Abstract
Objective:
Lumbar punctures (LPs) are important for obtaining CSF in neurology studies but are associated with adverse events and feared by many patients. We determined adverse event rates and pain scores in patients prospectively enrolled in two cohort studies who underwent LPs using a standardized protocol and 25g needle.
Methods:
809 LPs performed in 262 patients age ≥60 years in the MADCO-PC and INTUIT studies were analyzed. Medical records were monitored for LP-related adverse events, and patients were queried about subjective complaints. We analyzed adverse event rates, including headaches and pain scores.
Results:
There were 22 adverse events among 809 LPs performed, a rate of 2.72% (95% CI 1.71% – 4.09%). Patient hospital stay did not increase due to adverse events. Four patients (0.49%) developed a post-lumbar puncture headache (PLPH). Twelve patients (1.48%) developed nausea, vasovagal responses, or headaches that did not meet PLPH criteria. Six patients (0.74%) reported lower back pain at the LP site not associated with muscular weakness or paresthesia. The median pain score was 1 [0, 3]; the mode was 0 out of 10.
Conclusions:
The LP protocol described herein may reduce adverse event rates and improve patient comfort in future studies.
Keywords: lumbar puncture, protocol, pain, headache
INTRODUCTION
Lumbar punctures (LPs) can result in complications, such as back pain, headache, and post-lumbar puncture headache (PLPH) [1], as well as more serious complications, including infection and subarachnoid bleeding. Post-LP adverse event rates in memory clinics are ~31%; including lower back pain (17%), headache (19%), and PLPH (9%) [1]. High PLPH rates (17.5%) have also been reported in studies involving patients with neurologic diseases [2]. PLPH risk factors include previous headache history and the use of cutting type or larger bore spinal needles [1,3,4]. Additionally, procedural confidence may affect PLPH rates, since this may be a proxy for accessing the intrathecal space with fewer needle passes [5]. Minimizing PLPHs and patient discomfort are important, since many patients have concerns regarding LP safety that can decrease willingness to participate in research [6].
Here, we describe adverse event rates and pain scores in study patients who underwent LPs according to a standardized protocol. We hypothesized that adherence to this LP protocol would reduce adverse events and minimize pain.
METHODS
Patient Population
Lumbar punctures included in this analysis were performed on patients enrolled in the MADCO-PC study [7] and the first 100 patients enrolled in the INTUIT [8] study at Duke University Medical Center (Durham, NC). In the recently completed MADCO-PC study, surgical patients underwent lumbar punctures pre-operatively, 24 hours, six weeks, and 1-year post-operatively, and non-surgical controls underwent lumbar punctures at the same time intervals. The primary aim of the MADCO-PC study was to study the relationship between perioperative changes in cognition and CSF tau levels. In the ongoing INTUIT study, surgical patients underwent lumbar punctures at the same time points described above; the primary aim of the INTUIT study is to study the relationship between perioperative changes in cognition and neuroinflammation (as measured by CSF MCP-1 levels and the CSF monocyte/lymphocyte ratio). Both studies were approved by the Duke IRB (Pro00083288, Pro00045180) and registered with clinicaltrials.gov (NCT01993836, NCT03273335). Patients were eligible for both studies if they were age ≥60 years old, English speaking, and undergoing elective non-cardiac, non-neurologic surgery scheduled for ≥2 hours. Patients were excluded if they were on anticoagulants or chemotherapy [9]. In both studies, patients underwent LPs before surgery, and at 24 hours, 6 weeks, and 1 year after surgery.
Lumbar Puncture Protocol
Patients were seated upright and leaning forward or placed in the lateral decubitus position if unable to tolerate this position. We performed LPs at L4–L5 or one interspace above or below, based on anatomic landmarks. In anatomically complicated patients, a 60mm, 5–2 MHZ curvilinear ultrasound probe was used to visualize the dura and spinal anatomy (BK Medical; Peabody, MA) [10,11], and to determine best LP site and angle/trajectory. The lower back was sprayed with 20% benzocaine (available over the counter) and we waited 10-minutes for it to anesthetize the skin.
Next, an attending anesthesiologist (or a resident/fellow under attending supervision) performed the LP with strict sterile technique using a standard Portex spinal anesthesia kit (Smiths Medical; Dublin, OH). Up to 5 cc of 1% lidocaine was injected to create a skin wheal over the desired interspace and to anesthetize the anticipated needle tract. Occasionally, anatomic complications such as osteophytes or spinal stenosis would make the LP difficult to perform at a given level, which case another level was injected with 1% lidocaine again. We waited 2 full minutes for the lidocaine to take effect. Then, a 20-gauge, 1.25-inch introducer was inserted, into which a pencil-point 25-gauge spinal needle was inserted to perform the LP. After CSF flow was confirmed, CSF was gently aspirated using a 10-mL Luer-Lock polypropylene syringe (Becton & Dickinson, Franklin Lakes, NJ) [12]. Sterile gauze was then taped over the site and the patient was instructed to lie flat for 30 minutes.
In the first four MADCO-PC patients, a 25-gauge spinal catheter (Wiley Spinal; Johnstown, NY) was used to obtain CSF at the preoperative and 24 hr postoperative time points, but we had difficulty obtaining CSF through these 25-gauge lumbar catheters and thus switched to the method described above for remaining LPs. In less than 10 subsequent LPs, there was difficulty accessing the dura with a 25-gauge pencil-point needle (Smith’s Medical, Dublin, OH) typically due to anatomic abnormalities, in which case a 22-gauge Quinke needle was used [13].
Data Reporting
We monitored the electronic health record of all enrolled patients for adverse events related to the LP. Additionally, all patients were asked about subjective complaints after each LP and while they were on-site for their surgery and/or study visits. Each patient was given the study PI’s contact information (including cell phone number) and instructed (both verbally and in writing) to call at any time if they experienced headaches, nausea, fever, back pain, neck stiffness, or other symptoms of concern. Starting on March 21, 2018, pain scores (from 0 to 10) during the LP were recorded in INTUIT study patients.
Statistics
Study group characteristics were summarized using mean (SD) or median [Q1, Q3] for numeric variables and count (%) for categorical variables. Adverse event incidence was summarized with 95% Clopper-Pearson exact confidence intervals. A Kruskal-Wallis test was used to compare LP-associated pain scores by study visit/time point, since these pain scores were non-normally distributed.
RESULTS
Baseline patient demographics are listed in Table 1. A total of 809 LPs were performed: 553 LPs on 176 MADCO-PC patients and 256 LPs on 86 INTUIT patients. The maximum CSF volume collected was 13.5mL, with a median and mode of 10mL. Supplemental video 1 shows our lumbar puncture procedure. Supplemental Figure 2 shows an ultrasound image showing spine anatomy to guide needle placement during LP.
Table 1.
Summary of baseline patient characteristics for patients in the MADCO-PC and INTUIT studies. These data reflect 191 patients originally enrolled in the MADCO-PC study (of whom 15 refused LPs or dropped out of the study), and the first 100 patients enrolled in the INTUIT study (of whom 14 refused LPs or dropped out of the study). MMSE, mini mental status examination.
| Variable | MADCO-PC (n=176) | INTUIT (n=86) | Total (n=262) |
|---|---|---|---|
| Age, median [Q1, Q3] | 68 [64, 73] | 68 [64, 72] | 68 [64, 73] |
| Race, n (%) | |||
| Caucasian/White | 148 (84.1) | 72 (83.7) | 220 (84.0) |
| Black or African American | 27 (15.3) | 12 (14.0) | 39 (14.9) |
| Asian | 1 (0.6) | 2 (2.3) | 3 (1.1) |
| Gender (Female), n (%) | 71 (40.3) | 48 (55.8) | 119 (45.4) |
| Years of Education, median [Q1, Q3] | 16 [13, 18] | 16 [13, 17] | 16 [13, 18] |
| MMSE total Score, median [Q1, Q3] | 29 [28, 29] | 28 [27, 29] | 29 [27, 29] |
| MMSE Category, n (%) | |||
| <20 | 1 (0.6) | 1 (1.2) | 2 (0.8) |
| 20–24 | 9 (5.1) | 6 (7.0) | 15 (5.7) |
| 25–30 | 166 (94.3) | 79 (91.9) | 245 (93.5) |
There were 22 adverse events (Table 2), for a post-LP complication rate of 2.72% (95% CI 1.71% – 4.09%). There were no infections or subarachnoid hemorrhages, and no patients had their postoperative hospital stay extended (in days) due to post-LP complications. Four patients (0.49%) developed a PLPH, of which three resolved with conservative treatment (bed rest, increased fluid and caffeine intake) [14]. One patient with a PLPH required a blood patch (0.12%), after which the PLPH resolved. Importantly, all four patients returned for subsequent LPs in the study and none had a repeat PLPH. Twelve patients (1.48%) developed nausea, a vasovagal response, or a headache that did not meet PLPH criteria. If the same patient experienced more than one of the symptoms in this subgroup after a single LP (e.g. nausea and vasovagal response) this was counted as a single adverse event. No same patient reported an AE after more than one of the four LPs at different time points. Six patients (0.74%) reported lower back pain at the LP site not associated with muscular weakness, radiating pain or paresthesia(s).
Table 2.
Summary of adverse events following lumbar punctures in the MADCO-PC and INTUIT studies. Percentages reflect the number of adverse events over the total numbers of LPs performed in each study. PLPH, post-lumbar puncture headache.
| Adverse Events | MADCO-PC (n=553) | INTUIT (n=256) | Total (n=809) |
|---|---|---|---|
| Leptomeningeal infection | 0 | 0 | 0 |
| Subarachnoid hemorrhage | 0 | 0 | 0 |
| PLPH requiring blood patch | 1 | 0 | 1 |
| PLPH not requiring a blood patch | 1 | 2 | 3 |
| Non-PLPH headache, nausea, vagal responses | 6 | 6 | 12 |
| Lower back pain | 4 | 2 | 6 |
| Total Adverse Events (N) | 12 | 10 | 22 |
| Percentage | 2.17% | 3.91% | 2.72% |
| Event Rate (95% CI) | (1.13, 3.76) | (1.89, 7.07) | (1.71, 4.09) |
Pain during the procedure was rated ≤2 out of 10 during 125 of 165 LPs (73.3%; Figure 1). The median pain score was 1 out of 10 [0, 3] and the mode was 0 out of 10. There was no difference in LP-associated pain scores by pre-operative, 24 hours, six weeks, and 1-year post-operative time point (p=0.44) (Supplemental Figure X).
Figure 1.
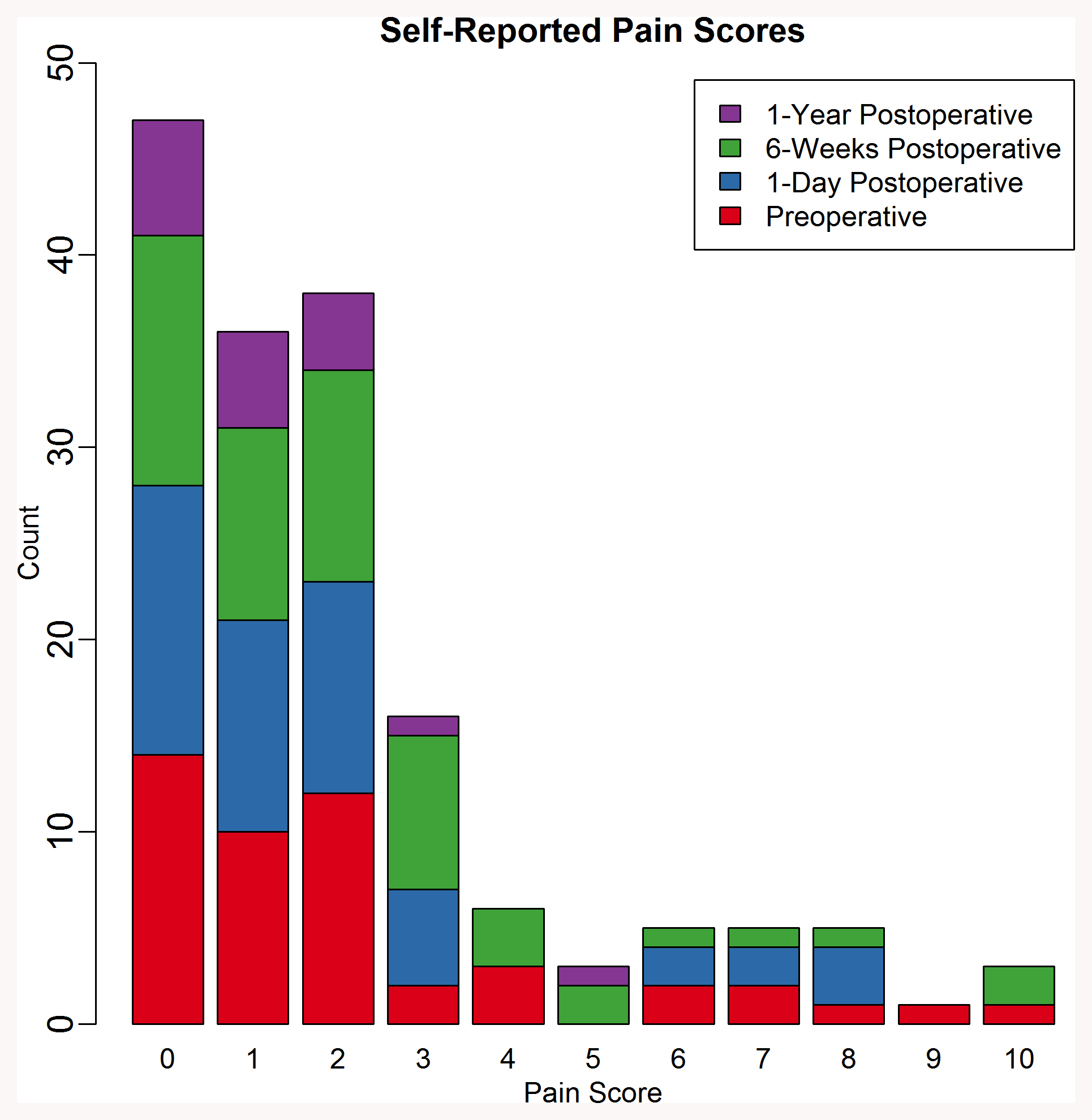
Distribution of self-reported pain scores of INTUIT patients following LP. Stacked bar chart demarcates if the LP was performed at the pre-operative, post-operative, 6-week follow up, or 1 year follow up time points.
Discussion
In >800 LPs performed in two prospective cohort studies, we report a lower post-LP adverse event rate (2.72%) than seen in prior studies with similar cohorts [1,2], and a lower PLPH rate (0.49%) than the 6–36% rates previously reported [15]. In comparing our study to other published papers, one major difference is standardization of the lumbar puncture needle. For instance, in the Monserrate et al study, which had a PLPH rate of 4.4%, they stated that an atraumatic 22G or 24G needle was part of the protocol, but non-standard needles were also used (20G, 23G, 25G, 26G). The use of non-standard needles in their study was associated with a significant risk for PLPH. Furthermore, their study found a 3.2% PLPH rate with a 22G needle. In comparison, our study used a spinal anesthesia kit with a 25G atraumatic needle and had a 0.62% PLPH rate.
These data support the safety and tolerability of the LP protocol described here, including standardized 25G atraumatic needle, topical benzocaine spray usage, timed waiting periods following topical analgesia, and ultrasound guidance when needed. Ultrasound usage has been correlated with decreased procedural pain [16–18] and topical benzocaine application has been shown to decrease pain of needle insertion [19]. Although the addition of benzocaine and ultrasound has not yet been studied for LPs, we believe that these can contribute to increased patient comfort.
One limitation of this study was the use of patient self-report and electronic health record monitoring to detect post-LP adverse events versus 24-hour follow up phone calls [20]. Nonetheless, we observed a lower PLPH rate (i.e. 0.49% vs 5.7–13.9%) than seen in other studies that similarly used patient self-report and EHR monitoring to define adverse events [15,21]. Here, all patients were given the study PI’s contact information and told to call anytime if they experienced headaches or any other symptoms of concern.
We are confident that there were no serious infections such as leptomeningitis, though we cannot rule out minor self-limited infections that may have gone undetected. The data reported here are from older adults (median age of 68 years) and PLPH rates decline with age [13]. However, the adverse event rate reported here is still 5-fold lower than in other studies in this age group [2].
Since the standardized LP protocol described here resulted in low adverse event rates and pain scores, it may be useful for reducing post-LP complications and improving patient comfort in future studies.
Supplementary Material
Supplemental Figure 1. Video showing the lumbar puncture procedure.
Supplemental Figure 3. Plot of pain scores compared by pre-operative (BL), 24-hour, 6-week, and 1-year post-operative LPs. Means are plotted with Q1, Q3 and SD. Non-parametric Kruskal-Wallis test was used to compare scores over time. There was no evidence of a difference in score by visit (p=0.44).
Supplemental Figure 2. Ultrasound image showing spine anatomy to guide needle placement during LP. AP: articular process L: lamina TP: transverse process AC: anterior complex (anterior dura and posterior longitudinal ligament of vertebrae) Red arrowheads: posterior complex (posterior dura and ligamentum flavum) Dotted line: distance from skin to posterior complex (in this case, about 3.2 cm) Solid line: Anteroposterior diameter of the spinal canal
Research Support
This work was supported by National Institutes of Health (Bethesda, Maryland) T32 grant No. GM08600 (to MB), an International Anesthesia Research Society (IARS; San Francisco, California) Mentored Research Award (to MB), National Institutes of Health R03 AG050918 (to MB), National Institutes of Health Beeson K76 AG057022 (to MB), a Jahnigen Scholars Fellowship award from the American Geriatrics Society (New York, New York) and the Foundation for Anesthesia Education and Research (to MB), additional support from National Institutes of Health P30AG028716, and Duke Anesthesiology departmental funds.
Footnotes
Ethical Standards
These studies were conducted with approval from the Duke IRB (Pro00083288, Pro00045180) and are registered with clinicaltrials.gov (NCT01993836, NCT03273335). All persons gave their informed consent prior to their inclusion in the study.
Declaration of Conflict of Interest
MB acknowledges income from a legal consulting cases related to postoperative cognition in older adults, and material support from Massimo for a study unrelated to the data presented here. MB has also taken part in a peer-to-peer consulting session for Massimo, for which his honorarium was donated (at his request) to the Foundation for Anesthesia Education & Research. The other authors declare that they have no conflict of interest.
REFERENCES
- 1.Duits FH, Martinez-Lage P, Paquet C, Engelborghs S, Lleo A, Hausner L, Molinuevo JL, Stomrud E, Farotti L, Ramakers I, Tsolaki M, Skarsgard C, Astrand R, Wallin A, Vyhnalek M, Holmber-Clausen M, Forlenza OV, Ghezzi L, Ingelsson M, Hoff EI, Roks G, de Mendonca A, Papma JM, Izagirre A, Taga M, Struyfs H, Alcolea DA, Frolich L, Balasa M, Minthon L, Twisk JWR, Persson S, Zetterberg H, van der Flier WM, Teunissen CE, Scheltens P, Blennow K (2016) Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 12 (2):154–163. doi: 10.1016/j.jalz.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Monserrate AE, Ryman DC, Ma S, Xiong C, Noble JM, Ringman JM, Morris JC, Danek A, Muller-Sarnowski F, Clifford DB, McDade EM, Brooks WS, Darby DG, Masters CL, Weston PS, Farlow MR, Graff-Radford NR, Salloway SP, Fagan AM, Oliver A, Bateman RJ (2015) Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA neurology 72 (3):325–332. doi: 10.1001/jamaneurol.2014.3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oosterhout WP, van der Plas AA, van Zwet EW, Zielman R, Ferrari MD, Terwindt GM (2013) Postdural puncture headache in migraineurs and nonheadache subjects: a prospective study. Neurology 80 (10):941–948. doi: 10.1212/WNL.0b013e3182840bf6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidoni ED, Morris JK, Raider K, Burns JM, Alzheimer’s Disease Neuroimaging I (2014) Reducing post-lumbar puncture headaches with small bore atraumatic needles. J Clin Neurosci 21 (3):536–537. doi: 10.1016/j.jocn.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connick RM, Connick P, Klotsas AE, Tsagkaraki PA, Gkrania-Klotsas E (2009) Procedural confidence in hospital based practitioners: implications for the training and practice of doctors at all grades. In: BMC Med Educ, vol 9 p 2. doi: 10.1186/1472-6920-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell JC, Parker MW, Watts KD, Kollhoff A, Tsvetkova DZ, Hu WT (2016) Research Lumbar Punctures among African Americans and Caucasians: Perception Predicts Experience. Front Aging Neurosci 8:296. doi: 10.3389/fnagi.2016.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giattino CM, Gardner JE, Sbahi FM, Roberts KC, Cooter M, Moretti E, Browndyke JN, Mathew JP, Woldorff MG, Berger M, Investigators M-P (2017) Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front Syst Neurosci 11:24. doi: 10.3389/fnsys.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger M, Oyeyemi D, Olurinde MO, Whitson HE, Weinhold KJ, Woldorff MG, Lipsitz LA, Moretti E, Giattino CM, Roberts KC, Zhou J, Bunning T, Ferrandino M, Scheri RP, Cooter M, Chan C, Cabeza R, Browndyke JN, Murdoch DM, Devinney MJ, Shaw LM, Cohen HJ, Mathew JP (2019) The INTUIT Study: Investigating Neuroinflammation Underlying Postoperative Cognitive Dysfunction. J Am Geriatr Soc 67 (4):794–798. doi: 10.1111/jgs.15770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, Brown DL, Heit JA, Mulroy MF, Rosenquist RW, Tryba M, Yuan CS (2010) Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Regional anesthesia and pain medicine 35 (1):64–101 [DOI] [PubMed] [Google Scholar]
- 10.Shaikh F, Brzezinski J, Alexander S, Arzola C, Carvalho JC, Beyene J, Sung L (2013) Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ (Clinical research ed) 346:f1720. doi: 10.1136/bmj.f1720 [DOI] [PubMed] [Google Scholar]
- 11.Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M (2017) Does Preprocedural Ultrasound Increase the First-Pass Success Rate of Epidural Catheterization Before Cesarean Delivery? A Randomized Controlled Trial. Anesthesia and analgesia 124 (3):851–856. doi: 10.1213/ane.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 12.Rembach A, Evered LA, Li QX, Nash T, Vidaurre L, Fowler CJ, Pertile KK, Rumble RL, Trounson BO, Maher S, Mooney F, Farrow M, Taddei K, Rainey-Smith S, Laws SM, Macaulay SL, Wilson W, Darby DG, Martins RN, Ames D, Collins S, Silbert B, Masters CL, Doecke JD (2015) Alzheimer’s disease cerebrospinal fluid biomarkers are not influenced by gravity drip or aspiration extraction methodology. Alzheimer’s research & therapy 7 (1):71. doi: 10.1186/s13195-015-0157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbari A, Alijanpour E, Mir M, Bani hashem N, Rabiea SM, Rupani MA (2013) Post spinal puncture headache, an old problem and new concepts: review of articles about predisposing factors. In: Caspian J Intern Med, vol 4 vol 1 pp 595–602 [PMC free article] [PubMed] [Google Scholar]
- 14.Hatfield MK, Handrich SJ, Willis JA, Beres RA, Zaleski GX (2008) Blood patch rates after lumbar puncture with Whitacre versus Quincke 22- and 20-gauge spinal needles. AJR Am J Roentgenol 190 (6):1686–1689. doi: 10.2214/AJR.07.3351 [DOI] [PubMed] [Google Scholar]
- 15.Barreras P, Benavides DR, Barreras JF, Pardo CA, Jani A, Faigle R, Bahouth MN (2017) A dedicated lumbar puncture clinic: performance and short-term patient outcomes. J Neurol 264 (10):2075–2080. doi: 10.1007/s00415-017-8597-6 [DOI] [PubMed] [Google Scholar]
- 16.Fredrickson MJ, Ball CM, Dalgleish AJ, Stewart AW, Short TG (2009) A prospective randomized comparison of ultrasound and neurostimulation as needle end points for interscalene catheter placement. Anesth Analg 108 (5):1695–1700. doi: 10.1213/ane.0b013e31819c29b8 [DOI] [PubMed] [Google Scholar]
- 17.Costantino TG, Parikh AK, Satz WA, Fojtik JP (2005) Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med 46 (5):456–461. doi: 10.1016/j.annemergmed.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 18.Grau T, Leipold RW, Conradi R, Martin E (2001) Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand 45 (6):766–771. doi: 10.1034/j.1399-6576.2001.045006766.x [DOI] [PubMed] [Google Scholar]
- 19.Rehman N, Qazi SR (2019) Efficacy of Topical Benzocaine in Maxilla: A Randomized Controlled Trial. Anesth Prog 66 (1):24–29. doi: 10.2344/anpr-66-01-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelborghs S, Niemantsverdriet E, Struyfs H, Blennow K, Brouns R, Comabella M, Dujmovic I, van der Flier W, Frölich L, Galimberti D, Gnanapavan S, Hemmer B, Hoff E, Hort J, Iacobaeus E, Ingelsson M, Jan de Jong F, Jonsson M, Khalil M, Kuhle J, Lleó A, de Mendonça A, Molinuevo JL, Nagels G, Paquet C, Parnetti L, Roks G, Rosa-Neto P, Scheltens P, Skårsgard C, Stomrud E, Tumani H, Visser PJ, Wallin A, Winblad B, Zetterberg H, Duits F, Teunissen CE (2017) Consensus guidelines for lumbar puncture in patients with neurological diseases. In: Alzheimers Dement (Amst), vol 8 pp 111–126. doi: 10.1016/j.dadm.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dakka Y, Warra N, Albadareen RJ, Jankowski M, Silver B (2011) Headache rate and cost of care following lumbar puncture at a single tertiary care hospital. Neurology 77 (1):71–74. doi: 10.1212/WNL.0b013e318220abc0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Video showing the lumbar puncture procedure.
Supplemental Figure 3. Plot of pain scores compared by pre-operative (BL), 24-hour, 6-week, and 1-year post-operative LPs. Means are plotted with Q1, Q3 and SD. Non-parametric Kruskal-Wallis test was used to compare scores over time. There was no evidence of a difference in score by visit (p=0.44).
Supplemental Figure 2. Ultrasound image showing spine anatomy to guide needle placement during LP. AP: articular process L: lamina TP: transverse process AC: anterior complex (anterior dura and posterior longitudinal ligament of vertebrae) Red arrowheads: posterior complex (posterior dura and ligamentum flavum) Dotted line: distance from skin to posterior complex (in this case, about 3.2 cm) Solid line: Anteroposterior diameter of the spinal canal


