Abstract
We compared resting state (RS) functional connectivity and task‐based fMRI to lateralize language dominance in 30 epilepsy patients (mean age = 33; SD = 11; 12 female), a measure used for presurgical planning. Language laterality index (LI) was calculated from task fMRI in frontal, temporal, and frontal + temporal regional masks using LI bootstrap method from SPM12. RS language LI was assessed using two novel methods of calculating RS language LI from bilateral Broca's area seed based connectivity maps across regional masks and multiple thresholds (p < .05, p < .01, p < .001, top 10% connections). We compared LI from task and RS fMRI continuous values and dominance classifications. We found significant positive correlations between task LI and RS LI when functional connectivity thresholds were set to the top 10% of connections. Concordance of dominance classifications ranged from 20% to 30% for the intrahemispheric resting state LI method and 50% to 63% for the resting state LI intra‐ minus interhemispheric difference method. Approximately 40% of patients left dominant on task showed RS bilateral dominance. There was no difference in LI concordance between patients with right‐sided and left‐sided resections. Early seizure onset (<6 years old) was not associated with atypical language dominance during task‐based or RS fMRI. While a relationship between task LI and RS LI exists in patients with epilepsy, language dominance is less lateralized on RS than task fMRI. Concordance of language dominance classifications between task and resting state fMRI depends on brain regions surveyed and RS LI calculation method.
Keywords: epilepsy, fMRI, functional laterality, language, magnetic resonance imaging, resting state, seizures
1. INTRODUCTION
Identifying eloquent cortex is a critical goal in epilepsy surgery planning. Reliable task‐based functional MRI (fMRI) methods have been developed (Binder, Swanson, Hammeke, & Sabsevitz, 2008; Gaillard et al., 2004; Lehericy et al., 2000) to lateralize (Szaflarski, Gloss, Binder, et al., 2017) and localize language function presurgically (Austermuehle et al., 2017; Benjamin et al., 2018; Szaflarski et al., 2017), as well as help predict postoperative language outcome (Bonelli et al., 2012; Rolinski et al., 2019; Sabsevitz, Swanson, & Hammeke, 2003; You, Zachery, Fanto, et al., 2019). However, spatial extent and strength of activations can vary with task paradigm (Binder et al., 2008; Gaillard et al., 2004) and are subject to participant performance. Task noncompliance can be affected by several factors, including task length, degree of cognitive demand, and underlying patient pathology.
Resting state fMRI is a promising alternative. In resting state fMRI, spontaneous low frequency (<0.1 Hz) fluctuations in baseline blood‐oxygen level dependent (BOLD) signal are measured to study functionally connected interregional neuronal activity (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox & Raichle, 2007). Resting state fMRI offers several advantages over task fMRI, including the ability to examine multiple functional networks in less scan time and collect data from children or patients unable to complete task paradigms.
Recent studies show seed‐based resting state fMRI lateralizes language function in healthy controls with moderate success (Joliot & Tzourio‐Mazoyer, 2016; Liu, Stufflebeam, Sepulcre, Hedden, & Buckner, 2009; Lou, Peck, Brennan, Mallela, & Holodny, 2017; Wang, Buckner, & Liu, 2014). Results in temporal lobe epilepsy have been inconsistent (Doucet, Pustina, et al., 2015; Teghipco, Hussain, & Tivarus, 2016). Methods comparable to task‐based fMRI for calculating laterality index (LI) at rest in patients with epilepsy are not well‐developed.
We compared functional connectivity from resting state to task‐based fMRI to lateralize language dominance in epilepsy patients, using two new approaches of calculating LI at rest. We hypothesized that numerical and categorical LI values from resting state FC would be comparable to task‐based fMRI LI language activation. Numerical language LI values from task fMRI activation and resting state FC were compared to evaluate whether resting state FC can adequately capture between‐subject variation in language lateralization (Liu et al., 2009; Wang et al., 2014). Categorical (left dominant, right dominant, bilateral) LI values were compared to evaluate the clinical relevance in relation to preoperative planning.
2. MATERIALS AND METHODS
2.1. Participants
Thirty adult, English‐speaking patients with drug‐resistant epilepsy referred to the National Institutes of Health for presurgical evaluation between 2014 and 2018 who had both language fMRI and resting state fMRI as part of their presurgical evaluation were included. All provided informed consent in accordance with the NIH Combined Neurosciences Institutional Review Board prior to participating in this study. All patients underwent ictal video‐EEG monitoring and one or two sessions of fMRI where they completed a T1‐weighted magnetization‐prepared rapid acquisition gradient echo (MPRAGE) anatomical MRI, 6‐min run of resting state fMRI, and a fMRI language task. If completed in two sessions, resting state and language tasks were collected separately.
2.2. fMRI task description
Language tasks were collected using echo‐planar imaging (EPI) BOLD signal using a block design with alternating 30 s control activation blocks for five epoch cycles. All patients completed an auditory description decision task (ADDT) language fMRI task, described previously (Gaillard et al., 2007). Briefly, during ADDT the activation block consisted of listening to 6–8 word descriptions of a word from the Boston Naming test and pressing a button when the definition was thought to be true. For example, 70% of targets in the active condition were true: “a large gray animal is an elephant.” The remaining 30% of targets were false: “Spaghetti is something you sit on.” The control block consisted of listening to reverse speech with intermittent tones to account for primary and secondary auditory processing (Gaillard et al., 2007). Patients were instructed to press the button when they heard a tone. ADDT is a complex task that requires decision making and allows for in‐scanner performance tracking. Although different fMRI paradigms can vary greatly in their pattern and extent of activation, this task is designed to require comprehension of a phrase and semantic decision‐making, controlling for first and second order auditory processing, attention, and motor response that in turn yields highly lateralized activation in receptive, Werincke's area and expressive, Broca's area language processing (Gaillard et al., 2004; Rosenberger et al., 2009). We have reported on this task previously in over 100 controls and 200 patients (Berl et al., 2014) and it has been validated by intracarotid amytal procedure (IAP) (Gaillard et al., 2004; Szaflarski et al., 2017), cortical stimulation mapping (Austermuehle et al., 2017), and postresection language performance (Sabsevitz et al., 2003).
2.3. Task fMRI acquisition and preprocessing
All imaging was done on a 3.0 Tesla scanner at the NIH Nuclear Magnetic Resonance Center. Due to scanner updates, 16 patients were imaged on a Siemens Skyra scanner and 14 on a GE Signa HDxt scanner during the task fMRI language paradigm. The functional language task was collected using gradient EPI. Scan parameters on the Siemens scanner were: echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; acquisition matrix = 64 × 64, 4 mm thickness, 37 slices, 150 volumes. Voxel size was 3.4 × 3.4 × 4.0 mm. Scan parameters on the GE scanner were: TE = 27 ms; TR = 2000 ms; acquisition matrix = 72 × 72, 3 mm thickness, 40 slices, 150 volumes. Voxel size was 3.0 × 3.0 × 3.0 mm. Previous studies found no differences between scanners using these acquisition parameters and this paradigm (Berl et al., 2014; You et al., 2011).
The fMRI language task was preprocessed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Processing of the input EPI dataset began with outlier detection, followed by slice timing correction, to synchronize timing across slices. T1‐weighted MPRAGE was aligned to an EPI registration base and transformed to MNI standard space using a 12‐parameter affine transformation. Remaining volumes in the EPI dataset were aligned to the EPI base volume and anatomical dataset in MNI space using a concatenated transformation. Volumes in the EPI dataset were blurred with a 4.0 mm full‐width half maximum Gaussian kernel and scaled to a voxelwise percentage of the mean (signal percent change). De‐meaned motion parameters were used in regression analysis to remove motion artifact. Time points where estimated motion exceeded 0.3 mm were censored from the linear regression model. The voxelwise response to the active condition given the input stimulus time series was modeled using a Generalized Least Squares linear regression.
2.4. Resting state fMRI acquisition and preprocessing
All resting state fMRI data were collected using a 3.0 Tesla Siemens scanner. A 6‐minute run was collected, during which the patient was instructed to fixate on a central crossbar. Functional images were collected using multi‐echo (ME) EPI with three echoes: TE =12,24,37 ms, TR = 2,500 ms, acquisition matrix = 64 × 64, 3 mm thickness, 40 slices, 150 volumes. Voxel size was 3.0 × 3.0 × 3.0 mm.
Resting state fMRI was preprocessed in AFNI (Cox, 1996). The T1 MPRAGE was intensity bias corrected, skull‐stripped, and transformed to MNI standard space using a nonlinear transformation. The initial two volumes were removed from the ME‐EPI data and slice timing corrected. The reverse gradient method was used to correct for distortion caused by magnetic field inhomogeneity (Chang & Fitzpatrick, 1992). Head motion was estimated using the first echo of the EPI data and applied to all echoes. While the same transformation was applied to all echoes, echoes were transformed to MNI space separately and visually checked for successful alignment before subsequent preprocessing (Cohen, Nencka, & Wang, 2018). Alignment failed in one case, and an EPI reference mask was segmented in SPM‐12 (http://www.fil.ion.ucl.ac.uk/spm) to improve the transformation. Once aligned, the three echoes of each run were used as input for the multiecho independent component analysis (ME‐ICA) denoising step, which was conducted on a run‐by‐run basis.
It is important to note that task fMRI data were collected with single‐echo acquisition, but resting state fMRI data with ME acquisition. We used single‐echo task fMRI to match previous studies and current language task fMRI guidelines for presurgical evaluation in patients with epilepsy (Szaflarski et al., 2017). In addition, single‐echo acquisition was deemed sufficient to capture fluctuations in BOLD signal during task due to signal averaging across activation and control blocks (Fox & Raichle, 2007). In contrast, resting state fMRI data does not follow a block design that facilitates signal averaging. Under these circumstances, we felt it was appropriate to implement a relatively newer ME acquisition approach to accomplish denoising and artifact removal from baseline BOLD fluctuations. Differences between task fMRI and resting state fMRI processing are based on differences in the nature of single‐echo versus ME signal acquisition. ME‐ICA has been shown to improve sensitivity, increase statistical power, and reduce the influence of head motion compared to standard single echo task activation and connectivity designs (Dipasquale, Sethi, Laganà, et al., 2017; Kundu et al., 2019). Use of ME acquisition and ME‐ICA techniques for artifact removal in resting state fMRI only may result in more robust connectivity measures that would bias results in favor of resting state fMRI.
2.5. ME‐ICA denoising and artifact removal
ME‐ICA is a denoising method for ME fMRI datasets that automatically identifies, and removes, noise sources in fMRI data. Briefly, while BOLD‐like fluctuations—in units of signal percent change—show a linear dependence with echo time (TE) in ME data, non‐BOLD fluctuations (e.g., noise) lacks such dependence. Therefore, ME‐ICA exploits the linear or flat dependence relationship of BOLD signal percent change units with TE to help separate signal from noise (Kundu, Inati, Evans, Luh, & Bandettini, 2012). ME‐ICA proceeds in three steps. First, it performs ICA decomposition to identify spatially independent fluctuations in the data. Next, it uses the above‐mentioned discrepancy in TE dependence profiles for BOLD and non‐BOLD fluctuations in order to identify non‐BOLD ICA. Finally, such noise components are removed from the data (Kundu et al., 2012). In addition to noise ICA components identified by ME‐ICA, we also regressed out head motion estimates and residual artifactual slow drifts (legendre polynomial up to fifth order). This resulted in a 4‐dimensional EPI dataset in units of signal percent change that was used as the input for connectivity analysis.
After automated processing was complete, components were visually inspected by a member of the research team to confirm successful artifact removal. In three runs, ME‐ICA identified as noise components whose spatial maps resemble typical resting state networks. Such errors were manually corrected.
2.6. Seed selection and functional connectivity analysis
Broca's area was chosen as the seed region of interest based on its involvement in expressive language functioning, in addition to several studies supporting its temporal reliability (Tomasi & Volkow, 2012; Zhu et al., 2014) and lateralizing effects in resting state fMRI (Doucet, Pustina, et al., 2015; Tomasi & Volkow, 2012; Zhu et al., 2014). Left and right Broca's area were identified using the Eickhoff–Zilles probability maps on MNI‐152 template from the postmortem analysis atlas available in AFNI (Amunts et al., 1999). Seeds were centered at the peak xyz coordinate in both left and right hemispheres of the Broca's area probability map and 6 mm spheres drawn around each center. The time series of all voxels contained within a sphere were averaged to produce a single representative time series per ROI subsequently used as input for FC analysis.
FC was computed between Broca's seed average time series and time series of all voxels contained within a set of predefined masks (Figure 1):
A broad language mask, including both frontal and temporal regions, created from an automated meta‐analysis of 885 activation and connectivity studies for the term “language” on the large‐scale, meta‐analysis platform Neurosynth (http://neurosynth.org). The mask generated from Neurosynth (NS) was binarized and mirrored on the opposite hemisphere to produce an identical mask on both brain hemispheres. Anatomical regions included in each mask are demonstrated in Figure 1 and a full list of regions can be found in Table S1.
Only the frontal portion of the NS mask.
Only the temporal portion of the NS mask.
FIGURE 1.
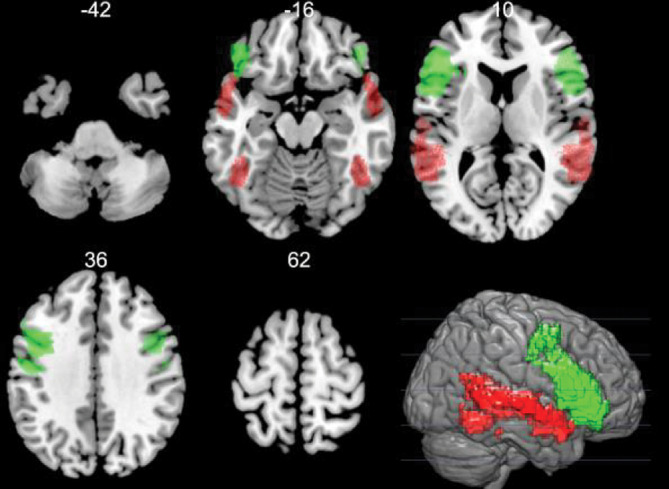
Regional language masks. Regional language masks created from an automated meta‐analysis of 885 studies for the term “language” on the large‐scale, meta‐analysis platform Neurosynth (http://neurosynth.org). Regional masks were used as inclusion masks for resting state fMRI FC analysis of LI and task fMRI activation LI. Regions examined included frontal (green), temporal (red), and combined frontal+temporal brain areas
2.7. Calculating and categorizing LI
LI was calculated for each patient's task fMRI activation using the LI Toolbox bootstrap method (Wilke & Schmithorst, 2006) (LI‐Toolbox for SPM12) in a set of predefined masks: (a) frontal+temporal, (b) frontal, (c) temporal. LI was calculated using the following equation:
where LH is thevoxel count in the left hemisphere and RH is the voxel count in the right hemisphere.
At rest, hemispheric language dominance was determined in three masks (frontal+temporal, frontal, and temporal) at four thresholds: p < .05, p < .01, p < .001, and the top 10% of connections. p‐values for thresholding correspond to the correlation coefficient of the connection between the seed time series and the time series of all other voxels included in the mask. The number of voxels within each hemisphere that survived above threshold was calculated and classified to produce an LI. Patients were considered left language dominant if LI ≥ 0.20, right language dominant if LI ≤ −0.20, and bilateral dominant if −0.20 < LI < 0.20 (Seghier, 2008).
2.8. Resting state LI methods
A current challenge to implementation of resting state fMRI for language lateralization is how to incorporate FC generated from both left and right hemisphere seeds in an unbiased way; therefore, LI was calculated in two separate methods that aimed to address this issue. In the first method, the language connectivity map in each mask (frontal, temporal, and frontal+temporal) and at each threshold was binarized. FC maps of each hemisphere ipsilateral to the seed location generated from left and right Broca's area seeds were combined into a composite thresholded connectivity map (Figure 2). LI was calculated on the combined ipsilateral to seed FC map using the following equation:
where LSLH is the voxel count from left seed, left hemisphere FC; RSRH is the voxel count from right seed, right hemisphere FC. This approach is supported by studies showing patients have greater intrahemispheric FC if their language dominant side is ipsilateral to the seed, and weaker intrahemispheric FC if their nondominant side is ipsilateral to the seed location (Doucet, Pustina, et al., 2015; Joliot & Tzourio‐Mazoyer, 2016; Wang et al., 2014). In the second method, LI for seeds in left and right Broca's areas were calculated as intra‐ minus interhemispheric FC voxels divided by the total voxels above threshold. Then the difference between the left seed LI minus the right seed LI was determined as the final LI value using the following equation:
where LSLH is the voxel count from left seed, left hemisphere FC; LSRH is the voxel count from left seed, right hemisphere FC; RSLH is the voxel count from right seed, left hemisphere FC; RSRH is the voxel count from right seed, right hemisphere FC. This method of determining LI in resting state fMRI relies on the assumption that there will be greater intrahemispheric than interhemispheric FC from the seed on the language dominant hemisphere. For example, if left Broca's seed shows greater intrahemispheric FC and right Broca's seed shows greater interhemispheric FC, there will be an additive effect and LI will be more strongly left dominant. If both left and right seeds show greater intrahemispheric FC than interhemispheric FC, the effect will be more bilateral. This method uses voxel counts to account for both intra‐ and interhemispheric FC from left and right seeds without the need for voxelwise matching. Since either seed could result in complete hemispheric dominance (LI = 1), the scale for this method ranges from −2 to 2.
FIGURE 2.
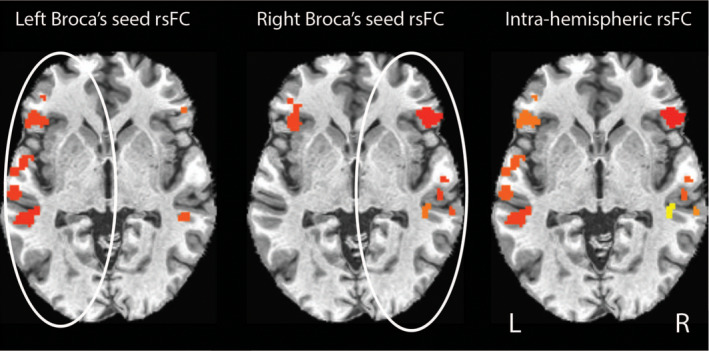
Intrahemispheric functional connectivity map. Example from a single patient using the method of calculating resting state laterality index (rs‐LI) from intrahemispheric functional connectivity of the ipsilateral seed. FC from left Broca's seed in the left hemisphere and FC from right Broca's seed in the right hemisphere were combined into a single intrahemispheric map, and rs‐LI was calculated from the intrahemispheric map. Colors indicate significant clusters that survived above threshold
2.9. LI comparison and statistical analysis
Correlation analysis was conducted between each patients' task LI (t‐LI) and resting state LI (rs‐LI) methods across three regional masks and four resting state FC thresholds (p < .05, p < .01, p < .001, top 10% connections). Correlation coefficient significance was determined using Spearman's Rho correlation test for nonparametric data. Concordance of LI categorization was assessed between rs‐LI and t‐LI with LI ≥ 0.2 as the cutoff to identify typical, left language dominance (Seghier, 2008). In addition, the effect of rs‐LI threshold on concordance rate was assessed. Factors of clinical significance including seizure onset age were also examined using Fisher's exact test. Relation to resection side and seizure outcomes after surgery was described in patients that had undergone surgery.
3. RESULTS
Patient demographics, including seizure onset age, resection type, and seizure outcome are included in Table 1 (mean age = 33; SD = 11; 12 female). Twenty‐five patients showed a consistent pattern of left dominance during ADDT fMRI on all three regional masks. Five patients had mixed or atypical language dominance (Figure 3). Two were strongly right (LI < −0.40) dominant across all regional masks, one had bilateral activation in frontal and combined frontal+temporal regions but right dominance in the temporal mask. Another was right dominant in frontal and combined frontal+temporal regions but had bilateral temporal activation. The last patient was left dominant in temporal and combined frontal+temporal regions and bilateral in the frontal mask (Table 2). Early seizure onset (<6 years old) was not associated with atypical language dominance during task fMRI (Fisher's exact test, p = 1), or any resting state fMRI laterality metric.
TABLE 1.
Patient demographics
| Patient | Sex | Age at fMRI | Age of seizure onset | Structural MRI | Resection type | Seizure outcome |
|---|---|---|---|---|---|---|
| 1 | M | 44 | 32 | Normal | R frontal topectomy | IA++ |
| 2 | M | 33 | 25 | Atrophy and nonspecific right frontal subcortical WM lesion | L ATL | IA+++ |
| 3 | F | 34 | 19 | Subtle increased FLAIR bilateral hippocampus | R ATL | IA++ |
| 4 | F | 31 | 26 | Normal | — | — |
| 5 | F | 56 | 41 | White matter signal abnormality | L ATL | IA |
| 6 | F | 28 | 18 | Left MTS | L ATL | IB++ |
| 7 | M | 34 | 3 | T2/FLAIR hyperintensity right occipital WM | R parietal topectomy | IIB++ |
| 8 | F | 40 | 25 | Right MTS | R ATL | IB++ |
| 9 | M | 25 | 19 | Normal | L frontal | IA+ |
| 10 | M | 27 | 21 | Normal | — | — |
| 11 | M | 21 | 18 | Normal | R frontal topectomy | IIIA |
| 12 | M | 18 | 9 | Normal | — | — |
| 13 | M | 57 | 32 | Right inferior temporal encephalomalacia | R ATL+ | IA+ |
| 14 | F | 33 | 18 mo | Right inferior medial frontal FCD | R frontal topectomy | IA+ |
| 15 | F | 36 | 17 | Left insular FCD | — | — |
| 16 | M | 20 | 17 | Normal | R ATL | IA+ |
| 17 | F | 53 | 50 | DVA in right parietal region | R ATL | IB+ |
| 18 | M | 20 | 18 | Normal | — | — |
| 19 | F | 21 | 13 | Right parietal encephalomalacia | R parietal topectomy | IIB |
| 20 | M | 18 | 5 | Normal | — | — |
| 21 | F | 33 | 13 | Possible malrotation left hippocampus | — | — |
| 22 | M | 32 | 3 | Normal | — | — |
| 23 | F | 30 | 16 | Right MTS | — | — |
| 24 | M | 32 | 23 | WM lesions | L ATL + WM lesion | IA++ |
| 25 | M | 52 | 45 | Normal | — | — |
| 26 | F | 27 | 21 | Mild right MTS | — | — |
| 27 | M | 32 | 15 | Bilateral nodular subependymal gray matter heterotopia | L ATL + incomplete lesion | IA+ |
| 28 | M | 44 | 38 | Right parietal FCD II and right MTS | R ATL | IB |
| 29 | M | 24 | 18 | Generalized atrophy and left hippocampal malrotation | — | — |
| 30 | M | 34 | 22 | Normal | — | — |
Note: IA—completely seizure free since surgery; IB—nondisabling simple partial seizures only since surgery; IIB—rare disabling seizures since surgery; IIIA—worthwhile seizure reduction; +, >12 months postoperation, ++, >24 months postoperation, +++, >36 months postoperation.
Abbreviations: ATL: anterior temporal lobectomy; DVA: developmental venous abnormality; FCD: focal cortical dysplasia; L: left; MTS: mesial temporal sclerosis; R: right; WM: white matter.
FIGURE 3.
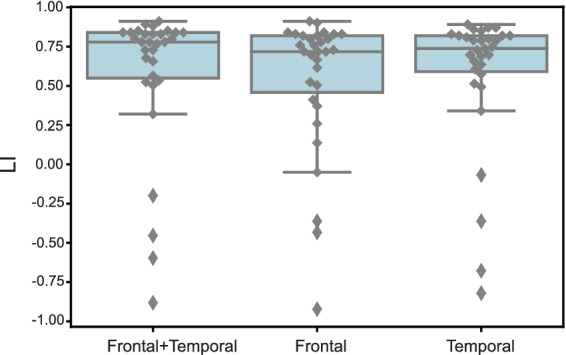
Language laterality index during task fMRI. Language laterality index (LI) distribution during task fMRI for patients with typical language dominance (LI ≥ 0.20) and atypical language dominance (LI < 0.20) across three regional masks
TABLE 2.
Task‐based and resting state language dominance classification in three regional masks
| Task LI | Resting state LI: Difference | Resting state LI: Intrahemispheric | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Frontal + temporal | Frontal | Temporal | Frontal + temporal | Frontal | Temporal | Frontal + temporal | Frontal | Temporal |
| 1 | L | L | L | R | R | R | BL | R | BL |
| 2 | L | L | L | L | L | L | BL | BL | BL |
| 3 | L | L | L | L | L | L | L | BL | L |
| 4 | L | L | L | BL | BL | L | BL | BL | BL |
| 5 | L | L | L | L | L | L | L | BL | BL |
| 6 | L | L | L | BL | L | BL | BL | BL | BL |
| 7 | L | L | L | BL | L | R | BL | BL | BL |
| 8 | L | L | L | BL | L | BL | BL | BL | BL |
| 9 | L | L | L | L | L | L | L | L | L |
| 10 | L | L | L | L | BL | L | BL | BL | BL |
| 11 | L | L | L | L | L | BL | BL | L | BL |
| 12 | L | L | L | R | R | R | BL | BL | BL |
| 13 | L | L | L | R | BL | R | R | BL | R |
| 14 | L | L | L | L | L | L | L | L | L |
| 15 | L | L | L | L | L | L | BL | BL | BL |
| 16 | L | L | L | L | L | BL | L | L | BL |
| 17 | L | L | L | BL | BL | L | BL | BL | BL |
| 18 | L | L | L | R | R | R | R | BL | BL |
| 19 | L | L | L | BL | BL | L | BL | BL | BL |
| 20 | L | L | L | L | L | R | BL | L | BL |
| 21 | L | L | L | L | BL | L | BL | BL | L |
| 22 | L | L | L | L | L | R | BL | L | BL |
| 23 | R | R | R | R | R | R | R | BL | R |
| 24 | R | R | R | R | R | R | BL | BL | BL |
| 25 | L | L | L | L | L | BL | BL | BL | BL |
| 26 | L | L | L | BL | L | L | BL | L | BL |
| 27 | BL | R | BL | R | BL | R | R | BL | R |
| 28 | L | L | L | R | R | R | R | R | R |
| 29 | R | BL | R | BL | BL | R | BL | BL | BL |
| 30 | L | L | BL | BL | BL | R | BL | BL | BL |
Abbreviations: BL, bilateral dominant; L, left dominant; R, right dominant.
For the intrahemispheric rs‐LI method, significant positive correlations were found between t‐LI and rs‐LI in all three regional masks when the threshold for resting state FC was set to the top 10% of connections (frontal+temporal: r = .47, p = .008; frontal r = .53, p = .002; temporal r = .41, p = .026). For the difference rs‐LI method, significant positive correlations were found between t‐LI and rs‐LI in all three regional masks when the threshold for resting state FC was set to the top 10% of connections (frontal+temporal: r = .43, p = .017; frontal r = .51, p = .004; temporal r = .51, p = .004). The relationship between LI from the intrahemispheric rs‐LI method and difference rs‐LI method was also examined. There was a strong significant positive correlation between the two rs‐LI methods in all three regional masks when the resting state FC threshold was set to the top 10% of connections (frontal+temporal: r = .97, p = 3.5 × 10−18; frontal: r = .96, p = 2.2 × 10−16; temporal: r = .90, p = 1.4 × 10−11). There were no significant correlations at any other threshold (Table 3). Resting state LI was also calculated as connectivity of left seed to all voxels included in the mask minus connectivity of right seed to all voxels included in the mask, divided by the sum of all voxels in connectivity above threshold. There were no significant correlations based on this resting state LI definition and task LI for any threshold or regional mask. This supports our other results demonstrating that the difference in connectivity across hemispheres is more indicative of language dominance during resting state fMRI than overall connectivity.
TABLE 3.
Resting state and task LI correlation analysis
| Mask type | Rs‐LI method and threshold | Spearman's rho | |
|---|---|---|---|
| Correlation (r) | p‐value | ||
| Frontal+temporal | t‐LI vs. intrahemispheric rs‐LI | ||
| Top 10% | .47 | .008 | |
| p < .05 | .12 | n.s. | |
| p < .01 | .18 | n.s. | |
| p < .001 | .22 | n.s. | |
| t‐LI vs. rs‐LI difference | |||
| Top 10% | .43 | .017 | |
| p < .05 | .14 | n.s. | |
| p < .01 | .23 | n.s. | |
| p < .001 | .21 | n.s. | |
| Intrahemispheric rs‐LI vs. rs‐LI difference | |||
| Top 10% | .97 | 3.5 × 10−18 | |
| p < .05 | .15 | n.s. | |
| p < .01 | −.10 | n.s. | |
| p < .001 | −.06 | n.s. | |
| Frontal | t‐LI vs. intrahemispheric rs‐LI | ||
| Top 10% | .53 | .002 | |
| p < .05 | .08 | n.s. | |
| p < .01 | .18 | n.s. | |
| p < .001 | .22 | n.s. | |
| t‐LI vs. rs‐LI difference | |||
| Top 10% | .51 | .004 | |
| p < .05 | .19 | n.s. | |
| p < .01 | .29 | n.s. | |
| p < .001 | .27 | n.s. | |
| Intrahemispheric rs‐LI vs. rs‐LI difference | |||
| Top 10% | .96 | 2.2 × 10−16 | |
| p < .05 | .08 | n.s. | |
| p < .01 | .05 | n.s. | |
| p < .001 | −.02 | n.s. | |
| Temporal | t‐LI vs. intrahemispheric rs‐LI | ||
| Top 10% | .41 | .026 | |
| p < .05 | .13 | n.s. | |
| p < .01 | .11 | n.s. | |
| p < .001 | .20 | n.s. | |
| t‐LI vs. rs‐LI difference | |||
| Top 10% | .51 | .004 | |
| p < .05 | .31 | n.s. | |
| p < .01 | .33 | n.s. | |
| p < .001 | .23 | n.s. | |
| Intrahemispheric rs‐LI vs. rs‐LI difference | |||
| Top 10% | .90 | 1.4 × 10−11 | |
| p < .05 | .25 | n.s. | |
| p < .01 | .32 | n.s. | |
| p < .001 | .12 | n.s. | |
Abbreviations: LI, laterality index; rs‐LI, resting state laterality index; t‐LI, task laterality index.
Concordance of language dominance classification between t‐LI and rs‐LI was examined at statistical thresholds showing significant correlation on Spearman's Rho test (Table 2; Figure 4). Using the intrahemispheric method of rs‐LI in the frontal+temporal mask at top10% threshold, 6 of 30 patients demonstrated concordance between t‐LI and rs‐LI. Twenty patients were discordant, showing left or right lateralization on t‐LI but bilateral on rs‐LI. An additional three discordant patients were left dominant on task but right dominant during resting state. One patient was bilateral on task and right dominant during resting state. This patient, Patient #27, showed mixed, atypical dominance across the three t‐LI regional masks. With the temporal mask using the intrahemispheric rs‐LI method, 7 patients were concordant between t‐LI and rs‐LI. Twenty‐one were left or right lateralized on t‐LI but bilateral on rs‐LI. Two were left dominant on task but showed right dominance during resting state. When FC was restricted to the frontal mask for the intrahemispheric method of rs‐LI, nine patients were concordant between t‐LI and rs‐LI. Nineteen were left or right lateralized on t‐LI but bilateral on rs‐LI. Two were left dominant on task but showed right dominance at rest. Cohen's Kappa statistics were calculated in addition to concordance rates and can be found in Table S2.
FIGURE 4.
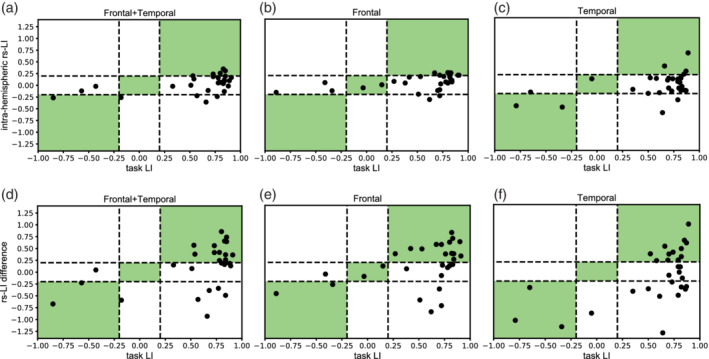
Concordance between task laterality indices and resting state laterality indices. Distribution of concordance between task laterality indices (t‐LI) and resting state laterality indices (rs‐LI) across three regional masks and two methods of calculating rs‐LI. Patients with LI ≥ 0.20 are left language dominant, LI ≤ −0.20 are right language dominant, and −0.20 < LI < 0.20 are bilateral language dominant. Concordant classifications are highlighted in green. Concordance between t‐LI and intrahemispheric rs‐LI method is shown in the top row in (a) frontal+temporal (b) frontal and (c) temporal regional masks. Concordance between t‐LI and rs‐LI difference is shown in the bottom row in (d) frontal+temporal (e) frontal and (f) temporal regional masks
A larger proportion of patients demonstrated concordance between t‐LI and rs‐LI using the difference method (Table 2; Figure 4). In the combined frontal+temporal mask, 15 patients were concordant between t‐LI and rs‐LI. Eight patients were left dominant on t‐LI but bilateral during resting state. Five patients were left dominant on t‐LI but showed right dominance on rs‐LI. One patient was bilateral on t‐LI and right dominant on rs‐LI, and another patient was right dominant on t‐LI and bilateral on rs‐LI. Both patients, patient #27 and #29, showed mixed, atypical dominance across the three regional masks for t‐LI. Looking at the frontal only mask, 19 patients demonstrated concordance between t‐LI and rs‐LI. Seven patients were discordant, showing left dominance on t‐LI and bilateral dominance during resting state. Another four patients were discordant, showing left dominance on t‐LI and right dominance on rs‐LI. In the temporal only mask, 15 patients demonstrated concordance between t‐LI and rs‐LI. Five patients were discordant, showing left dominance during task and bilateral dominance during resting state. Another nine patients were discordant, showing left dominance on t‐LI and right dominance on rs‐LI. Again, patient #29, who demonstrated mixed dominance across regional masks for t‐LI, was bilateral in the temporal mask for t‐LI but right dominant during resting state.
Concordance of language dominance classifications was also examined between the two rs‐LI methods (Table 2). In the combined frontal+temporal mask at top 10% threshold, 19 patients were concordant between the intrahemispheric rs‐LI method and the difference rs‐LI method. Five of the 19 were concordant left language dominant, five right language dominant, and nine bilateral. Eight patients were classified as left language dominant using one rs‐LI method but were classified as bilateral using the other rs‐LI method. Three patients were classified as right language dominant using one rs‐LI method but were classified as bilateral using the other rs‐LI method. Within the frontal only mask, 18 patients were concordant between the intrahemispheric rs‐LI method and difference rs‐LI method. Seven of the 18 were concordant left language dominant, two right language dominant, and none bilateral. Eight patients were classified as bilateral using one rs‐LI method and classified as left language dominant using the other rs‐LI method. Four patients were classified as bilateral using one rs‐LI method and classified as right language dominant using the other rs‐LI method. With the temporal only mask, 13 patients were concordant between the intrahemispheric rs‐LI method and the difference rs‐LI method. Four of the 13 were concordant left language dominant, four right language dominant, and five bilateral. Eight patients were classified as bilateral using one rs‐LI method and classified as left language dominant using the other rs‐LI method. Nine patients were classified as bilateral using one rs‐LI method and classified as right language dominant using the other rs‐LI method.
There was no difference in the rate of LI concordance between t‐LI and rs‐LI for patients scanned on 3T Siemens Skyra versus 3T GE Signa during task fMRI (parametric independent samples t‐test, p = .33). In addition to analysis of concordance, receiver operating characteristics (ROC) and area under the curve (AUC) analyses demonstrated both resting state LI methods have good classification performance of typical versus atypical language dominance compared to task LI as the standard of reference. These data can be found in Figure S1 and Table S3.
In an additional analysis, the cutoff for rs‐LI was varied while the cutoff for t‐LI remained constant at LI ≥ 0.2 for patients that were left language dominant. This was done to examine the effect of rs‐LI threshold on language dominance concordance rates between rs‐LI and t‐LI. Concordance rates generally declined as threshold for left language dominance increased from −1 to 1. Concordance rates were above 0.70 when then cutoff for left, typical dominance was set from −1 to 0. The largest decrease in concordance rate occurred after the rs‐LI cutoff for left language dominance exceeded 0 (Figure 5). The average concordance rate across masks and methods for calculating rs‐LI was 0.72 (range 0.57–0.80) when left language dominance was defined as LI ≥ 0.
FIGURE 5.
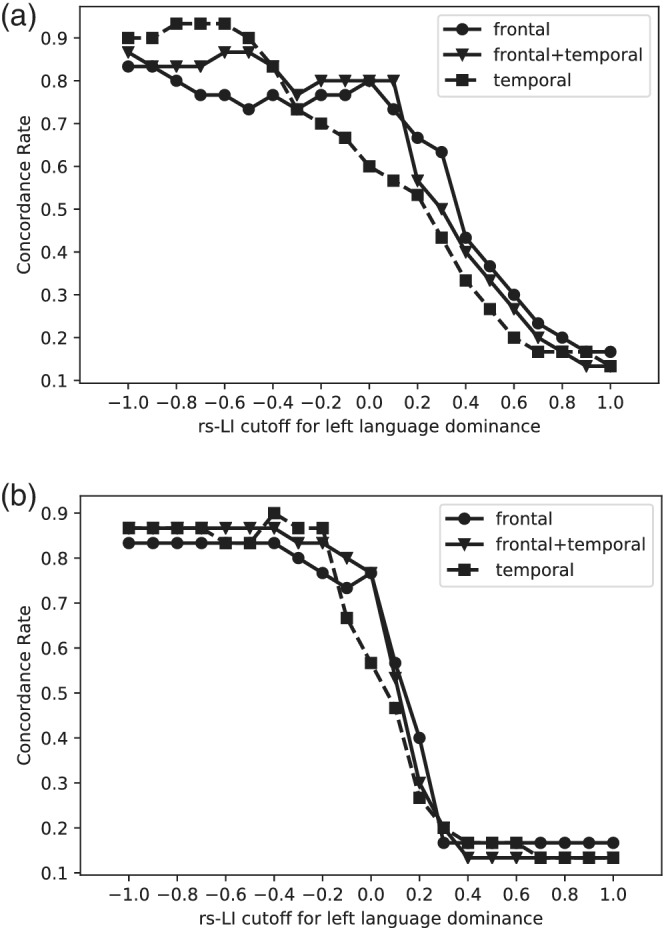
Effect of resting state LI threshold on concordance rate. The threshold for left language dominance for resting state laterality index (rs‐LI) varied while the threshold for left language dominance for task fMRI was held constant (LI ≥ 0.20). The rate of concordance for language dominance classification between rs‐LI and task LI (t‐LI) was examined as the threshold for typical, left language increased from −1 to 1 for rs‐LI. This relationship was examined for both rs‐LI methods (a) difference rs‐LI method and (b) intrahemispheric rs‐LI method. It was also examined across all three regional masks: 1) frontal+temporal 2) frontal only 3) temporal only
Seventeen patients had resections: six left‐sided and 11 right‐sided. We aimed to answer the question whether resection side, as a surrogate for focus localization, was related to the rate of concordance between t‐LI and rs‐LI. There was no difference in the frequency of concordant LI dominance classifications between patients with right‐sided resections and patients with left‐sided resections (parametric independent samples t‐test, p = .12). Nine patients were classified as left language dominant on task but showed right language dominance using one or more rs‐LI methods, regional masks, and threshold combinations that were significantly correlated with t‐LI. Four of these nine patients had a right‐sided seizure focus. Two of four reported complete seizure freedom (Engel class 1a) at least 12 months after surgery (mean follow‐up = 20 months). Patient #28 reported only nondisabling simple partial seizures since surgery (Engel class 1b) 10 months after surgery, and patient #6 reported only rare disabling seizures since surgery (Engel class 2b) 24 months after surgery (Table 1).
4. DISCUSSION
We found significant positive correlations between LI from task fMRI and two new methods of calculating LI with resting state fMRI. There was moderate concordance when comparing LI categorization from rs‐LI to t‐LI. Both methods of calculating LI with resting state fMRI yielded a larger proportion of patients that were bilateral, and LI at rest was less consistent across regional masks than t‐LI.
We used the fMRI ADDT task as our standard for comparison with resting state fMRI. This task reliably activates expressive frontal and receptive temporal processing regions associated with different domains of language function (Gaillard et al., 2007; Rosenberger et al., 2009). In addition, it is commonly used to identify language asymmetries with LI calculations in patients with epilepsy (Berl et al., 2014; Rosenberger et al., 2009; You et al., 2019). Laterality indices are used in a clinical context to help guide presurgical planning and predict postoperative language deficits (Janecek et al., 2013; Sabsevitz et al., 2003; Szaflarski et al., 2017; You et al., 2019). LI has also been used to compare the efficacy of task fMRI to the Wada and is now utilized to compare resting state to task fMRI (Desai et al., 2019; Liu et al., 2009; Teghipco et al., 2016).
We compared LI from task and resting state across three language masks. The first was a large mask encompassing both frontal and temporal regions derived from a meta‐analysis search of the term “language” on NeuroSynth (http://neurosynth.org). This mask was intended to capture the broad extent of FC networks seen at rest (Doucet, He, Sperling, Sharan, & Tracy, 2017) and to identify language networks potentially reorganized due to early injury or seizure onset age (Berl et al., 2014; Gaillard et al., 2007; Rasmussen & Milner, 1977). LI was also examined in frontal and temporal regional masks separately. By examining language lateralization on a regional basis, we aimed to capture the variability in typical and atypical dominance patterns. For example, some studies have shown a stronger association between early seizure onset and atypical language lateralization in temporal regions than frontal regions (Berl et al., 2014). We did not find an association between seizure onset age and atypical language dominance during task or resting state fMRI. The lack of association may be due to the small proportion of patients with seizure onset earlier than 6 years of age (13% vs. ~30% in previous studies of task fMRI) and smaller number of overall patients included (n = 31 vs. n > 100 in previous studies of task fMRI). Other reports of children with focal epilepsy have shown disruption of network organization during resting state fMRI (Ibrahim et al., 2015).
We introduced two new methods of calculating LI from seed‐based resting state fMRI and found significant correlations from both methods with task LI. A challenge of using seed‐based functional connectivity to calculate laterality indices is the need to incorporate connectivity measurements from selected regions of interest in both hemispheres. Previously proposed methods of calculating LI from seed‐based resting state fMRI were inadequate for patient populations (Teghipco et al., 2016). When language LI from resting state fMRI was correlated with language LI from four different task fMRI paradigms in both patients and healthy controls, a significant correlation was only observed during one task for a subset of patients with left hemisphere and left hemisphere temporal lobe lesions. In addition, this fMRI task generated bilateral language activation in the scanner and was deemed inappropriate for clinical consideration, therefore diminishing the clinical relevance of the relationship with resting state fMRI (Teghipco et al., 2016).
Conceptually, both resting state methods rely on observations of intrinsic connectivity seen previously in healthy volunteers and patients with epilepsy: subjects have greater intrahemispheric FC if their language dominant side is ipsilateral to the seed, and weaker intrahemispheric FC if their nondominant side is ipsilateral to the seed location (Doucet, Pustina, et al., 2015; Joliot & Tzourio‐Mazoyer, 2016; Wang et al., 2014). The major difference between the two methods of calculating LI is that one incorporates information from hemispheres both ipsilateral and contralateral to the seed (difference method) while the other uses only information from the hemisphere ipsilateral to the seed (intrahemispheric method). Interhemispheric FC is reportedly weak (Doucet, Pustina, et al., 2015; Joliot & Tzourio‐Mazoyer, 2016) and has been excluded from analyses in previous studies (Joliot & Tzourio‐Mazoyer, 2016). However, Doucet, Pustina, et al., 2015 found patterns of high intrahemispheric FC and low interhemispheric FC best predicted hemispheric language dominance. We designed and compared methods of calculating language LI during resting state fMRI using both strategies. Our intrahemispheric method weights all importance for language dominance on intrahemispheric FC and conceptually weights the influence of interhemispheric FC on language dominance as negligible. In other words, the language dominant hemisphere is completely responsible for determining language dominance and the nondominant hemisphere does not play a role. In contrast, the difference method grants equal importance to both intrahemispheric and interhemispheric FC on language dominance and incorporates how the disparity in FC between intra minus inter can influence language LI. While it is still unclear how large the nondominant hemisphere's role is in establishing language dominance, this method does acknowledge it as an important factor in understanding the overall language network at rest.
A significant correlation between rs‐LI and t‐LI was found only when resting state FC threshold was set to the top 10% of connections. This relatively new metric creates individualized language maps based on the amount of connections present in each patient and can be more reliable than conventional fixed statistical thresholds because it is more flexible to intersubject differences in connectivity compared to arbitrary cutoffs. A recent study found that the extent of top 10% fMRI language activation resected during temporal lobectomy predicted naming decline (You et al., 2019). Our results suggest that the top 10% threshold should be considered when examining language networks in resting state fMRI data.
Overall, we found only fair concordance between resting state and task fMRI, due, in part, to the large proportion of patients with left dominance on task and bilateral dominance at rest. This is consistent with other reports from seed‐based resting state methods. One study found a moderate correlation (r = .48, p < .005) between rs‐LI and t‐LI in 35 healthy controls; however, the maximum LI value at rest was 0.25 while the maximum LI value from task activation was 0.9. When their methodology was applied to an independent cohort of patients with epilepsy, 16 out of 17 patients showed left‐lateralized language (LI > 0) (Liu et al., 2009). In a different study using the same intrinsic LI calculation described in Liu et al., a significant correlation was only found between rs‐LI and the fMRI language task that elicited the most bilateral activation in their cohort (Teghipco et al., 2016). Another found language LI values from FC‐based networks were significantly less lateralized than activation during a verb generation task in healthy controls and patients with temporal lobe epilepsy (Doucet et al., 2017). Resting state networks were more bilateral, showing a wider network that encompassed and extended beyond brain regions activated during the task. While there appears to be a relationship between language network asymmetry at rest and during task, the degree of functional asymmetry may not be equivalent.
Studies of ICA‐based methods for resting state fMRI analysis demonstrated 96% language LI concordance with the intracarotid amobarbital procedure (IAP) in adults with intractable epilepsy (DeSalvo, Tanaka, Douw, et al., 2016) and 93% concordance with task‐based fMRI in children with intractable epilepsy (Desai et al., 2019). For task comparison in children, LI was determined using expert visual inspection. Cohorts examined and methods of determining LI were sufficiently different that it is challenging to evaluate why seed‐based reports show lower concordance rates without a direct comparison of ICA and seed‐based methods in a single study.
While LI values fall on a continuous scale, hemispheric language dominance is typically determined by comparing LI to a predefined threshold or cutoff. Language LI is usually set to 0.2 but has ranged from 0.0–0.4 in previous work (Liu et al., 2009; Seghier, 2008; You et al., 2019). Since language dominance is defined by LI threshold, it is important to consider when drawing comparisons between LI values of different clinical tests (i.e., task activation vs. IAP or task activation vs. resting state FC). While both task activation and resting state FC tests rely on changes in BOLD signal, it is unclear whether they should be held to the same LI threshold. Our data show the degree of laterality is less for FC, suggesting that a lower LI threshold may be more appropriate for this test; although this might introduce risk of overconfidence in determining dominance. Based on our analysis of rs‐LI threshold on language dominance concordance, a LI cutoff of LI ≥ 0 for left language dominance may be more appropriate in connectivity data. A larger study with step‐wise analysis of incremental thresholds in control populations would be beneficial in confirming our findings and elucidating the optimal LI threshold for FC data. In addition, clinical correlates, such as direct cortical stimulation and neuropsychological outcomes, are necessary to establish which LI thresholds may be clinically meaningful at rest in patient populations with epilepsy.
Consistency across regions was stronger for task than resting state fMRI. During task fMRI, 27 of 30 patients had the same dominance classification across frontal, temporal, and combined frontal+temporal regions. In contrast, only 12 patients and 14 patients showed the same dominance classification across all masks for the rs‐LI difference method and intrahemispheric rs‐LI method, respectively. It is not uncommon to have regional differences in LI classification, especially for patients with atypical language (Berl et al., 2014). However, this does not explain the high rate of discrepancy across regions seen in our results. It is possible that during a task anatomically distinct regions are more likely to be recruited and activated concurrently when they are located on the same hemisphere. In contrast, connectivity at rest may involve more diffuse connections in which subtle differences would affect language dominance patterns. Temporal regions had slightly lower concordance between t‐LI and rs‐LI than frontal regions. Lower concordance in temporal regions may be due to increased variability within this region related to disease processes. This is consistent with previous reports from fMRI language task activations (Rosenberger et al., 2009). Patterns of language activation previously observed in task activation studies appear to represent only a small portion of the complex connectivity observed at rest.
There are multiple factors to consider when deciding which rs‐LI method would be more appropriate to calculate language dominance. At higher LI thresholds standard to task fMRI (LI ≥ 0.2 for left language dominance), the difference rs‐LI method may be preferable due to its higher rate of concordance at this threshold. However, it also resulted in more patients being incorrectly identified as atypical language dominant. Overrepresentation of interhemispheric connections may be contributing to the inaccuracy of language classification in these patients. In contrast, the intrahemispheric rs‐LI method resulted in LI values that clustered more into what is traditionally seen as the bilateral dominance range (−0.2 < LI < 0.2). Adjusting the LI threshold to LI ≥ 0 for left language dominance may increase the concordance rate and result in more patients being correctly identified as left dominant. However, tighter clustering of all LI values may make accurate detection of atypical language more challenging. This may be due to the influence of interhemispheric connectivity being underrepresented in the language network model. As presented in this study, both methods demonstrate moderate concordance with task‐based LI but present risk of inaccurate dominance classification if applied to the context of clinical presurgical planning.
Clinical applications of resting state fMRI to epilepsy surgery include preoperative functional mapping to help guide surgical planning (Doucet, Pustina, et al., 2015; Liu et al., 2009; Mitchell, Hacker, & Breshears, 2013) and identifying prognostic markers of individual patients' relevant clinical outcomes after surgery (Boerwinkle, Mohanty, Foldes, et al., 2017; He et al., 2017; Negishi, Martuzzi, Novotny, Spencer, & Constable, 2011). There are limited data on the ability of resting state fMRI to predict neurocognitive outcomes after surgery, including language and memory (Audrain, Barnett, & McAndrews, 2018; Doucet, Rider, & Taylor, 2015; McCormick, Quraan, Cohn, Valiante, & McAndrews, 2013). In addition, others have shown that the best predictive model of verbal fluency change following dominant anterior temporal lobectomy was one that combined all three imaging modalities: task fMRI, resting state fMRI, and DTI (Osipowicz, Sperling, Sharan, & Tracy, 2016). While resting state fMRI continues to show potential in control and patient populations, its applicability to the clinical realm has yet to demonstrate equal promise to task‐based fMRI methods.
There are several limitations to this study. Task and resting state fMRI are both subject to technical factors (Szaflarski et al., 2017). Failure of patients to understand or comply with task instructions may affect task fMRI results. Motion and other non‐neuronal physiological fluctuations can cause poor signal to noise ratio. In an attempt to minimize the contribution of such nuisance factors to our resting data—which is more strongly affected by these undesired sources of fluctuation—we used a ME‐ICA denoising strategy previously shown to improve identification of intrinsic connectivity networks in resting state fMRI (Kundu et al., 2012; Kundu et al., 2013). In fact, one recent study found that while apparent left‐hemisphere language network dominance was seen across several processing approaches, laterality index values were higher when using an integrated ME processing approach (Amemiya, Yamashita, Takao, & Abe, 2019).
While we defined Broca's area as the seed in our model based on its reproducibility and lateralizing effects at rest (Doucet, Pustina, et al., 2015; Tomasi & Volkow, 2012; Zhu et al., 2014), there are inherent limitations to seed‐based resting state fMRI. FC patterns are confined to brain regions functionally connected to the seed, necessitating several seeds and analyses to map multiple networks. Seed selection is user‐dependent, and therefore can affect the interpretation of results. Patients with epilepsy can have functional reorganization of expressive and receptive language areas. Reorganization and compensation predominantly occurs in the right hemisphere homologs rather than through intrahemispheric reorganization. In addition, frontal language regions are more localized and reliable than temporal language regions in both patients and normal controls (Rosenberger et al., 2009). Functional reorganization was an important consideration in this study design that we attempted to address by choosing a seed in Broca's area based on a probabilistic atlas. However, an uncertainty remains that intrahemispheric reorganization could have occurred in an individual patient that would lead to the incorrect identification of Broca's area. Alternative approaches include data‐driven methods of calculating FC such as ICA and cluster analysis. These data‐driven techniques can uncover novel networks but may lead to spurious conclusions about network relationships in the absence of additional information or other a priori hypotheses. Another assumption in our model was that functional connectivity remains static across the 6‐minute resting state fMRI run. We extracted the FC language pattern that, on average, was the strongest and most stable across the run (Chen, Rubinov, & Chang, 2017). Nonetheless, failure to account for dynamic changes across a range of timescales may lead to oversimplification of observed network findings.
In conclusion, we introduced two new methods of calculating LI during resting state fMRI using seed‐based methods. There was a significant positive correlation and moderate concordance between language laterality indices from task and resting state fMRI in patients with epilepsy. Similar to task fMRI, LI dominance classifications during resting state fMRI depend on which regions of the brain are analyzed. We found that rs‐LI was generally less consistent and more bilateral across regional masks than t‐LI. Further investigation is required before resting state fMRI can be applied in the clinical context of presurgical functional mapping in epilepsy surgery.
Supporting information
Table S1 Regions included in task activation and resting state functional connectivity maps
Table S2 Cohen's Kappa statistics for comparison of fMRI task LI and resting state LI.
Figure S1 Receiver operating characteristics (ROC) curve analyses across three brain regions: Frontal+Temporal, Frontal, and Temporal. Language lateralization (LI) from task fMRI was used as the standard of reference to determine whether a patient had typical (left) or atypical (right or bilateral) language dominance. Classification as typical or atypical language dominance from intrahemispheric resting state LI and difference resting state LI were each compared to the standard task LI.
Table S3 Area under the Curve (AUC) values for ROC analysis.
Rolinski R, You X, Gonzalez‐Castillo J, et al. Language lateralization from task‐based and resting state functional MRI in patients with epilepsy. Hum Brain Mapp. 2020;41:3133–3146. 10.1002/hbm.25003
Funding information National Institutes of Health, Grant/Award Number: ZIANS002236
DATA AVAILABILITY STATEMENT
De‐identified data from this study, conducted between 2014 and 2018, may be shared as prescribed by NIH policy.
REFERENCES
- Amemiya, S. , Yamashita, H. , Takao, H. , & Abe, O. (2019). Integrated multi‐echo denoising strategy improves identification of inherent language laterality. Magnetic Resonance in Medicine, 81, 3262–3271. [DOI] [PubMed] [Google Scholar]
- Amunts, K. , Schleicher, A. , Burgel, U. , Mohlberg, H. , Uylings, H. B. M. , & Zilles, K. (1999). Broca's region revisited: Cytoarchitecture and intersubject variability. The Journal of Comparative Neurology, 412, 319–341. [DOI] [PubMed] [Google Scholar]
- Audrain, S. , Barnett, A. J. , & McAndrews, M. P. (2018). Language network measures at rest indicate individual differences in naming decline after anterior temporal lobe resection. Human Brain Mapping, 39, 4404–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austermuehle, A. , Cocjin, J. , Reynolds, R. , Agrawal, S. , Sepeta, L. , Gaillard, W. D. , … Theodore, W. H. (2017). Language functional MRI and direct cortical stimulation in epilepsy preoperative planning. Annals of Neurology, 81, 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, C. F. , Li, A. X. , Blumenfeld, H. , Constable, R. T. , Alkawadri, R. , Bickel, S. , … Hirsch, L. J. (2018). Presurgical language fMRI: Clinical practices and patient outcomes in epilepsy surgical planning. Human Brain Mapping, 39, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl, M. M. , Zimmaro, L. A. , Khan, O. I. , Dustin, I. , Ritzl, E. , Duke, E. S. , … Gaillard, W. D. (2014). Characterization of atypical language activation patterns in focal epilepsy. Annals of Neurology, 75, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , Swanson, S. J. , Hammeke, T. A. , & Sabsevitz, D. S. (2008). A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia, 49(12), 1980–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Boerwinkle, V. L. , Mohanty, D. , Foldes, S. T. , Guffey, D. , Minard, C. G. , Vedantam, A. , … Curry, D. J. (2017). Correlating resting‐state functional magnetic resonance imaging connectivity by independent component analysis‐based epileptogenic zones with intracranial electroencephalogram localized seizure onset zones and surgical outcomes in prospective pediatric intractable epilepsy study. Brain Connectivity, 7, 424–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, S. B. , Thompson, P. J. , Yogarajah, M. , Vollmar, C. , Powell, R. H. W. , Symms, M. R. , … Duncan, J. S. (2012). Imaging language networks before and after anterior temporal lobe resection: Results of a longitudinal fMRI study. Epilepsia, 53, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. , & Fitzpatrick, J. M. (1992). A technique for accurate magnetic resonance imaging in the presence of field inhomogeneities. IEEE Transactions on Medical Imaging, 11, 319–329. [DOI] [PubMed] [Google Scholar]
- Chen, J. E. , Rubinov, M. , & Chang, C. (2017). Methods of considerations for dynamic analysis of functional MR imaging data. Neuroimaging Clinics of North America, 27, 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. D. , Nencka, A. S. , & Wang, Y. (2018). Multiband multi‐echo simultaneous ASL/BOLD for task‐induced functional MRI. Plos One, 13, e0190427 10.1371/journal.pone.0190427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Desai, V. R. , Vedantam, A. , Lam, S. K. , Mirea, L. , Foldes, S. T. , Curry, D. J. , … Boerwinkle, V. L. (2019). Language lateralization with resting‐state and task‐based functional MRI in pediatric epilepsy. Journal of Neurosurgery. Pediatrics, 23, 171–177. [DOI] [PubMed] [Google Scholar]
- DeSalvo, M. N. , Tanaka, N. , Douw, L. , Leveroni, C. L. , Buchbinder, B. R. , Greve, D. N. , & Stufflebeam, S. M. (2016). Resting‐state functional MR imaging for determining language laterality in intractable epilepsy. Radiology, 281, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale, O. , Sethi, A. , Laganà, M. , Baglio, F. , Baselli, G. , Kundu, P. , … Cercignani, M. (2017). Comparing resting state fMRI de‐noising approaches using multi‐ and single‐echo acqusitions. Plos One, 12, e0173289 10.1371/journal.pone.0173289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. E. , He, X. , Sperling, M. R. , Sharan, A. , & Tracy, J. I. (2017). From “rest” to language task: Task activation selects and prunes from broader resting‐state network. Human Brain Mapping, 38, 2540–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. E. , Pustina, D. , Skidmore, C. , Sharan, A. , Sperling, M. R. , & Tracy, J. I. (2015). Resting state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normal. Human Brain Mapping, 36, 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. E. , Rider, R. , & Taylor, N. (2015). Presurgery resting‐state local graph‐theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia, 56, 517–526. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8(9), 700–711. [DOI] [PubMed] [Google Scholar]
- Gaillard, W. D. , Balsamo, L. , Xu, B. , McKinney, C. , Papero, P. H. , Weinstein, S. , … Theodore, W. H. (2004). fMRI language task panel improves determination of language dominance. Neurology, 63(8), 1403–1408. [DOI] [PubMed] [Google Scholar]
- Gaillard, W. D. , Berl, M. M. , Moore, E. N. , Ritzl, E. K. , Rosenberger, L. R. , Weinstein, S. L. , … Theodore, W. H. (2007). Atypical language in lesional and nonlesional complex partial epilepsy. Neurology, 69, 1761–1771. [DOI] [PubMed] [Google Scholar]
- He, X. , Doucet, G. E. , Pustina, D. , Sperling, M. R. , Sharan, A. D. , & Tracy, J. I. (2017). Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology, 88, 2285–2293. [DOI] [PubMed] [Google Scholar]
- Ibrahim, G. M. , Morgan, B. R. , Doesburg, S. M. , Taylor, M. J. , Pang, E. W. , Donner, E. , … Snead, O. C., III . (2015). Atypical language laterality is associated with large‐scale disruption of network integration in children with intractable focal epilepsy. Cortex, 65, 83–88. [DOI] [PubMed] [Google Scholar]
- Janecek, J. K. , Swanson, S. J. , Sabsevitz, D. S. , Hammeke, T. A. , Raghavan, M. , Mueller, W. , & Binder, J. R. (2013). Naming outcome prediction in patients with discordant Wada and fMRI language lateralization. Epilepsy & Behavior, 27, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot, M. , & Tzourio‐Mazoyer, N. (2016). And Mazoyer. Intra‐hemispheric intrinsic connectivity asymmetry and its relationship with handedness and language lateralization. Neuropsychologia, 93, 437–447. [DOI] [PubMed] [Google Scholar]
- Kundu, P. , Brenowitz, N. D. , Voon, V. , Worbe, Y. , Vertes, P. E. , Inati, S. J. , … Bullmore, E. T. (2013). Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences of the United States of America, 110, 16187–16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, P. , Inati, S. J. , Evans, J. W. , Luh, W. M. , & Bandettini, P. A. (2012). Differentiating BOLD and non‐BOLD signals in fMRI time series using multi‐echo EPI. NeuroImage, 60(3), 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, P. , Voon, V. , Balchandani, P. , Lombardo, M. V. , Poser, B. A. , & Bandettini, P. A. (2019). Multi‐echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage, 154, 59–80. [DOI] [PubMed] [Google Scholar]
- Lehericy, S. , Cohen, L. , Bazin, B. , Samson, S. , Giacomini, E. , Rougetet, R. , … Baulac, M. (2000). Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology, 54(8), 1625–1633. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Stufflebeam, S. M. , Sepulcre, J. , Hedden, T. , & Buckner, R. L. (2009). Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proceedings of the National Academy of Sciences of the United States of America, 106, 20499–20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, W. , Peck, K. K. , Brennan, N. , Mallela, A. , & Holodny, A. (2017). Left‐lateralization of resting state functional connectivity between the pre‐supplementary motor area and primary language areas. Neuroreport, 28, 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, C. , Quraan, M. , Cohn, M. , Valiante, T. A. , & McAndrews, M. P. (2013). Default mode network connectivity indicates episodic memory capacity in mesial temporal lobe epilepsy. Epilepsia, 54, 809–818. [DOI] [PubMed] [Google Scholar]
- Mitchell, T. J. , Hacker, C. D. , & Breshears, J. D. (2013). A novel data‐driven approach to preoperative mapping of functional cortex using resting‐state functional magnetic resonance imaging. Neurosurgery, 73, 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi, M. , Martuzzi, R. , Novotny, E. J. , Spencer, D. D. , & Constable, T. (2011). Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia, 52, 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipowicz, K. , Sperling, M. R. , Sharan, A. D. , & Tracy, J. I. (2016). Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. Journal of Neurosurgery, 124, 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, T. , & Milner, B. (1977). The role of early left‐brain injury in determining lateralization of cerebral speech functions. Annals of the New York Academy of Sciences, 299, 355–369. [DOI] [PubMed] [Google Scholar]
- Rolinski, R. , Austermuehle, A. , Wiggs, E. , Agrawal, S. , Sepeta, L. N. , Gaillard, W. D. , … Theodore, W. H. (2019). Functional MRI and direct cortical stimulation: Predictions of postoperative language decline. Epilepsia, 60, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger, L. R. , Zeck, J. , Berl, M. M. , Moore, E. N. , Ritzl, E. K. , Shamim, S. , … Gaillard, W. D. (2009). Interhemispheric and intraheispheric language reorganization in complex partial epilepsy. Neurology, 72, 1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz, D. S. , Swanson, S. J. , & Hammeke, T. A. (2003). Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology, 60, 1788–1792. [DOI] [PubMed] [Google Scholar]
- Seghier, M. L. (2008). Laterality index in functional MRI: Methodological issues. Magnetic Resonance Imaging, 26(5), 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Gloss, D. , Binder, J. R. , Gaillard, W. D. , Golby, A. J. , Holland, S. K. , … Theodore, W. H. (2017). Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Neurology, 88, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teghipco, A. , Hussain, A. , & Tivarus, M. E. (2016). Disrupted functional connectivity affects resting state based language lateralization. Neuroimage Clinical, 12, 910–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry, 17, 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Buckner, R. L. , & Liu, H. (2014). Functional specialization in the human brain estimated by in intrinsic hemispheric interaction. The Journal of Neuroscience, 34, 12341–12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke, M. , & Schmithorst, V. J. (2006). A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage, 33, 522–530. [DOI] [PubMed] [Google Scholar]
- You, X. , Adjouadi, M. , Guillen, M. R. , Ayala, M. , Barreto, A. , Rishe, N. , … Gaillard, W. D. (2011). Sub‐patterns of language network reorganization in pediatric localization related epilepsy: A multisite study. Human Brain Mapping, 32, 784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, X. , Zachery, A. , Fanto, E. J. , Norato, G. , Germeyan, S. C. , Emery, E. J. , … Theodore, W. H. (2019). fMRI prediction of naming change after adult temporal lobe epilepsy surgery: Activation matters. Epilepsia, 60, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Fan, Y. , Zou, Q. , Wang, J. , Gao, J. H. , & Niu, Z. (2014). Temporal reliability and lateralization of the resting‐state language network. PLoS One, 9, e85880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Regions included in task activation and resting state functional connectivity maps
Table S2 Cohen's Kappa statistics for comparison of fMRI task LI and resting state LI.
Figure S1 Receiver operating characteristics (ROC) curve analyses across three brain regions: Frontal+Temporal, Frontal, and Temporal. Language lateralization (LI) from task fMRI was used as the standard of reference to determine whether a patient had typical (left) or atypical (right or bilateral) language dominance. Classification as typical or atypical language dominance from intrahemispheric resting state LI and difference resting state LI were each compared to the standard task LI.
Table S3 Area under the Curve (AUC) values for ROC analysis.
Data Availability Statement
De‐identified data from this study, conducted between 2014 and 2018, may be shared as prescribed by NIH policy.


