. NeuroImage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Ray, R. R. , Hughes, B. , McRae, K. , Cooper, J. C. , Weber, J. , … Gross, J. J. (2009). Bottom‐up and top‐down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science, 20, 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Year in Cognitive Neuroscience, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero‐Gallagher, N. , Eickhoff, S. B. , Hoffstaedter, F. , Schleicher, A. , Mohlberg, H. , Vogt, B. A. , … Zilles, K. (2015). Functional organization of human subgenual cortical areas: Relationship between architectonical segregation and connectional heterogeneity. NeuroImage, 115, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, W. D. , Stephan, K. E. , Daunizeau, J. , Rosa, M. J. , Friston, K. J. , Schofield, T. M. , & Leff, A. P. (2010). Comparing families of dynamic causal models. PLoS Computational Biology, 6, e1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, W. D. , Stephan, K. E. , Mechelli, A. , & Friston, K. J. (2004). Comparing dynamic causal models. NeuroImage, 22, 1157–1172. [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , Delgado, M. R. , Nearing, K. I. , & LeDoux, J. E. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , O'Connor, K. J. , Gatenby, J. C. , Gore, J. C. , Grillon, C. , & Davis, M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4, 437–441. [DOI] [PubMed] [Google Scholar]
- Philippot, P. , & Segal, Z. (2009). Mindfulness based psychological interventions developing emotional awareness for better being. Journal of Consciousness Studies, 16, 285–306. [Google Scholar]
- Phillips, M. L. , Ladouceur, C. D. , & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(829), 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon, S. , de Gelder, B. , & Grezes, J. (2012). Threat prompts defensive brain responses independently of attentional control. Cerebral Cortex, 22, 274–285. [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. , Fletcher, P. C. , Henson, R. N. , Worsley, K. J. , Brett, M. , & Nichols, T. E. (2008). Guidelines for reporting an fMRI study. NeuroImage, 40, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez‐Mahaluf, J. P. , Perramon, J. , Otal, B. , Villoslada, P. , & Compte, A. (2018). Subgenual anterior cingulate cortex controls sadness‐induced modulations of cognitive and emotional network hubs. Scientific Reports, 8, 8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck, C. , Ames, D. R. , & Ochsner, K. N. (2016). The social regulation of emotion: An integrative, cross‐disciplinary model. Trends in Cognitive Sciences, 20, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler, K. J. , & Mayberg, H. S. (2007). Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience, 10, 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck, P. H. , Putnam, P. T. , Daniels, T. E. , Yang, T. , Mitz, A. R. , Rhodes, S. E. , & Murray, E. A. (2014). A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proceedings of the National Academy of Sciences of the United States of America, 111, 5391–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, D. , Grandjean, D. , & Scherer, K. R. (2005). A systems approach to appraisal mechanisms in emotion. Neural Networks: The Official Journal of the International Neural Network Society, 18, 317–352. [DOI] [PubMed] [Google Scholar]
- Scherer, K. R. (1982). Emotion as a process: Function, origin and regulation. Social Science Information, 21, 555–570. [Google Scholar]
- Schlosser, R. G. , Wagner, G. , Koch, K. , Dahnke, R. , Reichenbach, J. R. , & Sauer, H. (2008). Fronto‐cingulate effective connectivity in major depression: A study with fMRI and dynamic causal modeling. NeuroImage, 43, 645–655. [DOI] [PubMed] [Google Scholar]
- Sergerie, K. , Lepage, M. , & Armony, J. L. (2006). A process‐specific functional dissociation of the amygdala in emotional memory. Journal of Cognitive Neuroscience, 18, 1359–1367. [DOI] [PubMed] [Google Scholar]
- Sitaram, R. , Ros, T. , Stoeckel, L. , Haller, S. , Scharnowski, F. , Lewis‐Peacock, J. , … Sulzer, J. (2017). Closed‐loop brain training: the science of neurofeedback. Nature Neuroscience Reviews, 18, 86–100. [DOI] [PubMed] [Google Scholar]
- Sladky, R. , Friston, K. J. , Trostl, J. , Cunnington, R. , Moser, E. , & Windischberger, C. (2011). Slice‐timing effects and their correction in functional MRI. NeuroImage, 58, 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. P. , Stephan, K. E. , Rugg, M. D. , & Dolan, R. J. (2006). Task and content modulate amygdala‐hippocampal connectivity in emotional retrieval. Neuron, 49, 631–638. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stephan, K. E. , Penny, W. D. , Daunizeau, J. , Moran, R. J. , & Friston, K. J. (2009). Bayesian model selection for group studies. NeuroImage, 46, 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, K. E. , Penny, W. D. , Moran, R. J. , den Ouden, H. E. , Daunizeau, J. , & Friston, K. J. (2010). Ten simple rules for dynamic causal modeling. NeuroImage, 49, 3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, F. L. , Hurley, R. A. , & Taber, K. H. (2011). Anterior cingulate cortex: unique role in cognition and emotion. The Journal of Neuropsychiatry and Clinical Neurosciences, 23, 121–125. [DOI] [PubMed] [Google Scholar]
- Stoeckel, L. E. , Garrison, K. A. , Ghosh, S. , Wighton, P. , Hanlon, C. A. , Gilman, J. M. , … Evins, A. E. (2014). Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage. Clinical, 5, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer, J. , Haller, S. , Scharnowski, F. , Weiskopf, N. , Birbaumer, N. , Blefari, M. L. , … Sitaram, R. (2013). Real‐time fMRI neurofeedback: Progress and challenges. NeuroImage, 76, 386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. F. , & Liberzon, I. (2007). Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences, 11, 413–418. [DOI] [PubMed] [Google Scholar]
- Treadway, M. T. , & Zald, D. H. (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35, 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden, A. M. , Kuyken, W. , Wattar, U. , Crane, C. , Pallesen, K. J. , Dahlgaard, J. , … Piet, J. (2015). A systematic review of mechanisms of change in mindfulness‐based cognitive therapy in the treatment of recurrent major depressive disorder. Clinical Psychology Review, 37, 26–39. [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience, 6, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, B. A. , & Pandya, D. N. (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of Comparative Neurology, 262, 271–289. [DOI] [PubMed] [Google Scholar]
- Vrticka, P. , Sander, D. , & Vuilleumier, P. (2011). Effects of emotion regulation strategy on brain responses to the valence and social content of visual scenes. Neuropsychologia, 49, 1067–1082. [DOI] [PubMed] [Google Scholar]
- Vrticka, P. , Sander, D. , & Vuilleumier, P. (2012). Lateralized interactive social content and valence processing within the human amygdala. Frontiers in Human Neuroscience, 6, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. (2008). The roles of medial prefrontal cortex in emotion: Neuroimaging evidence for functional subdivisions and cortical‐subcortical pathways. Biological Psychiatry, 63, 151s–151s. [Google Scholar]
- Wager, T. D. , Barrett, L. F. , Bliss‐Moreau, E. , Lindquist, K. , Duncan, N. W. , Kober, H. , … Mize, J. (2008). The neuroimaging of emotion In Lewis M. (Ed.), Handbook of emotions (3rd ed., pp. 249–271), New York, NY: The Guilford Press. [Google Scholar]
- Wager, T. D. , Davidson, M. L. , Hughes, B. L. , Lindquist, M. A. , & Ochsner, K. N. (2008). Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, G. , Schachtzabel, C. , Peikert, G. , & Bar, K. J. (2015). The neural basis of the abnormal self‐referential processing and its impact on cognitive control in depressed patients. Human Brain Mapping, 36, 2781–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, D. M. , & Zanakos, S. (1994). Chronic thought suppression. Journal of Personality, 62, 615–640. [DOI] [PubMed] [Google Scholar]
- Weiskopf, N. , Hutton, C. , Josephs, O. , & Deichmann, R. (2006). Optimal EPI parameters for reduction of susceptibility‐induced BOLD sensitivity losses: A whole‐brain analysis at 3 T and 1.5 T. NeuroImage, 33, 493–504. [DOI] [PubMed] [Google Scholar]
- Weiskopf, N. , Hutton, C. , Josephs, O. , Turner, R. , & Deichmann, R. (2007). Optimized EPI for fMRI studies of the orbitofrontal cortex: Compensation of susceptibility‐induced gradients in the readout direction. Magnetic Resonance Materials in Physics, Biology and Medicine, 20, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Sun, H. , Xu, J. , Wu, Y. , Wang, C. , Xiao, J. , … Wang, J. (2016). Changed hub and corresponding functional connectivity of subgenual anterior cingulate cortex in major depressive disorder. Frontiers in Neuroanatomy, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Tsai, S. J. , & Li, C. R. (2020). Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: A meta‐analysis of the effects of valence of emotion and passive exposure versus active regulation. Brain Structure & Function, 225, 345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda, R. , Hoge, C. W. , McFarlane, A. C. , Vermetten, E. , Lanius, R. A. , Nievergelt, C. M. , … Hyman, S. E. (2015). Post‐traumatic stress disorder. Nature Reviews. Disease Primers, 1, 15057. [DOI] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Misaki, M. , Zotev, V. , Phillips, R. , Drevets, W. C. , & Bodurka, J. (2018). Altered task‐based and resting‐state amygdala functional connectivity following real‐time fMRI amygdala neurofeedback training in major depressive disorder. Clinical NeuroImage, 17, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , … Bodurka, J. (2017). Randomized clinical trial of real‐time fMRI amygdala Neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. The American Journal of Psychiatry, 174, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , Drevets, W. C. , & Bodurka, J. (2014). Real‐time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS ONE, 9, e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, J. , & Ochsner, K. N. (2012). The neuroscience of empathy: Progress, pitfalls and promise. Nature Neuroscience, 15, 675–680. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Hu, S. , Chao, H. H. , Ide, J. S. , Luo, X. , Farr, O. M. , & Li, C. S. (2014). Ventromedial prefrontal cortex and the regulation of physiological arousal. Social Cognitive and Affective Neuroscience, 9, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand, A. , Parvaz, M. A. , & Goldstein, R. Z. (2017). Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A Systematic Review. Neuroimage, 151, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Krueger, F. , Phillips, R. , Alvarez, R. P. , Simmons, W. K. , Bellgowan, P. , … Bodurka, J. (2011). Self‐regulation of amygdala activation using real‐time FMRI neurofeedback. PLoS ONE, 6, e24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Misaki, M. , Wong, C. K. , Wurfel, B. E. , Krueger, F. , … Bodurka, J. (2018). Real‐time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat‐related PTSD. NeuroImage. Clinical, 19, 106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Young, K. D. , Drevets, W. C. , & Bodurka, J. (2013). Prefrontal control of the amygdala during real‐time fMRI neurofeedback training of emotion regulation. PLoS ONE, 8, e79184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Yuan, H. , Misaki, M. , & Bodurka, J. (2014). Self‐regulation of human brain activity using simultaneous real‐time fMRI and EEG neurofeedback. NeuroImage, 85(Pt 3), 985–995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The authors do not have permission to share data.


