Abstract
We found no clear evidence of the clinical superiority of distal radius fracture surgery among older adults at one year.
Surgical treatment, however, may yield a faster recovery to previous level of activity in elderly patients.
With operative treatment, hardware-based problems may warrant secondary operations and implant removal, whereas in non-operative treatment, symptomatic loss of alignment and malunion can occur.
In elderly patients, non-operative treatment can be considered to be the gold standard.
Cite this article: EFORT Open Rev 2020;5:361-370. DOI: 10.1302/2058-5241.5.190060
Keywords: distal radius fracture, elderly, older adults, RCT, review
Introduction
Distal radius fracture (DRF) is the most common fall-related fracture and the most common fracture of the upper extremities.1,2 The age-adjusted overall incidence of DRF varies between 100 and 300 per 100,000 person-years.3,4 Despite the common occurrence of this injury, there seem to be challenges in our understanding of this complex condition. The main questions that remain to be answered are the following:
1) Which fractures should we treat non-operatively and what would be the best way to achieve this?
2) Which fractures should we treat operatively?
3) How can we predict fracture behaviour during non-operative treatment and based on what premises should we intervene to maximize the functional outcome?
4) What would be the most efficient way to recognize those patients who do not heal well and how should we rehabilitate them?
5) How can we do all this in an effective and cost-effective manner, taking into account the number of fractures?
Aetiology and risk factors
Risk factors for DRF vary depending on age, gender and living conditions,2,5,6 and women have a greater risk of sustaining a DRF than men.2,3,5 Moreover, the rate of osteoporosis is higher in elderly DRF patients, and can therefore be considered to be a substantial risk factor for both men and women.6 In countries with snow, there have also been reports of the significant seasonal variations in fracture rates. In winter, for example the rate of DRF is substantially higher and fracture epidemics can occur.3,7–10 Other risk factors for DRF include low body mass index (BMI) and the tendency to fall as the result of an individual’s reduced balance.6,11,12
Diagnostics and classification of DRFs
The gold standard in DFR diagnostics is anterior-posterior and lateral radiographs. However, computed tomography (CT)13 may yield additional benefits in evaluating the articular surface and comminution of the fracture, which may be a risk factor for osteoarthritis of the radiocarpal joint.13,14 The clinical examination of soft tissues in the wrist and hand is also important. Soft tissue injuries have been shown to be present in approximately 31% of cases,15 and they may have an effect on treatment decisions.15–17 If injury of the carpal ligaments is suspected, CT or high-resolution magnetic resonance imaging may be beneficial before a final treatment decision is made.18,19
Several classification systems, such as the AO and Fernandez and Frykman classifications20–22 exist for DRFs. These classification systems are commonly based on radiographs, fracture pattern and injury mechanism. Articular surface unity and fracture comminution are important factors in these classification systems. However, the utility of these in clinical practice is modest since patient characteristics, such as age, are not taken into account. In clinical practice, fractures are commonly classified as Colles’ fracture (Fig. 1), Smith’s fracture (Fig. 2), Barton’s fracture (Fig. 3) and Chauffeur’s fracture (Fig. 4). These classifications can help to distinguish between different fracture patterns. Smith’s and Barton’s fractures are generally considered to be unstable, warranting operative treatment in most cases. However, with Colles’ fracture, there is no classification to help in predicting instability.23
Fig. 1.
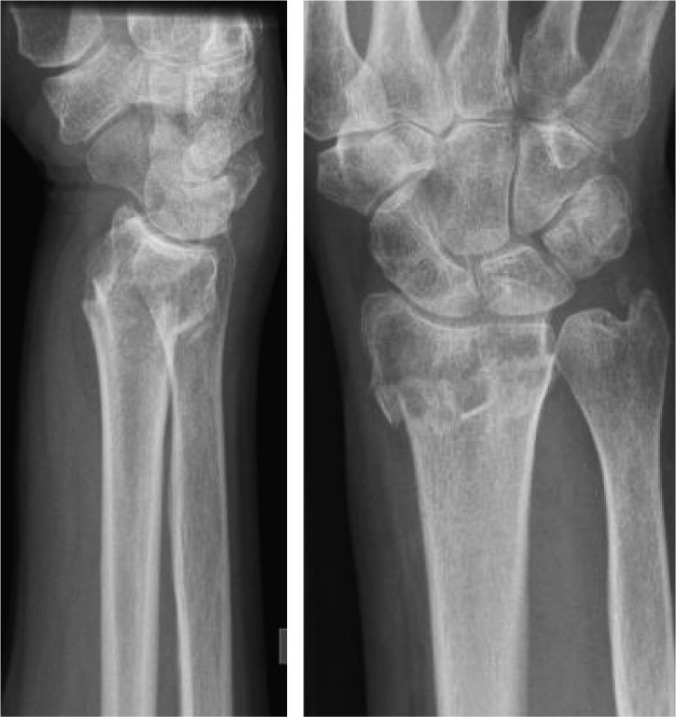
Lateral and antero-posterior view of the distal radius in a 74-year-old female showing displaced Colles’ fracture with a probable extension to the wrist joint.
Fig. 2.
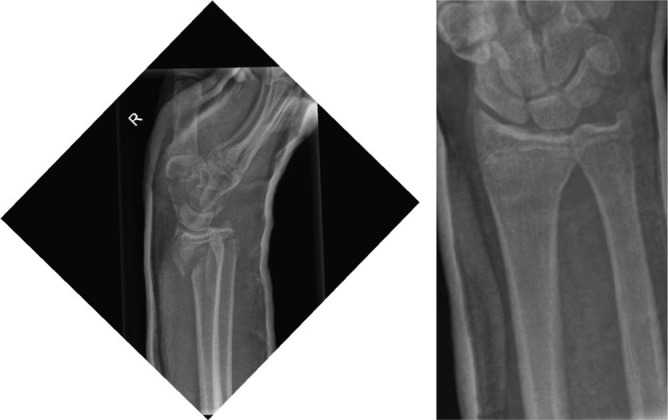
Lateral and antero-posterior view of the distal radius in a 28-year-old male showing displaced Smith’s fracture.
Fig. 3.
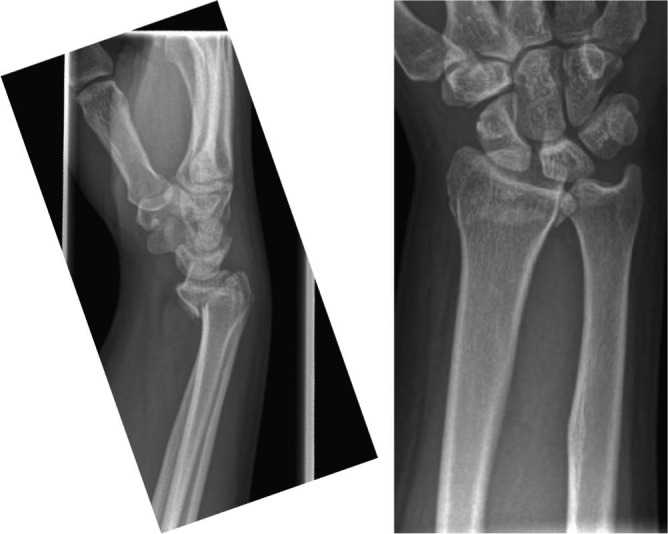
Lateral and antero-posterior view of the distal radius in a 52-year-old female showing displaced Barton’s fracture.
Fig. 4.
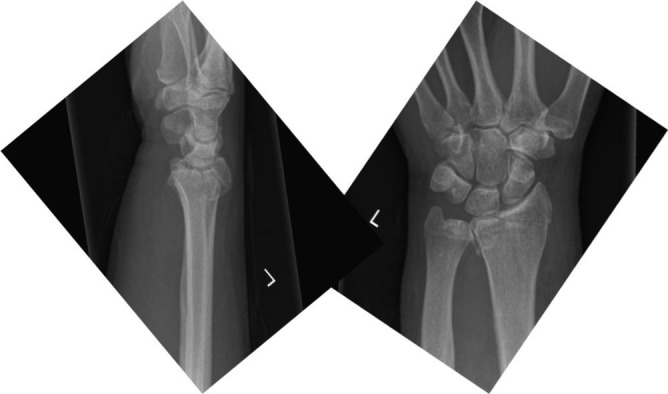
Lateral and antero-posterior view of the distal radius in a 60-year-old male showing non-displaced Chauffeur’s fracture.
Outcome predictors
Factors that predict radiological outcome
Studies that have explored the association between poor functional outcome and radiological parameters have generally used the following criteria for acceptable alignment: ≤ 10° to 15° dorsal angulation, ≤ 20° volar angulation, ≤ 2 to 4 mm ulnar variance, ≥ 15° radioulnar inclination angle and < 1 to 2 mm step-off or gap on the joint line.24–31 It has been stated that malunion represents a risk for a poor functional outcome. Loss of alignment can occur early (< 1 to 2 weeks) or late (between 2 and 6 weeks).24,29,32,33 Moreover, increasing age, initial dorsal angulation of the joint line, the collapse and initial variance of length between the radius and the ulna as well as metaphyseal comminution of the fracture are all predictors of the loss of alignment during cast treatment.24,25 Of these, patient age has been most consistently shown to be a statistically significant predictor for loss of alignment.25,28,34,35
Radiological factors that predict functional outcome
Shortening of the radius, articular step-off and gap in the joint line are the most significant radiological predictors of poor functional outcome.36–39 In younger patients, a shortening of the radius of more than 3 mm may cause functional deficit in terms of prolonged pain, reduced range of motion (ROM) and decreased grip strength.40,41 It seems, however, that over 1 mm step-off or gap on the joint line significantly increases the risk of secondary osteoarthritis and may cause prolonged pain and stiffness in younger patients. In addition, over 2 mm step-off or gap may also cause similar problems in elderly patients.39,40 Possible dorsal angulation may also have an effect on functional outcome. However, the role is not as clinically important as compared with the shortening of the radius.37,39,42–46
Wilcke and colleagues observed that when dorsal angulation was over 10° to 15°, radioulnar inclination was under 15° and the radius was shortened by more than 2 to 3 mm, the poor functional outcome was both statistically significant and clinically important in patients aged under 65 years when results were measured using Disabilities of Arm, Shoulder and Hand (DASH), Patient-Rated Wrist Evaluation (PRWE) and Visual Analogue Scale (VAS).41
Treatment
The aim of treatment is to recover function to a level as close as possible to the level preceding the fracture. Regardless of the treatment method, some patients will still have pain and stiffness to some extent. Unfortunately, the literature regarding non-operative treatment is both confusing and scarce. Traditionally, non-operative treatment has comprised the reduction of the fracture near to the anatomical position, followed by immobilization with a functional position cast for four to five weeks.47 However, a recent randomized controlled trial (RCT) shows no difference in three versus five weeks of cast treatment after minimally displaced DRF, although another recent RCT contradicts these findings.48,49 Periodical (every 1–2 weeks) radiographs have been used to check the fracture position. According to the current literature, the suggested acceptable values during non-operative treatment in persons younger than 65 years are as follows:
dorsal tilt less than 10° to 15°,
radioulnar inclination more than 15°,
radial shortening less than 2 to 4 mm and
joint gap or step-off less than 1 to 2 mm.50
These limit values are in accordance with the findings of previous studies for a good outcome.24–31
Operative techniques
Various surgical techniques have been described, such as dorsal plates, non-locking t-plates, fragment-specific plates, anatomical volar locking plates (VLP), external fixators, metal K-wires, nails and screws. Before the introduction of VLP, the most commonly used method was external fixation with or without K-wires. Surgery with VLP was introduced at the beginning of the 2000s, and it quickly gained popularity thereafter.51–57 The aim of locking plate surgery is to repair osteoporotic or comminute fractures by providing a stable construction that holds the joint in alignment until the fracture heals.52–55 It has been hypothesized that reducing the fracture to near the anatomical position would produce a superior functional outcome in the younger patient population.58 There have been a few studies that have compared percutaneous techniques (external fixator and K-wires) with VLP and reported similar functional outcomes in PRWE and DASH scores.59–61
Rehabilitation
The focus of DRF rehabilitation is to minimize the functional limitations caused by the fracture and treatment. Generally, the scientific evidence for rehabilitation protocols has been rated as very low quality, and thus most of the recommendations are based on expert opinions. In the latest Cochrane review of the rehabilitation of DRF, the authors stated that the evidence is insufficient to draw any conclusions and that further RCTs are warranted.62
In the majority of patients, DRF does not cause any limitations to daily activities and support, patient education, and supervision for active and passive mobilization exercises is sufficient in most cases. Patient education and supervision can be provided by physiotherapists or by the treating physician. Mobilization supervised by a physiotherapist is usually considered and applied when signs of prolonged immobilization, excessive oedema, complicated or comminuted fracture pattern, disproportioned pain, stiffness of the thumb and other fingers, increased activity of the sympathetic nervous system, pain, or the inability to use or fear of using the extremity, are present.
During the period of immobilization, both passive and active range-of-motion exercises should be initiated for the digits, elbow and shoulder. The aim is to prevent stiffness and reduce oedema. After cast removal, instructions for home exercises should be given. Additional therapy should be considered for patients with complications or serious functional impairment.
Patient-reported outcome measures and minimal clinical important difference
From the increased appreciation of patients’ subjective assessment of the health condition the need has risen for patient-reported outcome measures (PROMs). In DRF settings the most common PROMs (scale; number of questions) are PRWE (0–100; 15), DASH (0–100; 20), Quick-DASH (0–100; 11), and Pain (0–10; 1). While low in number, these scales enable the comparability of the results between studies.63 In the past there has been more frequent use of e.g. the Gartland and Werley measure, which combines subjective and objective assessments of the ability to function and of DRF.
Interpretation of PROMs is challenging and studies assessing the minimal clinical important differences (MCID) have become widely implemented, including in orthopaedic settings.64 There are two common methods to investigate MCID: the distributional approach (means and SDs of PROMs) and the anchor-based approach (relate the PROMs scores to external criterion at patient level).65 However, the estimates of MCID for particular PROMs have varied a lot between studies, partially explained by carrying methods, baseline of the PROMs score, disease condition and direction of the change in the health.64,66–68 In the clinical context of DRF, there seems to be little point in assessing PROMs scores at the time of the fracture, limiting patient-level comparisons between follow-up points. Thus, the mean scores of PROMs at different time-points have become a default way to interpret the results. Moreover, the patient-level MCID scores have become routinely used in the sample size calculations of orthopaedic clinical trials, while other factors could also have an influence.69
Distal radius fracture in elderly people
DRF is common in the elderly population with an incidence rate of between 200 and 1200 per 100,000 person-years.3,4 In patients aged over 65 years, distal radius fractures are the second most common fractures after hip fractures, and they account for almost one-fifth of all fractures in this age group.1,70 Of all DRFs, nearly 50% occur in patients aged over 65 years.1 After the age of 70 years, other fractures, such as proximal humerus and proximal femur fractures, become more common and the incidence rate of DRF decreases.1,2,70
In elderly patients, DRFs are typically caused by a fall from a standing height over an outstretched hand. Court-Brown et al reported that 80% of distal radius fractures are fall-related.2 Advanced age is one of the most important risk factors for DRF.1,70 As previously stated, patient age has been consistently shown to be a statistically significant predictor for loss of alignment in radiographs.25,28,34,35 However, it has been observed that persons aged over 65 years tolerate poorer radiological outcome rather well.41,46,71–78 and hence non-operative treatment with a cast has been suggested as the primary treatment modality. More recent randomized controlled trials have challenged this belief and suggest that it might be more beneficial to reduce the alignment with a volar locking plate (VLP) rather than non-operative treatment and reduce loss of reduction in people who are active and aged under 75 years.79,80 A recent systematic review by Mellstrand Navarro et al, however, found no differences in outcomes between different surgical techniques.81
The purpose of this article is to summarize the current evidence regarding DRFs and to closely review the latest RCTs that have compared operative and non-operative treatments options in elderly DRF patients.
Methods
We continued the literature search after the latest published systematic review of distal radius fracture by Mellstrand Navarro et al.81 The Patient, Intervention, Control, Outcome measure and Study design (PICOS) criteria used are presented in Table 1. The quality assessment was carried out for risk of bias after Furlan et al.82 A score of 6 points or more was regarded as indicating low risk of bias.
Table 1.
PICOS criteria for the search of relevant publications
| P | 60+, Distal radius fracture, follow-up minimum 1 year |
| I | Operative treatment with a volar locking plate |
| C | Non-operative treatment |
| O | Any functional or disability score |
| S | RCT |
Note. PICOS, Patient Intervention Control Outcome measure Study design; RCT, randomized controlled trial.
The clinical significance was regarded as each publication’s predefined MCID for each primary outcome. We compared the mean differences between study groups to these MCID estimates in the studies at one-year follow-up. When confidence interval (CI) of mean difference was not reported, CI was calculated from standard deviations (SD) of group means as described in Cochrane Handbook.83 Complications were recorded and reported for each trial.
Results
We found six RCTs published between 2011 and 2019 that compared locking plate surgery and non-operative treatment. The risk of bias was assessed following Furlan et al (Table 2).82 All of the publications had a low risk of bias, and therefore all of these trials were included. However, Bartl et al79 had a significant number of crossovers (42%) and, due to intention-to-treat principle, there is a risk of bias in the results.
Table 2.
Risk of bias after Furlan et al82
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arora et al, 201172 | yes | yes | no | no | no | yes | yes | unsure | yes | no | yes | yes | 7 |
| Bartl et al, 201479 | yes | yes | no | no | unsure | yes | yes | unsure | yes | no | no | yes | 6 |
| Martinez, 201880 | yes | yes | no | no | no | yes | yes | no | yes | no | yes | yes | 7 |
| Mulders et al, 201984 | yes | yes | no | no | no | yes | yes | no | yes | no | yes | yes | 7 |
| Sirniö et al, 201985 | yes | yes | no | no | n/a | yes | yes | no | yes | no | yes | yes | 7 |
| Saving et al, 201983 | yes | yes | no | no | no | yes | yes | no | yes | no | yes | yes | 7 |
1. Was the method of randomization adequate?
2. Was the treatment allocation concealed?
3. Was the patient blinded to the intervention?
4. Was the care provider blinded to the intervention?
5. Was the outcome assessor blinded to the intervention?
6. Was the drop-out rate described and acceptable?
7. Was intention-to-treat principle carried out?
8. All reports of the study free of suggestion of selective outcome reporting?
9. Were the groups similar at baseline regarding the most important prognostic indicators?
10. Were co-interventions avoided in all groups?
11. Was the compliance acceptable in all groups?
12. Was the timing of the outcome assessment similar in all groups?
Two studies included all except AO B type DRFs72,84 (AO A1–A3 and C1–C3), two studies included intra-articular fractures79,80 (AO C1–C3), one study included extra-articular fractures85 (A1–A3) and one study included all fractures except C3.86 The results of these trials are presented in Tables 3a, 3b and 3c.
Table 3a.
Basic characteristics of the trials
| Author, year | Patients in surgery group | Patients in non-surgery group | F-U time | Drop out % | Completed patients at F-U | Mean age surgery | Mean age non-surgery |
|---|---|---|---|---|---|---|---|
| Arora et al, 201172 | 45 | 45 | 12 Mo | 19% | 73 | 77 | 76 |
| Bartl et al, 201479 | 86 | 88 | 12 Mo | 14% | 149 | 74 | 75 |
| Martinez 201880 | 50 | 47 | 24 Mo | 0% | 97 | 70 | 67 |
| Mulders et al, 201984 | 47 | 43 | 12 Mo | 7% | 92 | 60 | 59 |
| Sirniö et al, 201985 | 38 | 42 | 24 Mo | 9% | 72 | 64 | 62 |
| Saving et al, 201983 | 68 | 72 | 12 Mo | 15% | 119 | 78 | 80 |
Note. F-U, follow-up; Mo, months.
Table 3b.
Mean scores according to PRWE over different time-points
| Publication | C 3M | I 3M | CI 3M | C 6M | I 6M | CI 6M | C 12M | I 12M | CI 12M | C 24M | I 24M | CI 24M | MCID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arora et al, 201172 | 54.4 | 33.7 | 5.1–31.2 | 31.4 | 27.7 | –11.4–18.8 | 14.6 | 12.8 | –8.9–12.5 | 10 | |||
| Bartl et al, 201479 | |||||||||||||
| Martinez 201880 | 30 | 17 | –21.3– –4.6 | 14 | |||||||||
| Mulders et al, 201984 | 32.5 | 11.0 | NA | 20.0 | 7.0 | NA | 10.0 | 4.0 | NA | 11.5 | |||
| Sirniö et al, 201985 | |||||||||||||
| Saving et al, 201983 | 34.2 | 20.6 | –21.5– –5.7 | 22.4 | 12.7 | –16.7– –2.7 | 10 |
Note. PRWE, Patient-Rated Wrist Evaluation; C, control; CI, confidence interval (95%) for mean difference; I, intervention; M, months; MCID, minimal clinical important difference.
Table 3c.
Mean scores according to DASH over different time-points
| Publication | C 3M | I 3M | CI 3M | C 6M | I 6M | CI 6M | C 12M | I 12M | CI 12M | C 24M | I 24M | CI 24M | MCID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arora et al, 201172 | 23.3 | 13.3 | 1.6–18.2 | 12.4 | 12.2 | –7.1–7.5 | 8.0 | 5.7 | –7.1–2.5 | 10 | |||
| Bartl et al, 201479 | 28.2 | 22.7 | –11.4–0.4 | 14 | 19.0 | –11.0–1.0 | 10 | ||||||
| Martinez 201880 | 28 | 16 | –19.5– –4.5 | ||||||||||
| Mulders et al, 201984 | 27.5 | 6.7 | NA | 14.2 | 5.8 | NA | 9.2 | 2.5 | NA | 10 | |||
| Sirniö et al, 201985 | 23.1 | 17.0 | –12.2–0.1 | 16.9 | 10.2 | –12.7–0.8 | 13.3 | 10.1 | –9.8–3.4 | 14.4 | 7.2 | –13–1.5 | 10 |
| Saving et al, 201983 | 30.2 | 21.2 | –16.2– –1.9 | NA | NA | 23.1 | 15.6 | –14.3– –0.7 | 10 |
Note. DASH, Disabilities of Arm, Shoulder and Hand; C, control; CI, confidence interval (95%) for mean difference; I, intervention; M, months;
MCID, minimal clinical important difference.
The mean age of patients in these studies varied between 59 and 80 years, and the follow-up was either 12 or 24 months. All studies used PRWE or DASH as the primary outcome measure. The mean values and SDs of PRWE and DASH differed between trials at 12 and 24 months, suggesting systematic differences between the study populations. The observed between-group mean difference at follow-up did not exceed the predefined MCID of the primary outcome at 12 or 24 months in any of the studies. MCID size of mean difference was included in 95 CI in four of five studies, as Mulders et al did not report SD or CI of means. Thus, the treatment effect over the MCID size to the benefit of operative treatment was compatible to the observed data in these four studies.
Several types of adverse events have been described in association with DRFs. Some are a consequence of the fracture itself and others are related to the treatment. Common complications reported are tendon problems, carpal tunnel syndrome, malunion, nonunion, infection related to surgery, complex regional pain syndrome (CRPS) and post-traumatic arthritis.87
The severe adverse events (SAE) reported in the reviewed studies are described in Table 4.
Table 4.
Severe adverse events (SAE)
| Loss of reduction/malunion warranting ORIF or corrective osteotomy | Implant removal due to malpositioning or other reason | Extensor tendon rupture/tenosynovitis | Flexor tendon rupture/tenosynovitis | Wound healing problem or infection | Carpal tunnel syndrome and/or release | Nerve injury | CRPS | Delayed uniond/ Nonunion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I |
| Arora et al, 201172 | NA | NA | 0/37 | 6/36 | 0/37 | 5/36 | NA | 4/36 | NA | NA | 0/37 | 1/36 | 0/37 | 0/36 | 5/37c | 2/36c | 6/37 | 2/36 |
| Bartl et al, 201479 | 37/90a | 0/84 | 2/90a | 4/90 | 1/90 | 0/84 | 1/90 | 0/84 | 0/90 | 1/84 | 2/90 | 1/84 | 3/90b | 0/84 | 1/90c | 0/84 | 0/90 | 0/90 |
| Martinez, 201880 | NA | NA | 0/47 | 1/50 | 0/47 | 1/50 | NA | NA | 0/47 | 0/50 | 0/47 | 1/50 | NA | NA | 1/47 | 0/50 | 0/47 | 1/50 |
| Mulders et al, 201984 | 18/60a | 0/59 | 1/60 | 9/59 | 5/60 | 5/59 | NA | NA | 1/60 | 3/59 | 3/60 | 0/59 | NA | NA | 4/60 | 1/59 | 0/60 | 1/59 |
| Sirniö et al, 201985 | 1/42 | 0/38 | NA | NA | NA | NA | 0/42 | 1/38 | NA | NA | 4/42 | 1/38 | NA | NA | NA | NA | NA | NA |
| Saving et al, 201983 | 3/63 | 0/56 | 0/63 | 4/56 | 0/63 | 0/56 | 0/63 | 3/56 | 0/63 | 2/56 | 5/63 | 2/56 | 5/63e | 7/56e | 2/63 | 0/56 | NA | NA |
|
Total
Mean %f |
59/255 23.0% (3.9%g) |
0/237 0.0% |
3/297 1.0% |
24/291 8.2% |
6/297 2.0% |
11/285 3.9% |
0/195 0.0% |
7/214 3.3% |
1/260 0.4% |
6/249 2.4% |
14/339 4.1% |
6/323 1.9% |
8/190 4.2% |
7/176 4.0% |
13/297 4.3% |
3/285 1.1% |
6/234 2.6% |
4/235 1.7% |
Note. ORIF, open reduction internal fixation; CRPS, complex regional pain syndrome; C, control; I, intervention; VLP, volar locking plates.
aAll were initially treated non-operatively but later operated with VLP due to loss of alignment during non-operative treatment. bAll neurological complications occurred after conversion in the surgical arm (two lesions of sensory radial nerve branches, one median nerve hypoesthesia). cCRPS type 1. dUnion not observed after six weeks in plain X-rays. eNerve numbness. fMean (%) of all complications. gCorrective osteotomies outside the initial treatment.
Discussion
None of the included trials observed between-group mean difference exceeding predefined MCID between VLP and non-operative treatment in primary outcomes at one year after treatment. However, most of the trials showed a small, statistically but not clinically significant benefit of operative treatment, and MCID was included in confidence intervals in four of five studies at the primary end-point. The surgery group had clinically and statistically better short-term results (3 and 6 months) but this effect diminished thereafter. We compared the mean difference between study groups to the pre-defined MCID, which was the basis of sample size calculation in included studies. In studies by Saving et al84 and Martinez-Mendez et al80 the included fractures were initially more severely displaced and there was a trend towards favourable results with VLP compared with non-operative treatment even at 12 months. There were some limitations but a low risk of bias in all of the analysed RCTs (Table 3). Results suggest that surgery yields statistically but not clinically better functional outcome at one-year follow-up. Treatment with VLP might benefit patients who have the need to gain the previous level of activity in a short period of time. However, it needs to be pointed out that the current literature does not provide an actual cut-off for age, fracture malalignment or other specific factors. Age stratification in current studies is lacking, and most challenging treatment decisions are among persons aged 65 to 74 years. In general, non-operative treatment can be considered to be the gold standard but shared decision-making between the patient and the treating physician is warranted.
According to the reviewed articles, the loss of acceptable fracture alignment seems to be the most common complication, affecting over 20% of the initially non-operatively treated patients. Most of these were caused by crossovers in the study by Bartl et al. The clinical significance of fracture malalignment is, however, unclear and in these trials only 4.2% of patients received corrective osteotomy within the follow-up period. In the surgery group, 8.6% of the patients required implant removal due to plate malpositioning or other reasons, such as extensor or flexor tendon rupture or tenosynovitis. Although it is known that problems with the plate may also appear later, in the reviewed studies, the follow-up of adverse events was limited to between 12 and 24 months. In operative treatment, extensor tendon rupture or synovitis are usually provoked by penetration of the distal screws through the dorsal cortex, whereas flexor tendon ruptures and tenosynovitis can be caused by the too distal positioning of the implant (distal to watershed line) irritating the flexor pollicis longus (FPL).88 These complications are at least partially avoidable with meticulous surgical technique. In addition, the Soong classification may help implant positioning, but tendon rupture is possible even after perfect plate positioning.88,89
Carpal tunnel syndrome (4.3% non-operative, 1.9% surgery) and CRPS (4.6% non-operative, 1.1% surgery) seems to be more common in non-operatively treated patients compared with surgery patients. However, as there was no reported severity scale for the conditions or predefined criteria for CRPS (such as International Association for Study of Pain – IASP criteria) we were not able to evaluate clinical significance of the observed difference nor make a meaningful statistical comparison between the groups.
Other outcome-affecting factors
Perfectly healed bone and good functional outcome do not necessarily correlate after DRF. About 20% of patients have reported some degree of symptom even years after DRF.90 Patients may report increased pain and loss of strength, which can be related to a fear of using the upper limb.90 In addition, depression, pain intensity and decreased motivation to do exercises may also have a significant effect on grip strength development, especially in elderly people.91–93 Furthermore, loss of grip strength has been shown in previous studies to define over 40% of the PRWE outcome two years after DRF, whereas the ROM of the joint with the same patients has not declined.94 Taking this into account, the treatment decision cannot be based solely on radiological parameters.
Strengths and limitations of this study
The strength of our review is the inclusion of the latest RCT studies with predefined PICOS, which were not included in the previous systematic review by Mellstrand Navarro et al.81 However, regardless of the findings of the six RCTs included in this study, we were not able to draw meaningful conclusions on limiting ages or give clear indications to distinguish between those patients suitable for operative treatment and those suitable for non-operative treatment. In addition, our study is not a rigorous meta-analysis, and, hence, there are several potential sources of bias in the included studies which may have an effect on the generalizability of the results. Firstly, there is a significant disparity in the types of included fractures (see Results section). However, it appears that the differences in the fracture morphology have little effect on the estimates of mean differences between the studies analysed. Secondly, some of the studies (such as Bartl et al79 and Mulders et al85) have a significant crossover from initial non-operative treatment to operative treatment which may have effect on the final outcome estimates. Thirdly, in Arora’s widely referenced study, the non-operatively treated patients healed radiographically in relatively well-aligned position (only 10.4° of dorsal angulation), which calls into question the heterogeneity of the included fractures.72
In conclusion, we found no clear evidence of the clinical superiority of distal radius fracture surgery among older adults at one-year follow-up. However, younger, more active individuals may benefit from surgery in terms of a faster recovery to previous levels of activity. In operative treatment, hardware-based problems may warrant secondary operations and implant removal, whereas in non-operative treatment, symptomatic loss of alignment and malunion can occur, and neurological deficits, such as carpal tunnel syndrome and CRPS, may be present. In elderly patients, non-operative treatment can be considered to be the gold standard, accepting slower return to former activities in some cases.
Footnotes
ICMJE Conflict of interest statement: MKL reports travel expenses to orthopaedic conference from Stryker, outside the submitted work.
APL reports support for travel to meetings for study or other purposes from Tampere University Hospital, relevant to the submitted work.
The other authors declare no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37:691–697. [DOI] [PubMed] [Google Scholar]
- 2. Court-Brown CM, Clement ND, Duckworth AD, Biant LC, McQueen MM. The changing epidemiology of fall-related fractures in adults. Injury 2017;48:819–824. [DOI] [PubMed] [Google Scholar]
- 3. Flinkkilä T, Sirniö K, Hippi M, et al. Epidemiology and seasonal variation of distal radius fractures in Oulu, Finland. Osteoporos Int 2011;22:2307–2312. [DOI] [PubMed] [Google Scholar]
- 4. Brogren E, Petranek M, Atroshi I. Incidence and characteristics of distal radius fractures in a southern Swedish region. BMC Musculoskelet Disord 2007;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lofthus CM, Frihagen F, Meyer HE, Nordsletten L, Melhuus K, Falch JA. Epidemiology of distal forearm fractures in Oslo, Norway. Osteoporos Int 2008;19:781–786. [DOI] [PubMed] [Google Scholar]
- 6. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radial fractures: a case-control study. J Bone Joint Surg Am 2011;93:348–356. [DOI] [PubMed] [Google Scholar]
- 7. Falch JA. Epidemiology of fractures of the distal forearm in Oslo, Norway. Acta Orthop Scand 1983;54:291–295. [DOI] [PubMed] [Google Scholar]
- 8. Kaukonen JP. Fractures of the distal forearm in the Helsinki district. Ann Chir Gynaecol 1985;74:19–21. [PubMed] [Google Scholar]
- 9. Solgaard S, Petersen VS. Epidemiology of distal radius fractures. Acta Orthop Scand 1985;56:391–393. [DOI] [PubMed] [Google Scholar]
- 10. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand 1990;61:457–459. [DOI] [PubMed] [Google Scholar]
- 11. Cameron ID, Murray GR, Gillespie LD, et al. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev 2010;1:CD005465. [DOI] [PubMed] [Google Scholar]
- 12. Compston JE, Watts NB, Chapurlat R, et al. ; Glow Investigators. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catalano LW, III, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J., Jr Displaced intra-articular fractures of the distal aspect of the radius: long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am 1997;79:1290–1302. [DOI] [PubMed] [Google Scholar]
- 14. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am 1994;19:720–727. [DOI] [PubMed] [Google Scholar]
- 15. Cooney WP, III, Dobyns JH, Linscheid RL. Complications of Colles’ fractures. J Bone Joint Surg Am 1980;62:613–619. [PubMed] [Google Scholar]
- 16. Leversedge FJ, Srinivasan RC. Management of soft-tissue injuries in distal radius fractures. Hand Clin 2012;28:225–233. [DOI] [PubMed] [Google Scholar]
- 17. Lindau T. Arthroscopic evaluation of associated soft tissue injuries in distal radius fractures. Hand Clin 2017;33:651–658. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida S, Yoshida K, Sakai K, Nakama K, Shiba N. Frequency of scapholunate ligament injuries associated with distal radius shearing fracture: correlation of fracture patterns and ligament tear. Hand Surg 2015;20:440–446. [DOI] [PubMed] [Google Scholar]
- 19. Desai MJ, Kamal RN, Richard MJ. Management of intercarpal ligament injuries associated with distal radius fractures. Hand Clin 2015;31:409–416. [DOI] [PubMed] [Google Scholar]
- 20. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function: a clinical and experimental study. Acta Orthop Scand 1967;suppl 108:3. [DOI] [PubMed] [Google Scholar]
- 21. Fernández DL. Fractures of the distal radius: operative treatment. Instr Course Lect 1993;42:73–88. [PubMed] [Google Scholar]
- 22. Muller ME, Nazarian SN, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. 1. 14th ed. Heidelberg: Springer-Verlag, 1990. [Google Scholar]
- 23. Luokkala T, Flinkkilä T, Paloneva J, Karjalainen TV. Comparison of expert opinion, majority rule, and a clinical prediction rule to estimate distal radius malalignment. J Orthop Trauma 2018;32:e97–e101. [DOI] [PubMed] [Google Scholar]
- 24. Wadsten MA, Sayed-Noor AS, Englund E, Buttazzoni GG, Sjödén GO. Cortical comminution in distal radial fractures can predict the radiological outcome: a cohort multicentre study. Bone Joint J 2014;96-B:978–983. [DOI] [PubMed] [Google Scholar]
- 25. Mackenney PJ, McQueen MM, Elton R. Prediction of instability in distal radial fractures. J Bone Joint Surg Am 2006;88:1944–1951. [DOI] [PubMed] [Google Scholar]
- 26. Nesbitt KS, Failla JM, Les C. Assessment of instability factors in adult distal radius fractures. J Hand Surg Am 2004;29:1128–1138. [DOI] [PubMed] [Google Scholar]
- 27. Makhni EC, Taghinia A, Ewald T, Zurakowski D, Day CS. Comminution of the dorsal metaphysis and its effects on the radiographic outcomes of distal radius fractures. J Hand Surg Eur Vol 2010;35:652–658. [DOI] [PubMed] [Google Scholar]
- 28. Tahririan MA, Javdan M, Nouraei MH, Dehghani M. Evaluation of instability factors in distal radius fractures. J Res Med Sci 2013;18:892–896. [PMC free article] [PubMed] [Google Scholar]
- 29. Leone J, Bhandari M, Adili A, McKenzie S, Moro JK, Dunlop RB. Predictors of early and late instability following conservative treatment of extra-articular distal radius fractures. Arch Orthop Trauma Surg 2004;124:38–41. [DOI] [PubMed] [Google Scholar]
- 30. Walenkamp MM, Aydin S, Mulders MA, Goslings JC, Schep NW. Predictors of unstable distal radius fractures: a systematic review and meta-analysis. J Hand Surg Eur Vol 2016;41:501–515. [DOI] [PubMed] [Google Scholar]
- 31. Walenkamp MM, Vos LM, Strackee SD, Goslings JC, Schep NW. The unstable distal radius fracture – how do we define it? A systematic review. J Wrist Surg 2015;4:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Altissimi M, Mancini GB, Azzarà A, Ciaffoloni E. Early and late displacement of fractures of the distal radius: the prediction of instability. Int Orthop 1994;18:61–65. [DOI] [PubMed] [Google Scholar]
- 33. Wichlas F, Haas NP, Lindner T, Tsitsilonis S. Closed reduction of distal radius fractures: does instability mean irreducibility? Arch Orthop Trauma Surg 2013;133:1073–1078. [DOI] [PubMed] [Google Scholar]
- 34. Abbaszadegan H, Jonsson U, von Sivers K. Prediction of instability of Colles’ fractures. Acta Orthop Scand 1989;60:646–650. [DOI] [PubMed] [Google Scholar]
- 35. Makhni EC, Ewald TJ, Kelly S, Day CS. Effect of patient age on the radiographic outcomes of distal radius fractures subject to nonoperative treatment. J Hand Surg Am 2008;33:1301–1308. [DOI] [PubMed] [Google Scholar]
- 36. Karnezis IA, Panagiotopoulos E, Tyllianakis M, Megas P, Lambiris E. Correlation between radiological parameters and patient-rated wrist dysfunction following fractures of the distal radius. Injury 2005;36:1435–1439. [DOI] [PubMed] [Google Scholar]
- 37. Leung F, Ozkan M, Chow SP. Conservative treatment of intra-articular fractures of the distal radius: factors affecting functional outcome. Hand Surg 2000;5:145–153. [DOI] [PubMed] [Google Scholar]
- 38. Nelson GN, Stepan JG, Osei DA, Calfee RP. The impact of patient activity level on wrist disability after distal radius malunion in older adults. J Orthop Trauma 2015;29:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trumble TE, Schmitt SR, Vedder NB. Factors affecting functional outcome of displaced intra-articular distal radius fractures. J Hand Surg Am 1994;19:325–340. [DOI] [PubMed] [Google Scholar]
- 40. Young BT, Rayan GM. Outcome following nonoperative treatment of displaced distal radius fractures in low-demand patients older than 60 years. J Hand Surg Am 2000;25:19–28. [DOI] [PubMed] [Google Scholar]
- 41. Wilcke MK, Abbaszadegan H, Adolphson PY. Patient-perceived outcome after displaced distal radius fractures: a comparison between radiological parameters, objective physical variables, and the DASH score. J Hand Ther 2007;20:290–298. [DOI] [PubMed] [Google Scholar]
- 42. Grewal R, MacDermid JC. The risk of adverse outcomes in extra-articular distal radius fractures is increased with malalignment in patients of all ages but mitigated in older patients. J Hand Surg Am 2007;32:962–970. [DOI] [PubMed] [Google Scholar]
- 43. Batra S, Gupta A. The effect of fracture-related factors on the functional outcome at 1 year in distal radius fractures. Injury 2002;33:499–502. [DOI] [PubMed] [Google Scholar]
- 44. Baruah RK, Islam M, Haque R. Immobilisation of extra-articular distal radius fractures (Colles type) in dorsiflexion: the functional and anatomical outcome. J Clin Orthop Trauma 2015;6:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture: a prospective review. J Bone Joint Surg Br 1987;69:635–638. [DOI] [PubMed] [Google Scholar]
- 46. McQueen MM, Hajducka C, Court-Brown CM. Redisplaced unstable fractures of the distal radius: a prospective randomised comparison of four methods of treatment. J Bone Joint Surg Br 1996;78:404–409. [PubMed] [Google Scholar]
- 47. Handoll HH, Madhok R. Conservative interventions for treating distal radial fractures in adults. Cochrane Database Syst Rev 2003;2:CD000314. [DOI] [PubMed] [Google Scholar]
- 48. Bentohami A, van Delft EAK, Vermeulen J, et al. Non- or minimally displaced distal radial fractures in adult patients: three weeks versus five weeks of cast immobilization – a randomized controlled trial. J Wrist Surg 2019;8:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christersson A, Larsson S, Sandén B. Clinical outcome after plaster cast fixation for 10 days versus 1 month in reduced distal radius fractures: a prospective randomized study. Scand J Surg 2018;107:82–90. [DOI] [PubMed] [Google Scholar]
- 50. Current Care Guidelines. Working group appointed by the Finnish Medical Society Duodecim the Finnish Society for Surgery of Hand and the Finnish Orthopaedic Association, 2016. www.kaypahoito.fi. (date last accessed 6 September 2019).
- 51. Hevonkorpi TP, Launonen AP, Huttunen TT, Kannus P, Niemi S, Mattila VM. Incidence of distal radius fracture surgery in Finns aged 50 years or more between 1998 and 2016: too many patients are yet operated on? BMC Musculoskelet Disord 2018;19:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drobetz H, Kutscha-Lissberg E. Osteosynthesis of distal radial fractures with a volar locking screw plate system. Int Orthop 2003;27:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg 2005;13:159–171. [DOI] [PubMed] [Google Scholar]
- 54. Musgrave DS, Idler RS. Volar fixation of dorsally displaced distal radius fractures using the 2.4-mm locking compression plates. J Hand Surg Am 2005;30:743–749. [DOI] [PubMed] [Google Scholar]
- 55. Wilcke MK, Hammarberg H, Adolphson PY. Epidemiology and changed surgical treatment methods for fractures of the distal radius: a registry analysis of 42,583 patients in Stockholm County, Sweden, 2004–2010. Acta Orthop 2013;84:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma 2011;71:939–942. [DOI] [PubMed] [Google Scholar]
- 57. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am 2009;91:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tarallo L, Mugnai R, Adani R, Catani F. Malunited extra-articular distal radius fractures: corrective osteotomies using volar locking plate. J Orthop Traumatol 2014;15:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Esposito J, Schemitsch EH, Saccone M, Sternheim A, Kuzyk PR. External fixation versus open reduction with plate fixation for distal radius fractures: a meta-analysis of randomised controlled trials. Injury 2013;44:409–416. [DOI] [PubMed] [Google Scholar]
- 60. Costa ML, Achten J, Parsons NR, et al. ; DRAFFT Study Group. Percutaneous fixation with Kirschner wires versus volar locking plate fixation in adults with dorsally displaced fracture of distal radius: randomised controlled trial. BMJ 2014;349:g4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mellstrand Navarro C, Ahrengart L, Törnqvist H, Ponzer S. Volar locking plate or external fixation with optional addition of K-wires for dorsally displaced distal radius fractures: a randomized controlled study. J Orthop Trauma 2016;30:217–224. [DOI] [PubMed] [Google Scholar]
- 62. Handoll HH, Elliott J. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev 2015;9:CD003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Changulani M, Okonkwo U, Keswani T, Kalairajah Y. Outcome evaluation measures for wrist and hand: which one to choose? Int Orthop 2008;32:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hao Q, Devji T, Zeraatkar D, et al. Minimal important differences for improvement in shoulder condition patient-reported outcomes: a systematic review to inform a BMJ rapid recommendation. BMJ Open 2019;9:e028777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:171–184. [DOI] [PubMed] [Google Scholar]
- 66. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90–94. [DOI] [PubMed] [Google Scholar]
- 67. Wang YC, Hart DL, Stratford PW, Mioduski JE. Baseline dependency of minimal clinically important improvement. Phys Ther 2011;91:675–688. [DOI] [PubMed] [Google Scholar]
- 68. Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life: a systematic review. J Clin Epidemiol 2017;89:188–198. [DOI] [PubMed] [Google Scholar]
- 69. Cook JA, Julious SA, Sones W, et al. DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. Trials 2018;19:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology 1996;7:612–618. [DOI] [PubMed] [Google Scholar]
- 71. Abbaszadegan H, Jonsson U. External fixation or plaster cast for severely displaced Colles’ fractures? Prospective 1-year study of 46 patients. Acta Orthop Scand 1990;61:528–530. [DOI] [PubMed] [Google Scholar]
- 72. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am 2011;93:2146–2153. [DOI] [PubMed] [Google Scholar]
- 73. Azzopardi T, Ehrendorfer S, Coulton T, Abela M. Unstable extra-articular fractures of the distal radius: a prospective, randomised study of immobilisation in a cast versus supplementary percutaneous pinning. J Bone Joint Surg Br 2005;87:837–840. [DOI] [PubMed] [Google Scholar]
- 74. Howard PW, Stewart HD, Hind RE, Burke FD. External fixation or plaster for severely displaced comminuted Colles’ fractures? A prospective study of anatomical and functional results. J Bone Joint Surg Br 1989;71:68–73. [DOI] [PubMed] [Google Scholar]
- 75. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg 2004;93:64–67. [DOI] [PubMed] [Google Scholar]
- 76. Roumen RM, Hesp WL, Bruggink ED. Unstable Colles’ fractures in elderly patients: a randomised trial of external fixation for redisplacement. J Bone Joint Surg Br 1991;73:307–311. [DOI] [PubMed] [Google Scholar]
- 77. Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement: a prospective, randomised study using Norian SRS. J Bone Joint Surg Br 2000;82:856–863. [DOI] [PubMed] [Google Scholar]
- 78. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma 2006;20:115–121. [DOI] [PubMed] [Google Scholar]
- 79. Bartl C, Stengel D, Bruckner T, Gebhard F, ORCHID Study Group. The treatment of displaced intra-articular distal radius fractures in elderly patients. Dtsch Arztebl Int 2014;111:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martinez-Mendez D, Lizaur-Utrilla A, de-Juan-Herrero J. Intra-articular distal radius fractures in elderly patients: a randomized prospective study of casting versus volar plating. J Hand Surg Eur Vol 2018;43:142–147. [DOI] [PubMed] [Google Scholar]
- 81. Mellstrand Navarro C, Brolund A, Ekholm C, et al. Treatment of radius or ulna fractures in the elderly: a systematic review covering effectiveness, safety, economic aspects and current practice. PLoS One 2019;14:e0214362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Furlan AD, Pennick V, Bombardier C, van Tulder M; Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929–1941. [DOI] [PubMed] [Google Scholar]
- 83. Cumpston M, Li T, Page MJ, Chandler J, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saving J, Severin Wahlgren S, Olsson K, et al. Nonoperative treatment compared with volar locking plate fixation for dorsally displaced distal radial fractures in the elderly: a randomized controlled trial. J Bone Joint Surg Am 2019;101:961–969. [DOI] [PubMed] [Google Scholar]
- 85. Mulders MAM, Walenkamp MMJ, van Dieren S, Goslings JC, Schep NWL, VIPER Trial Collaborators. Volar plate fixation versus plaster immobilization in acceptably reduced extra-articular distal radial fractures: a multicenter randomized controlled trial. J Bone Joint Surg Am 2019;101:787–796. [DOI] [PubMed] [Google Scholar]
- 86. Sirniö K, Leppilahti J, Ohtonen P, Flinkkilä T. Early palmar plate fixation of distal radius fractures may benefit patients aged 50 years or older: a randomized trial comparing 2 different treatment protocols. Acta Orthop 2019;90:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seigerman D, Lutsky K, Fletcher D, et al. Complications in the management of distal radius fractures: how do we avoid them? Curr Rev Musculoskelet Med 2019;12:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Azzi AJ, Aldekhayel S, Boehm KS, Zadeh T. Tendon rupture and tenosynovitis following internal fixation of distal radius fractures: a systematic review. Plast Reconstr Surg 2017;139:717e–724e. [DOI] [PubMed] [Google Scholar]
- 89. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am 2011;36:3–9. [DOI] [PubMed] [Google Scholar]
- 90. Steven JL, Buer N, Samuelsson L, Harms-Ringdahl K. Pain-related fear, catastrophizing and pain in the recovery from a fracture. Scand J Pain 2010;1:38–42. [DOI] [PubMed] [Google Scholar]
- 91. van Lier AM, Payette H. Determinants of handgrip strength in free-living elderly at risk of malnutrition. Disabil Rehabil 2003;25:1181–1186. [DOI] [PubMed] [Google Scholar]
- 92. van Milligen BA, Lamers F, de Hoop GT, Smit JH, Penninx BW. Objective physical functioning in patients with depressive and/or anxiety disorders. J Affect Disord 2011;131:193–199. [DOI] [PubMed] [Google Scholar]
- 93. Phillips HJ, Biland J, Costa R, Souverain R. Five-position grip strength measures in individuals with clinical depression. J Orthop Sports Phys Ther 2011;41:149–154. [DOI] [PubMed] [Google Scholar]
- 94. Karnezis IA, Fragkiadakis EG. Association between objective clinical variables and patient-rated disability of the wrist. J Bone Joint Surg Br 2002;84:967–970. [DOI] [PubMed] [Google Scholar]


