Abstract
Cancer remains the leading cause of death worldwide. Traditional treatments such as surgery, radiation, and chemotherapy have had limited efficacy, especially with late stage cancers. Cancer immunotherapy and targeted therapy have revolutionized how cancer is treated, especially in patients with late stage disease. In 2013 cancer immunotherapy was named the breakthrough of the year, partially due to the established efficacy of blockade of CTLA-4 and PD-1, both T cell co-inhibitory molecules involved in tumor-induced immunosuppression. Though early trials promised success, toxicity and tolerance to immunotherapy have hindered long term successes. Optimizing the use of co-stimulatory and co-inhibitory pathways has the potential to increase the effectiveness of T cell-mediated antitumor immune response, leading to increased efficacy of cancer immunotherapy. This review will address major T cell co-stimulatory and co-inhibitory pathways and the role they play in regulating immune responses during cancer development and treatment.
Keywords: Cancer immunotherapy, Co-stimulation, Co-inhibition, combination therapy, autoimmunity
Introduction:
Recent advancements in the field of tumor immunology have shown that harnessing the potential of the immune system is needed to eradicate cancer. Immune responses are modulated by a complex network of checks and balances, which include co-stimulatory and co-inhibitory pathways involved in regulating T cell activation and function. Activation of T cells requires at least two signals delivered by the antigen presenting cells (APC). The first signal is antigen presented in the form of peptides bound to a major histocompatibility complex (MHC). The peptide recognized by the T cell receptor and this interaction provides specificity to the T cell response. The second signal is provided by co-stimulatory ligands on APCs that interact with corresponding receptors on the T cell surface. Without co-stimulation T cells will either die or become anergic [1]. T cells require co-stimulatory signals for optimal proliferation, differentiation, and survival making co-stimulation necessary to induce productive immune responses. Antitumor immune responses depend on efficient presentation of tumor antigens and co-stimulatory signals by host antigen presenting cells. Cancer evades the immune system through various mechanisms including the use of regulatory cells, anti-inflammatory cytokines, decreased stimulatory receptors, defective antigen presentation, T cell tolerance, and apoptosis. Traditional therapies including chemotherapy, radiation, and surgery are often accompanied with transient immune suppression. This can increase a patient’s risk of infection along with decreased ability of immune responses to cancer [2]. Though these pillars of cancer treatment are still used today new strategies for tumor treatment are developing. New therapies include using cellular targets such as T regulatory cells and myeloid derived suppressor cells, vaccine therapy, and adoptive T cell transfer therapy.
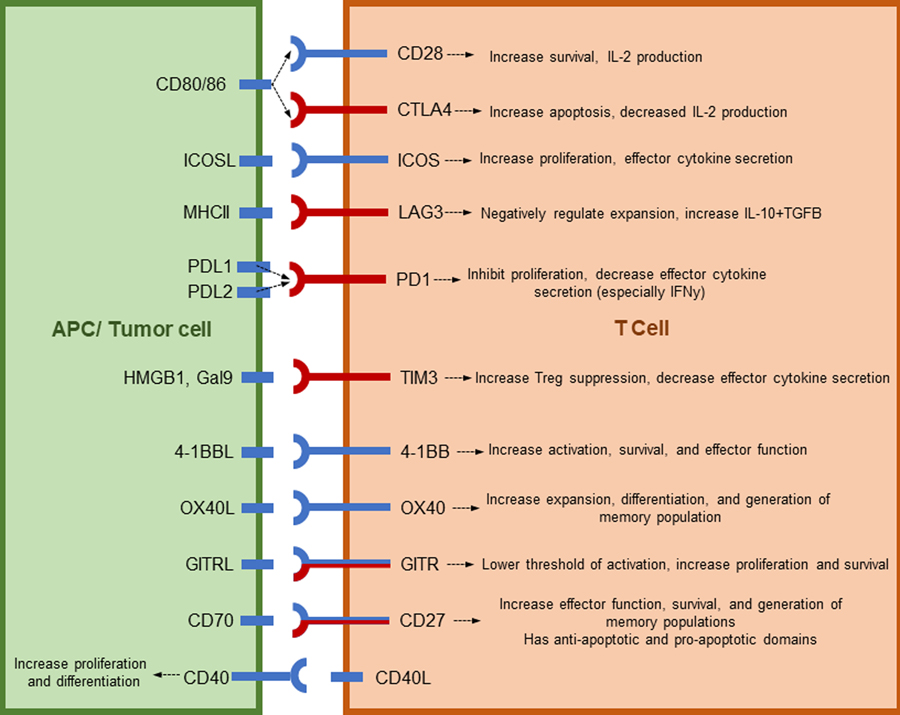
The latest tumor immunotherapy is molecular therapy directed against co-stimulatory and co-inhibitory markers in the tumor microenvironment. The expression of these markers is highly versatile and responsive to changes in the tissue environment, making them ideal candidates for tumor immunotherapy. Co-stimulatory and co-inhibitory signals can be broken down into a few major families, two of which are addressed in this review (Figure 1), the Ig family and the TNF family. Studies into CD28 were the first to explore and identify the Ig superfamily of receptor-ligand pairs. Interactions between receptors and ligands in the Ig family are key for T-B cell interaction and development of some secondary lymphoid organs. This family includes ICOS, PD1, LAG3, CTLA-4 and CD28 [3]. These receptors were first thought to only control the expansion and survival of naïve T cells, but more recently have been shown to have both co-stimulatory and co-inhibitory functions on naïve and activated T cells [4]. The TNF superfamily of ligands and receptors provide key communication between various cell types during development. This family consists of TNFR1 and 2, HVEM, CD30, 4-1BB, OX40, GITR, CD27, and CD40. Many members of the TNFR family share ligands and downstream signaling pathways creating a dense network of co-stimulatory and co-inhibitory pathways [5]. Understanding the mechanism of action of these receptors and ligands will help achieve therapeutic effectiveness in human diseases.
Figure 1:
Costimulatory and coinhibitory molecules discussed in this review.
Ig-Superfamily Receptors
CD28 and CTLA-4
Expression and Signaling
CD28 is one of the best characterized co-stimulatory molecules on T cells. Functional CD28 is critical for co-stimulation of naïve T lymphocytes and T regulatory (Treg) cells [6, 7]. Without CD28 co-stimulation T cell receptor (TCR) signaling often induces an anergic state or cell death [8]. CD28 plays a critical role in the survival of both effector T cells and Treg cells, as shown by the rapid expanse of T cells in culture after CD28 ligation [9]. The function of CD28 in activated T cells is counteracted by CTLA-4, which competes for ligand binding at the immune synapse. CD28 and CTLA-4 compete for binding to ligands CD80 and CD86. Since CTLA-4 displays a higher avidity for the ligands it displaces CD28 [10]. CTLA-4 dampens T cell responses, potentially through increasing the threshold for TCR signaling, which can protect against the development of autoimmune diseases, such as lupus and autoimmune thyroid disease [8]. CTLA-4 reduces IL-2 production and increases apoptosis, whereas CD28 increases IL-2 production and leads to increased cell survival [11]. In vitro assays show that CTLA-4 blocks the activation of transcription factors preferentially activated by CD28, including cJun and NFκB [12].
Immunotherapy
As humans age CD28 levels decrease on T cells, especially CD8+ populations, which may decrease the potential use for treatment in aging cancer populations [13]. Previous studies suggest that this decrease may be an adaptation of the immune system against chronic stimulation. It was noted that the change was due to a decrease in the percentage of CD28+ cells, not in the expression of CD28 per cell [14]. Early attempts at manipulating CD28 in disease were unsuccessful partially because of the low avidity of CD28 for its ligands and nonspecific polyclonal T cell activation. In contrast, CTLA-4 was very effective at binding CD80/86. CTLA-4 blocks the engagement of CD28 with CD80/86 and is able to inhibit the progression of cell cycle, differentiation, and survival making it an ideal treatment candidate for long-term organ graft survival [15–17]. Studies show that tumor cells transfected with CD80/86 become more immunogenic and are subsequently rejected, increasing interest in using this pathway for tumor immunotherapy [18, 19]. Early CD28 super-agonist trials were associated with serious toxicities and abandoned in phase I clinical trials [20, 21]. Since then, localized and targeted use of CD28 monoclonal antibodies (mAbs) has been tested for improved effects compared to early super-agonists [22].
Chimeric antigen receptor modified T cells (CAR-T) have been created in the hopes of harnessing the antibody specificity, homing, tissue penetration, and target destruction of T cells to fight B cell lineage malignancies. The chimeric receptor features the extracellular antigen binding domain from a tumor specific monoclonal antibody, typically anti-CD19. The transmembrane and intracellular domains of the receptor are derived from T cell signaling molecules, including CD3 and costimulatory signaling domains. The second generation of CAR-T cells used the CD28 co-stimulatory cytoplasmic domain to further enhance T cell function [23]. Studies have shown a complete response rate of over 90% when treating pediatric or adult acute lymphoblastic leukemia (ALL) with second-generation CAR-T cells. When treating solid tumors, the efficacy of CAR-T therapy is reduced. This may be due to several reasons including immunosuppressive factors present in the tumor microenvironment and T cell access to tumors. This immunosuppressive barrier has prompted further studies into third-generation CAR-T cells, which combine multiple intracellular costimulatory domains to enhance cytotoxicity and durability, and more recently T cells redirected for universal cytokine mediated killing (TRUCKs). TRUCK cells are developed from second-generation CARs with additional genes for cytokine production and release [24].
CD28 and CTLA-4 are critical regulators in autoimmune disease and tolerance to solid organ transplants. Animal models using CD28 deficient mice have shown a reduction of disease intensity in some autoimmune diseases [25–27]. In fact, CD28- T cells have been used in transplants to promote tolerance by tolerizing allogeneic antigen presenting cell (APCs). The interaction of CD28- T cells with allogeneic APCs induced the expression of inhibitory receptors and down-regulation of costimulatory molecules on the APCs. This in turn converted effector T cells into suppressive FOXP3+ T regulatory cells [28]. But, it is unclear to date whether CD28- T cells are the cause or consequence of infectious and inflammatory conditions [29, 30]. Studies of mutations in the CTLA-4 locus highlighted the importance of CTLA-4 in immune homeostasis. Patients with CTLA-4 mutations were observed to have decreased suppressive function in Treg cells and extensive CD4+ T cell infiltrate in several organs [31, 32]. Clinical trials using CTLA-4Ig for reversing autoimmune disease and inducing allograft tolerance are currently ongoing in addition to the proven use of CTLA-4Ig in arthritis and prevention of rejection of renal transplants [33–35]. Currently, the long-term benefits of CTLA-4Ig treatment outweigh the potential drawback of lymphoproliferative disorder [36, 37]. The use of CTLA-4 on Treg populations in vivo has shown contradictory results in solid organ transplant models. Some studies show no change to circulating Treg cells, but a significant increase in FoxP3/CD3 ratios in graft biopsies after CTLA-4 treatment [38]. Other studies show that CTLA-4 antibodies in vivo do not promote the expansion of Treg cells and will not induce tolerance in solid organ transplants [39]. Many clinical trials looking at different disease types are still ongoing for both CD28 and CTLA-4 (Table 1). Most of the completed trials used CTLA-4 blockade as a monotherapy, but ongoing and future clinical trials are increasingly combining this therapy with other traditional or molecular therapies. FDA has approved a monoclonal antibody targeting CTLA-4 (ipilimumab) to treat melanoma and more recently renal cell carcinoma. Unfortunately, treatment has been associated with severe and potentially fatal adverse side effects in about 10–20% of patients. Dermatologic toxicity, enterocolitis, hepatotoxicity, and neurological toxicity are the most commonly reported immune related adverse events following treatment [40, 41].
Table 1:
Ig Superfamily completed, ongoing, and upcoming clinical trials.
| Co-stimulatory Molecule | Number | Status | Main Condition Treated | Treatment | Early Phase 1 | Phase 1 | Phase 2 | Phase 3 | Phase 4 | N/A: | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD28 | 14 | Active, not-recruiting | Hematological tumors | Hepatitis | CAR-T therapy: 10 Anti-viral: 1 No interventions: 3 |
- | 10 | 2 | - | - | 1 |
| Glioblastoma | Other | ||||||||||
| 33 | Completed | Hematological tumors | Ovarian/Breast | Ex vivo T cells: 9 Anti-viral: 7 Other: 10 No intervention: 7 |
- | 7 | 10 | 3 | 2 | 8 | |
| Kidney | Lung | ||||||||||
| HIV/viral | Other | ||||||||||
| Autoimmune/Allergy | |||||||||||
| 50 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| CTLA-4 | 54 | Active, not-recruiting | Melanoma | Pancreatic | Monotherapy: 14 Combo-therapy: 37 No interventions: 3 |
2 | 18 | 24 | 9 | 1 | 1 |
| Renal | Autoimmune/Allergy | ||||||||||
| Liver | Breast/Ovarian | ||||||||||
| Head Neck | Hepatitis | ||||||||||
| Lung | Solid tumor | ||||||||||
| 79 | Completed | Melanoma | Autoimmune/Allergy | Monotherapy: 50 Combo-therapy: 25 No interventions: 4 |
1 | 27 | 32 | 21 | - | 2 | |
| Renal | Breast/Ovarian | ||||||||||
| Liver | Hepatitis | ||||||||||
| Hematological tumors | Pancreatic | ||||||||||
| Lung | Solid tumor | ||||||||||
| Prostate | |||||||||||
| 182 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| ICOS | 4 | Moving forward: Not yet recruiting/Recruiting | |||||||||
| LAG3 | 2 | Active, not-recruiting | Melanoma | Combo-therapy: 2 | - | 1 | 1 | - | - | ||
| Colorectal caner | |||||||||||
| 6 | Completed | Renal Cell Carcinoma | Hepatitis | Monotherapy: 5 Combo-therapy: 1 |
- | 6 | - | - | - | - | |
| Autoimmune/Allergy | Other | ||||||||||
| Breast | |||||||||||
| 39 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| TIM3 | 6 | Completed | Autoimmune/Allergy | Breast | Monotherapy: 2 Combo-therapy: 1 No interventions: 3 |
- | 1 | 1 | 1 | - | 2 |
| Sepsis | Lung | ||||||||||
| Glomerulonephritis | |||||||||||
| 23 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| PD1 | 176 | Active, not-recruiting | Hematological tumors | Liver | Monotherapy: 60 Combo-therapy: 38 No interventions: 2 |
2 | 74 | 78 | 36 | 1 | 2 |
| Breast/Ovarian | Melanoma | ||||||||||
| HIV/viral | Lung | ||||||||||
| Autoimmune/Allergy | Brain | ||||||||||
| Renal | Other | ||||||||||
| 65 | Completed | Hematological tumors | Colorectal | Monotherapy: 30 Combo-therapy: 17 No interventions: 18 |
- | 28 | 16 | 3 | 1 | 7 | |
| Breast/Ovariaon | Melanoma | ||||||||||
| HIV/viral | Lung | ||||||||||
| Autoimmune/Allergy | Other | ||||||||||
| 847 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| PDL1 | 98 | Active, not-recruiting | Breast/Ovarian | Lung | Monotherapy: 54 Combo-therapy: 41 No interventions: 3 |
- | 29 | 30 | 42 | - | - |
| Hematological tumors | Melanoma | ||||||||||
| Renal | HIV/viral | ||||||||||
| Colorectal caner | Other | ||||||||||
| Liver | |||||||||||
| 21 | Completed | Breast/Ovarian | Lung | Monotherapy: 11 Combo-therapy: 5 No interventions: 3 |
- | 9 | 6 | 2 | - | - | |
| HIV/viral | Pancreatic | ||||||||||
| Autoimmune/Allergy | Melanoma | ||||||||||
| Brain | Other | ||||||||||
| 227 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
ICOS and ICOS-L
Expression and Signaling
ICOS was named for being an inducible co-stimulatory molecule found mainly on activated CD4+ T cells. Expression has not been found on resting T cells but is rapidly induced on CD4+ and CD8+ T cells following TCR engagement [42–45]. The ligand for ICOS, ICOS-L, is expressed on professional APCs including B cells, DCs, and macrophages [46]. ICOS has a similar structure and signaling pathway to CD28, promoting T cell proliferation and cytokine secretion. Studies using CD28 knockout mice have shown that ICOS is a more potent activator of the PI3K/Akt pathway than CD28 and can lead to enhanced activation of downstream MAPKs cascade [43, 47–49]. ICOS-dependent CD4+ T cell proliferation is independent of IL-2 [43, 45, 50]. ICOS favors Th2 and Th17 lymphocytes, but is critical for the induction, maintenance, and homing of Tfh cells [42, 51–54]. Studies using ICOS deficient mice have shown that ICOS deficiency on T cells does not affect primary clonal expansion of memory CD4+ T cells, but these cells have defective reactivation in vivo [55]. Similarly, ICOS-deficient patients have decreased circulating CD4+ memory T cells [56].
Immunotherapy
ICOS can enhance or dampen Th1 or Th2 responses dependent on the pathogenic challenge [57]. Common variable immunodeficiency (CVID), characterized by low serum gamma globulins, occurs in humans that have a homozygous ICOS deficiency. Patients with ICOS gene mutations had increased susceptibility to infections, mainly due to defects in B cell compartments [58, 59]. Studies involving ICOS have mainly pursued its role in hypersensitivity and autoimmune diseases because of its expression on Th2 and Tfh cells [60]. Lupus patients treated with anti-ICOSL antibodies have diminished antibody production and decreased disease associated symptoms. Neutralization of ICOSL on innate lymphoid cells has also been shown to decrease release of cytokines central to airway hypersensitivity. Transplantation studies looking at modulating ICOS have had less definitive results. Cardiac allograft survival in rats increased after anti-ICOS treatment, but in kidney transplant models no effect was observed. In cardiac allograft models the combination of an ICOS blockade with a CD28 or a CD40 blockade increased long-term allograft survival [57, 61, 62]. Other studies have shown that a blockade of ICOS lessens GVHD, especially when used in combination with traditional transplant therapies or CD28 blockade [63, 64].
Since ICOS is only expressed on activated T cells, there is interest in using it to identify immunocompetent T cells within tumors [49]. Studies analyzing the peripheral blood from colon cancer patients identified ICOS expression, along with other B7 family members, as significantly decreased compared to control expression, suggesting that ICOS expression profile may serve as an early indicator of colon cancer [65]. ICOSL is highly expressed on human melanomas and may directly drive activation and expansion of Treg cells in the tumor microenvironment. Therefore blockade of ICOSL or ICOS may have a therapeutic benefit by decreasing intratumoral Treg cells [66]. In preclinical models of melanoma and pancreatic cancer, ICOS activation had a synergistic effect with CTLA-4 blockade. The combination therapy was able to reject established melanoma and prostate cancers in mice. The authors hypothesize that this occurs because CTLA-4 blockade enables activation of tumor-reactive T cells with up-regulation of ICOS. Then the engagement of ICOS, through the IVAX vaccine, a whole cell vaccine consisting of ICOS-L expressing B16 melanoma, is able to further enhance T cell proliferation, survival and migration into the tumor [67]. Though few trials using anti-ICOS therapies are ongoing, preclinical tumor data have led to four new trials that were recently proposed (Table 1).
LAG-3 (CD223)
Expression and Signaling
LAG-3 was once identified as a marker of Treg cells [68]. It was discovered to be upregulated on activated CD4+ and CD8+ T cells as well as NK cells [69]. LAG-3 resembles the CD4 co-receptor and binds to MHCII with a higher affinity, but it may also have additional ligands. The binding of CD4 and LAG-3 to MHC II is required for interaction with the APC [70, 71]. LAG-3 functions as a negative regulator of T cell responses, especially expansion, to MHC II restricted antigen presentation [70, 72, 73]. Studies using LAG-3 deficient mice have shown that signaling of LAG-3 decreases homeostatic expansion of both CD4+ and CD8+ T cells. Dysregulation of T cell homeostasis, due to the lack of LAG-3 expression, resulted in the expansion of other cell types including B cells, macrophages and dendritic cells [71]. LAG-3 is co-expressed with PD1 on exhausted CD8+ T cells. Antibody studies have shown that antagonism of LAG-3 and PD1 synergistically reactivated exhausted CD8+ T cells, suggesting a non-overlapping role of LAG-3 and PD1 in regulating immune responses [74, 75].
Immunotherapy
Since LAG-3 was identified on Treg cells, it was also studied in autoimmune disease onset. Though LAG-3 deficiency alone does not result in autoimmunity, when combined with a non-obese diabetic (NOD) mouse background it results in 100% diabetes onset at a time when other NOD mice exhibit only 15% disease onset. Studies have also shown that blocking LAG-3 can exacerbate type 1 diabetes [76, 77]. Animal models of allergen-specific immunotherapies show a positive correlation between LAG-3 expression and IL-10 production from Treg cells [78]. Together these studies suggest an immunoinhibitory role of LAG-3 on T cell function.
When studied in models of cancer and chronic viral diseases, LAG-3 is highly expressed on dysfunctional and exhausted T cells. These T cells have defects in proliferation and effector function [79]. Using LCMV clone 13 to model chronic infection, LAG-3 correlated strongly with severity of infection. Virus specific CD8+ T cells co-expressed PD1 and LAG-3. The blockade of LAG-3 alone had little effect on T cell activity, but when combined with PDL1 blockade T cell response was dramatically improved [74]. In the setting of chronic malaria infection, combined blockade improved parasite clearance mediated by CD4+ T cells. The combined blockade of PD-1 and LAG-3 was able to clear blood stage malaria as well as prevent chronic infection. This is believed to be due to the combination therapy increasing Tfh and plasma cells, which clear the infection [80]. Patients with melanoma and colorectal cancer often have high expression levels of LAG-3 on Treg cells expanded in PBMC, lymph nodes, and tumor tissue. These LAG-3 expressing Treg cells produce high levels of IL-10 and TGF-β [81]. Tumor specific T cells that were LAG-3+PD1+ had impaired IFNɣ and TNFα production compared to single positive cells. LAG-3 and PD1 are often co-expressed on tumor infiltrating lymphocytes (TILs), and the blockade has significantly improved anti-tumor T cell responses. Combination LAG-3 and PD1 blockade during T cell priming augmented the proliferation and cytokine production of tumor specific CD8+ T cells [75, 82]. In addition, increased LAG-3 expression on tumor infiltrating T cells is associated with poor prognosis of colorectal cancer as well as ovarian cancer [83]. Blockade of LAG-3 synergizes with anti-tumor vaccination to improve CD8+ T cell activation. This was shown to be a due to a direct role of LAG-3 on CD8+ T cells and independent of its role on CD4+ T cells [84]. Together, these promising preclinical data with viral and tumor models have led to increased interest in new clinical trials using LAG-3 blockade as a monotherapy and in combination with other existing immunotherapies (Table 1). However, future studies on LAG-3 still need to look into its function in modulating effector T cells, Treg cells, and NK cells in different diseases and disease stages.
PD1 and PDL1/PDL2
Expression and Signaling
After the immunosuppressive function of PD1 and its ligands was discovered, immense interest and investment have been placed into studies of the receptor/ligand pair. PD1 is expressed on T lymphocytes during thymic development. The expression of PD1 on early thymocytes weakens the interaction of autoreactive T cells with DCs presenting self-antigen [85, 86]. PD1 is also expressed on activated T cells, B cells, NKT cells, monocytes and some DC subsets. The expression of PD1 is upregulated upon antigenic stimulation of T cells, both CD4+ and CD8+, and B cells [87]. One ligand, PDL1, is expressed on resting T cells, B cells, DCs, macrophages, parenchymal cells, endothelial cells, and pancreatic islets. PDL1 can be expressed on hematologic and non-hematologic tissues. Antigen presenting cells often co-express CD80/86 with PDL1 [88]. The second ligand, PDL2, is more selectively expressed on activated DCs, macrophages, and B cell subsets [85, 87]. PDL2 has a 3-fold higher affinity than PDL1 for binding with PD1 [86]. Interaction of PD1 and its ligands leads to inhibition of T cell receptor mediated proliferation and cytokine secretion, thereby acting as a major mechanism of peripheral tolerance. The interaction can also decrease the cytolytic function of T cells and NKT cells [85, 87, 88]. PD1 is a member of the CD28 family of T cell regulators. The trans-membrane domain of PD1 resembles the Ig region of the CD28 family. Phosphorylation of PD1’s immunoreceptor tyrosine-based switch motif (ITSM) results in the direct dephosphorylation of signaling molecules directly downstream of the TCR. Ligation of PD1 augments PTEN inhibition of PI3K activity to decrease T cell proliferation, survival, protein synthesis, and IL-2 release [86].
Immunotherapy
PD1 expression and signaling have been shown to be important in many aspects of immune dysregulation such as autoimmunity, infectious disease, and cancer. PD1 helps regulate the balance between stimulatory and inhibitory signals needed for the maintenance of T cell homeostasis and immune responses. The complete knockdown of PD1 in animal models results in a breakdown in peripheral tolerance in hosts [88]. The high expression of PD1 and PDL1 on Treg cells enhances the expansion of these cells, which control the development, maintenance, and function of peripheral responses [87]. In viral models, PD1 is rapidly upregulated following viral infection, but downregulated as the virus is cleared. In cases of chronic infection, CD8+ T cells express PD1 constitutively, leading to the “exhausted” T cell phenotype [85]. Studies of HIV were among the first to show that a PD1/PDL1 blockade could regain effective immunity [89]. In HIV studies, it was discovered that PDL1 could be upregulated on neutrophils, and expression was induced by IFNα, TLR7/8, and LPS. The upregulation of PDL1 on neutrophils in HIV is correlated to T cell exhaustion and expression of PD1 on T cells [90]. Animal studies using mouse hepatitis virus (MHV) in PD1 deficient mice showed significant tissue damage, especially of the liver. Blockade of IFNɣ and TNFα in the infected mice led to reduced mortality, suggesting that a major role of PD1 in viral infection is controlling the release of pro-inflammatory cytokines [91]. A similar role for PD1 control of cytokine secretion was observed in models of autoimmune disease. In diabetes models, ligation of PD1 leads to the reduction of cytokine production through the truncation of TCR signaling. The blockade of PD1 or PDL1 led to increased onset of diabetes [92]. The role of PD1 in other autoimmune diseases is not as well defined. PD1 deficient mice often display lupus-like disease, but the blockade of PD1 in established lupus delays disease progression. In animal models of lupus, the expression of PDL1 on Treg cells suppresses autoreactive B cells that express PD1 [87, 93].
Many of the major breakthroughs using PD1 therapy have been in studies of cancer. Tumors often use this pathway to silence the immune system. Tumor cells can evade T cell killing by upregulating PDL1 and PDL2 following T cell infiltration and release of IFNɣ [85, 86]. Functional PDL1 expression on tumors can also suppress IL-2 production by T cells to further inhibit T cell proliferation and survival within the tumor microenvironment [94, 95]. In most tumors the expression of PDL1 is a poor prognosis for overall survival and tumor control, and early studies using an antibody blockade of PDL1 have had promising outcomes with higher response rates and durable responses [96, 97]. Blockade of this pathway in late stage cancers can significantly reduce tumor burden and promote a durable tumor regression as seen in studies of advanced melanoma and renal cell cancer [98, 99]. The blockade of this pathway has recently been used in many studies looking at the combination with co-stimulatory agonists on T cells. Though PD1 can limit proliferation of T cells, it is dependent on strength of signal delivered via the TCR and CD28. A strong enough TCR+CD28 signal can overcome PD1 mediated inhibition [88]. The use of PD1 and PDL-1/-2 therapy in clinical trials has been growing rapidly over the years. These treatments have been used in multiple cancer types, autoimmune diseases, viral infections, and much more. The excitement over this pathway can be seen in the dramatic increase in clinical trials that are currently recruiting or recently proposed (Table 1). Recently two anti-PD1 (pembrolizumab and nivolumab) and one anti-PDL1 (atezolizumab) antibodies have been approved by the FDA for use in various solid and hematological cancers, which include melanoma, non-small cell lung cancer (NSCLC), urothelial carcinoma and Hodgkin’s lymphoma [100]. Unfortunately, PD1 and PDL1 are also expressed on cardiomyocytes which have recently led to substantial adverse effects. Some patients treated with PD1 or PDL1 inhibitors have developed severe and sometimes lethal myocarditis and heart failure [101, 102].
TIM family Receptors
TIM3 (HAVCR2)
Expression and Signaling
TIM3 was initially discovered as a cell surface molecule that was selectively expressed on IFNɣ producing Th1 and CD8+ T cells. More recently TIM3 has been identified on DCs, NK cells, and monocytes [103]. TIM3 is upregulated on mature and functional NK cells, serving as a marker of IFNɣ producing NK cells [104, 105]. There are currently several ligands known to interact with TIM3 such as Gal9, HMGB1, and CEACAM1 [106–108]. When co-expressed with PD1 on CD8+ T cells, TIM3 identifies highly dysfunctional cells that are unable to respond to antigen stimulation [109]. These T cells are typically referred to as exhausted T cells. A role for TIM3 in regulating the function of Treg cells has recently been proposed. Studies have shown that TIM3+ Treg cells have superior suppressive function compared to TIM3- Treg cells [110]. TIM3+ Treg cells have been identified at tumor sites as well as within allografts [111–115]. TIM3 has also recently been shown to indirectly suppress immune function through MDSCs. Overexpression of TIM3 on T cells promoted the expansion of MDSCs [116]. Therefore, when considering a TIM3 blockade on human tumors, it is important to remember that a blockade would affect multiple target cell types. This includes CD4+, CD8+, Treg cells, NK cells, DCs, and MDSCs [117].
Immunotherapy
In autoimmunity and chronic viral infections, TIM3 is a prognostic indicator of disease course and a negative regulator of type 1 immunity. Mouse models have shown that TIM3 blockade can lead to spontaneous autoimmunity. This is due at least partially to dampening the immunosuppressive function of Treg cells. Mice treated with anti-TIM3 antibody showed hyperproliferation of Th1 cells and increased Th1 cytokine release. These studies suggest that TIM3 might act as an inhibitory molecule that serves to reduce cytokine driven inflammation [118, 119]. Treatment with anti-TIM3 antibody in mice also led to development of hyper-acute EAE, which was accompanied by uncontrolled macrophage activation [103]. TIM3 in both humans and mice has a role in regulating monocyte and macrophage function. Downregulation of TIM3 increases macrophage production of IL-1B, IL-6, IL-10, IL-12, and TNFα, which suggest a role of TIM3 as a regulator of pro- and anti-inflammatory immune responses [112, 120]. TIM3 can also inhibit DC activation by limiting expression of pro-inflammatory cytokines, which leads to reduced inflammation. Studies of mouse and human tumors show that TIM3 is highly expressed on tumor associated DCs. When tumors were treated with a DNA vaccine, TIM3 diminished the efficacy of the vaccine by interfering with the recruitment of nucleic acids into the DC endosomes. This decreased the immunogenicity of tumor cells [121]. Altogether, TIM3 appears to be protective in autoimmune disorders although its expression is poorly defined; in contrast in cancer models TIM3 is highly expressed but contributes to the dampening of protective immunity.
TIM3 marks dysfunctional T cells in cancer [122]. The frequency of TIM3+ T cells correlates with cancer severity and poor prognosis in several cancer types, including NSCLC, and follicular lymphoma (FL) [111, 112]. In models of melanoma, NSCLC, and FL, TIM3 blockade improves T cell function [122, 123]. The effect of TIM3 blockade was further enhanced when used in combination with a 4-1BB agonist [124]. The increased efficacy of the combination treatment may be due to the interaction of TIM3 and Gal9 on tumor cells [125, 126]. The cells that are TIM3+ and PD1+ are often the most dysfunctional in tumor studies [122]. Studies of chronic viral infections have offered insights to how TIM3 blockade may enhance tumor clearance. Using a model of chronic LCMV, TIM3 marks virus specific CD8+ T cells that have the worst defects in pro-inflammatory cytokine production. These virus specific T cells co-express PD1 and TIM3. It was observed that a co-blockade of PD1 and TIM3 was most effective in chronic LCMV models [109]. Blockade of TIM3 in HIV was shown to restore proliferative potential to T cells in response to HIV peptides [127]. The restoration of T cell function after using TIM3 blockade has been repeated in several other models of chronic viral infections [128–131]. In HIV TIM3 can be found on T cells that lack PD1, suggesting that TIM3 and PD1 have non-redundant and synergistic functions in inhibiting T cell responses [127]. This finding has driven studies into using combination TIM3 and PD1 therapy to treat advanced cancers. Though only a few clinical trials have been completed using TIM3 blockade many more have been proposed and are now recruiting patients (Table 1).
TNF-Superfamily Receptors
4-1BB and 4-1BBL
Expression and Signaling
4-1BB (CD137) was first discovered on activated T lymphocytes [132]. 4-1BB has been found on all subsets of T cells including Treg cells. It has more recently been discovered on NK cells, B cell, neutrophils, and cells within the myeloid lineage [133, 134]. Ligation with 4-1BBL was found to induce T cell activation, survival and effector functions [133]. 4-1BB and 4-1BBL ligation especially favored proliferation through IL-2 production [135, 136]. 4-1BB is expressed rapidly after T cell activation and expression remains detectable on activated T cells [137]. Like many of members of the tumor necrosis factor receptor superfamily (TNFRSF), 4-1BB uses TRAF adapter proteins, namely TRAF2 and TRAF5, that result in increased NFκB and MAP-kinase signaling [138–140]
Immunotherapy
The use of a 4-1BB agonist has shown a role in maintaining immune cell homeostasis. Studies using knockout mice to look at precursor cell turnover in primary and secondary lymphoid organs have shown that 4-1BB also plays a role in myelopoiesis [133]. Agonizing antibodies to 4-1BB in mouse studies have been shown to increase graft versus host disease (GVHD), accelerate rejection of cardiac allografts, and eradicate established tumors. In tumor models, studies suggest that this is due to 4-1BB preferentially stimulating CD8+ T cells, but largely not affecting CD4+ T cells [141, 142]. Agonists to 4-1BB have recently entered phase 1 trials. Phase 1 studies looking at serum samples from patients with solid tumors and B cell non-Hodgkin’s lymphoma showed that with agonist 4-1BB treatment tumor infiltrating CD8+ T cells and NK cells significantly increased while effector CD4+ and CD4+FoxP3+ Treg cells decreased. An increase in pro-inflammatory cytokines was also noted after agonist treatment. The specific agonistic activity increased T cell and NK proliferation and activity to eradicate remaining tumor cells [22, 143]. Targeted therapies, such as cetuximab (EGFR- targeting mAb), can lead to upregulation of 4-1BB on NK cells. NK mediated cytotoxicity of tumor cells following cetuximab therapy was further enhanced when combined with a 4-1BB agonist. This combination therapy is dependent on NK and CD8+ T cells, as depletion of either cell type abrogated therapy efficacy [144]. The role of NK cells in 4-1BB function remains controversial: some murine tumor models show the requirement of NK cells for the functionality of the 4-1BB agonists, while studies using different tumor models show no reliance on NK cells [142, 145]. Combination studies involving radiotherapy and chemotherapy have reported synergism with 4-1BB agonists. When looking at poorly immunogenic tumors, including murine lung carcinomas, spinal tumors, and melanomas, they often became refractory to 4-1BB treatment alone. Yet tumor regression could be achieved even in established tumors.by ‘educating’ cytotoxic T lymphocytes (CTLs) with a tumor antigen vaccine in combination with 4-1BB agonism. Tumor regression of established tumors was also observed when 4-1BB therapy was combined with an IL-12 secreting vaccine. In studies combining chemotherapy and 4-1BB agonism, mice also rejected a re-challenge with the original tumor, suggesting a long-lasting tumor-specific memory [146–150]. However, treatment with the agonists does result in adverse effects including mild liver inflammation, which is dependent on the presence of 4-1BB, IFNɣ, and TNFα [151].
The cytoplasmic tail of 4-1BB has also recently been used in CAR-T cell therapy development. This addition of the 4-1BB tail is critical to support the persistence and expansion of the modified CAR-T lymphocytes. The addition of the cytoplasmic domain of 4-1BB also reprogrammed the CAR-T cells for increased cytokine secretion of predominantly Th1 cytokines, with low or undetectable levels of anti-inflammatory cytokines [152]. Second generation CAR-T therapy generally combined CD28 or 4-1BB cytoplasmic domains with a CD3 intracellular domain in order in increase intratumoral persistence and function, but third generation CARs are now combining more T cell co-stimulatory domains to further enhance persistence. Recent studies including ICOS domain along with a 4-1BB domain saw increased anti-tumor effects and increased persistence in vivo compared to second generation CARs. This study also determined that the placement of each domain in the CAR-T cells determines the in vivo functions, only when ICOS was proximal to the cell surface was the third generation CAR most effective [153].
Few clinical trials have been completed using 4-1BB and 4-1BBL therapies, but many more have recently begun recruiting patients. The ongoing or recently completed trials have used 4-1BB as an important component in CAR-T therapy in both hematological tumors and solid tumors (Table 2). Despite initial successes with 4-1BB expressing CAR-T cells in hematological tumor, these cells still face a barrier overcoming the immunosuppressive microenvironment in solid tumors [154].
Table 2:
TNF Superfamily completed, ongoing, and upcoming clinical trials.
| Co-stimulatory Molecule | Number | Status | Condition | Treatment | Early Phase 1 | Phase 1 | Phase 2 | Phase 3 | Phase 4 | N/A: | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 41BB | 2 | Active, not-recruiting | Hematological cancer | CAR-T therapy | 1 | - | 1 | - | - | - | |
| Pancreatic Cancer | |||||||||||
| 1 | Completed | Multiple Myeloma | CAR-T therapy | 1 | - | - | - | - | - | ||
| 17 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| 41BBL | 3 | Moving forward: Not yet recruiting/Recruiting | |||||||||
| Ox40 | 9 | Active, not-recruiting | Advanced cancer | Colon | Monotherapy: 5 Combo-therapy: 2 CAR-T therapy: 2 |
- | 8 | 1 | - | - | - |
| Autoimmune/Allergy | Breast | ||||||||||
| Head and Neck | Other | ||||||||||
| 10 | Completed | Advanced cancers | Melanoma | Monotherapy: 4 Combo-therapy: 1 CAR-T therapy: 1 No interventions: 4 |
- | 5 | 1 | - | - | 3 | |
| Autoimmune/Allergy | Hepatitis | ||||||||||
| Glomerulonephritis | Prostate | ||||||||||
| 18 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| Ox40L | 3 | Completed | Autoimmune/Allergy | Monotherapy: 2 No interventions: 1 |
- | 1 | 1 | - | - | 1 | |
| 2 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| GITR | 2 | Active, not-recruiting | Metastatic/Advanced tumors | Monotherapy: 1 | - | 2 | - | - | - | - | |
| 3 | Completed | Hepatitis | No interventions: 3 | - | - | - | - | - | 3 | ||
| Glomerulonephritis | |||||||||||
| Uveitis | |||||||||||
| 7 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| CD27 | 2 | Active, not-recruiting | Refractory solid tumors | Monotherapy: 1 Combo-therapy: 1 |
- | 1 | - | - | - | 1 | |
| Hepatitis | |||||||||||
| 25 | Completed | Hematological tumors | Kidney | Monotherapy: 15 Combo-therapy: 5 Vaccination: 2 No interventions: 3 |
1 | 3 | 10 | 3 | 1 | 2 | |
| Autoimmune/Allergy | HIV/viral | ||||||||||
| Colon Cancer | Other | ||||||||||
| Ovarian/Breast | |||||||||||
| 22 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| CD70 | 1 | Active, not-recruiting | Advanced cancers | Monotherapy | - | 1 | - | - | - | - | |
| 5 | Completed | Renal Cancer | Monotherapy: 5 | - | 5 | - | - | - | - | ||
| Melanoma | |||||||||||
| Nasopharyngeal Carcinoma | |||||||||||
| 4 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
| CD40 | 11 | Active, not-recruiting | Lymphoma | Melanoma | Monotherapy: 8 Combo-therapy: 2 No interventions: 1 |
- | 5 | 4 | 1 | - | - |
| Non-Small Cell Lung Cancer | Respiratory disorder | ||||||||||
| 153 | Completed | Melanoma | Diabetes | Monotherapy: 116 Combo-therapy: 22 No interventions: 15 |
1 | 48 | 61 | 18 | 14 | 19 | |
| Kidney | GVHD | ||||||||||
| Solid Tumors | HIV/viral | ||||||||||
| 46 | Moving forward: Not yet recruiting/Recruiting | ||||||||||
OX40 and OX40L
Expression and Signaling
OX40 was first discovered on activated CD4+ T cells [155, 156]. Transiently expressed, OX40 appears 12 hours after activation and decreases by 4 days [157]. OX40 expression is important to the survival, expansion and memory formation of T cells [157, 158]. It is also important for the reactivation of CD4+ and CD8+ memory populations [159]. More recently expression of OX40 has been seen on NK cells, NKT cells and neutrophils [160–162]. OX40L has been identified on activated APCs, hematopoietic, non-hematopoietic cells, and activated endothelium cells [163–167]. The ligand is the limiting factor in OX40 signaling [168]. Ligation of OX40 recruits TRAF2 and TRAF3, which activate both the canonical and non-canonical NFκB pathways. This leads to enhanced T cell expansion, differentiation, and generation of long lived memory cells [169, 170].
Immunotherapy
Stimulation of OX40 can cause pro-inflammatory or pro-survival effects dependent on disease type and expression pattern. In animal models of sepsis, anti-OX40L treatment was able to decrease disease severity and improve survival. In this context OX40L activation was macrophage dependent and T cell independent. Patients with sepsis have elevated OX40L on neutrophils and monocytes that correlates to disease severity [171]. Several disease models have been used to determine the role of OX40 in disease progression including asthma, allergies, colitis, GVHD and diabetes. In each of these models, blockade of OX40 has played an important role in reducing disease severity [172–175].
In cancer immunotherapy, agonism of OX40 is thought to play an important role in the reduction of Treg-mediated immunosuppression. In animal models of colon cancer, it was observed that intratumoral injection of an OX40 agonist was able to induce tumor rejection in a CD8+ T cell-dependent manner. The authors show that both Treg cells and effector T cells must be triggered by OX40 for tumor rejection [176]. It has also been shown that OX40 agonist treatment can reactivate memory T cell population, therefore playing an important role in tumor antigen recall and anti-tumor activity. Studies show that in both new and established tumors, OX40 was critical for early priming of CD8+ T cells by APCs and recall response of previously primed CD8+ T cells. OX40 plays a role in the survival and accumulation of these primed CD8+ T cells at the tumor site [177–179]. The agonism of OX40 promotes the proliferation and survival of activated T cells and generates a CD8+ T cell response with increased secretion of IFNɣ and granzyme B [180, 181]. Preclinical models using OX40 agonist have shown effectiveness in sarcomas, CT26 colon carcinomas, breast cancer, and melanoma. Many of these models study the use of OX40 agonists in combination with other tumor treatments. When combined with chemotherapy agents, OX40 agonism was able to provide potent antitumor immunity and decrease the size of established animal tumor models. This combination therapy resulted in a significant decrease in tumor-infiltrated Treg cells, but interestingly an increase in peripheral Treg cells. When used in combination with radiation, OX40 agonists worked by increasing proliferation of tumor antigen specific CD8+ T cells. The combination with radiation significantly increased disease-free survival [182, 183]. Preclinical ovarian cancer models using checkpoint inhibitor combinations have proven successful by engaging multiple immune subsets to combat the tumor challenge. A murine model combining TIM3 blockade and OX40 agonism showed that the combination treatment was able to inhibit ovarian cancer growth significantly better than either mono-treatment. A similar study looking at the combination with anti-PD1 saw tumor growth inhibited in the majority of host mice. This is due to the increase in CD4+ and CD8+ effector T cells combined with a decrease in Treg and myeloid derived suppressor cell (MDSC) populations [124, 184]. From these preclinical models, two humanized anti-OX40 mAbs are currently on the verge of clinical development. Ongoing clinical trials using OX40 monoclonal or combination trials have mainly focused on use in advanced stages of cancers (Table 2).
GITR and GITR-L
Expression and Signaling
GITR was first discovered as a TNF family receptor induced by glucocorticoids [185]. GITR is unique in that it is constitutively expressed on T regulatory cells, but only expressed on effector CD4+ and CD8+ T cells after TCR engagement [186–190]. Expression of GITR has also been reported on dendritic cells, monocytes, and NK cells [188, 191]. The ligand for GITR, GITRL, is highly expressed on activated DCs, B cells, macrophages, and endothelial cells at the site of inflammation [192, 193]. The ligation of GITR recruits TRAF2 and TRAF5 to its cytoplasmic tail, activating MAP-kinase and NFκB signaling pathways [194]. GITR is important in CD28-driven activation of CD8+ T cells by lowering the threshold of activation. GITR is crucial for CD28-mediated NFκB activation; activated GITR-/- T cells had impaired nuclear translocation of NFκB compared to GITR+/+ T cells when stimulated with anti-CD3 and anti-CD28 [195].
Immunotherapy
GITR has a critical role in supporting effector T cell activity by inducing T cell proliferation, effector function, and survival. Agonizing GITR on T cells increased effector function by increasing cytotoxic killing of a mouse mastocytoma cell line [195, 196]. In animal models of melanoma, agonism of GITR at early timepoints in tumor growth was able to decrease tumor burden. This is believed to be due to the decreased suppressive function of Treg cells after ligation of GITR [197]. The mechanism of increased effector T cells in the tumor microenvironment is due to direct targeting of GITR on Treg cells. This may work through directly depleting intratumoral Treg cells or via targeting GITRL on suppressive myeloid cells. Agonizing GITR was shown to decrease the stability of intratumoral Treg cells and result in the loss of FoxP3 expression as well as decreased infiltration into the tumor [198, 199]. Agonists to GITR have shown synergism when combined with vaccines, TLR agonists, and other immunostimulatory mAbs. Combined GITR agonism with a DNA vaccination was able to offer increased protection from a lethal challenge with melanoma. This combination treatment led to prolonged persistence of the antigen-specific CD8+ T cells and enhanced recall response to a booster vaccine. These findings are supported by additional tumor models, including myeloma [200, 201]. When downregulation of Treg activity is critical for success, this pathway has recently been shown to be effective when combined with other therapies, such as PD-1 and CTLA-4 blockade. The combined treatments have shown a durable effect associated with CD4+ and CD8+ memory response. PD-1 combined with GITR therapy resulted in increased frequency of IFNɣ producing cells and decreased MDSCs. The tumor microenvironment shifted from immunosuppressive to immunostimulatory [202, 203]. Launched from these preclinical data, a few clinical trials studying the role of GITR have been completed. A number of new trials have been proposed to use GITR therapy on solid and hematological cancers (Table 2).
CD27 and CD70
Expression and Signaling
CD27 is unique as a T cell co-stimulatory molecule in that it is constitutively expressed at significant levels on the majority of T cells, including naïve T cells and Treg cells. CD27 expression is lost on T cells following differentiation and activation but is retained on memory cells [204–206]. CD27 is also expressed on early hematopoietic cells, memory B cells, plasma cells and a subset of NK cells [206–208]. CD27 has recently been shown to drive the cytolytic activity of some subsets of NK cells [209]. Expression of CD27 on naïve T cells helps T cells with low affinity TCRs enter the cell cycle [210]. This pathway is important for sustained effector functions, T cell survival, and development of memory T cells [211, 212]. CD70, the ligand for CD27, regulates the interaction with CD27 because it is only transiently expressed on activated APCs, T cells, NK cells [208, 213, 214]. During chronic inflammation it has been observed that CD70 can become constitutively expressed causing dysregulation of this pathway [215]. The chronic signaling of CD27 has been shown to lead to T cell exhaustion [216]. The ligation of CD27 recruits TRAF2 and TRAF5, which activate the NFκB and c-Jun pathways to promote cell survival, enhance expansion, and increase effector functions [217, 218]. As a co-stimulatory molecule, CD27 counteracts apoptosis by increasing anti-apoptotic Bcl-xl and decreasing FasL on CD4+ and CD8+ T cells [219, 220]. Signaling through CD27 also rapidly induces the expression of Pim-1, which is known to increase aerobic glycolysis and protein translation [221]. SIVA1 can also be recruited to the cytoplasmic tail of CD27. This protein is known to promote caspase dependent apoptosis [222]. Together these findings suggest that under certain circumstances CD27 can act as either a co-stimulatory or a co-inhibitory receptor.
Immunotherapy
Therapies involving the CD27-CD70 pathway must appreciate the pathways inhibitory and stimulatory effects under different immune contexts. During acute challenges CD27 has thus far been regarded as important for the formation and function of effector and memory T cells. Studies analyzing influenza and acute viral challenges are examples of the co-stimulatory role CD27-CD70 have on primary T cell responses. CD27+ and CD70+ cells often secrete higher levels of IFNɣ and are therefore more effective at inhibiting viral replication [220]. This is in contrast to studies looking at CD27 in the context of strong and persistent immune challenges, such as chronic infections or GVHD, where CD27-CD70 have been shown to play an inhibitory role on T cell responses [216, 223–225]. The role of CD27 in diseases is also dependent on tissue context and duration of expression [22]. Use of antagonists or agonists will require precise triggering in specific tissues and cell types.
The CD27-CD70 pathway has been studied in several different autoimmune diseases. In models of lupus high levels of soluble CD27 and CD27+ plasma cells correlate to an increased disease index. Patients also have increased CD70+CD4+ T cells with a memory-like phenotype. In rheumatoid arthritis patients, CD70 is found in higher levels on naïve and memory CD4+ T cells compared with healthy patients. These T cells also secrete higher levels of IFNɣ and IL-17. In mouse models, treatment with an anti-CD70 antibody was able to ameliorate autoimmune disease. Animal studies using an anti-CD70 treatment in multiple sclerosis decreased TNFα production and prevented disease. But in similar models of multiple sclerosis CD27 or CD70 deficiency was seen to exacerbate disease. Increased sCD27 in cerebrospinal fluid has been validated as a biomarker of intrathecal T cell activation in neuro-immunological diseases [226].
Solid and hematological tumors have been reported to have overexpression of CD70 at high levels [227, 228]. CD70 has been identified as a biomarker for renal cell cancers as well as several hematological malignances [229]. Using an anti-CD70 antibody against the malignant T and B cells has been shown to regulate their expansion. Neutralization of CD70 has also been shown to inhibit the signaling of CD27, therefore blocking the activation of and proliferation of Treg cells [228, 230]. In some models of lymphoma expression of CD70 on tumor cells and APCs improves anti-tumor immunity. Unfortunately, in other tumor models intact CD27/CD70 signaling has been associated with decreased anti-tumor immune responses and increased intratumoral Treg cells through the reduction of Treg apoptosis and increased production of IL-2 necessary for Treg survival [231]. Recently, the use of a CD27 agonist has shown protection against intravenous injection of two lymphoma cell lines. The agonism of CD27 induced proliferation and cytokine production from T cells [232]. Since this discovery, ongoing clinical trials for use of a CD27 agonist in combination with PD1 antibodies have been approved for non-Hodgkin lymphoma and some solid tumors [233]. Prostate cancer bearing mice treated with a combination of a DC vaccine and CD27 agonist showed decreased tumor growth and increased T cell proliferation and effector function when compared to controls. A CD27 agonist is also being used to treat B-cell lymphomas and melanomas [232, 234]. Combined results from these studies suggest that the agonism of CD27 can lead to improved anti-tumor immunity and favors long term persistence of TILs [232, 235–237].
Recently preclinical models using CD70 CAR-T cell therapies to treat cancers expressing CD70 have been tested, and this therapeutic strategy has been approved for phase I/II clinical trials. In normal tissue, CD70 is expressed only on activated lymphocytes, therefore targeting this pathway with CD70 expressing tumors may spare toxicity to other normal tissues. Creating CARs with the extracellular binding portion of CD27 along with intracellular co-stimulatory domains of CD28 and 4-1BB in animal models was able to cure tumor bearing mice with limited toxicities [229]. The use of CD70 targeting CAR-T cells has also recently been tested in preclinical models of gliomas. These preclinical studies show that CD70 expression is associated with poor prognosis in patients with recurrent tumors. This led authors to identify CD70 as an immunosuppressive mediator in part because of its production of pro-tumor chemokines and inducing CD8+ T cell death [238].
Several clinical trials using anti-CD27 as a monotherapy in hematological tumors and autoimmune disease treatment have recently been completed. After early successes, new trials using CD27 and CD70 therapies are actively recruiting or recently proposed (Table 2).
CD40 and CD40L
Expression and Signaling
CD40 was initially characterized on B cells. Expression of CD40 has since been discovered on DCs, monocytes, platelets, macrophages and some non-hematopoietic cells such as myofibroblasts, epithelial and endothelial cells [239, 240]. Depending on cell type and context CD40 can be constitutively or inducibly expressed. CD40L (CD154) is primarily expressed on activated CD4+ T cells. CD40L expression has also been seen on B cells and platelets. In inflammatory settings CD40L can be expressed on monocytic cells, NK cells, mast cells, and basophils [241, 242]. The CD40 receptor has no enzymatic activity and therefore must directly bind TRAF family proteins for signal transduction [243]. TRAF2, TRAF3, TRAF5 and TRAF6 have been shown to directly interact with CD40 receptor. This interaction results in the activation of MAPK signaling, cytokine secretion, proliferation and differentiation [240]. Ligation of CD40 on dendritic cells promotes the induction of other co-stimulatory molecules and facilitates cross-presentation of antigens to the T cells [244]. Signaling of CD40 on DCs results in the heightened expression and increased stability of the MHC-antigen complex [245] and production of pro-inflammatory cytokines. On B cells, CD40 signaling promotes isotype switching, formation of germinal centers, and formation of long lived plasma cells [246]. The CD40-CD40L pathway plays a critical role in the survival of germinal center B cell, DCs, and endothelial cells in both normal and inflammatory conditions.
Immunotherapy
CD40 plays a major role in the initiation of T cell dependent autoimmune disease. CD40 functions during T cell selection in the thymus by promoting medullary thymic epithelial cells (mTECs), which leads to the development of self-tolerance [247]. A disruption of CD40 in the thymus can lead to failure of central tolerance. Signaling of the CD40-CD40L pathway results in the production of proinflammatory cytokines including IL-6. This signaling pathway can also skew differentiation towards Th17 cells [248]. The aberrant expression of CD40 in tissues where it is normally undetectable is a major contributing factor in autoimmune disease initiation [249]. In Grave’s disease, an autoimmune disease of the thyroid, CD40 is abnormally expressed on thyroid epithelial cells. This leads to the presentation of autoantigens to T cells [250]. Blocking with anti-CD40L antibodies was able to prevent experimental thyroiditis in mouse models [251]. Antagonistic anti-CD40L antibodies have also been tested in the models of inflammatory bowel disease (IBD). Given at the time of colitis induction, blocking CD40L prevented disease onset, blocked lymphocytic infiltration of the gut, and decreased IFNɣ production by gut T cells [252, 253]. Production of IFNɣ in the gut can cause upregulation of CD40 on normal colonic fibroblasts. CD40+ cells including DCs, B cells and macrophages are found within the intestinal mucosa of colitis patients [254, 255]. CD40 signaling in these cell types leads to the production of IL-6, IL-12, and IL-23, which contribute to disease initiation [248]. The CD40-CD40L pathway has been shown to be critical for IBD induction but not required for ongoing inflammatory responses [252]. Patients with active Lupus disease have overexpression of CD40L on CD4+ and CD8+ T cells [256]. Disease activity can be correlated to serum levels of CD40L [257]. Mouse models of lupus have shown that blocking CD40L prior to disease onset can prolong survival and ameliorate kidney disease [258, 259]; If treatment of these mice was stopped disease symptoms returned. Autoimmune models have shown that blocking CD40L prevents the relapse of ongoing disease or halts pathogenic progression in rheumatoid arthriris, lupus, multiple sclerosis, IBD and diabetes [260]. However, the blocking antibody has been ineffective in treatment of some established diseases. Ruplizumab was the first CD40L blockade used in clinical trials [261]. Patients with Lupus and Crohn’s disease showed partial therapeutic responses, but trials had to be halted due to development of thromboembolism [260]. Studies of allograft rejection sought to exploit the CD40 pathways because it was believed that the blockade of CD40L would limit APC maturation and decrease CD28 signaling resulting in T cell anergy [262]. Unfortunately, anti-CD40L blocking antibodies failed as a monotherapy in inducing allograft tolerance [263, 264]. This was due to the inability of the therapy to block rejection mediated by CD8+ T cells. Nonetheless, the combination of CD40L blockade and immunosuppressive drugs, such as rapamycin, promotes long term graft acceptance [265].
CD40-CD40L is critical for the development of protective anti-tumor immunity. Since CD40 is expressed on a wide variety of normal tissue it has also been discovered on many tumor types. CD40 was first discovered on bladder carcinoma [266, 267]. More recently it has been discovered on melanoma, prostate cancer, lung cancer, cervical, lymphoma, leukemia, and myeloma [268–271]. Human CD40+ breast tumors have been shown to co-express CD40L that may increase proliferation, motility and invasion of tumor cells [266]. CD40 ligation in B cell malignancies has been shown to cause an increase in expression of anti-apoptotic factors such as Bcl-XL [272]. This protects the malignant cells from apoptosis. In vitro studies of non-Hodgkin’s lymphoma show that low level constitutive signaling of CD40 leads to increased malignant cell proliferation [273]. Whereas in vitro and in vivo studies of Burkitt’s lymphoma show that treatment with sCD40L resulted in reduced proliferation of tumor cells [270]. Soluble CD40L alone or in combination with chemotherapy in models of breast and ovarian cancer can significantly inhibit the growth of the tumors and increase overall survival [274]. When used in combination with tumor vaccines, anti-CD40L blocking treatment inhibited the generation of protective immune responses and decreased potency of the vaccine [275]. This led to an increased interest in studying agonistic CD40 antibodies, which act as powerful adjuvants for inducing tumor immunity. Agonizing CD40 enhances anti-tumor immune responses by promoting DC maturation, survival, and proinflammatory cytokine secretion [276, 277]. CD40 stimulation also induces the upregulation of other co-stimulatory molecules that promote antigen presentation, priming, and cross-priming of T cells. In mouse tumor models CD40 agonism lead to increased survival of mice to primary tumor challenges, but decreased secondary responses [276]. However, the antibody therapy did induce liver toxicity after prolonged treatment. In addition, combination therapies with other co-stimulatory and co-inhibitory molecules and cytokines have been performed in mouse disease models with promising results that encourage clinical trials in patients [278–281].
Phase I clinical trials in patients with non-Hodgkin’s lymphoma treated with recombinant CD40L showed partial to complete responses three months after treatment [282]. Also, promising results from early clinical trials in chromic lymphatic leukemia and multiple myeloma encouraged development of CD40 mAbs to be used in combination therapy.
Concluding remarks
In the past 30 years, studies of co-stimulatory and co-inhibitory pathways have led to several breakthroughs in understanding of human diseases and development of immunotherapies. These co-stimulatory and co-inhibitory molecules equip immune cells a mechanism to sense environmental conditions and respond appropriately. These molecules create complex interactions due the vast number of receptor/ligand pairs and downstream signaling pathways. As more co-signaling molecules are discovered, many with unique and non-overlapping functions, the simplistic “signal two” model will likely be replaced by a complex tidal model of co-signaling. Therefore, designing combination therapies that target multiple co-signaling pathways as well as the tumor microenvironment may offer the greatest chance of successful treatment of cancer.
Acknowledgement
This work attempts to comprehend most studies on the co-stimulatory/co-inhibitory pathways relevant to cancer immunotherapy. However, we apologize for not being able to be comprehensive in including all publications on this topic due to limitation of space.
Funding
This work was supported by NIH Grant R01 HL135325 (to XC)
Abbreviations:
- TCR
T cell receptor
- MHC
major histocompatibility complex
- APC
antigen presenting cell
- mAb
monoclonal antibodies
- Treg
T regulatory cell
- MDSC
Myeloid derived suppressor cell
- CAR
chimeric antigen receptor
- DC
dendritic cell
Footnotes
Conflict of Interest
Authors have declared that there is no conflict of interest.
References
- 1.Sharpe AH, Abbas AK: T-Cell Costimulation — Biology, Therapeutic Potential, and Challenges. New England Journal of Medicine 2006, 355(10):973–975. [DOI] [PubMed] [Google Scholar]
- 2.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H et al. : Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in cancer biology 2015, 35 Suppl:S185–s198. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe AH, Freeman GJ: The B7–CD28 superfamily. Nature Reviews Immunology 2002, 2:116. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe AH: Mechanisms of Costimulation. Immunological reviews 2009, 229(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward-Kavanagh L, Lin WW, Šedý JS, Ware CF: The TNF Receptor Superfamily in Costimulating and Coinhibitory Responses. Immunity 2016, 44(5):1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA: Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev 2011, 241(1):180–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA: An obligate cell-intrinsic function for CD28 in Tregs. The Journal of clinical investigation 2013, 123(2):580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd CE, Taylor A, Schneider H: CD28 and CTLA-4 coreceptor expression and signal transduction. Immunological reviews 2009, 229(1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R: A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol 2001, 2(4):325–332. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R: Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1994, 1(9):793–801. [DOI] [PubMed] [Google Scholar]
- 11.Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R: A Single Amino Acid Alteration in Cytoplasmic Domain Determines IL-2 Promoter Activation by Ligation of CD28 but Not Inducible Costimulator (ICOS). The Journal of experimental medicine 2003, 197(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochman Y, Yukawa M, Kartashov AV, Barski A: Functional Characterization of Human T Cell Hyporesponsiveness Induced by CTLA4-Ig. PLoS ONE 2015, 10(4):e0122198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F: CD28 expression in T cell aging and human longevity. Experimental gerontology 1998, 33(3):267–282. [DOI] [PubMed] [Google Scholar]
- 14.Strioga M, Pasukoniene V, Characiejus D: CD8(+) CD28(-) and CD8(+) CD57(+) T cells and their role in health and disease. Immunology 2011, 134(1):17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluestone JA, Clair EW, Turka LA: CTLA4Ig: Bridging the Basic Immunology with Clinical Application. Immunity 2006, 24(3):233–238. [DOI] [PubMed] [Google Scholar]
- 16.Adams AB, Ford ML, Larsen CP: Costimulation Blockade in Autoimmunity and Transplantation: The CD28 Pathway. Journal of immunology (Baltimore, Md : 1950) 2016, 197(6):2045–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbins J, Gagnon E, Godec J, Pyrdol J, Vignali DAA, Sharpe AH, Wucherpfennig KW: Binding of the cytoplasmic domain of CD28 to the plasma membrane inhibits Lck recruitment and signaling. Science signaling 2016, 9(438):ra75–ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS: Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992, 71(7):1093–1102. [DOI] [PubMed] [Google Scholar]
- 19.Townsend SE, Allison JP: Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science (New York, NY) 1993, 259(5093):368–370. [DOI] [PubMed] [Google Scholar]
- 20.Hunig T: The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nature reviews Immunology 2012, 12(5):317–318. [DOI] [PubMed] [Google Scholar]
- 21.Tabares P, Berr S, Romer PS, Chuvpilo S, Matskevich AA, Tyrsin D, Fedotov Y, Einsele H, Tony HP, Hunig T: Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. European journal of immunology 2014, 44(4):1225–1236. [DOI] [PubMed] [Google Scholar]
- 22.Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I: Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS. Seminars in oncology 2015, 42(4):640–655. [DOI] [PubMed] [Google Scholar]
- 23.Tang X-Y, Sun Y, Zhang A, Hu G-L, Cao W, Wang D-H, Zhang B, Chen H: Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 2016, 6(12):e013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maus MV, June CH: Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2016, 22(8):1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA: B7/CD28 Costimulation Is Essential for the Homeostasis of the CD4+CD25+ Immunoregulatory T Cells that Control Autoimmune Diabetes. Immunity 2000, 12(4):431–440. [DOI] [PubMed] [Google Scholar]
- 26.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH: Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3(5):541–547. [DOI] [PubMed] [Google Scholar]
- 27.Lenschow DJ, Zeng Y, Thistlethwaite Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA: Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science (New York, NY) 1992, 257(5071):789. [DOI] [PubMed] [Google Scholar]
- 28.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N: Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. International Immunology 2004, 16(8):1055–1068. [DOI] [PubMed] [Google Scholar]
- 29.Parish ST, Wu JE, Effros RB: Sustained CD28 Expression Delays Multiple Features of Replicative Senescence in Human CD8 T Lymphocytes. Journal of Clinical Immunology 2010, 30(6):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mou D, Espinosa J, Lo DJ, Kirk AD: CD28 Negative T cells: is their loss our gain? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014, 14(11):2460–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel J-N, Tran DQ, Stoddard J, Zhang Y et al. : Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science (New York, NY) 2014, 345(6204):1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen B-S, Schäffer AA, Grüning BA et al. : Autosomal-dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nature medicine 2014, 20(12):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I et al. : Abatacept for Rheumatoid Arthritis Refractory to Tumor Necrosis Factor α Inhibition. New England Journal of Medicine 2005, 353(11):1114–1123. [DOI] [PubMed] [Google Scholar]
- 34.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K et al. : Costimulation Blockade with Belatacept in Renal Transplantation. New England Journal of Medicine 2005, 353(8):770–781. [DOI] [PubMed] [Google Scholar]
- 35.Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, Klempnauer J, Lang P, Larsen CP, Mühlbacher F et al. : Five-Year Safety and Efficacy of Belatacept in Renal Transplantation. Journal of the American Society of Nephrology : JASN 2010, 21(9):1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G et al. : Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012, 12(1):210–217. [DOI] [PubMed] [Google Scholar]
- 37.Ford ML, Adams AB, Pearson TC: Targeting co-stimulatory pathways: transplantation and autoimmunity. Nature reviews Nephrology 2014, 10(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F: The Effect of Costimulatory and Interleukin 2 Receptor Blockade on Regulatory T Cells in Renal Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2008, 8(10):2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levitsky J, Miller J, Huang X, Chandrasekaran D, Chen L, Mathew JM: INHIBITORY EFFECTS OF BELATACEPT ON ALLOSPECIFIC REGULATORY T CELL GENERATION IN HUMANS. Transplantation 2013, 96(8):689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fecher LA, Agarwala SS, Hodi FS, Weber JS: Ipilimumab and its toxicities: a multidisciplinary approach. The oncologist 2013, 18(6):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR: Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. Journal for ImmunoTherapy of Cancer 2015, 3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH: The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Science translational medicine 2010, 2(55):55ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA: ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999, 397(6716):263–266. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M, Hara Y, Tanabe K, Toma H, Abe R: A distinct role for ICOS-mediated co-stimulatory signaling in CD4+ and CD8+ T cell subsets. International Immunology 2005, 17(3):269–278. [DOI] [PubMed] [Google Scholar]
- 45.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK et al. : Mouse Inducible Costimulatory Molecule (ICOS) Expression Is Enhanced by CD28 Costimulation and Regulates Differentiation of CD4<sup>+</sup> T Cells. The Journal of Immunology 2000, 165(9):5035. [DOI] [PubMed] [Google Scholar]
- 46.Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA: Characterization of Human Inducible Costimulator Ligand Expression and Function. The Journal of Immunology 2000, 164(9):4689. [DOI] [PubMed] [Google Scholar]
- 47.van Berkel ME, Oosterwegel MA: CD28 and ICOS: similar or separate costimulators of T cells? Immunology letters 2006, 105(2):115–122. [DOI] [PubMed] [Google Scholar]
- 48.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS: Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proceedings of the National Academy of Sciences of the United States of America 2002, 99(18):11790–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capece D, Verzella D, Fischietti M, Zazzeroni F, Alesse E: Targeting Costimulatory Molecules to Improve Antitumor Immunity. Journal of Biomedicine and Biotechnology 2012, 2012:926321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong C, Juedes AE, Temann U-A, Shresta S, Allison JP, Ruddle NH, Flavell RA: ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 2001, 409:97. [DOI] [PubMed] [Google Scholar]
- 51.Shilling RA, Clay BS, Tesciuba AG, Berry EL, Lu T, Moore TV, Bandukwala HS, Tong J, Weinstock JV, Flavell RA et al. : CD28 and ICOS play complementary non-overlapping roles in the development of Th2 immunity in vivo. Cellular immunology 2009, 259(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh W-K: Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America 2009, 106(48):20371–20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wikenheiser DJ, Ghosh D, Kennedy B, Stumhofer JS: The co-stimulatory molecule ICOS regulates host T(H)1 and T(FH) cell differentiation in response to Plasmodium chabaudi chabaudi AS infection. Journal of immunology (Baltimore, Md : 1950) 2016, 196(2):778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W et al. : Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496:523. [DOI] [PubMed] [Google Scholar]
- 55.Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D: The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. European journal of immunology 2007, 37(7):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee S-K, Mizutani S, Morio T: Impaired CD4 and CD8 Effector Function and Decreased Memory T Cell Populations in ICOS-Deficient Patients. The Journal of Immunology 2009, 182(9):5515. [DOI] [PubMed] [Google Scholar]
- 57.Wikenheiser DJ, Stumhofer JS: ICOS Co-Stimulation: Friend or Foe? Frontiers in Immunology 2016, 7:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Dräger R, Eibel H, Fischer B, Schäffer AA, Mages HW et al. : Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunology 2003, 4:261. [DOI] [PubMed] [Google Scholar]
- 59.Yong PF, Salzer U, Grimbacher B: The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev 2009, 229(1):101–113. [DOI] [PubMed] [Google Scholar]
- 60.Coyle AJ, Gutierrez-Ramos JC: The role of ICOS and other costimulatory molecules in allergy and asthma. Springer seminars in immunopathology 2004, 25(3–4):349–359. [DOI] [PubMed] [Google Scholar]
- 61.Guillonneau C, Aubry V, Renaudin K, Seveno C, Usal C, Tezuka K, Anegon I: Inhibition of chronic rejection and development of tolerogenic T cells after ICOS-ICOSL and CD40-CD40L co-stimulation blockade. Transplantation 2005, 80(4):546–554. [PubMed] [Google Scholar]
- 62.Guo L, Fujino M, Kimura H, Funeshima N, Kitazawa Y, Harihara Y, Tezuka K, Makuuchi M, Suzuki S, Li XK: Simultaneous blockade of co-stimulatory signals, CD28 and ICOS, induced a stable tolerance in rat heart transplantation. Transplant immunology 2003, 12(1):41–48. [DOI] [PubMed] [Google Scholar]
- 63.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR: Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 2005, 105(8):3372. [DOI] [PubMed] [Google Scholar]
- 64.Sato M, Storb R, Loretz C, Stone D, Mielcarek M, Sale GE, Rezvani AR, Graves SS: Inducible costimulator (ICOS) up-regulation on activated T cells in chronic graft-versus-host disease after dog leukocyte antigen-nonidentical hematopoietic cell transplantation: a potential therapeutic target. Transplantation 2013, 96(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H, Kim JH, Yang SY, Kong J, Oh M, Jeong DH, Chung J-i, Bae KB, Shin JY, Hong KH et al. : Peripheral blood gene expression of B7 and CD28 family members associated with tumor progression and microscopic lymphovascular invasion in colon cancer patients. Journal of Cancer Research and Clinical Oncology 2010, 136(9):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Orozco N, Li Y, Wang Y, Liu S, Hwu P, Liu Y-J, Dong C, Radvanyi L: Melanoma Cells Express ICOS Ligand to Promote the Activation and Expansion of T-Regulatory Cells. Cancer Research 2010, 70(23):9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP: Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. The Journal of experimental medicine 2014, 211(4):715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI et al. : Role of LAG-3 in Regulatory T Cells. Immunity 2004, 21(4):503–513. [DOI] [PubMed] [Google Scholar]
- 69.Triebel FJS, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. : LAG-3, a novel lymphocyte activation gene closely related to CD4. The Journal of experimental medicine 1990, 171(5):1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F: CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. European journal of immunology 1995, 25(9):2718–2721. [DOI] [PubMed] [Google Scholar]
- 71.Workman CJ, Vignali DAA: Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223). The Journal of Immunology 2005, 174(2):688. [DOI] [PubMed] [Google Scholar]
- 72.Workman CJ, Vignali DA: The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. European journal of immunology 2003, 33(4):970–979. [DOI] [PubMed] [Google Scholar]
- 73.Workman CJ, Cauley LS, Kim I-J, Blackman MA, Woodland DL, Vignali DAA: Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. The Journal of Immunology 2004, 172(9):5450. [DOI] [PubMed] [Google Scholar]
- 74.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ: Coregulation of CD8(+) T cell exhaustion during chronic viral infection by multiple inhibitory receptors. Nature immunology 2009, 10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano D, Vogel P, Liu CL et al. : Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T cell function to promote tumoral immune escape. Cancer Research 2012, 72(4):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DAA: Cutting Edge: Accelerated Autoimmune Diabetes in the Absence of LAG-3. The Journal of Immunology 2011, 187(7):3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okazaki T, Okazaki I-m, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T et al. : PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. The Journal of experimental medicine 2011, 208(2):395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, Carney LJ, Gough J, Strobel S, Wraith DC: Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nature Communications 2014, 5:4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wherry EJ, Kurachi M: Molecular and cellular insights into T cell exhaustion. Nature reviews Immunology 2015, 15(8):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT: Therapeutic PD-L1 and LAG-3 blockade rapidly clears established blood-stage Plasmodium infection. Nature immunology 2012, 13(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L, Castelli C: LAG-3 Expression Defines a Subset of CD4<sup>+</sup>CD25<sup>high</sup>Foxp3<sup>+</sup> Regulatory T Cells That Are Expanded at Tumor Sites. The Journal of Immunology 2010, 184(11):6545. [DOI] [PubMed] [Google Scholar]
- 82.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P et al. : Tumor-infiltrating NY-ESO-1–specific CD8(+) T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 2010, 107(17):7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Chen Z: The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Medical oncology (Northwood, London, England) 2014, 31(8):82. [DOI] [PubMed] [Google Scholar]
- 84.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC et al. : LAG-3 regulates CD8(+) T cell accumulation and effector function in murine self- and tumor-tolerance systems. The Journal of clinical investigation 2007, 117(11):3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharon E, Streicher H, Goncalves P, Chen HX: Immune checkpoint inhibitors in clinical trials. Chinese Journal of Cancer 2014, 33(9):434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A: Programmed death-1 pathway in cancer and autoimmunity. Clinical immunology (Orlando, Fla) 2014, 153(1):145–152. [DOI] [PubMed] [Google Scholar]
- 87.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH: Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine 2006, 203(4):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al. : Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. The Journal of experimental medicine 2000, 192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicholas KJ, Zern EK, Barnett L, Smith RM, Lorey SL, Copeland CA, Sadagopal S, Kalams SA: B Cell Responses to HIV Antigen Are a Potent Correlate of Viremia in HIV-1 Infection and Improve with PD-1 Blockade. PLoS ONE 2013, 8(12):e84185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowers NL, Helton ES, Huijbregts RPH, Goepfert PA, Heath SL, Hel Z: Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway. PLoS Pathogens 2014, 10(3):e1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Wu S, Guo G, Fei L, Guo S, Yang C, Fu X, Wu Y: Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection. PLOS Pathogens 2011, 7(7):e1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ansari MJI, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ et al. : The Programmed Death-1 (PD-1) Pathway Regulates Autoimmune Diabetes in Nonobese Diabetic (NOD) Mice. The Journal of experimental medicine 2003, 198(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong M, Cava AL, Hahn BH: Blockade of Programmed Death-1 (PD-1) in young (New Zealand Black × New Zealand White)F(1) mice promotes the suppressive capacity of CD4(+) regulatory T cells protecting from lupus-like disease. Journal of immunology (Baltimore, Md : 1950) 2013, 190(11):5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY: PD-L1: PD-1 Interaction Contributes to the Functional Suppression of T-Cell Responses to Human Uveal Melanoma Cells In Vitro. Investigative ophthalmology & visual science 2008, 49(6):2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dotti G: Blocking PD-1 in cancer immunotherapy. Blood 2009, 114(8):1457. [DOI] [PubMed] [Google Scholar]
- 96.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ et al. : Tumor Infiltrating PD1-Positive Lymphocytes and the Expression of PD-L1 Predict Poor Prognosis of Soft Tissue Sarcomas. PLoS ONE 2013, 8(12):e82870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H et al. : Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chinese Journal of Cancer Research 2014, 26(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. : Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. The New England journal of medicine 2013, 369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. : Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. The New England journal of medicine 2012, 366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, Marone G, Tocchetti CG: Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017, 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohé C: Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer 2016, 99:117–119. [DOI] [PubMed] [Google Scholar]
- 102.Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, Mohri M: Acute Lymphocytic Myocarditis With Anti-PD-1 Antibody Nivolumab. Circulation: Heart Failure 2016, 9(10):e003514. [DOI] [PubMed] [Google Scholar]
- 103.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA et al. : Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415:536. [DOI] [PubMed] [Google Scholar]
- 104.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A et al. : Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119(13):3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL: Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119(16):3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH: TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunological reviews 2010, 235(1):172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang Y-H, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen B-S, Melum E, Pertel T et al. : CEACAM1 regulates TIM–3–mediated tolerance and exhaustion. Nature 2015, 517(7534):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK: The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunology 2005, 6:1245. [DOI] [PubMed] [Google Scholar]
- 109.Jin H-T, Anderson AC, Tan WG, West EE, Ha S-J, Araki K, Freeman GJ, Kuchroo VK, Ahmed R: Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America 2010, 107(33):14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gautron A-S, Dominguez-Villar M, de Marcken M, Hafler DA: Enhanced Suppressor Function of TIM-3(+) FoxP3(+) Regulatory T cells. European journal of immunology 2014, 44(9):2703–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B: TIM-3 Expression Characterizes Regulatory T Cells in Tumor Tissues and Is Associated with Lung Cancer Progression. PLoS ONE 2012, 7(2):e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Z-Z, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM: IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. The Journal of clinical investigation 2012, 122(4):1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, Anderson AC: TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013, 2(4):e23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan J, Zhang Y, Zhang J-P, Liang J, Li L, Zheng L: Tim-3 Expression Defines Regulatory T Cells in Human Tumors. PLoS ONE 2013, 8(3):e58006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, Strom TB: Allograft rejection is restrained by short-lived TIM-3(+)PD-1(+)Foxp3(+) Tregs. The Journal of clinical investigation 2012, 122(7):2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabrilovich DI, Nagaraj S: Myeloid-derived-suppressor cells as regulators of the immune system. Nature reviews Immunology 2009, 9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anderson AC, Joller N, Kuchroo VK: Lag-3, Tim-3, and TIGIT co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016, 44(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nature Immunology 2003, 4:1102. [DOI] [PubMed] [Google Scholar]
- 119.Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK et al. : Tim-3 inhibits T helper type 1–mediated auto- and alloimmune responses and promotes immunological tolerance. Nature Immunology 2003, 4:1093. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ: Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14(+) monocytes. Journal of Leukocyte Biology 2012, 91(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD et al. : Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature immunology 2012, 13(9):832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC: Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine 2010, 207(10):2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK et al. : Coexpression of Tim-3 and PD-1 identifies a CD8(+) T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117(17):4501–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, Zhang S: Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. Journal of Translational Medicine 2013, 11:215–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madireddi S, Eun S-Y, Lee S-W, Nemčovičová I, Mehta AK, Zajonc DM, Nishi N, Niki T, Hirashima M, Croft M: Galectin-9 controls the therapeutic activity of 4-1BB–targeting antibodies. The Journal of experimental medicine 2014, 211(7):1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK: Emerging Tim-3 functions in anti-microbial and tumor immunity. Trends in immunology 2011, 32(8):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM et al. : Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of experimental medicine 2008, 205(12):2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR: Negative Immune Regulator Tim-3 Is Overexpressed on T Cells in Hepatitis C Virus Infection and Its Blockade Rescues Dysfunctional CD4(+) and CD8(+) T Cells. Journal of Virology 2009, 83(18):9122–9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR: Tim-3 expression on PD-1(+) HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. The Journal of clinical investigation 2010, 120(12):4546–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J et al. : Upregulation of the Tim-3/Galectin-9 Pathway of T Cell Exhaustion in Chronic Hepatitis B Virus Infection. PLoS ONE 2012, 7(10):e47648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z: Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. European journal of immunology 2012, 42(5):1180–1191. [DOI] [PubMed] [Google Scholar]
- 132.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS: Inducible T cell antigen 4-1BB: Analysis of expression and function. Journal of Immunology 1993, 150(3):771–781. [PubMed] [Google Scholar]
- 133.Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS: Immune Responses in 4-1BB (CD137)-Deficient Mice. The Journal of Immunology 2002, 168(11):5483. [DOI] [PubMed] [Google Scholar]
- 134.Vinay DS, Kwon BS: 4-1BB signaling beyond T cells. Cellular And Molecular Immunology 2011, 8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Melero I, Murillo O, Dubrot J, Hervás-Stubbs S, Perez-Gracia JL: Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends in Pharmacological Sciences 2008, 29(8):383–390. [DOI] [PubMed] [Google Scholar]
- 136.Hendriks J, Xiao Y, Rossen JWA, van der Sluijs KF, Sugamura K, Ishii N, Borst J: During Viral Infection of the Respiratory Tract, CD27, 4-1BB, and OX40 Collectively Determine Formation of CD8<sup>+</sup> Memory T Cells and Their Capacity for Secondary Expansion. The Journal of Immunology 2005, 175(3):1665. [DOI] [PubMed] [Google Scholar]
- 137.Vinay DS, Kwon BS: Role of 4-1BB in immune responses. Seminars in Immunology 1998, 10(6):481–489. [DOI] [PubMed] [Google Scholar]
- 138.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH: ERK-Dependent Bim Modulation Downstream of the 4-1BB-TRAF1 Signaling Axis Is a Critical Mediator of CD8 T Cell Survival In Vivo. The Journal of Immunology 2008, 180(12):8093. [DOI] [PubMed] [Google Scholar]
- 139.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y et al. : CD28-independent, TRAF2-dependent Costimulation of Resting T Cells by 4-1BB Ligand. The Journal of experimental medicine 1998, 187(11):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martinez-Forero I, Azpilikueta A, Bolaños-Mateo E, Nistal-Villan E, Palazon A, Teijeira A, Perez-Chacon G, Morales-Kastresana A, Murillo O, Jure-Kunkel M et al. : T Cell Costimulation with Anti-CD137 Monoclonal Antibodies Is Mediated by K63–Polyubiquitin-Dependent Signals from Endosomes. The Journal of Immunology 2013, 190(12):6694. [DOI] [PubMed] [Google Scholar]
- 141.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP et al. : 4-1BB Costimulatory Signals Preferentially Induce CD8(+) T Cell Proliferation and Lead to the Amplification In Vivo of Cytotoxic T Cell Responses. The Journal of experimental medicine 1997, 186(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, Lynch DH: 4-1BB-Specific Monoclonal Antibody Promotes the Generation of Tumor-Specific Immune Responses by Direct Activation of CD8 T Cells in a CD40-Dependent Manner. The Journal of Immunology 2002, 169(4):1792. [DOI] [PubMed] [Google Scholar]
- 143.Chester C, Chang S, Kurland JF, Sagiv-Barfi I, Czerwinski D, Rajapaksa A, Waller E, Sadaram M, Richards L, Cohen LJ et al. : Biomarker characterization using mass cytometry in a phase 1 trial of urelumab (BMS-663513) in subjects with advanced solid tumors and relapsed/refractory B-cell non-Hodgkin lymphoma. Journal of Clinical Oncology 2014, 32(15_suppl):3017–3017. [Google Scholar]
- 144.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R et al. : Targeting CD137 enhances the efficacy of cetuximab. The Journal of clinical investigation 2014, 124(6):2668–2682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L: NK1.1 Cells Express 4-1BB (CDw137) Costimulatory Molecule and Are Required for Tumor Immunity Elicited by Anti-4-1BB Monoclonal Antibodies. Cellular immunology 1998, 190(2):167–172. [DOI] [PubMed] [Google Scholar]
- 146.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L: Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. The Journal of clinical investigation 2002, 109(5):651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quetglas JI, Dubrot J, Bezunartea J, Sanmamed MF, Hervas-Stubbs S, Smerdou C, Melero I: Immunotherapeutic Synergy Between Anti-CD137 mAb and Intratumoral Administration of a Cytopathic Semliki Forest Virus Encoding IL-12. Molecular Therapy 2012, 20(9):1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, Hervas-Stubbs S, Sangro B, Ochoa C, Rouzaut A et al. : Combined Immunostimulatory Monoclonal Antibodies Extend Survival in an Aggressive Transgenic Hepatocellular Carcinoma Mouse Model. Clinical Cancer Research 2013, 19(22):6151. [DOI] [PubMed] [Google Scholar]
- 149.Shi W, Siemann DW: Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Research 2006, 26(5 A):3445–3453. [PubMed] [Google Scholar]
- 150.Ju SA, Cheon SH, Park SM, Tam NQ, Kim YM, An WG, Kim BS: Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. International journal of cancer 2008, 122(12):2784–2790. [DOI] [PubMed] [Google Scholar]
- 151.Dubrot J, Milheiro F, Alfaro C, Palazón A, Martinez-Forero I, Perez-Gracia JL, Morales-Kastresana A, Romero-Trevejo JL, Ochoa MC, Hervás-Stubbs S et al. : Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunology, Immunotherapy 2010, 59(8):1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM et al. : Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences 2009, 106(9):3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guedan S, Posey AD, Shaw C, Wing A, Da T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M et al. : Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 2018, 3(1):e96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beatty GL, Moon EK: Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 2014, 3(11):e970027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mallett S, Fossum S, Barclay AN: Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. The EMBO journal 1990, 9(4):1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Calderhead DM, Buhlmann JE, van den Eertwegh AJ, Claassen E, Noelle RJ, Fell HP: Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. The Journal of Immunology 1993, 151(10):5261. [PubMed] [Google Scholar]
- 157.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M: The OX40 Costimulatory Receptor Determines the Development of CD4 Memory by Regulating Primary Clonal Expansion. The Journal of Immunology 2000, 165(6):3043. [DOI] [PubMed] [Google Scholar]
- 158.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF: Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Molecular Immunology 1987, 24(12):1281–1290. [DOI] [PubMed] [Google Scholar]
- 159.Salek-Ardakani S, Song J, Halteman BS, Jember AG-H, Akiba H, Yagita H, Croft M: OX40 (CD134) Controls Memory T Helper 2 Cells that Drive Lung Inflammation. The Journal of experimental medicine 2003, 198(2):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU: Functional expression of CD134 by neutrophils. European journal of immunology 2004, 34(8):2268–2275. [DOI] [PubMed] [Google Scholar]
- 161.Zaini J, Andarini S, Tahara M, Saijo Y, Ishii N, Kawakami K, Taniguchi M, Sugamura K, Nukiwa T, Kikuchi T: OX40 ligand expressed by DCs costimulates NKT and CD4(+) Th cell antitumor immunity in mice. The Journal of clinical investigation 2007, 117(11):3330–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang Y-H, Ye Y, Sikora AG et al. : Plasmacytoid dendritic cells induce NK cell–dependent, tumor antigen–specific T cell cross-priming and tumor regression in mice. The Journal of clinical investigation 2008, 118(3):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baum PR, Gayle RB 3rd, Ramsdell F, Srinivasan S, Sorensen RA, Watson ML, Seldin MF, Baker E, Sutherland GR, Clifford KN et al. : Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. The EMBO journal 1994, 13(17):3992–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG: Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. The Journal of experimental medicine 1994, 180(2):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stüber E, Neurath M, Calderhead D, Perry Fell H, Strober W: Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity 1995, 2(5):507–521. [DOI] [PubMed] [Google Scholar]
- 166.Weinberg AD, Wegmann KW, Funatake C, Whitham RH: Blocking OX-40/OX-40 Ligand Interaction In Vitro and In Vivo Leads to Decreased T Cell Function and Amelioration of Experimental Allergic Encephalomyelitis. The Journal of Immunology 1999, 162(3):1818. [PubMed] [Google Scholar]
- 167.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G: Expression and function of OX40 ligand on human dendritic cells. The Journal of Immunology 1997, 159(8):3838. [PubMed] [Google Scholar]
- 168.Croft M: Control of Immunity by the TNFR-Related Molecule OX40 (CD134). Annual Review of Immunology 2010, 28(1):57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M: OX40 Promotes Bcl-xL and Bcl-2 Expression and Is Essential for Long-Term Survival of CD4 T Cells. Immunity 2001, 15(3):445–455. [DOI] [PubMed] [Google Scholar]
- 170.Song J, So T, Cheng M, Tang X, Croft M: Sustained Survivin Expression from OX40 Costimulatory Signals Drives T Cell Clonal Expansion. Immunity 2005, 22(5):621–631. [DOI] [PubMed] [Google Scholar]
- 171.Karulf M, Kelly A, Weinberg AD, Gold JA: OX40 Ligand Regulates Inflammation and Mortality in the Innate Immune Response to Sepsis. The Journal of Immunology 2010, 185(8):4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M et al. : In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. The Journal of clinical investigation 2007, 117(12):3868–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Higgins LM, McDonald SAC, Whittle N, Crockett N, Shields JG, MacDonald TT: Regulation of T Cell Activation In Vitro and In Vivo by Targeting the OX40-OX40 Ligand Interaction: Amelioration of Ongoing Inflammatory Bowel Disease with an OX40-IgG Fusion Protein, But Not with an OX40 Ligand-IgG Fusion Protein. The Journal of Immunology 1999, 162(1):486. [PubMed] [Google Scholar]
- 174.Martin-Orozco N, Chen Z, Poirot L, Hyatt E, Chen A, Kanagawa O, Sharpe A, Mathis D, Benoist C: Paradoxical Dampening of Anti-Islet Self-Reactivity but Promotion of Diabetes by OX40 Ligand. The Journal of Immunology 2003, 171(12):6954. [DOI] [PubMed] [Google Scholar]
- 175.Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K: Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood 2000, 95(7):2434. [PubMed] [Google Scholar]
- 176.Piconese S, Valzasina B, Colombo MP: OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. The Journal of experimental medicine 2008, 205(4):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song A, Song J, Tang X, Croft M: Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. European journal of immunology 2007, 37(5):1224–1232. [DOI] [PubMed] [Google Scholar]
- 178.Song A, Tang X, Harms KM, Croft M: OX40 and Bcl-x<sub>L</sub> Promote the Persistence of CD8 T Cells to Recall Tumor-Associated Antigen. The Journal of Immunology 2005, 175(6):3534. [DOI] [PubMed] [Google Scholar]
- 179.Schaer DA, Hirschhorn-Cymerman D, Wolchok JD: Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. Journal for Immunotherapy of Cancer 2014, 2:7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adler AJ, Vella AT: Betting on improved cancer immunotherapy by doubling down on CD134 and CD137 co-stimulation. Oncoimmunology 2013, 2(1):e22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA et al. : OX40 is a potent immune stimulating target in late stage cancer patients. Cancer research 2013, 73(24):7189–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN: OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. The Journal of experimental medicine 2009, 206(5):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, Hu HM, Redmond WL, Holland J, Weinberg AD: Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. Journal of immunotherapy (Hagerstown, Md : 1997) 2010, 33(8):798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S: PD-1 Blockade and OX40 Triggering Synergistically Protects against Tumor Growth in a Murine Model of Ovarian Cancer. PLoS ONE 2014, 9(2):e89350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Olffen RW, Koning N, van Gisbergen KPJM, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RAW, Nolte MA: GITR Triggering Induces Expansion of Both Effector and Regulatory CD4<sup>+</sup> T Cells In Vivo. The Journal of Immunology 2009, 182(12):7490. [DOI] [PubMed] [Google Scholar]
- 186.Gurney AL, Marsters SA, Huang A, Pitti RM, Mark M, Baldwin DT, Gray AM, Dowd P, Brush J, Heldens S et al. : Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Current Biology 1999, 9(4):215–218. [DOI] [PubMed] [Google Scholar]
- 187.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C: GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. European journal of immunology 2004, 34(3):613–622. [DOI] [PubMed] [Google Scholar]
- 188.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S: Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nature Immunology 2002, 3:135. [DOI] [PubMed] [Google Scholar]
- 189.Petrillo MG, Ronchetti S, Ricci E, Alunno A, Gerli R, Nocentini G, Riccardi C: GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmunity Reviews 2015, 14(2):117–126. [DOI] [PubMed] [Google Scholar]
- 190.Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C: GITR-GITRL System, A Novel Player in Shock and Inflammation. TheScientificWorldJOURNAL 2007, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clouthier DL, Watts TH: Cell-specific and context-dependent effects of GITR in cancer, autoimmunity, and infection. Cytokine Growth Factor Rev 2014, 25(2):91–106. [DOI] [PubMed] [Google Scholar]
- 192.Schaer DA, Murphy JT, Wolchok JD: Modulation of GITR for cancer immunotherapy. Current Opinion in Immunology 2012, 24(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hanabuchi S, Watanabe N, Wang Y-H, Wang Y-H, Ito T, Shaw J, Cao W, Qin FX-F, Liu Y-J: Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood 2006, 107(9):3617. [DOI] [PubMed] [Google Scholar]
- 194.Snell LM, McPherson AJ, Lin GHY, Sakaguchi S, Pandolfi PP, Riccardi C, Watts TH: CD8 T Cell-Intrinsic GITR Is Required for T Cell Clonal Expansion and Mouse Survival following Severe Influenza Infection. The Journal of Immunology 2010, 185(12):7223. [DOI] [PubMed] [Google Scholar]
- 195.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C: Glucocorticoid-Induced TNFR-Related Protein Lowers the Threshold of CD28 Costimulation in CD8<sup>+</sup> T Cells. The Journal of Immunology 2007, 179(9):5916. [DOI] [PubMed] [Google Scholar]
- 196.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M: Costimulation via Glucocorticoid-Induced TNF Receptor in Both Conventional and CD25<sup>+</sup> Regulatory CD4<sup>+</sup> T Cells. The Journal of Immunology 2004, 172(12):7306. [DOI] [PubMed] [Google Scholar]
- 197.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Houghton AN: Concomitant Tumor Immunity to a Poorly Immunogenic Melanoma Is Prevented by Regulatory T Cells. The Journal of experimental medicine 2004, 200(6):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA et al. : Agonist Anti-GITR Monoclonal Antibody Induces Melanoma Tumor Immunity in Mice by Altering Regulatory T Cell Stability and Intra-Tumor Accumulation. PLOS ONE 2010, 5(5):e10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM: Engagement of Glucocorticoid-Induced TNFR Family-Related Receptor on Effector T Cells by its Ligand Mediates Resistance to Suppression by CD4<sup>+</sup>CD25<sup>+</sup> T Cells. The Journal of Immunology 2004, 173(8):5008. [DOI] [PubMed] [Google Scholar]
- 200.Cohen AD, Diab A, Perales M-A, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP et al. : Agonist Anti-GITR Antibody Enhances Vaccine-Induced CD8<sup>+</sup> T-Cell Responses and Tumor Immunity. Cancer Research 2006, 66(9):4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoffmann C, Stanke J, Kaufmann AM, Loddenkemper C, Schneider A, Cichon G: Combining T-cell vaccination and application of agonistic anti-GITR mAb (DTA-1) induces complete eradication of HPV oncogene expressing tumors in mice. Journal of immunotherapy (Hagerstown, Md : 1997) 2010, 33(2):136–145. [DOI] [PubMed] [Google Scholar]
- 202.Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X: Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. Journal of Translational Medicine 2014, 12(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S: Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3<sup><strong>+</strong></sup>CD25<sup><strong>+</strong></sup>CD4<sup><strong>+</strong></sup> regulatory T cells. The Journal of experimental medicine 2005, 202(7):885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van Lier RAW, Borst J, Vroom TM, Klein H, Van Mourik P, Zeijlemaker WP, Melief CJ: Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. Journal of Immunology 1987, 139(5):1589–1596. [PubMed] [Google Scholar]
- 205.Jung J, Choe J, Li L, Choi YS: Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. European journal of immunology 2000, 30(8):2437–2443. [DOI] [PubMed] [Google Scholar]
- 206.de Jong R, Loenen WA, Brouwer M, van Emmerik L, de Vries EF, Borst J, van Lier RA: Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. The Journal of Immunology 1991, 146(8):2488. [PubMed] [Google Scholar]
- 207.Nolte Martijn A, Van Olffen Ronald W, Van Gisbergen Klaas PJM, Van Lier René AW: Timing and tuning of CD27–CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunological Reviews 2009, 229(1):216–231. [DOI] [PubMed] [Google Scholar]
- 208.Borst J, Hendriks J, Xiao Y: CD27 and CD70 in T cell and B cell activation. Current Opinion in Immunology 2005, 17(3):275–281. [DOI] [PubMed] [Google Scholar]
- 209.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K: CD27-Mediated Activation of Murine NK Cells. The Journal of Immunology 2000, 164(4):1741. [DOI] [PubMed] [Google Scholar]
- 210.van Gisbergen Klaas PJM, Klarenbeek Paul L, Kragten Natasja AM, Unger P-Paul A, BB Nieuwenhuis Marieke, Wensveen Felix M, ten Brinke A, Tak Paul P, Eldering E, Nolte Martijn A et al. : The Costimulatory Molecule CD27 Maintains Clonally Diverse CD8+ T Cell Responses of Low Antigen Affinity to Protect against Viral Variants. Immunity 2011, 35(1):97–108. [DOI] [PubMed] [Google Scholar]
- 211.Lens SMA, Tesselaar K, van Oers MHJ, van Lier RAW: Control of lymphocyte function through CD27–CD70 interactions. Seminars in Immunology 1998, 10(6):491–499. [DOI] [PubMed] [Google Scholar]
- 212.Hendriks J, Xiao Y, Borst J: CD27 Promotes Survival of Activated T Cells and Complements CD28 in Generation and Establishment of the Effector T Cell Pool. The Journal of experimental medicine 2003, 198(9):1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H et al. : Characterization of murine CD70 by molecular cloning and mAb. International Immunology 1998, 10(4):517–526. [DOI] [PubMed] [Google Scholar]
- 214.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA: Characterization of murine CD70, the ligand of the TNF receptor family member CD27. The Journal of Immunology 1997, 159(10):4959. [PubMed] [Google Scholar]
- 215.Matter M, Odermatt B, Yagita H, Nuoffer J-M, Ochsenbein AF: Elimination of chronic viral infection by blocking CD27 signaling. The Journal of experimental medicine 2006, 203(9):2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Penaloza-MacMaster P, Rasheed AU, Iyer SS, Yagita H, Blazar BR, Ahmed R: Opposing Effects of CD70 Costimulation during Acute and Chronic Lymphocytic Choriomeningitis Virus Infection of Mice. Journal of Virology 2011, 85(13):6168–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gravestein LA, Amsen D, Boes M, Calvo CR, Kruisbeek AM, Borst J: The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. European journal of immunology 1998, 28(7):2208–2216. [DOI] [PubMed] [Google Scholar]
- 218.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D et al. : CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. The Journal of biological chemistry 1998, 273(21):13353–13358. [DOI] [PubMed] [Google Scholar]
- 219.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MHJ, Eldering E, van Lier RAW: CD27–CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. International Immunology 2007, 19(6):713–718. [DOI] [PubMed] [Google Scholar]
- 220.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD: Late Signals from CD27 Prevent Fas-Dependent Apoptosis of Primary CD8<sup>+</sup> T Cells. The Journal of Immunology 2008, 180(5):2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Peperzak V, Veraar EAM, Keller AM, Xiao Y, Borst J: The Pim Kinase Pathway Contributes to Survival Signaling in Primed CD8<sup>+</sup> T Cells upon CD27 Costimulation. The Journal of Immunology 2010, 185(11):6670. [DOI] [PubMed] [Google Scholar]
- 222.Prasad KVS, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF: CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. PNAS 1997:6346–6351. [DOI] [PMC free article] [PubMed]
- 223.Nolte MA, van Lier RAW: The price of the CD27–CD70 costimulatory axis: you can’t have it all. The Journal of experimental medicine 2006, 203(11):2405–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.O’Neill RE, Du W, Mohammadpour H, Alqassim E, Qiu J, Chen G, McCarthy PL, Lee KP, Cao X: T Cell–Derived CD70 Delivers an Immune Checkpoint Function in Inflammatory T Cell Responses. The Journal of Immunology 2017, 199(10):3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Leigh ND, O’Neill RE, Du W, Chen C, Qiu J, Ashwell JD, McCarthy PL, Chen GL, Cao X: Host-Derived CD70 Suppresses Murine Graft-versus-Host Disease by Limiting Donor T Cell Expansion and Effector Function. The Journal of Immunology 2017, 199(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Han BK, Olsen NJ, Bottaro A: The CD27–CD70 pathway and pathogenesis of autoimmune disease. Seminars in Arthritis and Rheumatism 2016, 45(4):496–501. [DOI] [PubMed] [Google Scholar]
- 227.Grewal IS: CD70 as a therapeutic target in human malignancies. Expert Opinion on Therapeutic Targets 2008, 12(3):341–351. [DOI] [PubMed] [Google Scholar]
- 228.Silence K, Dreier T, Moshir M, Ulrichts P, Gabriels SME, Saunders M, Wajant H, Brouckaert P, Huyghe L, Van Hauwermeiren T et al. : ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. mAbs 2014, 6(2):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang QJ, Yu Z, Hanada K-i, Patel K, Kleiner D, Restifo NP, Yang JC: Preclinical Evaluation of Chimeric Antigen Receptors Targeting CD70-Expressing Cancers. Clinical Cancer Research 2017, 23(9):2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lens SM, Drillenburg P, den Drijver BF, van Schijndel G, Pals ST, van Lier RA, van Oers MH: Aberrant expression and reverse signalling of CD70 on malignant B cells. British journal of haematology 1999, 106(2):491–503. [DOI] [PubMed] [Google Scholar]
- 231.Claus C, Riether C, Schürch C, Matter MS, Hilmenyuk T, Ochsenbein AF: CD27 Signaling Increases the Frequency of Regulatory T Cells and Promotes Tumor Growth. Cancer Research 2012, 72(14):3664. [DOI] [PubMed] [Google Scholar]
- 232.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ: Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood 2007, 109(11):4810. [DOI] [PubMed] [Google Scholar]
- 233.Infante JR, Dees EC, Olszanski AJ, Dhuria SV, Sen S, Cameron S, Cohen RB: Phase I Dose-Escalation Study of LCL161, an Oral Inhibitor of Apoptosis Proteins Inhibitor, in Patients With Advanced Solid Tumors. Journal of Clinical Oncology 2014, 32(28):3103–3110. [DOI] [PubMed] [Google Scholar]
- 234.Sakanishi T, Yagita H: Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochemical and Biophysical Research Communications 2010, 393(4):829–835. [DOI] [PubMed] [Google Scholar]
- 235.He L-Z, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, Pilsmaker CD, Round SM, Tutt A, Glennie MJ et al. : Agonist Anti-Human CD27 Monoclonal Antibody Induces T Cell Activation and Tumor Immunity in Human CD27–Transgenic Mice. The Journal of Immunology 2013, 191(8):4174. [DOI] [PubMed] [Google Scholar]
- 236.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR et al. : Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clinical Cancer Research 2011, 17(13):4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wei S-M, Fei J-X, Tao F, Pan H-L, Shen Q, Wang L, Wu Y-J, Zhou L, Zhu S-X, Liao W-B et al. : Anti-CD27 Antibody Potentiates Antitumor Effect of Dendritic Cell–Based Vaccine in Prostate Cancer–Bearing Mice. International Surgery 2015, 100(1):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jin L, Ge H, Long Y, Yang C, Chang Y, Mu L, Sayour EJ, De Leon G, Wang QJ, Yang JC et al. : CD70, a novel target of CAR T-cell therapy for gliomas. Neuro-Oncology 2018, 20(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kooten Cv, Banchereau J: Functions of CD40 on B cells, dendritic cells and other cells. Current Opinion in Immunology 1997, 9(3):330–337. [DOI] [PubMed] [Google Scholar]
- 240.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ: TRAF proteins in CD40 signaling. Advances in experimental medicine and biology 2007, 597:131–151. [DOI] [PubMed] [Google Scholar]
- 241.Carbone E, Ruggiero G, Terrazzano G, Palomba C, Manzo C, Fontana S, Spits H, Kärre K, Zappacosta S: A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. The Journal of experimental medicine 1997, 185(12):2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Katsiari CG, Liossis S-NC, Souliotis VL, Dimopoulos AM, Manoussakis MN, Sfikakis PP: Aberrant Expression of the Costimulatory Molecule CD40 Ligand on Monocytes from Patients with Systemic Lupus Erythematosus. Clinical Immunology 2002, 103(1):54–62. [DOI] [PubMed] [Google Scholar]
- 243.Bishop GA: The multifaceted roles of TRAFs in the regulation of B-cell function. Nature Reviews Immunology 2004, 4:775. [DOI] [PubMed] [Google Scholar]
- 244.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ: CD40/CD154 Interactions at the Interface of Tolerance and Immunity. Annual Review of Immunology 2004, 22(1):307–328. [DOI] [PubMed] [Google Scholar]
- 245.Frleta D, Lin JT, Quezada SA, Wade TK, Barth RJ, Noelle RJ, Wade WF: Distinctive Maturation of In Vitro Versus In Vivo Anti-CD40 mAb-Matured Dendritic Cells in Mice. Journal of Immunotherapy 2003, 26(1):72–84. [DOI] [PubMed] [Google Scholar]
- 246.Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E: In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. The Journal of experimental medicine 1993, 178(5):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM et al. : The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity 2008, 29(3):423–437. [DOI] [PubMed] [Google Scholar]
- 248.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M: CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America 2009, 106(3):876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peters AL, Stunz LL, Bishop GA: CD40 and autoimmunity: the dark side of a great activator. Seminars in immunology 2009, 21(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Smith TJ, Sciaky D, Phipps RP, Jennings TA: CD40 Expression in Human Thyroid Tissue: Evidence for Involvement of Multiple Cell Types in Autoimmune and Neoplastic Diseases. Thyroid 1999, 9(8):749–755. [DOI] [PubMed] [Google Scholar]
- 251.Carayanniotis G, Masters SR, Noelle RJ: Suppression of murine thyroiditis via blockade of the CD40-CD40L interaction. Immunology 1997, 90(3):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stuber E, Strober W, Neurath M: Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. The Journal of experimental medicine 1996, 183(2):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu Z, Geboes K, Colpaert S, Overbergh L, Mathieu C, Heremans H, de Boer M, Boon L, D’Haens G, Rutgeerts P et al. : Prevention of Experimental Colitis in SCID Mice Reconstituted with CD45RB<sup>high</sup> CD4<sup>+</sup> T Cells by Blocking the CD40-CD154 Interactions. The Journal of Immunology 2000, 164(11):6005. [DOI] [PubMed] [Google Scholar]
- 254.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ: Characteristics of Intestinal Dendritic Cells in Inflammatory Bowel Diseases. Gastroenterology 2005, 129(1):50–65. [DOI] [PubMed] [Google Scholar]
- 255.Liu Z, Colpaert S, D’Haens GR, Kasran A, Boer Md, Rutgeerts P, Geboes K, Ceuppens JL: Hyperexpression of CD40 Ligand (CD154) in Inflammatory Bowel Disease and Its Contribution to Pathogenic Cytokine Production. The Journal of Immunology 1999, 163(7):4049. [PubMed] [Google Scholar]
- 256.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK: Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. The Journal of clinical investigation 1996, 97(9):2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG: Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. Journal of Autoimmunity 2006, 26(3):165–171. [DOI] [PubMed] [Google Scholar]
- 258.Wang X, Huang W, Schiffer LE, Mihara M, Akkerman A, Hiromatsu K, Davidson A: Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. Arthritis & Rheumatism 2003, 48(2):495–506. [DOI] [PubMed] [Google Scholar]
- 259.Early GS, Zhao W, Burns CM: Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. The Journal of Immunology 1996, 157(7):3159. [PubMed] [Google Scholar]
- 260.Law C-L, Grewal IS: Therapeutic Interventions Targeting CD40L (CD154) and CD40: The Opportunities and Challenges In: Therapeutic Targets of the TNF Superfamily. Edited by Grewal IS. New York, NY: Springer New York; 2009: 8–36. [DOI] [PubMed] [Google Scholar]
- 261.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A: A short course of BG9588 (anti–CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis & Rheumatism 2003, 48(3):719–727. [DOI] [PubMed] [Google Scholar]
- 262.Ranheim EA, Kipps TJ: Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. The Journal of experimental medicine 1993, 177(4):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ: CD40-CD40 Ligand-Independent Activation of CD8<sup>+</sup> T Cells Can Trigger Allograft Rejection. The Journal of Immunology 2000, 165(2):1111. [DOI] [PubMed] [Google Scholar]
- 264.Lehnert AM, Mottram PL, Han W, Walters SN, Patel AT, Hawthorne WJ, Cowan PJ, d’Apice AJF, O’Connell PJ: Blockade of the CD28 and CD40 pathways result in the acceptance of pig and rat islet xenografts but not rat cardiac grafts in mice. Transplant immunology 2001, 9(1):51–56. [DOI] [PubMed] [Google Scholar]
- 265.Li Y, Zheng XX, Li XC, Zand MS, Strom TB: Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation 1998, 66(10):1387–1388. [DOI] [PubMed] [Google Scholar]
- 266.Baxendale AJ, Dawson CW, Stewart SE, Mudaliar V, Reynolds G, Gordon J, Murray PG, Young LS, Eliopoulos AG: Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene 2005, 24:7913. [DOI] [PubMed] [Google Scholar]
- 267.Cooke PW, James ND, Ganesan R, Wallace M, Burton A, Young LS: CD40 expression in bladder cancer. The Journal of Pathology 1999, 188(1):38–43. [DOI] [PubMed] [Google Scholar]
- 268.Tan J, Town T, Mori T, Obregon D, Wu Y, DelleDonne A, Rojiani A, Crawford F, Flavell RA, Mullan M: CD40 is expressed and functional on neuronal cells. The EMBO journal 2002, 21(4):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Altenburg A, Baldus SE, Smola H, Pfister H, Hess S: CD40 Ligand-CD40 Interaction Induces Chemokines in Cervical Carcinoma Cells in Synergism with IFN-γ. The Journal of Immunology 1999, 162(7):4140. [PubMed] [Google Scholar]
- 270.Pellat-Deceunynck C, Amiot M, Robillard N, Wijdenes J, Bataille R: CD11a-CD18 and CD102 Interactions Mediate Human Myeloma Cell Growth Arrest Induced by CD40 Stimulation. Cancer Research 1996, 56(8):1909. [PubMed] [Google Scholar]
- 271.Aldinucci D, Poletto D, Nanni P, Degan M, Rupolo M, Pinto A, Gattei V: CD40L induces proliferation, self-renewal, rescue from apoptosis, and production of cytokines by CD40-expressing AML blasts. Experimental Hematology 2002, 30(11):1283–1292. [DOI] [PubMed] [Google Scholar]
- 272.Choi MSK, Boise LH, Gottschalk AR, Quintans J, Thompson CB, Klaus GGB: The role of bcl-xL in CD40-mediated rescue from anti-μ-induced apoptosis in WEHI-231 B lymphoma cells. European journal of immunology 1995, 25(5):1352–1357. [DOI] [PubMed] [Google Scholar]
- 273.Pham LV, Tamayo AT, Yoshimura LC, Lo P, Terry N, Reid PS, Ford RJ: A CD40 Signalosome Anchored in Lipid Rafts Leads to Constitutive Activation of NF-κB and Autonomous Cell Growth in B Cell Lymphomas. Immunity 2002, 16(1):37–50. [DOI] [PubMed] [Google Scholar]
- 274.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, Stone MJ: Growth-inhibitory Effects of CD40 Ligand (CD154) and Its Endogenous Expression in Human Breast Cancer. Clinical Cancer Research 2001, 7(3):691. [PubMed] [Google Scholar]
- 275.Mackey MF, Gunn JR, Ting PP, Kikutani H, Dranoff G, Noelle RJ, Barth RJ: Protective Immunity Induced by Tumor Vaccines Requires Interaction between CD40 and Its Ligand, CD154. Cancer Research 1997, 57(13):2569. [PubMed] [Google Scholar]
- 276.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH et al. : IFN-γ mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nature Medicine 2007, 13:354. [DOI] [PubMed] [Google Scholar]
- 277.van Mierlo GJD, den Boer AT, Medema JP, van der Voort EIH, Fransen MF, Offringa R, Melief CJM, Toes REM: CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proceedings of the National Academy of Sciences of the United States of America 2002, 99(8):5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vincenti F: Costimulation blockade in autoimmunity and transplantation. Journal of Allergy and Clinical Immunology 2008, 121(2):299–306. [DOI] [PubMed] [Google Scholar]
- 279.Mai G, Del Rio M-L, Tian J, Ramirez P, Buhler L, Rodriguez-Barbosa J-I: Blockade of the PD-1/PD-1L pathway reverses the protective effect of anti-CD40L therapy in a rat to mouse concordant islet xenotransplantation model. Xenotransplantation 2007, 14(3):243–248. [DOI] [PubMed] [Google Scholar]
- 280.Lee J-h, Ha J, Kim S-h, Kim SJ: IL-2 pathway blocking in combination with anti-CD154 synergistically establishes mixed macrochimerism with limited dose of bone marrow cells and prolongs skin graft survival in mice. Journal of Korean medical science 2006, 21(6):1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wang Y, Dai H, Liu Z, Cheng X, Tellides G, Dai Z: Neutralizing IL-7 Promotes Long-Term Allograft Survival Induced by CD40/CD40L Costimulatory Blockade. American Journal of Transplantation 2006, 6(12):2851–2860. [DOI] [PubMed] [Google Scholar]
- 282.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ et al. : Phase I Study of Recombinant Human CD40 Ligand in Cancer Patients. Journal of Clinical Oncology 2001, 19(13):3280–3287. [DOI] [PubMed] [Google Scholar]