Abstract
Background
Although drug-eluting stents (DES) have reduced the rates of in-stent restenosis (ISR) compared with bare-metal stents (BMS), DES related ISR (DES-ISR) still occurs and outcomes of DES-ISR remain unclear. The objective of this meta-analysis was to investigate the long-term clinical outcomes of patients with DES-ISR compared with patients with BMS related ISR (BMS-ISR) after the treatment of DES or drug-eluting balloon (DEB). Methods and results. We searched the literature in the main electronic databases including PUBMED, EMBASE, Cochrane Library, and Web of Science. The primary endpoints were target lesion revascularization (TLR) and target vessel revascularization (TVR). The secondary endpoints included all cause death (ACD), cardiac death (CD), myocardial infarction (MI), stent thrombosis or re-in-stent restenosis (ST/RE-ISR), and major adverse cardiovascular events (MACEs). A total of 19 studies with 6256 participants were finally included in this meta-analysis. Results showed that the rates of TLR (P < 0.00001), TVR (P < 0.00001), CD (P=0.02), ST/RE-ISR (P < 0.00001), and MACEs (P < 0.00001) were significantly higher in the DES-ISR group than in the BMS-ISR group. No significant differences were found between the two groups in the rates of MI (P=0.05) and ACD (P=0.21).
Conclusions
Our study demonstrated that patients with DES-ISR had worse clinical outcomes at the long-term follow-up than patients with BMS-ISR after the treatment of DES or DEB, suggesting that DES and DEB may be more effective for BMS-ISR than that for DES-ISR. Positive prevention of DES-ISR is indispensable and further studies concentrating on detecting the predictors of outcomes of DES-ISR are required.
1. Introduction
Although the use of drug-eluting stents (DES) has significantly reduced the rates of in-stent restenosis (ISR) compared with bare-metal stents (BMS) [1,2], DES related ISR (DES-ISR) still occurs and the prognosis of patients with DES-ISR, which may be different from patients with BMS-ISR due to the different pathological features, remains unclear [3,4]. Recently, several studies investigated the long-term clinical outcomes of DES-ISR versus BMS-ISR after treated by DES or drug-eluting balloon (DEB), but the results were inconsistent [7–25]. Therefore, we enrolled these studies to conduct a meta-analysis to evaluate the results.
2. Methods
We searched the relevant literature in the main electronic databases (PUBMED, EMBASE, Cochrane Library, and Web of Science), using combinations of the following key words: “outcome” OR “prognosis” OR “result” AND “in-stent restenosis” OR “bare-metal in-stent restenosis” OR “drug-eluting in-stent restenosis.” Two authors independently performed the studies selection according to the titles or abstracts first, and then full texts of the relevant articles were evaluated according to the selection criteria. The inclusion criteria were as follows: (1) studies comparing the clinical outcomes of DES-ISR versus BMS-ISR; (2) treatment for ISR being DES or DEB; (3) follow-up time of at least six months; (4) studies including at least 30 participants; and (5) randomized clinical trials or observational studies. The exclusion criteria were as follows: (1) studies not comparing the clinical outcomes of DES-ISR versus BMS-ISR; (2) treatment for ISR including bare-metal stent (BMS), balloon angioplasty (BA) or coronary artery bypass surgery; (3) follow-up time being less than six months; (4) participants less than 30; and (5) case reports, reviews, and comments. A study was enrolled for the meta-analysis if it was eligible. Furthermore, we also searched the reference lists of all identified literatures to retrieve additional articles.
Two investigators independently performed the data extraction, using a standardized data extraction form including the following information: first author, year of study, type of study, number of participants, treatment of ISR, follow-up time, outcomes of ISR, and baseline characteristics of the enrolled patients. We tried to contact the authors by e-mails for the required data which was missing from the original published articles. Two reviewers independently assessed the risk of bias by using the Newcastle-Ottawa Scale [5]. Discrepancies were resolved by team discussion.
The primary endpoints were target lesion revascularization (TLR) and target vessel revascularization (TVR). The secondary endpoints included all cause death (ACD), cardiac death (CD), myocardial infarction (MI), stent thrombosis or re-in-stent restenosis (ST/RE-ISR), and major adverse cardiovascular events (MACEs). ISR is defined as recurrent diameter stenosis >50% at the stent segment or its edges. ACD is defined as death due to any cause. The definitions of “TLR,” “TVR,” “CD,” “MI,” “ST,” and “MACEs” were in accordance with the Academic Research Consortium criteria [6].
Effect sizes expressed as risk ratios (RR) with 95% confidence intervals (CIs) were calculated for each study. Statistical heterogeneity was evaluated by the Cochrane Q test and the I2 statistic. A random effect model was utilized if P values <0.1 and I2 values > 50%; conversely, a fixed effect model was used. Furthermore, to investigate the potential heterogeneity across studies, we also conducted subgroup analyses based on the treatment of ISR (DES or DEB). All analyses were carried out by the REVIEW MANAGER VERSION 5.3.
3. Results
A total of 2626 potential articles were screened at the first screening, and 19 observational studies with 6256 participants were finally included. A flow diagram depicting the process of literature search strategy is shown in Figure 1. Among the 19 studies enrolled, 7 studies investigated the clinical outcomes of DES-ISR versus BMS-ISR after the treatment of DEB, and the remaining 12 studies compared the clinical outcomes of DES-ISR with BMS-ISR after the treatment of DES. Among the participants enrolled, 2514 patients were with DES-ISR, and 3742 patients were with BMS-ISR. Table 1 describes the main characteristics of the included studies. The mean follow-up time ranged from 8 to 72 months. Baseline characteristics of the enrolled patients are shown in Table 2. Risk of bias assessment is listed in Table 3.
Figure 1.

Flow diagram of literature search strategy process.
Table 1.
Main characteristics of the included studies.
| First author | Published year | Study type | TPN | Treatment | FU time | Endpoints |
|---|---|---|---|---|---|---|
| Berta | 2014 | Observational | 82 | DEB | 28 months | TLR, ST, MI, MACE, death |
| Lee | 2016 | Observational | 230 | DEB | 12 months | RE-ISR, MI, MACE, death, CD |
| Alfonso | 2017 | Observational | 249 | DEB | 12 months | TLR, TVR, ST, MI, MACE, death, CD |
| Beatriz | 2011 | Observational | 126 | DEB | 12 months | TLR, ST, MI, MACE, death, CD |
| Markus | 2016 | Observational | 135 | DEB | 12 months | RE-ISR, TLR |
| Christoph | 2012 | Observational | 81 | DEB | 12 months | TLR, TVR, ST, MI, MACE, death, CD |
| Ralph | 2014 | Observational | 918 | DEB | 13 months | TLR, TVR, ST, MI, MACE, death, CD |
| Daniel | 2009 | Observational | 238 | DES | 12 months | TVR, ST, MI, MACE, death |
| Robert | 2013 | Observational | 650 | DES | 12 months | TLR, ST, MI, MACE, death |
| Negar | 2012 | Observational | 194 | DES | 12 months | TLR, TVR, MI, MACE, CD |
| Jose | 2009 | Observational | 216 | DES | 72 months | TLR, ST, MI, CD |
| Heng | 2010 | Observational | 97 | DES | 28 months | TVR, MI, MACE, death |
| Fernando | 2016 | Observational | 249 | DES | 12 months | TLR, TVR, ST, MI, MACE, death, CD |
| Mohammad | 2012 | Observational | 94 | DES | 12 months | TLR, ST, MI, MACE, death, CD |
| Cheol | 2008 | Observational | 295 | DES | 32 months | TLR, ST, MI, death, CD |
| Yan | 2013 | Observational | 388 | DES | 42 months | TLR, TVR, ST, MI, MACE, death, CD |
| Kensaku | 2010 | Observational | 158 | DES | 8 months | TLR, TVR, RE-ISR, MACE |
| Alexandre | 2012 | Observational | 1590 | DES | 12 months | TLR, ST, MI, MACE, death |
| Gert | 2013 | Observational | 266 | DES | 24 months | TLR, TVR, ST, MI, MACE, death, CD |
TPN: total patient number; FU: follow-up; DEB: drug-eluting balloon; DES: drug-eluting stent; TLR: target lesion revascularization; TVR: target vessel revascularization; CD: cardiac death; MI: myocardial infarction; ST: stent thrombosis; RE-ISR: re-in-stent restenosis; MACE: major adverse cardiovascular event.
Table 2.
Basic characteristics of the enrolled patients.
| Study | Type of ISR | PN | Age (years) | Male (%) | HTN (%) | DM (%) | HLP (%) | Smoke (%) | ACS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Berta | BMS | 47 | 63.6 ± 10.2 | 51.1 | 97.9 | 38.3 | 87.2 | 23.4 | 25.6 |
| DES | 35 | 62.7 ± 10.0 | 45.7 | 100.0 | 34.3 | 94.3 | 22.9 | 14.3 | |
| Lee | BMS | 115 | 65.1 ± 10.4 | 77.4 | 75.7 | 50.4 | 67.0 | 43.5 | 82.6 |
| DES | 115 | 63.5 ± 10.3 | 76.5 | 75.7 | 57.4 | 66.1 | 36.5 | 77.4 | |
| Fernando | BMS | 95 | 67.0 ± 11.0 | 86.0 | 72.0 | 32.0 | 73.0 | 59.0 | 40.0 |
| DES | 154 | 66.0 ± 10.0 | 82.0 | 71.0 | 49.0 | 71.0 | 58.0 | 52.0 | |
| Beatriz | BMS | 65 | 66.2 ± 11.9 | 78.5 | 69.2 | 27.7 | 60.0 | 30.8 | 66.2 |
| DES | 61 | 64.4 ± 10.2 | 88.5 | 80.3 | 39.3 | 77.0 | 29.5 | 34.4 | |
| Markus | BMS | 65 | 59.9 ± 9.4 | 76.9 | 89.2 | 36.9 | — | — | — |
| DES | 70 | 65.0 ± 8.7 | 71.4 | 87.1 | 41.4 | — | — | — | |
| Christoph | BMS | 43 | 65.0 ± 8.8 | 79.1 | 81.4 | 25.6 | 81.4 | — | 14.0 |
| DES | 38 | 67.0 ± 10.1 | 76.3 | 94.8 | 29.0 | 94.7 | — | 15.8 | |
| Ralph | BMS | 499 | 66.9 ± 10.8 | 76.4 | 85.8 | 30.7 | 84.8 | 60.7 | 33.7 |
| DES | 419 | 66.8 ± 10.5 | 72.1 | 84.7 | 38.2 | 86.2 | 57.8 | 31.3 | |
| Daniel | BMS | 119 | 63.4 ± 10.9 | 68.9 | 90.8 | 40.5 | 93.2 | 16.8 | 63.9 |
| DES | 119 | 64.4 ± 11.4 | 60.5 | 95.8 | 42.7 | 96.6 | 19.3 | 71.4 | |
| Robert | BMS | 200 | 64.2 ± 10.6 | 78.5 | 54.0 | 29.0 | 56.0 | 11.0 | — |
| DES | 450 | 66.7 ± 10.6 | 76.7 | 72.4 | 36.0 | 75.8 | 12.0 | — | |
| Negar | BMS | 114 | 57.5 ± 9.9 | 67.5 | 48.2 | 25.4 | 75.4 | 21.9 | 51.9 |
| DES | 80 | 56.4 ± 11.0 | 66.3 | 42.5 | 26.3 | 70.0 | 23.8 | 58.7 | |
| Jose | BMS | 158 | 62.6 ± 11.5 | 72.8 | 78.5 | 32.9 | 67.1 | 10.8 | 24.1 |
| DES | 58 | 59.5 ± 9.8 | 71.7 | 75.8 | 36.1 | 79.3 | 24.1 | 43.1 | |
| Heng | BMS | 56 | 63.7 ± 11.9 | 80.4 | 64.3 | 26.8 | — | — | 48.2 |
| DES | 41 | 65.7 ± 9.3 | 70.7 | 78.0 | 43.9 | — | — | 41.4 | |
| Fernando | BMS | 94 | 64.0 ± 12.0 | 87.0 | 72.0 | 20.0 | 66.0 | — | 45.0 |
| DES | 155 | 66.0 ± 10.0 | 84.0 | 78.0 | 42.0 | 78.0 | — | 51.0 | |
| Mohammad | BMS | 64 | 67.9 ± 10.6 | 87.5 | 76.5 | 28.1 | 31.2 | 53.1 | — |
| DES | 30 | 66.8 ± 11.9 | 73.3 | 86.0 | 43.3 | 16.6 | 50.0 | — | |
| Cheol | BMS | 224 | 59.9 ± 10.6 | 76.3 | 50.0 | 31.9 | — | 22.3 | 41.5 |
| DES | 71 | 58.7 ± 10.9 | 66.2 | 47.9 | 22.5 | — | 19.7 | 38.0 | |
| Yan | BMS | 244 | 58.0 ± 10.9 | 85.2 | 66.0 | 27.5 | 52.9 | 53.7 | 59.9 |
| DES | 144 | 57.4 ± 9.0 | 81.9 | 68.1 | 25.0 | 54.9 | 44.4 | 59.7 | |
| Kensaku | BMS | 109 | 66.6 ± 10.8 | 84.0 | 73.0 | 33.0 | 41.0 | 17.0 | — |
| DES | 49 | 67.0 ± 8.3 | 84.0 | 73.0 | 57.0 | 57.0 | 12.0 | — | |
| Alexandre | BMS | 1235 | 63.2 ± 10.8 | 73.3 | 76.9 | 26.9 | 81.1 | 54.1 | 43.5 |
| DES | 355 | 63.7 ± 10.5 | 70.4 | 71.3 | 39.4 | 79.8 | 54.1 | 44.2 | |
| Gert | BMS | 196 | 65.5 ± 10.4 | 75.0 | — | 29.1 | — | — | 45.4 |
| 7 | 65.6 ± 10.6 | 82.9 | — | 32.9 | — | — | 45.7 |
BMS: bare-metal stent; DES: drug-eluting stent; PN: patient number; HTN: hypertension; DM: diabetes mellitus; HLP: hyperlipemia; ACS: acute coronary syndrome; ISR: in-stent restenosis; -: not available.
Table 3.
Risk of bias assessment.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Berta | A | A | A | A | A | B | A | A | 8 |
| Lee | A | A | A | B | A | B | A | A | 7 |
| Fernando | A | A | A | B | A | B | A | A | 7 |
| Beatriz | A | A | A | A | A | B | A | A | 8 |
| Markus | A | A | A | B | A | B | A | A | 7 |
| Christoph | A | A | A | A | A | B | A | B | 8 |
| Ralph | A | A | A | A | A | B | A | B | 8 |
| Daniel | A | A | A | B | A | B | A | A | 7 |
| Robert | A | A | A | B | A | B | A | A | 7 |
| Negar | A | A | A | B | A | B | A | A | 7 |
| Jose | A | A | A | B | A | B | A | A | 7 |
| Heng | A | A | A | B | A | B | A | A | 7 |
| Fernando | A | A | A | B | A | B | A | A | 7 |
| Mohammad | A | A | A | B | A | B | A | A | 7 |
| Cheol | A | A | A | A | A | B | A | A | 8 |
| Yan | A | A | A | B | A | B | A | A | 7 |
| Kensaku | A | A | A | A | A | B | A | A | 8 |
| Alexandre | A | A | A | B | A | B | A | C | 6 |
| Gert | A | A | A | B | A | B | A | B | 7 |
1: representativeness of the exposed cohort; 2: selection of the nonexposed cohort; 3: ascertainment of exposure; 4: outcome of interest was not present at the beginning of study; 5: comparability of cohorts; 6: assessment of outcome; 7: long enough follow-up; 8: adequacy of follow-up; A: 1 score; B: 0/1 score; C: 0 score.
In terms of the clinical outcomes, 16 studies including 5478 patients contributed to analysis of the overall rate of TLR, which was significantly higher in the DES-ISR group than in the BMS-ISR group (RR: 0.53, 95% CI: 0.45–0.64, P < 0.00001, Figure 2(a)); 10 studies with 2784 patients contributed to analysis of the overall rate of TVR, which was significantly higher in the DES-ISR group than in the BMS-ISR group (RR: 0.51, 95% CI: 0.40–0.63, P < 0.00001, Figure 2(b)); 15 studies including 5354 patients contributed to analysis of the overall rate of ACD, which was similar between the two groups (RR: 0.83, 95% CI: 0.62–1.11, P=0.21, Figure 3(a)); 12 studies with 3252 patients contributed to analysis of the overall rate of CD, which was significantly higher in the DES-ISR group than in the BMS-ISR group (RR: 0.58, 95% CI: 0.36–0.93, P=0.02, Figure 3(b)); 17 studies with 5750 patients reported the rates of MI, and results showed that patients with DES-ISR had higher rates of MI than patients with BMS-ISR, although not statistically significant (RR: 0.73, 95% CI: 0.53–1.00, P=0.05, Figure 4(a)); 17 studies reported the incidences of ST or RE-ISR, and the results showed that the rates were significantly higher in the DES-ISR group than in the BMS-ISR group (RR: 0.57, 95% CI: 0.44–0.74, P < 0.0001, Figure 4(b)); 16 studies with 5417 patients contributed to the analysis of the overall rate of MACEs, which was markedly higher in the DES-ISR group compared with the BMS-ISR group (RR: 0.63, 95% CI: 0.55–0.72, P < 0.00001, Figure 5).
Figure 2.
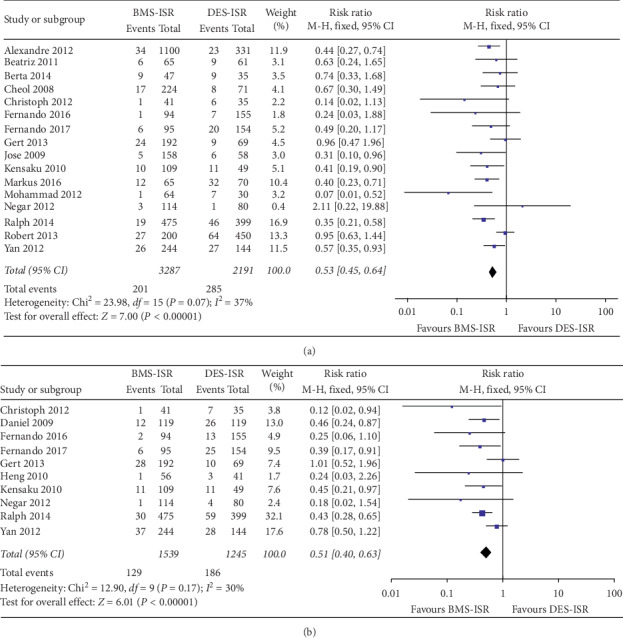
Forest plot with RR for BMS-ISR versus DES-ISR: (a) TLR, (b) TVR.
Figure 3.
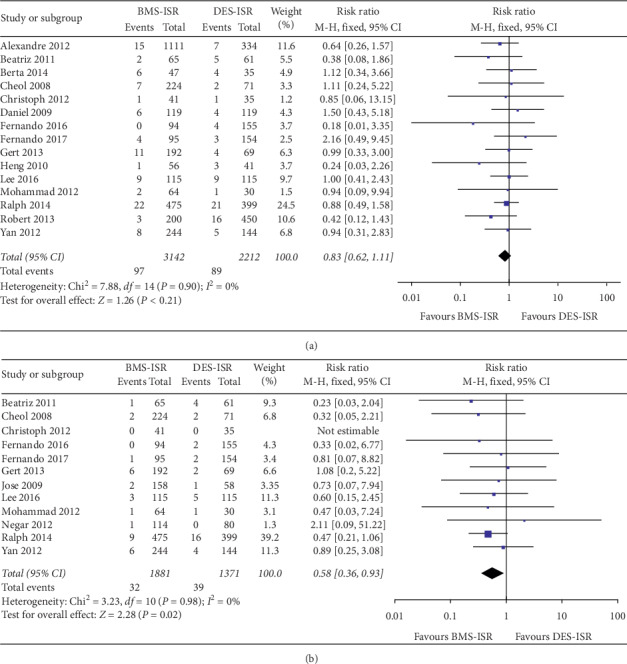
Forest plot with RR for BMS-ISR versus DES-ISR: (a) ACD, (b) CD.
Figure 4.

Forest plot with RR for BMS-ISR versus DES-ISR: (a) MI, (b) ST/RS-ISR.
Figure 5.

Forest plot with RR for BMS-ISR versus DES-ISR : MACEs.
Subgroup analyses showed that the incidences of TLR (P < 0.00001, Supplemental Figure 1A), TVR (P=0.0003, Supplemental Figure 1B), ST/RE-ISR (P=0.01, Supplemental Figure 2C), and MACEs (P < 0.00001, Supplemental Figure 3) at the long-term follow-up were markedly higher in the DES-ISR group than in the BMS-ISR group after treated by DES, but the rates of ACD (P=0.15, Supplemental Figure 1C), CD (P=0.42, Supplemental Figure 2A) and MI (P=0.21, Supplemental Figure 2B) were similar between the two groups.
Similarly, patients with DES-ISR had higher rates of TLR (P < 0.00001, Supplemental Figure 4A), TVR (P < 0.00001, Supplemental Figure 4B), CD (P=0.02, Supplemental Figure 5A), ST/RE-ISR (P=0.0007, Supplemental Figure 5C), and MACEs (P < 0.00001, Supplemental Figure 6) at the long-term follow-up than patients with BMS-ISR after treated by DEB, but no significant differences were found between the two groups in the rates of ACD (P=0.74, Supplemental Figure 4C) and MI (P=0.013, Supplemental Figure 5B).
4. Discussion
This is the first meta-analysis to investigate the long-term clinical outcomes after treatment for DES-ISR compared with BMS-ISR. What we found was that patients with DES-ISR had poorer clinical outcomes than patients with BMS-ISR after treated by DES or DEB.
The reasons of these findings are not fully understood, and possible explanations are as follows: first, different pathological features of the two types of ISR lesion may result in different outcomes. The homogeneous type mainly composed of the smooth muscle cells with collagen fibers is predominant in the BMS-ISR lesions, while layered type that comprises proteoglycans, inflammatory cells, and fibrinoids is the main pattern of the DES-ISR lesions. Besides, neoatherosclerosis occurs more frequently and earlier in DES-ISR lesions than in BMS-ISR lesions [27, 28]. Nagoshi et al. [26] evaluated the efficiency of BA for homogeneous and layered lesions, and the results showed that after BA, reduction in neointimal tissue area was significantly smaller in homogeneous lesions than in layered lesions, suggesting that layered ISR tissue may respond better to BA than those homogeneous ISR tissue. Based on this concept, one speculated that different patterns of neointimal tissue may also have different responses to DES or DEB (DES or DEB is more effective in homogeneous type but might be less effective in layered type tissue and more effective in classical neointimal proliferation but less effective in neoatherosclerosis). Further studies with optical coherence tomography (OCT) or intravascular ultrasound (IVUS) are required to confirm these speculations.
Another possible explanation is that the vascular wall of DES-ISR may have poorer response to the repeated anti-inflammatory and antiproliferative drugs which were covered by DES or DEB after the wall shows resistance to the beneficial effects of DES in a de novo lesion by developing ISR. However, the lesions of BMS-ISR are “drug-naive,” which may create a potential milieu for the anti-inflammatory and proliferative drugs to play their roles richly after DES or DEB implanted to the lesions [8,9,14].
Finally, the selection bias of patients may also lead to the difference of outcomes between DES-ISR and BMS-ISR. As we known, the use of DES has significantly reduced the incidence of ISR compared to BMS, but with the growing application of DES in the complex circumstances, DES-ISR has also increased [1, 3]. Therefore, the majority of DES-ISR patients in our enrolled studies are usually those who have more adverse characteristics such as diabetes, ACS, and more complex or severe lesions than BMS-ISR patients, which may impair the efficiency of DES or DEB for patient with DES-ISR [9, 24].
The main findings of our study suggest that we should pay more attention to how to prevent patients with DES-ISR from undergoing unfavorable outcomes after treated by DES or DEB. In other words, concentrating on the predictors of outcomes of DES-ISR after treated by DES or DEB is required. Abizaid et al. [24] found that the independent predictors of TLR after using SES for the treatment of DES-ISR were diabetes mellitus in advanced stage (P=0.001), postprocedure diameter stenosis <20% (P < 0.001), bifurcation lesion treated with no less than 2 stents (P=0.004), and the total number of lesions treated (P=0.009). Besides, independent predictors of MACEs after using SES for the treatment of DES-ISR were diabetes mellitus in advanced stage (P < 0.001), postprocedure residual stenosis (P=0.001), and bifurcation lesion treated with 2 stents (P < 0.015). In another study, the associations of TLR following the use of DEB for the treatment of patients with DES-ISR included end stage renal disease on maintenance hemodialysis (P=0.047) and previous DEB failure (P < 0.001) [7]. Moreover, it is also indispensable to prevent DES-ISR from occurring. There are different kinds of risk factors associated with DES-ISR, including female gender, diabetes mellitus, renal failure, and complex lesions such as type C lesions, calcified lesion, long lesion, and small diameter vessel. For patients with high risk of DES-ISR, OCT, IVUS, and fractional flow reserve (FFR) may be helpful for clinicians to decide whether a DES implantation or not and to avoid the procedure-related factors such as stent fracture and stent underexpansion [3,29,30].
Although our study demonstrated that DES and DEB had less efficiency and safety for DES-ISR than for BMS-ISR, there are no other better choices than DES and DEB. A meta-analysis comparing the efficacy of DES, DEB, and BA for DES-ISR showed that both DES and DEB were superior to BA, but there were no significant differences between the DES group and the DEB group [31]. Another meta-analysis found no differences in the rates of TLR, CD, MI, ST, and MACEs between the DEB group and the DES group, but with meta-analysis of clinical trials only, the TLR rate was significantly reduced in the DES group (P=0.015) [32]. Recently, several studies were conduct to investigate the efficiency of DEB versus DES stratified by the generation for DES-ISR, but there was no enough evidence to confirm which is better. PEPCAD China ISR trial was designed to compare first-generation DES (FG-DES) versus DEB for DES-ISR showed that the clinical outcomes at 1-year follow-up were similar between the two groups [33]. A multicenter randomized study involving 309 patients demonstrated that the rates of TLR, TVR, and MACEs at 1 year and 3 years were significantly lower in patients treated with second-generation DES (SG-DES) than those treated with DEB [34,35]. However, in another multicenter randomized trial enrolling 172 patients, the rates of TLR, TVR, ACD, MI, and ST at 1-year follow-up were comparable between the SG-DES group and the DEB group [36]. Recently, a meta-analysis comparing DEB versus SG-DES for the management of ISR was conducted and the subgroup analysis of DES-ISR showed that second-generation DES was associated with lower risk of TLR (P=0.004), TVR (P=0.012), and MACEs (P=0.043) than DEB, but the sample size of the subgroup is so small that the statistical power to evaluate the effective size may be not enough to properly compare the efficacy and safety of DEB and SG-DES in DES-ISR patients [37]. Whether DES (including FG-DES and SG-DES) or DEB is more effective for DES-ISR remains unclear. Further large-scale randomized trials are required to found out the answers.
Recently, several studies were conducted to investigate whether there were outcomes differences when DES-ISR treated by different types of DES. In the ISAR-DESIRE-2 study, 450 patients with sirolimus DES-ISR were randomly divided into resirolimus DES treatment group and paclitaxel DES treatment group, and the results showed that the rates of TLR (P=0.52), ACD (P=0.6), MI (P=0.53), and ST (P=0.67) at 1-year follow-up were similar between the two groups [38]. Similarly, in the RIBS III study, there were also no marked differences of the clinical outcomes between the hetero-DES and homo-DES group [39]. Whether using a different DES or a similar DES when DES-ISR occurs remains controversial. Besides, there were limited studies conducted to investigated differences between FG-DES and SG-DES for DES-ISR. The study of Song et al. [40] which included patients with diffuse type DES-ISR demonstrated that implantation of SES or EES had comparable efficiency and safety for the treatment of DES-ISR in terms of clinical outcomes at 1-year follow-up.
There are a number of alternative DEB devices that are available for DES-ISR. Colleran et al. [41] compared two different kinds of paclitaxel-coated balloons for DES-ISR; the results demonstrated that the clinical outcomes including TLR (P=0.91), ACD (P=0.73), MI (P=0.73), ST (P=0.34), and MACEs (P=0.91) at 1 year were similar between the BTHC-based PEB group and iopromide-based PEB group. In a multicenter randomized trial enrolling 50 patients with DES-ISR, the incidence of TLR, ACD, ST, and MACES up to 12 months did not differ between the sirolimus-coated balloon group and paclitaxel-coated balloon group [42].
Overall, with regard to the treatment for DES-ISR, whether DES or DEB, which kind of DES and DEB is more appropriated for DES-ISR remains unclear. Large-scale randomized trials are needed to determine the optimal strategies for DES-ISR.
4.1. Limitations
Firstly, the studies pooled in this analysis were all observational studies, which may decrease the validity of the study to a certain extent. Besides, there was a level of heterogeneity between the included studies due to different initial DES types. Finally, we did not analyze the angiographic outcomes because the pattern of quantitative coronary assessment was inconsistent, some were by in-segment pattern, and others were by in-stent pattern.
4.2. Conclusions
Our study demonstrated that patients with DES-ISR had worse clinical outcomes at the long-term follow-up than patients with BMS-ISR after the treatment of DES or DEB, suggesting that DES and DEB may be more effective for BMS-ISR than that for DES-ISR. Positive prevention of DES-ISR is indispensable and further studies concentrating on detecting the predictors of outcomes of DES-ISR are required.
Acknowledgments
This work was supported, in part, by National 135 Key Research and Development Program in 2016 (no. 2016YFC1301203); Major Science and Technology Projects of Tianjin Science and Technology Commission in 2016 (no. 16ZXMJSY00150); the Key Project of Healthcare Industry of Tianjin in 2016 (no. 16KG131); and the Science and Technology Project of Tianjin Jinnan District Science and Technology Commission (no. 20171514).
Data Availability
All data used to support the findings of our study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplement Figure 1: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES: (A) TLR, (B) TVR, and (C) ACD; Supplement Figure 2: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES: (A) CD, (B) MI, and (C) ST/RE-ISR; Supplement Figure 3: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES : MACES; Supplement Figure 4: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB: (A) TLR, (B) TVR, and (C) ACD; Supplement Figure 5: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB: (A) CD, (B) MI, and (C) ST/RE-ISR; Supplement Figure 6: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB : MACES.
References
- 1.Waldo S. W., O’Donnell C. I., Prouse A., et al. Incidence, procedural management, and clinical outcomes of coronary in-stent restenosis: insights from the National VA CART Program. Catheterization and Cardiovascular Interventions. 2018;91(3):425–433. doi: 10.1002/ccd.27161. [DOI] [PubMed] [Google Scholar]
- 2.Farooq V., Gogas B. D., Serruys P. W. Restenosis. Circulation: Cardiovascular Interventions. 2011;4(2):195–205. doi: 10.1161/circinterventions.110.959882. [DOI] [PubMed] [Google Scholar]
- 3.Dangas G. D., Claessen B. E., Caixeta A., Sanidas E. A., Mintz G. S., Mehran R. In-stent restenosis in the drug-eluting stent era. Journal of the American College of Cardiology. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Chieffo A., Foglieni C., Nodari R. L., et al. Histopathology of clinical coronary restenosis in drug-eluting versus bare metal stents. The American Journal of Cardiology. 2009;104(12):1660–1667. doi: 10.1016/j.amjcard.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Wells G. A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, Canada: The Ottawa Hospital Research Institute; 2016. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 6.Cutlip D. E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials. Circulation. 2007;115(17):2344–2351. doi: 10.1161/circulationaha.106.685313. [DOI] [PubMed] [Google Scholar]
- 7.Berta B., Jambrik Z., Kohar K., et al. Efficacy of drug-eluting balloon in patients with bare-metal or drug-eluting stent restenosis. Hellenic Journal of Cardiology. 2014;55:369–377. [PubMed] [Google Scholar]
- 8.Lee W.-C., Fang Y.-N., Fang C.-Y., et al. Comparison of clinical results following the use of drug-eluting balloons for a bare-metal stent and drug-eluting stent instent restenosis. Journal of Interventional Cardiology. 2016;29(5):469–479. doi: 10.1111/joic.12327. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso F., Pérez-Vizcayno M. J., García del Blanco B., et al. Usefulness of drug-eluting balloons for bare-metal and drug-eluting in-stent restenosis (from the RIBS IV and V randomized trials) The American Journal of Cardiology. 2017;119(7):983–990. doi: 10.1016/j.amjcard.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Vaquerizo B., Serra A., Miranda-Guardiola F., et al. One-year outcomes with angiographic follow-up of paclitaxel-eluting balloon for the treatment of in-stent restenosis: insights from Spanish multicenter registry. Journal of Interventional Cardiology. 2011;24(6):518–528. doi: 10.1111/j.1540-8183.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- 11.Resch M., Ostheim P., Endemann D. H., et al. Drug coated balloon is less effective for treatment of DES in-stent restenosis both in native coronary arteries and saphenous vein grafts: results from a bicenter registry. Journal of Interventional Cardiology. 2016;29(5):461–468. doi: 10.1111/joic.12324. [DOI] [PubMed] [Google Scholar]
- 12.Hehrlein C., Dietz U., Kubica J., et al. Twelve-month results of a paclitaxel releasing balloon in patients presenting with in-stent restenosis First-in-Man (PEPPER) trial. Cardiovascular Revascularization Medicine. 2012;13(5):260–264. doi: 10.1016/j.carrev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Toelg R., Merkely B., Erglis A., et al. Coronary artery treatment with paclitaxel-coated balloon using a BTHC excipient: clinical results of the international real-world DELUX registry. EuroIntervention. 2014;10(5):591–599. doi: 10.4244/eijv10i5a102. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg D. H., Gaglia M. A., Pinto Slottow T. L., et al. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. The American Journal of Cardiology. 2009;103(4):491–495. doi: 10.1016/j.amjcard.2008.09.107. [DOI] [PubMed] [Google Scholar]
- 15.Byrne R. A., Cassese S., Windisch T., et al. Differential relative efficacy between drug-eluting stents in patients with bare metal and drug-eluting stent restenosis; evidence in support of drug resistance: insights from the ISAR-DESIRE and ISAR-DESIRE 2 trials. EuroIntervention. 2013;9(7):797–802. doi: 10.4244/eijv9i7a132. [DOI] [PubMed] [Google Scholar]
- 16.Faramarzi N., Salarifar M., Kassaian S. E., et al. Mid-term follow-up of drug-eluting stenting for in-stent restenosis: bare-metal stents versus drug-eluting stents. The Journal of Tehran Heart Center. 2013;8(1):14–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Ribamar C. J., Sousa A. G., Moreira A., et al. Comparison of the very long term (>1 year) outcomes of drug-eluting stents for the treatment of bare-metal and drug-eluting stent restenosis. EuroIntervention. 2009;5:448–453. doi: 10.4244/eijv5i4a71. [DOI] [PubMed] [Google Scholar]
- 18.Ge H., Zhang Q., Zhou W., He Q., Han Z.-H., He B. Efficacy and safety of drug-eluting stent implantation for the treatment of in-stent restenosis occurring within bare-metal stent and drug-eluting stent. Journal of Zhejiang University Science B. 2010;11(8):553–560. doi: 10.1631/jzus.b1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfonso F., Pérez-Vizcayno M. J., García D. B. B., et al. Everolimus-eluting stents in patients with bare-metal and drug-eluting in-stent restenosis: results from a patient-level pooled analysis of the RIBS IV and V trials. Circulation: Cardiovascular Interventions. 2016;9 doi: 10.1161/circinterventions.115.003479. [DOI] [PubMed] [Google Scholar]
- 20.Almalla M., Pross V., Marx N., Hoffmann R. Effectiveness of everolimus-eluting stents in the treatment of drug-eluting stent versus bare-metal stent restenosis. Coronary Artery Disease. 2012;23(7):492–496. doi: 10.1097/mca.0b013e328358a58f. [DOI] [PubMed] [Google Scholar]
- 21.Whan L. C., Kim S. H., Suh J., et al. Long-term clinical outcomes after sirolimus-eluting stent implantation for treatment of restenosis within bare-metal versus drug-eluting stents. Catheterization and Cardiovascular Interventions. 2008;71:594–598. doi: 10.1002/ccd.21399. [DOI] [PubMed] [Google Scholar]
- 22.Yan R. Q., Chen J. L., Gao L. J., et al. Sirolimus-eluting stents for treatment of drug-eluting versus bare-metal stents restenosis: 42-month clinical outcomes from a Chinese single center. Chinese Medical Journal. 2012;125(19):3398–3403. [PubMed] [Google Scholar]
- 23.Nishihira K., Shibata Y., Ishikawa T., et al. Repeated sirolimus-eluting stent implantation to treat sirolimus-eluting stent and bare-metal stent restenosis. Circulation Journal. 2010;74(11):2329–2333. doi: 10.1253/circj.cj-10-0210. [DOI] [PubMed] [Google Scholar]
- 24.Abizaid A., Costa J. R., Banning A., et al. The sirolimus-eluting cypher select coronary stent for the treatment of bare-metal and drug-eluting stent restenosis. JACC: Cardiovascular Interventions. 2012;5(1):64–71. doi: 10.1016/j.jcin.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Richardt G., Leschke M., Abdel-Wahab M., et al. Clinical outcomes of the resolute zotarolimus-eluting stent in patients with in-stent restenosis. JACC: Cardiovascular Interventions. 2013;6(9):905–913. doi: 10.1016/j.jcin.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Nagoshi R., Shinke T., Otake H., et al. Qualitative and quantitative assessment of stent restenosis by optical coherence tomography. Circulation Journal. 2013;77(3):652–660. doi: 10.1253/circj.cj-12-0610. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa G., Otsuka F., Nakano M., et al. The pathology of neoatherosclerosis in human coronary implants. Journal of the American College of Cardiology. 2011;57(11):1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xhepa E., Byrne R. A., Rivero F., et al. Qualitative and quantitative neointimal characterization by optical coherence tomography in patients presenting with in-stent restenosis. Clinical Research in Cardiology. 2019;108(9):1059–1068. doi: 10.1007/s00392-019-01439-5. [DOI] [PubMed] [Google Scholar]
- 29.Kastrati A., Dibra A., Mehilli J., et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113(19):2293–2300. doi: 10.1161/circulationaha.105.601823. [DOI] [PubMed] [Google Scholar]
- 30.Rathore S., Terashima M., Katoh O., et al. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention. 2009;5(3):349–354. doi: 10.4244/v5i3a55. [DOI] [PubMed] [Google Scholar]
- 31.Goel S. S., Dilip Gajulapalli R., Athappan G., et al. Management of drug eluting stent in-stent restenosis: a systematic review and meta-analysis. Catheterization and Cardiovascular Interventions. 2016;87(6):1080–1091. doi: 10.1002/ccd.26151. [DOI] [PubMed] [Google Scholar]
- 32.Bajraktari G., Jashari H., Ibrahimi P., et al. Comparison of drug-eluting balloon versus drug-eluting stent treatment of drug-eluting stent in-stent restenosis: a meta-analysis of available evidence. International Journal of Cardiology. 2016;218:126–135. doi: 10.1016/j.ijcard.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 33.Xu B., Gao R., Wang J. A., et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis. JACC: Cardiovascular Interventions. 2014;7(2):204–211. doi: 10.1016/j.jcin.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Alfonso F., Pérez-Vizcayno M. J., Cárdenas A., et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents. Journal of the American College of Cardiology. 2015;66(1):23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 35.Alfonso F., Pérez-Vizcayno M. J., Cuesta J., et al. 3-year clinical follow-up of the RIBS IV clinical trial: a prospective randomized study of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis in coronary arteries previously treated with drug-eluting stents. JACC. Cardiovascular Interventions. 2018;11(10):981–991. doi: 10.1016/j.jcin.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 36.Wong Y. T. A., Kang D. Y., Lee J. B., et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized RESTORE trial. American Heart Journal. 2018;197:35–42. doi: 10.1016/j.ahj.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Gao L., Wang Y. B., Jing J., Zhang M., Chen Y. D. Drug-eluting balloons versus new generation drug-eluting stents for the management of in-stent restenosis: an updated meta-analysis of randomized studies. Journal of Geriatric Cardiology: JGC. 2019;16(6):448–457. doi: 10.11909/j.issn.1671-5411.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehilli J., Byrne R. A., Tiroch K., et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents. Journal of the American College of Cardiology. 2010;55(24):2710–2716. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Alfonso F., Pérez-Vizcayno M. J., Dutary J., et al. Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis: results from a prospective multicenter study (RIBS III [restenosis intra-stent: balloon angioplasty versus drug-eluting stent]) JACC: Cardiovascular Interventions. 2012;5 doi: 10.1016/j.jcin.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Song H.-G., Park D.-W., Kim Y.-H., et al. Randomized trial of optimal treatment strategies for in-stent restenosis after drug-eluting stent implantation. Journal of the American College of Cardiology. 2012;59(12):1093–1100. doi: 10.1016/j.jacc.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 41.Colleran R., Joner M., Kufner S., et al. Comparative efficacy of two paclitaxel-coated balloons with different excipient coatings in patients with coronary in-stent restenosis. International Journal of Cardiology. 2018;252:57–62. doi: 10.1016/j.ijcard.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 42.Ali R. M., Abdul Kader M. A. S. K., Wan Ahmad W. A., et al. Treatment of coronary drug-eluting stent restenosis by a sirolimus- or paclitaxel-coated balloon. JACC: Cardiovascular Interventions. 2019;12(6):558–566. doi: 10.1016/j.jcin.2018.11.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES: (A) TLR, (B) TVR, and (C) ACD; Supplement Figure 2: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES: (A) CD, (B) MI, and (C) ST/RE-ISR; Supplement Figure 3: forest plot with RR for BMS-ISR versus DES-ISR after treated by DES : MACES; Supplement Figure 4: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB: (A) TLR, (B) TVR, and (C) ACD; Supplement Figure 5: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB: (A) CD, (B) MI, and (C) ST/RE-ISR; Supplement Figure 6: forest plot with RR for BMS-ISR versus DES-ISR after treated by DEB : MACES.
Data Availability Statement
All data used to support the findings of our study are included within the article.


