Abstract
Recently, checkpoint inhibition of the PD-1/PD-L1 axis has been shown to be therapeutically relevant in urothelial carcinoma (UC). To evaluate the predictive and prognostic value of PD-L1 on response and survival in UC patients after cystectomy, chemotherapy, or anti-PD-1/PD-L1 therapy, a systematic review of PubMed, Embase, Web of Science, and the Cochrane Library was performed. A total of 2154 patients from 14 published studies were included. In all UC patients after cystectomy, tumour cell (TC) PD-L1 expression was not associated with the OS or PFS. For the subset of patients with organ-confined disease, TC PD-L1 expression significantly predicted OS after cystectomy (P = 0.0004). There was no significant evidence of an association between TC PD-L1 status and ORR or OS for UC patients treated with platinum-based chemotherapy. For UC patients treated with anti-PD-1/PD-L1 therapy, TC PD-L1 expression ≥ 5% could predict the response (P = 0.005), but not for the 1% cut-off (P ≥ 0.05). As for PD-L1 expression in tumour-inflating immune cells (TIICs), both subsets with IC2/3 vs. IC0/1 and IC1/2/3 vs. IC0 were associated with ORR to anti-PD-1/PD-L1 therapy. In the TIIC subset, IC2/3 vs. IC0/1 of PD-L1 was associated with higher CR (P = 0.002), PR (P = 0.04), and PD (P = 0.007). Further, higher TIIC PD-L1 status benefited from longer PFS (P < 0.001), but was not associated with OS in UC patients with anti-PD-1/PD-L1 therapy. Our study suggested that TIIC PD-L1 expression with 5% cut-off was valuable as a predictive and prognostic biomarker for ORR and PFS in UC patients with anti-PD-1/PD-L1 therapy.
1. Introduction
Urothelial carcinoma (UC) is regarded as an aggressive tumour, with unfavorable clinical survival in advanced stages and metastatic diseases. Radical cystectomy (RC) is the gold-standard treatment for muscle-invasive organ-confined UC, providing efficacy of local control and better disease-free survival (DFS) [1, 2]. With high expression level of programmed death-ligand 1 (PD-L1), UC appears to be immunogenic, demonstrating the potential value of PD-L1 as a promising biomarker in UC after RC [3]. Although the upregulated PD-L1 was associated with tumour-infiltrating immune cell (TIIC) response and advanced disease, clinical efficiency for UC patient survival was characterized by different degrees of uncertainty [4].
Patients with metastatic UC usually had a poor prognosis; perioperative cisplatin-based chemotherapy in addition to RC could benefit a response of 50% and prolong survival [5]. However, Tsao and colleagues performed a meta-analysis raising serious doubt about the predictive value of PD-L1 expression in prognosis and response for adjuvant chemotherapy in early stage non-small cell lung cancer (NSCLC). They indicated that PD-L1 status showed neither prognostic nor predictive value of benefits from adjuvant chemotherapy in patients with partial pneumonectomy [6]. Therefore, it remained contentious whether PD-L1 could serve as a valuable biomarker in UC patients with adjuvant chemotherapy.
Recently, blocking immune checkpoints with anti-PD-1/PD-L1 monoclonal antibodies has demonstrated promising clinical efficacy for advanced UC [7]. The effect of PD-1 on T-cells with its ligand PD-L1 on tumour cell and immune cell interaction inhibited the function of effector T-cells [8]; therefore, tumours could escape from T-cell regulated immune response by blocking the PD-1/PD-L1 signaling pathway [9]. PD-1/PD-L1 inhibitors have shown survival benefits in various advanced cancers, including melanoma, lymphoma, NSCLC, renal cell carcinoma, and UC [9–12]. PD-L1 status has been demonstrated to significantly correlate with response and survival improvement from anti-PD-1/PD-L1 immunotherapy in UC patients [13], while there is no convincing evidence whether PD-L1 expression in tumour cells (TCs) or TIICs with a cut-off value of 5% or 1% could predict the prognosis and response.
To clarify the available evidence, we conducted this meta-analysis of eligible literatures to determine the predictive and prognostic significance of PD-L1 expression in UC patients receiving cystectomy, chemotherapy, or anti-PD-1/PD-L1 immunotherapy.
2. Material and Methods
2.1. Search Strategy
All methods for this systematic review and meta-analysis are outlined in a prospectively registered protocol available online (PROSPERO identifier CRD42019130411). Our meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. Studies published before January 2019 were electronically retrieved from the PubMed, Web of Science, Embase, and Cochrane Library databases. The following terms were used: urothelial carcinoma or urothelial tumour or urothelial neoplasm or bladder cancer or bladder tumour; PD-L1 or programmed cell death ligand 1 or B7-H1 or CD274. The reference lists were also screened to obtain other eligible studies by correspondence with study investigators. Each study was evaluated independently by two reviewers for the inclusion. Any disagreement in the articles was resolved by discussing with a third reviewer. Figure 1 shows the flow diagram of the study selection.
Figure 1.
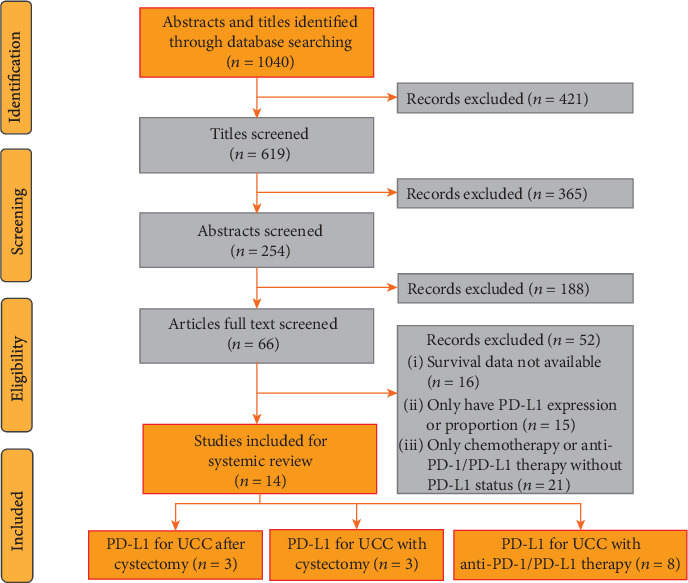
Flow diagram for study selection.
2.2. Selection Criteria
Included publications should satisfy the following criteria: (1) The studies reported PD-L1 expression on urothelial carcinoma. (2) The results showed the association of PD-L1 status and any of the following outcomes: objective response rate, PFS or OS after cystectomy, and chemotherapy or anti-PD-1/PD-L1 immunotherapy. (3) Only clinical trials, including prospective or retrospective cohort studies or comparative series, were eventually enrolled. Exclusion criteria were the following: (1) studies only reported the PD-L1 expression in urothelial carcinoma patients; (2) studies only studied the molecular mechanism of PD-L1 and its biological function in bladder cancer; (3) animal or in vitro studies; (4) studies did not report or no data available on response rate, PFS, or OS; and (5) articles not in English, case reports, comments, letters, editorials, congress reports, and review papers. When several papers from the same center were available, the one with the newest information, the longest follow-up, and the most participants was finally included in our meta-analysis.
2.3. Data Extraction and Study Quality
The following data was extracted independently by two trained reviewers (TK and LHR) using a prior-designed form: last name of authors; publication year; study design; country; participants' inclusion and exclusion criteria; mean or median age of participants; sample size; tumour stage; PD-L1 status; assigned to treatment with cystectomy, chemotherapy, or anti-PD-1/PD-L1 immunotherapy; length of follow-up; and primary endpoints including response rate, PFS, and OS. Missing, unclear, but important supplementary data were requested from primary study authors. All discrepancies were adjudicated by a third reviewer and solved by discussion (YZQ). RevMan software version 5.3. (Cochrane, London, UK) was used to perform risk of bias graph and summary.
2.4. Statistical Analysis
All data analysis was conducted using RevMan software version 5.3. Efficacy data from assigned patients were calculated on an intention-to-treat basis in all enrolled studies [15]. The concerned endpoints included the response rates, PFS, and OS. In terms of both OS and PFS, the pooled HRs and their 95% CI were calculated. A subgroup analysis was performed for patients receiving anti-PD-1/PD-L1 immunotherapy by PD-L1 cut-off value (IC2/3 vs. IC0/1 or IC1/2/3 vs. IC0). Statistical heterogeneity was quantified by the Q and I2 tests. Based on the absence or presence of interstudy heterogeneity, pooled odds ratio (OR) and hazard ratio (HR) estimates were obtained by use of a fixed or random effects model. P values < 0.05 indicated statistical significance.
3. Results
3.1. Baseline Characteristics
Fourteen publications were selected for inclusion; three trials reported the association of PD-L1 status and concerned endpoints in UC patients after cystectomy, three trials reported platinum-based chemotherapy, and eight trials reported anti-PD-1/PD-L1 immunotherapy (Figure 1). All the included studies detected PD-L1 expression by immunohistochemistry (IHC), eight studies only detected the PD-L1 expression in tumour cells (TCs), four trials only in tumour-inflating immune cells (TIICs), and two studies measured PD-L1 in both locations. Three studies recruited UC patients after cystectomy, and one study was excluded without reporting survival HR value and 95% CI [16]. Two of three studies evaluated PD-L1 expression both in all UC patients and organ-confined disease. Three studies reported OS, and two showed CSS and DFS (Table 1). The baseline characteristics of three studies that reported chemotherapy are outlined in Table 2, and all three studies were about platinum-based preoperative treatment. For patients receiving anti-PD-1/PD-L1 immunotherapy, most of the published trials set 1% or 5% as the cut-off value, and one study was excluded with a cut-off value of 25% [17]. Among the eight trials, only one study was prospective but nonrandomized, and the others were multicenter RCTs. Four studies used atezolizumab, two used nivolumab, one used avelumab, and one used pembrolizumab. PD-L1-positive proportion has been noted ranging from 10.8% to 46.2% (5% cut-off) and 37.3% to 81.5% (1% cut-off) in UC patients. The ORR to anti-PD-1/PD-L1 immunotherapy in UC patients with PD-L1-positive expression ranged from 26.0% to 43.3% (5% cut-off) and 17.9% to 30.2% (1% cut-off) (Tables 3 and 4).
Table 1.
Characteristics of the included studies on PD-L1 status predicting the prognosis in UC patients after cystectomy.
| Study | Boorjian et al. (2008) [4] | Wang et al. (2009) [18] | Xylinas et al. (2014) [19] |
|---|---|---|---|
| Country | USA | China | USA |
| Study interval | 1990-1994 | 2000-2002 | 1988-2003 |
| Age (years) | 69 (37-90) | 62 (42-78) | 66 (61-72) |
| Male/female | 259/59 | 40/10 | 244/58 |
| Management | RC | RC | RC |
| PD-L1 expression | Tumour cells | Tumour cells | Tumour cells |
| Detection method | IHC | IHC | IHC |
| Cut-off value | 5% | 10% | 5% |
| Follow-up (mons.) | 164 (1-210) | 28 (6-52) | 120 (78-125) |
| Clinical outcomes | Receipt of BCG, tumour stage, TIL | Tumour grade, tumour stage, recurrent UC | None∗ |
| All UC patients | |||
| PD-L1+ | 12.4% | 72.0% | 25.2% |
| PD-L1+/- | 39/275 | 36/14 | 76/226 |
| OS, HR(95% CI) | 1.06(0.71-1.58) | 2.24(1.16-4.38) | 0.98(0.73-1.31) |
| P value | 0.88 | 0.01 | 0.79 |
| CSS, HR(95% CI) | 0.82(0.42-1.63) | na | 0.79(0.53-1.61) |
| P value | 0.23 | na | 0.58 |
| DFS, HR(95% CI) | 0.83(0.43-1.58) | na | 0.74(0.50-1.11) |
| P value | 0.15 | na | 0.56 |
| Organ-confined disease | na | ||
| PD-L1+ | 16.2% | na | 25.0% |
| PD-L1+/- | 27/140 | na | 24/72 |
| OS, HR(95% CI) | 2.18(1.26-3.77) | na | 1.93(1.09-3.43) |
| P value | 0.02 | na | 0.005 |
| CSS, HR(95% CI) | 1.59(0.56-4.49) | na | 1.21(0.47-3.13) |
| P value | 0.68 | na | 0.38 |
| DFS, HR(95% CI) | 1.24(0.44-3.45) | na | 1.27(0.50-3.26) |
| P value | 0.62 | na | 0.69 |
UC: urothelial carcinoma; PD-L1: programmed death-ligand 1; +/-: positive/negative; RC: radical cystectomy; TIL: tumour-inflating lymphocyte; IHC: immunohistochemistry; BCG: Bacillus Calmette-Guerin; OS: overall survival; CSS: cancer-specific survival; DFS: disease-free survival; HR: hazard ratio; CI: confidence interval; na: data not available. ∗No association of PD-L1 expression with clinicopathologic features.
Table 2.
Characteristics of the included studies on predictive and prognostic value of PD-L1 status in UC patients treated with platinum-based chemotherapy.
| Study | Baras et al. (2016) [21] | Bellmunt et al. (2015) [3] | Erlmeier et al. (2016) [20] |
|---|---|---|---|
| Country | USA | USA | Germany |
| UC patients | MIUC | Metastatic UC | Locally advanced |
| Tumour stage | All stage | pT2-T4 | pT3/pT4 |
| Management | TURBT | TURBT, RC | RC |
| Chemotherapy | Neoadjuvant platinum-based | Adjuvant platinum-based | Adjuvant platinum-based |
| PD-L1 expression | Tumour cells | Tumour cells/TIICs | Tumour cells |
| Detection method | IHC | IHC | IHC |
| Cut-off value | 1%, 5% | 5% | 10% |
| PD-L1+ | 20.6% | 16.3% | 35.5% |
| PD-L1+/- | 7/34 | 14/86 | 11/31 |
| ORR (PD-L1+/-) | 42.9%/38.2% | na | 37.5%/35.7% |
| OS rate (PD-L1+/-) | na | 64.3%/39.5% | 54.5%/51.6% |
UC: urothelial carcinoma; MIUC: muscle-invasive UC; PD-L1: programmed death-ligand 1; +/-: positive/negative; TURBT: transurethral resection of bladder tumour; RC: radical cystectomy; TIICs: tumour-inflating immune cells; IHC: immunohistochemistry; ORR: objective response rate; OS: overall survival; pT: physical stage; na: data not available.
Table 3.
Characteristics of the included studies on predictive and prognostic value of tumour-infiltrating immune cell PD-L1 status in UC patients treated with anti-PD-1/PD-L1 therapy.
| Study | Balar et al. (2017) [13] | Powles et al. (2014) [24] | Rosenberg et al. (2016) [28] | Petrylak et al. (2018) [27] |
|---|---|---|---|---|
| Trial name | IMvigor210 | PCD4989g | NCT02108652 | NCT01375842 |
| Study design | MRCT | MRCT | MRCT | MRCT |
| Trial phase | Phase 2 | Phase 1 | Phase 2 | Phase 1 |
| Study interval | 2014-2015 | 2011-2013 | May 2014-Nov 2014 | Mar 2013-Aug 2015 |
| UC patients | Locally advanced and metastatic UC | Metastatic UC | Locally advanced and metastatic UC | Metastatic UC |
| Age (years) | 73 (51-92) | 65 (36-86) | 66 (32-91) | 66 (36-89) |
| Male/female | 96/23 | 46/19 | 241/69 | 72/23 |
| Immunotherapy | Atezolizumab | Atezolizumab | Atezolizumab | Atezolizumab |
| Target | Anti-PD-L1 | Anti-PD-L1 | Anti-PD-L1 | Anti-PD-L1 |
| Treatment | 1200 mg, iv every 3 weeks | 15 mg/kg, iv every 3 weeks | 1200 mg, iv every 3 weeks | 15 mg/kg, iv every 3 weeks |
| PD-L1 expression | TIICs | Tumour cells/TIICs | TIICs | TIICs |
| Detection method | IHC | IHC | IHC | IHC |
| Cut-off value | 1%, 5% | 1%, 5% | 1%, 5% | 5% |
| PD-L1+ (5%/1%) | 67.2%/26.9% | 81.5%/46.2% | 66.8%/32.3% | 52.6% |
| PD‐L1 ≥ 5% vs. PD‐L1 < 5% | ||||
| No. of PD-L1+/- | 32/87 | 30/35 | 100/210 | 50/44 |
| ORR (PD-L1+/-) | 28.1%/20.7% | 43.3%/11.4% | 26.0%/9.0% | 40.0%/11.0% |
| CR (PD-L1+/-) | 12.5%/8.0% | na | 11.0%/1.9% | 16.0%/2.0% |
| PR (PD-L1+/-) | 15.6%/12.6% | na | 15.0%/7.1% | 24.0%/9.0% |
| SD (PD-L1+/-) | na | 26.7%/37.1% | 16.0%/20.5% | 18.0%/21.0% |
| PD (PD-L1+/-) | na | 26.7%/37.1% | 44.0%/54.8% | 34.0%/55.0% |
| PD‐L1 ≥ 1% vs. PD‐L1 < 1% | ||||
| No. of PD-L1+/- | 80/39 | 53/12 | 207/103 | na |
| ORR (PD-L1+/-) | 23.8%/20.5% | 30.2%/8.3% | 17.9%/7.8% | na |
| CR (PD-L1+/-) | 10.0%/7.7% | na | 6.3%/1.9% | na |
| PR (PD-L1+/-) | 13.8%/12.8% | na | 11.6%/5.8% | na |
| SD (PD-L1+/-) | na | 30.2%/41.7% | 1.9%/24.3% | na |
| PD (PD-L1+/-) | na | 30.2%/41.7% | 51.7%/50.5% | na |
| PFS(PD-L1+/-) (mon.) | 4.1 ± 2.4/2.4 ± 0.6 | na | 4.0 ± 0.6/2.6 ± 0.3 | 5.5(2.7-10.8)/1.4(1.3-2.7) |
| OS rate (PD-L1+/-) | 52%/59% | na | 48%/30% | 42%/10% |
| Follow-up (mons.) | 17.2 (0.2-23.5) | 4.2 (1.1-8.5) | 11.7 (11.4-12.2) | 37.8 (0.7-44.4) |
TCs: tumour cells; TIICs: tumour-inflating immune cells; UC: urothelial carcinoma; PD-L1: programmed death-ligand 1; +/-: positive/negative; MRCT: multicenter randomized controlled trial; IHC: immunohistochemistry; ORR: objective response rate; CR: completed response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival; na: data not available.
Table 4.
Characteristics of the included studies on predictive and prognostic value of tumour PD-L1 status in UC patients treated with anti-PD-1/PD-L1 therapy.
| Study | Plimack et al. (2017) [23] | Powles et al. (2014) [24] | Sharma et al. (2016) [25] | Sharma et al. (2017) [26] | Patel et al. (2018) [22] |
|---|---|---|---|---|---|
| Trial name | KEYNOTE-012 | PCD4989g | CheckMate 032 | CheckMate 275 | NCT01772004 |
| Study design | MRCT | MRCT | MRCT | Nonrandomised | MRCT |
| Trial phase | Phase 1b | Phase 1 | Phase 1/2 | Phase 2 | Phase 1 |
| Study interval | May 2013-Dec 2013 | 2011-2013 | 2014-2015 | Mar 2015-Oct 2015 | Sept 2014-Mar 2016 |
| UC patients | Locally advanced or metastatic UC | Metastatic UC | Recurrent metastatic UC | Metastatic UC | Locally advanced or metastatic UC |
| Age (years) | 70 (44-85) | 65 (36-86) | 65.5 (31-85) | 66 (38-90) | 68 (63-76) |
| Male/female | 23/10 | 46/19 | 54/24 | 211/59 | 178/71 |
| Immunotherapy | Pembrolizumab | Atezolizumab | Nivolumab | Nivolumab | Avelumab |
| Target | Anti-PD-1 | Anti-PD-L1 | Anti-PD-1 | Anti-PD-1 | Anti-PD-L1 |
| Treatment | 10 mg/kg, iv every 2 weeks | 15 mg/kg, iv every 3 weeks | 3 mg/kg, iv every 2 weeks | 3 mg/kg, iv every 2 weeks | 10 mg/kg, iv every 2 weeks |
| PD-L1 expression | Tumour cells | Tumour cells/TIICs | Tumour cells | Tumour cells | Tumour cells |
| Detection method | IHC | IHC | IHC | IHC | IHC |
| Cut-off value | 1% | 1%, 5% | 1%, 5% | 1% | 5% |
| PD-L1+ (5%/1%) | 84.0% | 43.1%/10.8% | 46.0%/30.6% | 37.3% | 33.0% |
| PD‐L1 ≥ 5% vs. PD‐L1 < 5% | |||||
| No. of PD-L1+/- | 7/58 | 81/184 | na | 63/76 | |
| ORR (PD-L1+/-) | na | 28.6%/25.9% | 28.4%/15.8% | na | 24%/13% |
| CR (PD-L1+/-) | na | na | 4.9%/1.1% | na | 10%/3% |
| PR (PD-L1+/-) | na | na | 23.5%/14.7% | na | 14%/11% |
| SD (PD-L1+/-) | na | 14.3%/34.5% | 28.4%/20.1% | na | 29%/20% |
| PD (PD-L1+/-) | na | 57.1%/29.3% | 25.9%/45.1% | na | 29%/50% |
| PD‐L1 ≥ 1% vs. PD‐L1 < 1% | |||||
| No. of PD-L1+/- | 21/4 | 28/37 | 122/143 | 25/42 | na |
| ORR (PD-L1+/-) | 23.8%/0% | 28.6%/24.3% | 23.8%/16.1% | 24.0%/26.2% | na |
| CR (PD-L1+/-) | na | na | 1.6%/0.7% | 16.0%/2.4% | na |
| PR (PD-L1+/-) | na | na | 19.7%/15.4% | 8.0%/23.8% | na |
| SD (PD-L1+/-) | na | 28.6%/35.1% | 28.7%/17.5% | 32.0%/26.2% | na |
| PD (PD-L1+/-) | na | 35.7%/29.7% | 30.3%/46.9% | 32.0%/42.9% | na |
| PFS(PD-L1+/-) (mon.) | na | na | na | na | 11.9(6.1-18)/6.1(5.9-8) |
| OS rate (PD-L1+/-) | na | na | na | na | 59%/51% |
| Follow-up (mons.) | 13 (5-23) | 4.2 (1.1-8.5) | 15.2 (12.9-16.8) | 7.0 (3.0-8.8) | 9.9 (4.3-12.1) |
TCs: tumour cells; TIICs: tumour-inflating immune cells; UC: urothelial carcinoma; PD-L1: programmed death-ligand 1; +/-: positive/negative; MRCT: multicenter randomized controlled trial; IHC: immunohistochemistry; ORR: objective response rate; CR: completed response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival; na: data not available.
3.2. PD-L1 Expression in TCs Predicted Poor Survival after Cystectomy for Patients with Organ-Confined Tumours (but Not All UC)
Three studies considering OS and CSS as the primary endpoints were included [4, 18, 19]. For all patients treated with cystectomy, pooled results indicated that the expression of PD-L1 in TCs was not related with the OS (HR, 1.10; 95% CI, 0.88-1.38; P = 0.40; Figure 2(a)), CSS (HR, 0.80; 95% CI, 0.57-1.12; P = 0.19; Figure 2(b)), or PFS (HR, 0.76; 95% CI, 0.54-1.07; P = 0.12; Figure 2(c)). However, for organ-confined UC, PD-L1 expression in TCs significantly predicted all-cause mortality (OS) after cystectomy (HR, 2.06; 95% CI, 1.38-3.06; P = 0.0004; Figure 3(a)), but was not significant in terms of CSS (HR, 1.37; 95% CI, 0.68-2.76; P = 0.38; Figure 3(b)) and DFS (HR, 0.26; 95% CI, 0.63-2.51; P = 0.52; Figure 3(c)).
Figure 2.
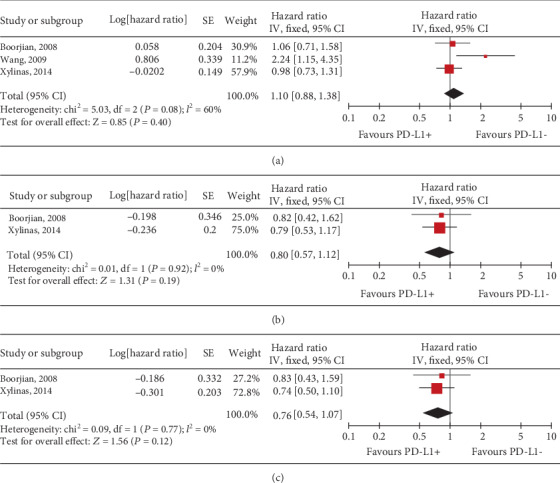
Forest plots of tumour cell PD-L1 expression predicted (a) OS, (b) CSS, and (c) DFS for all UC patients after cystectomy. PD-L1: programmed death-ligand 1; +/-: positive/negative; OS: overall survival; CSS: cancer-specific survival; DFS: disease-free survival; CI: confidence interval.
Figure 3.
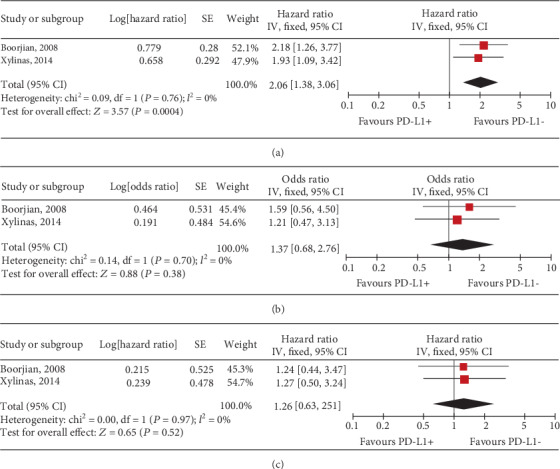
Forest plots of tumour cell PD-L1 expression for predicting (a) OS, (b) CSS, and (c) DFS for patients with organ-confined tumours after cystectomy. PD-L1: programmed death-ligand 1; +/-: positive/negative; OS: overall survival; CSS: cancer-specific survival; DFS: disease-free survival; CI: confidence interval; IV: inverse variance; SE: standard error.
3.3. TC PD-L1 Status Showed No Obvious Relationship with the Prognosis or Response to Platinum-Based Chemotherapy of UC Patients
In terms of the previously described association of PD-L1 expression and clinical outcomes in UC, it was investigated whether pretreatment PD-L1 status could predict the response to chemotherapy from three eligible studies [3, 20, 21]. As shown in Figure 4(a), there was no statistical difference of PD-L1 status in the tumour cell membrane between responders and resistant cases (OR, 1.15; 95% CI, 0.34-3.88; P = 0.82). Also, the result showed no significant evidence of a relationship with PD-L1 expression and OS (OR, 1.89; 95% CI, 0.78-4.58; P = 0.16; Figure 4(b)) for UC patients receiving platinum-based chemotherapy.
Figure 4.
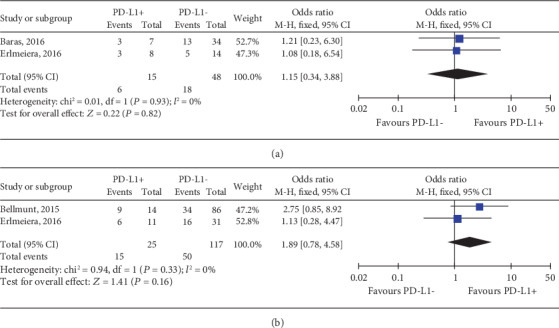
Forest plots of tumour cell PD-L1 status for predicting the (a) response and (b) prognosis to platinum-based chemotherapy in UC patients. PD-L1: programmed death-ligand 1; +/-: positive/negative; ORR: objective response rate; OS: overall survival; CI: confidence interval; M-H: Mantel-Hansel; SD: standard deviation.
3.4. TC PD-L1 Expression Failed to Predict the Response to Anti-PD-1/PD-L1 Immunotherapy in UC Patients
Among the 8 trials, 5 RCTs of elevated TC PD-L1 status predicting response to the PD-1/PD-L1 blockade therapy were pooled in this meta-analysis [22–26]. TC PD-L1 status with 5% as the cut-off value could predict the ORR to anti-PD-1/PD-L1 immunotherapy in UC patients (Figure 5(b)). TC PD − L1 ≥ 5% was correlated with higher completed response (CR) (OR, 4.24; 95% CI, 1.29-13.96; P = 0.02; Figure 6(a)). Otherwise, TC PD-L1 status with a cut-off value of 5% but not 1% failed to predict the partial response (PR), stable disease (SD), or progressive disease (PD) (Figure 6) in UC patients receiving anti-PD-1/PD-L1 immunotherapy.
Figure 5.
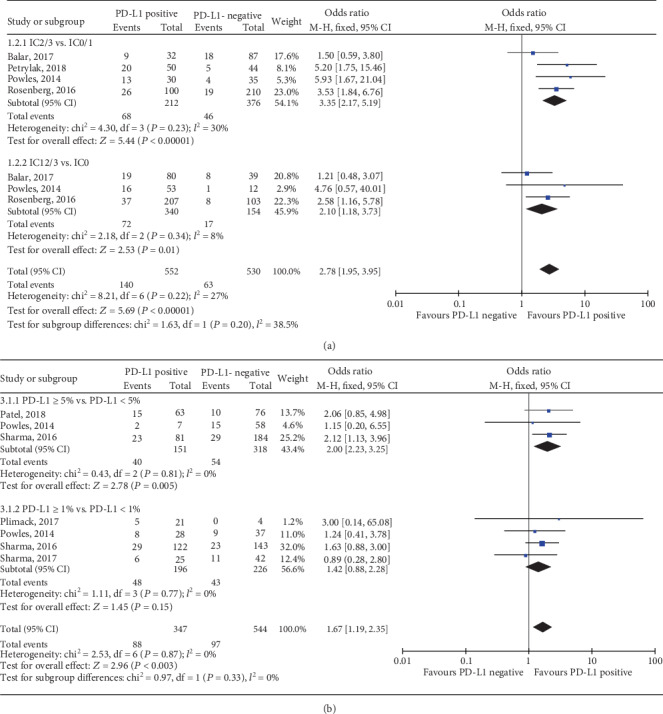
Forest plots of (a) immune cell and (b) tumour cell PD-L1 status with cut-off values of 5% and 1% in predicting the response to anti-PD-1/PD-L1 immunotherapy. PD-L1: programmed death-ligand 1; ORR: objective response rate; IC: immune cell; CI: confidence interval; M-H: Mantel-Hansel; SD: standard deviation.
Figure 6.
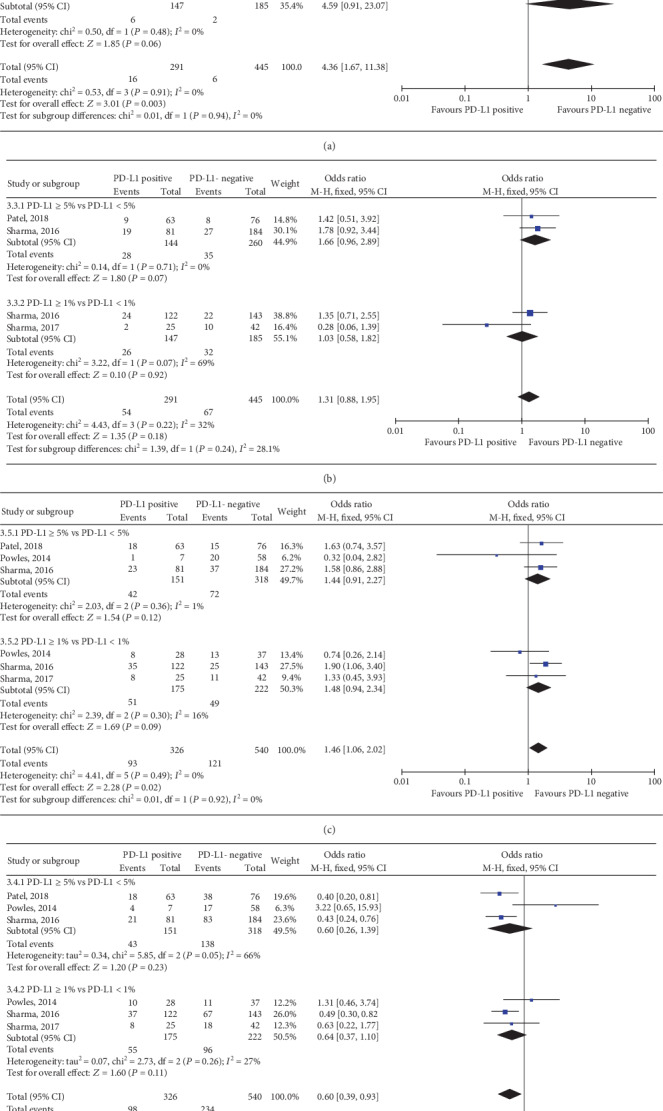
Forest plots of tumour cell PD-L1 status with cut-off values of 5% and 1% in predicting the (a) CR, (b) PR, (c) SD, and (d) PD to anti-PD-1/PD-L1 immunotherapy. PD-L1: programmed death-ligand 1; CR: completed response; PR: partial response; SD: stable disease; PD: progressive disease; CI: confidence interval; M-H: Mantel-Hansel; SD: standard deviation.
3.5. TIIC PD-L1 Expression Predicted Response to Anti-PD-1/PD-L1 Immunotherapy in UC Patients
Among the 6 trials, 4 RCTs of elevated TIIC PD-L1 status predicting the response to anti-PD-1/PD-L1 immunotherapy were pooled in this meta-analysis [13, 24, 26, 27]. Both TIIC PD-L1 status with IC2/3 vs. IC0/1 and IC1/2/3 vs. IC0 showed significant relationship with ORR to anti-PD-1/PD-L1 immunotherapy and a higher PD-L1 expression was correlated with a better response (IC2/3 vs. IC0/1: OR, 3.35; 95% CI, 2.17-5.19; P < 0.001; Figure 5(a); and IC1/2/3 vs. IC0: OR, 2.10; 95% CI, 1.18-3.73; P = 0.01; Figure 5(b)). In the TIIC subset, the positive expression (score of 2-3) versus the negative (score of 0-1) of PD-L1 was correlated with higher CR (OR, 4.21; 95% CI, 1.97-9.02; P = 0.0002; Figure 7(a)), PR (OR, 2.16; 95% CI, 1.24-3.74; P = 0.006; Figure 7(b)), and PD (OR, 0.59; 95% CI, 0.40-0.87; P = 0.007; Figure 7(d)); however, it could not predict the SD (OR, 0.73; 95% CI, 0.45-1.19; P = 0.21; Figure 7(c)). In the subset of IC1/2/3 vs. IC0, results indicated that PD-L1 expression level had no significant association with the CR (OR, 2.17; 95% CI, 0.80-5.91; P = 0.13; Figure 7(a)), PR (OR, 1.65; 95% CI, 0.81-3.37; P = 0.17; Figure 7(b)), SD (OR, 0.19; 95% CI, 0.02-1.79; P = 0.15; Figure 7(c)), or PD (OR, 0.99; 95% CI, 0.63-1.54; P = 0.95; Figure 7(d)).
Figure 7.

Forest plots of immune cell PD-L1 status with cut-off values of 5% and 1% in predicting the (a) CR, (b) PR, (c) SD, and (d) PD to anti-PD-1/PD-L1 immunotherapy. PD-L1: programmed death-ligand 1; IC: immune cell; CR: completed response; PR: partial response; SD: stable disease; PD: progressive disease; CI: confidence interval; M-H: Mantel-Hansel; SD: standard deviation.
3.6. TIIC PD-L1 Status Predicted PFS and OS in UC Patients Receiving Anti-PD-1/PD-L1 Immunotherapy
Further, only three trails reported the relationship of TIIC PD-L1 status and prognosis in UC patients receiving anti-PD-1/PD-L1 immunotherapy [13, 27, 28]. As shown in Figure 8, pooled results showed that elevated TIIC PD-L1 expression level benefited from improved PFS (IC2/3 vs. IC0/1: WMD, 2.40; 95% CI, 0.59-4.21; P = 0.009; and IC1/2/3 vs. IC0: WMD, 0.39; 95% CI, 0.29-0.49; P < 0.001), but was not correlated with OS in UC patients.
Figure 8.
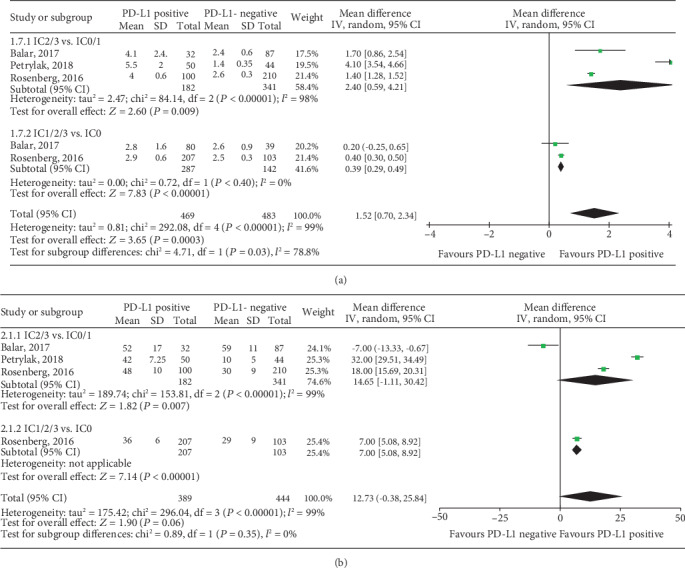
Forest plots of immune cell PD-L1 status with cut-off values of 5% and 1% in predicting the (a) PFS and (b) OS for UC patients with anti-PD-1/PD-L1 immunotherapy. PD-L1: programmed death-ligand 1; IC: immune cell; PFS: progression-free survival; OS: overall survival; CI: confidence interval; IV: inverse variance; SD: standard deviation.
4. Discussion
Currently, this was the first meta-analysis discussing the predictive and prognostic significance of PD-L1 expression in UC patients treated with cystectomy, chemotherapy, or anti-PD-1/PD-L1 immunotherapy. We confirmed that TC PD-L1 status could predict reduced survival after cystectomy for organ-confined UC patients, but not all UC patients. However, we found that TC PD-L1 expression was neither a predictive biomarker for survival benefit or response to adjuvant platinum-based chemotherapy nor a biomarker for response to anti-PD-1/PD-L1 immunotherapy. At the same time, we demonstrated that higher PD-L1 expression of TIICs but not TCs showed significant relationship with better response to the PD-1/PD-L1 blockade therapy. Furthermore, our results found that immune cell PD-L1 expression could serve as a prognostic biomarker for PFS but not OS in patients receiving anti-PD-1/PD-L1 immunotherapy.
Evidence suggested that UC was an immunogenic disease; in addition, presence of tumour-infiltrating lymphocytes (TILs) often correlated with immune response against the tumour and favorable clinical outcomes [29]. Aberrant expression of T-cell coregulatory molecule PD-L1 interacted with T-cell PD-1 that resulted in tumour-specific T-cell apoptosis, which might evade host immune surveillance, and was related with unfavorable outcomes in tumours [30]. Furthermore, PD-L1-positive expression has demonstrated a significant correlation with increased risk of disease progression and cancer death in various tumours [31–33]. Previously, two meta-analyses focused on PD-L1 and UC survival were reported. Wu et al. [34] indicated that PD-L1 status was related with worse 3-year overall survival in UC, and Wang et al. [35] revealed that PD-L1 status could predict the clinical stage of UC. Our pooled results with HR value and 95% CI raised a doubt and showed different results with them. We demonstrated that TC PD-L1 status was not correlated with the OS, CSS, or DFS in UC patients treated with cystectomy. However, for organ-confined UC, TC PD-L1 status could predict OS after cystectomy. Our findings were consistent with the published results of Boorjian et al. [4] and Xylinas et al. Increased tumour cell PD-L1 expression was related with advanced tumour stage, which could be an explanation for the predictive role of mortality in organ-confined but not local control tumours [4]. Of note, no study elevated the prognostic significance of immune cell PD-L1 status for UC patients receiving cystectomy, especially for early-stage tumours. Therefore, further trials are needed to explore whether PD-L1 expression in the immune cell has a prognostic role for UC patients who underwent cystectomy.
The recommended first-line therapy for metastatic UC is cisplatin-based chemotherapy, and nearly 50% of patients could respond to the treatment [36]. The improved prediction of clinical outcomes for advanced UC patients with platinum-based chemotherapy has recently attracted great interest [37, 38]. A recent RCT failed to demonstrate the p53 mutation either as a predictive biomarker of survival or as a response to adjuvant chemotherapy in UC patients who underwent RC [39]. PD-L1 status could predict postoperative outcomes in organ-confined UC patients and might provide better implications for the management of metastatic UC patients with chemotherapy. Our pooled results found that the expression of PD-L1 had no association with prognostic or predictive benefit from platinum-based chemotherapy. It was similar with that of Tsao et al. [6], who performed a pooled analysis using three pivotal adjuvant chemotherapy trials, and found that TC PD-L1 had neither prognostic nor predictive value from adjuvant chemotherapy in NSCLC. We concluded the nonsignificant role of tumour cell PD-L1; however, further trials are needed to assess whether PD-L1 expression in the immune cell could be a prognosticator for UC patients with chemotherapy.
Cisplatin-based chemotherapies were associated with several substantial toxicities, and only 10% of participants responded to the second-line single-agent chemotherapy [6]. Immune checkpoint blockade is a promising new way to cancer therapy via the activation of therapeutic tumour immunity. It was reported that PD-1/PD-L1 inhibitors had regulatory efficacy for metastatic UC patients whose disease progressed following platinum-based chemotherapy [40]. Recently, Bellmunt and his colleagues [7] reported that pembrolizumab (antibody against PD-1) could improve overall survival (by nearly 3 months) and have less therapy-related adverse events than chemotherapy for platinum-resistant advanced UC. Moreover, in a multicenter phase 1 and 2 cohort trial, nivolumab (one PD-1 inhibitor) raised a response in 24.4% of metastatic UC patients who had received previous chemotherapy without regard to TC PD-L1 expression [13, 25, 26, 28]. And their findings also provided that PD-L1 status in immune cells was a promising predictor for selected UC patients treated with atezolizumab or nivolumab. Our results demonstrated that higher PD-L1 expression of TIICs but not TCs with a cut-off value of 5% showed better response to anti-PD-1/PD-L1 immunotherapy. When T-cells were activated by antigen, they produced several cytokines which could increase the expression of PD-L1 in adjacent tumour and immune cells [41]. PD-L1 expression in TIICs as well as TILs was tumour antigen-specific, and their response to the tumour could be an explanation for this. Balar et al. reported a median PFS of 4.1 months in IC2/3 patients which was longer than 2.1 months in IC1 patients and 2.6 months in IC0 patients. However, IC2/3 patients did not benefit from OS with a median of 15.9 months versus 19.1 months in IC0/1 patients [13]. Finally, our meta-analysis convinced us that immune cell PD-L1 status was useful as a prognostic biomarker for PFS but not OS in UC patients receiving anti-PD-1/PD-L1 immunotherapy. Our results supported that PD-L1 expression in immune cells might serve as a promising predictor for immune checkpoint blockade therapies in UC. Furthermore, PD-L1-negative patients also responded to the PD-1/PD-L1 blockade therapy, which highlighted the need for better response biomarkers for immunotherapies [13]. In addition, few studies with short-term follow-up resulted in lack of power for the analysis of positive IC PD-L1 status on survival benefit. Patients should be followed up to assess the response and other long-term survival.
Notably, several limitations still existed in our study. The main limitation of our meta-analysis reflected the drawbacks of the literatures concerning this topic; several available publications were out-of-date or enrolled a relatively small sample size, and only three RCTs were methodologically qualified. The second limitation was the different locations of PD-L1 protein expressed; in most cases, they were only measured in tumour cells. However, there was no study reporting on immune cell PD-L1 status and survival after cystectomy, and only one study evaluated this in UC patients with chemotherapy. We could not pool the conclusion of immune cell PD-L1 status for predicting response and prognosis in patients treated with cystectomy or chemotherapy. The third limitation of our study was few comparative data for survival, leading to the lack of power for the analysis of survival benefit based on the limited studies with small sample sizes and short-term follow-up period. Patients should be followed up to assess the response and other long-term survival. Finally, other limits mainly included the different populations enrolled, the different drugs used, and the varying durations of treatment in the different studies included. Nevertheless, this systematic review offered a comprehensive overview of the predictive and prognostic significance of PD-L1 expression in UC patients after cystectomy, chemotherapy, or anti-PD-1/PD-L1 immunotherapy for data extraction with a robust search strategy. Furthermore, we applied a rigorous inclusion/exclusion criterion to identify studies, different subgroup analyses, full outcomes of interest (ORR, CR, PR, SD, PD, OS, CSS, and DFS), and advanced meta-analysis using HR and corresponding 95% CI for survival. Here, we provided up-to-date information of predictive and prognostic significance of PD-L1 in UC which was worthy for reference to the ongoing clinical trials.
Tumour cell PD-L1 expression showed significant association with advanced UC and could predict survival after cystectomy for organ-confined UC patients. Tumour cell PD-L1 status had no predictive or prognostic benefit from platinum-based chemotherapy. Higher PD-L1 expression of TIICs but not TCs with a cut-off value of 5% predicted better response to anti-PD-1/PD-L1 immunotherapy. TIIC PD-L1 status was useful as a prognostic biomarker for PFS but not OS in UC patients receiving anti-PD-1/PD-L1 immunotherapy. However, further RCTs with longer follow-up and a larger sample size should be conducted to verify whether the tumour immune cell PD-L1 as a biomarker has predictive and prognostic value in advanced UC patients treated with immune checkpoint inhibitors.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (81270788, 81470935, 81370805, 81900645, and 81402087). The authors thank Kehua Jiang and Hua Xu for giving significant suggestions for the study design.
Conflicts of Interest
The authors have declared no competing interests.
Authors' Contributions
Haoran Liu and Tao Ye contribute equally to this work. The study concept and design were handled by TK and LRH; data extraction by TK and YT; data analysis by TK, LHR, YT, LP, ZH, and WXL; manuscript preparation by TK and LRH; manuscript revision by TK, LHR, and YZQ; and study supervision by TK.
References
- 1.Alfred Witjes J., Lebret T., Compérat E. M., et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. European Urology. 2017;71(3):462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Tang K., Li H., Xia D., et al. Laparoscopic versus open radical cystectomy in bladder cancer: a systematic review and meta-analysis of comparative studies. PloS One. 2014;9(5, article e95667) doi: 10.1371/journal.pone.0095667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellmunt J., Mullane S. A., Werner L., et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Annals of Oncology. 2015;26(4):812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 4.Boorjian S. A., Sheinin Y., Crispen P. L., et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clinical Cancer Research. 2008;14(15):4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 5.Grossman H. B., Natale R. B., Tangen C. M., et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England Journal of Medicine. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 6.Tsao M.-S., Le Teuff G., Shepherd F. A., et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Annals of Oncology. 2017;28(4):882–889. doi: 10.1093/annonc/mdx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellmunt J., de Wit R., Vaughn D. J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. The New England Journal of Medicine. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst R. S., Soria J. C., Kowanetz M., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell S. M., Lesokhin A. M., Borrello I., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England Journal of Medicine. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J., Reckamp K. L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. The New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C., Long G. V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF Mutation. The New England Journal of Medicine. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 13.Balar A. V., Galsky M. D., Rosenberg J. E., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein M., Hedges L. V., Higgins J. P., Rothstein H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunology, Immunotherapy. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massard C., Gordon M. S., Sharma S., et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. Journal of Clinical Oncology. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhuang Q., Zhou S., Hu Z., Lan R. Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. Journal of Huazhong University of Science and Technology [Medical Sciences] 2009;29(1):77–79. doi: 10.1007/s11596-009-0116-2. [DOI] [PubMed] [Google Scholar]
- 19.Xylinas E., Robinson B. D., Kluth L. A., et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. European Journal of Surgical Oncology. 2014;40(1):121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Erlmeier F., Seitz A. K., Hatzichristodoulou G., et al. The role of PD-L1 expression and intratumoral lymphocytes in response to perioperative chemotherapy for urothelial carcinoma. Bladder Cancer. 2016;2(4):425–432. doi: 10.3233/BLC-160067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baras A. S., Drake C., Liu J. J., et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5(5, article e1134412) doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel M. R., Ellerton J., Infante J. R., et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. The Lancet Oncology. 2018;19(1):51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plimack E. R., Bellmunt J., Gupta S., et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. The Lancet Oncology. 2017;18(2):212–220. doi: 10.1016/S1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]
- 24.Powles T., Eder J. P., Fine G. D., et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P., Callahan M. K., Bono P., et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. The Lancet Oncology. 2016;17(11):1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P., Retz M., Siefker-Radtke A., et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. The Lancet Oncology. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 27.Petrylak D. P., Powles T., Bellmunt J., et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncology. 2018;4(4):537–544. doi: 10.1001/jamaoncol.2017.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg J. E., Hoffman-Censits J., Powles T., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carosella E. D., Ploussard G., LeMaoult J., Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. European Urology. 2015;68(2):267–279. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 30.Dong H., Strome S. E., Salomao D. R., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Medicine. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Calderaro J., Rousseau B., Amaddeo G., et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64(6):2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 32.Danilova L., Wang H., Sunshine J., et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(48):E7769–E7777. doi: 10.1073/pnas.1607836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masugi Y., Nishihara R., Yang J., et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–1473. doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu P., Wu D., Li L., Chai Y., Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PloS One. 2015;10(6, article e0131403) doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y. U., Liu A. N., Zhao S. H. Association between B7-H1 expression and bladder cancer: a meta-analysis. Genetics and Molecular Research. 2015;14(1):1277–1286. doi: 10.4238/2015.February.13.6. [DOI] [PubMed] [Google Scholar]
- 36.von der Maase H., Hansen S. W., Roberts J. T., et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. Journal of Clinical Oncology. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 37.Groenendijk F. H., de Jong J., Fransen van de Putte E. E., et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. European Urology. 2016;69(3):384–388. doi: 10.1016/j.eururo.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Plimack E. R., Dunbrack R. L., Brennan T. A., et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. European Urology. 2015;68(6):959–967. doi: 10.1016/j.eururo.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadler W. M., Lerner S. P., Groshen S., et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. Journal of Clinical Oncology. 2011;29(25):3443–3449. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellmunt J., Powles T., Vogelzang N. J. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treatment Reviews. 2017;54:58–67. doi: 10.1016/j.ctrv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Bald T., Landsberg J., Lopez-Ramos D., et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discovery. 2014;4(6):674–687. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]


