Abstract
In recent years, as living standards have continued to improve, the number of diabetes patients in China, along with the incidence of complications associated with the disease, has been increasing. Among these complications, diabetic foot disease is one of the main causes of disability and death in diabetic patients. Due to the differences in economy, culture, religion and level of medical care available across different regions, preventive and treatment methods and curative results for diabetic foot vary greatly. In multidisciplinary models built around diabetic foot, the timely assessment and diagnosis of wounds and appropriate methods of prevention and treatment with internal and external surgery are key to clinical practice for this pathology. In 2019, under the leadership of the Jiangsu Medical Association and Chinese Diabetes Society, the writing group for the Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition) was established with the participation of scholars from the specialist areas of endocrinology, burn injury, vascular surgery, orthopedics, foot and ankle surgery and cardiology. Drawing lessons from diabetic foot guidelines from other countries, this guide analyses clinical practices for diabetic foot, queries the theoretical basis and grades and gives recommendations based on the characteristics of the pathology in China. This paper begins with assessments and diagnoses of diabetic foot, then describes treatments for diabetic foot in detail, and ends with protections for high-risk feet and the prevention of ulcers. This manuscript covers the disciplines of internal medicine, surgical, nursing and rehabilitation and describes a total of 50 recommendations that we hope will provide procedures and protocols for clinicians dealing with diabetic foot.
Registry number: IPGRP-2020cn124
Keywords: Diabetic foot disease, Diabetic peripheral neuropathy, Peripheral arterial disease, Diabetic foot osteomyelitis, Diabetic foot infection, Diabetic complication, Ankle-brachial index, Transcutaneous oxygen pressure, Recommendation, Randomized controlled trials
Highlights.
Comprehensiveness: Carry out the whole course of treatment for patients, and propose multi-disciplinary teamwork
Detail: detail the process of diabetic foot treatment
Practicability: Combining Chinese guidelines and Chinese medical configuration, according to the situation of Chinese diabetic foot patients, and starting from the actual situation, it has a strong operability
Background
Diabetic foot disease represents a spectrum of complications in patients with diabetes, including lower extremity infection, ulcer formation and/or deep tissue damage, caused by a combination of neuropathy and varying degrees of vascular disease. Epidemiological studies have shown that diabetic foot ulcers (DFUs) have a prevalence of 5–10% and an incidence of 6.3% (95% confidence interval (CI), 5.4–7.3%) annual incidence of 1–4%; in China, the incidence is 4.1% (95% CI, 3.1–5.2%), and diabetic foot disease is the most common cause of hospitalization for diabetes [1]. Common etiologies of DFU include neuropathic (approximately 55%), arterial (10%) and neuroischemic causes (approximately 35%). The healing rate of DFUs after 12 weeks of treatment is 24–82% [2], and the recurrence rate is as high as 60% [3]. The prognosis of DFUs is poor: this disease is debilitating to quality of life, often leading to nontraumatic lower extremity amputation and even mortality. A study conducted by the Diabetes Amputation Research Group of the Chinese Diabetes Society found that, compared with those of nondiabetic patients, hospital stays were significantly longer (33.5 days vs. 22.0 days) and more expensive ($5,932 vs. $4,101) in patients with diabetes [4]. It is estimated that the medical cost of diabetes treatment in China will increase from the current $4.9 billion to over $7.4 billion in 2030. Based on the assumption that DFUs account for 20% of the total medical costs associated with diabetes, this would impose a heavy economic burden on society [5].
Patients with diabetic foot have a higher risk of amputation and death, and thus it is important to standardize their diagnosis and treatment. The development and practice of diabetic foot guidelines can effectively improve this standardization. The organizing committee of the International Working Group on the Diabetic Foot (IWGDF) published the ‘IWGDF guidelines on the prevention and treatment of diabetic foot disease’ [6] in 2019 after many years of thorough discussion involving experts from multiple countries and disciplines. In tandem with this, the Chinese Diabetes Society (CDS) also released the ‘Chinese Diabetes Foot Prevention Guideline (2019 edition)’ as their inaugural clinical practice guidelines [7]. Compared with the CDS guidelines, the IWGDF guidelines include discussion of the clinical issues around the use of the PICO format for the evaluation of current evidence in order to formulate recommendations. Meanwhile, the CDS guidelines focus on a detailed elaboration on issues relating to diabetic foot and serve as a comprehensive and practical guide; however, neither of these guidelines include a recommendation for an intensity classification. Both guidelines lack a discussion of the practical steps and methods for clinicians to diagnose and treat diabetic foot patients. For example, there is no guidance on how clinicians should decide on surgical interventions for different ulcers and their associated perioperative risk.
In 2019, under the leadership of the Jiangsu Medical Association and the Diabetes Society of the Chinese Medical Association, a writing group for the ‘Guidelines on a multidisciplinary approach for the prevention and management of diabetic foot disease (2020 edition)’ was established. These guidelines include contributions from scholars from the specialist areas of endocrinology, burn injury, vascular surgery, orthopedics, foot and ankle surgery and cardiology. This article makes recommendations for the screening, diagnosis, treatment and prevention of diabetic foot (see online supplementary material for details). Under the multidisciplinary model established for diabetic foot, the timely evaluation and diagnosis of wounds and the utilization of appropriate medical and surgical preventive and management methods are key to the clinical management of diabetic foot recommended in this article. We hope that this article, prepared by frontline clinicians, will aid fellow medical staff in China in improving the management of diabetic foot.
Methods
The ‘Guidelines on a multidisciplinary approach for the prevention and management of diabetic foot disease (2020 edition)’ was compiled by professional scholars from the specialist areas of endocrinology, burn injury, vascular surgery, orthopedics, foot and ankle surgery and cardiology, and we invited renowned domestic burn specialists Fu Xiaobing (an academic) and endocrinologists Ran Xingwu, Xu Zhangrong, Liu Chao, Xue Yaoming, Tang Zhengyi and Bao Junmin to act as lead judges. This article is based on domestic and foreign guidelines, combined with the clinical experience and research results of Chinese specialists and is written with an emphasis on practicability and feasibility. Each recommendation in this article represents a consensus from the editorial board, and a detailed description of the methodology, background and summary of the evidence can be found online. However, due to the lack of sufficient large-scale randomized controlled trial (RCT) evidence in China and a comprehensive understanding from the editorial board, many of the opinions recommended in this article are only preliminary, and further evidence is needed before they can be fully recommended. As such, we welcome scholars to scrutinize and comment on these recommendations. This article follows the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system for providing each recommendation and its corresponding theoretical basis (see Table 1). The recommendation strength classification considers four key factors: balance of interests, quality of evidence, values and willingness to choose and resource allocation. Importantly, a Level 1 recommendation is based on the prognosis of the patient and thus may also be based on poor-quality evidence.
Table 1.
Grades of Recommendation, Assessment, Development and Evaluation system evidence grades
| Quality of evidence | |
|---|---|
| A (high quality) | Very confident that the true effect value approximates the effect estimate |
| B (moderate quality) | There is a moderate degree of confidence in the effect estimates, and it is possible that the true value is close to the estimates, but there is still a possibility that the two are quite different |
| C (poor quality) | The degree of confidence in the effect estimate is limited and the true value may be quite different from the estimate |
| D (very low quality) | There is little confidence in the estimates of effects and the true values are likely to be very different from the estimates |
| Strength of recommendations | |
| Level 1 recommendation (strong) | The benefits clearly outweigh the risks and the credibility of both clinician implementation and population acceptance is high |
| Level 2 recommendation (weak) | The benefits are equal to the risks, depending on the specific clinical situation. In general, the preferences of doctors and patients play a more important role in the decision-making process |
Recommendations
Evaluation and diagnosis of diabetic foot disease
Diabetic foot is frequently a challenging condition in clinical practice, with issues including infection, neuropathy and vascular lesions, but the underlying soft tissue and bone healing abnormalities should also be taken into consideration, especially in people with a long duration of foot disease and glycated hemoglobin A1c (HbA1c) >7%. Therefore, the assessment should be comprehensive and thorough, with special focus on infections, lower extremity peripheral vascular disease, preoperative risk stratification and treatment risk assessment.
Performing a comprehensive medical evaluation
Recommendation 1: A comprehensive medical evaluation should be performed on all patients diagnosed with diabetes, especially for important organs such as the heart, brain and kidney and their levels of risk, and an assessment of lower limb disease should be made (Grades of Recommendation: Strong Levels of Evidence: Low).
Studies have shown that a comprehensive evaluation and early intervention can help to identify populations at high risk of diabetic foot and reduce the possibility of hospitalization and amputation. Such practices can also aid physicians in obtaining an accurate diagnosis and developing an appropriate treatment process to improve the prognosis for diabetic foot.
Medical history taking is the foundation of every physician’s understanding of his or her patients. All patients are unique, regardless of the similarities in their medical conditions. A detailed medical history should include both the general medical history and foot-specific history. The general medical history should include the following. (1) age, gender, occupation, types of physical activities, occupational needs and footwear habits. (2) Social history and lifestyle, including diet, exercise and nutritional status, smoking, alcohol, recreational drug use, prescription drug use (particularly corticosteroids and metformin). Cultural, psychological, social and economic factors are important considerations in any treatment plan, as they could contribute to the understanding of the patient’s compliance and level of expectation from the treatment, the treatment risks and prognosis [8]. (3) Diabetes and its related complications, such as cerebrovascular, cardiovascular and peripheral vascular disease and diabetic-related nephropathy, the worst complication of which is chronic renal failure, which further hinders wound healing. The percentage of amputation recovery is only 50–60% and there is a high risk of hemorrhage and hematoma formation after hemodialysis [9]. Moreover, diabetes itself is one of the major risk factors of cardiovascular and cerebrovascular diseases, causing a 2-4–fold increase in these conditions. The incidence of heart failure from DFUs can reach up to 39% and is related to the severity of the foot disease [10]. (4) Other systemic diseases: many systemic diseases can cause foot and ankle lesions, especially autoimmune diseases, inflammatory arthritis and central system or peripheral nervous disease. Podiatric medical history should include any previous history of foot and ankle surgery, amputation, Charcot neuroarthropathy, foot ulceration, foot infection, peripheral neuropathy, peripheral arterial disease (PAD), footwear, musculoskeletal foot deformity, callosities, corns or gangrene (see Table 2).
Table 2.
Medical history of diabetic foot patients
| General medical history | (1) Occupational needs, (2) family history of diabetes, (3) previous hospitalization, (4) surgical history (5) allergies, (6) adverse reactions to anesthetics, (7) nutritional status, (8) quality of life, (9) alcohol, tobacco, depression, (10) diabetes duration, (11) current medications, (12) glycemic management, (13) diabetic complications, (14) diabetic comorbidities, (15) other systemic diseases, (16) patient compliance, (17) doctors | |
| Foot-specific history | General foot history | (1) Foot disease history, (2) treatment history of foot disease, (3) footwear, (4) foot warmth, (5) mechanical or chemical contact, (6) acupuncture or pain in the lower limbs, (7) proximal leg muscle atrophy and weakness, (8) foot deformity, (9) abnormal foot pressure and callosity, (10) lack of joint range of motion, (11) claudication or pain at rest (12) bilateral or unilateral edema |
| Wound/ulcer history | (1) Incentives (stimulus time or trauma), (2) duration, (3) recurrence, (4) location, (5) wound care, (6) wound size (length, width and depth), (7) interference with wound care (family or social), (8) offloading techniques | |
Physical examination begins with the measurement of basic parameters such as height and weight, vital signs and urine output. Physicians should check for any airway obstruction, jugular vein dilation (indicating congestive heart failure), carotid murmur (cerebrovascular disease) or signs of jugular vein intubation. Next, the examiner should proceed with routine cardiopulmonary and abdominal examinations and a musculoskeletal examination of the whole body. The latter should include muscle strength and tone, signs of paresthesia, etc. Following this, physicians should shift focus towards the lower limbs. Lower limb examinations comprise dermatological, neurological, vascular and musculoskeletal assessments. Skin texture and integrity, hair growth and nails form the dermatological assessment. Lower limb swelling or edema can be due to multiple etiologies: cellulitis, cardiac insufficiency, venous insufficiency, renal impairment and deep tissue infection. Anhidrotic skin is often a complication of autonomic neuropathy and can result in fissures and ulcerations. Proper musculoskeletal assessment of the foot and ankle should include muscle strength, structural bone alignment and range of motion of the ankle and subtalar, midtarsal and the first metatarsophalangeal joints. Neurological and vascular assessment of the lower limb will be covered in a later section (see Table 3).
Table 3.
Lower limb physical examination
| Dermatologic examination | • Color, turgor, wetness, hair growth, chap |
| • Nail atrophy or hypertrophy | |
| • Calluses and subcallus hemorrhage | |
| • Ulcers (location, size, depth, infection status), gangrene | |
| • Others: (1) tinea pedis (fungal infection), paronychia (bacterial infection), itchy with scratch marks (yeast infection),(2) microvascular change, light brown, scaly patches (diabetic dermatopathy),(3) diabetic steatosis, bullous disease,(4) eruptive xanthomatosis, distal sclerosis, disseminated granuloma annulare (5) anaphylaxis | |
| Vascular examination | • Absence of hair growth, onychodystrophy, thinning skin (parchment-like skin), cyanosis and erythema, postural color change |
| • Temperature gradient (ipsilateral and contralateral extremity) | |
| • Abdominal artery to dorsal foot artery auscultation, palpation of femoral artery to dorsal foot artery | |
| • Handheld doppler examination | |
| Neurologic and musculoskeletal examination | • Vibration perception: tuning fork 128 cps, biothesiometer |
| • Light pressure: Semmes-Weinstein 10-gram monofilament | |
| • Light touch: cotton wool, two-point discrimination | |
| • Pain: pinprick (sterile needle) | |
| • Temperature perception: cold and hot | |
| • Deep tendon reflexes: patellar and ankle reflexes, clonus testing, Babinski test, Romberg test | |
| • Biomechanical abnormalities: (1) structural deformities: hammertoe, bunion, tailor’s bunion, hallux limitus, flat or high-arched feet, Charcot deformities, postsurgical deformities (including prior amputation); (2) limited joint mobility; (3) plantar pressure assessment: callus, corns, skin pressure red and other manifestations, computerized devices, Harris ink mat, pressure sensitive foot mat |
Laboratory investigations should include the following: (1) blood sugar level, hemoglobin, plasma albumin and prealbumin and blood lipids to determine nutritional status; (2) liver and kidney function, electrolytes, lactic acid, blood gas analysis, atrial natriuretic peptide and myocardial enzyme spectrum to assess vital organ function and internal environment homeostasis; (3) platelet, prothrombin time, international standardized ratio (INR; normal value approximately 1–2) and activated partial thromboplastin time (APTT; may be preferred over INR when heparin is used) to determine coagulation function; (4) full blood count, erythrocyte sedimentation rate, C-reactive protein (CRP), procalcitonin and blood culture to assess the level of inflammatory biomarkers; and (5) tissue and/or bone culture for microscopy culture sensitivity and histopathological study to guide antibiotic regimens for infected tissue. The interpretation of the results from any laboratory investigations should correlate with other clinical findings. For example, when the foot infection is severe, the level of white blood cells (WBCs) may not increase proportionately. In terms of noninvasive clinical examinations, the role of a resting ECG examination in functional heart evaluation is limited. High-risk groups need 24-hour ambulatory electrocardiography, echocardiography and even coronary angiography when required. Radiological imaging of the foot and plantar pressure measurements are necessary to assess the structural abnormality of the foot and ankle and identify any high-pressure points.
Assessment and diagnosis of diabetic foot infections
Recommendation 2: Diabetic foot infection (DFI) is a clinical diagnosis based on local signs (erythema, swelling, warmth, pain). Systemic inflammatory symptoms may be present in severe infection (strong; moderate).
Recommendation 2.1: If the clinical examination is ambiguous or cannot be explained, consider using inflammatory biomarkers such as CRP, erythrocyte sedimentation rate (ESR), Procalcitonin (PCT), etc. to aid in the diagnosis of DFI (strong; low).
Recommendation 2.2: Tissue and/or bone cultures should be collected from infected ulcers to identify pathogenic bacteria and guide antibiotic regimens (strong; moderate).
In 2012, the Infectious Diseases Society of America (IDSA) updated their clinical practice guidelines on the diagnosis and treatment of DFI. DFI is considered a clinical diagnosis for which the diagnostic criteria are based on cardinal signs of inflammation, including localized swelling, erythema <2 cm, increased skin temperature, pain and the formation of purulent discharge. Commonly, patients with diabetes-related foot issues may develop symptoms such as fever, chills, delirium, anorexia, vomiting, sweating, hemodynamic instability (e.g. tachycardia, hypotension) and metabolic disorders (e.g. acidosis, hyperglycemia, dyslipidemia, electrolyte disturbances, worsening azotemia). Clinical findings include leukocytosis, left-band shift, elevated inflammatory markers (such as ESR and CRP) and elevated procalcitonin, all of which elaborate the possibility of deep tissue infection [11]. The latest IWGDF guidelines discuss the role of inflammatory serum biomarkers in diagnosing DFI. These guidelines suggest that the correlation between WBC count and the severity of infection is small; approximately half of individuals with DFI exhibit normal WBC counts. Although ESR has been shown to have some value in detecting the possibility of bone infection when it is >70 mm, the diagnostic accuracy of CRP and PCT is higher than that of ESR, as CRP levels peak rapidly during infection and quickly subside when the infection is resolved [6]. Physicians should consider the following when collecting bacterial culture: (1) specimens should be collected before starting patients on empirical antibiotics; (2) deep tissue specimens should be preferred over swab specimens; (3) microscopy culture should include aerobic and anaerobic species with their corresponding antimicrobial sensitivity tests; (4) two sets of blood and urine cultures should be taken from each patient, regardless of whether they are symptomatic; (5) the use of molecular microbiology techniques, temperature measurements of plantar foot (‘hot spots’) using infrared thermography and quantitative analysis of microorganisms are not recommended as first-line methods for identifying pathogens and defining DFI; and (6) the accuracy of the culture results depends on the quality of the sample processing (including collection, transport and culture). Therefore, this article proposes that a diagnosis of DFI be based on local or systemic inflammatory symptoms and signs, with inflammatory indicators to evaluate its extensiveness and tissue culture to provide a bacterial profile of the pathogenic microbes causing the infection.
Recommendation 3: A diagnosis of osteomyelitis should be considered, unless proven otherwise, when there are signs of deep wounds in the sinus tract that probe to the bone, exposed bones or chronic nonhealing ulcers despite standard medical care. Further investigations, such as laboratory tests, diagnostic imaging (plain radiography, magnetic resonance imaging (MRI), WBC-labeled scintigraphy) and bone culture or biopsy can aid in the confirmation of osteomyelitis (strong; moderate).
Diabetic foot osteomyelitis (DFO) is an infection of the bone caused by bacterial invasion into the cortical bone and bone marrow cavity, eventually leading to nontraumatic lower extremity amputation. DFO may be suspected in wounds involving deep structures of the joints or bone that do not improve despite adequate arterial perfusion and appropriate offloading. Clinicians should also consider the possibility of DFO when there are signs of soft tissue infection (localized erythema, swelling, warmth, pain), spreading lymphangitis or exposed bone, and a positive sign in a probe to bone (PTB) test [12]. Among them, PTB assessment has a pooled sensitivity of 38–87% and specificity of 85% in the diagnosis of DFO. Lavery et al. found that the negative predictive value of the PTB test was as high as 96–98%, but the positive predictive value was only 57–62%, indicating that if the PTB result is negative, other additional tests are needed to exclude DFO. Commonly used clinical parameters in the diagnosis of DFO include: (1) bone exposure (sensitivity, 38–87%; specificity, 85–91%); (2) an ulcer area >2 cm2 (sensitivity, 56%; specificity, 92%); (3) an ulcer depth >3 mm (sensitivity, 74%; specificity, 77%); (4) an ESR >70 mm/h (sensitivity, 90%; specificity, 100%); (5) alkaline phosphatase (ALP) >135 Just U/L (specificity, 100%); and (6) a ‘sausage toe’ appearance (i.e. swollen toe) to the ulcer.
Recommendation 3.1: In all patients suspected of having DFIs, plain film radiography of the foot is recommended to determine bone abnormalities (deformities, damage), soft tissue gases and foreign bodies (strong; moderate).
Plain film radiography (PFR) is commonly used as first-line medical imaging in the assessment of the musculoskeletal structure of the lower extremities. Apart from being affordable, convenient and relatively fast, it also offers clinicians a bird’s eye view of any abnormalities in the bones and soft tissues (fractures, dislocations, malalignments, variant or accessory bones, the presence of foreign bodies, soft tissue gases) and allows them to dynamically monitor disease progression and the perioperative assessment of recovery, develop a roadmap with other medical imaging modalities and assess vascular calcification, neuropathic osteoarthropathy and bone deformities. The presence of foreign bodies or soft tissue gases (also called subcutaneous emphysema), often associated with necrotizing fasciitis, is a medical emergency that requires rapid treatment [13]. When PFR is used in DFO, it shows soft tissue swelling, cortical bone destruction or loss, periosteal reaction, bone mineral loss and, in some chronic cases, sequestrum may be evident. However, its sensitivity and specificity are low, reported to be 54% and 68%, respectively. It is often ineffective in differentiating between DFO and Charcot neuroarthropathy. As such, a PR examination has difficulty in confirming a DFO diagnosis. Furthermore, early radiographs often show negative signs and lag behind clinical signs by at least 2 weeks. Bone mineral losses of 30–50% or inflammatory response ranges extending >1 cm may be required to observe significant changes in PFR.
Recommendation 3.2: When DFO is diagnosed, advanced medical imaging, such as MRI (strong; low), is recommended; for those patients with contraindications for MRI, bone scans combined with WBC-labeled or antigranulocyte scans should be considered (weak; low).
MRI is the preferred and most advanced imaging method for aiding in the diagnosis of DFO. In active DFO, the bone marrow exhibits a hypointense signal on T1-weighted images and a hyperintense signal on T2-weighted images. In contrast, both the T1-weighted and T2-weighted images show reduced signal intensity during the chronic phase. The sensitivity of MRI is 90% (range, 77–100%), which is better than that of PR, technetium-99m (99mTc) bone scan or leukocyte scintigraphy. These bone marrow abnormalities are also observed in bone fractures, malignancies and other systemic inflammatory conditions, such as inflammatory arthritis, bone infarction and neuro-osteoarthropathy; thus, a differential diagnosis of other conditions must be considered. The advantages of MRI over other advanced imaging modalities include the absence of ionizing radiation, superior visualization of soft tissue structures (including the exploration of sinuses, deep tissue necrosis, abscesses and other inflammatory changes) and high sensitivity at early stages where features of bone marrow edema could be indicative of an infection. Disadvantages include a low specificity (79–82.5%), interference from metal products which can attenuate magnetic resonance (MR) signal, incompatibility with pacemakers (as they are subject to the magnetic field and radio-frequency (RF) pulse interference of the MRI system, leading to arrhythmia and tissue damage) and high maintenance costs.
Nuclear medicine scans (NMSs) may have some value in the diagnosis of DFO, especially when MRI is contraindicated. Direct scintigraphy and two-dimensional processed images can be used in combination with various radioisotopes and increase DFO diagnosis accuracy [12]. Typical findings of hyperperfusion, hyperemia and bone resorption are suggestive of DFO. NMSs exhibit higher sensitivity but lower specificity and poor anatomical localization compared with MRI. Single-photon emission computed tomography and positron emission tomography can overcome the poor anatomical localization of NMSs by generating three-dimensional slice imaging and have found their role in bone and WBC scanning. However, their applications are limited by their lack of practicability and cost-effectiveness. Hence, many practice guidelines generally do not recommend their use in routine imaging [14]. The traditional three-phase bone scan using 99mTc or indium (111In) has a sensitivity of 94% but a low specificity of 33%. When a labeled leukocyte scan is combined with a three-phase bone scan, the specificity for the diagnosis of acute infection can be increased to 80–90% [15].
Recommendation 3.3: Bone biopsy and bone cultures should be considered the gold standard for the diagnosis of DFO (strong; high).
Confirmation of the diagnosis of DFO is largely based on the isolation of bacteria in bone tissue, the discovery of osteonecrosis and histopathological findings of inflammatory cell infiltration. Bone biopsy is performed under surgical debridement or percutaneous puncture under fluoroscopy or computed tomography (CT) guidance and is considered the gold standard for the diagnosis of DFO [16]. The sensitivity and specificity of bone biopsy can reach 95% and 90%, respectively. Bone biopsy has not only played a role in the diagnosis of DFO but also provided guidance in the identification of pathogenic bacteria and their antibiotic sensitivity. The disadvantages lie mainly in the cost, the availability of equipment and the need for expertise training. Additionally, factors such as antibiotic interference, sampling errors (false negatives), contamination (false positives), invasiveness and abnormal clinical responses have been suggested to influence bone biopsy culture results, and bone biopsies are generally avoided in people with lower limb ischemia.
In summary, this article recommends that the PTB test be used to assist in the diagnosis of DFI in patients with open wounds. Second, plain radiographs should be used to identify bone abnormalities as well as soft tissue gas and radiopaque foreign bodies; MRI is recommended if a soft tissue abscess or DFO is suspected. Alternatively, 111In-labeled WBC scans combined with bone scans are recommended for patients with contraindications for MRI. Confirmation of DFO should be based on bone biopsy or bone culture for microscopy culture and histopathology.
Recommendation 4: The assessment of the severity of DFI is recommended based on the diagnostic criteria of the IWGDF/IDSA. Furthermore, the diagnostic criteria of sepsis are recommended according to the guidelines of the Chinese Society of Critical Care Medicine (strong; moderate)
Over the past decade, the severity of DFIs has been assessed by the IDSA/IWGDF classification system. On many occasions, this system has provided convenience in clinical application, as it only requires routine clinical examination, laboratory investigation and medical imaging, which are helpful for direct diagnosis and decision-making for infection treatment. Furthermore, this system has been widely accepted by researchers and practitioners. In addition, other existing classification schemes have not been developed or verified specifically for DFI. In 2014, the IWGDF/IDSA DFI classification criteria were updated as follows: (1) no sign of infection and without symptoms of either local or systemic infection (local swelling, erythema, warmth, pain or pus discharge); (2) mild, localized skin/subcutaneous infection (at least two signs of inflammation), surrounding erythema <2 cm; (3) moderate local infection involving deeper structures (tendon, muscle, joint or bone) or lymphangitis, erythema >2 cm or gangrene; and (4) severe localized infection associated with signs of systemic inflammatory response syndrome (SIRS) (hyperthermia or hypothermia, hypotension, tachycardia or severe unexplained hyperglycemia; it should be noted that >50% of limb-threatening infections have no systemic symptoms or signs). Lavery et al. re-evaluated the IDSA classification system for DFI in 294 patients and reclassified them as having moderate or severe infection. DFO had a much worse prognosis than soft tissue infection, including antibiotic duration (63.8 ± 55.1 days vs. 32.5 ± 46.8 days; p < 0.01), surgical requirements (99.4% vs, 55.5%; p < 0.01), number of operations (3.3 ± 2.3 vs. 2.1 ± 1.3; p < 0.01), percentage of amputations (83.4% vs. 26.3%; p < 0.01), reinfection (56.7% vs. 38.0%; p < 0.01), percentage of acute kidney injuries (49.7% vs. 37.2%; p = 0.04) and length of hospital stay (22.6 ± 19.0 days vs. 14.5 ± 14.9 days; p < 0.01). There were no differences in the prognosis of patients with moderate soft tissue infection and DFO, except for the number of operations (2.8 ± 2.1 vs. 4.1 ± 2.5; p < 0.01) and length of hospital stay (18.6 ± 17.5 vs. 28.2 ± 17.7; p < 0.01). These findings suggest that the IDSA classification of DFI can reflect the patient’s prognosis [17]. The diagnostic criteria for sepsis were adopted from the guidelines for the treatment of severe sepsis/septic shock published by the Chinese Critical Medical Association in 2014. A clear or suspected infection has the following clinical characteristics. (1) General clinical features: (a) body temperature of >38.3°C or <36°C; (b) heart rate >90 beats/min or >2 standard deviations from normal values at different ages; (c) shortness of breath; (d) change in mental state; (e) significant edema or positive liquid balance (>20 ml/kg in 24 h); (f) hyperglycemia (blood glucose >7.7 mmol/L) and no history of diabetes. (2) Inflammatory response indicators: (a) WBC count >12 × 109/L or <4 × 109/L; (b) normal WBC count but total number of immature leukocytes exceeding 10%; (c) plasma CRP 2 standard deviations greater than normal; (d) plasma procalcitonin 2 standard deviations greater than normal. (3) Hemodynamic parameters: hypotension as defined by systolic blood pressure <90 mmHg and mean arterial pressure <70 mmHg or a drop in systolic blood pressure for adults by >40 mmHg or 2 standard deviations below the normal value for the age of the patient. (4) Indicators of organ dysfunction: (a) arterial hypoxemia: PaO2/FiO2 <300 mmHg; (b) acute onset of oliguria: urine output <0.5 ml/kg/h and lasting for at least 2 hours even after sufficient fluid intake; (c) serum creatinine >4.2 μmol/L; (d) abnormal blood coagulation: INR >1.5 or APTT >60 s; (e) intestinal obstruction; (f) thrombocytopenia as defined by a platelet count <100 × 109/L; (g) hyperbilirubinemia as defined by a total plasma bilirubin >70 μmol/L. (5) Tissue perfusion indicators: (a) hyperlactatemia as defined by a blood lactate level >1 mmol/L; (b) reduced capillary reperfusion ability or ecchymosis. Therefore, this article recommends that the severity of DFIs be assessed according to the IWGDF/IDSA classification system. Meanwhile, sepsis should be diagnosed according to the standards developed by the Chinese Society of Critical Care Medicine.
Assessment and diagnosis of PAD
Recommendation 5: All patients with diabetes (regardless of the presence or absence of ulceration) should undergo peripheral arterial assessment at least annually, including an updating of the medical history and pedal pulse palpation. Patients aged >50 years who had a previous history of DFUs, cardio-cerebral atherosclerosis, previous vascular intervention, bypass surgery or abnormal lower extremity blood vessel conditions should have peripheral assessment performed at least once every 1–3 months (strong; low).
PAD is an independent risk factor for diabetic foot complications that can cause lower limb ischemia, tissue necrosis and delayed wound healing [18]. The prevalence of PAD in diabetic populations increases with advancing age and duration of diabetes. The diagnosis of PAD is based on the Ankle Branchial index (ABI) <0.9, Guan Ye et al. found that the incidence of PAD in people with diabetes aged >50 years in China was as high as 19.47%. The prevalence among those with diabetes in ``high- and middle-income countries is as high as 50 while neuropathic ulcers are more common in low-income countries. DFUs with PAD have a worse prognosis than many common cancers, with a 5-year mortality rate of up to 50% [19]. Typical manifestations of PAD include symptoms of intermittent claudication, nocturnal resting pain, cold, pale feet, weak or absent pedal pulses (dorsalis pedis and posterior tibial arteries), a monophasic waveform on Doppler ultrasound, a positive Buerger’s test and delayed capillary refill. It should be noted that the skin temperature is the temperature at which the arteries contract or relax to maintain balance and that can be used to determine the blood flow rate of the dermal blood vessels. This examination needs to be compared between segments (proximal and distal) on both the ipsilateral and contralateral sides. Temperature differences can provide a rough estimation of the blocked segment, but they can be easily confused when the bilateral limbs are diseased. As with the IWGDF guidelines, this article recommends that all patients with diabetes (even those without foot ulcers) have their peripheral arteries examined at least annually through a medical history and pedal pulse palpation [20]. When PAD is present, assessment review period should be increased as follows: (1) to at least every 6–12 months; (2) to every 3–6 months when there is a combination of the loss of protective sensation and foot deformity; (3) to every 1–3 months in high-risk patients with active foot ulcers, a previous history of foot ulcers, lower limb amputations and/or end-stage renal disease; or (4), in those patients aged >50 years who present with a previous history of DFUs, cardio-cerebral atherosclerosis, vascular intervention or bypass surgery, or a history of abnormalities in the vascular examination of the lower extremities, to at least once every 1–3 months, or even more often.
Recommendation 6: The ABI is currently the first choice for evaluating PAD; together with the toe-brachial index (TBI), Doppler ultrasound of the dorsal or posterior tibial artery and transcutaneous oxygen pressure can improve the diagnostic accuracy of lower limb ischemia (strong; moderate).
Peripheral artery assessment always starts with a detailed medical history of the patient, a family history, checks for any symptoms of cramping in the calf during ambulation (intermittent claudication), claudication distance, if any and checks for any symptoms of resting pain, lower extremity discomfort and decreased walking speed. Nonetheless, it is difficult to diagnose diabetes-related PAD, as the majority of patients are asymptomatic. Notably, the first clinical indication is often tissue necrosis or tissue loss and may also include severe calcification of blood vessels, local infection and edema. In some cases, peripheral neuropathy will interfere with the results of physical examinations. Palpation and auscultation of the lower extremity arteries can provide valuable information for diabetes-related PAD. Studies have shown that pedal pulse palpation and auscultation of the femoral arteries are 93.8% accurate and 98.3% specific, while providing 94.9% negative prediction for diagnosing or excluding diabetic arterial lesions; however, clinical misdiagnosis and missed diagnosis are still common [21].
ABI is a noninvasive clinical test, described by Winsor in the 1950s and characterized by its simplicity, affordability, high reproducibility and strong specificity. The sensitivity and specificity of diagnosing diabetes-related PAD can reach 68–84% and 84–99%, respectively [22]. The normal reference value of the ABI is 0.9–1.30. Values >1.30 indicate incompressible arteries secondary to vascular calcification [23] (especially in people with diabetes and chronic kidney disease); those between 0.5 and 0.90 indicate vascular stenosis; those between 0.3 to 0.5 indicate severe stenosis; and those <0.3 indicate the possibility of gangrene. If the ABI examination suggests an abnormality, the patient may require advanced medical imaging such as computed tomography angiography (CTA), magnetic resonance angiography (MRA) or digital subtraction angiography (DSA) of the lower extremities to plan the revascularization strategy. However, even though an ABI of 0.91–0.99 is acceptable, cardiovascular risks, including stroke, coronary heart disease may be increased [24].
The TBI is currently preferred for assessing arterial perfusion in the forefoot, as the digital arteries are less likely to be calcified. The TBI was first introduced to evaluate PAD in 1965. The exact thresholds remain controversial. In general, values >0.7 are considered normal; those <0.7 suggest arterial occlusion and may indicate symptoms of intermittent claudication; values <0.2 may be associated with resting pain; and a toe pressure <55 mmHg indicates poor wound healing [25]. Brooks et al. published a case–control study in 2001 and compared 174 diabetic patients with 53 nondiabetic patients in the use of the ABI and TBI to determine lower limb perfusion. The results showed that the TBI is not superior to the ABI, except in cases where the ABI is >1.3, in which the TBI performs significantly better.
Transcutaneous oxygen pressure (TcPO2) is a measure of skin perfusion that is not affected by calcification of the medial arteries. In 2008, Meijer et al. found that TcPO2 was significantly associated with diabetes patients (correlation coefficient = 0.258; p = 0.004), with an average value of 50.02 ± 8.92 mmHg. TcPO2 is different from the ABI in that it can effectively predict the prognosis of diabetic foot (even in cases of recanalization failure) (p = 0.015) [26], and the sensitivity and specificity of peripheral vascular lesions are better than those of ABI (specificity, 0.72 (95% CI, 0.61–0.81) vs. 0.48 (95% CI, 0.36–0.61); sensitivity, 0.86 (95% CI, 0.68–0.95) vs. 0.52 (95% CI, 0.42–0.63)) [27].
Factors influencing accurate TcPO2 measurement include (1) inadequate patient preparation, emotional instability, smoking history, the consumption of antihypertensive drugs, obesity and improper posture; (2) improper operation, such as leaks caused by incorrect placement of the electrode over the bony prominence of the plantar sole or examiners who lack the ability to correctly judge the TcPO2 curve; (3) excess equipment use, lack of preheating to 45°C before testing, excess electrode pad heating; and (4) other factors, such as ambient temperature, 75% ethanol not being used for disinfection or not allowed to dry sufficiently before placement of the electrode probes.
At present, there is no single test that has proven to be optimal; particularly, there is no clear threshold indicating normal lower limb blood vessels. Generally, the possibility of PAD is lower when the ABI is 0.9–1.3, the TBI is <0.75 and a triphasic waveform is seen on Doppler ultrasound. The presence of any of the following indicators suggests an increase in the healing rate of at least 25% in DFU patients with PAD [6]: skin perfusion pressure ≥40 mmHg, TBI ≥30 mmHg or TcPO2 ≥25 mmHg. Therefore, this article proposes the ABI as the first-line indicator to evaluate PAD, combined with the TBI, Doppler ultrasound of the dorsalis pedis or posterior tibial artery and TcPO2 to improve the accuracy of detecting lower limb ischemia.
Recommendation 7: When noninvasive examinations indicate the presence of ischemia, further investigation may be needed to aid in the planning of a lower limb revascularization approach (strong; moderate).
When the aforementioned clinical tests indicate the presence of ischemia and clinical considerations for lower extremity revascularization are underway, color duplex ultrasound (CDU), CTA, MRA or DSA can be used to obtain anatomical information and evaluate arterial circulation throughout the lower extremities, especially the lower knee and foot arteries.
CDU, also known as diagnostic ultrasound or diagnostic medical ultrasound, is an imaging method that uses a handheld sensor to introduce high-frequency (1–30 MHz) sound waves into a blood vessel to evaluate its structure and function. Type B, continuous-wave, pulsed-wave Doppler and two-dimensional ultrasound are used in the evaluation process. A prospective blinded comparative study showed that ultrasound evaluation of lower limb arteries had 88% sensitivity, 79% specificity and 95% accuracy [28]. Other studies have suggested that the sensitivity and specificity of ultrasound diagnosis of PAD is higher. Most ultrasounds are performed using probes placed over the skin surface. The advantage of ultrasound is that low-power sound waves are not harmful to the human body; however, they cannot provide images of the lungs or head in great detail, as sound waves cannot be transmitted through the air or bones.
CTA is a multidetector computer tomography technology that can cover a large area at high speeds while maintaining high resolution. It can provide the number, length, lumen diameter and morphology of arterial lesions in the lower limbs, the severity of calcification and the status of the distal runoff vessels, allowing accurate preoperative planning in terms of surgical path, balloon selection and long-term patency expected after intervention. At the same time, the status of collateral vessels can be adequately evaluated and occlusion of the arterial segments can be clearly displayed. In 2003, Ofer et al. found that the sensitivity, specificity and accuracy of CTA in the evaluation of arterial occlusive disease in the lower limbs were >90%. This finding is inconsistent with that of another study: in 2005, Edwards et al. found that the sensitivity of CTA for arterial stenosis was 70–80%. However, this study also found that about 7.3% of the lower segment of the artery occlusion plane was invisible to DSA while CTA was visible. Dual-energy CTA can improve the sensitivity and specificity of PAD diagnosis in diabetic patients, reaching 100% and 93.1% after multilevel reconstruction and 99% and 91.8% after maximum intensity projection, respectively [29]. A single-center, nonrandom observational prospective study showed that CTA can effectively determine lower limb ischemia and suggest left heart dysfunction (r = −0.54; p <0.0001) [30]. A retrospective study also concluded that CTA is similar to DSA in treatment guidance, such as intravascular and open surgery rates (intravascular and open surgery rates p = 0.305) and reintervention rates (21% CTA and 16% DSA; p = 0.517). [31]. The advantage of CTA is its ability to provide the details of the vascular network from the renal arteries to the feet; the disadvantage is that severe calcification may prevent the assessment of smaller arteries, potential allergic reactions and contrast-induced nephropathy (A history of kidney disease or heart failure).
MRA does not require arterial puncture and is considered a less invasive and more acceptable test than CTA or DSA. The data show that MRA has excellent sensitivity and specificity for inguinal stenosis and occlusion, estimated to be 94.7% and 95.6%, respectively. In contrast, the infrapopliteal sensitivity and specificity can reach 92.2% and 93.3%, respectively, and MRA can more easily identify distal vessels than DSA [32]. However, the accuracy of MRA for diabetic patients (especially those with knee disease) is unclear. A 2013 study by Healy et al. found that the sensitivity of MRA on infrapopliteal vessels was 86% (95% CI, 0.86–0.91) and the specificity was 93% (95% CI, 0.90–0.95), based on studies involving 83 patients published between January 1998 and June 2012. This finding suggests that MRA as a tool to guide bloodstream reconstruction would cause considerable misjudgment and that it may be more suitable for screening than diagnosis. Gadolinium is commonly used as a contrast agent for MRA and images can be obtained from the abdominal aorta to the foot, but there are limitations: (1) previous stent and artificial products will limit the resolution of the images; (2) the procedure is contraindicated for patients who have pacemakers or claustrophobia; and (3) patients with severe renal insufficiency (creatinine clearance <30 ml/min) are not allowed to use gadolinium-containing contrast agents, as they are at risk of developing renal-derived systemic fibrosis. In such cases, the use of nonthallium drugs, such as ultrasmall superparamagnetic iron oxide particles, can be considered. It is worth noting that to avoid the side effects of contrast agents and obtain high-quality images, many nonenhanced MRA systems, including static interval single-shot MRA, are increasingly used to assess the severity of PAD and can achieve good results.
DSA is an invasive technique. Contrast agent is commonly injected after femoral artery puncture to obtain the highest spatial resolution and image quality with DSA. It is widely considered the gold standard technique for simultaneously diagnosing and treating arterial disease. A 2005 study by Lapeyre et al. revealed that DSA has an advantage over CTA in determining the severity of lower limb ischemia and vascular density, especially in Trans-Atlantic Inter-Society Consensus (TASC) grade C or D distal segment lesions (25% in the DSA group and 0% in the CTA group; p = 0001) and lower limb vascular deficiency (72% in the DSA group and 26% in the CTA group; p = 0.001). The advantage of DSA is that it allows endovascular treatment to be performed at the same time, while the disadvantages are the use of iodine contrast agents, the need for invasive surgery, potential arterial puncture complications and sometimes allergic reactions.
In summary, anatomical information about the lower extremity arteries should be obtained to assess the presence, severity and distribution of arterial stenosis or occlusion. In diabetic patients, the need for detailed infrapopliteal and foot arterial imaging (especially for the special assessment of foot circulation) is important. Therefore, this article recommends that, in cases of noninvasive clinical examinations suggesting the presence of ischemia and clinical considerations of lower extremity revascularization, further imaging investigations are warranted and need to be appropriately selected depending on the patient’s condition.
Assessment and diagnosis of peripheral neuropathy
Diabetic neuropathy is one of the many complications associated with diabetes. The most common type is diabetic peripheral neuropathy (DPN). The main clinical manifestations are divided into positive symptoms and negative symptoms. Positive symptoms include subjective paresthesia (such as tingling, hyperalgesia, burning pain, formication), while negative symptoms usually require clinical and objective examination to be found and include sensory dysfunction, numbness, lower limb muscle atrophy, etc. Loss of protective sensation (LOPS) caused by DPN is an important cause of DFUs. A 2002 study by Reiber et al. showed that 45–60% of foot ulcers are caused solely by neuropathy. DPN increased the risk of foot ulcers by 15%, and the annual incidence of DPN-induced foot ulcers reached 5–7.5%. The risk of ulceration increased 7-fold and the incidence of recurrent ulceration in the DPN group was >3.5 times that in healthy individuals.
Recommendation 8: The assessment of DPN should include a light touch sensation test with a 10-gram monofilament and other neurological tests, such as vibration perception, sharp/blunt sensation, hot/cold temperature sensation and ankle reflex (strong; moderate).
Peripheral nerves can be divided according to the thickness of the nerve fibers: large fibers with a diameter of 6–12 μm, which mediate ankle reflex, touch, pressure, vibration and proprioception; and small fibers with a diameter of ≤5 μm, which account for pain, temperature and autonomic function. The most commonly encountered DPN in clinical practice is loss of protective sensation (LOPS). Interestingly, the early onset of LOPS is attributed to large fiber neuropathy, which is an important cause of DFUs and amputations. The main purpose of neurological examination is the early detection of LOPS. The significant of another common form of diabetic neuropathy, diabetic autonomic neuropathy, is often overlooked for in foot health. It is caused by deranged regulation of blood vessels, resulting in skin changes such as decreased elasticity, dryness and clefts, as well as the shortening of neurotrophic blood vessels short and microcirculation disorders, which are important pathological processes that occur and aggravate the pathogenesis of DPN. Furthermore, cardiovascular autonomic neuropathy, gastrointestinal autonomic neuropathy and urogenital autonomic neuropathy all have a large impact on quality of life and, in some cases, mortality. During the neurological assessment of the lower extremities, physicians should also perform appropriate assessments in the prevention of autonomic neuropathy. Diabetes can also cause motor neuropathy, manifested as claw-shaped toes, deepening of the arch of the foot and atrophy of the small muscles of the feet, all of which can cause increased peak plantar pressure over the sole of the foot, leading to the formation of callosities, musculoskeletal deformities and ulcerations. The presence of these clinical findings during physical examination indicates a high risk for foot disease that requires specialist work-up plans. [33]
Peripheral neurological assessment should include a 10-gram monofilament light touch test and other tests (vibration perception, ankle reflex, sharp/blunt and cold/heat sensory tests). These tests are simple and inexpensive and are suitable for screening in primary medical units or populations. However, performing all these clinical tests may be time-consuming, the examiner needs to have adequate formal training to be competent and the patients need to have appropriate levels of hearing, cognition and comprehension to understand the examination procedures. In addition, their results are often poorly reproducible. To avoid errors in the results and the need for standardization, we recommend using the IWGDF method and a combination of multiple tools for a comprehensive evaluation.
First, a light touch sensation test is conducted using a 5.07 Semmes-Weinstein monofilament, which is calibrated to provide a 10-gram force of pressure upon bending of the filament fiber. The specific steps are as follows: (1) the patient lies in a relaxed supine position with his or her eyes closed; (2) the examiner performs a trial test on the back of the patient’s hand to anticipate the amount of pressure he or she is expected to experience in the actual test; (3) the locations of the test site include the plantar aspect of the distal first toe and of the first and fifth metatarsophalangeal joints (Figure 1), while avoiding areas of callosities, ulceration or necrotic tissue; and (4) the examiner places the monofilament perpendicular to the skin surface and applies a force sufficient to buckle the monofilament for <2 seconds (Figure 2). The test is conducted three times and the patient is asked to identify where on the foot a light touch sensation was felt. The response is graded as ‘present’ when the subject is sensate at all test locations and ‘absence’ when the subject cannot identify the sensation at one or more locations. During the procedure, the examiner must not slide the filament across the skin or test on the same site repeatedly. The time spent on each test location should not be extensive or the force of the flexion of the monofilament will be lost. Light touch pressure sensation is an effective tool for assessing advanced neuropathy. Dros et al. reported the sensitivity and specificity of the monofilament test to be 41–93% and 68–100%, respectively. Smieja and his team demonstrated that the sensitivity and specificity could be improved to 80–93% and 86–100%, respectively, when the assessment is performed on the plantar aspect of the first toe and the first metatarsal heads. Several authors have found that an increased pressure threshold is associated with a 10–20% increased risk of foot ulceration (for a threshold 2.5–8 times greater than that in healthy individuals) and a 5–15% increased risk of amputation (for a threshold 1.5–15 times greater than that in healthy individuals) [34].
Figure 1.
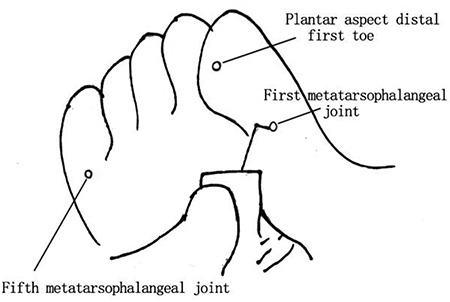
Monofilament test sites include plantar aspect of the distal first toe and the plantar aspects of the first and fifth metatarsophalangeal joints
Figure 2.
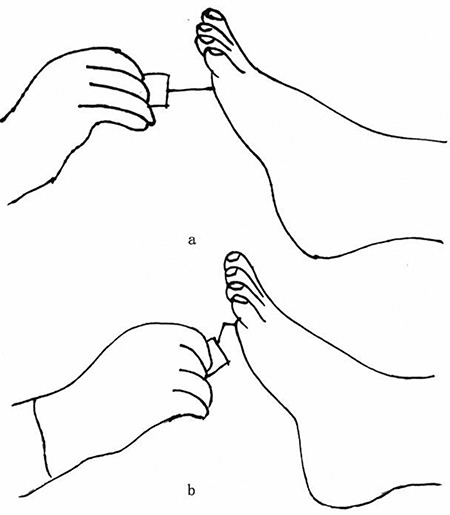
(a) The examiner places the monofilament perpendicular to the skin surface. (b) The examiner applies enough force to buckle the monofilament
Vibration perception is often assessed with a 128 Hz tuning fork. The specific steps are as follows: (1) the patient is maintained in the supine position with his or her eyes closed in a quiet and calm room; (2) before the actual test, the examiner places a vibrating tuning fork over the bony prominences of the wrist and elbow to allow the patient to become familiarized with the vibration sensation; and (3) the tuning fork is then placed over the distal aspect of the first toe (Figure 3). This test is conducted three times, at least once without the tuning fork, and the examiner asks the patient if he or she can feel any vibration and on which foot the vibration is felt. The response is graded as: (1) normal when there is a positive result ≥2 times; (2) reduced when there are <2 positive results—patients in this category are at risk of foot ulceration; or (3) absent. In such cases, to minimize the possibility of false-positive results, the examiner should consider repeating the test on other bony prominence locations, such as the ankle and tibial tuberosity. It should be noted that positive feedback and encouragement by the examiner may be considered during the test. The sensitivity and specificity of monofilament light touch and tuning fork vibration sensation can achieve a diagnostic accuracy of 90% in the diagnosis of peripheral neuropathy. [35]
Figure 3.
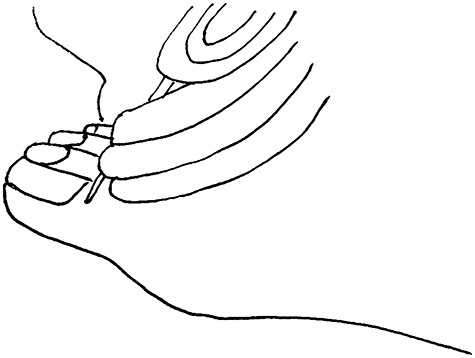
The tuning fork is held perpendicular to the skin and placed over the distal aspect of the first toe
Pain sensitivity is commonly evaluated with a disposable 40-grams needle or pin. The specific steps are as follows: (1) the examiner positions the needle/pin on the dorsal surface of the hallux, starting from the proximal nail fold and proceeding to the distal ends of the toenails; (2) the pressure exerted should be a light force that just deforms the skin; and then (3) the examiner then asks if there is any pain and, if so, the degree of the pain (Figure 4). The response is graded as ‘normal’, ‘weakened’ or ‘absent’. A ‘gloves and socks’ distribution can occur at multiple levels: the toes, forefoot, ankle, midcalf or knee. The examiner must not use too much force, as this could puncture the skin.
Figure 4.
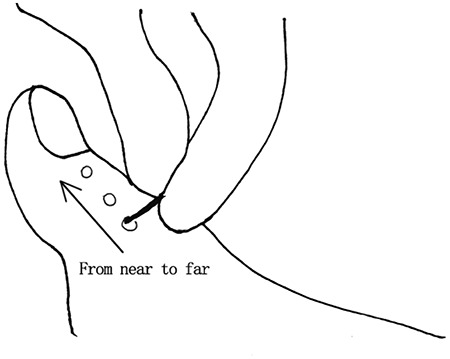
The examiner evaluates pain by pressing the patient's skin from proximal to toenail with a disposable 40-g pressure needle or pin
Achilles tendon reflex assessment is performed by percussion of the Achilles tendon with a reflex hammer. Three procedures can be performed to assess the reflex. In the first, the patient can be maintained in the supine position with the knee flexed and abducted. The examiner holds the patient’s toe with slight dorsiflexion, and then taps the Achilles tendon with the reflex hammer. In the second variation, the patient can sit upright with the lower limb hanging freely from the side of the examination bed while the examiner holds the subject’s toes in a slightly flexed position, and the examiner then strikes the Achilles tendon with the hammer. Finally, in the third variation, the patient can kneel backwards on a chair with both feet suspended off the edge of the seat while the examiner holds one of the feet in a slightly dorsiflexed position. The results can be a normal reaction, which involves gastrocnemius contraction and foot flexion to the plantar surface (Figure 5), the absence of a reaction or a hyperactive reaction, with clonus of the ankle reflex considered an abnormal response. The accuracy and specificity of this test are not as good as those of the monofilament or vibration perception tests.
Figure 5.
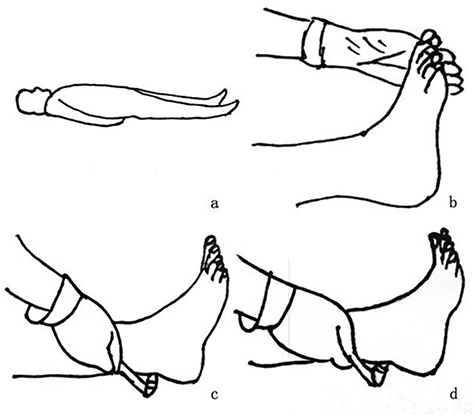
Specific steps for assessing the Achilles tendon reflex. (a) Patient is maintained in a supine position with the knee flexed and abducted. (b) The examiner holds the patient's toe in slight dorsiflexion. (c) The examiner taps the Achilles tendon with a tendon hammer. (d) The normal reaction is gastrocnemius contraction and foot flexion to plantar surface
Temperature sensitivity is commonly evaluated with the use of a Tip-Therm®, which is composed of two ends: one side feels warmer (34–45°C), while the other side feels colder (5–10°C). The examiner places both the warm and cold ends over the skin surfaces of the instep consecutively and asks the subject if her or she feels either of them. The response is graded as normal, reduced or absent (Figure 6).
Figure 6.
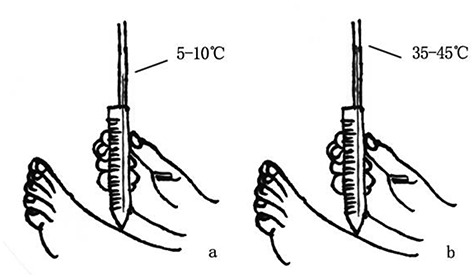
Tip-Therm specific steps. (a) The examiner places the warm end (34–45°C) over the skin surface of the instep. (b) The examiner places the cold end (5–10°C) over the skin surface of the instep
A simpler screening tool called the Ipswich touch test was designed by Rayman and his team at Ipswich Hospital. Prior to performing the test, the examiner explains the details of the test procedure to the subject. The examiner then asks the subject to close his or her eyes and indicate with a ‘yes’ when he or she feels a touch sensation after the examiner lightly touches the apexes of the hallux and the third and fifth toes of the foot for a duration of 1–2 seconds. This novel screening tool has been reported to be in almost perfect agreement with the 10-gram monofilament [36], achieving a sensitivity of 78.3% and a specificity of 93.9% when assessed by nonhealthcare practitioners [37].
Alternative inspection:
(1) Vibration perception threshold (VPT) measurement by a neurothesiometer can replace the tuning fork test. The pooled sensitivity and specificity of the VPT in diagnosing DPN ranges from 77.3% to 100% and from 72.8% to 81.0%, respectively. A high VPT of >25 Volt is associated with a 7-fold increased risk of ulceration, while a VPT of <15 V suggests a low risk. In a four-year prospective study by Young et al., the authors reported that every 1-V increment in the VPT carries a significant increase in the foot ulcer risk of 5.6%. A VPT of >25 V has also been reported as an independent risk factor for DFU with an odds ratio (OR) of 12.05, more than that of the presence of PAD or LOPS [3]. Compared with the 10-gram monofilament, the VPT was observed to have a higher positive predictive value.
(2) Use DPN examination instead of electromyogram detection.
(3) Neuroelectrophysiological examination, nerve conduction measurement are widely considered as the gold standard for the diagnosis of DPN. However, their use is limited by availability and time, and they are even less desirable in primary medical units. Therefore, they are generally not recommended as routine examinations, and are only used when the symptoms are atypical and differential diagnosis is required. These instruments are more convenient, faster, and can provide better repeatable detection results, operators only need simple training to master the use.
Recommendation 9: The diagnostic criteria of DPN are partly adapted from the ‘Guidelines for the Prevention and Treatment of Type 2 Diabetes in China’ and the American Diabetes Association’s (ADA) Position Statement (strong; moderate).
DPN has various clinical manifestations and can be examined with different techniques. A gold standard for diagnosing DPN has yet to be established, however. While numerous case–control studies suggest the use of neurophysiological examination, such as nerve conduction velocity (NCV), as a reference assessment tool, as it can provide objective and reliable results for the identification of large fibrous neuropathy, others believe that the gold standard for diagnosis should be skin biopsy and nerve fiber density measurements because they are more sensitive in the detection of early DPN. This article recommends the following in the diagnosis of DPN, partly adapted from the China Guidelines for the Prevention and Treatment of Type 2 Diabetes and the ADA Position Statement [38, 39]: (1) a known history of diabetes mellitus; (2) neuropathy that occurs at the time of or after the diagnosis of diabetes, although early peripheral neuropathy can also occur in prediabetes [40]; and (3) clinical symptoms and signs consistent with DPN, although some patients may be asymptomatic. A diagnosis of DPN can be confirmed in patients who present with clinical symptoms (pain, numbness, paresthesia, etc.) and abnormal results for all of the five clinical examinations (ankle reflex, acupuncture pain, vibration, pressure and temperature); a likely diagnosis of DPN can be made in asymptomatic patients if any two of the five clinical examinations are abnormal. Physicians should exclude neuropathy caused by conditions other than diabetes and work on differential diagnosis instead, especially in those who present with the following characteristics: symptoms that are acute or subacute, non-length-dependent manifestations that are asymmetric in distribution or those symptoms predominantly involving motor or autonomic function [41].
The framework principles and workflow for the diagnosis of diabetic foot
Recommendation 10: After a comprehensive evaluation, diagnoses and differential diagnoses should be assigned according to diagnostic principles and procedures (strong; low).
The diagnosis of diabetic foot involves excluding other diseases according to the following guidelines: (1) the condition meets the diagnostic criteria for diabetes mellitus; (2) the condition possesses the characteristics of diabetic foot, including (a) a history of previous ulcers, amputations and vascular intervention, (b) the presence of peripheral neuropathy, (c) the presence of peripheral vascular lesions, and (d) the presence of foot infections, ulcers and/or deep tissue loss. A diagnosis of infection is based on clinical observations of bacterial cultures, the severity of which is evaluated by the extent and depth of the wounds and the systemic conditions after the removal of nonviable and necrotic tissue. Globally, numerous medical associations and institutions have issued clinical practice guidance on the diagnosis and management of diabetic foot, such as ‘The management of diabetic foot: A Clinical Practice Guide’, developed by the American Academy of Vascular Surgery in collaboration with the American Podiatric Medical Association and the Vascular Medical Science Association [20]; the ‘Diabetes Foot Diagnosis and Treatment Process’ of the British National Institute of Health and Clinical Optimization [42]; and guidance from the Diabetes Foot Prevention Network of Australia [43]. Therefore, this article recommends that, after comprehensive evaluation, physicians follow stepwise framework principles and processes to arrive at an appropriate diagnosis and differential diagnosis. Based on these guidelines and our own clinical experience, we have proposed diagnostic procedures for diabetic foot (Figure 7).
Figure 7.
The diagnostic procedure for diabetic foot
Recommendation 11: Re-evaluation of management strategies with a focus on differential diagnosis is needed when DFUs are encountered in uncommon areas, have an atypical appearance or do not respond well to conventional treatment (strong; moderate).
The causes of lower extremity ulcerations are multifactorial (see Table 4), and include vascular causes (venous, arterial and mixed), neurological causes (diabetes-related neuropathy, spinal cord, syringomyelia), metabolic causes (diabetes, gout, proline peptidase deficiency), hematological causes (sickle cell disease, cryoglobulinemia), trauma (stress, trauma, burns), neoplastic (basal cell carcinoma, squamous cell carcinoma), infection (bacteria, fungi, protozoa), panniculitis (fat progressive necrosis, fat necrosis), pyoderma (gangrene pyoderma) and others, such as hypertension. Koerber et al. found that the majority of ulcerations are due to venous insufficiency (70%), arterial disease (4%) and mixed arteriovenous conditions (15%), and the remaining 13.5% are caused by vascular inflammation and other rare conditions. Inappropriate treatment of acute traumatic wounds is also a common cause and is sometimes even considered a major cause of chronic ulcers. Labropoulos et al. evaluated 19 uncommon ulcerations that were mostly concentrated in the middle of the lower leg. Among the causes were 5 cases of malignancy, 3 cases of chronic inflammation, 2 cases of sickle cell disease, 2 cases of vasculitis, 1 case of rheumatoid arthritis, 1 case of pyoderma and 1 case caused by hydroxyurea.
Table 4.
Different types of ulcers and their clinical manifestation
| Subjects | Venous ulcer | Arterial ulcer | Neurogenic ulcer |
|---|---|---|---|
| High risk factors | Venous valve dysfunction,deep venous thrombosis,prolonged standing,pregnancy, exercise less, obesity, family history |
High cholesterol, arteriosclerosis, hypertension, diabetes, aging, smoker, thromboangiitis obliterans, arteriovenous fistula |
Diabetes, peripheral nerve injury |
| Limb change | Edema, hyperpigmentation, superficial varicose veins, dry scaly skin, eczematous dermatitis, lymphedema | Toenail thickening,pale and dry skin, intermittent lameness, peripheral arterial pulse weaken or disappear, capillary reperfusion time is prolonged(>3–4 s), Pale skin appeared 1 minute after leg elevation of 45° | Sensory dysfunction, foot deformity |
| Location | Medial malleolar region: malleolar region, tibia, lower limb under a third | Pressure parts or extremity (toe): tiptoe, head of phalanx of toes, lateral malleolus or metatarsal |
Pressure parts |
| Characteristics | Wide range, irregular edges, shallow ulcer, red granulation tissue, less necrotic tissue and more exudate | Small scope and clear boundary, deep ulcer, basal paleness, black necrotic tissue, less exudate | Deep ulcer with reddish base and easy bleeding |
| Surrounding ulcer skin | Hemosiderosis(severe), lipodermatosclerosis | Adermotrophia and hair loss, mild pigmentation | Thick callosity |
| Pain | Mild or moderate pain, pain lessens with lower limb elevation | Pain obviously, pain lessens at rest or when the lower limbs are lowered | No obvious pain |
| Pulse | Normal pulse and skin temperature | Lower limbs pulse weakens or disappear and cold skin | The pulsating test is not reliable |
A differential diagnosis is required when DFUs appear in uncommon areas, have an atypical appearance and/or do not respond well to conventional treatment (>6 weeks). In addition to the common causes, special attention should be paid to the following. (1) Arterial hypertension ulcers. Hafner et al. proposed the concept of ischemic subcutaneous atherosclerosis with typical presentations of skin necrosis over the anterolateral lower limbs and medial calcinosis of the subcutaneous arterioles on histopathological skin biopsy. They are often misdiagnosed as pyoderma grangrenosum or necrotizing vasculitis. This newly proposed concept gives rise to 4 types of ulcers that share a common pathophysiological characteristic: nonuremic distal Martorell hypertensive ischemic leg ulcers, proximal nonuremic calcified ulcers and distal and proximal calcified ulcers with renal insufficiency [44]. (2) Arteriovenous mixed ulcers are venous leg ulcers complicated by arterial lesions that require arterial reconstruction combined with superficial venous reflux surgery. (3) Non-hyperkeratosis ulcer is not caused by common neuropathic lesions in diabetic foot. It should be paid attention to and pathologic examination should be made when diagnosis. (4) Lipid progressive necrotic ulcers, first described by Dr. Urbach in 1932, are a rare noninfective granulomatous skin disease that can result in cutaneous manifestations in people with insulin resistance or, in some cases, nondiabetes-related conditions such as rheumatoid arthritis. This type of ulcer occurs in approximately 0.3% of people with diabetes, and the ratio of females to males is approximately 3:1. Their incidence is independent of diabetes control and is related to the occlusion or stenosis of blood vessels near the anterior tibias of the lower extremities [45]. (5) Cancerous ulcers, most commonly squamous cell carcinomas or basal cell carcinomas, which can be primary or metastatic, or Marjolin ulcers, which can derive from the malignant changes of chronic benign ulcers. Marjolin ulcers were first described by French doctor Jean-Nicolas Marjolin in 1827. The median time to evolution is 25 years. The most common pathological changes are basal cell carcinoma, squamous cell carcinoma and melanoma, with an annual incidences of 75–100 per 100,000 people, 23–33 per 100,000 people and 5–20 per 100,000 people, respectively. The pathogenesis is complex, and several proposed mechanisms include: (a) increased expression of proto-oncogenes involved in cell proliferation and transformation; (b) overexpression of p53 and p21WAF/CIP1; (c) external factors, such as ultraviolet radiation; and (d) chronic inflammation and infections (including bullous epidermolysis bullosa malnutrition) that can also lead to malignancy due to repeated tissue stress. Therefore, we recommend re-evaluation of management strategies with a focus on differential diagnosis when DFUs are encountered in uncommon areas, have an atypical appearance or do not respond well to conventional treatment.
Classification and grading of diabetic foot ulceration
Recommendation 12: Among the numerous and varied wound classification systems, the University of Texas (UT) DFU classification system, which takes into account wound etiology and severity, has been widely validated and recommended for clinical practice (strong; moderate).
There are many different wound classification systems, including the Meggitt–Wagner classification system, the China Air Force General Hospital classification method, the Texas classification system, the S(AD) SAD scoring system, the PEDIS system, the DUSS system, the Kobe classification method, the SINBAD classification and the WIFI classification, as well as the SIANM classification method, which was developed by a local provincial foot and ankle center [46]. These classification systems are incredibly different, and it is impossible to suggest which is the best among them; therefore, their utilization largely depends on clinical indications. The newly published IWGDF guidelines recommend the SINBAD system for interprofessional communication, the IDSA/IWGDF classification for infection assessment and the WIFI system for perfusion and revascularization assessment.
The Texas DFU classification and grading method, proposed by the University of Texas Health Science Center in 1996, is based on depth, infection and vascular status. For example, Texas C3 (Grade 3, Stage C) indicates a noninfected ischemic wound that penetrates to the bone or joint space (see Table 5). The efficacy of this classification system has been evaluated in several studies. A 1998 study by Armstrong et al. found a significant increase in the amputation rate in deeper and higher-grade wounds. Deep wounds that probed to bone carry an 11-fold greater risk of high-level amputation (midfoot and higher) than superficial wounds. This risk increased significantly to 90 times when the wounds were further complicated by infection and ischemia. Oyibo et al. compared this classification with the Wagner classification system using a multicenter prospective case study method in 2001 and concluded that the two classification systems provided comparable clinical prognoses. In both classification systems, a higher amputation rate was observed in wounds with higher grading and stages, but the Texas classification appeared to be better at predicting healing outcome. The study also showed that the higher the grade and stage under the Texas system, the lower the cure rate and recovery rate and the higher the amputation rate were and ulcer depth and bone tissue amputation rate increased 11 times, if infection and ischemia were both present, the rate of amputation increased nearly 90 times [47]. Therefore, this article recommends the use of the UT DFU classification method, as it has been widely validated as taking into account both the etiology and the severity of the lesion.
Table 5.
Texas classification
| Texas classification | Grade 0: the epidermis is intact before and after ulcer formation | Grade 1 :superficial ulcers, not involving tendons, joint sacs or bone | Grade 2: the wound involves the tendon or capsule of the joint | Grade 3: the wound involves bone or joint |
|---|---|---|---|---|
| Stage A: no ischemia or infection | A0 | A1 | A2 | A3 |
| Stage B: infection | B0 | B1 | B2 | B3 |
| Stage C: ischemia | C0 | C1 | C2 | C3 |
| Stage D: ischemia and infection | D0 | D1 | D2 | D3 |
Preoperative risk stratification
Recommendation 13: The risk of deep vein thrombosis (DVT), bleeding, pressure sore and anesthesia should be evaluated in patients with diabetic foot prior to surgery to improve their surgical safety and outcomes (strong; moderate).
The pathogenesis of venous thromboembolism (VTE) in patients with diabetic foot complications is likely due to the following proposed mechanisms: (1) hyperglycemia and a high level of fibrinogen—Petrauskiene et al. suggested their possible role, as they reported that the risk of diabetes-related VTE increased by approximately 1.4 times for diabetic foot patients with these conditions (95% CI, 1.27-1.63); (2) a combination of venous dilatation and endothelial cell disruption; (3) iatrogenically damaged veins as a consequence of surgery; (4) the use of tourniquets, which constrict arterial circulation, resulting in venous dilation and increasing the risk of venous endothelial microtearing; (5) the heat generated during the polymerization of polymethyl methacrylate, which damages the integrity of the endothelium; (6) loss of vessels during peripheral vascular reconstruction (incidence of DVT within 1 year, 8.73%); (7) the use of anesthesia drugs, which can cause lower extremity veins to dilate; and (8) other factors, such as advanced age, comorbidities and delay in hospitalization. To date, there is no specific method for VTE risk assessment in diabetic foot populations. The Caprini scores, adopted in many orthopedic and vascular surgeries since 2005, stratify patients into four categories according to their corresponding risk of VTE: low, intermediate, high and very high risk. It is recommended that patients with Caprini scores ≥7 and beyond 8 be administered pharmacologic prophylaxis, which can reduce the relative incidence of VTE by 50% and the absolute risk by 1.2–4.5%.
The increased risk of bleeding in patients with diabetic foot may be related to the following factors. Long-term oral non-steroidal anti-inflammatory drugs, antiplatelet and anticoagulant drugs, and stress gastrointestinal ulcers. Among them, warfarin increases the risk of hemorrhage in patients by about 2 times. Although it is more common in clinical practice to have extracranial blood such as gastrointestinal bleeding, intracranial hemorrhage events are usually more destructive (the mortality rate of patients with warfarin associated intracranial hemorrhage is nearly 50%). Some patients were observed to have perioperative bleeding events after PCI procedures [48]. For surgical debridement of DFUs, as most patients are in a hypercoagulable state and intraoperative bleeding can be seen locally, perioperative bleeding can be addressed promptly in a controllable manner; however, it is still recommended to maintain the pre-existing anticoagulant and vasodilator dosages but to pay attention to the choice of administration of anesthesia. In addition to hyperglycemia, the presence of complex diabetes complications and other comorbidities such as age, hypertension, congestive heart failure, cerebrovascular disease and liver and kidney disease increases the risk of bleeding, especially intracranial bleeding. Both the puncture and nonpuncture sites may bleed during vascular intervention and should not be ignored. This is mainly limited to the operative procedure and the types of anticoagulative and antithrombotic agents used during treatment. A 1978 postoperative PCI study conducted by Branden et al. showed that puncture and operative technique-related bleeding accounted for 42.1% and nonpuncture site bleeding accounted for 57.9% (including gastrointestinal bleeding (16.6%), retroperitoneal hemorrhage (13.3%), urogenital tract bleeding (5.0%) and other bleeding (23.0%)). There have been few studies on bleeding during surgical intervention in patients with diabetic foot. Moreover, the Air Force Hospital from the Eastern Theater of PLA reported 6 cases of severe bleeding (1 case from the lateral iliac artery, 3 cases from the puncture site and 2 cases of spontaneous bleeding from the abdominal wall artery) in 150 patients with lower limb ulcers that underwent surgical intervention. To date, there is no specific method for the risk stratification of perioperative bleeding in patients with diabetic foot. As such, comprehensive information such as medical history, clinical symptoms, signs, routine blood tests, coagulation function and thromboelastography are needed. Specifically, for those patients who are taking warfarin and other anticoagulants, there are many bleeding risk scoring methods to predict bleeding episodes. One such example is the European CRUSADE scoring method, which was also cited by the Chinese PCI guidelines in 2016. A score of 35 or higher indicates a sensitivity of detecting patients with bleeding of 70% (95% CI, 55.57–82.43), a specificity of 77.41% (95% CI, 72.23–82.58), a positive predictive value of 40.78% (95% CI, 30.80–50.75) and a negative predictive value of 92.07% (95% CI, 88.34–95.81) [49].
Hospital-acquired pressure ulcer (HAPU) prevention remains an important clinical challenge. A 1997 survey of family doctors revealed that 70% believe they are not adequately prepared to care for HAPU patients. In contrast, good HAPU preventive measures can reduce the incidence of pressure ulcers (PUs) from 12.6% to 2.6% within 6 years (p < 0.001). While patients with diabetic foot are forced to stay in bed, the latest IWGDF guidelines point out the need to prevent the onset of pressure sore in the contralateral foot; the prevalence of HAPU depends on the type of operation, the timing of the operation and the recovery time from the effect of anesthesia. At present, there is no specific assessment method for the risk of HAPU in the diabetic foot population. The Braden scale, developed in 1987 (total score of 23 points: ≤9 points indicates extreme high risk; 10–12 points, high risk; 13–14 points, moderate risk; and 15–18 points, low risk), has a reported sensitivity and specificity of 70–80%. Many domestic nursing studies have adopted this scale in their clinical practice. Note that because the Braden score does not include age, body mass index and some comorbidities, the assessment of the HAPU risk for diabetic foot should be based on clinical conditions.
During the preoperative anesthesia risk assessment for diabetic foot patients, the choice of appropriate anesthetic agent is made according to the type of operation, the design of the operative techniques used and the duration of the operation. Some studies have proven the safety and operating procedures of peripheral nerve block (PNB) [50], and prospective RCT studies have shown that PNB is effective in stabilizing hemodynamics and managing perioperative pain. It is commonly the first choice for lower limb surgical anesthesia for diabetic patients [51] (Figure 8). Epidural anesthesia is not recommended. Although it has little effect on blood glucose, epidural anesthesia can inhibit the release of catecholamines and hinder the occurrence of stress-induced hyperglycemia. Most importantly, its usage is associated with calcification of ligaments, bone hyperplasia, narrowing of the intervertebral space, reduction of the success rate of spinal anesthesia puncture and increased risk of bleeding and nerve damage. Furthermore, there are reported episodes of hypotension and cardiovascular and cerebrovascular accidents [52]. Therefore, epidural anesthesia is not safe, and ensuring adequate anesthesia and analgesic effects may be a challenge while maintaining stable hemodynamics. There are currently no specific management strategies for the mitigation of anesthesia risk in patients with diabetes who require surgical interventions. Although the position statement of the American Society of Anesthesiologists’ classification of physical status (ASAPS) has been revised many times since 1941, it is worthy of reference and can be used as an independent predictor of the incidence and mortality of perioperative anesthesia accidents. A retrospective observational study revealed that both the ASAPS and its latest revision can predict anesthesia-related death and that the latter has a stronger effect (the area under the correlation curve for death prediction within 30 days increased by 4.7% to 0.848 ± 0.008; p <0.00001). However, this finding still requires future prospective research to support the observation [53].
Figure 8.
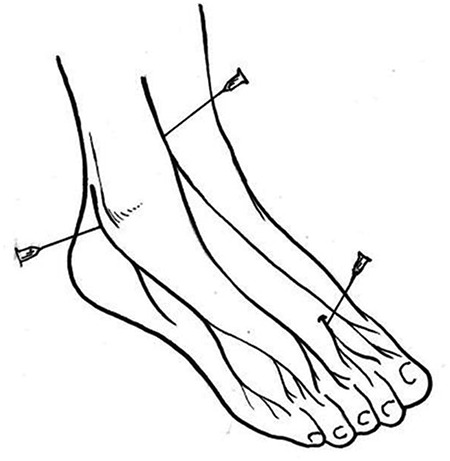
Foot nerve block
Therefore, in cases of a moderate level of evidence, it is strongly recommended that the risk of DVT, bleeding, pressure ulcers and anesthesia be evaluated in patients with diabetic foot before surgery to improve its safety.
Management of diabetic foot
Formation of a multidisciplinary limb protection team
Recommendation 14: Establish a multidisciplinary limb protection team comprising endocrinologists, foot and ankle (podiatric) surgeons, vascular surgeons and infectious disease physicians; if necessary, promptly request referral to a specialist diabetic foot treatment center to help reduce amputation and mortality rates in patients with diabetic foot disease (strong; low).
Research published by Singh and Armstrong et al. in the Journal of the American Medical Association in 2005 showed that patients with diabetes have a 25% risk of developing foot ulcers in their lifetime; 50% will be infected and require hospitalization and 20% will need amputation. The mortality rate is higher than that in those with malignant tumors. The management of diabetic foot requires a dedicated collaboration from different medical specialties. Among them, podiatric and vascular surgeons play a predominant role in the limb salvage team. Podiatric surgeons (also known as podiatrists) are often described as the gatekeepers of a team of diabetic foot experts who identify susceptible risk factors for preventing ulcers and perform biomechanical analysis of the feet and ankles to prevent ulcer recurrence. A 2005 study by Driver et al. showed that the presence of a podiatrist reduced the amputation rate in diabetic foot patients by 82% during a 4-year follow-up. The involvement of podiatric physicians resulted in substantial cost savings of $4,271.30 per year under the National Medical Insurance plan and $19,686 under a private insurance plan [54].
A retrospective study demonstrated that multidisciplinary teams significantly improve the clinical diagnosis and prognosis (infection control, wound healing) of DFUs (neuropathic, ischemic or mixed) [55]. The results showed that in 95% of referrals for which the etiology needed to be recategorized (p <0.001), the diagnostic accuracy for the etiology (85.71% vs. 6.12%; p <0.001), the grade of healing (100% vs. 44.90%; p <0.001), the judgment of vascular disease (14.28% vs. 2.04%; p = 0.03), bacterial identification (71.4% vs. 42.86%; p = 0.04), pain evaluation (100% vs. 8.16%; p = 0.001), footwear offloading evaluation (100% vs. 30.60%; p <0.001), wound closure (30.0%, vs. 4.08%; p <0.001) and the frequency of replacement of accessories (1.90% vs. 1.69%; p = 0.035) improved after the implementation of a multidisciplinary team [55]. Globally, many medical centers have adopted similar methods of diabetic limb salvage and prevention. Anichini and his team in 2007 presented a reduction in major amputation rates from 10.7 per 100,000 to 6.24 per 100,000 inhabitants 5 years after the inception of a multidisciplinary care team. In 2008, Krishnan and colleagues observed a reduction of 62% in major amputation and an overall 40% reduction in all amputations for an 11-year follow-up duration after the establishment of a diabetic foot care service. In Asia, Nather et al. evaluated the clinical impact of multidisciplinary teams and found that the rate of major amputations was reduced by one-third and the length of hospital stay was reduced from 20.36 days to 12.20 days (p < 0.05) [56]. In China, Wang et al. reported improvements in major amputation rates from 9.5% to <5% following the introduction of a team-based approach [57]. In particular, the major amputation rates involving Wagner ulcers of grades 3–4 were reduced from 35.7% in 2004 to 4.4% after 2007.
According to the requirements of the IWGDF, diabetic foot centers must have specialists for diagnosis and treatment, rapid access to equipment to enable this and the ability to provide vascular reconstruction procedures for both endovascular and open surgery. Such centers should provide education at multiple levels, from the patients with diabetic foot complications and their caregivers to healthcare professionals within the centers, frequent foot screening for those at risk and rapid and effective treatment of any foot ulcer or related infection. Finally, design a comprehensive treatment system for patients in need of long-term treatment, rather than simply dealing with emergencies. The latest IWGDF guidelines [6] indicate that, in all countries, it is best to have at least three levels of diabetic foot centers: level 1 consisting of podiatrists, GPs and nurses; level 2 consisting of endocrinologists (with shoe technicians, orthopedics or prosthetists), surgeons (general surgery, orthopedics, foot and ankle and vascular surgery), infectious disease physicians, podiatric physicians and diabetes nurses; and level 3 built on level 2 centers with medical specialists working together in a multidisciplinary approach and functioning as tertiary referral centers. The 2007 China Guidelines for the Prevention and Treatment of Type 2 Diabetes clearly stipulate that timely referral or consultation will help reduce the amputation rate and medical costs. Medical professionals who do not specialize in diabetic foot disease should be familiarized with the clinical indications that warrant rapid consultation or referral. Urgent referrals should be made to diabetic foot specialists or to relevant specialists when patients present with the following: skin color changes, increasing tenderness, presence of active ulcers, ulcers that deteriorate or those involving deep structures that probe to bone, signs of localized infection, cellulitis with ascending lymphangitis, signs of systemic infection or and suspected DFO.
In view of the moderate level of evidence, this article strongly recommends the establishment of multiple levels of multidisciplinary diabetic foot teams and specialist centers and emphasizes the importance of rapid consultation and/or referral to multidisciplinary teams or diabetic foot centers according to the severity of the patient's condition. Referrals to level 1 or 2 diabetic foot centers should be made when there are clinical signs of calluses, blisters, lower limb cellulitis, foot or ankle deformities, ingrown nails, toenail dystrophy or onychomycosis toe infection in the feet of patients with diabetes. Urgent referrals to level 3 diabetic foot centers are required when there are one or more severe open wounds, severe infections, the onset of ischemia or gangrene symptoms, early signs of Charcot's neuroarthropathy (redness, fever, swelling of the midfoot or ankle) or new or increased pain without a history of trauma.
Management of blood glucose, blood pressure and lipids in patients with diabetic foot
Recommendation 15: Good glycemic control (avoiding hypoglycemia) enables the healing of DFUs and reduces the risk of foot ulcer infection and amputation in patients (strong; moderate).
The wound healing process of DFUs is often affected by hyperglycemia. An observational study found that HbA1c is an important clinical predictor of the wound healing rate. For every 1% increase in HbA1c level, the wound healing rate decreases by 0.028 cm2/day (95% CI, 0.003–0.054) [58]; patients with higher HbA1c had significantly longer ulcer healing times [59]. Blood glucose is also related to the prognosis of patients with diabetic foot. One study analysed 9 patients with type 2 diabetes and found that intensive glycemic control (HbA1c 6–7.5%) resulted in a low amputation rate (risk ratio (RR), 0.65; 95% CI, 0.45–0.94; I2 = 0%) and a slow decline in the sensory threshold (mean difference, −8.27; 95% CI, −9.75–6.79) [60]. Hyperglycemia was also associated with wound-related infection, showing a rate of infection approximately 3.4 times that of the control group [61]. However, a recent review concluded that in the absence of randomized controlled trials, whether intensive glycemic control has a positive or negative effect on the healing of DFUs remains inconclusive [62]. Furthermore, some research findings that hypoglycemia has also been suggested to be an independent predictor of diabetic foot amputation, related to endothelium dysfunction caused by hypoglycemia and impaired wound healing [63]. In summary, in cases of moderate-quality evidence, good blood glucose control (avoiding hypoglycemia) is strongly recommended to promote the healing of DFUs and reduce the risk of foot ulcer infection and amputation.
Recommendation 16: For patients with diabetes-related comorbidities, such as hypertension, blood pressure control should be defined as (1) a more stringent target of <130/80 mmHg or (2) a less stringent target of <140/90 mmHg for elderly or critically ill (strong; moderate).
Diabetes-related hypertension is one of the most common complications in diabetes patients. In a multicenter prospective cohort study comparing 452 diabetic foot patients and 881 diabetic patients in China, it was found that diabetic foot patients were more likely to develop hypertension than diabetic patients (58.6% vs. 49.6%; p = 0.002) [64]. Other studies have found that hypertension can significantly increase the risk of foot ulcers and gangrene by inducing PAD [65]. A recent retrospective cohort study found that systolic blood pressure is closely related to diabetic foot. Excessive systolic blood pressure can increase the incidence of DFUs. It has also been observed that calcium channel blockers can reduce the occurrence of DFUs, possibly by stabilizing systolic blood pressure [66]. Although we understand that patients with diabetic foot require good blood pressure control, the exact control target has not yet been determined. In the prevention of diabetic foot disease, references can be made to standardized blood pressure control targets for patients with diabetes to formulate an individualized antihypertensive drug treatment plan.
In setting the blood pressure control goals for diabetic patients, the 2019 Guidelines for the Prevention and Treatment of Diabetes Mellitus in China recommended referencing the ADA’s position statement ‘Standard of Medical Care in Diabetes’. In this statement, the ADA recommends strict blood pressure control of <130/80 mmHg for patients who showed high cardiovascular risk (complicated with atherosclerosis or ≥15% risk of atherosclerosis risk within 10 years). A less strict blood pressure control of <140/90 mmHg is recommended for patients who have low cardiovascular risk (<15% risk of atherosclerosis within 10 years) [67]. Some experts even recommend that young people and individuals with proteinuria and/or hypertension with one or more risk factors for atherosclerosis should adhere to the stringent blood pressure control of <130/80 mmHg. Many studies have shown that every 10 mmHg reduction in blood pressure will lead to a reduction in the mortality of diabetic patients and in the absolute risk of cardiovascular disease [68–70], which results in the reduction of all-cause death by 13% that persists when the systolic blood pressure is <130 mmHg [71]. Systolic blood pressure should not be lower than 120 mmHg. With the participation of 4733 diabetic patients and an average follow-up of 4.7 years, the results of the ACCORD BP test found that patients with intense antihypertensive control goals set to 120 mmHg had no better fatal and nonfatal major cardiovascular incidence rates and composite outcomes than patients with a target of 140 mmHg [72]. Due to the risk of adverse effects caused by intense antihypertensive treatment, elderly patients with chronic kidney disease and frail and critically ill patients may benefit from a relatively broad blood pressure standard to improve quality of life [73]. Starting in 2020, the ADA recommends that the blood pressure control target for this population be 140/90 mmHg [35].
Therefore, this guideline strongly recommends that patients with diabetic foot with hypertension should be prescribed antihypertensive drugs on an individual basis and have their blood pressure control target set according to their condition.
Recommendation 17: Patients with diabetes-related comorbidities such as hyperlipidemia should be instructed to adopt lifestyle adjustments and have the intensity and dose of statins determined based on age and risk factors for atherosclerosis (strong; medium).
Diabetic patients are susceptible to atherosclerotic cardiovascular disease (ASCVD), where dyslipidemia is one of the main risk factors. Patients with diabetic foot often have dyslipidemia, and high triglycerides have been shown to be an independent risk factor for amputation in patients with diabetes [74]. A 2008 meta-analysis by Kearney et al. included data from >18,000 diabetic patients with an average follow-up period of 4.3 years. They showed that for every 39 mg/dl drop in LDL-C low-density lipoprotein cholesterol, all-cause mortality decreased by 9% and vascular-related mortality decreased by 13% [75]. A multicenter prospective cohort study found that high-density lipoprotein (HDL) was inversely related to the development of DFUs [64]. Another meta-analysis of four studies on the relationship between lipoproteins and diabetic foot found that a significant reduction in HDL was associated with the occurrence of diabetic foot and that there were significant differences in the levels of lipoproteins (including HDL, low-density lipoprotein, cholesterol and triglycerides) between the DFU and nonulcer groups (p < 0.005) [76]. The 2007 American Heart Association (AHA) and ADA consensus statement concludes that controlling individual cardiovascular risk factors can effectively prevent or delay increases in the incidence of ASCVD in diabetic patients. Therefore, actively controlling the blood lipid level of patients with diabetic foot is of great significance to their disease development and prognosis.
Diabetic foot patients with hyperlipidemia need comprehensive treatment. The 2019 Guidelines for the Prevention and Treatment of Diabetic Foot in China should be implemented according to the 2017 Guidelines for the Prevention and Treatment of Type 2 Diabetes in China. Meanwhile, the strategy of lipid control for patients with diabetes should be adopted from the ADA’s position statement ‘Standard of Medical Care in Diabetes’. In this statement, the ADA recommends: (1) a reduction of saturated fats, trans fats and cholesterol while increasing dietary intake of n–3 fatty acids, fiber and plant sterols; (2) triglycerides should be ≥1.7 mmol/L and HDL <1.0 mmol/L in males or <1.3 mmol/L in females to improve their lifestyle and blood glucose control. For patients 40–75 years old but without cerebral vasospasm risk factors, the use of moderate-intensity statins is recommended. For high-risk diabetes patients, patients with ASCVD risk factors or patients 50–70 years old, high-intensity statins are recommended. For all patients with diabetes and ASCVD risk factors, lifestyle interventions and high-intensity statins should be used [67]. For those who cannot tolerate high-intensity statin therapy, the combined use of moderate-intensity statins and ezetimibe can be considered [67]. This strategy has been confirmed by the IMPROVE-IT study. In addition, compared with 40 mg simvastatin alone, 40 mg vastatin combined with 10 mg ezetimibe can reduce the absolute risk of major cardiovascular adverse events by approximately 5% and the RR by 14% (RR = 0.86; 95% CI, 0.78–0.94) [77]. Therefore, this guideline strongly recommends that when diabetic foot patients have hyperlipidemia and lifestyle adjustments, the intensity and dose of statins should be determined according to patient age and the presence or absence of atherosclerotic risk factors.
The treatment of atherosclerosis in diabetes foot disease
Recommendation 18: For patients with diabetic foot disease and comorbidities, such as coronary heart disease/peripheral atherosclerosis, a 100 mg dosage of aspirin every night is recommended; if aspirin is not tolerated, 75 mg/day clopidogrel should be substituted (strong; moderate).
People with an ASCVD risk of <5% tend to be those who are younger than 50 years of age without family history of early cardiovascular disease or other risk factors including hypertension, smoking, dyslipidemia, or proteinuria. Aspirin has been shown to be effective in reducing cardiovascular morbidity and mortality (secondary prevention) in high-risk populations with a history of myocardial infarction or stroke. However, for people with diabetes without a history of cardiovascular events, the net benefit of aspirin is highly controversial. In 2010, a position statement from the ADA, the AHA and the American College of Cardiology Foundation suggested 75–162 mg/day of low-dose aspirin for the prevention of cardiovascular events in patients with diabetes and no history of vascular disease, but with a high risk of ASCVD (10-year ASCVD diabetes population with an increased event risk of >10%) and no increased risk of bleeding [78]. Those with diabetes at increased risk of ASCVD include men aged ≥50 years and women aged ≥60 years with one or more additional major risk factors, such as smoking, hypertension, dyslipidemia or a family history of early-onset ASCVD [78]. Patients who are intolerant of 100 mg/night aspirin can be given 75 mg/day clopidogrel [79]. People with diabetic foot, especially those with lower extremity ischemia, often present with high blood coagulation. Thrombosis can be prevented with anticoagulation therapy, including: (1) antiplatelet drugs to prevent platelet aggregation and thrombosis; (2) vasodilator drugs, which dilate blood vessels to reduce peripheral vascular resistance and the need for percutaneous endovascular angioplasty and/or stents, extend the patency time of transplanted blood vessels and facilitate stem cell differentiation; and (3) drugs that reduce the effectiveness of fibrinogen, which is often higher than normal in diabetic foot populations [80]. Therefore, this article strongly recommends that patients with diabetic foot with coronary heart disease or peripheral atherosclerosis be given 100 mg/night aspirin with reference to a 10-year ASCVD event risk exceeding 10%, and those who cannot tolerate aspirin should be given 75 mg/day clopidogrel instead.
Treatment of heart failure in diabetic foot disease
Recommendation 19: Patients with diabetic foot disease and comorbidities such as cardiac insufficiency who have been treated by oxygen inhalation, sedation, vasodilation and diuretic medications should be immediately referred to the cardiology department or intensive care unit to continue treatment (strong; low).
The management of comorbidities such as heart failure cannot be fully achieved unless the following have been established. (1) Elimination of stimulus and control of cardiovascular risk factors. (2) Provision of inhalation oxygen therapy: the effect of an oxygen mask is better than that of a nasal catheter and, in severe cases, the patient should be given Positive End Expirtory Pressure (PEEP) or continuous positive airway pressure oxygen, where the level of PEEP is gradually increased from a low level of between 3–5 cmH2O to a suitable level. (3) Sedation, where, in cases of extreme irritability, 3–5 mg of morphine can be administered intravenously. (4) Sufficient fluid replenishment when the effective circulating blood volume is insufficient to compensate for the lack of heart blood volume and central venous pressure monitoring to accurately replenish fluids and prevent overdose. (5) Vasodilator drug administration: when the systolic blood pressure is 100 mmHg or above, nitroglycerin can be administered sublingually or intravenously to reduce the cardiac afterload quickly but may cause hypotension. Therefore, medication should be withheld when systolic blood pressure is below 90 mmHg. (6) Intravenous diuretic administration: furosemide or torasemide should be administered to reduce preloading of the heart, but with caution in people with low blood pressure, especially those with acute myocardial infarction or aortic stenosis. (7) Effective brain natriuretic peptide (BNP)/NT-proBNP monitoring during treatment: (a) studies show that N-terminal pro-B-type natriuretic peptide (NT-proBNP) has high sensitivity and specificity for the diagnosis of cardiac insufficiency and is also a quantifiable indicator for anti-heart failure treatment [81]; (b) the latest Chinese guidelines emphasize that in stage A heart failure (pre-heart failure stage) and stage B (pre-clinical heart failure stage), as a form of early intervention for those with risk factors and asymptomatic left ventricular systolic dysfunction, BNP/NT-proBNP screening and intervention are recommended for people at high risk of heart failure, which can greatly prevent or delay disease progression; (c) however, in the case of renal insufficiency, atrial fibrillation or advanced age, the level of NT-proBNP will increase without clinical manifestations of cardiac insufficiency. Clinicians are required to closely monitor heart function without the need for immediate intervention, except for patients who are on hemodialysis. In cases of the latter, elevated NT-proBNP indicates the possibility of insufficient hemodialysis and/or cardiac insufficiency, which requires active hemodialysis and/or anti-heart failure treatment [82]. (8) Other treatments: referral to a cardiologist or intensive care unit for further treatment. Therefore, this article recommends that patients with diabetes-related cardiac insufficiency be treated with oxygen inhalation, sedation, vasodilation and diuresis. If necessary, the patient should be referred to cardiology or the intensive care unit for further investigation and management.
Risk prevention during the diabetic foot perioperative period
Recommendation 20: Perform perioperative risk stratification for VTE, postoperative bleeding, pressure ulcers and anesthesia risk in patients with diabetic foot who have undergone recent surgery (strong; moderate).
The choice of treatment of either thromboprophylaxis or a preventive option for those considered high-risk for VTE depends on the balance between thrombosis and bleeding and should follow a series of evidence-based procedures and guidelines. Basic preventive measures include the provision of sufficient preoperative patient education, standardization of surgical operations during surgery to reduce endometrial damage and appropriate use of tourniquets during surgery. Following surgery, the use of anticoagulants in sequence, graded compression socks and intermittent pressure compression devices, early ambulation, elevation of the patient’s limb perioperatively to promote venous return and moderate fluid replacement to reduce the risk of blood coagulation should all be performed.
Commonly used pharmacotherapeutics include heparin, low-molecular-weight heparin (LMWH), factor Xa inhibitors (such as rivaroxaban, fondaparinux sodium, etc.), vitamin K antagonists (warfarin) and antiplatelet drugs (aspirin), all of which have different sites of action and require different monitoring methods during their use. For example, a dose adjustment of oral warfarin is required every 3 days until the INR is 1.3–1.5, after which monitoring can be performed weekly. After the stabilization of INR or the elimination of contraindications, many studies recommend a subcutaneous injection of relatively LMWH, a major inhibitor of factor Xa, oral factor Xa inhibitors or aspirin, [83]. In 2007, Segal et al. concluded that there is strong evidence that LMWH is superior to unfractionated heparin in the prevention of recurrent DVT and pulmonary embolism. In addition, LMWH has been frequently used in hospital or outpatient settings due to its cost-effectiveness.
Regarding physical precautions: first, there is moderate-to-high-quality research evidence that early use of elastic graded compression stockings can effectively prevent thrombosis and post-thrombotic syndrome (PTS). In 1997, a study by Branjas et al. found that in near-stage DVT patients who started using custom stockings for at least 2 years within the first month of surgery, the prevalence of PTS was significantly lower after 5 years (mild-to-moderate PTS decreased from 47% to 20%; severe PTS decreased from 23% to 11%). Prandoni et al. performed an RCT in 2004 and found that the incidence of PTS was 25% after 2 years in patients wearing stretch socks and 49% in the control group. The risk ratio was 0.49 (95% CI, 0.29–0.84), and the patient group had a severe PTS incidence of 3% (11% in the control group) and a cumulative incidence of PTS in the first 6 months of 21% (40% in the control group). Second, intermittent pneumatic compression with a device that exerts a compression pressure of 35–40 mmHg at a rate of approximately 10 times/minute onto the calf and/or thigh muscles to simulate the muscle-pumping effect of walking can promote fibrinolysis and has been proven to reduce the risk of VTE [84]. Third, venous foot pumps mimic normal walking by compressing the plantar vein plexus to generate pulsating flow into the veins to increase venous outflow and reduce venous stasis. They have been shown to effectively reduce asymptomatic DVT after orthopedic surgery, but there is no evidence that they can reduce the risk of symptomatic DVT [85]. Compared with general anesthesia, localized nerve block anesthesia (either via single injection or continuous infusion) of the sympathetic nerve causes vasodilation and reduces the possibility of VTE formations. Finally, Young et al. 2007 showed that vena cava filters are only effective in preventing pulmonary embolism. In cases where a patient’s limb is unable to undergo the physical measures described above, these preventive measures can be performed on the contralateral limb. Nonetheless, special attention is needed for patients with congestive heart failure, pulmonary edema, DVT of the lower extremities, pulmonary embolism or thrombophlebitis in the surgical area, severe arteriosclerosis or stenosis of the lower extremity blood vessels.
The basic prevention measures for those with a risk of postoperative bleeding are mainly used for low-risk patients. For high-risk patients, coagulation and hemoglobin function should be closely monitored and anticoagulation programs should be adjusted accordingly. Specific clinical measures include the following. (1) Reasonable use of antithrombotic and anticoagulant drugs. (a) Aspirin does not pose a risk if local anesthesia is performed, but clopidogrel should be discontinued 7 days before local nerve block anesthesia. (b) Patients who use aspirin for a long time should choose an enteric preparation before bedtime but not with meals to reduce the risk of gastrointestinal tract injury. (c) When aspirin and clopidogrel are used in combination with dual antibodies, their use should be maintained for 6–12 months. The use of proton pump inhibitors, such as pantoprazole, rabeprazole, etc., should be considered for those at higher risk of gastrointestinal bleeding (note that some proton pump inhibitors can competitively inhibit clopidogrel's antiplatelet effect and increase the risk of thrombosis through the P450 metabolic pathway). (d) LMWH (such as enoxaparin) and unfractionated heparin have similar safety and efficacy profiles of anticoagulation and can be used immediately after vascular reconstruction, but the simultaneous use of both drugs should be avoided during the initial perioperative period. A study by White et al. in 2006 showed that, after PCI, the proportion of patients with major bleeding increased after the crossover from enoxaparin to unfractionated heparin (from 3.7% to 7.8%) and unfractionated heparin to enoxaparin (from 2.5% to 8.6%). (2) Optimized interventional procedures to reduce blood vessel-related bleeding. Femoral artery puncture is usually performed 2 cm below the groin skin folds. To reduce injuries and bleeding caused by penetrating the femoral artery, an antegrade approach is usually preferred. However, retrograde approaches are preferred over antegrade in patients who are obese because the antegrade approach can be more difficult in terms of the compression required, and there may be a risk of pelvic bleeding. (3) Patients undergoing major surgery, such as lower limb arterial repair, bypass or plaque resection, rehydration and blood preparation, should be given fluid infusion to reduce the effects of hypoperfusion, low volume and ischemia on the body. A prospective, randomized, multicenter study by Scheeren et al. showed that intraoperative fluid therapy can reduce the incidence of postoperative wound infection and reduce postoperative organ dysfunction [86]. The Cochrane Peripheral Vascular Disease group reviewed the data from 38 randomized controlled trials and concluded that fluid type (crystals and colloids) did not affect the prognosis [87]. Bunn and Trivedi analysed randomized controlled trials of critically ill patients and surgical patients, and no evidence was found that noncolloida solutions were better, more effective or safer than any other (except for hydrated ethyl starch products, as evidence suggests that they can cause acute kidney damage with risks greater than benefits) [88].
Patients considered at high risk of HAPU should be given various measures to prevent pressure ulcers. Three aspects related to HAPUs need to be considered: whether nurses use effective risk assessment and intervention checklists, the accuracy of HAPU risk assessments and the HAPU prevention strategies used. The following measures can be taken into consideration for people at high risk of diabetic foot disease. (1) Screening of nutritional status and development of personalized diabetes nutrition treatments, such as sufficient protein to maintain positive nitrogen balance, fluid replacement and other energy intake. (2) Posture changes to relieve or redistribute pressure to avoid direct pressure on the pressure ulcer and ensure that the heel is not under pressure. (3) The use of topical preparations, as some studies have observed that locally purified omentum lipid preparations (cream, liquid, emulsion and detergent) at different concentrations (10–25%) can prevent pressure ulcer in patients with diabetes [89]. (4) The use of mattresses to minimize the intensity and duration of pressure on vulnerable skin parts of high-risk HAPU patients: an RCT study from the UK showed that alternating pressure mattresses and high-specification foam mattresses could have a preventive effect on high-risk pressure ulcer patients [90]. (5) The use of various auxiliary materials on patient undergoing vascular surgery: one study observed that the use of 5 layers of silicone foam dressings resulted in only 1 patient developing HAPU in the dressing group (The incidence of HAPU in the dressing group was 2%, in the control group was >50%, p=0.0001). After further correction for age, gender and other factors, the use of 5 layers of silicone foam dressing was shown to significantly reduce the likelihood of a new HAPU [91].
The anesthesia risk for patients with diabetic foot disease largely depends on their blood glucose control and the method of anesthesia delivery. The total amputation rate for DFU patients in China is approximately 19.03%, of which large and small amputations comprise 2.14% and 16.88%, respectively [92]. The challenge in pain management lies in the effort to ensure the adequacy of anesthetic and analgesic effects while maintaining stable hemodynamics and reducing the risk of anesthesia. First, it is important to ensure that the blood sugar level is within a reasonable range. Surgery will be withheld when instances of hyperglycemia arise. Although there are no evidence-based recommendations, in principle, elective surgery should not be performed when ketoacidosis or hypertonic coma is present. Second, the choice of anesthesia method plays a crucial role. PNB should be the first choice, as it can stabilize hemodynamics [50] and control postoperative pain better than other methods of anesthesia. Some RCTs have compared PNB with the effect of spinal anesthesia (subarachnoid block (SAB)) on hemodynamics and pain in patients with diabetic foot and found that the SAB group had more hypotension (14 patients vs. 1 patient; p = 0.001) and needed more vasopressin treatment (6 patients vs. 0 patients). The postoperative pain-free time was longer in the PNB group (9 hours vs. 4.54 hours; p = 0.05), and the pain score was lower 1 day after the operation (3.63 points vs. 4.69 points; p = 0.01) [51]. A retrospective study examined the effects of general anesthesia (GEA) and PNB on postoperative pain and hemodynamic stability in patients with diabetic foot amputation. The amount of pethidine administered to the PNB group was reduced within 6 hours after the operation (27 ± 28 vs. 9 ± 18 mg; p = 0.013), and the average blood pressure in the GEA group was still low (Bonferroni-corrected p < 0.01) despite receiving more ephedrine (p < 0.01). There was a significant increase in patients who developed postoperative pneumonia (p = 0.030) and more patients requiring intensive care unit management (p = 0.038) among those in the GEA group [93]. Hence, this guide also recommends PNB as the first choice. Epidural anesthesia is not safe as it will inhibit the release of catecholamines that hinder the occurrence of surgical stress-induced hyperglycemia, and issues regarding ligamentous calcification, bone hyperplasia and narrowing of the intervertebral space reduce success rate of spinal anesthesia puncture while increasing the risk of bleeding and nerve damage. In addition, epidural anesthesia can also induce hypotension, eventually leading to cardiovascular and cerebrovascular events [52]. Therefore, this guide does not condone the use of epidural anesthesia.
Treatment of peripheral neuropathy
In recent years, significant progress has been made in the management of symptomatic DPN, but there is still no effective means to address the cause, delay the progression or even reverse the onset of DPN. Moreover, some drugs have been developed, but there is little evidence from RCTs to support their use for the treatment of DPN.
Recommendation 21: Management of hyperglycemia and optimization of blood glucose control are the foundation for the treatment of DPN (strong; high).
For type 1 diabetes, large clinical studies have proven that good blood glucose control helps reduce neurological complications. For example, the DCCT/EDIC study revealed that, compared with conventional therapy, neuropathy in patients with type 1 diabetes (assessed by NCV testing) was significantly reduced following 5 years of ‘intensive’ treatment. This study also observed that intensive treatment was beneficial in delaying the progression of autonomic neuropathy, and the effect was still observable 13 years after the completion of the study [94]. However, in type 2 diabetes, through a meta-analysis of large clinical studies, strict glycemic control was found to be helpful for treating neurological complications, but not significantly so. In some subsequent analyses, it was found that intensive intervention to lower blood glucose can improve both nerve conduction and vibration sensation thresholds, which may suggest a more complex pathogenesis of type 2 diabetes requiring comprehensive metabolic management (blood glucose, blood pressure, blood lipids, weight, etc.) to improve the prognosis of neuropathy. Bragd et al. [95], in 2008, demonstrated that blood glucose fluctuations had an impact on the occurrence and development of DPN. Presently, there is no consensus on the optimal level of blood sugar for the management of DPN. Nevertheless, most guidelines recommend a fasting blood glucose of <7.8 mmol/L and a random blood glucose of <10 mmol/L. However, these recommendations are not specific to the management of peripheral neuropathy. Intensive intervention of blood glucose can increase the risk of weight loss and hypoglycemia, and if blood sugar reduces rapidly within a short period it may likely induce treatment-related DPN (or insulin-induced neuritis), so its risk–benefit ratio needs to be explored. In particular, patients with diabetic foot disease typically have a longer duration of diabetes, poorer islet function and a lower incidence of hypoglycemia than patients with diabetes without foot complications. Additionally, these patients often have cardiac autonomic neuropathy. Once hypoglycemia occurs, the ability of hormones to perform regulatory functions also declines in a mechanism called hypoglycemia-related autonomic nervous failure. This leads to a significant increase in the risk of cardiovascular and cerebrovascular events in patients and requires increased monitoring. In light of the above evidence, this article strongly recommends active management of hyperglycemia and optimization of blood glucose control for the treatment of DPN.
Recommendation 22: According to the pathophysiological changes of DPN, treatment in microcirculation lesions, neurotrophic and abnormal repair can improve peripheral nerve function to a certain extent (weak; low).
Basic and clinical studies have confirmed that oxidative stress and activation of aldose reductase in the polyol pathway play important roles in the pathophysiological changes and pathogenesis of DPN. In theory, antioxidants and aldose reductase inhibitors should be able to significantly improve DPN prognosis, but this is not exactly the case. First, alpha lipoic acid (ALA) is an antioxidant that has been proven by current evidence-based medicine to have definite efficacy. Ziegler et al. conducted a test to verify whether 600 mg per day of ALA for three weeks could be effective in treating symptomatic DPN in a meta-analysis of four randomized double-blind placebo-controlled studies involving 1258 patients in 2004. They showed that lipoic acid treatment performed better than placebo in terms of clinical symptoms, the Total Symptom Score (TSS) and the Neuropathy Impairment Score in the Lower Limbs (NIS-LL). However, it was not clear whether the nerve conduction speed and nerve fiber density had improved. In studies conducted by Ziegler et al. and Aladin et al. in 1995 and 1999, respectively, they used oral lipoic acid for 3 weeks followed by oral administration for the next 6 months. The observational indicators, such as the TSS, Neurological Disability Score (NDS), Neurological Symptom Score (NSS) and the pain adjective list (Hamburg Pain Adjective List), did not yield positive results. Separately, in the 2006 SYDNEY 2 study, the authors observed several parameters (TSS, Neurological Symptom Change (NSC) score) following 5 weeks of ALA. The results showed that a patient's symptom score could be improved after 5 weeks of oral administration of ALA. Second, epalrestat is currently the only aldose reductase inhibitor on the market. Studies have shown that epalrestat (50 mg 3 times a day) can improve the pathological structure and electrophysiological abnormalities of peripheral nerves, thereby improving DPN symptoms and certain neuroelectrophysiological indicators. The multicenter the Aldose Reductase Inhibitor-Diabetes Complications Trial (ADCT) study conducted by Hotta et al. in 2006 involved >600 patients who took oral epalrestat (50 mg 3 times a day) for 3 years, while the control group received standard treatment. Objective study parameters included the NCV, the shortest F wave latency and VPT. Their results suggested that long-term oral epalrestat treatment improved the objective study parameters. It was also found that epalrestat improved retinopathy and kidney disease [96]. However, due to the lack of other RCTs, medical evidence is still insufficient, and as liver enzymes may be elevated, epalrestat has not yet been approved for marketing in Europe and the United States. Third, acetyl-L-carnitine (ALC), an acetylated derivative of carnitine, which is found in the mitochondria, plays an important role in the production process and thus has cytoprotective, antioxidative and antiwithering effects on the nervous system. It can also play an analgesic role by reducing the synaptic glutamate concentration, promoting regeneration and repair after nerve trauma and enhancing the role of nerve growth factor. An RCT by Grandis et al. in 2002 involved 333 DPN patients randomly given intramuscular ALC 1000 mg/day for 10 days followed by oral ALC 2000 mg/day for 1 year. They found that ALC could improve peripheral NCV and pain Visual Analogue Scale/Score. In a multicenter RCT by Sima et al., 500 mg ALC 3 times a day and 1000 mg ALC 3 times a day were used to treat a total of 1257 patients over 52 weeks. The number of gastrocnemius nerve fibers and regenerating nerve fiber bundles increased significantly, but the nerve conduction speed did not improve; in the 1000 mg 3 times a day group, the pain level was appreciably improved. In another large-scale, multicenter, randomized, double-blind, positive-control study in China, 232 cases of DPN were treated with either 500 mg ALC 3 times a day or 0.5 mg mecobalamin 3 times a day for 24 weeks. Symptom scores and neurophysiological parameters showed comparable efficacy between the two treatments [97]. Since ALC is marketed at home and abroad and approved for the short-term treatment of diabetic neuropathy, long-term large-scale RCTs are required for verification of its clinical efficacy.
Microcirculatory lesions play an important role in the development of diabetic foot. During the occurrence of DPN, microcirculation disorders around and within the nerve can cause microcirculation occlusion or short circuiting between the neurotrophic arteriovenous and venous systems, which leads to reduced or even lost oxygen supply to the nerve fibers. Prostaglandin E1 (PGE1), by increasing cAMP in vascular smooth muscle cells, exerts vasodilator effects, inhibiting platelet aggregation and activation, reducing blood viscosity and improving microcirculation. A small-scale RCT by Akahori et al. in 2004 found that alprostadil 10 mg/day for 2 weeks could alleviate DPN symptoms and improve sensory thresholds; another small-scale RCT found that 40 μg beraprost sodium 3 times a day for 8 weeks could improve the DPN symptom score [98]. Pancreatic kininogenase degrades kininogen, generates kinin, expands small blood vessels and capillaries and activates plasminogen and converts it to plasmin, thereby improving circulation. A case–control study by Ma et al. compared the efficacy of pancreatic kininogenase and PGE1 on DPN. The Michigan Neuropathy Screening Instrument, neurological symptoms and NCV were used as observation indicators. The authors found that pancreatin kallikrein 40 Unit/day could also improve DPN symptoms and NCV, and the efficacy was comparable to, but better than that of the control group [99]. Other circulating drugs (cilostazol, pentoxifylline, etc.) have some clinical effects, but due to lack of sufficient evidence, this article does not currently recommended their use.
Nutrition for nerve regeneration is equally important in the pathogenesis and healing of diabetic foot. There are currently two major classes of drugs that can repair and improve DPN nutrition, namely B vitamins (such as methylcobalamin) and nerve growth factors. Methylcobalamin, an endogenous form of vitamin B12, can promote axonal transport and regeneration and the formation of the myelin sheath, prevent axonal mutation and repair damaged nerve tissue. The earliest RCT was performed by Yaqub and colleagues in 1992 on 50 patients treated with 0.5 mg oral mecobalamin 3 times per day continuously for 4 months. The peripheral nervous system NCV was used as an observation indicator. The results showed significant improvement in clinical symptoms and nerve conduction. In a randomized, double-blind, double-simulated phase 2 clinical study comparing L-carnitine with mecobalamin, it was found that 0.5 mg mecobalamin 3 times a day for 24 weeks significantly improved the NDS, NSS and NCV [97]. Recombinant human nerve growth factor (rhNGF) has also been introduced for the treatment of DPN. In 2000, McArthur et al. found that it influences the relief of DPN symptoms and nerve conduction. However, a large-scale phase 3 RCT performed by Apfel et al. in 2000 evaluated the efficacy of rhNGF in 1019 DPN patients. Observation indicators were the NIS-LL score, Quantitative Sensory Test score, NSC score, Patient Benefit Questionnaire (PBQ) score, Overall Symptom Score, NCV and occurrence of new foot ulcers. After 12 months of treatment, except for on the Overall Symptom Score and individual PBQ results, rhNGF was slightly effective and other observational indicators were not found to have significant differences. Therefore, the role of rhNGF in DPN treatment requires further study. In view of the above studies, this article only makes a weak recommendation for mecobalamin for the treatment of DPN and currently does not recommend rhNGF.
Ultimately, the use of traditional Chinese medicine for DPN lacks sufficient clinical and basic research to prove its effectiveness and safety, and the clinical results of most studies are not objective, so it is not recommended in this article.
The above medication evidence and recommendations are not specifically targeted at patients with diabetic foot disease because long-term treatment is often required to improve diabetic neuropathy and delays in the initiation of treatment and shorter courses of treatment will worsen the effect. Therefore, these drugs are recommended for the prevention of diabetic foot deformities and ulcers. There is not enough evidence on whether the use of such drugs in the acute and recovery phases of diabetic foot disease can improve the prognosis. Due to the low level of evidence, this article only makes weak recommendations. The treatment of pathophysiological changes, microcirculatory lesions and neurotrophic and abnormal repair in DPN can improve peripheral nerve function to a certain extent.
Recommendation 23: When DPN patients have pain, some anticonvulsants and antidepressants should be used as first-line medications; opioids cannot be recommended as first-line or second-line treatments (strong; moderate).
The pain caused by DPN is neuropathic pain. After excluding the pain caused by vascular stenosis and occlusion, the following analgesics can be selected. (1) Anticonvulsants. Pregabalin and gabapentin are effective for neuropathic pain, but their effects may be dose-dependent. For example, negative results are usually encountered when pregabalin is used at a dosage of 150 mg/day but these show improvement when a higher dosage of 300–600 mg/day is used. In addition, Freeman et al. (2008) found that 300–600 mg/day pregabalin not only effectively relieved pain but also improved sleep disorders caused by pain. Somnolence (26%), dizziness (24%), peripheral edema (13%) and weight gain (11%) were common adverse reactions to pregabalin. Backonja et al. in 1998 found that 900–3600 mg/day of gabapentin alone achieved good results for the treatment of diabetic neuralgia. Compared with placebo, gabapentin can significantly improve pain, quality of life and mood. The main adverse reactions included dizziness (24%), sleepiness (23%) and mental confusion (8%). (2) Antidepressants, including duloxetine, amitriptyline, imipramine and citalopram. A meta-analysis involving 23 clinical studies confirmed that 60 mg/day duloxetine for 6 months in patients with diabetic neuropathic pain was significantly better than placebo. In another randomized double-blind head-to-head study of amitriptyline, duloxetine and pregabalin, patients were initially given lose-dose medication (amitriptyline 25 mg twice daily, duloxetine 60 mg every morning, pregabalin 150 mg twice daily) for 2 weeks, followed by higher-dose medications (amitriptyline 25 mg every morning and 50 mg every night; duloxetine 60 mg twice daily; pregabalin 300 mg twice daily) for the next 2 weeks. The results showed that, compared with the control treatments, neither treatment regimen (i.e. the lower and higher dosages) significantly improved neuropathic pain [100, 101]. (3) Opioids, including tramadol and oxycodone. A meta-analysis of 9 clinical trials by Eisenberg et al. in 2006 found that opioids can effectively reduce the degree of neuropathic pain and improve stimulation pain, such as mechanical hyperalgesia and temperature hyperalgesia. Among them, controlled-release oxycodone tablets have a significant effect on diabetic neuralgia. The main adverse reactions are nausea, constipation and drowsiness, with an overall incidence >30%. (4) Capsaicin is a topical preparation mainly used for localized pain, has fewer systemic adverse reactions and is suitable for patients who cannot swallow oral medicine. Capsaicin tablets with a latex coating are available over the counter in Europe and has been approved for the treatment of painful DPN, but only a few studies have found pain relief effects. A meta-analysis of 6 clinical studies has also shown that low-concentration latex-coated capsaicin tables are not very effective in reducing neuropathic pain [102]. Capsaicin patches with higher concentrations of the drug have not been approved for diabetic patients in Europe and the United States, and the main adverse reactions are skin lesions and dermatitis.
At present, domestic and foreign guidelines (American Association of Clinical Endocrinologists (AACE) guidelines, American Academy of Neurology (AAN) guidelines, European Federation of Neurological Societies (EFNS) guidelines, National institute for Health and Care Excellence (NICE) guidelines, the Toronto consensus and China's Guidelines for the Prevention and Treatment of Type 2 Diabetes) mostly recommend pregabalin (300–600 mg daily), gabapentin (900–1800 mg daily), fluoxetine (60–120 mg daily) and amitriptyline (50–200 mg daily) as first-line drugs. When conventional doses of these first-line drugs are not effective, one can switch to another first-line drug, increase the dose of the original drug or use a combination of other first-line drugs with different mechanisms of action. Therefore, this article recommends that when DPN patients experience pain, they are prescribed first-line drugs that have both anticonvulsant and antidepressant effects. However, opioids, which are mostly used after other drugs have failed, are not recommended as first-line or second-line drugs. Meanwhile, this article proposes referencing the ‘Guidelines for the Prevention and Treatment of Diabetic Foot in China (2019 Edition)’ for the treatment of pain in DPN.
Considerations and methods for the treatment of lower extremity vascular disease
Recommendation 24: When DFUs do not show signs of healing after 6 weeks of appropriate treatment, regardless of the results of noninvasive examination, direct angiography and, if necessary, vascular reconstruction may be considered (strong; low).
Recommendation 25: For diabetic foot patients who have undergone previous revascularization, or where perioperative examination is still suggesting moderate-to-severe lower limb ischemia, re-evaluation of the lower limb vascular status is required to determine if further investigation is necessary (strong; low).
The 2019 IWGDF guidelines indicate that angiography and vascular reconstruction should be considered when the following conditions occur in people with diabetic foot disease: (1) toe pressure <30 mmHg or transdermal oxygen pressure <25 mmHg; (2) foot ulcers showing no improvement after 4–6 weeks of active treatment, regardless of the bedside test results, as when microvascular lesions are present, they cannot be viewed as the sole cause of nonhealing of foot ulcers, and other possible factors should be considered; or (3) ankle pressure <50 mmHg or ABI <0.5, in which emergency angiography should also be considered [6]. It is worth noting that ABI has little value in predicting ulcer healing, but an increased risk of amputation is predicted when ABI <0.5 and/or ankle pressure <50 mmHg. Furthermore, the healing rate of ischemic DFUs was highest at 8 weeks after revascularization. Limbs with severe ischemia that were unable to undergo vascular reconstruction in time had an increased amputation rate, especially when the delay was >2 weeks [103]. Therefore, in combination with actual clinical work, this article recommends that people with DFUs complicated by lower extremity arterial ischemia, in which ulcers do not improve after 4–6 weeks of appropriate treatment, be considered for angiography or vascular reconstruction.
Recommendation 26: Surgeons need to integrate cardiovascular risk assessment into surgical planning and consider whether the benefit of surgery is greater than the risk. Preoperative and intraoperative precautions should be discussed with patients and their families to allow patients to make informed decisions ahead of their proposed surgery (strong; low).
People with ischemic diabetic foot complicated by infection need to be urgently evaluated and treated. Treatment is not only difficult but also risky (perioperative mortality is 5%) and the risk of amputation or even mortality is high. Studies have shown that after vascular reconstruction the 1-year limb salvage rate is approximately 70% and the 1-year mortality rate is approximately 40% [104]. Several observational studies have shown that in people with severe ischemic DFUs who have not undergone vascular reconstruction the ulcer healing rate (with or without small amputations) is only approximately 50%. Therefore, when the risk of vascular reconstruction is high, the risk–benefit ratio is unclear and full consideration should be given to whether the patient will benefit from the procedure. The plan should be discussed before surgery in detail and the surgical plan and precautions should be communicated with the patient and his or her family members. Preoperative risk assessment, including cardiovascular-related examinations, electrocardiogram, cardiac ultrasound and, if necessary, CTA or coronary angiography, should be performed. The clinical indications for surgery must be from the patient’s perspective, and revascularization should be avoided in populations with an unfavorable risk–benefit ratio of surgical success [42, 105]. Therefore, this article suggests the clinical need to fully evaluate the patient's cardiovascular risk factors: surgeons should consider whether the surgical benefit is greater than the surgical risk and discuss the preoperative plan in detail and communicate the surgical plan and precautions with the patient and family. When the risk clearly outweighs the benefits for surgical intervention, but the possibility of wound healing is low or amputation is unavoidable, revascularization should not be considered.
Recommendation 27: The goal of revascularization in patients with DFUs and PAD is to restore direct blood flow to at least one foot artery, preferably an artery within the ulcer (strong; low).
Recommendation 28: In patients with diabetic foot disease complicated by PAD, bypass grafting and endovascular treatment can be used for revascularization and there is no sufficient evidence to prove which method is better (strong; moderate).
According to the angiosome theory, the foot tissue can be divided into three large pieces, each with its own blood supply artery. Direct blood flow reconstruction leads to blood supply in the foot ulcer area. Flow recovery and indirect revascularization restores the blood supply in the areas of foot ulcers via adjacent collateral vessels [106]. A 2016 study by Brownrigg et al. showed that achieving a skin perfusion pressure ≥40 mmHg, a toe pressure ≥30 mmHg or a TcPO2 ≥25 mmHg would result in the healing rate of DFUs increasing by at least 25%. Lower limb vascular reconstruction can result in a patient limb salvage rate of 80–85% and an ulcer healing rate >60% within 12 months. Acin F et al. [107] conducted a study of 101 patients who received infrapopliteal endovascular reconstruction in 2014. Ischemic ulcer healing was achieved at 12 months, limb salvage was achieved at 24 months and no difference was found between single or multiple revascularizations. This indicates that for patients who had ischemic ulcers or critical limb ischemia (CLI), good results can be achieved if at least a single vessel outflows into the foot. In another study by Söderström et al. in 2013, it was reported that the healing rate of foot ulcers after direct and indirect revascularization was significantly increased (69% and 47%, respectively), but there was no difference in limb repair; and similar results were found in other studies [108].
Revascularization methods for ischemic limbs include bypass surgery (surgical bypass, the most common of which is femoral–inferior popliteal artery inverted saphenous vein bypass, see Figure 9) and percutaneous endovascular treatment (endovascular therapy). The Trans-Atlantic Inter-Society Consensus (TASC II) notes that CLI populations showing ischemic resting pain, ulcers or gangrene should be revascularized, but there is still no evidence on which is the best method. There is currently only one clinical RCT (bypass vs. angioplasty in severe ischemia of the leg, BASIL) comparing the efficacy of bypass grafting and percutaneous transluminal angioplasty on CLI and it found no differences between the two treatments in terms of amputation-free survival, treatment costs and quality of life [109]. The current trend has shifted towards minimally invasive endovascular treatment, but retrospective literature shows that there is an increased probability of reintervention in the later stage of endovascular treatment, especially for people with long-segment arterial occlusive disease. In addition, factors such as the experience of the attending doctor and the instruments and equipment in different hospitals also directly affect the treatment effect of the final revascularization. The clinical needs should be based on the characteristics of the population's disease, especially the comorbidities and body veins available for transplantation. They must also be determined according to the doctor's skills, local medical equipment, medical level and surgical methods.
Figure 9.
Femoral–inferior popliteal artery inverted saphenous vein bypass
In summary, this article suggests that DFUs with PAD can be bypassed and endovascularly treated for revascularization. The goal should be to restore direct blood flow to at least one foot artery, preferably within the anatomy of the ulcer.
Recommendation 29: Lower extremity arterial reconstructive surgery is a highly technical procedure that requires medical professionals who have performed a certain number of vascular reconstruction cases per year at either a specialist hospital or diabetic foot center (recommended, >20 cases/year) (strong; low).
The latest IWGDF guidelines note that there is an increased risk of perioperative death in patients undergoing vascular revascularization (perioperative mortality 5%, 1-year mortality 40% and 1-year limb salvage rate approximately 70%) [6]. A large study evaluated 226,501 patients with lower extremity vascular revascularization (10,4491 under endovascular angioplasty and 122,010 under open vascular bypass surgery). The results showed that the patient safety indicators (PSIs) of the two groups were significantly different (7.74% (open) vs. 8.51% (intraluminal); p < 0.0001). The incidence of postoperative hemorrhage or hematoma in patients undergoing endoplasty was 4.74%, increasing the probability of patient death almost 3-fold. PSI predictors included advancing age, female sex, dark complexion, congestive heart failure (OR, 1.83; 95% CI, 1.72–1.96), diabetes (OR, 1.20; 95% CI, 1.12–1.28), renal failure (OR, 2.31; 95% CI, 2.14–2.50), hospital teaching status and larger hospitals [110]. Lower extremity vascular reconstruction surgery is safe and effective and can significantly improve clinical symptoms and effectively avoid or reduce the level of amputation, but, currently, only a few hospitals in China are permitted to perform the procedure. Any diabetic foot treatment center should have their own PAD expertise and rapid access to the facilities and technologies (including endovascular and bypass surgery) needed for diagnosis and treatment to ensure that patients can undergo revascularization surgery and comprehensive treatment from a multidisciplinary team after surgery. Therefore, this article vigorously proposes the promotion of lower limb vascular reconstruction technology in hospitals that have the proper medical talent, equipment and technology and strongly recommends that professionals perform the procedure in a diabetic foot center with a certain number of lower limb vascular reconstruction cases per year (at least 20 cases/year according to the recommended model given by the European Society of Vascular Surgery 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms [111]). A large sample RCT was performed to confirm this recommendation. In short, the diagnosis and treatment of diabetic foot disease complicated by PAD should be planned according to the patient’s medical condition.
Selection of antibiotics and management of sepsis in DFI
Recommendation 30: The antibiotic regimen (dose form, dosage, method of administration and course of treatment) for DFIs should be based on a comprehensive judgment of the severity of the clinical infection, bacterial culture and drug sensitivity results and liver and kidney function of the body (strong; high).
Based on bacterial culture and drug sensitivity, narrow-spectrum, large-dose, short-term treatments are ideal antibacterial methods for DFIs; early experience is often used empirically [112]. There are many factors that can lead to failure of antibiotic treatment, including inaccurate tissue sampling, delayed or inaccurate culture results, drug allergies and the emergence of multidrug-resistant organisms, which can complicate and change antibiotic selection [113]. Even if the antibiotic is selected properly, there may be problems such as the emergence of drug-resistant microorganisms and double infection. Any antibiotic, including β-lactams, lincosamides, quinolone and carbapenem antibiotics (especially third-generation cephalosporins, clindamycin and piperacillin/tazobactam), will cause Clostridium difficile infection (CDI), especially in those over 65 years old, immunosuppressed patients on hospital admission, patients taking proton pump inhibitors and patients with previous CDI infections [113]. All choice of β-lactams, quinolones, and macrolides has been associated with the production of methicillin-resistant staphylococcus aureus (MRSA), which has not been shown to be more virulent than non-MRSA but has been shown to be less effective in antimicrobial therapy Carbapenem treatment leads to overgrowth of Candida species and promotes the formation of resistant coliforms [114].
The use of antibiotics, including the choice of drug form, dosage, administration method and course of treatment, needs to be considered with a variety of factors, including the degree of infection, the history of antibiotic use in the previous 3 months and whether to incorporate DFO and infections with pathogenic microorganisms (anaerobic bacteria, multidrug resistance bacteria, such as MRSA, local antibiotic resistance, etc.) [6], the patient's own condition (antibiotic allergies, impaired immune status, patient's willingness and compliance with treatment, renal or liver dysfunction, gastrointestinal malabsorption, PAD affecting the limb, risk of Multiple Drug Resistant Organism (MDROs) or unusual pathogens), etc. According to the 2012 IDSA guidelines for the treatment of DFO, patients should receive antibiotic treatment for 2–5 days preoperatively after surgical debridement and without signs of residual tissue infection; patients with residual soft tissue infection should receive antibiotic treatment for 2–4 weeks; and patients with residual (but survivable) bone infections should be treated with antibiotics for 4–6 weeks [115]. Multiple studies have confirmed the rationality of the antibiotic treatment recommendations in these guidelines, including a retrospective (nonrandomized) report by Sadat et al. in 2008. This study investigated the difference between 5-day antibiotics and 24-hour antibiotics in 40 diabetic foot patients who had undergone amputation and found that the 5-day antibiotic group had a shorter hospital stay (22 vs. 34 days; p = 0.001). A 1998 study found that for 302 patients postvascular reconstruction, the wound infection rate was lower in the multiple antibiotic use group than in the single antibiotic use group (10% vs. 18%; p = 0.04).
This article recommends that the use of antibiotics (formulations, dosages, administration methods and treatment courses) for DFIs be determined based on the comprehensive judgment of the severity of the clinical infections, bacterial culture and drug sensitivity results and the patient’s liver and kidney function. (1) All clinically infected diabetic foot wounds should undergo antibacterial treatment, but uninfected wounds should not. (2) Before bacterial culture and drug susceptibility examinations are performed, an empirical antibiotic regimen should be given based on the clinical manifestations of the infection, hematological indicators, liver and kidney function and other comprehensive evaluations. For example, anaerobic bacteria are often found in ischemic foot infections. Fourth-generation moxifloxacin or a combination of third-generation cephalosporin/metronidazole should be administered. At the same time, oral or intravenous antifungal therapy should be provided immediately. The antibiotic regimen should be adjusted as appropriate according to bacterial culture and drug sensitivity results. (3) Infections with staphylococcus aureus and Streptococcus dysgalactiae are commonly observed in patients with mild infection and good nutritional status who have not received antibiotics before admission; penicillin should be the first-choice antibiotic. The course of treatment should be 1–2 weeks. (4) In moderate-to-severe infections, gram-negative bacteria such as Proteus, Escherichia coli and Pseudomonas aeruginosa are common. In such cases, aminoglycosides, third-generation cephalosporins and carbapenem are preferred. Often, there is a need for additional antibiotics to provide gram-positive bacterial coverage as well. Once culture and drug sensitivity results are available, the antibiotic regimen should be adjusted. Parenteral treatment is required initially, and then oral preparations should be selected after the infection has been stabilized. (5) Consistent with international guidelines, this article does not particularly recommend topical wound dressing materials for anti-infection purposes; there are many such materials on the market, including iodine preparations (with a broad antibacterial spectrum), silver sulfadiazine and various silver-containing dressings (for Staphylococcus, but even MRSA and Pseudomonas are effectively treated), mupirocin (good for gram-positive bacteria, including MRSA) and polymyxin B (for Pseudomonas aeruginosa, large intestine Bacillus species, etc.) However, without exception, they lack large RCTs to support their use, and further research on inhibiting wound growth is needed.
Recommendation 31: For patients with severe infection and sepsis, both the local and systemic treatment of foot ulcers should be actively managed, including intensive care and an urgent referral to a hospital with a diabetic foot specialist (strong; moderate).
When diabetic foot is complicated by severe infection, sepsis often occurs. Sepsis is a SIRS caused by an infection that can develop into severe sepsis and septic shock. Early diagnosis of sepsis is an important prerequisite to reduce the mortality from multiple organ dysfunction caused by the condition. A meta-analysis of 30 clinical trials showed that the sensitivity of procalcitonin in diagnosing sepsis was 77% (95% CI, 0.72–0.81), the specificity was 79% (95% CI, 0.74–0.84) and the Area Under The Curve (AUC) was 0.85 (95% CI, 0.81–0.88), suggesting that procalcitonin is an effective indicator of the early diagnosis of sepsis in severe patients [116]. If severe sepsis or septic shock is diagnosed, antibacterial drugs and fluid resuscitation should be administered intravenously as soon as possible. A 2013 study by Moore et al. showed that early identification and treatment of severe sepsis or septic shock can reduce sepsis-related mortality and improve prognosis. Based on the above evidence, this article suggests that people with diabetic foot and sepsis should receive active local and systemic treatment for foot ulcers, including intensive care and urgent referral to a hospital with a diabetic foot specialist.
Debridement and bone reconstruction for DFUs
Severe DFIs require thorough debridement in addition to antibiotics and supportive care. However, inexperienced surgeons tend to underestimate the extent of infection and perform inadequate debridement, leading to problems such as the need for revisional surgery, high hospitalization rates and extended periods of hospitalization.
Debridement of DFUs
Recommendation 32: The choice of debridement method for DFUs should be individualized (strong; moderate).
Wound debridement involves irrigation and drainage of pus and removal of all necrotic and infected tissues. The different debridement methods can be classified as: mechanical, chemical, sharp, autolytic, selective and nonselective. Studies have shown that the number of debridement procedures is correlated with patient admission rates, repeated debridement rates, length of stay and cost of hospitalization [117]. Among them, sharp debridement and gentle debridement are used according to the intensity of debridement required. (1) Sharp debridement is used to clean keratinized edges and ulcer bases with a surgical blade until bleeding stops, which aids in removing necrotic tissue and debris from the wound bed. (2) Gentle debridement is a selective mechanical and/or instrument debridement that results in little tissue damage [118] and consists of the following techniques. (a) The wet-to-dry method: the wound bed is first covered with wet gauze; when the gauze becomes dry, the inactivated tissue adheres to the gauze and is removed along with the adherent material. This is a standard mechanical debridement technique that cannot distinguish good tissue from necrotic tissue and may cause pain. (b) Autolytic debridement and (c) enzymatic debridement. Necrotic or inactivated tissues and fibrinogen are liquefied, softened and removed using wound lysozymes or exogenous enzymes with proteolytic coverings without damaging adjacent normal tissues to achieve debridement. The commonly used dressings for autolytic debridement are hydrogel, hydrocolloid or alginate dressings; common auxiliary materials for enzymatic debridement are subtilase and collagenase. (d) Water knife debridement: removal of necrotic tissue, tissue fragments, foreign matter and impurities to clean the wound by means of water flow and instrument scraping. (e) Ultrasonic debridement: the cavitation and hemostatic effects of ultrasonic waves are used to remove bacteria and fungi on the surface and deep layers of the wound, which can wipe out foreign matter contaminating the wound, effectively removing bacteria and promoting wound healing. (f) Biological debridement: the sterile pupae of Cercidium lucidum are placed directly on the infected wounds, and Ascaris species are used to digest necrotic tissues and pathogens for debridement. This technique is more suitable for open wounds near large blood vessels or suspected cancerous wounds. A systematic study retrospectively analysed 13 intervention studies (10 RCTs and 3 nonrandomized studies). In the case of moderate risk of bias, 3 RCTs considered that autolytic debridement had a higher healing rate than standard debridement (RR = 1.89; 95% CI, 1.35–2.64), 4 other controlled studies found that, compared to standard debridement, maggot debridement reduced the amputation rate but did not improve the healing rate, and one RCT found that surgical debridement has a shorter healing time than conventional treatment. Both ultrasonic and water knife debridement have advantages over standard treatment [119]. Therefore, this article recommends that the clinical situation should be based on the cardiopulmonary function and other basic conditions of diabetic foot patients, the degree of foot tissue damage, the depth and size of the wound, the presence of limb ischemia, physician expertise and material availability, patient tolerance and selection and cost-effectiveness to personally choose the debridement method, frequency and range.
Recommendation 33: Urgent surgical debridement should be performed for some moderately and all severely infectious DFUs, especially when there is the presence of abscesses, wet (gas) gangrene or necrotizing fasciitis (strong; moderate).
From the surgeon's perspective, it is important to distinguish between infections that threaten the limb and those that do not, and early aggressive surgical intervention can reduce above-the-ankle amputations. A retrospective study by Tan et al. in 1996 divided 112 patients with DFIs into two groups: one group received antibiotics alone for the first 3 days, and another received antibiotics plus surgical intervention for the first 3 days. The ankle amputation rate of the combined treatment group was significantly higher than that of the antibiotics alone group (27.6% vs. 13%; p <0.01), with length of hospital stay of at least 6 days, indicating that early debridement treatment helps to save limbs, shorten the length of hospital stay and save medical expenses [20]. Therefore, this article recommends that some moderate and all severely infectious diabetic foot wounds, especially foot infections with abscesses, wet (gas) gangrene, or necrotizing fasciitis, should be debrided as soon as possible to open the wound for drainage purposes while avoiding squeezing to prevent the spread of infection.
At this time, special attention should be paid to the following: (1) no antibiotic can replace the need for debridement and drainage, and the timing of such procedures cannot be delayed; and (2) it is necessary to carefully judge whether dried wounds are stable, especially whether there is necrotic tissue and the presence of abscesses. Comprehensive judgment can be made based on local fluctuance, redness and swelling of the surrounding tissues, examination of abnormal inflammatory indexes, ultrasound exploration or X-ray examination results. Once instability and dryness are suspected, incision and drainage should be immediately performed to reduce the pressure in the wound.
Recommendation 34: In cases of infection complicated by limb ischemia, incision, drainage and clearing of necrotic tissue (not expansion) should be performed to control acute infections, and thorough debridement should be performed after the blood supply to the lower limbs is restored (strong; moderate).
In cases of DFI, timely and adequate debridement, particularly the excision of infected areas, can reduce the possibility of major amputation. However, in cases of lower limb ischemia, the timing, method and scope of debridement are questionable. The 2012 IDSA guidelines state that patients with DFUs infected with ischemia should be promptly referred to a vascular surgeon for vascular assessment and treatment. Many studies, including a 1996 RCT by Chang et al., suggest that in cases of lower limb ischemia, antibiotics may have poor curative effects, and the following situations may also arise: (1) inappropriate sharp debridement without assessing the limb ischemia status may cause microvascular thrombosis and deepen and worsen the ulcer; (2) if the ischemia is resolved but debridement is delayed, foot infections may worsen and sepsis may even occur [118]. At this time, the best surgical treatment would be combined (multiprofessional), multiple or staged surgery. Therefore, this article recommends that when DFUs are accompanied by severe ischemia of the limbs, the physician should incision and drainage first and cleanup of necrotic tissue (not expansion) to control acute infection, followed by thorough debridement after the blood supply to the lower limbs is restored.
Recommendation 35: If there is bone destruction and conservative treatment is ineffective, the scope of surgical bone resection should be determined based on preoperative imaging examination and histopathology or bacteriological results, and the bone reconstructive method should be chosen based on existing changes in foot biomechanics. Bone defects caused by infection should, if necessary, be temporarily filled with materials containing sensitive antibiotics (bone cement, bioceramics, etc.) at the bone resection site, followed by bone reconstruction surgery (strong; moderate).
Surgical debridement can clear the infective DFO lesions, promote wound healing, reduce the incidence of amputations above the ankle joint and provide stability to nonfunctioning feet through corresponding orthopedic methods. Regarding the surgical treatment of DFO, there are also different views. A prospective RCT of DFO for nonischemic or necrotizing soft tissue infections found that there was no difference in the efficacy between 90 and 10 days of antibiotics use during the surgical and perioperative period [120].
Bone Reconstruction
Forefoot bone lesion processing
Recommendation 35.1: When there is evidence of destruction of the phalanges, the affected bone segments should be surgically removed. Additionally, soft tissue release and interphalangeal joint fusion should be considered to stabilize the toe (strong; low).
Diabetic motor neuropathy leads to atrophy of the intrinsic muscles of the foot, followed by deformities such as mallet toe, hammer toes and claw toes (Figure 10). Toe deformities are divided into two categories: soft and stiff. The former are caused by soft tissue contracture and can be corrected by flexor tenectomy, and the latter are caused by extensive soft tissue contracture caused by bone and joint disease and require removal of proximal toe bones for soft tissue release. A 1987 study by Cavanagh et al. found that if long flexor tenectomy, joint capsule release or flexor tendon transposition could not correct the deformity, it is necessary to perform partial toe or even proximal toe resection to reset the toe and reduce foot pressure. For deep toe ulcers or DFO, the toe or part of the toe must be removed through the articular surface. For toe ulcers that do not respond well to conservative treatment or present with DFO, partial or total removal of the phalanges can be performed, but not all toe amputations must be at the level of the metatarsophalangeal joint. In some cases, partial toe amputation can be performed to remove the infected bone segment while enough of the toes are retained as a buffer between adjacent toes. When closing the wound, the stump should retain a large enough skin flap to cover the stump of the bone. Therefore, an incision is sometimes made on the edge of the necrosis or ulcer, and there must be a balance between the preservation of bone, sufficient skin and soft tissue. The general principle is to ensure that the wound is closed without tension, and some bone can be sacrificed if necessary (Figure 11). In short, this article suggests that when the toe bone is damaged, the affected toe tissue should be surgically removed; soft tissue release and interphalangeal joint fusion should be considered.
Figure 10.
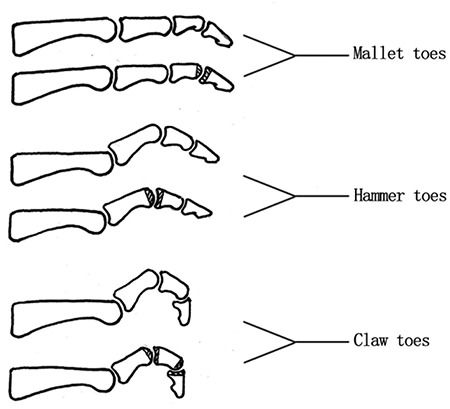
Mallet toe, hammer toe and claw toe (up) and operation method (down) which is to remove the shadow
Figure 11.
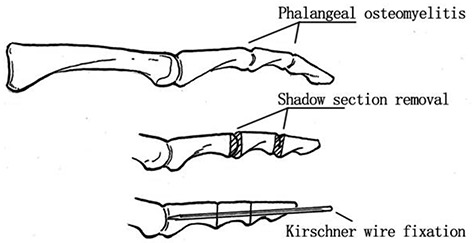
Osteotomy for phalangeal osteomyelitis after removal of shaded section, using Kirschner wire fixation
Recommendation 35.2: For first metatarsophalangeal joint destruction, surgical removal of the proximal base of the phalanges, cartilage of the first metatarsal head, the involved flexor tendon and sesamoid bone is recommended; first metatarsophalangeal arthroplasty may also be considered (strong; moderate).
DPN causes degeneration of fibrous connective tissues such as tendons and ligaments with changes in forefoot structure, abnormal plantar pressure and osteoarthritis of the first interphalangeal joint and the first metatarsophalangeal joint, all of which manifest as limited joint movement, excessive plantar flexion, internal rotation, weight-bearing posterior condyle formation, hallux valgus and pressure ulcers (the most common being plantar first metatarsophalangeal ulcers, accounting for approximately 22% of all ulcers), and DFO can result in serious consequences, such as amputation. A prospective cohort of diabetic forefoot ulcers conducted from October 2005 to October 2010 included 330 patients with DFO (and 1808 patients without DFO in the control group), followed up for 1 year, with an average age of 56.3 years. The ratio of males to females was 3:1; in the DFO group, 82.1% of ulcers penetrated to the bone or joints, 15.8% required major amputations (versus only 3.4% in the control group) and 10.6% required big toe amputations. Ulcer recurrence rates were similar in both groups (12.1%) [121].
First metatarsophalangeal joint (MTPJ) or first interphalangeal joint resection can restore joint activity. MTPJ resection originated with William Keller’s treatment of hallux valgus in 1912. It is widely used in the treatment of ulcers at the MTPJ with obvious effects, such as a 91% ulcer heal rate, and there is no recurrence of toe ulcers during the follow-up of 2 years. Other studies have shown that the average toe dorsiflexion before surgery is 46°, and the ulcers that had not healed for an average of 5 months after surgery did heal after an average of 3.1 weeks. One-year follow-up revealed that 22% of the patients had a recurrence of hallux toe ulcer within 3–12 months and the remaining 78% did not relapse after 2 years of follow-up [120]. In a few cases, ulcers caused by the dorsomedial enlargement of the hallux or the interphalangeal bone (usually over the plantar aspect of the interphalangeal joint) required that the cartilage of the proximal base of the toe bone and the first metatarsal head be removed, along with the tendons and sesamoid bone. In such cases, MTPJ angioplasty is performed to stabilize the joints and prevent recurrence of ulcers. However, it is worth noting that MTPJ angioplasty has the potential to cause joint instability and acute onset of Charcot (Figure 12). Therefore, this article recommends that when the first metatarsophalangeal joint is damaged, the proximal base of the phalanx, the first metatarsal head cartilage and the affected flexor tendon and sesamoid bone should be surgically removed. Alternatively, first metatarsophalangeal arthroplasty may be considered.
Figure 12.
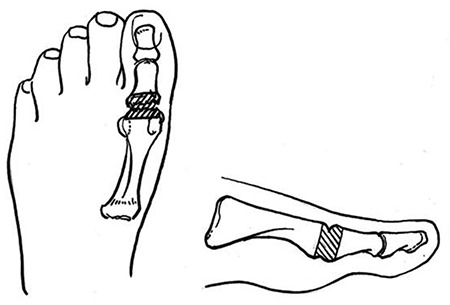
First metatarsophalangeal joint resection
Recommendation 35.3: For infections of the second to fifth metatarsal bones, partial or complete metatarsal head resection is recommended; partial tarsal head resection or V-shaped or Weil osteotomy may be considered when no ulcers have formed on the soles of the feet or if the ulcers have not invaded the bone or joint space (strong; moderate).
Diabetes can cause abnormal mechanical structuring of the foot, dislocation of the metatarsophalangeal joint and abnormal joint activity. The area under the metatarsal head is a common location for DFUs. Single metatarsal head resection is suitable for infectious ulcers below the metatarsal head or those involving the metatarsophalangeal joints. Wieman et al. reported on 101 cases of single or multiple metatarsal head resections in 1998 with an average follow-up of 35 months. Their results showed that the effective rate of surgery was 94%, 52% of patients had new ulcers in other parts and 8% of patients had recurrence of ulcers in situ. It is worth noting that simple metatarsal head resection may cause ulcers to form in adjacent areas. Therefore, only when DFO occurs in the entire metatarsal head and there is no other method of treatment should this surgical method be considered. Partial metatarsal head resection is suitable for DFUs where a single metatarsal head protrudes to the sole of the foot, leading to increased local pressure without ulceration or without infection. DuVrues first reported metatarsal condyle resection in 1953. Coughlin and Mann modified this technique by removing the protruding part of the metatarsal until it was parallel to the longitudinal axis of the metatarsal while simultaneously removing the deep ulcer and performing soft tissue debridement. Weil osteotomy of the metatarsal head is suitable for DFUs with relatively normal arches and flat feet and can significantly reduce the pressure on plantar ulcers and create conditions for ulcer healing. Vandeputte et al., in 2000, found that the pressure of the plantar foot of the patient was reduced by an average of 52% after Weil osteotomy. During an average follow-up of 30 months, in 5% of the patients their foot ulcers did not heal and 74% of the cured patients did not relapse. Only 11% of healed patients developed metastatic foot ulcers. Notably, because this operation cannot adequately offload the forefoot structurally, it is not suitable for patients with forefoot ulcers caused by high-arched feet (Figure 13). (4) V-shaped osteotomy of the metatarsal head can correct curvatures of the metatarsals and significantly reduce the pressure of forefoot ulcers. It is suitable for patients with diabetes and high-arched feet who have not developed plantar ulcers or noninfectious DFUs. It was discovered in 1989 that after V-shaped osteotomy of the sacrum head, 9% of patients still had lesions in other parts of the foot and 4% of patients had not improved, so the indications for this operation still need to be carefully selected.
Figure 13.
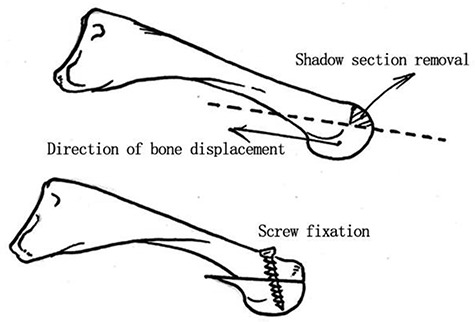
Weil osteotomy of the metatarsal head
Recommendation 35.4: For extensive soft tissue and/or bone destruction of the forefoot, or if new ulcers appear after partial metatarsal head resection, if local treatment fails, multiple metatarsal head resection (forefoot angioplasty) or trans-sacral amputation should be considered (weak; high).
Forefoot surgery is based on the principle of partial phalange/metatarsal amputation and maximum retention of stump length to minimize loss of the weight-bearing area. The more distal the amputation level is, the higher the requirements for soft tissue management, the more meticulous the postoperative care and the more likely the stump is to crack. Removal of the metatarsal and midfoot bones without resection of the toes is referred to as ‘hidden’ amputation. Although there is no difference in biomechanics, patients tend to be more agreeable to the procedure and there is no neuroma or pain in the affected limb [123].
Multiple metatarsal head resection (MMHR), also known as forefoot angioplasty, is suitable for extensive soft tissue destruction and/or bone destruction of the forefoot for new ulcerations after partial metatarsal head resection or when local, repeated treatment has failed. In 1993, Professor Giurini of Harvard University and others reported a group of 34 patients who underwent MMHR. The average observation period was 20.9 months. The results showed that 61% of patients were completely cured after one operation (the total cure rate was 97%). The common postoperative complications were pain and osteogenesis at the osteotomy site, so maintaining a normal parabolic curve of the metatarsal stump after osteotomy is key for this operation (the osteotomy surface must be smooth to prevent the formation of a new plantar high-pressure zone) (Figure 14).
Figure 14.
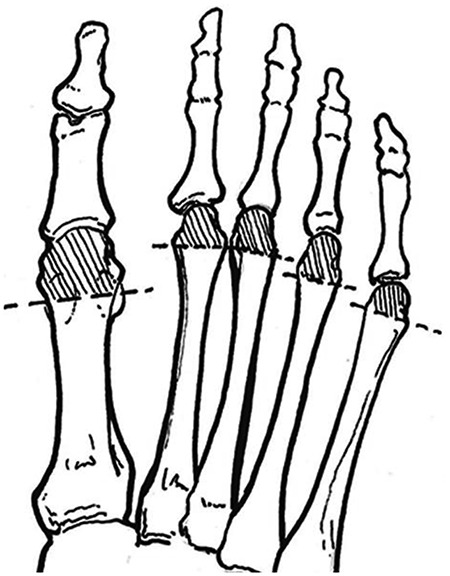
Multiple metatarsal head resection. The shaded part is cut off and the dotted line is the cut line
Transmetatarsal amputation (TMA), including transmetatarsal basal amputation, is generally considered to be a more accurate osteotomy than other small metatarsal amputation methods and is suitable for DFU patients with extensive skin and soft tissue destruction of the forefoot, involving multiple bone tissues, DFO and/or osteonecrosis or persistent forefoot ulcer recurrence. However, research also found that patients that undergo this procedure can still end up with a high rate of more proximal amputation. A search of the Medline, CINAHL and Cochrane Central databases yielded 159 abstracts. A total of 24 studies were included in the analysis. A total of 1453 TMA cases were included, and 391 (26.9%) patients required reoperation. It was reported that after 365 TMA procedures, 152 (29.7%) patients needed more proximal amputation. Another study observed 1146 patients after TMA and the number of large amputations above the ankle was 380 (up to 33.2%). A random-effects model estimated that the reoperation rate was 24.43% (95% CI, 11.64%–37.21%), the reamputation rate was 28.37% (95% CI, 19.56%–37.19%) and the large amputation rate was 30.16% (95% CI, 23.86%–36.47%); therefore, the author doubted the traditional practice of using TMA to replace other small amputations (such as partial first enucleation) and suggested that the choice of surgical method should be determined according to individual patient factors [124].
In short, although the level of evidence is high, the long-term effects of surgery are quite different and therefore uncertain. This article makes weak recommendations for the use of two surgical methods, MMHR and TMA, for diabetic forefoot ulcers and emphasizes that: (1) the surgery must take into account the balance of ankle muscle strength, and, if possible, soft tissue reconstruction surgery, such as gastrocnemius fascia elongation and Achilles tendon elongation, should be considered; and (2) patients with foot ulcer healing should wear custom shoes for decompression.
Management of osteopathy of the midfoot
Recommendation 35.5: In cases where the bone and joint of the forefoot are severely damaged and TMA cannot be performed or would be ineffective after implementation, tarsometatarsal joint (Lisfranc) amputation and transverse tarsal joint (Chopart) amputation should be considered (weak; moderate).
Transtarsal amputation (TA) should be considered when the bone and joint of the forefoot are severely damaged and TMA cannot be implemented or would be ineffective after implementation. TA includes tarsometatarsal joint (Lisfranc) amputation and transverse tarsal joint (Chopart) amputation. Lisfranc amputation is suitable for patients who have failed transmetatarsal amputation or whose extensive soft tissue loss on the forefoot is not sufficient for TA. Chopart amputation is mainly used for patients with severe midfoot infections and can ensure that the heel pad survives. Stone [125] reported in 2005 that 74 diabetic patients underwent 77 TMA procedures due to tissue loss and/or infection with an average follow-up of 20 months. The results showed that 32 patients with TMA who did not heal were treated with Chopart amputation (n = 22) or Lisfranc midfoot amputation (n = 10): 23 patients finally achieved functional walking goals, 14 (44%) patients healed after other wound repair treatments and only 6 patients needed above-the-knee amputation. TA surgery can save more than half of patients whose foot ulcers still cannot heal after TMA. The advantages of these two procedures are that patients can walk with shoes while wearing ankle and foot orthosis devices. The disadvantage is that even if a tendon balance operation is performed at the same time, the incidence of postoperative equinus foot deformities is still high. Therefore, in cases of ensuring sufficient foot blood perfusion, this article recommends the active use of TA surgery to save the foot so that the patient can maintain standing or even walking after the operation. Soft tissue reconstruction techniques such as Achilles tendon and gastrocnemius muscle extension, reconstruction of the tibialis anterior muscle and short peroneus tendon at the same time as TA joint dissection should be considered to prevent residual foot deformity.
Recommendation 35.6: When multiple midfoot joints are unstable, individual osteotomies (with or without fascial flaps), one-stage arthrodesis or multiplanar rearrangement of osteotomies should be selected individually to correct foot deformities, reduce pressure in any high-pressure area of the foot and maintain foot stability (weak; moderate).
Instability of the midfoot metatarsophalangeal joint and intertarsal joint and deformation of the plantar sole (mostly caused by subluxation and dislocation of the first metatarsal–medial cuneiform joint, which causes the plantar bone to protrude and become squeezed) are more common in diabetes with Charcot joint disease. A 1986 study by Leventen et al. showed that during the coalescence and remodeling period of Charcot, surgery to correct deformities and dislocations and reconstruct foot stability can effectively prevent or treat plantar ulcers. There are several surgical methods available to treat this condition. (1) Simple osteotomy an incision is made directly from the medial plantar ulcer or through the apex of the bone deformity (proximal metatarsal, medial cuneiform bone, etc.), resulting in a simple osteotomy (with or without fascial flap) for incision while preventing new bone processes from forming. Gatanzariti et al., in 2000, reported a group of 27 patients with osteotomies, 9 of which had lateral foot ulcers and 18 of which had midfoot ulcers, with a total cure rate of 74%. Eighty-five percent of the uncured patients had lateral ulcers and 55% had to undergo another orthopedic operation, wherein the adjacent soft tissue was used to cover and fill the lesions. (2) Medial column fusion surgery (Figure 15) is performed when the joint is unstable or when Simple osteotomy fails. Stabilization of the first metatarsal–medial cuneiform bone joint is a better choice. Specifically, a medial incision is sufficient to expose the dorsal and metatarsal joints, and the bilateral articular cartilage can be removed so that the first metatarsal bone plane is slightly from the proximal dorsal to the distal side. This allows reconstruction of the medial longitudinal arch of the foot to restore weight-bearing function. (3) Multiplanar rearrangement of bone resection. The most common deformity of Charcot’s foot neuroarthropathy is simultaneous deformation of the coronal and sagittal positions, in which case the deformed edge of the bone needs to be removed. A biplanar closed or open wedge osteotomy is performed and stabilized using suitable internal and external fixation. A prospective study by Early et al. in 1996 analysed 18 patients (21 feet) who underwent surgical reconstruction due to diabetes combined with Charcot's midfoot collapse from 1985 to 1993. Reconstruction procedures included reduction and fusion of the collapsed joints; the internal fixation restored the shape of the foot and maintained the weight-bearing position in the center. The results showed that, after an average of 28 months of follow-up, 18 feet were saved and the average healing time was 5 months. Of the 15 patients who underwent successful reconstruction, 13 patients had their shoes fitted. Healing degree and walking status improved: 47% of cases had no complications after surgery, 70% of foot ulcers healed without incident, and there was no recurrence of midfoot ulcers (Figure 15).
Figure 15.
Medial column fusion surgery
In short, the level of evidence for surgical reconstruction when multiple joints of the midfoot are unstable is low, but due to the difficulty of the operation and the high demands on the surgeon, this article makes a weak recommendation for the use of such surgery for diabetic feet. If necessary, it is recommended to refer patients to an experienced orthopedic center.
Management of osteopathy of the hindfoot
Recommendation 35.7: Midfoot and/or rearfoot joint fusion should be considered when a Lisfranc injury occurs/the tarsal joint is severely damaged and conservative treatment is ineffective (weak; moderate).
In the diabetic foot population, most cases are related to Charcot joint disease; a few are caused by trauma and are related to the poor tissue healing ability of the diabetic population. The most common lesions from Charcot's arthropathy are in the metatarsus and tarsus of the posterior foot (formed by metatarsal base, cuneiform, cuboid and the ligaments that connect the bones). The pathogenesis starts after ligament rupture with or without fracture and, due to an insensate foot secondary to peripheral neuropathy, the patient continues to walk on unstable feet, causing further damage, displacement and instability of the Lisfranc fracture. Initial treatment of the Lisfranc injury/tarsal joint, including weight bearing, fixation and bracing and surgical intervention should be considered in cases where conservative treatment has failed. When planning for surgical intervention, the surgeon should take note of the following. (1) Clinical indications for surgery include poor-healing ulcers with potential hindfoot deformity or instability, severe hindfoot instability and chronic heel ulcers with potential DFO. (2) The surgical method should mainly include midfoot and/or midfoot–rearfoot joint fusion (including talar joint fusion and triple articular fusion) and partial or full calcaneal resection if necessary. Triple articular joint fusion fixes the talar joint, heel joint and subtalar joint (Figure 16). The purpose of this procedure is to enhance the stability and reduce the deformity of the foot. It is suitable for patients with Charcot’s foot and severe instability of the tarsal joint (talar, calcaneal or subtalar joint). Surgery should be avoided during the acute phase of Charcot’s foot and delayed until the chronic remodeling phase; if an open ulcer is present, the operation should be postponed until active infections are addressed and, if necessary, the procedure should be combined with Achilles tendon extension to reduce late forefoot pressure. Partial or full calcaneal resection is performed to remove an infected calcaneus and necrotic tissue to promote wound healing. It is suitable for heel ulcers with chronic infection and DFO, adequate local blood flow, wide and deep lesions and patients who require removal of the diseased calcaneus. Cook et al. reported 51 patients (39 partial resections and 12 total resections) in 2007, with an average follow-up of 33 months. The results showed that 69% of the patients were cured; of these, 65% of patients were able to walk on their own and 35% of patients needed to undergo skin flap transfer or skin graft treatment.
Figure 16.
Triple articular joint fusion
In short, the level of evidence for midfoot and/or midfoot–rearfoot joint fusion is moderate. Due to the difficulty of the operation and the high demands on the surgeon, this article only makes a weak recommendation for the use of this type of surgery in diabetic feet and it is recommended to refer patients to an experienced orthopedic center if necessary. Additionally, note that due to the high risk of these types of surgery, all conservative treatment measures should be exhausted before surgery or until amputation is the only consideration.
Recommendation 35.8: When the ankle and subtalar joints are severely damaged and conservative treatment is not effective, ankle and subtalar fusion and talar joint fusion should be considered (weak; low).
Charcot neuroarthropathy around the talus usually causes ankle frailty. This deformity may be due to the total collapse of the talar body, a fracture of the medial/lateral ankle or both. People with these types of fractures are often found walking directly on the medial or lateral malleolus. This inherent instability will lead to the development of chronic ulcers. To save the limbs, fusion of the ankle and subtalar joint is necessary. Talar periarticular fusion is defined as a form of three-joint fusion. The optimal time for the operation is controversial. Thirty-three cases of different Eichenholtz periods were studied prospectively. The types of surgeries performed included ankle fusion, autologous iliac bone graft and subtalar joint fusion, with or without subtalar joint fusion, internal fixation with intramedullary nails, additional steel plates or cancellous bone screws. The results showed that after an average follow-up of 40 months (12–76 months), there were no significant differences in hindfoot scores or salvage, amputation or complication rates among patients who underwent surgery at different Eichenholtz periods [126]. In short, the level of evidence on talar joint fusion is weak and, due to the high demands for the surgeon and difficulty of the operation, this article only makes a weak recommendation for the use of this type of surgery in diabetic foot, and it is recommended that the patient be referred, if necessary, to an experienced center.
Recommendation 35.9: Heel ulcers that require thorough resection of soft tissue and bone from infected lesions resulting in bone defects can be temporarily filled with materials containing sensitive antibiotics (bone cement, bioceramics, etc.), while autologous iliac bone grafts and/or skin flaps may be considered at a later stage (strong; low).
Heel ulcers, a common manifestation in people with diabetes, are often associated with infection or Charcot’s joint disease. When calcaneal ulcers are complicated by osteomyelitis, pressure ankle ulcers or joint deformities and pain caused by Charcot to avoid below-knee amputations, partial calcaneal resection should be considered to remove all necrotic and infected tissue, deformed bone or osteophytes (note that no infected tissue or plantar bone protrusions should be left behind) [127]. Bone biological materials (BBMs) include bone conduction materials, such as bone grafts, calcium ceramics, hydroxyapatite and collagen and osteoinductive materials, such as platelet gel concentrates, demineralized bone matrix and bone morphogenetic proteins. The application of BBMs is explained in detail in the 2012 book ‘Surgery Reconstruction of Diabetic Foot and Ankle’ translated by Professor Xu Zhangrong. Autogenous bone transplantation has been the gold standard for bone grafting for many years, as it results in no immune rejection and is relatively easy to perform. The disadvantage is the impact on the donor site, such as donor site pain, fractures, infection and adjacent blood vessel, nerve and organ injury. The iliac crest, proximal and distal tibia, fibula or calcaneus are all considered potential donor sites, but the decision on the choice of donor site is made according to the surgical plan, the blood supply of the donor area, the bone graft requirements and the structural characteristics of the bone graft area. The understanding of the characteristics of each implanted bone and bone biomaterial and the selection of bone biomaterials in combination with autogenous bone grafts according to the characteristics of the operation can help to reduce bone removal complications. In short, although the level of evidence for the treatment of calcaneal ulcers is weak, calcaneal ulcers are a high-risk factor for large amputations at the ankle and require effective and timely intervention.
This article still strongly recommends the application of bone biomaterials combined with autologous bone grafting in diabetic feet; it also recommends the wearing of special foot and ankle orthoses to reduce pressure on the heel bone postoperatively.
Recommendation 35.10: When complex bone deformities of the hindfoot cannot be maintained by nonsurgical treatment, external fixation joint fusion or even ankle amputation should be considered (strong; moderate).
External fixation is used to correct deformities and fuse joints. Deformities of the hindfoot with a large amount of bone loss makes it impossible to use an internal fixation device reliably. The presence of open ulcers and DFO also contraindicate the use of an internal fixator. Severely damaged ankle joints in Charcot's disease can cause complex deformities and require external fixation combined with retrograde intramedullary nail internal fixation. Patients who have external fixation joint fusion combined with severe soft tissue infections or severe bone defects are unsuitable for internal fixation. In such cases, only external fixation devices can be considered. Moreover, an external fixation device allows easy assessment of open wounds, flaps and incisions for inspection and wound management. The compression and pulling of the fixing device can also help promote ankle joint fusion. For the orthopedic treatment of the hindfoot, no studies have directly compared the effects of surgical reconstruction of the bone structure and early amputation. Studies have shown the effect of partial calcaneal resection combined with Ilizarov external fixation on diabetic calcaneal ulcers. Among 23 patients who had this procedure, 18 (78%) wounds healed, 3 (13%) partially healed and 2 (9%) patients underwent below-the-knee amputation. Thus, this surgical procedure may effectively reduce the possibility of amputation in patients with DFUs with calcaneal DFO [128].
Above-the-ankle amputation at an early stage has been proven to be a favorable treatment option for people with: (1) severe ankle or hind foot bone damage; (2) extensive DFO; (3) a combination of multiple basic diseases and walking functional difficulties; and (4) poor compliance. Syme amputation through the ankle joint preserves the entire limb and the patient can partially bear weight. However, due to the continuous advancement of calf prosthetic technology, Syme amputation has no advantages and numerous side effects. Patients with diabetic foot complications are more suitable for a modified Pirogoff amputation (under the control of an image intensifier, the calcaneus is fixed to the tibia or below the knee with two 6.5 mm hollow screws). A prospective study by Larsson et al. in 1995 showed that among the 187 patients who underwent amputation, all of whom had DPN, versus 171 with PAD, 74 patients had subankle amputation healing, 88 patients had upper ankle amputation healing and 25 patients died without healing. Further research found that upper ankle amputations were related to older age, living in nursing homes and other institutions, difficulty walking, cerebrovascular disease, congestive heart failure and low hemoglobin levels. At the same time, ankle amputation was shown to be related to the diagnosis of diabetes and the course of diabetes before the age of 30. Surgeons should consider many factors before deciding whether a patient needs upper ankle amputation, including life-threatening limb infections, extensive bone destruction in the feet and ischemia in the lower limbs, a blood supply that cannot be reconstructed, intolerance of pain, family financial conditions that would make it difficult to adhere to long-term nonsurgical treatment or when the indication for amputation is strong. It is emphasized that the call for amputation of the ankle should be based more on physiological/clinical indications rather than on patient age. The choice of amputation plane should be based on the tissue necrosis or vascular occlusion plane, as well as age, gender, occupation, lifestyle and other factors, combined with the results of TcPO2 at the incision and the results of vascular imaging examination. A 2009 study by Fife et al. showed that wound healing was different with different readings of tissue TcPO2: <20 mmHg indicated that the amputation stump could not heal and lower limb vascular reconstruction would be needed to improve blood flow, whereas >40 mmHg indicated that the amputation stump could heal; finally, a TcPO2 of 20–40 mmHg suggested healing may or may not take place and lower limb vascular reconstruction may be required. In short, research on surgical methods for treating complex bone deformities of the hindfoot is moderate in level. This article strongly recommends the application of external fixation joint fusion and even ankle amputation in the diabetic foot. Summarizing the above content on the treatment of osteopathy of the diabetic foot, this guideline recommends that clinicians fully evaluate the condition of the diabetic foot population, clearly diagnose the osteopathy of the patient and develop individualized treatment plans for each patient and at the appropriate time.
Principles and methods of wound healing
Wound bed preparations for diabetic foot wounds involve the evaluation of various etiologies and the formulation of a strategy to create a healthy wound bed without signs of infection and an adequate amount of wound exudation and blood supply, thereby stimulating the process of endogenous wound repair and regeneration.
Recommendation 36: The progression of DFUs should be evaluated and monitored every 1–4 weeks and the wound bed should be prepared according to the TIME principle (strong; low).
The ‘TIME principle’ for wound bed preparation was proposed by Falanga in 2000 and Schultz et al. in 2003 and comprises four major aspects: tissue management, inflammation and infection control, moisture balance and the edge of the wound. Subsequently, this wound bed preparation framework has been recognized by many wound treatment experts and discussed in numerous relevant academic conferences. A 2012 retrospective study by Xu Yuan et al. retrieved 498 related documents and confirmed that the TIME principle is a valuable tool for wound treatment. In 2013, Qiu Tieying and colleagues concluded that the TIME principle makes the wound treatment process scientific and standardized, following the current direction of wound treatment efforts and research. However, Ligresti highlighted that if the wound does not heal after >60 days, the ‘TIME-H principle’ should be used; that is, for special wounds, in addition to ‘TIME’, healing (H) has not been achieved. The factors that delay wound healing are identified, re-evaluated and appropriately managed. In addition, the healing trajectory of DFUs tends to change dynamically. A 2006 study by Steed et al. showed that, when following the TIME-H principle, the ulcer area decreased by 10–15% within 1 week or >50% within 4 weeks and the possibility of reinfection and amputation was significantly reduced. This shows that the percentage of wound area reduction per unit time has an early predictive value for treatment efficacy. Although no studies have evaluated the benefits and usefulness of the interval between wound examinations, the sizes of wounds can be measured every 1 or 4 weeks, the progress of healing can be recorded and clinicians can be guided to adjust the treatment plan according to the TIME principle. A healthy wound bed appears pink and the margins of its wound edges are relatively vague and irregular. On the other hand, unhealthy wound margins may show infection, edema or hypertrophy, dark red and frail granulation tissues appear over the wound surface and the marginal tissues have poor elasticity. It is necessary to combine sharp debridement with oral antibiotics while maintaining humidity balance to promote wound healing. Therefore, this article recommends that the size and progression of DFUs be evaluated and measured every 1–4 weeks and wound preparations be performed in accordance with the TIME principle.
Recommendation 37: The appropriate soft tissue reconstructive procedure and adequate preoperative preparations should be selected based on the patient’s age, cardiopulmonary function and other vital functions, as well as the degree of the baseline wound bed characteristics (size, depth, location and blood supply) (strong; low).
Soft tissue constructive procedures ranges from simple to complex: primary suture, secondary suture, negative-pressure wound treatment, skin graft, dermal matrix, local flap, distant flap, tissue expansion and, finally, local fascial flap, myofascial flap, island flap or free-tissue transplantation [129]. The selection of wound repair techniques should be carried out in a stepwise manner in accordance with the above sequence and strive to solve complex problems with simple methods while taking into account the following basic principles: (1) evaluate the bone structure (important for checking for the presence of fracture), stability and treatment of related bone injuries and weight-bearing ability; (2) comprehensively assess the suitability of reconstruction and rehabilitation, especially lower limb blood supply, degree of infection and possible recovery of protective sensation; (3) properly prepare the wound bed, including debridement and antimicrobial control; and (4) consider the anatomical soft tissue characteristics of the foot, for example, the ankle and the back of the foot are covered with thin and flexible soft tissue, and the sole of the foot is fixed to the plantar bones and ligaments with thick fibrous connective tissue for resisting high-strain forces [130]. This article recommends the following treatment options: (1) for superficial wounds that cover larger areas with healthy granulation tissue growth, skin grafting can be selected; (2) for small but deeper wounds with healthy granulation wound beds, exposed vessel bundles and bones, the choice should be transfer flaps or free flaps, according to the condition of the patient (Figure 17–19).
Figure 17.
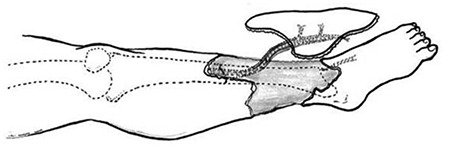
Free anterior tibial flap
Figure 19.
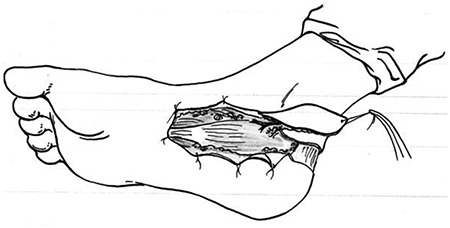
Medial island flap of the foot
Figure 18.
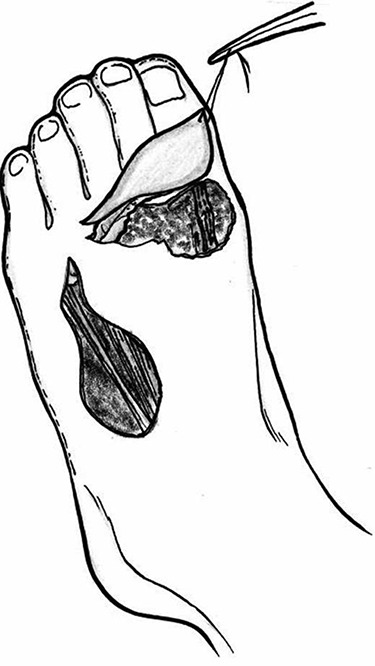
Dorsal island flap of foot
Any soft tissue reconstruction surgery requires a detailed preoperative plan described as follows. (1) The general condition of the patient, blood supply to the flaps and grafts, surgical techniques and minimization of the creation of new wounds should be considered. Other considerations include patient compliance, vascular status and bone quality. (2) The design of the flap, based on the concept of blood supply from deep to shallow, which has been an important aspect since its description by Milton in 1961. Milton pointed out that the blood supply at the bottom of the flap determines the success of the flap, but not its size. However, a 1986 study by Hidalgo et al. showed that a local plantar flap can be designed to include sensory and blood supply without the need for subfascial detachment.
The incision line of the flap should be parallel to the relaxed skin tension lines (RSTLs), which can minimize the lateral force on the skin (Figure 20); however, if bone surgery is performed at the same time, the RSTLs can be ignored. The extracted flap should be movable in one direction without lateral rotation. The factors that lead to the failure of flap transplantation are mainly bacterial infections, especially S.aureus, Pseudomonas and β-hemolytic streptococcus. There are also many complications of flap transplantation, including mechanical shear force, insufficient blood supply, seroma and hematoma formation and technical/surgical errors.
Figure 20.
Relax tension skin lines
Therefore, this article recommends that the selection of soft tissue reconstruction procedure be decided according to the age of the patient, cardiopulmonary function and other basic conditions, as well as the degree of trauma (size, depth, location and blood supply), and that it highlights the importance of performing preoperative risk assessment.
Recommendation 38: Dressing products should be used reasonably, the wound bed should be kept moist, exudation should be controlled to avoid complete skin infiltration around the wound and an epithelial growth environment should be created (strong; low).
It was originally thought that dry wounds were beneficial for avoiding bacterial reproduction but, in 1987, Eaglistein et al. found that wounds healed 40% faster in wet environments than in air, and warmer and humid environments were better for the epithelium in terms of migration. Maintaining balanced wound humidity can promote wound healing. A 2005 study by Fletcher showed that if the wound surface was too moist, the wound margin and surrounding skin would be impregnated, proteolytic enzymes and matrix metalloenzymes in the exudate would increase, extracellular matrix proteins and growth factors would be destroyed and senescence or apoptosis of the new fibroblasts would not be conducive to wound healing; if the wound was too dry, pain or itching may occur and the epidermal cells of the wound margin would slow down, limiting epidermal regeneration.
Traditional clinical dressings include gauze, bandages, cotton pads, etc. Their production processes are simple, the prices are low and they are widely used in clinical applications. The disadvantages are easy adhesion to wounds (secondary damage caused by dressing change) and easy penetration. Advanced dressings mainly include wet dressings, active ingredients (platelet gel, cell growth factor and stem cells), skin replacement products and collagen dressings [131]. At present, the most commonly used moisturizing dressings in the clinic include hydrocolloids, hydrogels, alginates and foam dressings. The benefits of the moisturizing dressing included the following. (1) They provide a more humid environment for wounds. Necrotic tissue can be hydrated by the exudate to release the tissue cells’ own plasmin and other proteolytic enzymes, which hydrolyse necrotic tissue and facilitate absorption to achieve a debridement effect. (2) The formation of hypoxic tension in the local microenvironment of the wound can obviously promote the proliferation of wound fibroblasts, stimulate the release of growth factors by macrophages and accelerate the formation of new blood vessels, shortening the healing time of the wound. (3) Moisturizing dressings protect wounds, isolate microorganisms in the external environment and reduce the infection rate. The appropriate wet dressing should be chosen according to the amount of fluid exudate in the clinic; for example, hydrocolloids and hydrogels for dry wounds, translucent dressings and hydrophilic fiber dressings for wounds with a small amount of exuded fluid, calcium alginate dressings and foam dressings for medium-permeation wounds and alginate dressings, foam dressings, vacuum-assisted closure (VAC), etc. for wounds with a large amount of exudate. An RCT compared the effects of multiple dressings on the treatment of DFU in a population of 2159 people from 1993 to 2017. Amniotic membranes were shown to be superior to alginate dressings, contact wound dressings, foam dressings, hydrocolloids and iodine-containing gauze dressings. Hydrogel dressings were superior to contact wound dressings, and according to the probability of the ranking results, amniotic membranes and hydrogel dressings could most effectively promote DFU healing and should be the first choice for DFUs [132]. Although the level of evidence is low, this article still strongly recommends the reasonable use of dressing products to keep the wound bed moist, control exudation to avoid intact skin infiltration around the wound and create an environment that promotes epithelial growth.
Adjuvant wound treatment
A retrospective study by Sheehan et al. in 2003 showed that a period of 4 weeks was sufficient to assess the tendency of simple neuropathic foot ulcers to heal. After at least 4 weeks of standardized treatment, if the DFU still does not improve (area reduction >50%), the wound and previous treatment options should be re-evaluated and adjuvant treatment should be considered simultaneously [20].
Recommendation 39: Negative-pressure wound therapy (also known as VAC) should be used to assist in the healing of wounds in patients with adequate peripheral blood circulation and after any infected or nonviable tissue has been completely removed (strong; low).
As early as 1947, some physicians began to explore the use of a negative-pressure suction device (now known as negative-pressure wound therapy (NPWT)) to remove accumulated blood, bile and secretions during surgical procedures. A few years later, this technology was introduced in the clinical setting: perioperative complications were minimized and hospital stays were shortened. A study performed by Hinman et al. in 1963 showed that closed wounds were easier to close than exposed wounds. Different mechanisms of action of NPWT in diabetic foot wounds have been described. (1) Deformation of the sponge brings the wound edges closer to each other and induces hypoxia, which leads to upregulation of vascular endothelial growth factor (VEGF) and the promotion of angiogenesis. (2) Continuous or interstitial negative pressure allows exudate to be sucked away quickly, effectively improving wound drainage. (3) NPWT effectively keeps the wound clean and inhibits bacterial growth. (4) The growth of granulation is stimulated. (5) In some cases, the wound can be mechanically washed by an irrigation solution and, when combined with closed negative pressure, this can reduce the bioburden load (if antimicrobial irrigation solution is used), moisturize the wound, prevent clogging of the exudate and accelerate the dissolution of necrotic tissue. An analysis report by Forbes et al. in 2008 reviewed four low-to-medium-quality RCTs and showed that NPWT can promote granulation and improve the wound healing rate. Among the studies reviewed, some concluded that the wound healing rate was increased by 20% (OR, 2.0%; 95% CI, −1.0−4.0) and the amputation rate was reduced by approximately 7.9% in the NPWT group, better than that of the control group (weak evidence level); the patients were quite satisfied with the NPWT therapy and no adverse events were reported. A 2008 study by Gregor et al. analysed 7 randomized controlled trials (n = 324) and 10 nonrandomized controlled trials (n = 278), among which 2 RCTs and 4 nonrandomized controlled trials proved that NPWT can improve the wound healing rate and shorten the wound healing time. When applying NPWT, clinicians should: (1) avoid setting the pressure too high, as this could induce tissue ischemia; and (2) understand the importance of adequate wound debridement prior to installation of the device. Therefore, there is a strong need for larger-scale trials to assess the effects and side effects of NPWT treatment in different groups of diabetic populations when different clinical goals and parameters are adopted. This article strongly recommends the use of NPWT in diabetic feet.
Recommendation 40: When there is no improvement in DFUs (area reduction of <50%) within 4–6 weeks, other adjuvant treatments, such as hyperbaric oxygen, cytokines and bioengineered skin alternatives, may be considered (weak; moderate).
Hyperbaric oxygen therapy (HBOT) has been used in many studies on diabetic foot wounds. Most people believe that it can not only improve wound inflammation and microcirculation but also promote wound healing and reduce the risk of amputation [133]. The proposed mechanisms of effect following introduction of pressurized oxygen involve: (1) increasing tissue oxygenation, which is beneficial to wound healing of chronic ulcers that have shown resistance against conventional treatments; (2) increasing oxygen partial pressure, helping to meet the energy requirements required for the normal healing process and reducing the incidence of infection; and (3) increasing oxygen, which, in general, leads to the production of active substances with hormonal activity that act on the signal transduction pathways that regulate the synthesis of inflammatory mediators, antioxidants and growth factors and facilitate the healing process. In an RCT reviewed by Roeckl-Wiedmann et al. in 2005, the authors concluded that HBOT does not exert a significant benefit on wound healing and minor amputation. A systematic review and meta-analysis searched multiple databases, namely, Medline, EMBASE, CINAHL, PubMed, Wiley's Cochrane Library and Biosis, and obtained 12 studies related to HBOT with a total of 531 patients with DFU; 6 of the studies were RCTs. In the 205 patients who used HBOT, the healing rate of foot ulcers was significantly higher than that of the control group at 6 weeks, but the long-term (one-year follow-up) effect was not obvious, and there was no difference in the major amputation rate (RR = 2.3; 95% CI, 1.19–4.62; p = 0.01) [134]. In summary, to date, the level of evidence for all HBOT studies is only low-to-moderate and this article therefore makes only a weak recommendation for the use of HBOT in diabetic feet.
Exogenous cytokines currently used in clinical applications and formulated into wound biologics include fibroblast growth factor, epidermal growth factor, endothelial growth factor, platelet-derived growth factor (PDGF), transforming growth factor, etc. The PDGF becaplermin gel is the only FDA-approved drug to date for the treatment of DFUs. Many clinical studies have shown that it can improve the healing rate of DFUs. Steed performed a 20-week observational study in 1995 and showed that the healing rate of DFUs in the PDGF group was 48%, versus 25% in the control group. The results from a 1998 study by Wieman et al. also showed that, following 6 weeks of treatment, the healing rate of DFUs in the PDGF group was 50%, while that in the control group was 35%, and that PDGF could improve the healing rate of ulcers by approximately 43%. The healing time for DFUs in the PDGF group was approximately 86 days, and that in the placebo group was 127 days; that is, PDGF can shorten the healing time of ulcers by approximately 32%. Autologously derived platelet-rich plasma gel (PRPG) has been used in the field of chronic wound treatment in recent years. In 2001, Margolis and others began to use it in the research of diabetic foot wounds and achieved good results. At present, it is believed that the main role of PRP-G lies in the WBCs and growth factors contained therein. (1) Leukocytes have anti-infective and immunoregulatory effects; (2) leukocytes can also induce the release of various growth factors leading to the release of VEGF, and VEGF has an important role in promoting angiogenesis; (3) autologous platelet gel fibrin produces fibronectin, which has adhesion properties and can promote wound healing; and (4) platelets in the gel contain high-concentration growth factors and release antibacterial peptides upon activation. Li et al. conducted a meta-analysis of 15 RCTs (a total of 829 people) involving the use of PRPG for the treatment of diabetic chronic skin ulcers and compared the results with those from standard medical care. They found a significantly increased healing rate of ulcers (RR = 1.39; 95% CI, 1.29–1.50; p < 0.00001), a shortened healing time (Mean difference = −9.18; 95% CI, −11.3−7.05; p < 0.00001) and a reduced incidence of infection (RR = 0.34; 95% CI, 0.15-0.77; p = 0.009) [135]. In summary, to date, the level of evidence for all growth factor (GF) treatment studies is only low-to-moderate, and this article makes a weak recommendation for their use in diabetic foot.
Bioengineered skin substitutes can be divided into three categories: epidermis, dermis or compound transplants. Individual products vary depending on the source of the cellular material, the method of delivery and the presence of auxiliary matrices such as fibroblasts or matrix proteins. Currently, bioengineering has developed artificial skins that can be used for diabetic wound repair to integrate somatic cells into bioengineered scaffolds; however, over the past 10 years, some small clinical trials have suggested that the clinical benefit of this effect is only moderate [136]. In contrast, in the area of burns and trauma, the main research focuses on the application of pre-epidermal cells, mesenchymal matrix/stem cells, adipose tissue-derived stem cells and induced pluripotent stem cells (IPS). Although still in its early stages, its potential applications are promising.
Stem cell transplantation (SCT): stem cells have the ability to self-renew and differentiate into other cell types and can be divided into adult stem cells, progenitor cells, embryonic stem cells and IPS. Unlike somatic cells, they have very high proliferative potential and can be genetically manipulated. Although the mechanism of action has not been fully elucidated, they are thought to exert the following properties: (1) differentiation into specialized cells, such as those of the dermis and epidermis; (2) paracrine or autocrine effects; and (3) immune regulatory factors. The stem cells of autologous SCT are derived from autologous bone marrow, peripheral blood or modified bone marrow and, as a result, there are no immune rejection or ethical problems. They have been popularized to some extent in China and have achieved good results. The stem cells of allogeneic SCT are derived from cord blood and mesenchymal and embryonic stem cells; cord blood and mesenchymal stem cells have also been tested in clinical practice. Guo and his colleagues searched library databases including PubMed, EMBASE, Web of Science and Cochrane, from which six RCTs were screened for meta-analysis and the endpoint of measurement was DFU healing [137]. The results showed that SCT can significantly promote the healing of diabetic ulcers (MD, 0.52; 95% CI, 0.38–0.65; p < 0.00001), and the effect of ulcer size and population age was similar [137]. In 2011, the European Medicines Agency published a ‘Reflection Paper on Stem Cell Drug Products’ and proposed that stem cell products should be produced and clinically tested according to international standards, including good laboratory practice, good manufacturing practice and good clinical practice. In short, stem cell therapy has certain effects, but in view of the safety of the population, it cannot be used as a standard method for the treatment of lower extremity disease in China. This article does not recommend stem cell therapy as a routine clinical treatment.
Extracellular matrix products (ECMPs). Wound healing is a dynamic process. Skin cells, GFs and extracellular matrix (ECM) interact to restore damaged tissue structure. In some chronic wounds, especially DFUs, the inflammatory cells reduce the production of ECM and growth factors, and also reduce the number of cells needed for wound healing. At present, in addition to the abovementioned cell therapy, ECMPs have also been developed to treat chronic DFUs. These products can provide cells, GFs and other key elements as scaffolds to promote fibroblasts, keratin cells or both in the wound bed, and increased amounts of growth factors are conducive to epithelialization and revascularization of the wound [138]. In a randomized, double-blind, controlled trial comparing the best standard of care (SOC), SOC plus Dermagraft® (bioengineered ECM with living cell components) and SOC plus Oasis® (biologically engineered ECM without living cell components), the results show that the three methods had similar effects on wound healing [139]. In addition, allogeneic acellular dermis can be used for the treatment of DFUs. Because this type of product is biological material that removes cells and antigen components in skin tissues and retains the extracellular matrix through special treatment of allogeneic skin, it is free from toxicity and irritation and possesses nonimmunogenic characteristics. These factors make it increasingly popular for therapeutic use in patients with large tissue defects requiring significant repair. However, it is imperative to ensure adequate blood circulation and debridement of necrotic or other nonviable tissue. To date, the level of evidence for all ECMP studies has been low-to-moderate, and this article only makes weak recommendations for the use of ECMP in diabetic foot.
Collagen wound dressing has been in clinical use for >50 years, including soluble collagen injections, liquid reconstituted solid structures and acellular collagen matrices. According to the material it contains, collagen wound dressings can be divided into single-layer collagen scaffold sponges, composite collagen scaffold sponges and model collagen materials. In recent years, many studies have been conducted on the role of collagen material in the construction of tissue engineering scaffolds. They are considered to have natural hemostasis, good cell growth and chemotactic properties, biocompatibility, low immunogenicity and controlled biodegradation [140]. Owing to its equal efficacy to that of biological material, it is mainly used as a scaffold material for deep wounds and a cover material for shallow wounds. A single-arm, open-ended, multicenter clinical observation was initiated to study the possible role of a new flowable matrix gel formulated from fibrous recombinant human type I collagen (rhCollagen) purified from tobacco plants for wound treatment in 20 people with a mean age of approximately 63 years. The proportions of neurological ulcers, posttraumatic ulcers, postoperative ulcers and venous ulcers were 45%, 35%, 10% and 10%, respectively. After 4 weeks of rhCollagen treatment, the average wound area decreased by 95% and a wound closure rate of 70% was achieved. No significant side effects were found [141]. At present, the level of evidence for all research on collagen excipients is only low-to-moderate. This article only makes weak recommendations for the use of collagen products for the treatment of DFUs.
Offloading of DFUs
Recommendation 41: An irremovable total contact cast (TCC) should be the first-line treatment for plantar DFUs (strong; high).
TCCs are a nonremovable pressure-relief medical devices. They are well-molded and minimally padded casts made of a fiberglass composite that maintain close contact with the entire plantar aspect of the foot and the lower leg. They are designed to reduce plantar peak pressure over wounds or high-pressure areas, allow the lower limbs and feet to share the loading forces, limit ankle rotation and reduce shear forces in an attempt to promote ulcer healing. Since the introduction of the TCC in India in the 1930s, and its use in Hansen's disease, a number of improvements have been made, such as the addition of Sifoam at the plantar metatarsal area, additional padding beneath the foot ulcer (wound isolation TCC), 6 mm of slow-rebound cellular urethane and 6 mm of soft cellular urethane (cushion-modified TCC) and semicompressed felt padding over ulcerated lesions, bone processes (medial and lateral malleolus) and the anterior border of the lower limb from the tibial tuberosity to the dorsal midfoot. A TCC operates through three mechanisms of action. (1) The plaster material can increase the contact area between the sole and the ground. Studies have shown that, compared with short shoes, TCCs can increase the contact area between the plantar sole and the ground for the whole foot (5%), midfoot (8%) and forefoot (6%) (p < 0.05) [142], so that the pressure load on the sole of the foot can be redistributed. (2) One-third of the pressure is transferred from the plantar foot to the plaster material over the shin or hindfoot region [142]. (3) The TCC restricts ankle motion.
The application of TCCs in DFUs has been proven to effectively reduce plantar pressure and promote healing of DFUs. A TCC can reduce the peak pressure in the forefoot by approximately 70% to 87%; however, with the removal of the ‘shank’ portion of the cast material to the ankle, the peak plantar pressure increases significantly by approximately 53% (p < 0.05), 17.5 kPa (13%) in the forefoot and 8.9 kPa (8%) in the midfoot. At the same time, the maximum pressure in the midfoot also increases by 13.2 N (6%; p <0.05), which effectively illustrates the efficacy of a full-length TCC in reducing peak plantar pressure [142]. In a study by Mueller et al. in 1989, compared with standard treatment, treatment with a TCC yielded high healing of foot ulcers and a low infection rate. The ulcer healed after an average of 42 days (control group 65 days on average, χ2 = 12.4; p < 0.05) and the incidence of infection was low (χ2 = 4.1; p < 0.05). Armstrong et al. reported similar results, in which the healing rate of DFUs after 5 to 7 weeks was 72% to 100%. A meta-analysis that evaluated several randomized controlled trials revealed that nonremovable walkers (such as TCCs or instant total contact casts (ITCCs)) promoted better DFU healing than removable cast walker (RCW) (RR = 1.09; 95% CI, 1.09–2.58; p = 0.004) [143]; however, there was no difference in the healing rate of DFUs among nonremovable walkers, including TCCs. Therefore, several international guidelines recommend TCCs as the ‘gold standard’ for decompression in DFUs.
Despite evidence of it offering the best offloading, TCCs are not commonly seen in routine clinical use. A 2008 study by Wu et al. found that only 1.7% of the 895 centers surveyed used TCCs and more than half of them did not even consider TCCs as a first-line treatment for noninfected DFUs. Fife et al. reported that only 6% of the patients retrieved from the US wound registry database received TCC treatment across 18 outpatient centers. From the results of a multicenter Eurodiale study involving 1232 physicians at 14 centers across 10 countries, TCC was only offered to approximately 18% of patients (n = 25), while 77% of the physicians had failed to perform effective offloading treatment (41% were treated with offloading, but only half of them were correctly treated) on patients who had neuropathic plantar forefoot or midfoot ulcers. Most of them used temporary shoes and the utilization rate of TCC varies significantly between countries or even between different centers in the same country. In 2008, Prompers et al. found that in a multivariable model, male sex (OR = 0.356; 95% CI, 0.151–0.840; p = 0.007), ulcer size (p = 0.038) and walking status (p = 0.001) were independent predictors of the use of TCCs and other such treatments. There are many reasons for the low utilization of TCCs; they require trained doctors or technicians and the application of the device is time-consuming. Some patients may develop skin irritation, and patients with the following conditions are contraindicated for the use of a TCC: (1) active deep tissue infections, DFO or gangrene; (2) persistent swelling or excessive exudation; (3) severe or extensive PAD (ABI <0.4); (4) ataxia; (5) blindness or severe obesity; (6) fungal infection of the skin or toenails, toenail malformation or dermatitis; and (7) claustrophobia.
In summary, to date, all TCC studies have a high level of evidence, so this guideline strongly recommends the use of TCCs in plantar ulcers in people with diabetes.
Recommendation 42: When the use of TCCs or nonremovable walking boots is either contradicted or not tolerated by the patient, a full-length RCW should be considered for patients with plantar foot ulcers (strong; moderate).
A full-length RCW can redistribute the pressure distribution of the foot and limit the movement of the foot and ankle joints. It can also be easily removed for regular wound dressing changes while allowing patients to perform their daily activities. It is suitable for those who are contraindicated or intolerant of TCC use. Several types of RCW have been used to evaluate their effectiveness in treating neuropathic DFUs. These include the Aircast diabetic RCW (Aircast, USA), Aircast Pneumatic Walker (XP Diabetic Walker), Stabil-D cast walker (Podartis, Italy) and DH Offloading Walker™ (Össur®, USA). The effectiveness of RCWs in the reduction of plantar pressure for the treatment of neuropathic DFUs has been proven. For example, in 2011, Gutekunst et al. showed that people using removable plaster walking boots (a type of RCW) had significantly lower forefoot pressure, pressure–time integral, maximum force and force–time integral than barefoot individuals, achieving a similar effect to using a TCC (but with a poorer ulcer healing rate). The peak pressure reduction in the midfoot was more effective than that obtained using a TCC (77% vs. 63%; p = 0.036), which promoted the healing of plantar ulcers. In 1996, Lavery et al. showed that an RCW could achieve a foot ulcer healing rate similar to that of a TCC, and the effect of a DH Offloading Walker™ on ulcers on the the base of the big toe were even better than that of a TCC. A 2010 study by Faglia et al. also concluded that the use of certain RCWs (such as a Stabil-D cast walker) resulted in no better ulcer healing rate or healing time than a TCC. However, some studies have suggested that RCWs do not promote the healing of DFUs as well as TCCs. For example, a prospective study by Armstrong et al. in 2001 suggested that, compared with TCCs, RCWs resulted in a lower foot ulcer healing rate (89.5 vs. 61.4%; p = 0.026; OR = 5.4; 95% CI, 1.1–26.1) and a longer healing time (p = 0.033).
The modified RCW is a kind of wrapped RCW with an adhesive or plaster bandage, making it a nonremovable RCW that can exert effects similar to those of TCCs while avoiding their shortcomings; consequently, modified RCWs are also called ITCCs. Evaluating the efficacy of this novel device for the treatment of neuropathic foot ulcers, a 2005 study by Armstrong et al. found that the healing rate of foot ulcers in the ITCC group was 84%, which was significantly higher than that of the RCW group (58.3%; p = 0.04), and the healing time of foot ulcers in the ITCC group was faster than that in the RCW group (41.6 ± 18.7 days vs. 58.0 ± 15.2 days; p = 0.02). Following this, Katz et al. in 2005 found that there was no significant difference in healing speed, average healing time or complications between the ITCC group and the traditional TCC group. A relevant meta-analysis showed that there was no difference between TCCs and ITCCs in DFU healing (RR = 1.06; 95% CI, 0.88–1.27; p = 0.31) [143]. The above results show that the offloading effect provided by this improved RCW is equivalent to that of a conventional TCC, far better than that of a conventional RCW, yet cheaper and simpler to apply. It is worth noting that population compliance plays an important role in the difference in offloading effects provided by TCCs, RCWs and therapeutic shoes. A 2001 study by Armstrong et al. demonstrated that the average daily steps of patients who used nonremovable TCC were 22% less than that of patients who used RCW and 59% less than that of patients who used therapeutic shoes. Interestingly, further investigation actually found that, compared with TCC users, patients who used RCWs only did so only one-third of the time yet walked a significantly greater number of steps.
Therefore, this article recommends that when the use of a TCC or other nonremovable walker is contraindicated, or the patient cannot tolerate it, the use of full-length RCW should be considered in foot ulcer patients.
Recommendation 43: When the use of a full-length RCW is either unsuitable or not tolerated by the patient, healing sandals or half shoes (heel or forefoot offloading footwear) is recommended (strong; moderate).
There is little evidence to suggest the role of half shoes or healing sandals in providing adequate pressure offloading on the plantar ulcers. The therapeutic shoe is a personalized shoe with a unique rocker outsole. From the perspective of biomechanics, the bottom aspect of this rocker can reduce metatarsophalangeal joint dorsiflexion under dynamic conditions, thereby reducing the pressure load on the metatarsal head. Half shoes are commonly used perioperatively to reduce plantar pressure in the foot. It has two designs: one is missing part of the sole (Heel Wedge ® DARCO (Europe) GmbH, Germany) from the heel, and the other is missing the part from the forefoot near the metatarsal sole (Ortho Wedge ® DARCO (Europe) GmbH, Germany).
Therapeutic offloading shoes can reduce forefoot pressure in diabetic populations. A 1996 study based on laboratory gait analysis by Lavery et al. concluded that the therapeutic shoe's effect on reducing plantar pressure was 9 times that of a TCC or an RCW. In-shoe pressure analysis found that treatment shoes had a significant effect on preventing the recurrence of foot ulcers (p = 0.045) and were superior to ordinary footwear (RR = 0.34; 95% CI, 0.15–0.79; p = 0.012), but, due to the small sample size, heterogeneous research methods and results, the quality of evidence was considered low. However, offloading footwear are less effective than TCCs in treating ulcers. A study by Caravaggi et al. in 2000 compared a special shoe (therapeutic shoe group) with a rocker bottom and 8 mm thick plastic soft elastic insole and a glass fiber material with variable stiffness. The authors found that the area of DFUs in the TCC group decreased more rapidly (p = 0.0004) and the healing rate was 50% at 30 days (20.8% for the treatment shoes).
Half shoes have a higher foot ulcer healing rate than traditional wound treatment. A 1993 study by Chantelau et al. reported that the healing time of DFUs in the half-shoe wound treatment group was 70 days, which was significantly shorter than the healing time of DFUs in the wound treatment group alone (118 days). Similarly, the half-shoe group had a lower hospitalization rate of 4% versus the control group with 41%. Later, a comparative study conducted by Fleischli et al. in 1997 found that the half-shoe was less effective than a TCC or RCW at the first metatarsophalangeal joint of the forefoot. Some studies have also suggested that although traditional or standard therapeutic shoes can reduce plantar pressure by approximately 44–64%, they have no obvious advantage in healing foot ulcers. Noncontrol study results show that ankle-high cast shoes, half shoes and forefoot decompression shoes can heal DFUs in a short period of time, with a healing rate of 70–96% and an average healing time of 34–79 days [144].
In general, offloading shoes are time-consuming and technically difficult to manufacture, and their offloading effect is greatly reduced owing to their removable nature, which has obvious disadvantages compared with other more available offloading modalities. In 2000, a researcher developed a device that mixed the properties of an RCW and therapeutic shoes called ‘MABAL’ shoes. These are designed to provide a greater foot contact area than traditional therapeutic shoes. A 2000 study by Hissink et al. conducted a retrospective study to confirm the effectiveness of the shoe. After using MABAL shoes for 23 patients with DFUs, the healing rate of the foot ulcers was 91% (21 of 23 ulcers were effectively healed) and the average healing time was 34 days (7–75 days). The authors argued that their result was similar to that obtained with TCCs, but technical expertise was required for the manufacture and application of the new device.
Therefore, all studies on therapeutic shoes or half shoes (heel or forefoot offloading shoes) have moderate evidence. This guideline still highly recommends the use of therapeutic shoes or half shoes (heel or forefoot decompression shoes) for people who are not suitable for or cannot tolerate RCWs.
Protection of high-risk foot and prevention of ulcer recurrence
The IWGDF clearly states five key elements in the prevention of diabetic foot ulceration: (1) identifying the at-risk feet; (2) regularly inspecting and assessing the at-risk feet; (3) educating the patient, family and healthcare providers; (4) wearing appropriate supportive shoes daily; and (5) treating the risk factors for ulceration. This article also provides guiding recommendations for the above five aspects.
Risk assessment and stratification of diabetic foot disease
Recommendation 44: Diabetic foot risk stratification is based on its risk factors (strong; moderate).
The risk factors for foot disease in patients with diabetes include PAD and DPN, with the latter being the most common risk factor. A 2004 study by Boulton et al. reported that DPN can increase the risk of foot ulcers by 9–32 times. Intrinsically, the risk of ulceration is associated with bone prominence, limited joint mobility, joint deformity—which results in high-pressure areas leading to callosity—altered tissue performance, a previous history of foot surgery and neuro-osteoarthropathy. Meanwhile, the presence of extrinsic factors such as inappropriate footwear, walking barefoot, falls and accidents, foreign bodies in shoes and strenuous physical activity increase the risk of plantar foot ulceration [145]. PAD is usually asymptomatic, and it is common in patients older than 50 years (especially those older than 65 years) and those with diabetes mellitus, hypertension, hyperlipidemia, a familial history of PAD, a smoking history, a history of diabetic foot disease and abnormal vascular examination results. Patients with a history of vascular intervention or bypass surgery and a history of atherosclerosis may also exhibit decreased long-distance walking function (approximately one-third of asymptomatic people can only walk less than six blocks per week), which is correlated with impaired wound healing, increased risk of infection and nontraumatic lower extremity amputation. A Chinese epidemiological study showed that 78.8% of people with diabetes have DPN and 48% of people have PAD. In this paper, the authors also observed that neuroischemic ulcers were found in 53.1% of the patients who had foot ulcerations, 23% had ischemic ulcers and 21.2% had neuropathic ulcers [92].
There are different classification methods for stratifying diabetic foot risk according to their corresponding risk factors. In 2008, the ADA developed a comprehensive foot examination and risk assessment tool. In this assessment, the foot ulceration risk is divided into 4 categories (0 to 3), and a higher risk category reflects a greater risk of plantar foot ulceration. The Scottish Diabetes Foot Action Group in collaboration with the Scottish Care Information-Diabetes Group conceptualized the Diabetic Foot Risk Stratification and Trial system, also known as the ‘traffic-light’ system. In this system, the risk is categorized as low, moderate or high, which are color-coded with green, yellow and red, respectively. The system was confirmed in a prospective observational study by Boulton et al. in 2008, involving >3,00 people; the authors found that the risk of foot ulcers in the high-risk group increased 83 times, while the probability of remaining ulcer-free after 2.4 years of follow-up was 99.7% in the low-risk group. The ‘active’ risk category was later added above the high-risk category to reflect the urgent need for specialist referral, as it is considered a sign of current ulcers, active infection, spreading infection with lymphangitis, CLI, gangrene or other clinical signs (sudden onset of redness, warmth or swelling of the midfoot or ankle without history of trauma and in absence of pain). A 2016 study by Stang et al. revised the traffic-light foot risk stratification again to include kidney disease as one of the risk factors and included the ‘remission period’ group in the high-risk category. The UT classification system classifies foot risk factors for diabetic patients into 6 levels: 0–3 are foot ulcer risk factors and 3–6 are amputation risk factors. The IWGDF classifies diabetic foot risk factors into 3 levels: level 1 refers to patients with DPN but no PAD, no foot deformity or no restricted joint motion; level 2 refers to those with DPN and foot deformity or restricted joint motion and/or PAD; while level 3 refers to an active foot ulcer, a previous history of amputation or Charcot neuroarthropathy. In summary, this article suggests that the risk of diabetic foot ulceration should be stratified according to the risk factors.
Recommendation 45: All patients with diabetes should have a comprehensive assessment of the foot, at least once a year, regardless of their diabetic foot risk stratification (strong; low).
After performing diabetic foot risk stratification, all patients with diabetes should undergo a comprehensive foot examination and evaluation annually. This assessment should be performed by a podiatric physician, doctor or other healthcare professional trained in diabetic foot care. Although the quality of the evidence is low, IWGDF still strongly recommends annual diabetic foot examinations to identify the risk of ulceration, especially indications of the presence of neuropathy and PAD. Patients who are considered low-risk or IWGDF category 0—that is, those presenting without DPN, PAD, foot deformity or limited joint mobility—require annual diabetic foot screening. Patients who are at risk of ulceration should be accessed more frequently. For example, patients with DPN, PAD and/or resultant foot deformity, who are classified as IWGDF category 2, should undergo assessment 2–4 times a year; patients at high risk of ulceration or those who had previous ulceration or amputation are category 3 and require more frequent review, 4–12 times a year [146]. For screening methods and evidence, see the sections on peripheral neuropathy and PAD. The quality of evidence for the provision of diabetic foot assessment and the risk stratification according to the risk factors for patients with diabetes is high. The key to prevention lies in early detection and intervention. As such, we recommend annual comprehensive diabetic foot assessments be conducted on all patients known to have a history of diabetes by medical or other trained healthcare professionals. More frequent review is warranted for patients at high risk of foot ulceration.
Diabetic Foot Health Education: Content and Significance
Recommendations 46: Diabetes foot health education can effectively prevent the occurrence of diabetic foot complications (strong; moderate).
Diabetes education has been widely advocated and implemented in clinical practice. We recommend the inclusion of foot care sections into general diabetes education led by a team of doctors, nutritionists, rehabilitators and brace engineers, itself led by a diabetes specialist education nurse, to provide proper foot care education for patients and their families. The contents of diabetic foot care education should include: (1) general foot care, such as maintaining good foot hygiene, self-assessment of the foot, regular application of moisturizers over dry areas and appropriate toenail trimming techniques; (2) footwear recommendations, such as wearing soft shock-absorbing slippers at home, avoiding walking barefoot at all times and using good supportive footwear for outdoor activities; and (3) principles of ulcer management, detecting the signs and symptoms of infection and emphasizing rest and the importance of regular dressings. In clinical practice, education is often combined with other preventive interventions aimed at directly improving the health of patients at the population level; for example, those who are at low risk (without obvious signs of sensory neuropathy or peripheral vascular conditions) are advised to regularly perform nail trimming. In patients who have complications of autonomic neuropathy, the sweat glands are often denervated, resulting in dry feet and eventually leading to chap. It is recommended that people apply moisturizing cream on these dry areas to avoid this condition. Presently, most studies exploring the role of education in the prevention and treatment of diabetic foot lack both a control group and bias analysis. For example, a 2001 study by Calle-Pascua et al. noted that the incidence of ulcers was significantly lower in people who adhered to 90–120 minutes of foot care education than in those who did not (3.1% vs. 31.6%; p < 0.001). In a larger, noncontrolled study conducted by Viswanathan et al. in 2005, the authors showed that following the provision of comprehensive diabetic foot care education, the incidence of ulcers in people who adhered to foot care for at least 5 days per week decreased by 5% versus an up to 26% incidence of ulcers in people who did not adhere to foot care (p < 0.0001). Moreover, RCTs evaluated by Gershater et al. in 2011 and Lincoln et al. in 2008 found no significant difference in the prevention of ulceration between patients who had received a 12-month period of 60-minute diabetic foot care education and those who had received standard care. This finding is contrary to those reported by Malone et al., who demonstrated a significant effect of foot care education after a 1-year follow-up duration, with a relative risk of amputation of 0.33 (95% CI, 0.15–0.76) and a relative risk of ulcers of 0.31 (95% CI, 0.14–0.66). However, it should be noted that the risk of bias in this study was high and the educational role may have been overestimated. In summary, although there is no conclusive evidence that education can directly influence the incidence of DFUs and amputation rates, this article still strongly recommends that clinical attention be paid to the role and implementation of health education for diabetic foot.
The use of offloading devices in high-risk patients for the prevention of DFUs
Recommendation 47: Low-risk patients without structural deformity or peripheral neuropathy can wear ordinary shoes and there is no requirement for the use of therapeutic shoes (strong; low).
Recommendation 48: For patients who are high-risk with a history of foot ulcers, major or minor amputations and Charcot foot, the use of offloading therapeutic footwear to prevent foot ulcers and recurrence of ulcers is recommended (strong; moderate).
The IWGDF guidelines point out that the use of therapeutic footwear can reduce the relative risk of foot ulcers in high-risk populations such as patients with Charcot arthrosis by 70.2% and therefore recommend its use in patients with a history of foot ulcers, partial amputation and foot deformities. Customized therapeutic footwear should be used to prevent the occurrence of DFUs. In contrast, low-risk patients are not recommended to use such shoes; conventional shoes are sufficient, but guidance should be provided on their proper use. Foot pressure measurement plays an important role in identifying high-pressure points and improving decompression footwear. Many prospective studies have confirmed that barefoot walking in diabetic patients leads to a significant increase in plantar mechanical pressure, which is an independent risk factor for ulcers [18]. Decompression shoes, insoles or other customized adjustable orthotics can be effective in relieving anterior plantar pressure. For example, Ulbrecht et al. used peak plantar pressure in 2014 to design custom orthopedic appliances and proved that such appliances could significantly reduce the recurrence of plantar ulcers (p = 0.003).
There are varying results regarding the effectiveness of footwear in preventing the recurrence of ulcers. Several reports of RCTs have shown that the use of therapeutic shoes can reduce the relative risk of foot ulcers in diabetic populations. For example, Rizzo and colleagues found that the recurrence of foot ulcers in people wearing therapeutic shoes was significantly lower than in those wearing standard shoes during 1-, 3- and 5-year follow-up sessions [147]; however, Reiber et al. reported in 2002 that there was no significant difference in the recurrence of ulcers among the use of custom-made therapeutic shoes, prefabricated therapeutic shoes or ordinary shoes within 2 years. Many studies conclude that the use of customized insoles can effectively prevent the occurrence or recurrence of DFUs, such as the 2014 study by Ulbrecht and others (9.1% vs. 25.0%; p = 0.007). A 2012 study by Lavery et al. found that insole treatment and the history of foot complications were significant influencing factors in a Cox regression model. The risk of recurrence of ulcers using ordinary insoles was 3.5 times that using custom insoles (RR = 3.47; 95% CI, 0.96– 12.67). A suitable shoe should have a wide and deep toe and soft leather material to minimize stress and adapt to toe deformation. It should be noted that, in many cases, the population's compliance with footwear is very low, especially at home, and many people do not even realize that they should use therapeutic footwear at home. For others, there may be many difficulties when using or disassembling shoes—they may feel too hot or too heavy, or there may be cultural or religious objections. At the same time, because custom shoes are more expensive than ordinary shoes, it is also costly to go regularly to the doctor's office to replace wound dressings, so expense is also a major issue. Interestingly, Zimny et al., in 2003, found that felted foam dressings can be used in the treatment of DFUs and are especially suitable for forefoot plantar neuropathic ulcers. These dressings are an effective, economic and simple plantar ulcer treatment but have not been widely used.
In short, despite the low-to-medium evidence, this article recommends that low-risk patients wear ordinary shoes and that it is unnecessary to use therapeutic shoes, while high-risk or very high-risk patients are recommended to use decompression orthopedic therapeutic shoes to prevent the formation and recurrence of foot ulcers.
Management of preulcerative signs in the prevention of diabetic foot ulceration
Recommendation 49: Any signs of preulceration should be identified and treated in all patients with diabetes in the prevention of foot ulcers. These signs include plantar callosity, corns, ingrown nails, fungal infection, blisters and chap formation (strong; moderate).
Structural foot deformities, such as toe abduction and long second metatarsals, occur half of the time in patients with diabetes, often leading to elevated peak plantar pressure over the forefoot and the formation of callosities/corns. Subsequent walking on these thickened callosities can result in 18,600 kg of pressure per day (based on 10,000 steps per day). The formation of callosities and corns is a physiological protective response to elevated plantar peak pressure and frictional forces from tight-fitted footwear. However, the continual build-up of callosities or corns induces pain, extravasation of blood capillaries beneath the skin and subsequent plantar foot ulceration. Patients who are found to be at risk of foot ulcerations should be protected from abnormal biomechanical forces. Presently, there is a general consensus that the regular surgical removal of callosities or corns with a scalpel can effectively prevent foot ulcers but the frequency at which this should be performed is not clear. A 1999 study by Pitei et al. suggested that surgical removal can effectively reduce plantar pressure by approximately 30% and that the trimming should be decided according to the dynamic pressure changes in the sole of the shoe but not on the weight of the callus or calluses. In 1992, Young et al. demonstrated that surgical removal could achieve up to a 60% reduction in forefoot peak plantar pressure (p < 0.001) and reduce the stride duration by 150 ms (p < 0.05). A prospective study by Slater and coworkers in 2006 showed that the combination of surgical removal and offloading silicon orthotic braces enhanced pressure reduction by 59%, while the use of offloading braces alone or surgical removal achieved reductions of 30% and 29%, respectively. Patients with ingrown toenails, fungal infections and blisters should seek medical treatment promptly, including partial toenail removal, the use of topical antifungal drugs and blister drainage. We recommend against the use of ointments or potions, as the active ingredients of these products may contain acids that can corrode the surrounding skin. Finally, patients should be educated about the signs and symptoms of local infections and they should seek immediate medical attention if they are unwell. Despite the moderate level of evidence, this article still strongly recommends the elimination of preulcerative signs for all patients with diabetes.
The role of elective surgery in the prevention of diabetic foot complications
Recommendation 50: Surgical offloading is an effective method for the prevention and treatment of DFUs (weak; moderate).
The incidence of DFUs is high and the recurrence rate can reach 40% within 4 months, reaching as high as 60% within 3 years. Diabetic foot ulcerations, both new and recurrent, are mostly caused by elevated peak plantar pressure secondary to structural osseous deformity [3]. Surgical resection of bony prominences can effectively prevent the occurrence and recurrence of DFUs, as shown through many retrospective studies. In view of inconclusive evidence, however, the latest IWGDF guidelines do not take a clear position on the effectiveness and safety of surgical offloading modalities in the prevention of DFUs. Therefore, this article only makes weak recommendations. At present, there are several surgical methods commonly used for the prevention of diabetic foot, some of which have sufficient theoretical evidence, while some are insufficient.
Achilles tendon lengthening (ATL) is an operation performed by making multiple small incisions to elongate the Achilles tendon. A 2003 study by Mueller et al. evaluated the efficacy of ATL by comparing it with TCC in an RCT and found no difference in the ulcer healing rate but a significantly lower ulcer recurrence rate in favor of ATL at the 7-month and 2-year follow-up periods (7-month follow-up: 15% in the ATL group vs. 59% in the TCC group; p = 0.001; 2-year follow-up: 38% in the ATL group vs. 81% in the TCC group; p = 0.002). Ankle dorsiflexion was significantly increased for ATL compared with TCC and remained increased even at 7-month follow-up (p < 0.01) Furthermore, the authors suggested that Achilles tendon lengthening can reduce the risk of recurrence of foot ulcers by 75% in 7 months and 52% in 2 years, especially in patients who present with neuropathic forefoot ulcers complicated by a limited ankle dorsiflexion of <5° (Figure 21).
Figure 21.
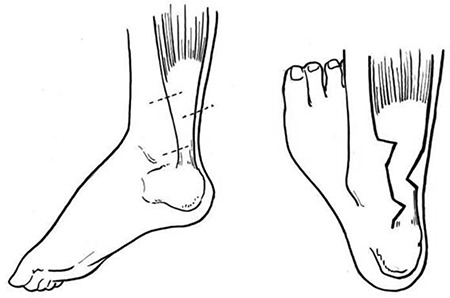
Achilles tendon lengthening (ATL); the dotted line on the left represents the incision line and the figure on the right represents the operation after ATL
Dorsiflexion metatarsal osteotomy (DMO). In 2000, Cavanagh et al. examined the clinical usefulness of DMO in 20 patients who had nonhealing foot ulcers with a mean duration of 13 months. The operation involved irrigation, debridement and basilar closing wedge metatarsal osteotomy via an incision made over the dorsal foot. The authors observed a healing rate of 95% over a mean duration of 40 days. Perioperative complications were reported in 15 patients, such as acute Charcot foot (32%), deep wound infection (14%) and transfer of lesions to the adjacent plantar metatarsal head (9%) (Figure 22).
Figure 22.
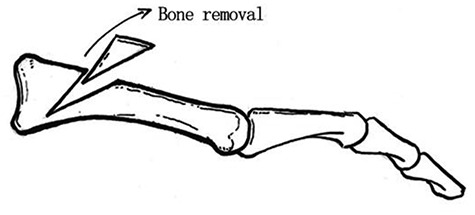
Dorsiflexion metatarsal osteotomy
Percutaneous flexor tenotomy (PFT) has been suggested to be a safe and effective surgical method in the management and prevention of diabetic toe ulcers. One retrospective study retrieved 42 articles, 5 of which met the inclusion criteria. A total of 163 people underwent 250 flexor tendon incisions. The ulcer cure rate was high (92–100% within 2 months), the recurrence rate was low (0–18% within a month) and the incidence of infections or new deformities was low; the authors concluded that there was level 4 evidence that flexor tenotomy can help ulcer healing and prevent recurrence [148]. However, the methodological design of several studies involving the use of PFT for diabetic toe ulcers was mostly flawed due to the lack of a control group, the use of a nonrandom design and inconsistent reporting during the follow-up period after intervention (Figure 23).
Figure 23.
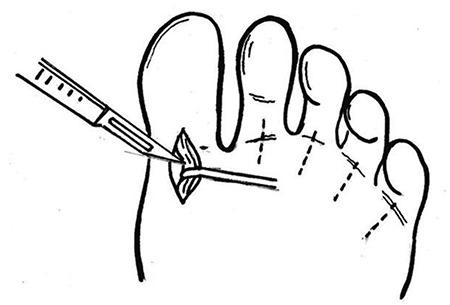
Percutaneous flexor tenotomy
Distal metatarsal metaphyseal osteotomy (DMMO) and distal metatarsal diaphyseal osteotomy (DMDO) are commonly used to relieve surgically offloading ulcers of the first to fifth metatarsal heads. A 1998 study by Piaggesi compared the effects of DMMO with those of standard nonsurgical treatments in patients with neuropathic ulcers. The results showed that surgical intervention could significantly improve the healing rate and healing time while reducing infection complications and ulcer recurrence rates at the 6-month follow-up (8/24 vs. 3/22; p < 0.01). Although the follow-up duration of 6 months may have been too short to evaluate the true results in the long term, it is worth noting that no ulcers recurred following the surgical intervention. The author believes that bone resection works on the principles of load transfer to adjacent areas, which may predispose other areas to developing new ulcers. However, given this probability, the incidence of new ulcers is lower than the risk of ulcer recurrence, which was treated conservatively. In a similar method to reduce plantar pressure over the metatarsal heads, DMDO is a percutaneous, minimally invasive procedure where an incision is made into the neck of the metatarsal. Biz et al. evaluated the safety and clinical effectiveness of this method in a prospective case series of 35 chronic DFUs in the forefoot region, most of which were classified as type B3 according to the UT wound classification system. The average healing time was 7.9 ± 4.0 weeks (range, 4–17 weeks), and the OFAS score increased from 55.3 to 81.4 points (p < 0.001). No recurrence of ulceration was observed over the follow-up period of 25.3 months (range, 18–71 months) [149]. In both studies, the authors presented a case in point that minimally invasive DMMO and DMDO are safe and effective methods to promote the healing of forefoot DFUs by reducing plantar pressure under the metatarsal head and effectively improving function and structure with few complications (Figure 24–25).
Figure 24.
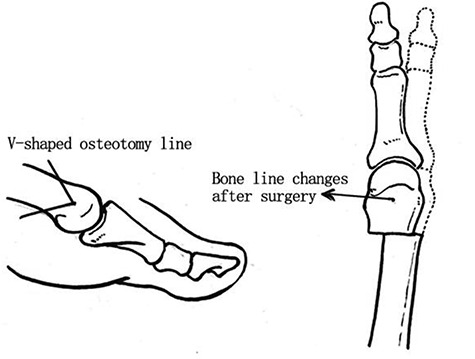
Distal metatarsal metaphyseal osteotomy
Figure 25.
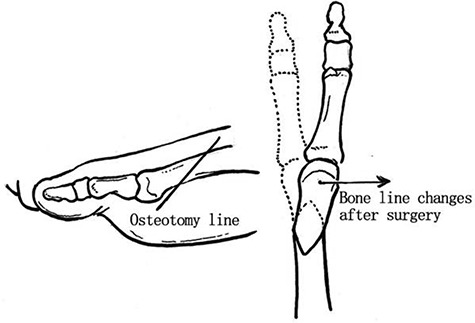
Distal metatarsal diaphyseal osteotomy
Modified Keller resection arthroplasty has also been suggested to treat plantar ulcers over the interphalangeal joint of the hallux. It involves the resection of one-quarter of the proximal phalanges of the hallux (the initial description called for half or one-third resection of the proximal phalanges of the hallux), which can achieve a therapeutic effect while minimizing excessive osteotomy that would result in unnecessary shortening of the hallux, deformity, muscular weakness and poor stability. In a retrospective case series conducted by Berner and coworkers in 2005, the authors studied the effect of the modified Keller surgery on 13 ulcers on the plantar aspect of the hallux in 11 patients. All ulcers healed within 6 months after undergoing the surgery. The recurrence rate was 38% (5 out of 13) within 1 year postoperatively. In addition, the modified Keller resection is also an effective treatment for DFUs. In short, despite the insufficient level of evidence, minimally invasive surgical methods such as ATL and metatarsophalangeal arthroplasty have been used clinically and have achieved varying effects. As such, this article concludes that surgical offloading treatment may be promising for the prevention and treatment of DFUs. However, the choice of surgical option needs to correlate with the clinical indications to prevent possible complications. This article specifically notes that in the process of diabetic foot prevention, comprehensive foot care should be combined with the following five elements: diabetic foot risk stratification, regular inspections, education, reasonable use of footwear and treatment of ulcer precursor lesions. Meanwhile, the treatment plan should be tailored to each patient’s unique condition and provide the patient with appropriate devices or materials, such as insoles, offloading padding and even beveled-out material from the insoles or footwear in areas of high plantar pressure in the patient’s feet to optimize offloading.
Conclusion
This clinical practice guideline discusses the evaluation, diagnosis, treatment and prevention of diabetic foot, with special focus on clinical operability and an emphasis on the use of comprehensive treatment modalities in a multidisciplinary approach. The timing and type of surgical techniques are key to the treatment and prevention of DFUs. It is unavoidable that there are still some limitations in this article, and many questions have not been answered. We hope that colleagues in the academic community can provide comments and suggestions to jointly promote the standardization of diabetic foot treatment.
Funding
Fund program: The National Natural Science Foundation of China (grant number: 81770810)
Conflicts of interest
None declared.
Supplementary Material
References
- 1. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. 2017;49:106–16. [DOI] [PubMed] [Google Scholar]
- 2. Bus SA, van Netten JJ, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016;32:16–24. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–75. [DOI] [PubMed] [Google Scholar]
- 4. Wang A, Xu Z, Mu Y, Ji L. Clinical characteristics and medical costs in patients with diabetic amputation and nondiabetic patients with nonacute amputation in central urban hospitals in China. Int J Low Extrem Wounds. 2014;13:17–21. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes A Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Working Group on the Diabetic Foot IWGDF guidelines on the prevention and management of diabetic foot disease [Internet]. Available from: https://iwgdfguidelines.org/wp-content/uploads/2019/05/IWGDF-Guidelines-2019.
- 7. Chinese Diabetes Society, Society of Infectious Disease, Chinese Medical Association of Organization Repair and Regeneration Branch . Chinese guidelines for the prevention and treatment of diabetic foot (2019 edition) (in Chinese ). Chinese Journal of Diabetes. 2019;11:92–108. [Google Scholar]
- 8. Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raspovic KM, Ahn J, La Fontaine J, Lavery LA, Wukich DK. End-stage renal disease negatively affects physical quality of life in patients with diabetic foot complications. Int J Low Extrem Wounds 2017;16:135–42. [DOI] [PubMed] [Google Scholar]
- 10. Lee WS, Kim J. Diabetic cardiomyopathy: where we are and where we are going. Korean J Intern Med. 2017;32:404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozer Balin S, Sagmak Tartar A, Ugur K, Kilinc F, Telo S, Bal A, et al. Pentraxin-3: a new parameter in predicting the severity of diabetic foot infection?. Int Wound J. 2019;16:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013;103:2–7. [DOI] [PubMed] [Google Scholar]
- 13. Fridman R, Bar-David T, Kamen S, Staron RB, Leung DK, Rasiej MJ. Imaging of diabetic foot infections. Clin Podiatr Med Surg. 2014;31:43–56. [DOI] [PubMed] [Google Scholar]
- 14. Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. 2010;12:335–42. [DOI] [PubMed] [Google Scholar]
- 15. Saeed S, Zafar J, Khan B, Akhtar A, Qurieshi S, Fatima S, et al. Utility of (9)(9) mTc-labelled antimicrobial peptide ubiquicidin (29-41) in the diagnosis of diabetic foot infection. Eur J Nucl Med Mol Imaging. 2013;40:737–43. [DOI] [PubMed] [Google Scholar]
- 16. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132–73. [DOI] [PubMed] [Google Scholar]
- 17. Lavery LA, Ryan EC, Ahn J, Crisologo PA, Oz OK, La Fontaine J, et al. The infected diabetic foot: re-evaluating the IDSA diabetic foot infection classification. Clin Infect Dis. 2020;70:1573–79. 10.1093/cid/ciz489. [DOI] [PubMed] [Google Scholar]
- 18. Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev. 2012;28:574–600. [DOI] [PubMed] [Google Scholar]
- 19. Hinchliffe RJ, Brownrigg JR, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32:37–44. [DOI] [PubMed] [Google Scholar]
- 20. Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American podiatric medical association and the Society for Vascular Medicine. J Vasc Surg. 2016;63:3S–21S. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DW, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can J Cardiol. 2010;26:e346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the Management of Patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e726–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 24. Del Conde I, Benenati JF. Noninvasive testing in peripheral arterial disease. Interv Cardiol Clin. 2014;3:469–78. [DOI] [PubMed] [Google Scholar]
- 25. Andersen CA. Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg. 2010;52:76S–80S. [DOI] [PubMed] [Google Scholar]
- 26. Redlich U, Xiong YY, Pech M, Tautenhahn J, Halloul Z, Lobmann R, et al. Superiority of transcutaneous oxygen tension measurements in predicting limb salvage after below-the-knee angioplasty: a prospective trial in diabetic patients with critical limb ischemia. Cardiovasc Intervent Radiol. 2011;34:271–9. [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Hasan R, Firwana B, Elraiyah T, Tsapas A, Prokop L, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. 2016;63:29S–36S.e1–2. [DOI] [PubMed] [Google Scholar]
- 28. Eiberg JP, Gronvall Rasmussen JB, Hansen MA, Schroeder TV. Duplex ultrasound scanning of peripheral arterial disease of the lower limb. Eur J Vasc Endovasc Surg. 2010;40:507–12. [DOI] [PubMed] [Google Scholar]
- 29. Schabel C, Bongers MN, Ketelsen D, Syha R, Thomas C, Homann G, et al. Diagnostic accuracy of dual energy CT angiography in patients with diabetes mellitus. Radiologe. 2015;55:314–22. [DOI] [PubMed] [Google Scholar]
- 30. Jeremias Z, Rat N, Benedek I, Rapolti E, Ratiu M, Muresan A, et al. High iliac calcium score is associated with increased severity and complexity of peripheral arterial disease and predicts global atherosclerotic burden. Vasa. 2018;47:377–86. [DOI] [PubMed] [Google Scholar]
- 31. Dias-Neto M, Marques C, Sampaio S. Digital subtraction angiography or computed tomography angiography in the preoperative evaluation of lower limb peripheral artery disease - a comparative analysis. Rev Port Cir Cardiotorac Vasc. 2017;24:174. [PubMed] [Google Scholar]
- 32. Menke J, Larsen J. Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Ann Intern Med. 2010;153:325–34. [DOI] [PubMed] [Google Scholar]
- 33. Vinik AI. CLINICAL PRACTICE. Diabetic sensory and motor neuropathy. N Engl J Med. 2016;374:1455–64. [DOI] [PubMed] [Google Scholar]
- 34. Tan LS. The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract. 2010;90: 1–7. [DOI] [PubMed] [Google Scholar]
- 35. Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940–51. [DOI] [PubMed] [Google Scholar]
- 36. Rayman G, Vas PR, Baker N, Taylor CG Jr, Gooday C, Alder AI, et al. The Ipswich touch test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care. 2011;34:1517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma S, Kerry C, Atkins H, Rayman G. The Ipswich touch test: a simple and novel method to screen patients with diabetes at home for increased risk of foot ulceration. Diabet Med. 2014;31:1100–3. [DOI] [PubMed] [Google Scholar]
- 38. Chinese Diabetes Society Guidelines for the prevention and Treatment of type 2 diabetes in China (2017 edition) (in Chinese). Chinese Journal of Diabetes. 2018;10:4–67. [Google Scholar]
- 39. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu B, Hu J, Wen J, Zhang Z, Zhou L, Li Y, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes - ShangHai diabetic neuRopathy epidemiology and molecular genetics study (SH-DREAMS). PLoS One. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA. 2015;314:2172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan T, Shaw EJ, Siddiqui F, Kandaswamy P, Barry PW, Baker M, et al. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280. [DOI] [PubMed] [Google Scholar]
- 43. Bergin SM, Gurr JM, Allard BP, Holland EL, Horsley MW, Kamp MC, et al. Australian diabetes foot network: management of diabetes-related foot ulceration - a clinical update. Med J Aust. 2012;197:226–9. [DOI] [PubMed] [Google Scholar]
- 44. Hafner J, Nobbe S, Partsch H, Lauchli S, Mayer D, Amann-Vesti B, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961–8. [DOI] [PubMed] [Google Scholar]
- 45. Suarez-Amor O, Perez-Bustillo A, Ruiz-Gonzalez I, Rodriguez-Prieto MA. Necrobiosis lipoidica therapy with biologicals: an ulcerated case responding to etanercept and a review of the literature. Dermatology. 2010;221:117–21. [DOI] [PubMed] [Google Scholar]
- 46. Zhongyang S, Xinjuan S, Jin'an C, Wei W, Lan L, Yinchen C, et al. SIANM assessment: a new assessment method for diabetic foot (in Chinese). Journal of Trauma Surgery. 2017;19:869–72. [Google Scholar]
- 47. Eggert JV, Worth ER, Van Gils CC. Cost and mortality data of a regional limb salvage and hyperbaric medicine program for Wagner grade 3 or 4 diabetic foot ulcers. Undersea Hyperb Med. 2016;43:1–8. [PubMed] [Google Scholar]
- 48. Lv HH, Wu S, Liu X, Yang XL, Xu JF, Guan YT, et al. Comparison of VerifyNow P2Y12 and thrombelastography for assessing clopidogrel response in stroke patients in China. Neurol Sci. 2016;37:277–82. [DOI] [PubMed] [Google Scholar]
- 49. Abu-Assi E, Raposeiras-Roubin S, Garcia-Acuna JM, Gonzalez-Juanatey JR. Bleeding risk stratification in an era of aggressive management of acute coronary syndromes. World J Cardiol. 2014;6:1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hocking G, Mitchell CH. Optimizing the safety and practice of ultrasound-guided regional anesthesia: the role of echogenic technology. Curr Opin Anaesthesiol. 2012;25:603–9. [DOI] [PubMed] [Google Scholar]
- 51. Lai HY, Foo LL, Lim SM, Yong CF, Loh PS, Chaw SH, et al. The hemodynamic and pain impact of peripheral nerve block versus spinal anesthesia in diabetic patients undergoing diabetic foot surgery. Clin Auton Res. 2020;30:53–60. [DOI] [PubMed] [Google Scholar]
- 52. Choi YS, Shin HJ, Park JY, Kim HJ, Yun SH. Ultrasound-guided femoral and popliteal sciatic nerve blocks for below knee surgery in patients with severe cardiac disease. Korean J Anesthesiol. 2015;68:513–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Visnjevac O, Davari-Farid S, Lee J, Pourafkari L, Arora P, Dosluoglu HH, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg. 2015;121:110–6. [DOI] [PubMed] [Google Scholar]
- 54. Carls GS, Gibson TB, Driver VR, Wrobel JS, Garoufalis MG, Defrancis RR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc. 2011;101:93–115. [DOI] [PubMed] [Google Scholar]
- 55. Somayaji R, Elliott JA, Persaud R, Lim M, Goodman L, Sibbald RG. The impact of team based interprofessional comprehensive assessments on the diagnosis and management of diabetic foot ulcers: a retrospective cohort study. PLoS One. 2017. 10.1371/journal.pone.0185251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nather A, Siok Bee C, Keng Lin W, Xin-Bei Valerie C, Liang S, Tambyah PA, et al. Value of team approach combined with clinical pathway for diabetic foot problems: a clinical evaluation. Diabet Foot Ankle. 2010. 10.3402/dfa.v1i0.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang C, Mai L, Yang C, Liu D, Sun K, Song W, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team in patient with diabetes foot ulcer. BMC Endocr Disord. 2016. 10.1186/s12902-016-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011;131:2121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Markuson M, Hanson D, Anderson J, Langemo D, Hunter S, Thompson P, et al. The relationship between hemoglobin a(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009;22:365–72. [DOI] [PubMed] [Google Scholar]
- 60. Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63:22S-28S.e2. [DOI] [PubMed] [Google Scholar]
- 61. Kee KK, Nair HKR, Yuen NP. Risk factor analysis on the healing time and infection rate of diabetic foot ulcers in a referral wound care clinic. J Wound Care. 2019;28:S4–S13. [DOI] [PubMed] [Google Scholar]
- 62. Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M, et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2016;1:CD010764 10.1002/14651858.CD010764.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peled S, Pollack R, Elishoov O, Haze A, Cahn A. Association of Inpatient Glucose Measurements with amputations in patients hospitalized with acute diabetic foot. J Clin Endocrinol Metab. 2019;104:5445–52. [DOI] [PubMed] [Google Scholar]
- 64. Jiang Y, Wang X, Xia L, Fu X, Xu Z, Ran X, et al. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regen. 2015;23:222–30. [DOI] [PubMed] [Google Scholar]
- 65. Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015. 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brennan MB, Guihan M, Budiman-Mak E, Kang H, Lobo JM, Sutherland BL, et al. Increasing SBP variability is associated with an increased risk of developing incident diabetic foot ulcers. J Hypertens. 2018;36:2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. American Diabetes Association Cardiovascular disease and risk management: standards of medical Care in Diabetes-2020. Diabetes Care. 2020;43:S111–34. [DOI] [PubMed] [Google Scholar]
- 68. Adamsson Eryd S, Gudbjornsdottir S, Manhem K, Rosengren A, Svensson AM, Miftaraj M, et al. Blood pressure and complications in individuals with type 2 diabetes and no previous cardiovascular disease: national population based cohort study. BMJ. 2016;354:i4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–43. [DOI] [PubMed] [Google Scholar]
- 70. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–15. [DOI] [PubMed] [Google Scholar]
- 71. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- 72. The ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sink KM, Evans GW, Shorr RI, Bates JT, Berlowitz D, Conroy MB, et al. Syncope, hypotension, and falls in the Treatment of hypertension: results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc. 2018;66:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dietrich I, Braga GA, de Melo FG, da Costa Silva Silva ACC. The diabetic foot as a proxy for cardiovascular events and mortality review. Curr Atheroscler Rep. 2017;19 10.1007/s11883-017-0680-z. [DOI] [PubMed] [Google Scholar]
- 75. Cholesterol Treatment Trialists’ Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. [DOI] [PubMed] [Google Scholar]
- 76. Pei E, Li J, Lu C, Xu J, Tang T, Ye M, et al. Effects of lipids and lipoproteins on diabetic foot in people with type 2 diabetes mellitus: a meta-analysis. J Diabetes Complications. 2014;28:559–64. [DOI] [PubMed] [Google Scholar]
- 77. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- 78. Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Standards of medical Care in Diabetes-2016: summary of revisions. Diabetes Care. 2016;39:S4–5. [DOI] [PubMed] [Google Scholar]
- 80. American Diabetes A Standards of medical care in diabetes--2014. Diabetes Care. 2014, 2014;37:S14–80. [DOI] [PubMed] [Google Scholar]
- 81. de Antonio M, Lupon J, Galan A, Vila J, Urrutia A, Bayes-Genis A. Combined use of high-sensitivity cardiac troponin T and N-terminal pro-B type natriuretic peptide improves measurements of performance over established mortality risk factors in chronic heart failure. Am Heart J. 2012;163:821–8. [DOI] [PubMed] [Google Scholar]
- 82. Liu H, Zhang YZ, Gao M, Liu BC. Elevation of B-type natriuretic peptide is a sensitive marker of left ventricular diastolic dysfunction in patients with maintenance haemodialysis. Biomarkers. 2010;15:533–7. [DOI] [PubMed] [Google Scholar]
- 83. Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jara-Palomares L, Marin-Romero S, Asensio-Cruz MI, Elias-Hernandez T, Otero-Candelera R. Intermittent pneumatic compression plus pharmacological thromboprophylaxis to prevent deep vein thrombosis. J Thorac Dis. 2019;11:1731–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hou H, Yao Y, Zheng K, Teng H, Rong Z, Chen D, et al. Does intermittent pneumatic compression increase the risk of pulmonary embolism in deep venous thrombosis after joint surgery?. Blood Coagul Fibrinolysis. 2016;27:246–51. [DOI] [PubMed] [Google Scholar]
- 86. Scheeren TW, Wiesenack C, Gerlach H, Marx G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput. 2013;27:225–33. [DOI] [PubMed] [Google Scholar]
- 87. Toomtong P, Suksompong S. Intravenous fluids for abdominal aortic surgery. Cochrane Database Syst Rev. 2010;1:CD000991 10.1002/14651858.CD000991.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bunn F, Trivedi D. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev. 2012;7:CD001319 10.1002/14651858.CD001319.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Romanelli M, Dini V, Milani M. Topical purified omental lipid formulations in the prevention of skin ulcers: a narrative review. J Wound Care. 2019;28:284–90. [DOI] [PubMed] [Google Scholar]
- 90. Nixon J, Brown S, Smith IL, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Comparing alternating pressure mattresses and high-specification foam mattresses to prevent pressure ulcers in high-risk patients: the PRESSURE 2 RCT. Health Technol Assess. 2019;23:1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Riemenschneider KJ. Prevention of pressure injuries in the operating room: a quality improvement project. J Wound Ostomy Continence Nurs. 2018;45:141–5. [DOI] [PubMed] [Google Scholar]
- 92. Jiang Y, Ran X, Jia L, Yang C, Wang P, Ma J, et al. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds. 2015;14:19–27. [DOI] [PubMed] [Google Scholar]
- 93. Kim NY, Lee KY, Bai SJ, Hong JH, Lee J, Park JM, et al. Comparison of the effects of remifentanil-based general anesthesia and popliteal nerve block on postoperative pain and hemodynamic stability in diabetic patients undergoing distal foot amputation: a retrospective observational study. Medicine (Baltimore). 2016. 10.1097/MD.0000000000004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2:CD009122 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bragd J, Adamson U, Backlund LB, et al. , Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade?. [J]. Diabetes Metab. 2008;34:612–6. doi: 10.1016/j.diabet.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 96. Hotta N, Kawamori R, Fukuda M, Shigeta Y, Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–33. 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li S, Chen X, Li Q, Du J, Liu Z, Peng Y, et al. Effects of acetyl-L-carnitine and methylcobalamin for diabetic peripheral neuropathy: a multicenter, randomized, double-blind, controlled trial. J Diabetes Investig. 2016;7:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shin S, Kim KJ, Chang HJ, Lee BW, Yang WI, Cha BS, et al. The effect of oral prostaglandin analogue on painful diabetic neuropathy: a double-blind, randomized, controlled trial. Diabetes Obes Metab. 2013;15:185–8. [DOI] [PubMed] [Google Scholar]
- 99. Jin YP, Su XF, Li HQ, Wu JD, Ding B, Sun R, et al. The therapeutic effect of pancreatic Kininogenase on Treatment of diabetic peripheral neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2016;124:618–21. [DOI] [PubMed] [Google Scholar]
- 100. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32:1005–10. [DOI] [PubMed] [Google Scholar]
- 101. Boyle J, Eriksson ME, Gribble L, Gouni R, Johnsen S, Coppini DV, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35:2451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012;9:CD010111 10.1002/14651858.CD010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Elgzyri T, Larsson J, Nyberg P, Thorne J, Eriksson KF, Apelqvist J. Early revascularization after admittance to a diabetic foot center affects the healing probability of ischemic foot ulcer in patients with diabetes. Eur J Vasc Endovasc Surg. 2014;48:440–6. [DOI] [PubMed] [Google Scholar]
- 104. Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36:1815–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Elgzyri T, Larsson J, Thorne J, Eriksson KF, Apelqvist J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur J Vasc Endovasc Surg. 2013;46:110–7. [DOI] [PubMed] [Google Scholar]
- 106. Alexandrescu VA. Commentary: myths and proofs of angiosome applications in CLI: where do we stand?. J Endovasc Ther. 2014;21:616–24. [DOI] [PubMed] [Google Scholar]
- 107. Acin F, Varela C, Lopez de Maturana I, et al. , Results of infrapopliteal endovascular procedures performed in diabetic patients with critical limb ischemia and tissue loss from the perspective of an angiosome-oriented revascularization strategy[J]. Int J Vasc Med. 2014;2014:270539. doi: 10.1155/2014/270539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kabra A, Suresh KR, Vivekanand V, Vishnu M, Sumanth R, Nekkanti M. Outcomes of angiosome and non-angiosome targeted revascularization in critical lower limb ischemia. J Vasc Surg. 2013;57:44–9. [DOI] [PubMed] [Google Scholar]
- 109. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. [DOI] [PubMed] [Google Scholar]
- 110. Vogel TR, Dombrovskiy VY, Haser PB, Graham AM. Evaluating preventable adverse safety events after elective lower extremity procedures. J Vasc Surg. 2011;54:706–13. [DOI] [PubMed] [Google Scholar]
- 111. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Corrigendum to 'European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2020;59:454. [DOI] [PubMed] [Google Scholar]
- 112. Lipsky BA, Aragon-Sanchez J, Diggle M, Embil J, Kono S, Lavery L, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32:45–74. [DOI] [PubMed] [Google Scholar]
- 113. Barwell ND, Devers MC, Kennon B, Hopkinson HE, McDougall C, Young MJ, et al. Diabetic foot infection: antibiotic therapy and good practice recommendations. Int J Clin Pract. 2017. 10.1111/ijcp.13006. [DOI] [PubMed] [Google Scholar]
- 114. Hesstvedt L, Arendrup MC, Poikonen E, Klingpor L, Friman V, Nordoy I, et al. Differences in epidemiology of candidaemia in the Nordic countries- what is to blame?. Mycoses. 2017;60:11–9. [DOI] [PubMed] [Google Scholar]
- 115. Johnson SW, Drew RH, May DB. How long to treat with antibiotics following amputation in patients with diabetic foot infections? Are the 2012 IDSA DFI guidelines reasonable?. J Clin Pharm Ther. 2013;38:85–8. [DOI] [PubMed] [Google Scholar]
- 116. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426–35. [DOI] [PubMed] [Google Scholar]
- 117. Ahluwalia R, Vainieri E, Tam J, Sait S, Sinha A, Manu CA, et al. Surgical diabetic foot debridement: improving training and practice utilizing the traffic light principle. Int J Low Extrem Wounds. 2019;18:279–86. [DOI] [PubMed] [Google Scholar]
- 118. Wang Z, Zuze W, Xiaobing F. Diagnosis and treatment of diabetic foot (in Chinese). Beijing: People's Medical Publishing House, 2016, 276. [Google Scholar]
- 119. Elraiyah T, Domecq JP, Prutsky G, Tsapas A, Nabhan M, Frykberg RG, et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J Vasc Surg. 2016;63:37S–45S.e2. [DOI] [PubMed] [Google Scholar]
- 120. Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care. 2014;37:789–95. [DOI] [PubMed] [Google Scholar]
- 121. Widatalla AH, Mahadi SE, Shawer MA, Mahmoud SM, Abdelmageed AE, Ahmed ME. Diabetic foot infections with osteomyelitis: efficacy of combined surgical and medical treatment. Diabet Foot Ankle. 2012. 10.3402/dfa.v3i0.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tamir E, Tamir J, Beer Y, Kosashvili Y, Finestone AS. Resection Arthroplasty for resistant ulcers underlying the hallux in insensate diabetics. Foot Ankle Int. 2015;36:969–75. [DOI] [PubMed] [Google Scholar]
- 123. Baumgartner R. Forefoot and midfoot amputations. Oper Orthop Traumatol. 2011;23:254–64. [DOI] [PubMed] [Google Scholar]
- 124. Thorud JC, Jupiter DC, Lorenzana J, Nguyen TT, Shibuya N. Reoperation and Reamputation after Transmetatarsal amputation: a systematic review and meta-analysis. J Foot Ankle Surg. 2016;55:1007–12. [DOI] [PubMed] [Google Scholar]
- 125. Stone PA, Back MR, Armstrong PA, et al. , Midfoot amputations expand limb salvage rates for diabetic foot infections[J]. Ann Vasc Surg. 2005;19:805–11. doi: 10.1007/s10016-005-7973-3. [DOI] [PubMed] [Google Scholar]
- 126. Sundararajan SR, Srikanth KP, Nagaraja HS, Rajasekaran S. Effectiveness of Hindfoot arthrodesis by stable internal fixation in various Eichenholtz stages of neuropathic ankle Arthropathy. J Foot Ankle Surg. 2017;56:282–6. [DOI] [PubMed] [Google Scholar]
- 127. Dalla Paola L, Carone A, Boscarino G, Scavone G, Vasilache L. Combination of open subtotal Calcanectomy and stabilization with external fixation as limb salvage procedure in Hindfoot-infected diabetic foot ulcers. Int J Low Extrem Wounds. 2016;15:332–7. [DOI] [PubMed] [Google Scholar]
- 128. Akkurt MO, Demirkale I, Oznur A. Partial calcanectomy and Ilizarov external fixation may reduce amputation need in severe diabetic calcaneal ulcers. Diabet Foot Ankle. 2017. 10.1080/2000625X.2017.1264699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Janis JE, Kwon RK, Attinger CE. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg. 2011;127:205S–12S. [DOI] [PubMed] [Google Scholar]
- 130. Hahn HM, Jeong KS, Park MC, Park DH, Lee IJ. Free-flap transfer for coverage of Transmetatarsal amputation stump to preserve residual foot length. Int J Low Extrem Wounds. 2017;16:60–5. [DOI] [PubMed] [Google Scholar]
- 131. Xiaobing F. Treatment of diabetic foot and associated chronic refractory wounds (in Chinese) [M]. Beijing: Military Science Publishing House, 2013, 81–2. [Google Scholar]
- 132. Zhang X, Sun D, Jiang GC. Comparative efficacy of nine different dressings in healing diabetic foot ulcer: a Bayesian network analysis. J Diabetes. 2019;11:418–26. [DOI] [PubMed] [Google Scholar]
- 133. Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg. 2014;47:647–55. [DOI] [PubMed] [Google Scholar]
- 134. O'Reilly D, Pasricha A, Campbell K, Burke N, Assasi N, Bowen JM, et al. Hyperbaric oxygen therapy for diabetic ulcers: systematic review and meta-analysis. Int J Technol Assess Health Care. 2013;29:269–81. [DOI] [PubMed] [Google Scholar]
- 135. Li Y, Gao Y, Gao Y, Chen D, Wang C, Liu G, et al. Autologous platelet-rich gel treatment for diabetic chronic cutaneous ulcers: a meta-analysis of randomized controlled trials. J Diabetes. 2019;11:359–69. [DOI] [PubMed] [Google Scholar]
- 136. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–5. [DOI] [PubMed] [Google Scholar]
- 137. Guo J, Dardik A, Fang K, Huang R, Gu Y. Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res Ther. 2017. 10.1186/s13287-017-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hughes OB, Rakosi A, Macquhae F, Herskovitz I, Fox JD, Kirsner RS. A review of cellular and Acellular matrix products: indications, techniques, and outcomes. Plast Reconstr Surg. 2016;138:138S–47S. [DOI] [PubMed] [Google Scholar]
- 139. Lev-Tov H, Li CS, Dahle S, Isseroff RR. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013. 10.1186/1745-6215-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shekhter AB, Fayzullin AL, Vukolova MN, Rudenko TG, Osipycheva VD, Litvitsky PF. Medical applications of collagen and collagen-based materials. Curr Med Chem. 2019;26:506–16. [DOI] [PubMed] [Google Scholar]
- 141. Wiser I, Tamir E, Kaufman H, Keren E, Avshalom S, Klein D, et al. A novel recombinant human collagen-based Flowable matrix for chronic lower limb wound management: first results of a clinical trial. Wounds. 2019;31:103–7. [PubMed] [Google Scholar]
- 142. Begg L, McLaughlin P, Vicaretti M, Fletcher J, Burns J. Total contact cast wall load in patients with a plantar forefoot ulcer and diabetes. J Foot Ankle Res. 2016. 10.1186/s13047-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Morona JK, Buckley ES, Jones S, Reddin EA, Merlin TL. Comparison of the clinical effectiveness of different off-loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2013;29:183–93. [DOI] [PubMed] [Google Scholar]
- 144. Bus SA, Armstrong DG, van Deursen RW, Lewis JE, Caravaggi CF, Cavanagh PR, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev. 2016;32:25–36. [DOI] [PubMed] [Google Scholar]
- 145. Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr, Armstrong DG. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010;52:23S–7S. [DOI] [PubMed] [Google Scholar]
- 146. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ, International Working Group on the Diabetic F . The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32:2–6. [DOI] [PubMed] [Google Scholar]
- 147. Rizzo L, Tedeschi A, Fallani E, Coppelli A, Vallini V, Iacopi E, et al. Custom-made orthesis and shoes in a structured follow-up program reduces the incidence of neuropathic ulcers in high-risk diabetic foot patients. Int J Low Extrem Wounds. 2012;11:59–64. [DOI] [PubMed] [Google Scholar]
- 148. Scott JE, Hendry GJ, Locke J. Effectiveness of percutaneous flexor tenotomies for the management and prevention of recurrence of diabetic toe ulcers: a systematic review. J Foot Ankle Res. 2016;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Biz C, Gastaldo S, Dalmau-Pastor M, Corradin M, Volpin A, Ruggieri P. Minimally invasive distal metatarsal Diaphyseal osteotomy (DMDO) for chronic plantar diabetic foot ulcers. Foot Ankle Int. 2018;39:83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


