Abstract
Immune mediated necrotizing myopathy (IMNM) is part of the inflammatory myopathies group of diseases and presents with muscle weakness, myalgias and elevated serum creatine phosphokinase (CPK). Statin-induced IMNM is a rare complication. We present a patient with IMNM secondary to simvastatin use. The patient presented with proximal myopathy, dysphagia, and elevated creatinine kinase levels, and was subsequently found to have anti-3- hydroxy-3-methylglutaryl-CoA reductase (HMGCR) autoantibodies with a necrotizing process on muscle biopsy. This patient’s case was further complicated by sequelae of multiple disease processes, ultimately leading to deterioration of his health.
Key words: Immune mediated necrotizing myopathy, statins, statin-induced IMNM, inflammatory myopathy
Introduction
Over the last decade immune mediated necrotizing myopathy (IMNM) has emerged as an immune mediated process, distinct from other idiopathic inflammatory myopathies, such as dermatomyositis (DM), polymyositis (PM) and inclusion body myositis (IBM). It has been characterized histologically by a prominent necrosis and myophagocytosis in the relative absence of lymphocytic infiltrates.1 More importantly, recent literature has shown an association of IMNM with statin use, particularly in anti-HMGCR positive myopathy.2 With a cited prevalence as rare as 1-2 cases per million, it is considered a very rare complication of statin use.3 Prompt and accurate diagnosis is crucial, as symptoms can persist and progress despite statin discontinuation. IMNM can occur within months of initiation of a statin, however in most reported cases patients have been on statin therapy for years before the onset of myopathy.3,4 This can further complicate the diagnosis, and highlights the need for high clinical suspicion. We report a case of anti-HMGCR positive IMNM secondary to simvastatin. The case was complicated by persistent dysphagia, which resulted in aspiration, respiratory failure and subsequently death.
Case Report
A 73-year-old man with a history of diabetes, hypertension, and hyperlipidemia presented with a 3-week history of progressive muscle weakness and dysphagia. He has been taking insulin detemir, metformin, lisinopril, and simvastatin for the last several years. On admission, he was found to have CPK of 12,000 U/L and elevated inflammatory markers, concerning for inflammatory myopathy and rhabdomyolysis. Thyroid function tests, ANA and creatinine were within normal limits. Given that the patient’s CPK level did not improve after administration of IV fluids, immune mediated necrotizing myopathy secondary to statin use was suspected.
Other workup included Anti-Jo-1, anti- Ro-52, anti-SRP, anti-Mi-2, acetylcholine receptor antibody and muscle-specific tyrosine kinase antibody, which were all negative. Subsequent evaluation revealed positive HMG-CoA reductase antibodies, with titers greater than 200 (Normal 19), consistent with IMNM associated with statin use. Muscle biopsy revealed a necrotizing process with type 2 myofiber atrophy, consistent with immune myopathy (Figure 1). He initially received Prednisone 30 mg daily for 2 days, which was later changed to IV methylprednisolone 15 mg every 12 h secondary to confusion and agitation. He was started on methotrexate 20 mg subcutaneous once a week, which resulted in improvement in his CPK level. The patient was also treated with one course of IV immunoglobulin 2 g/kg over two days. Later, methotrexate was discontinued secondary to newly elevated LFTs (AST, ALT). He received Azathioprine for 2 days, but this was discontinued due to development of infection. The patient’s hospital course was complicated by persistent dysphagia and aspiration events leading to sepsis and hypoxic respiratory failure which ultimately led to death.
Discussion
Immune mediated necrotizing myositis (IMNM) is part of the inflammatory myopathies group of diseases. Although it shares similarities with other idiopathic inflammatory myopathies (IIM) such as dermatomyositis (DM), polymyositis (PM), and inclusion body myositis (IBM), it was recognized as a distinct entity in 2003, and has been characterized by muscle cell necrosis with lack of significant lymphocytic inflammatory infiltrates.4 In 2010, novel autoantibodies with specificity to a pair of protein with weights of 200/100-kd were identified in patients with IMNM and history of statin use.5 HMGCR was subsequently identified as the 100-kd autoantigen.2 Since then, IMNM has been increasingly associated with statin use. However, it remains a very a rare complication.
Patients with statin-induced IMNM often present with progressive proximal muscle weakness and significantly elevated CPK levels.6 CPK levels are usually >5 times upper limit of normal.7 It is typically a subacute process, with myopathy developing over weeks to months. The process is typically symmetric and lacks sensory involvement. Limb weakness and myalgias are the most common features, but other reported features include truncal weakness, facial weakness, dysphagia, fatigue, and weight loss.1 Symptoms and CPK elevation typically persist, despite discontinuation of statin, and usually show improvement with immunosuppressive therapy. This usually distinguishes statin-induced IMNM from myopathy secondary to direct statin toxicity, which typically improves with statin discontinuation. 8,9
The diagnosis of statin-induced IMNM can be challenging for a multitude of reasons. For one, statin-induced IMNM shares many similarities with other myopathies, which also present with elevated CPK levels and proximal muscle weakness. Furthermore, although it can present within months of statin initiation, the large majority of patients develop symptoms years after initiating statin therapy. Hence, high clinical suspicion is required for prompt diagnosis. The gold-standard for diagnosis of IMNM is generally based on muscle biopsy.10 Muscle biopsies reveal muscle fiber necrosis and inflammatory infiltrates composed of macrophages.6 However, as with other IIM, autoantibodies are now considered a hallmark in the diagnosis of IMNM.7 Not only does this allow other IIMs to be ruled out, but it also allows for subclassification. The most recent European Neuromuscular Centre (ENMC) workshop in 2016 identified three subgroups of IMNM; anti-HMGCR myopathy; anti-signal recognition particles (SRP) myopathy, and seronegative IMNM.11 Anti-HMGCR antibody is present in about half of patients with IMNM.7 It has been proposed that anti- HMGCR positive IMNM is frequently associated with statin use. This has been confirmed by subsequent literature.2 It has also been demonstrated to have high sensitivity and specificity for statin-induced IMNM, which has been reported to be as high as 94.4% and 99.3%, respectively.12 Anti-SRP positive IMNM represents 22-39% of IMNM, but unlike anti-HMGCR myopathy, has not been shown to have an association with statin use.7 With commercially available antibody testing now more readily available, biopsy is no longer required for a diagnosis of seropositive IMNM.13 However, seronegative IMNM accounts for 25-40% of IMNM, and presents with elevated CPK, muscle weakness, and a biopsy demonstrating necrotizing myopathy despite no antibody detection. Hence, muscle biopsy remains necessary for a diagnosis of seronegative IMNM, and for this reason is still often employed as part of the diagnostic work up. The association of seronegative IMNM with statins remains poorly defined in the literature.
HMGCR is the rate limiting enzyme of cholesterol synthesis and statins act as competitive inhibitors of HMGCR. The ensuing reduction in hepatic intracellular LDL results in an increase in LDL receptor expression and receptor mediated endocytosis, ultimately lowering serum LDL levels.14 However, the exact pathogenesis of statin induced anti-HMGCR myopathy remains poorly understood and is an active area of investigation. It is thought that the muscle damage in statin-induced IMNM is mediated by autoantibodies against HMGCR.6 It has been demonstrated that muscle expression of HMGCR is increased with statin exposure.2,15 Although largely a subcellular protein localized to the endoplastic reticulum, studies have proposed that its expression is also increased in regenerating muscle fibers and may be present on cell surfaces. 16 Therefore, the continued muscle expression of HMGCR may sustain the immune-mediated muscle necrosis caused by anti-HMGCR autoantibodies associated with statin therapy even after its discontinuation. 17 Figure 2 illustrates this proposed mechanism.
Treatment of statin-induced IMNM requires prompt discontinuation of statin therapy and aggressive treatment with immunosuppressive agents. However, there is a lack of prospective studies and clinical trials in the literature to guide therapy. Following the recent 2016 ENMC workshop, high dose prednisone/prednisolone or pulsed IV methylprednisolone remains the recommended first-line treatment. A second agent can be added, and either azathioprine 3 mg/kg/actual body weight or methotrexate 20-25 mg per week orally or subcutaneously can be used.11 Although considered third-line therapy, the 2016 workshop also suggested rituximab as a possible alternative, and this can be combined with methotrexate in severe cases. In cases of anti-HMGCR positive myopathy, as in our case, IVIG should be considered, particularly in patients with severe disease or refractory disease at 6 months. IVIG has also been reported to be used as first-line therapy in half of reported cases.11 Little literature guides IVIG therapy in this setting, but IVIG 2g/kg/m, 3-6 times has been suggested as an induction dose.18 Cycles of every 2 weeks to 4 weeks have been reported during maintenance therapy, and can be stopped and tapered as tolerated.19
Figure 1.

In this cryostat section, a pale necrotic myofiber is seen in the center of the field (hematoxylin & eosin, 400x).
Figure 2.
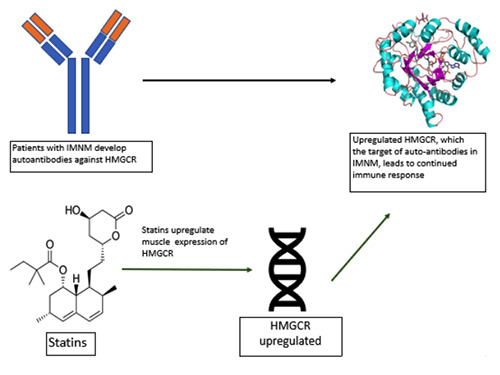
Proposed mechanism of immune mediated muscle injury in statin-induced immune mediated necrotizing myopathy (IMNM). HMGCR, hydroxy-3-methylglutaryl- CoA reductase.
Treatment with disease modifying agents should only be discontinued after 2-3 years of well-controlled disease and minimal corticosteroid use.1 CPK has been shown to correlate with disease activity, and hence treatment to normal range has been suggested as a target. Increases in CPK should warrant consideration of increased disease activity.
The prognosis of statin-induced IMNM is generally good. Although long term immunotherapy is usually required, symptom resolution is reported in most cases.6 The most commonly reported complication is persistent muscle weakness, which can be present even two years after onset. We report a case of anti-HMGCR positive statin-induced IMNM resulting in death secondary to associated dysphagia. Dysphagia has only been reported in 16-30% of statin-induced IMNM.16 It is not readily recognized as a characteristic symptom of the disease. However, in our case, despite down-trending CK values with immunotherapy (Figure 3), persistent dysphagia led to aspiration, and subsequent respiratory failure and death. A review of recent literature yielded only two reported cases with death as a complication of statininduced IMNM.13 Of these two cases, one described dysphagia as a presenting symptom. Interestingly, this case was also complicated by respiratory distress requiring ventilator support, and ultimately death from ventilator-acquired pneumonia.20 Furthermore, at least three of four recently published cases of statin-induced IMNM and associated dysphagia also reported the need for nutritional support with gastrostomy tube.20 Hence, although a less commonly associated symptom, dysphagia may result in increased risk of morbidity and mortality in patients with IMNM. Our case further highlights the need for prospective studies to better guide therapy in patients presenting with statin-induced IMNM. It further expands on the increasing, but still sparse literature on statin-induced IMNM, and emphasizes that complications can prove fatal.
It is also important to recognize the challenge that statin-induced IMNM can pose in patients with high cardiovascular risk. In a recently published review of 92 cases of statin-induced IMNM, atorvastatin was reported in 80% of cases, and simvastatin in 24%.13 Other statins also reported included rosuvastatin (17%) and pravastatin (3%). These are among the most commonly used statins in patients at risk for cardiovascular disease. Despite the increase in prevalence of statin-induced IMNM over the last decade, there is a lack of literature to guide management of patients with a history of statin induced IMNM who are also at high risk for cardiovascular disease. Statin rechallenge with the same or a different statin has shown to be largely unsuccessful and often results in disease flare up.19,6 A recent case series at Johns Hopkins University found that PCSK9 inhibitors were well tolerated in 7 out of 8 patients with a previous history of statin-induced IMNM.19 As a result PCSK9 may be an alternative medication for patients with high cardiovascular risk and IMNM. However, data is limited, thus highlighting an area requiring further research.
Conclusions
Statins are widely used and lower the risk of death from cardiovascular causes. Statin-induced myalgias and myopathies improve with withdrawal of statin therapy and supportive care. By contrast, statininduced IMNM is a potentially fatal autoimmune disease that follows an aggressive clinical course and often presents with progressive muscle weakness, elevated CPK levels, myonecrosis, and anti-HMGCoA reductase antibodies. Treatment includes discontinuation of the statin and initiation of immunosuppressive agents. Clinicians should be aware of this devastating complication of statin use, especially in patients recently discontinued off statins with persistently elevated CPK levels, as early detection is vital and allows for prompt initiation of treatment.
Figure 3.

Down trending creatine phosphokinase (CPK) levels with immunotherapy.
Key points
Statin-induced immune mediated necrotizing myopathy (IMNM) is a potentially fatal autoimmune disease. Recognized cases generally follow an aggressive clinical course if not quickly recognized. This case report provides an overview of statin-induced IMNM, including its common presentations and appropriate treatment options:
i) Statins are widely used agents that lower the risk of death from cardiovascular disease but their usage can present with adverse effects including myalgias and myopathies that improve with withdrawal of statin therapy and with supportive care.
ii) Statin-induced IMNM is an autoimmune disease with an aggressive clinical course, characterized by progressive muscle weakness, elevated CPK levels, myonecrosis on muscle biopsy, and anti-HMG-CoA reductase antibodies.
iii) Treatment of IMNM includes discontinuation of statin, immunosuppressive agents to prevent permanent damage, and often results in complete recovery.
References
- 1.Day JA, Limaye V. Immune-mediated necrotising myopathy: A critical review of current concepts. Semin Arthritis Rheum 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3- hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohassel P, Mammen AL. Statin-associated autoimmune myopathy and anti- HMGCR autoantibodies. Muscle Nerve 2013;48:477-83. [DOI] [PubMed] [Google Scholar]
- 4.Grable-Esposito P, Katzberg HD, Greenberg SA, et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010;41:185-90. [DOI] [PubMed] [Google Scholar]
- 5.Christopher-Stine L, Casciola-Rosen LA, Hong G, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immunemediated necrotizing myopathy. Arthritis Rheum 2010;62:2757-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazir S, Lohani S, Tachamo N, et al. Statin-associated autoimmune myopathy: a systematic review of 100 cases. J Clin Rheumatol 2017;23:149-54. [DOI] [PubMed] [Google Scholar]
- 7.Musset L, Allenbach Y, Benveniste O, et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: A history of statins and experience from a large international multi-center study. Autoimmun Rev 2016;15:983-93. [DOI] [PubMed] [Google Scholar]
- 8.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532-61. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;372:1734-47. [DOI] [PubMed] [Google Scholar]
- 10.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004;14:337-45. [DOI] [PubMed] [Google Scholar]
- 11.Allenbach Y, Mammen AL, Benveniste O, Stenzel W. 224th ENMC International Workshop:: Clinico-seropathological classification of immunemediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord 2018;28:87-99. [DOI] [PubMed] [Google Scholar]
- 12.Shovman O, Gilburd B, Chayat C, et al. Anti-HMGCR antibodies demonstrate high diagnostic value in the diagnosis of immune-mediated necrotizing myopathy following statin exposure. Immunol Res 2017;65:276-81. [DOI] [PubMed] [Google Scholar]
- 13.Essers D, Schäublin M, Kullak-ublick GA, Weiler S. Statin-associated immune-mediated necrotizing myopathy: a retrospective analysis of individual case safety reports from VigiBase. Eur J Clin Pharmacol 2019;75:409-16. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan CJ, Gotto AM, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 2000;35:1-10. [DOI] [PubMed] [Google Scholar]
- 15.Morikawa S, Murakami T, Yamazaki H, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J Atheroscler Thromb 2005;12:121-31. [DOI] [PubMed] [Google Scholar]
- 16.Mohassel P, Mammen AL. Anti- HMGCR Myopathy. J Neuromuscul Dis 2018;5:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selva-O'callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol 2018;14:215-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep 2018;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/kexin type 9 inhibitors in statin-associated immunemediated necrotizing myopathy: a case series. Arthritis Rheumatol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Ajiboye O, Manesh M, Asmi N, Mba B. Atypical presentation of necrotising autoimmune myopathy. BMJ Case Rep 2019;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


